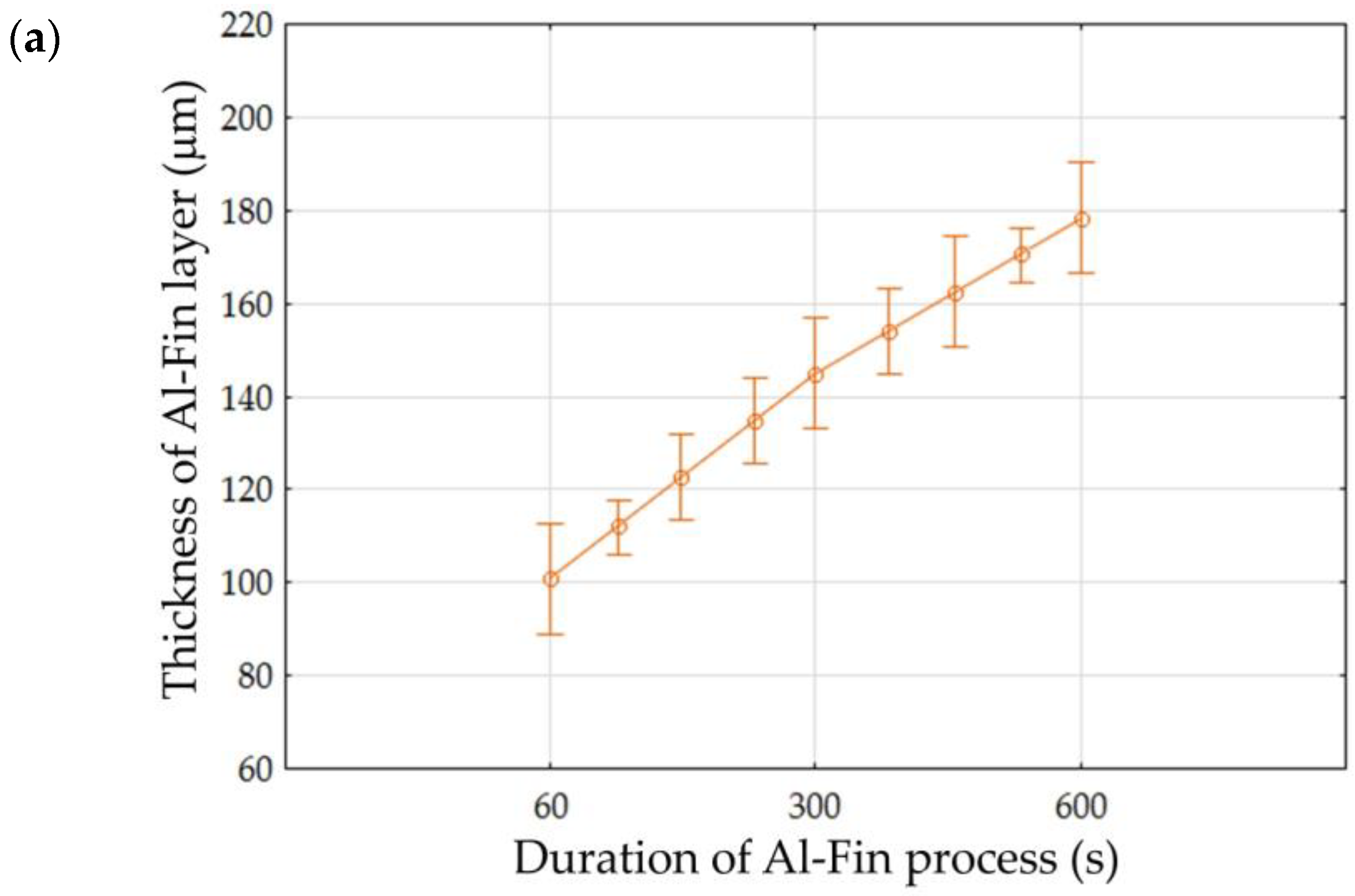
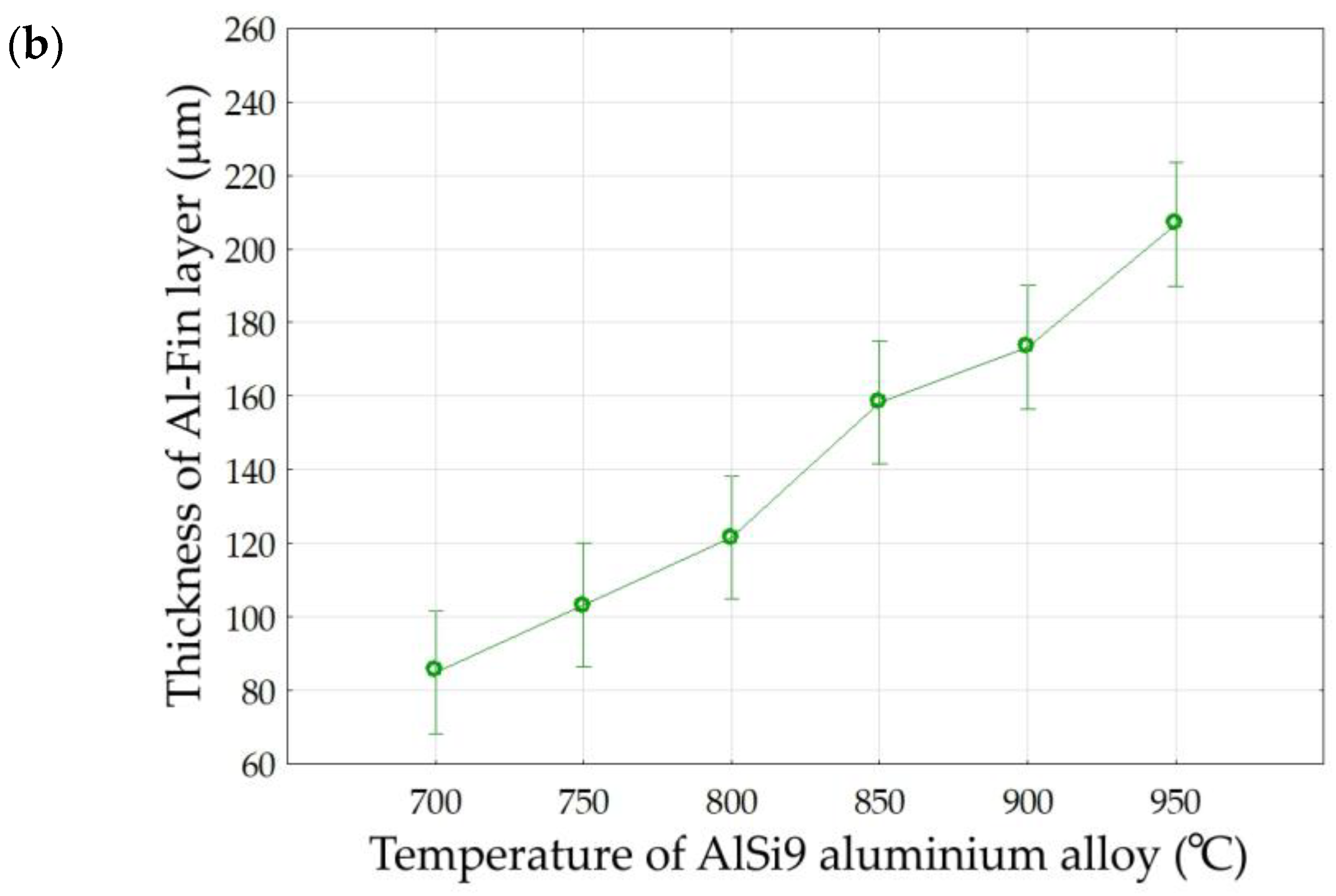
Abstract
The aim of this work was to determine the effect of the duration and temperature of the Al-Fin process for casting the bimetallic joint on the formation of the metallurgical bond between the AlSi9 aluminium alloy piston and the cast iron insert. Knowledge about the formation of individual bonding layers has a direct impact on the bonding strength between the aluminium alloy and the EN-GJLA-XNiCuCr15-6-2 austenitic cast iron ring. In the performed tests, the Al-Fin casting process was carried out for different temperatures of the liquid AlSi9 alloy. The temperature of the AlSi9 aluminium alloy varied in the range 700–950 °C in steps of 50 °C. The duration of the Al-Fin process ranged from 1 to 10 min. An optical microscope and a scanning electron microscope were used to study the microstructure of the bimetallic joints. The analysis of the microstructure of the bimetallic bond showed that characteristic layers are formed between the aluminium alloy piston and the cast iron insert: a transition layer, in which iron and aluminium atoms from both joined materials are mixed, and a diffusion layer, in which aluminium and silicon atoms penetrate the surface layer of the joined metals. The thickness of the intermetallic diffusion layer formed between the aluminium alloy and the cast iron insert is thinner and does not depend on the heating time of the aluminium insert in the bath. However, there is a significant effect of both the annealing time and the temperature of the AlSi9 aluminium alloy on the thickness of the transition zone.
1. Introduction
Castings that combine the properties of two metallic materials are called bimetallic castings [1], and their use seems to be an ideal solution wherever the materials’ strength requirements limit their use alone. For example, if high material hardness is required while maintaining low weight and good thermal conductivity, the solution is a bimetallic material consisting of a combination of steel and an aluminium alloy [2]. The bimetallic casting process is a technology in which two metals form a permanent intermetallic bond. Thanks to relatively low production costs, this method has found wide application in the production of bimetallic materials [3,4,5,6], consisting of material pairs such as aluminium and copper, aluminium and magnesium and aluminium and cast iron [7,8,9,10]. With the current development of the efficiency of high-power internal combustion engines, the pistons produced and used in them have reinforcements in the form of cast iron inserts. Through this solution, the piston combines high strength, good creep resistance and wear resistance. In addition, the use of a bimetallic joint improves the piston’s resistance to long-term exposure to high pressure and high temperatures inside the combustion chamber of the engine. The most important aspect of the production (casting) of such a piston is that the cast iron material from which the insert is made is properly bonded with the piston made of an aluminium alloy.
There is a special procedure for casting such a joint called the Al-Fin casting process [11,12]. This is a special casting technique which allows the formation of a metallurgical bond at the insert/alloy interface [13]. The Al-Fin casting process is used to prepare the ferrous surface before casting aluminium alloy pistons [14]. The production of pistons made with bimetallic technology is carried out using the commonly used technology of joining iron alloys and aluminium alloys. From an environmental perspective, aluminium is only good if recycled. Otherwise, the claimed advantages of aluminium for this type of component are, more or less, ruined. Bimetallic castings can thus be a problem for the aluminium industry in the long run. Separation of aluminium pistons and cast iron inserts is easy due to the difference in the melting point of the insert and piston materials.
The Al-Fin bonding process is used to bond a nonferrous aluminium alloy and Ni-resist ring carriers [15]. An important stage in the joining of two different metals in the casting process is the preparation of the surface of the cast iron inserts through their proper mechanical treatment (i.e., shot peening and sand blasting) in order to ensure the required purity and surface roughness. According to Aguado et al. [16] the surface roughness is a prerequisite for the formation of a correct bond. Moreover, according to the research of Suryadarma et al. [17] it is necessary to heat the insert for the appropriate length of time in order to get rid of moisture before the Al-Fin process. The Al-Fin process itself consists of two successive stages. The first stage is the initial preparation of the cast iron insert for joining with the aluminium alloy. The process itself consists of immersing cast iron inserts in an Al-Fin alloy for the time needed to create a joint between the two materials. This time, as shown by the results of research conducted by Manasijevic et al. [18], has a large impact on the diffusion of atoms between the materials to be joined and the final formation of the intermetallic layer built of AlxFey [19]. The presence of an intermetallic layer positively affects the mechanical properties of the bimetallic joint, which was confirmed on the basis of research conducted by Viala et al. [11]. A liquid aluminium alloy in a pre-heated form is then poured into the prepared inserts, where the temperature of the casting alloy, as indicated by the research of Ramadan et al. [20], plays a huge role in the quality of the bimetallic joint, affecting the dissolution of the Al-Fin layer and increasing diffusion, resulting in the formation of a regular and continuous intermetallic layer. Intermetallic phases (IPs) are characterised by the separation of the crystal lattice from the lattice of the elements they are composed of, as well as specific positions in the lattice being occupied by the atoms [19,21,22,23]. IPs can be formed during liquid solidification or in the solid state. Their presence in an alloy usually affects its mechanical and physical properties.
Typically, the presence of IPs increases brittleness and hardness. Austenitic cast iron is usually used as the material for the inserts that are used to manufacture the pistons. Since cast iron oxidises very quickly, the surface of the insert must be abrasively machined to remove oil and rust from it. Sandblasting is one of the most commonly used treatments to obtain a rough surface and remove metal oxides [24,25,26]. The shot peening time is short, and the steel balls used in this process are made of stainless steel. After the shot peening process, the cast iron insert has a roughened surface as well as the presence of graphite, which is concentrated on the insert surface. This is the result of the balls hitting the insert surface at high speed. The sandblasting process is widely used to strengthen the surface, modify it, clean it and remove oxides, etc. [27,28]. Thanks to the use of bimetallic technology in the production of pistons, it is possible to combine the durability and abrasion resistance of cast iron with the good thermal conductivity of aluminium alloys. However, due to the discontinuity of the material resulting from the use of two materials, the joint is vulnerable to damage. For this reason, the metals joined in bimetallic technology must have a similar thermal expansion value. This is especially important for joints that will be exposed to variable temperatures. Rapid changes in temperature cause expansion and contraction of the joined materials with different values and velocities, thus exposing the bimetallic joint to the possibility of damage [29]. Uthayakumar et al. [30] aimed to find the optimum dipping time for the Al-Fin process. They achieved bimetallic pistons without damaging the bonding between the aluminium and cast iron and increased the wear resistance. In highly loaded diesel engines, the shape of the geometric profile of piston grooves and piston rings changes as a result of wear. Dolata et al. [14] studied the tribological properties of aluminium matrix materials used in cooperation with piston rings. They found that AlSi12/silicon carbide composite with glassy carbon particles showed a lower coefficient of friction compared to commercial AlSi12CuNiMg alloy. In another article, Dolata et al. [31] found that increasing the hard silicon carbide particle content significantly decreased the volume loss of un-reinforced matrix under dry friction conditions. Piątkowski and Czerepak [32] investigated the effect of varying Fe content on the crystallization process of the AlSi9 alloy for the Al-Fin processing of iron ring supports. After Al-Fin processing of a ring insert made of AlSi9 alloy, the β-Al5FeSi phase causing local cracking in the ring–piston interface was identified.
