Acid Leaching of Al- and Ta-Substituted Li7La3Zr2O12 (LLZO) Solid Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. LLZO Samples
2.1.2. Chemicals
2.2. Methods
2.2.1. Characterization of the LLZO Samples
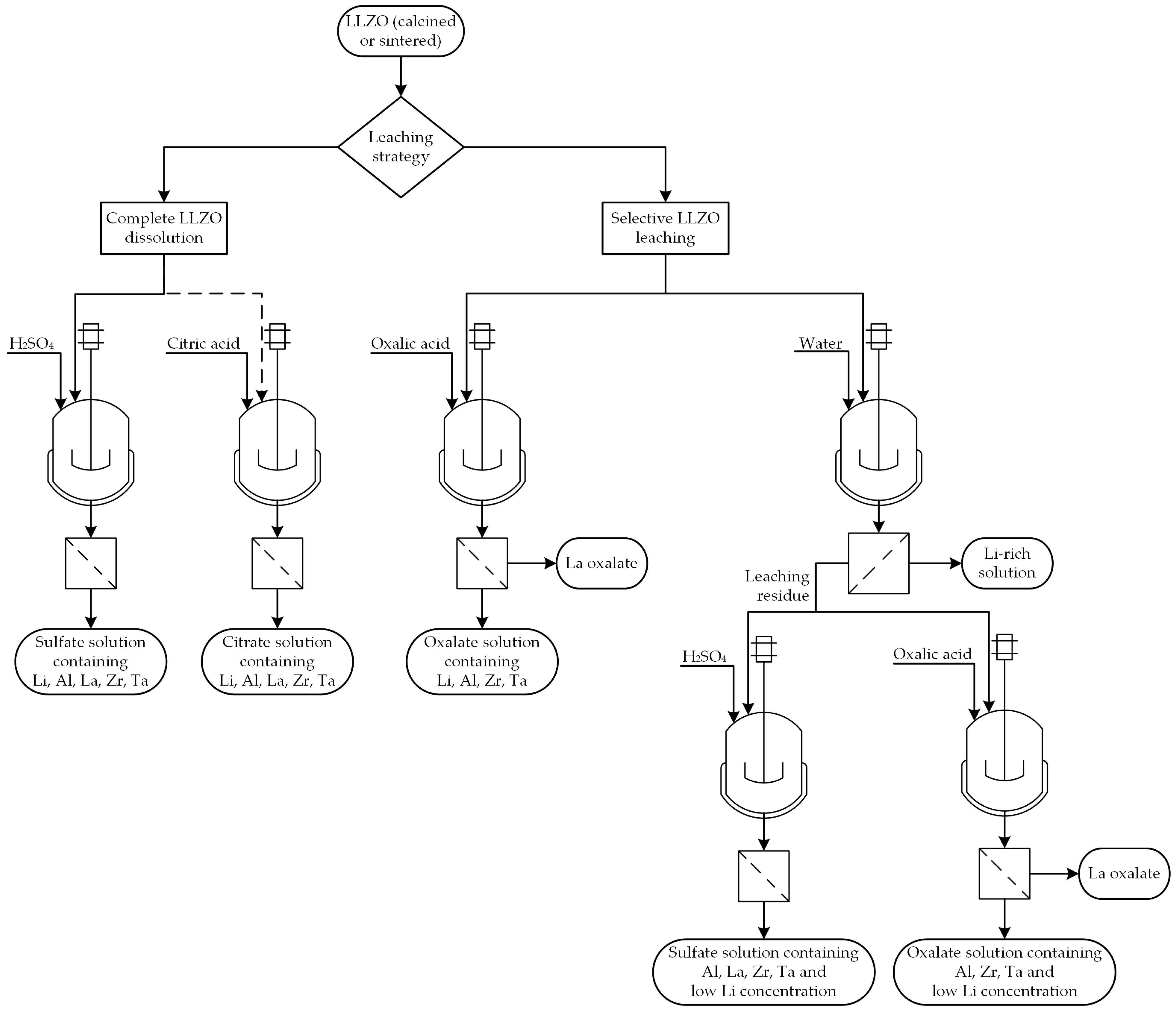
2.2.2. Leaching Experiments