Although many methods have been developed to join aluminium with aluminium and aluminium with Fe-based alloys, such as rolling [33], riveting [34], conventional laser welding [35,36], high-speed fibre laser welding [37], oscillating laser welding [38] and friction stir welding [39,40] it is hard to claim that these methods are easy to implement when joining elements with complex-shaped components [41]. The casting process, by means of which liquid metals can be joined with solid metals in the metallurgical process, has great flexibility when joining elements with complex shapes [42]. For example, Liu et al. [43] developed a new method of casting composites involving hot dipping in a Zn–7Si 2.2 wt.% alloy for metallurgical bonding of aluminium alloys to mild steels. Guo et al. [44] developed the method of fabrication of Al–7Si alloy/cast iron bimetallic composites. A new bonding interface between graphite and Al–Fe phases at the ferrite–graphite interface was established. Most methods of joining bimetallic materials focus mainly on joining aluminium alloys with steel. Methods of joining aluminium alloys with cast iron are rarely described.
In this paper, the effect of the duration of the Al-Fin process, and the temperature of AlSi9 aluminium alloy when casting the bimetallic joint, on the formation of the metallurgical bond between the aluminium alloy piston made of S2N aluminium and the EN-GJLA-XNiCuCr15-6-2 austenitic cast iron insert was investigated. In the performed tests, the Al-Fin process was carried out for different temperatures of the liquid AlSi9 alloy. The temperature of the AlSi9 aluminium alloy varied in the range 700–950 °C in steps of 50 °C. The duration of the Al-Fin process ranged from 1 to 10 min. The test results show that it is possible to obtain a metallic bond between the aluminium alloy piston and the austenitic cast iron ring without additional thermal/chemical treatments. The formation of an intermetallic bond layer is a very complex and not fully understood process. The literature lacks detailed descriptions of the phenomena that occur in relation to the formation of the intermetallic phases in bimetallic Al–cast iron joints.
2. Materials and Methods
2.1. Manufacturing of the Piston
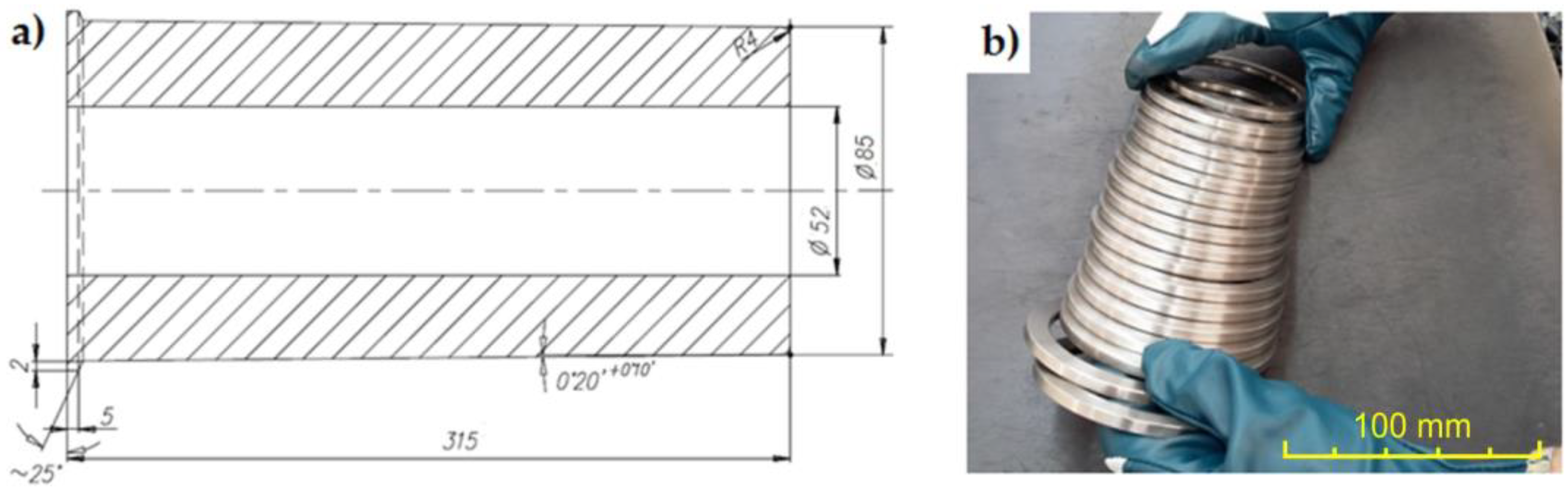
The preparation of the material necessary to conduct the research consisted of several stages of work. The first stage included the process of casting a semi-finished product for making the cast iron inserts embedded in the pistons in the Al-Fin process (Figure 1).
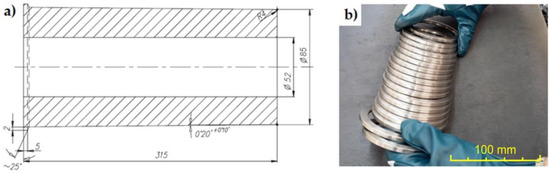
Figure 1.
(a) Production drawing of the semi-finished product (dimensions in mm); (b) cast iron inserts obtained by cutting a sleeve in the shape of a thick-walled pipe.
The semi-finished product was cast from EN-GJLA-XNiCuCr15-6-2 cast iron. This is an iron–carbon alloy with an austenitic microstructure, containing nickel, manganese, chromium and copper. Higher chromium content leads to an increase in gas corrosion resistance and hardness and a decrease in the coefficient of thermal expansion.
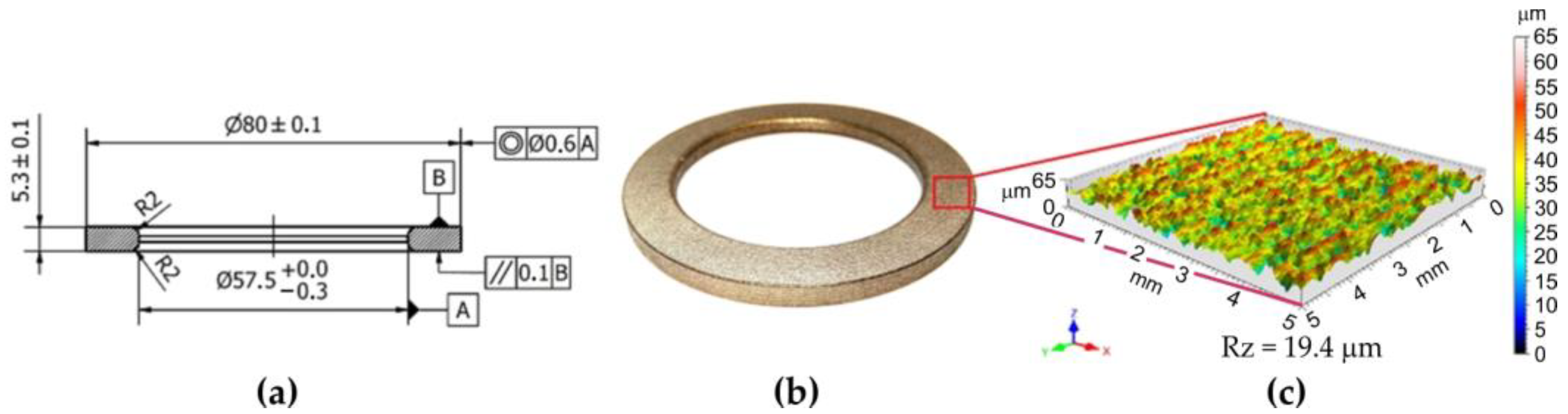
Then, the inserts (Figure 2a,b) were subjected to a surface modification process in order to obtain a surface free from impurities and moisture but, at the same time, characterised by the appropriate surface roughness. To remove impurities, grease and oxides from the surface of the cast iron inserts and to ensure the specified value of the roughness parameter, Rz > 15 µm (according to the company standard of Federal Modul Gorzyce Sp. z o.o.), the inserts were initially cleaned by sandblasting. Then, they were subjected to a shot blasting process in a rotary shot blasting machine for about 6 min, obtaining a ten-point height of surface (Figure 2c) as a result of plastic deformation. Sandblasting is the most common methods used for surface roughening [45].

Figure 2.
(a) Production drawing of insert (dimensions in mm); (b) cast iron insert; (c) surface topography.
The surface topography of the insert obtained in this way directly affects the quality of the joint and indirectly affects the bond strength in the bimetallic joint between the aluminium alloy and the cast iron insert. Shot peening of the cast iron ring causes larger deformations on the surface, which also supports the joining of the ring with the aluminium piston. After the shot blasting process, the inserts were heated at 230 °C for 2.5 h. The pistons were fabricated from S2N aluminium alloy, which is the basic material used in the production of pistons.
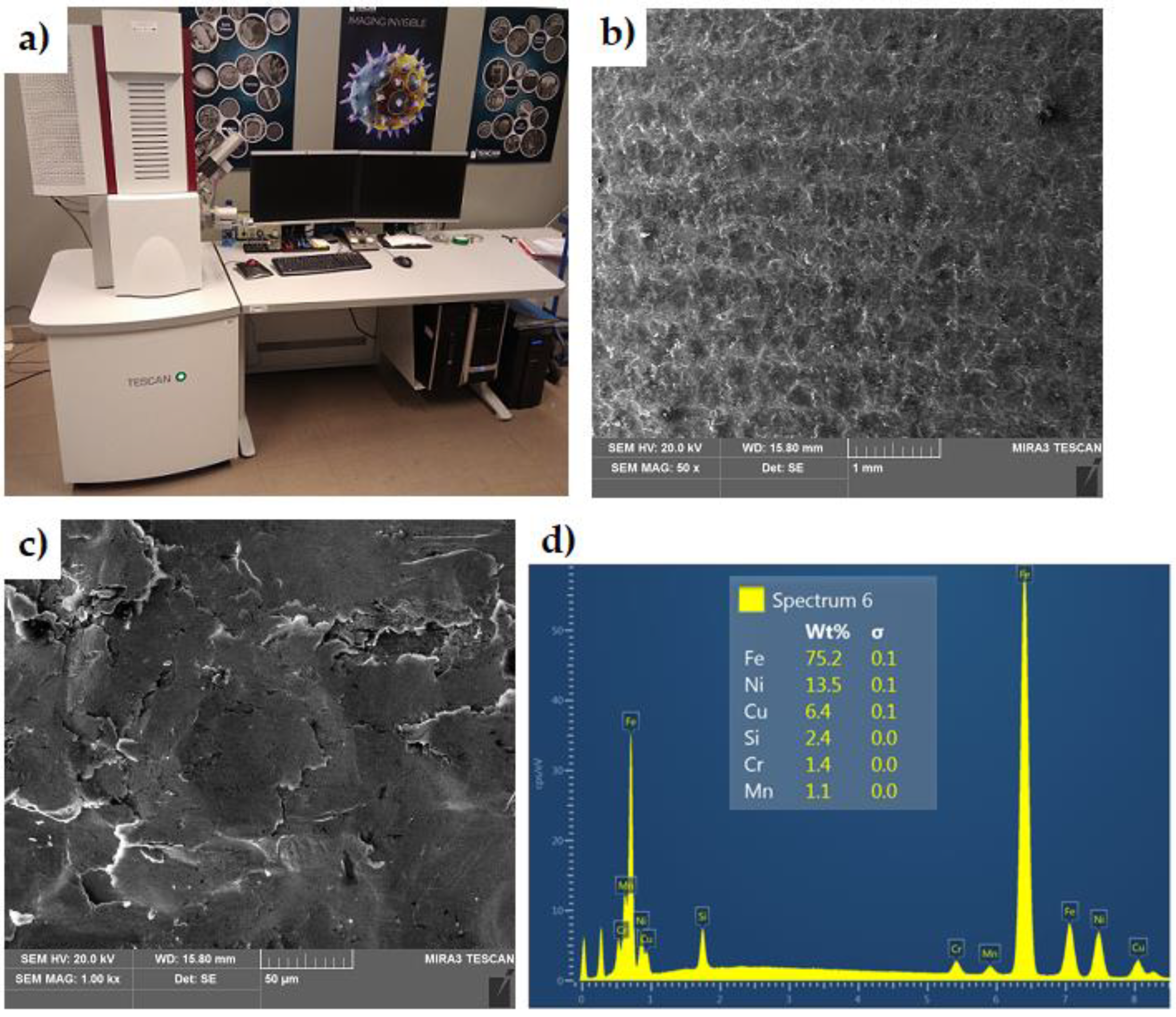
The chemical composition of the insert material (Figure 3) was determined using a scanning electron microscope (SEM) MIRA3 (Tescan, Brno, Czechia) equipped with an Energy Dispersive X-ray Spectroscopy (EDS) attachment. The chemical composition of the EN-GJLA-XNiCuCr15-6-2 austenitic cast iron and the S2N aluminium alloy are presented in Table 1 and Table 2, respectively.
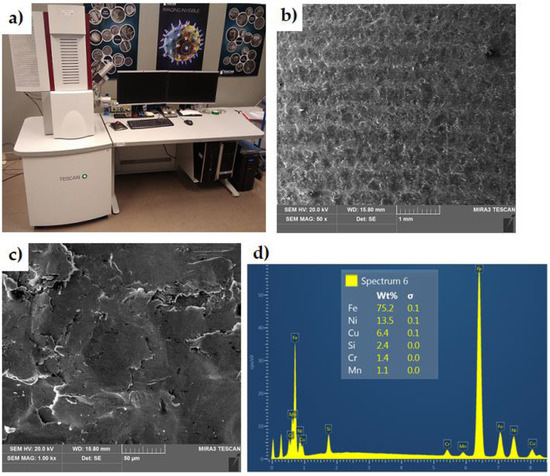
Figure 3.
(a) Scanning electron microscope, (b,c) SEM micrographs of EN-GJLA-XNiCuCr15-6-2 surface at magnification of (b) ×50 (c) and ×1000, and (d) EDS spectrum.

Table 1.
Chemical composition (wt.%) of the EN-GJLA-XNiCuCr15-6-2 (EN-JL3011) cast iron (insert).

Table 2.
Chemical composition (wt.%) of the S2N aluminium alloy (piston).
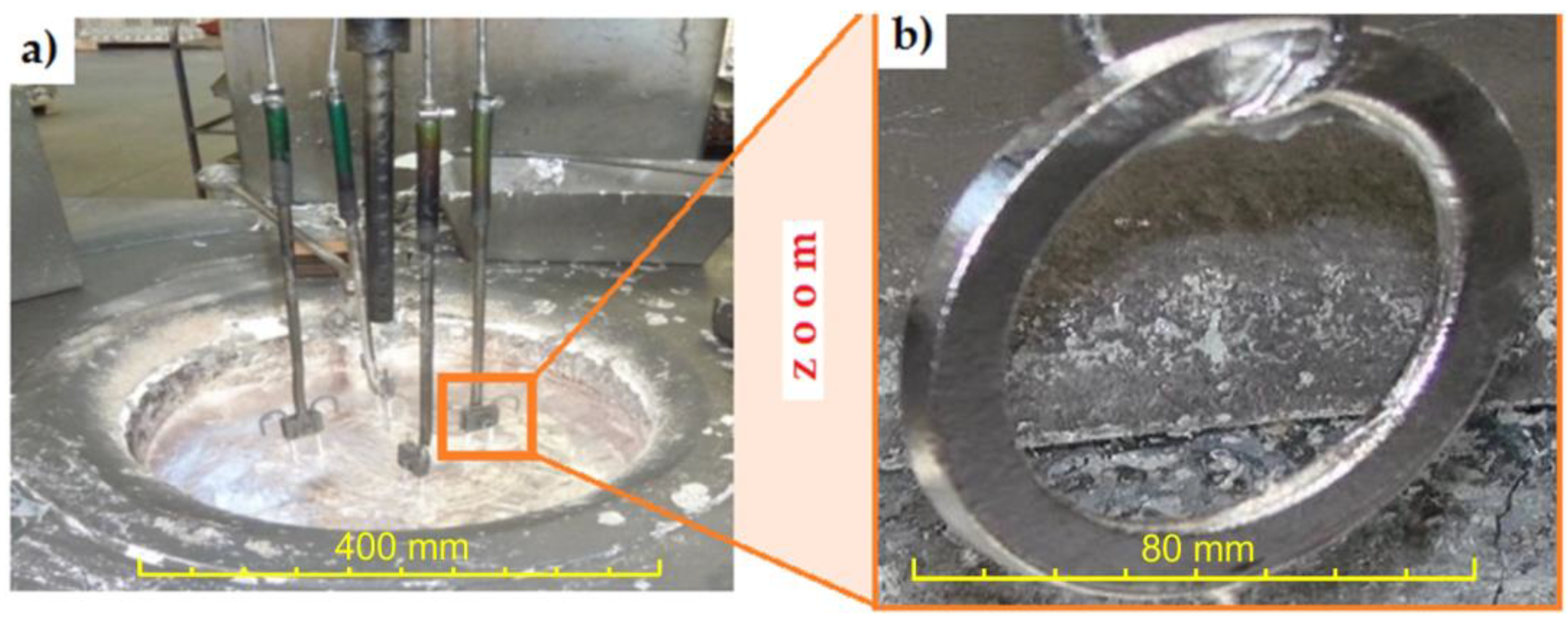
The next stage of preparing the material for testing consisted of the Al-Fin process (Figure 4) for joining the previously prepared inserts to the AlSi9 aluminium alloy with the chemical composition shown in Table 3.
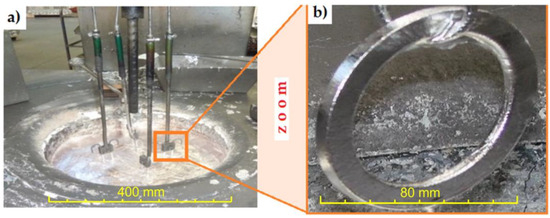
Figure 4.
(a) Al-Fin process and (b) cast iron insert after the Al-Fin process.

Table 3.
Chemical composition (wt.%) of the liquid AlSi9 aluminium alloy (EN 1706:2010) (Al-Fin layer).
In the performed tests, the Al-Fin process was carried out for different temperatures of the liquid AlSi9 aluminium alloy. The temperature of the AlSi9 aluminium alloy varied in the range 700–950 °C in 50 °C steps with an accuracy of ±10 °C. The duration of the Al-Fin process ranged from 1 to 10 min in increments of 1 min. After reaching the required time, the inserts were placed in metal moulds heated to 180 °C, into which the S2N aluminium alloy, at a temperature of 760 °C ± 10 °C, was poured. The castings prepared in this way were left to solidify for about 10 min, after which they were cooled in bathtubs filled with water, with a constant flow of water at a temperature of 50 °C.
After cooling, the elements of the gating system were removed from the cast pistons (Figure 5a). Then, mechanical processing was performed to obtain the finished product (Figure 5b).
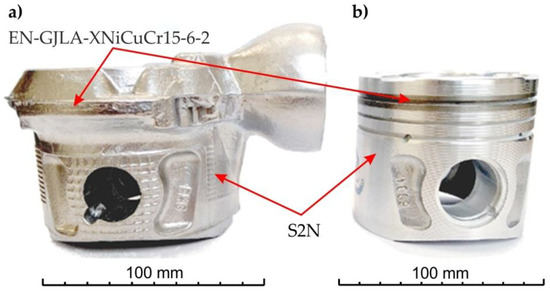
Figure 5.
(a) Piston casting and (b) piston after mechanical treatment.
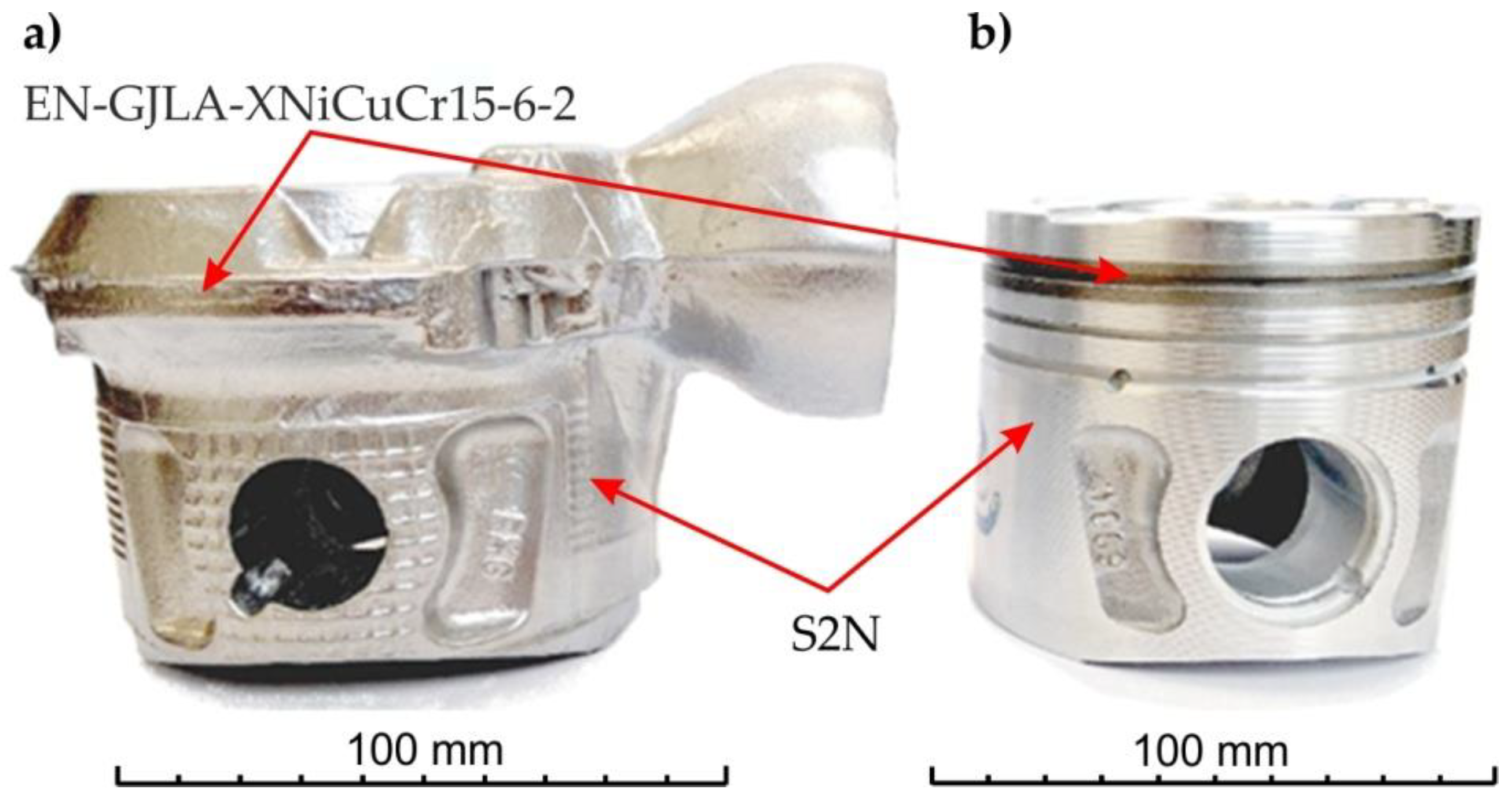
Figure 6 shows the location of the cast iron insert in the finished piston. The insert is located in the place where the first ring, the so-called sealing ring, is mounted. This is the place most exposed to thermal influences from the engine’s combustion chamber.
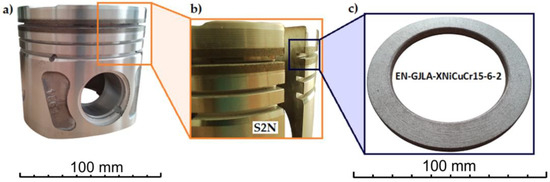
Figure 6.
(a) Piston; (b,c) cast iron insert.
2.2. Preparation of the Specimens
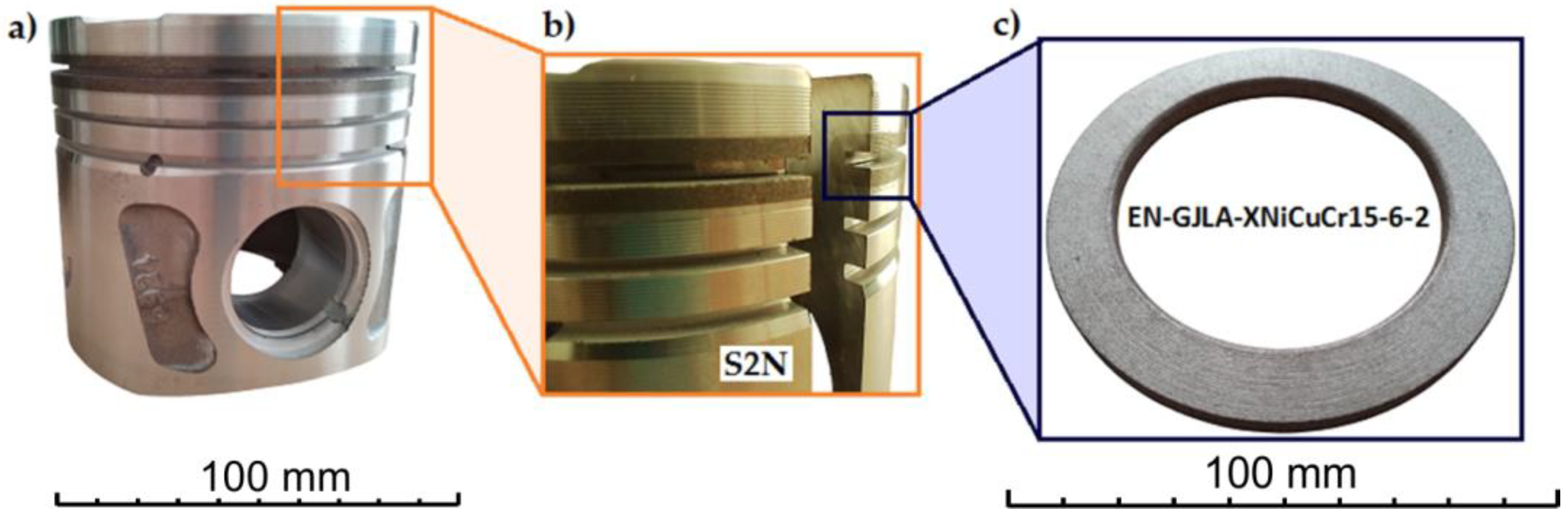
In order to observe and analyse the microstructure formed between the cast iron insert and the AlSi9 aluminium alloy, a sample was cut from the piston casting. The piston was cut parallel to its axis (i.e., perpendicular to the Fe–Al joint) using a BP95d electrical discharge machining (EDM) machine (Zakład Automatyki Przemysłowej, Kutno, Poland) (Figure 7).