3. Results and Discussion
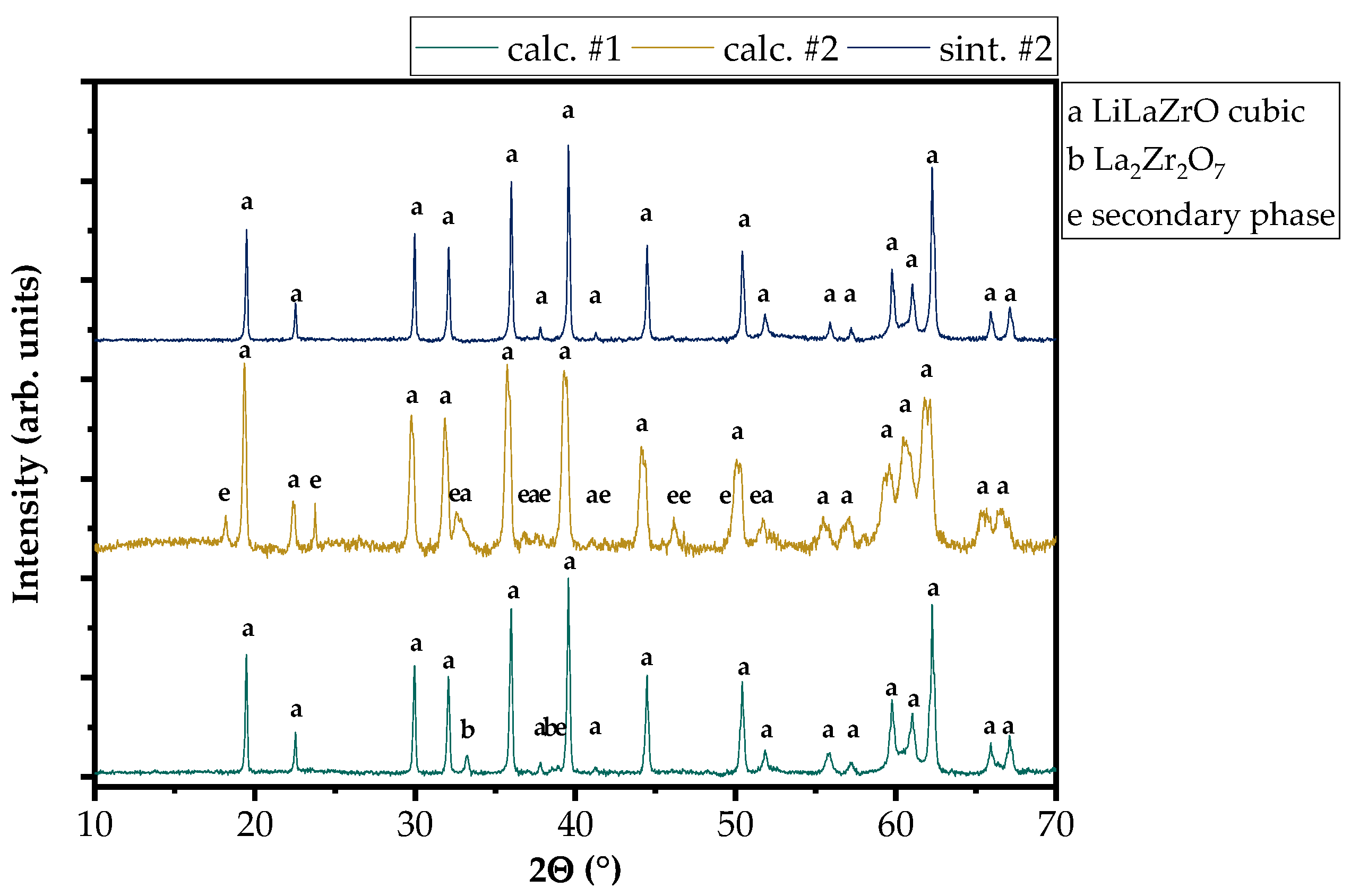
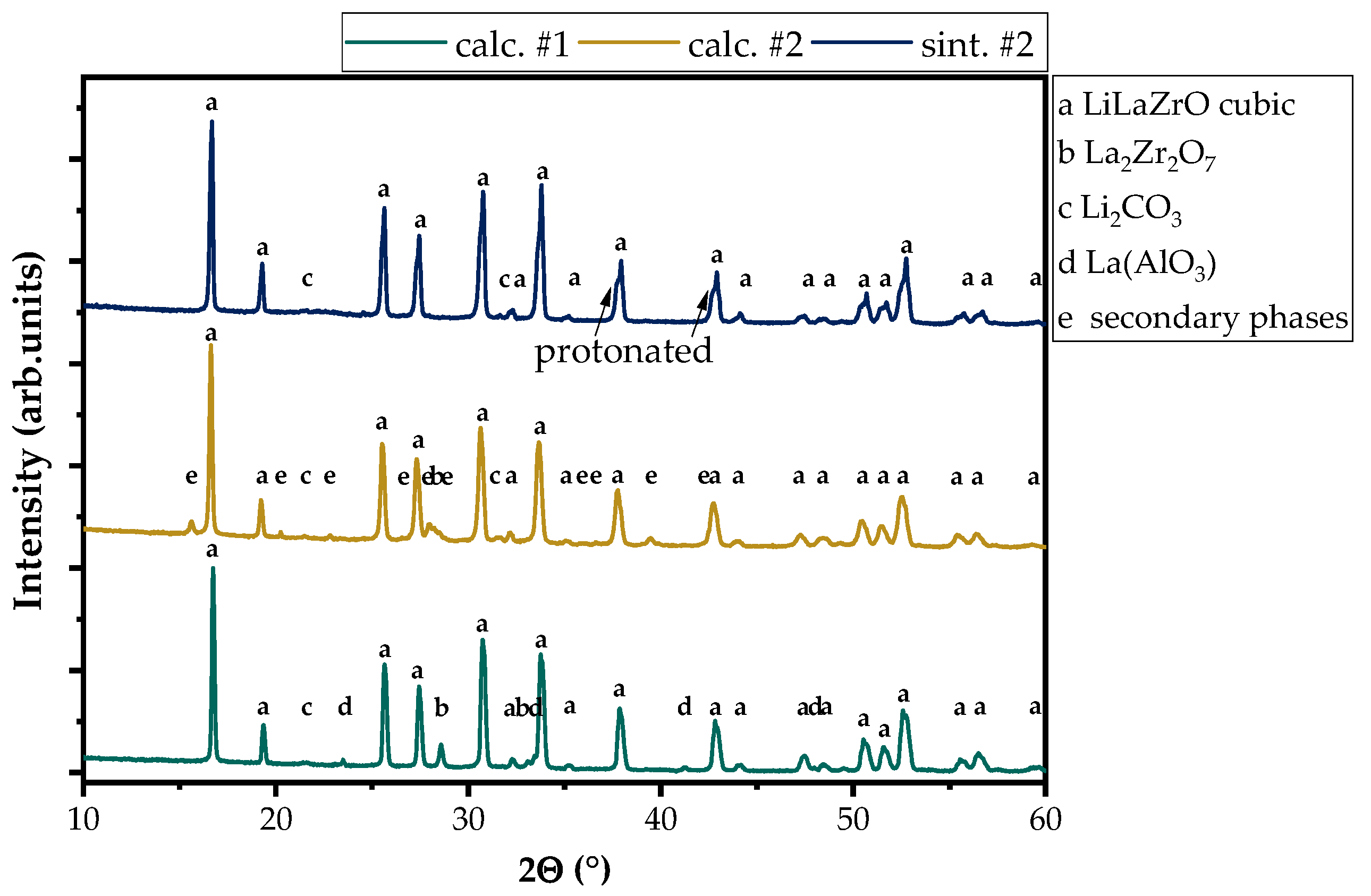