Figure 7.
Preparation of the test samples: (a) the place where the sample was cut from the piston casting; (b,c) BP95d EDM machine.
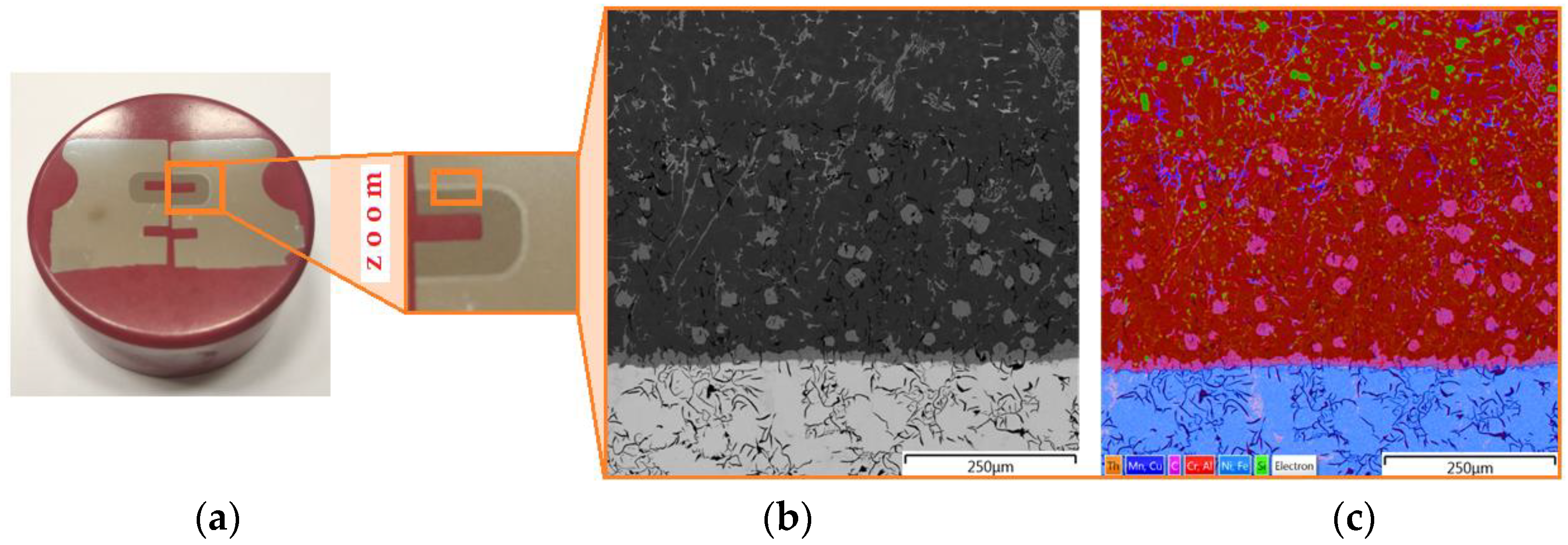
Characterisation of the microstructure of the bimetallic joint was carried out using an Olympus BX51M (Olympus, Tokyo, Japan) optical microscope nd a scanning electron microscope MIRA3 (Tescan, Brno, Czechia). The place in the piston from which the samples for analysis were cut is shown in Figure 8. The samples were positioned in epoxy resin and the sample was wet polished with discs with a grain size of 120 to 2500 and then polished with a diamond paste (grain size 3 μm and 1 μm). In order to reveal the microstructure, the samples were etched with Mi3Fe Pikral (chemical composition: 3 g C6H3N3O7, 100 cm3 C2H6O) and Mi1Al (chemical composition: 0.5 cm3 HF, 99.5 cm3 H2O). Metallographic observations were carried out using a BX51M optical microscope. The test sample and the location of the places where the joint structure was analysed are shown in Figure 9a. The joint zone is characterised by a clear transition layer (Figure 9b,c).

Figure 8.
Piston and specimen positioned in epoxy resin.
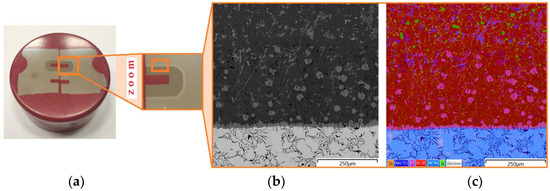
Figure 9.
(a) Sample for analysis with (b) the microstructure of the bimetallic joint and (c) the surface distribution of elements.
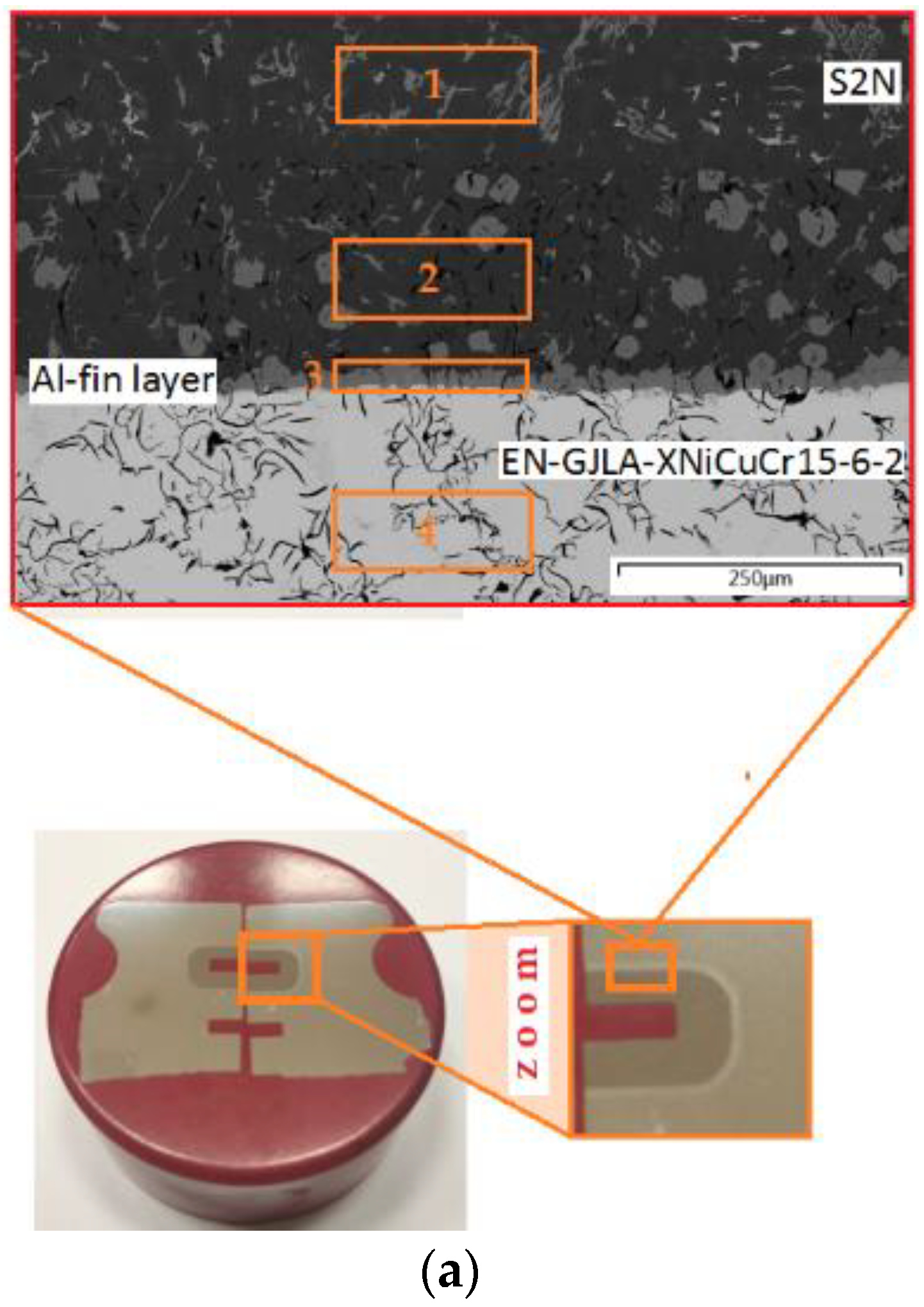
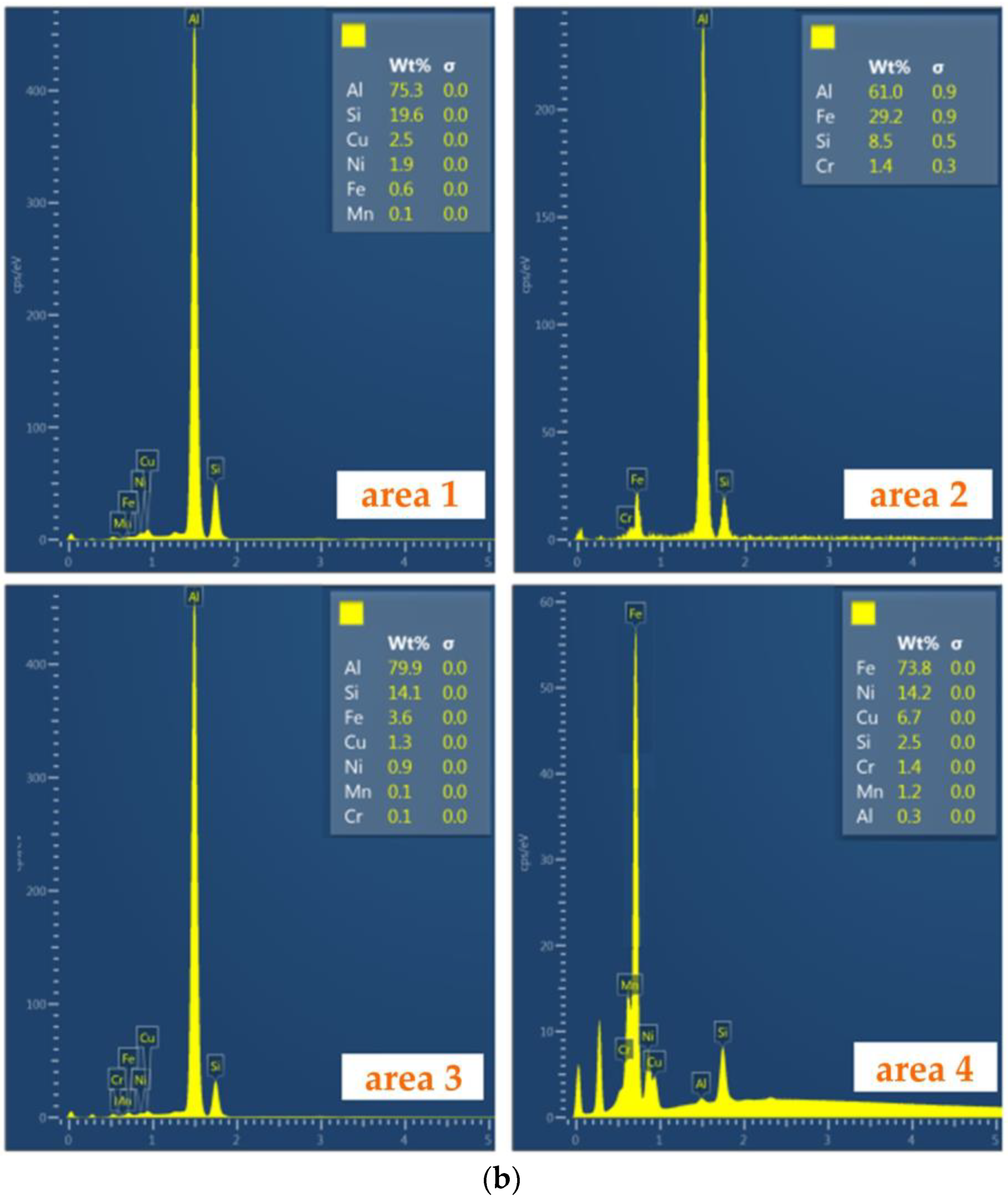
Then, for each of the tests (different temperatures of the AlSi9 alloy and duration of the Al-Fin process), EDS spectra (Figure 10b) were performed at specific locations in the bimetallic joint (Figure 10a). In this way, the distribution of elements in specific areas of the connection was obtained. The chemical composition of the transition layer was identified based on the SEM-EDS analysis (area 2 in Table 4).
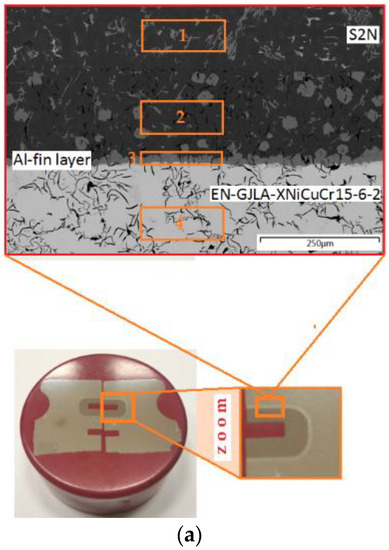
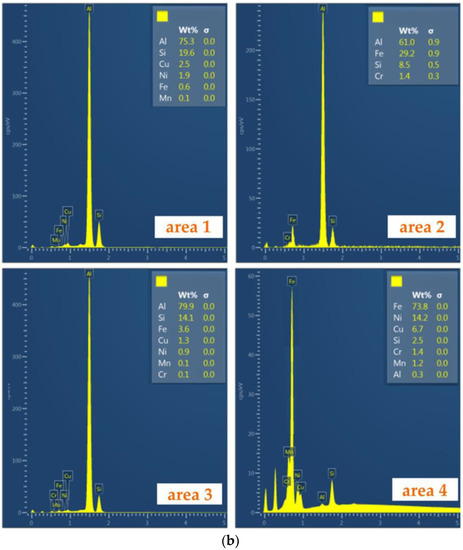
Figure 10.
(a) Characteristic zone in the joint and (b) the EDS spectra of the bimetallic joint fabricated at an Al-Fin process duration of 4 min and an AlSi9 alloy temperature of 750 °C ± 10 °C: areas od EDS analysis: area 1—piston material (S2N), area 2—piston material (S2N) near Al-Fin layer, area 3—Al-Fin layer (AlSi9) and area 4—insert material (EN-GJLA-XNiCuCr15-6-2).

Table 4.
Chemical composition of areas 1–4 shown in Figure 10a (wt.%).
3. Results
3.1. Optical Microscopy
Each bimetallic joint between a cast iron insert and an aluminium alloy cast for all the analysed parameters (the duration of the Al-Fin process and the temperature of the AlSi9 alloy) was subjected to microstructure analysis. Figure 11 shows an example of the cross-sectional microstructure of the bimetallic joint. Three zones can be clearly distinguished in the cross-sectional microstructure of the analysed bimetallic joint: austenitic cast iron zone (insert material), diffusion zone and transition zone. On the other hand, the term Al-Fin layer should be understood as the sum of the diffusion layer and the transition layer.

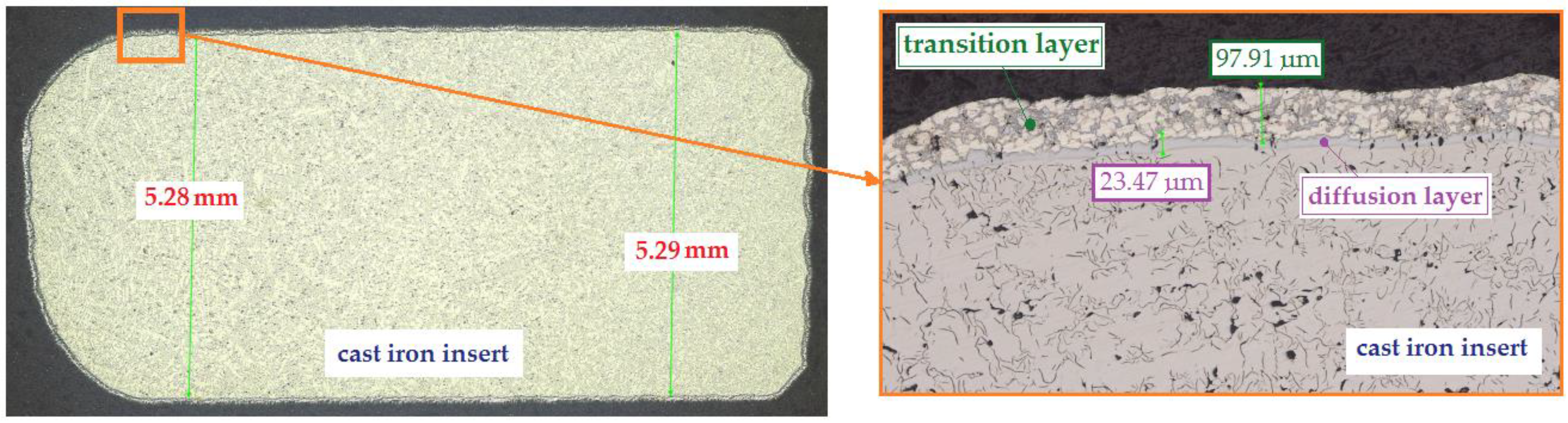
Figure 11.
Bimetallic joint between the cast iron insert and the aluminium alloy cast obtained for the following parameters: duration of the Al-Fin process—4 min., temperature of the AlSi9 alloy—750 °C ± 10 °C.
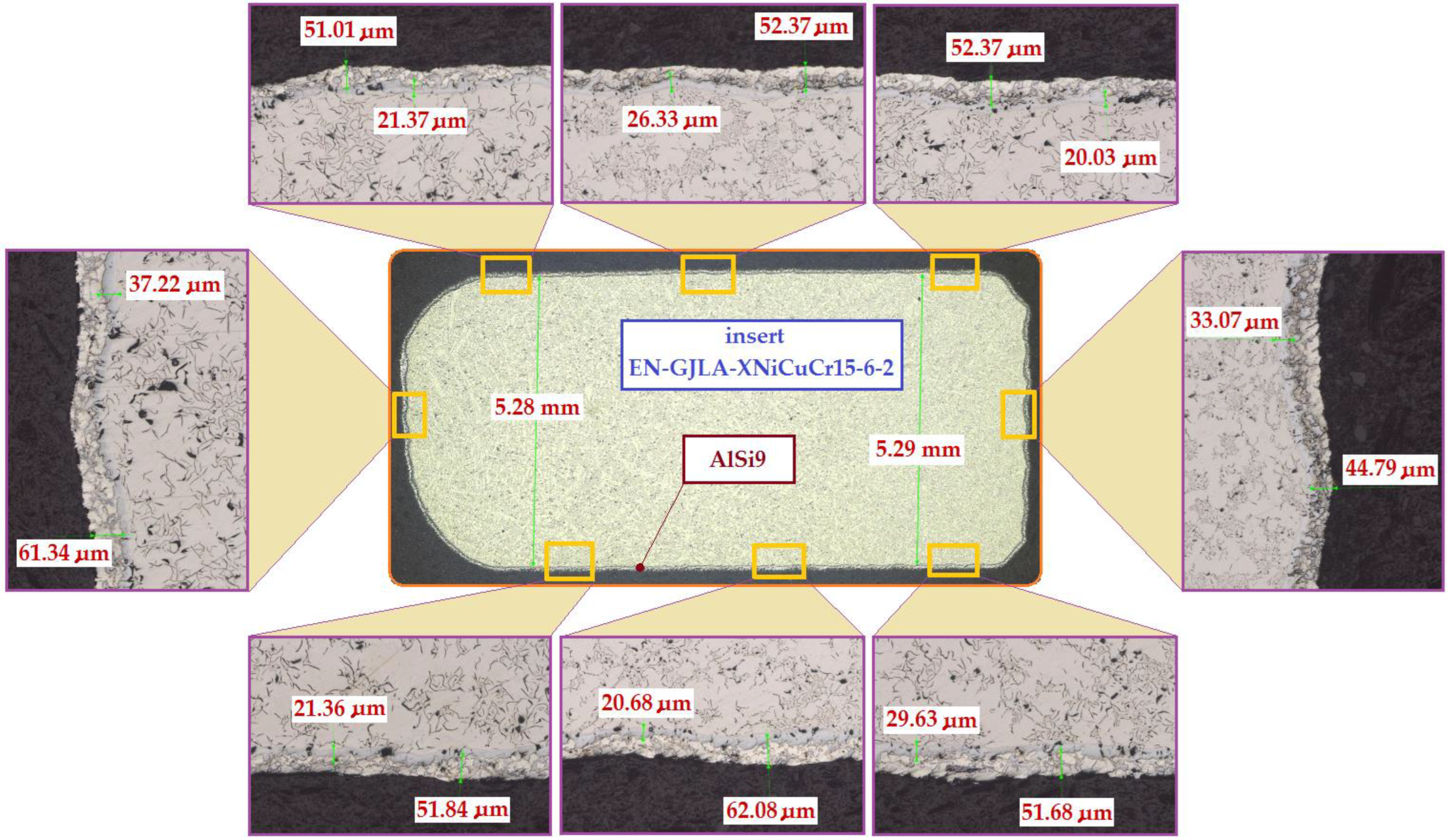
When the cast iron insert (ring) is immersed in the hot aluminium alloy (AlSi9) during the Al-Fin process, the convection and mixing processes that occur start to activate the Al atoms for adsorption onto the surface of the cast iron insert. At the same time, the Fe atoms from the cast iron insert begin to dissolve in the liquid AlSi9 aluminium alloy. Part of the dissolved Fe from the cast iron passes into the diffusion layer forming the Al–Fe intermetallic compound. Both the diffusion layer and the transition layer form the bypass of the insert for the joint with the base aluminium alloy of the piston (S2N) and transfer the shear stresses generated when the insert is embedded in the piston during pouring of the mould. In Figure 11, it can be seen that the thickness of the diffusion layer is about 23.47 μm and the thickness of the transition layer is 97.91 μm. Figure 12 shows an exemplary bimetallic joint on which is marked how both the diffusion layer and the transition layer on the circumference of the cast iron insert change after the Al-Fin process. The thickness of the diffusion layer varies from about 20.03 μm (the upper surface of the insert) to 37.22 μm (the lateral surface of the insert). The thickness of the transition layer varies from 62.08 μm (the bottom surface of the insert) to 44.79 μm (the lateral surface of the insert). Similar characteristics of the thickness distribution for the location on the perimeter of insert were obtained in the other tests carried out (of course, the thicknesses of the individual layers changed in value).
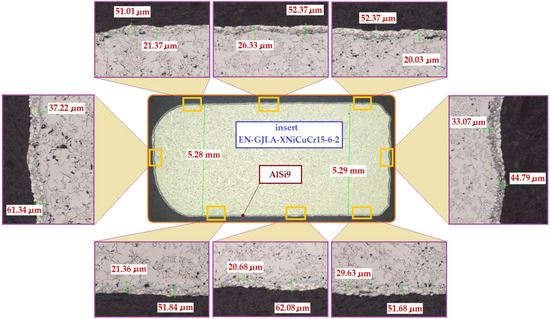
Figure 12.
Distribution of the thickness of the diffusion and transition layers in the bimetallic joint obtained following the Al-Fin process at an AlSi9 alloy temperature of 750 °C ± 10 °C (process duration 60 s).
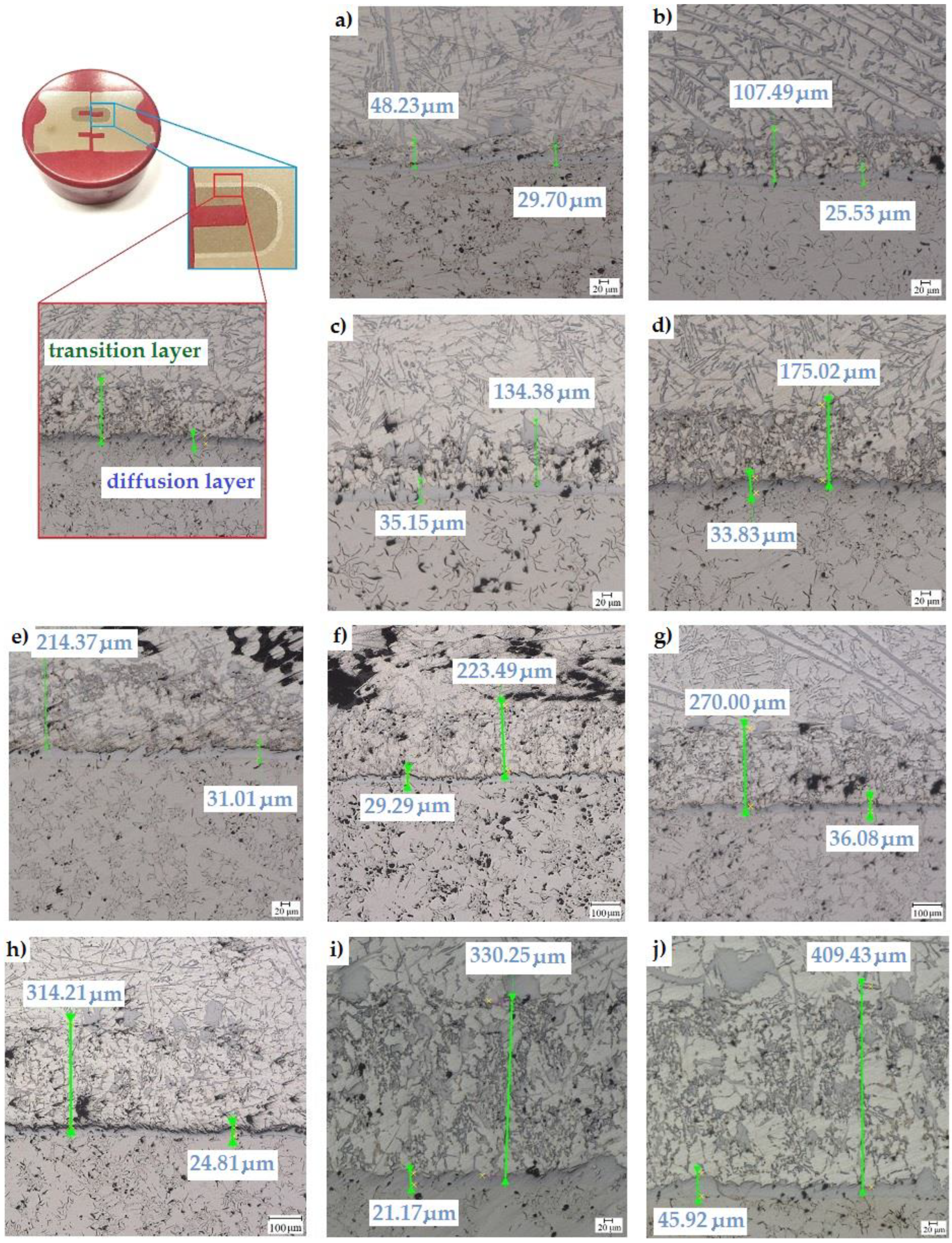
It was also observed that reducing the thickness of the transition layer significantly reduced the amount of graphite in the diffusion layer. During the Al-Fin process, the content of elements in the AlSi9 alloy changes due to the diffusion of the alloy elements from the inserts into the piston material. This is particularly evident in the case of changes in the iron content of the alloy, the content of which increases until reaching the upper limit established for the process, which is a maximum of 3.5%. In the conducted tests, it was noticed that both the temperature of the AlSi9 alloy and the duration of the Al-Fin process significantly affect the thickness of the diffusion and the transition layers obtained (Figure 13). However, it should be emphasised that only the transition layer, on which there is a significant influence of both analysed parameters of the Al-Fin process, is significantly affected. However, no significant effect of the duration of the Al-Fin process and the temperature of the AlSi9 alloy on the thickness of the diffusion layer was observed (Figure 13).
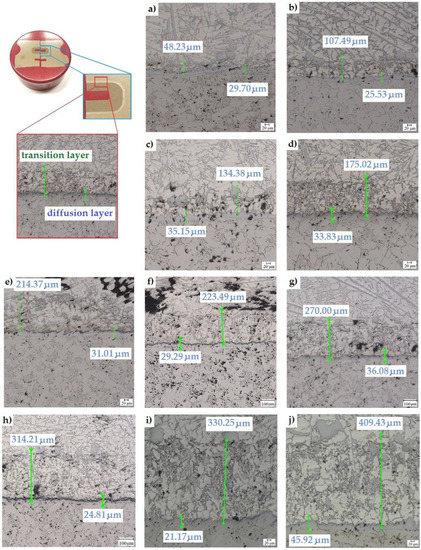
Figure 13.
Thickness of the diffusion and transition layers of bimetallic joints made at an AlSi9 alloy temperature of 850 °C ± 10 °C for different durations of the Al-Fin process (in minutes): (a) 1, (b) 2, (c) 3, (d) 4, (e) 5, (f) 6, (g) 7, (h) 8, (i) 9 and (j) 10.
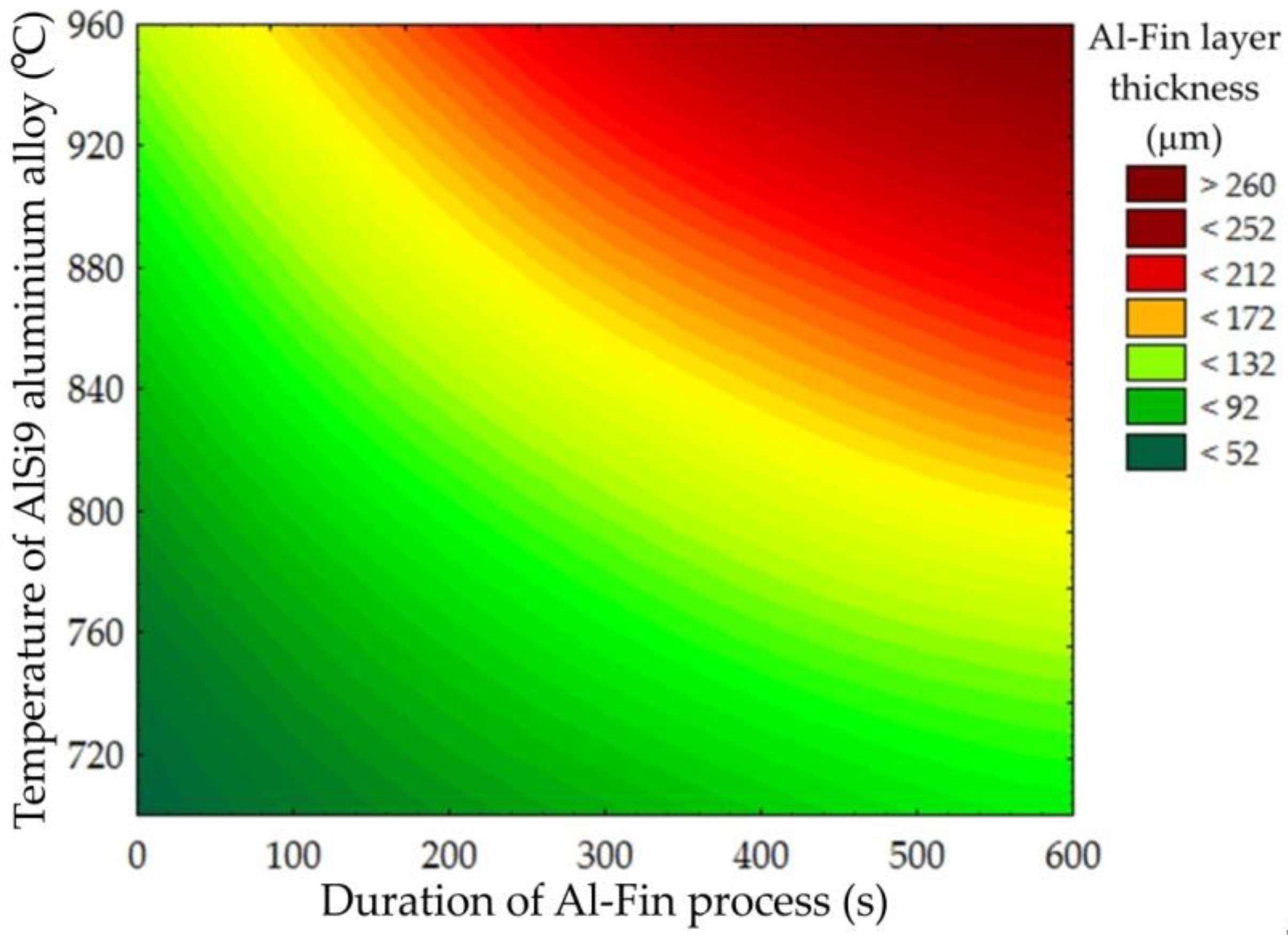
Figure 14 shows, in graphical form, the influence of both the temperature of the AlSi9 alloy and the duration of the Al-Fin process on the thickness of the Al-Fin layer (the sum of the thicknesses of the diffusion and transition layers) for all the tests carried out. The analysis shows that, in order to obtain the desired thickness of the Al-Fin layer, (a) higher temperatures of the AlSi9 alloy and a short immersion time in the liquid alloy bath should be used, or (b) lower temperatures of the AlSi9 alloy and a longer immersion time should be used.
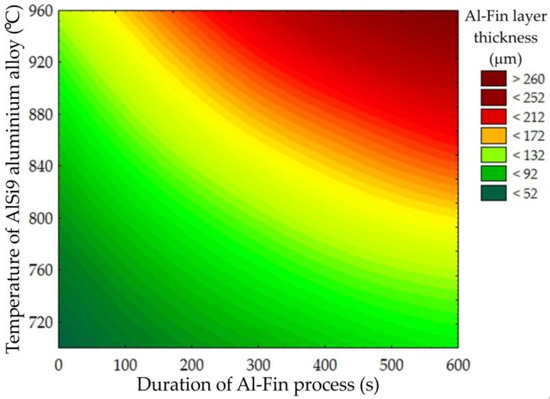
Figure 14.
Influence of the duration of the Al-Fin process and the temperature of the AlSi9 alloy on the thickness of the Al-Fin layer (contour diagram).
3.2. SEM Analysis
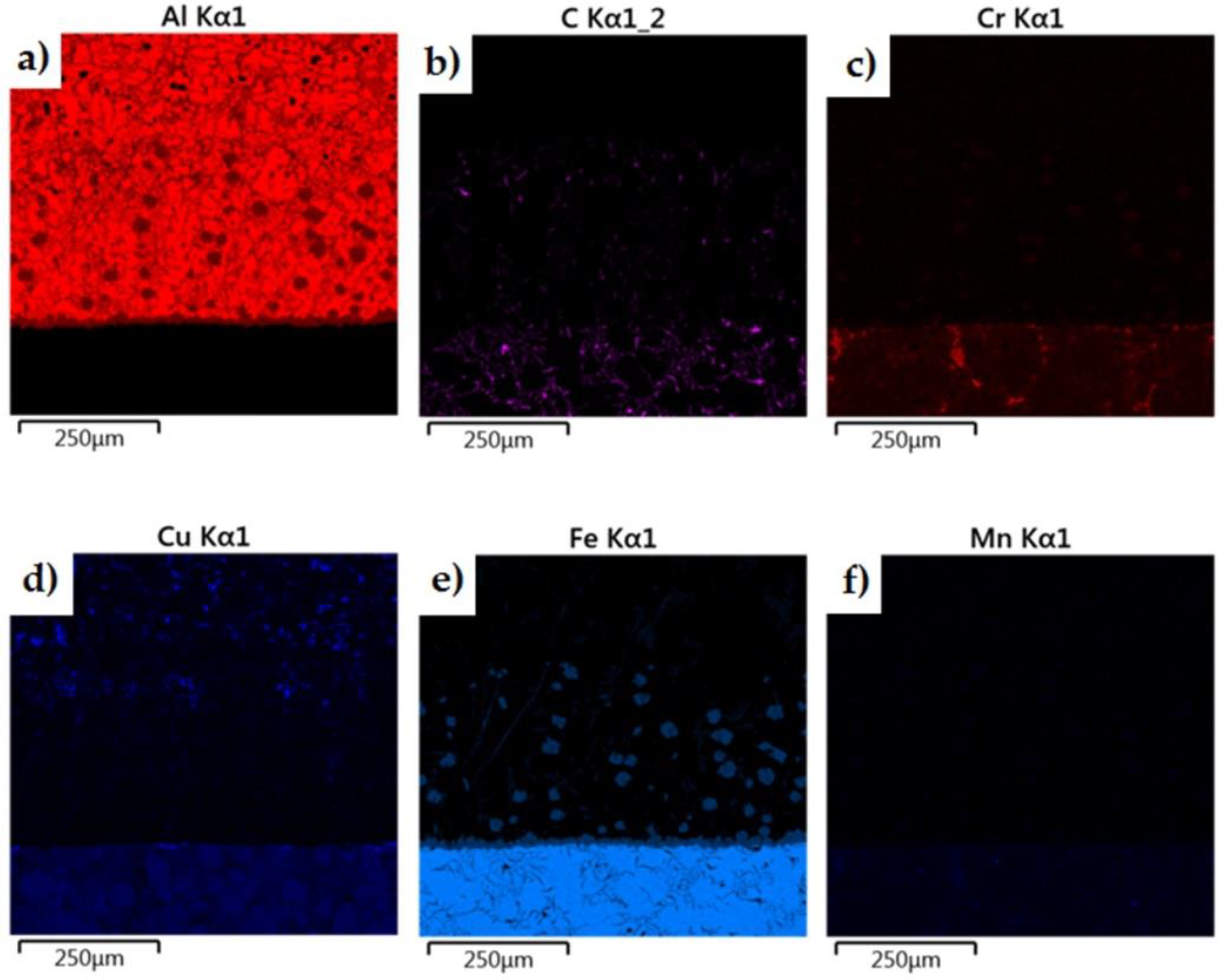
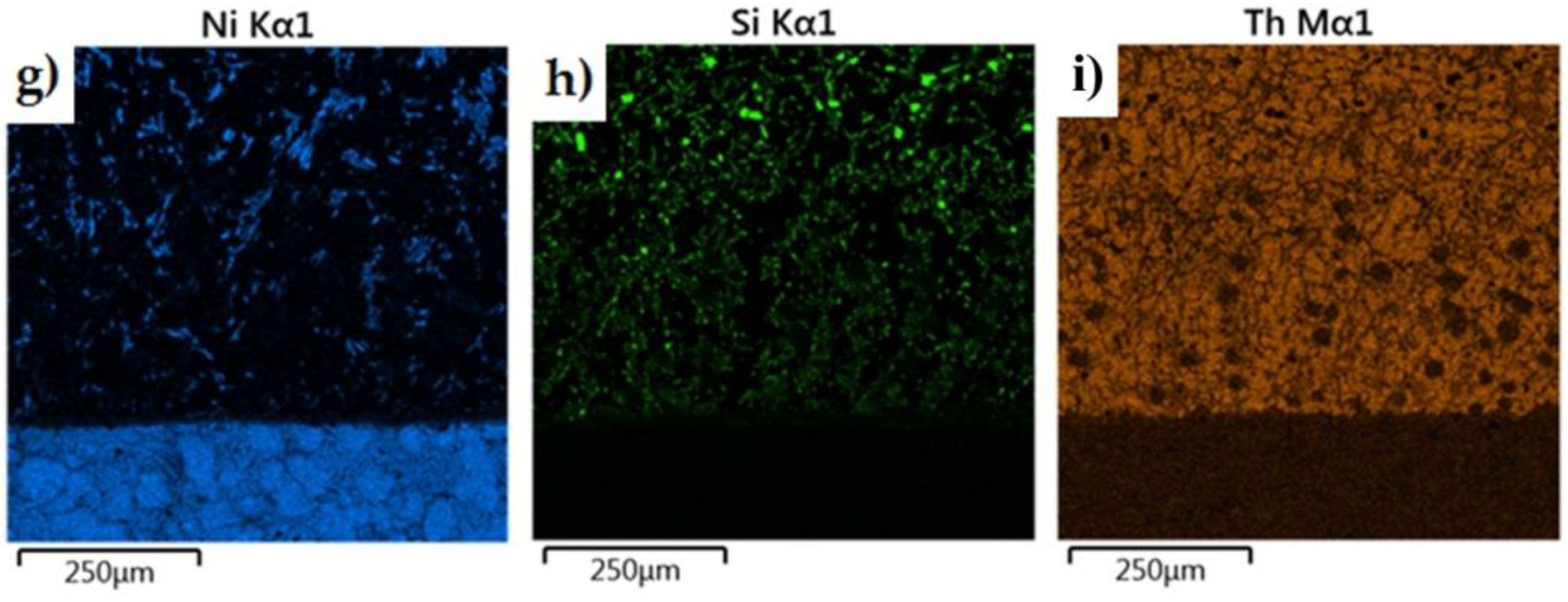
The morphology of the microstructure of the joint between the cast iron insert and the piston alloy was observed in samples of the finished piston casting, which were cut parallel to the piston axis (i.e., perpendicular to the Fe/Al interface) (Figure 7a). The phases formed in the bimetallic joint were characterised in chemical terms in order to determine the character of the resulting intermetallic compound formed at the Al–Fe interface. Detailed surface distributions of the elements in the analysed areas are shown in Figure 15.
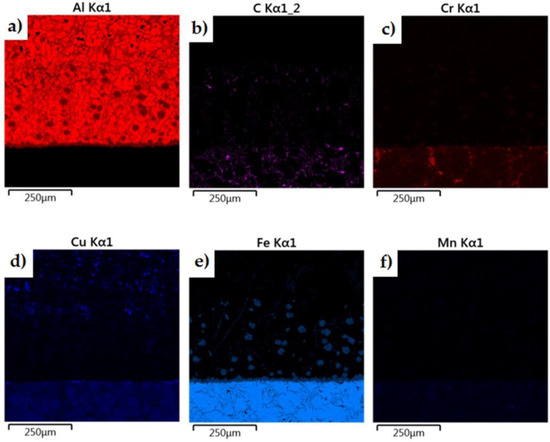
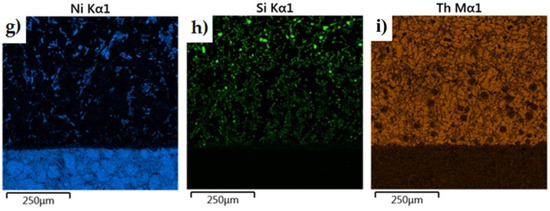
Figure 15.
Elemental maps of the common elements in a joint fabricated in the Al-Fin process at a duration of 4 min and an AlSi9 alloy temperature of 850 °C ± 10 °C: (a) test area of bimetallic joint, (b) distribution of all elements, and distribution of (c) Fe, (d) C, (e) Ni, (f) Si, (g) Al, (h) Cu and (i) Cr.
The analysis shows a significant share of iron and aluminium in the grey, uniformly coloured, irregular formations of the diffusion layer (Figure 15). Analysis of the microstructure of the transition layer shows a matrix consisting of aluminium characterised by a uniform distribution and inclusions of Fe atoms coming from the base material (the cast iron insert).
The mechanism of interfacial compound formation, according to the SEM analysis, reveals that the diffusion of Fe atoms influenced the formation of intermetallic compounds, and the development of intermetallic compounds is significantly dependent on temperature. Typically, the sample casting process takes only dozens of seconds from pouring to natural cooling. As a result, there is not enough time for the iron to diffuse. On the other hand, increasing the casting temperature can accelerate the duration and rate of diffusion.
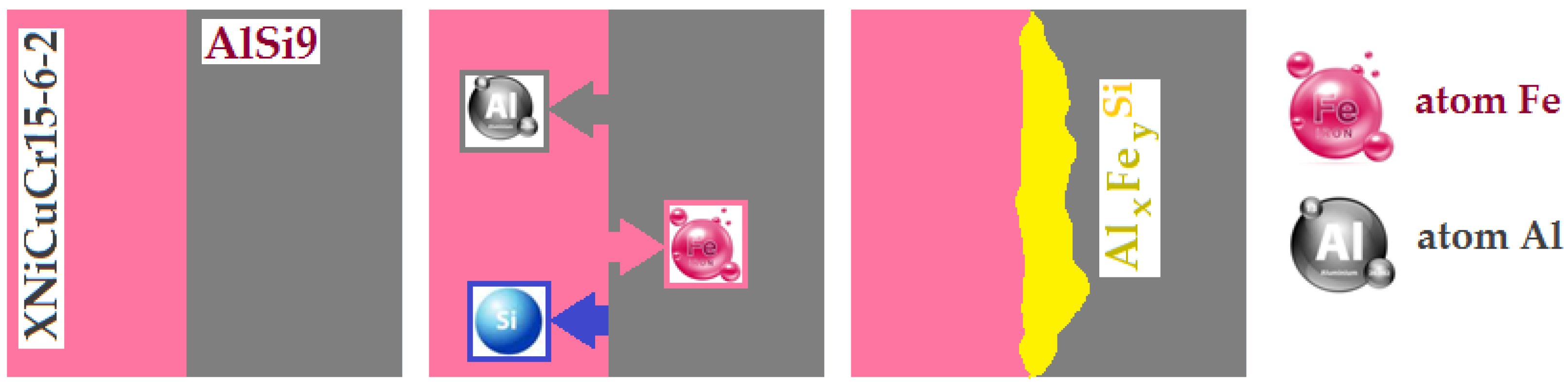
According to the results of the EDS analysis of the interfacial phases, the results reveal a diffusion layer containing the elements Fe, Si and Al between the cast iron and the aluminium (as shown in Figure 16). According to the Fe–Al phase diagram [46], iron dissolves in solid form in aluminium at high temperature, aluminium in these conditions is diffused and dissolved in molten aluminium. In addition, silicon has a strong affinity for iron, which increases the concentration of silicon near the surface of the cast iron insert. The reaction of iron, silicon and aluminium leading to the formation of intermetallic phases is shown in Figure 16. The Al-Fin bond has a chemical composition close to FexAly [47]. According to the Al-Si phase diagram, at a temperature of 595 °C, dendritic crystallisation of the AlSi9 aluminium alloy exists. The eutectic phase, α(Al)+Fe+β(Si), includes the intermetallic α-Al8Fe2Si phases [32].
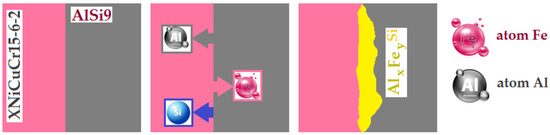
Figure 16.
The phenomenon of diffusion occurring in the process of Al–Fe bimetallic casting.
Microhardness tests were carried out along the line from steel to aluminium. According to the results, cast iron has an average hardness of 220 HB, whereas the aluminium alloy has a hardness of 115 HB. The hardest layer between the cast iron insert and the aluminium alloy piston is the intermetallic layer, which has a hardness of about 260 HB.
3.3. Analysis of Variance
Statistical analysis of the results was performed using the multivariate analysis of variance (ANOVA) using the STATISTICA 13.3 program (StatSoft, Tulsa, OK, USA). The multivariate analysis of variance allows checking of the significance of the influence of several independent variables on the dependent variable. In addition, the multivariate ANOVA allows the synergistic effect of the product of multiple variables to be taken into account in the statistical model. Taking into account the adopted significance level of p = 0.05, the statistical significance of individual groups of variables and individual variables is determined.
The results of the ANOVA analysis allow us to reject, at the significance level of p = 0.000, the hypothesis that the parameter ‘Duration of Al-Fin process’ has no effect on the thickness of the transition layer. However, no statistically significant effect of duration of Al-Fin process on the thickness of the diffusion layer was observed. The influence of ‘Duration of Al-Fin process’ at the significance level of p = 0.6187 on the thickness of the diffusion layer is negligible. However, in the case of the influence of ‘Temperature of AlSi9 alloy’ on the thickness of the diffusion and transition layer, the results of the ANOVA statistical analysis allow us to reject, at the significance level of p = 0.000, the hypothesis that the ‘Temperature of AlSi9 alloy’ has no effect on the thickness of the transition and diffusion layer. A statistically significant influence of ‘Duration of Al-Fin process’ on diffusion layer thickness at the significance level of p = 0.008 was also observed.
The results of the analysis (Table 5) allow us to reject, at the significance level of p = 0.000, the hypothesis that the parameters ‘Duration of Al-Fin process’ and ‘Temperature of AlSi9 alloy’ have no effect on the thickness of the Al-Fin layer. In the case of interactions between the analysed factors, a statistically significant effect was observed. It is evident that increasing either the duration of the Al-Fin process or the temperature of the AlSi9 alloy leads to an increase in the thickness of the Al-Fin layer (Figure 17).

Table 5.
Significance level of the effect of the Al-Fin parameters on the average thickness of the Al-Fin layer.
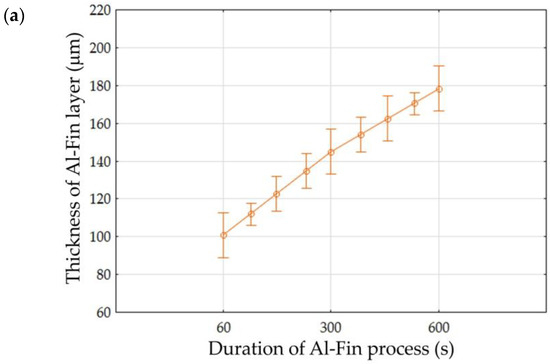
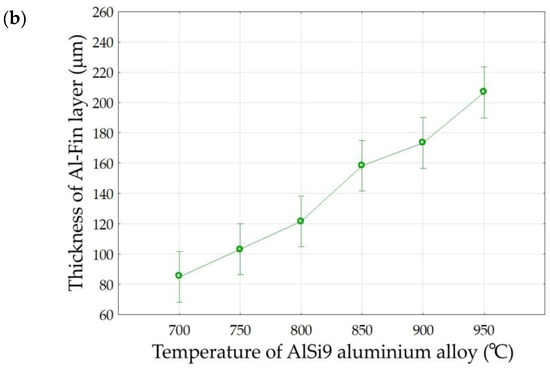
Figure 17.
The effect of (a) the duration of the Al-Fin process and (b) the temperature of the AlSi9 alloy on the average thickness of the Al-Fin layer.
4. Discussion
The bimetallic connection area between the cast iron insert and the aluminium alloy can be divided into three zones: the insert zone (austenitic cast iron area 1), the intermetallic layer (area 3) and the AlSi9 aluminium alloy layer (area 3) (Figure 10a). When the rings are immersed in the molten AlSi9 aluminium alloy, a process of convection and mixing causes the activation, adsorption and diffusion of aluminium atoms into the cast iron ring. At the same time, the iron atoms from the ring material begin to dissolve in the liquid aluminium. Part of the dissolved iron forms a diffusion intermetallic compound layer. In area 3 (Figure 10b), the content of elements such as Fe and Al, whose mass ratio is 29.2:79.9, exceeds the value of 1:2.5, and therefore this intermetallic phase can be defined as the Fe2Al5 phase. The boundary between the intermetallic layer and the aluminium alloy zone in the piston is jagged, whereas the morphology of the layer on the boundary of the cast iron insert is flat, which means that the intermetallic phases grow mainly through the diffusion of elements in the cast iron. When the aluminium alloy of the piston is poured into the mould, the reaction conditions between the cast iron ring and the piston alloy are similar to those that occur with the ring immersed in a liquid and hot aluminium alloy. As a result of transferring the ring from the hot AlSi9 alloy immersion bath into the mould, the residual molten aluminium bonded to the ring does not have enough time to fuse. In area 2 (the S2N aluminium alloy), as shown in Figure 10a, diffusion of Fe atoms towards the S2N aluminium alloy in the form of flake-shaped clusters can be observed. This microstructure is superimposed onto the intermetallic layer. The saturation of the layer with iron atoms depends on the duration of Al-Fin process. The intermetallic layer determines the quality of the joint between the cast iron insert and the S2N aluminium alloy of the piston.
In a diffusion-type joint, there should be no intermetallic compounds that are hard and brittle, because this results in a loss of quality. The diffusion layer should contain intermetallic phases of low hardness. Important factors are the parameters of the Al-Fin process (duration and temperature). The thickness of the diffusion layer is usually 0.02 to 0.03 mm. A thickness not exceeding 0.03 mm has a positive effect on the properties of the transition layer, as it is relatively thin. Its microstructure contains phases of low hardness, mostly FeAl (approx. 750 µHV). The formation of more brittle compounds with greater hardness is associated with an increase in layer thickness and the presence of two phases, e.g., FeAl and Fe2Al5 (approx. 1150 µHV).
5. Conclusions
In this paper, the microstructure of the bimetallic joints between the cast iron insert and the AlSi9 alloy cast obtained after the Al-Fin process was examined. It is possible to carry out the Al-Fin process for the material pairing of EN-GJLA-XNiCuCr15-6-2 with the AlSi9 aluminium alloy. Casting trials were successfully carried out directly and without any heat treatment. A good metallic bond can be formed between the cast iron insert and the aluminium alloy piston. The following key points of the current research can be drawn:
- The thickness of the diffusion layer formed between the piston alloy (AlSi9) and the cast iron does not increase with the increase in the duration of the Al-Fin process.
- It was found that the change in temperature of the AlSi9 alloy and the duration of the Al-Fin process did not significantly affect the thickness of the intermetallic layer.
- A statistically significant effect of both the duration of the Al-Fin process and the temperature of the AlSi9 alloy on the thickness of the transition layer of the bimetallic joint was observed.
- The highest value of hardness was noted in the resulting intermetallic compound Al2Fe5. It is higher compared to the hardness of the joined materials.
- The content of flake graphite in the resulting intermetallic compound decreases with increasing duration of the Al-Fin process. The thicker the transition layer becomes, the less graphite is present in the resulting intermetallic compound.
- The diffusion of Fe atoms towards the AlSi9 aluminium alloy in the form of flake-shaped clusters was observed.
- The saturation of the diffusion layer with iron atoms depends on the duration of the Al-Fin process.
- The SEM-EDS analysis showed the presence of the FeAl3 and Fe2Al5 intermetallic phases in the resulting bimetallic joint. The intermetallic layer determines the quality of the joint between the cast iron insert and the S2N alloy of the piston. The boundary between the Fe2Al5 intermetallic layer and the aluminium alloy zone of the piston is jagged.
- The morphology of the layer on the boundary between the cast iron insert and the AlSi9 layer is flat, which means that the intermetallic phases grow mainly through the diffusion of elements in the cast iron.
Author Contributions
Conceptualization, K.S. and J.Z.-S.; methodology, K.S. and J.Z.-S.; validation, K.S., J.Z.-S. and T.T.; formal analysis, K.S. and T.T.; investigation, K.S.; data curation, K.S. and J.Z.-S.; writing—original draft, K.S., J.Z.-S. and T.T.; writing—review and editing, K.S. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szajnar, J.; Wróbel, P.; Wróbel, T. Model castings with composite surface layer—Application. Arch. Foundry Eng. 2008, 8, 105–110. [Google Scholar]
- Żaba, K.; Trzepieciński, T. The Role of Al-10%Si Coating in the Manufacture and Use of Aluminized Open-Joint Steel Tubes. Materials 2022, 15, 4210. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, J.; Szajnar, J.; Wróbel, P. Study on theoretical bases of receiving composite alloy layers on surface of cast steel castings. J. Mater. Process. Technol. 2004, 157–158, 679–682. [Google Scholar] [CrossRef]
- Tayal, R.K.; Kumar, S.; Singh, V.; Garga, R. Characterization and Microhardness Evaluation of A356/Mg Joint Produced by Vacuum-Assisted Sand Mold Compound Casting Process. Int. J. Met. 2019, 13, 392–406. [Google Scholar] [CrossRef]
- Tayal, R.K.; Kumar, S.; Singh, V.; Gupta, A.; Ujjawal, D. Experimental Investigation and Evaluation of Joint Strength of A356/Mg Bimetallic Fabricated Using Compound Casting Process. Int. J. Met. 2019, 13, 686–699. [Google Scholar] [CrossRef]
- Bae, J.H.; Prasada Rao, A.K.; Kim, K.H.; Kim, N.J. Cladding of Mg alloy with Al by twin-roll casting. Scr. Mater. 2011, 64, 836–839. [Google Scholar] [CrossRef]
- Hajjari, E.; Divandari, M.; Razavi, S.H.; Homma, T.; Kamado, S. Intermetallic compounds and antiphase domains in Al/Mg compound casting. Intermetallics 2012, 23, 182–186. [Google Scholar] [CrossRef]
- Paramsothy, M.; Srikanth, N.; Gupta, M. Solidification processed Mg/Al bimetal macrocomposite: Microstructure and mechanical properties. J. Alloy. Compd. 2008, 461, 200–208. [Google Scholar] [CrossRef]
- Divandari, M.; Vahid Golpayegani, A.R. Study of Al/Cu rich phases formed in A356 alloy by inserting Cu wire in pattern in LFC process. Mater. Des. 2009, 30, 3279–3285. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, Y.J.; Park, K.T.; Nersisyan, H.H.; Jeong, H.G.; Lee, J.H. Controlling Al/Cu composite diffusion layer during hydrostatic extrusion by using colloidal Ag. J. Mater. Process. Technol. 2013, 213, 487–494. [Google Scholar] [CrossRef]
- Viala, J.C.; Peronnet, M.; Barbeau, F.; Bosselet, F.; Bouix, J. Interface chemistry in aluminium alloy castings reinforced with iron base inserts. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1417–1420. [Google Scholar] [CrossRef]
- Chen, G.; He, Y.H.; Shen, P.Z. Research actualities on materials and processes of engine piston parts and cylinder liner. Mater. Sci. Eng. Powder Metall. 2009, 14, 205–212. [Google Scholar]
- Uthayakumar, M.; Prabhakaran, P.; Aravindan, S.; Sivaprasad, J.V. Precision Machining of an Aluminum Alloy Piston Reinforced with a Cast Iron Insert. Int. J. Precis. Eng. Manuf. 2009, 10, 7–13. [Google Scholar] [CrossRef]
- Dolata, A.J.; Wieczorek, J.; Dyzia, M.; Starczewski, M. Assessment of the Tribological Properties of Aluminum Matrix Composites Intended for Cooperation with Piston Rings in Combustion Engines. Materials 2022, 15, 3806. [Google Scholar] [CrossRef]
- Manasijević, S.; Radiša, R.; Brodarac, Z.Z.; Dolić, N.; Djurdjevic, M. Al-Fin bond in aluminum piston alloy & austenitic cast iron insert. Int. J. Met. 2015, 9, 27–32. [Google Scholar]
- Aguado, E.; Baquedano, A.; Uribe, U.; Fernandez-Calvo, A.I.; Niklas, A. Comparative Study of Different Interfaces of Steel Inserts in Aluminium Castings. Mater. Sci. Forum 2013, 765, 711–715. [Google Scholar]
- Suryadarma, E.H.E.; Ai, T.J.; Bawono, B.; Siswantoro, A.T. Improving Bimetal Bond Quality Between Cast Steel and Aluminum Alloys Using Response Surface Methodology. Int. J. Met. 2022, 16, 1432–1441. [Google Scholar] [CrossRef]
- Manasijević, S.; Dolić, N.; Zovko-Brodarac, Z.; Djurdjević, M.; Radiš, R. An Analysis of Intermetallic Bonding between a Ring Carrier and an Aluminum Piston Alloy. Metall. Mater. Trans. 2014, 45, 4580–4587. [Google Scholar] [CrossRef]
- Acar, F.; Ozturk, F.; Bayrak, M. Effects of Variations in Alloy Content and Machining Parameters on the Strength of the Intermetallic Bonding Between a Diesel Piston and a Ring Carrier. Mater. Technol. 2010, 44, 391–395. [Google Scholar]
- Ramadan, M.; Khaliq, A.; Hafez, K.M.; Alghamdi, A.S.; Fathy, N.; Harraz, F.A.; Ayadi, B.; Abdel Halim, K.S. Super Bonding Strength of Al2O3 Nanoparticles Reinforced Sn Interlayer Steel/Aluminum Bimetal Casting. Crystals 2022, 12, 324. [Google Scholar] [CrossRef]
- Li, L.; Sang, G.; Jiang, G. Current researches and status of hot dip aluminizing. Mater. Rev. 2008, 22, 76–78. [Google Scholar]
- Li, C.; Xiong, J.; Sun, L.; Liao, Z.; Peng, M.C. Effect of Si content in hot dipping aluminium bath on Al–Fe bonding layer of aluminium piston with reinforced cast iron ring. Mater. Sci. Technol. 2012, 28, 953–958. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysserpyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low carbon steel and aluminium alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Kennedy, D.M.; Vahey, J.; Hanney, D. Micro shot blasting of machine tools for improving surface finish and reducing cutting forces in manufacturing. Mater. Des. 2005, 26, 203–208. [Google Scholar] [CrossRef]
- Javier, G.F.; Planell, J.A.; Padrós, A.; Aparicio, C. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar] [CrossRef]
- Khan, S.A.; Bhuiyan, M.S.; Miyashita, Y.; Mutoh, Y.; Koike, T. Corrosion fatigue behavior of die-cast and shot-blasted AM60 magnesium alloy. Mater. Sci. Eng. A 2011, 528, 1961–1966. [Google Scholar] [CrossRef]
- Lee, T.C.; Deng, J.X. Mechanical surface treatments of edged ceramic composites for improved strength and reliability. J. Eur. Ceram. Soc. 2002, 22, 545–550. [Google Scholar] [CrossRef]
- Raykowski, A.; Hader, M. Blasting cleaning of gas turbine components: Deposit removal and substrate deformation. Wear 2001, 249, 127–132. [Google Scholar] [CrossRef]
- Piątkowski, J.; Kamiński, P. Chosen Aspects of Quality Defects of ”Alphin” Inserts in Combustions Pistons. Arch. Foundry Eng. 2015, 15, 61–64. [Google Scholar] [CrossRef]
- Uthayakumar, M.; Prabhakaran, G.; Aravindan, S.; Sivaprasad, J.V. Influence of cutting force on bimetallic piston machning by a cubic boron nitride (CBN) tool. Mater. Manuf. Process. 2012, 27, 1078–1083. [Google Scholar] [CrossRef]
- Dolata, A.J.; Dyzia, M.; Wieczorek, J. Tribological Properties of Single (AlSi7/SiCp, AlSi7/GCsf) and Hybrid (AlSi7/SiCp + GCsf) Composite Layers Formed in Sleeves via Centrifugal Casting. Materials 2019, 12, 2803. [Google Scholar] [CrossRef]
- Piątkowski, J.; Czerepak, M. The Crystallization of the AlSi9 Alloy Designed for the Alfin Processing of Ring Supports in Engine Pistons. Arch. Foundry Eng. 2020, 11, 65–70. [Google Scholar]
- Stolbchenko, M.; Makeieva, H.; Grydin, O.; Frolov, Y.; Schaper, M. Strain parameters at hot rolling of aluminum strips reinforced with steel netting. J. Sandw. Struct. Mater. 2020, 22, 2009–2029. [Google Scholar] [CrossRef]
- Karathanasopoulos, N.; Mohr, D. Strength and Failure of Self-Piercing Riveted Aluminum and Steel Sheet Joints: Multi-axial Experiments and Modeling. J. Adv. Join. Process. 2022, 5, 100107. [Google Scholar] [CrossRef]
- Kuryntsev, S. A Review: Laser Welding of Dissimilar Materials (Al/Fe, Al/Ti, Al/Cu)—Methods and Techniques, Microstructure and Properties. Materials 2022, 15, 122. [Google Scholar] [CrossRef]
- Meco, S.; Cozzolino, L.; Ganguly, S.; Williams, S.; McPerson, N. Laser welding of steel to aluminium: Thermal modelling and joint strength analysis. J. Mater. Process. Technol. 2017, 247, 121–133. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, X.; Huang, Y.; Yu, L. Numerical analysis of the influence of molten pool instability on the weld formation during the high speed fiber laser welding. Int. J. Heat Mass Transf. 2020, 160, 120103. [Google Scholar] [CrossRef]
- Ai, Y.; Yu, L.; Huang, Y.; Liu, X. The investigation of molten pool dynamic behaviors during the ”∞” shaped oscillating laser welding of aluminum alloy. Int. J. Therm. Sci. 2022, 173, 107350. [Google Scholar] [CrossRef]
- Ahmed, M.M.Z.; El-Sayed Seleman, M.M.; Fydrych, D.; Çam, G. Friction Stir Welding of Aluminum in the Aerospace Industry: The Current Progress and State-of-the-Art Review. Materials 2023, 16, 2971. [Google Scholar] [CrossRef]
- Mahto, R.P.; Pal, S.K. Friction Stir Welding of Dissimilar Materials: An Investigation of Microstructure and Nano-Indentation Study. J. Manuf. Process. 2020, 55, 103–118. [Google Scholar] [CrossRef]
- Basak, S.; Das, H.; Pal, T.K. Characterization of intermetallics in aluminum to zinc coated interstitial free steel joining by pulsed MIG brazing for automotive application. Mater. Charact. 2016, 112, 229–237. [Google Scholar] [CrossRef]
- Tayal, R.K.; Singh, V.; Kumar, S.; Garg, R. Compound casting—A literature review. In Proceedings of the National Conference on Trends and Advances in Mechanical Engineering, YMCA University of Science and Technology, Faridabad, India, 19–20 October 2012. [Google Scholar]
- Liu, Y.; Bian, X.F.; Yang, J.; Zhang, K.; Feng, L.; Yang, C.C. An investigation of metallurgical bonding in Al–7Si/gray iron bimetal composites. J. Mater. Res. 2013, 28, 3190–3198. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, M.; Bian, X.; Liu, M.; Li, J. An Al–7Si alloy/cast iron bimetallic composite with super-high shear strength. J. Mater. Res. Technol. 2019, 8, 3126–3136. [Google Scholar] [CrossRef]
- Osak, P.; Maszybrocka, J.; Zubko, M.; Rak, J.; Bogunia, S.; Łosiewicz, B. Influence of Sandblasting Process on Tribological Properties of Titanium Grade 4 in Artificial Saliva for Dentistry Applications. Materials 2021, 14, 7536. [Google Scholar] [CrossRef]
- Li, X.; Scherf, A.; Heilmaier, M.; Stein, F. The Al-Rich Part of the Fe-Al Phase Diagram. J. Phase Equilib. Diffus. 2016, 37, 162–173. [Google Scholar] [CrossRef]
- Manasijević, S.; Dolić, N.; Djurdjevic, M.; Mišić, N.; Davitkov, N. The intermetallic bonding between a ring carrier and aluminum piston alloy. Rev. Metal. 2015, 51, e048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).