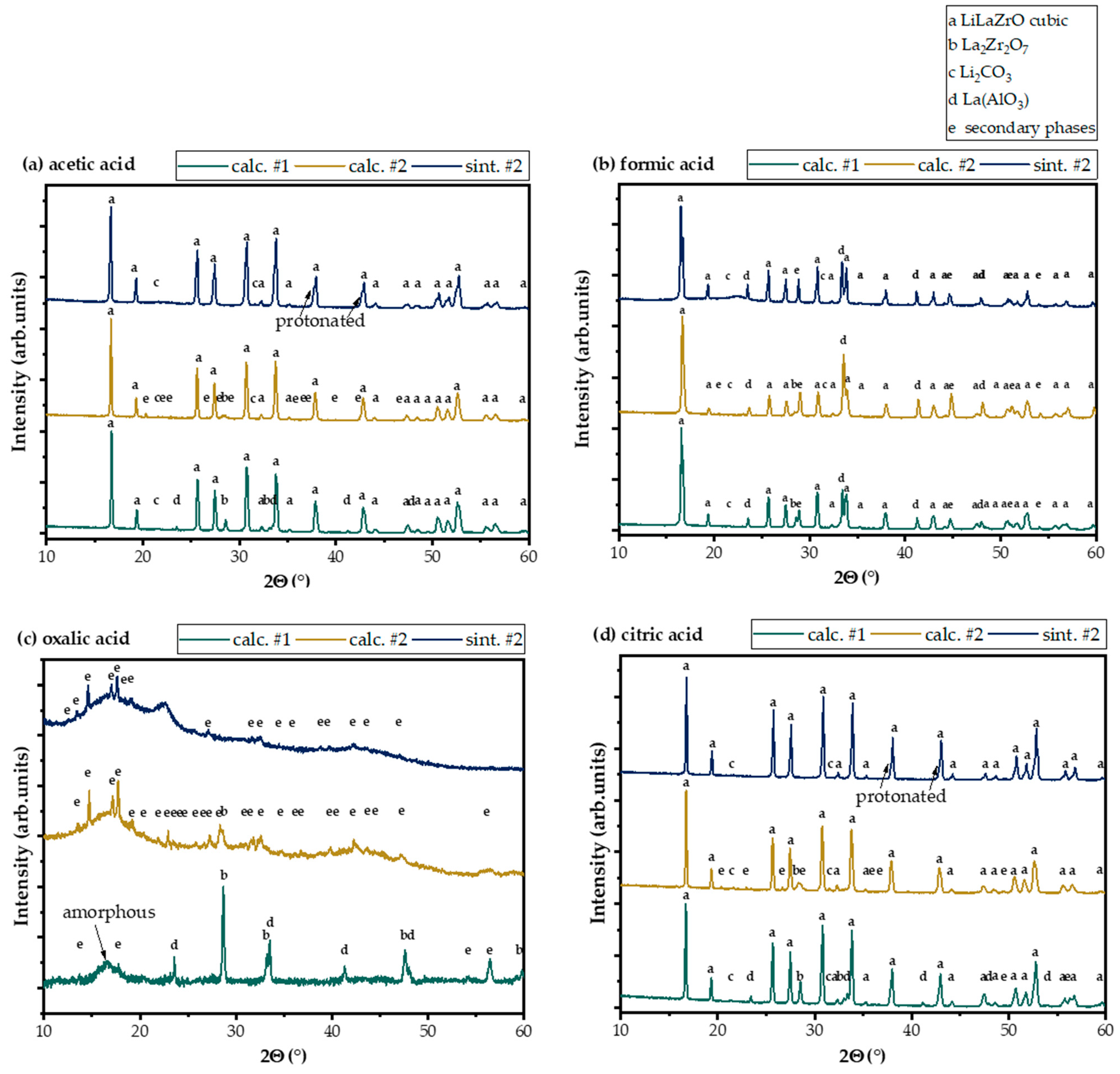
3.1. Characterization of the LLZO Samples
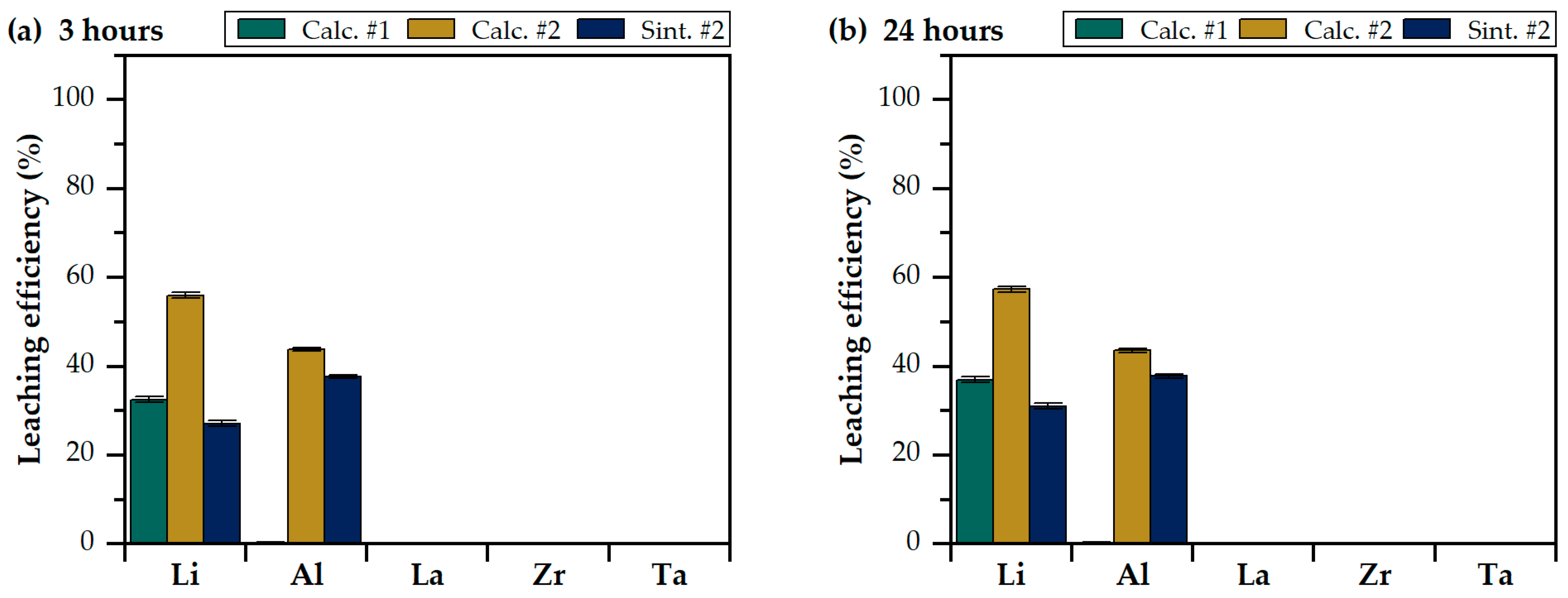
3.2. Leaching in Water
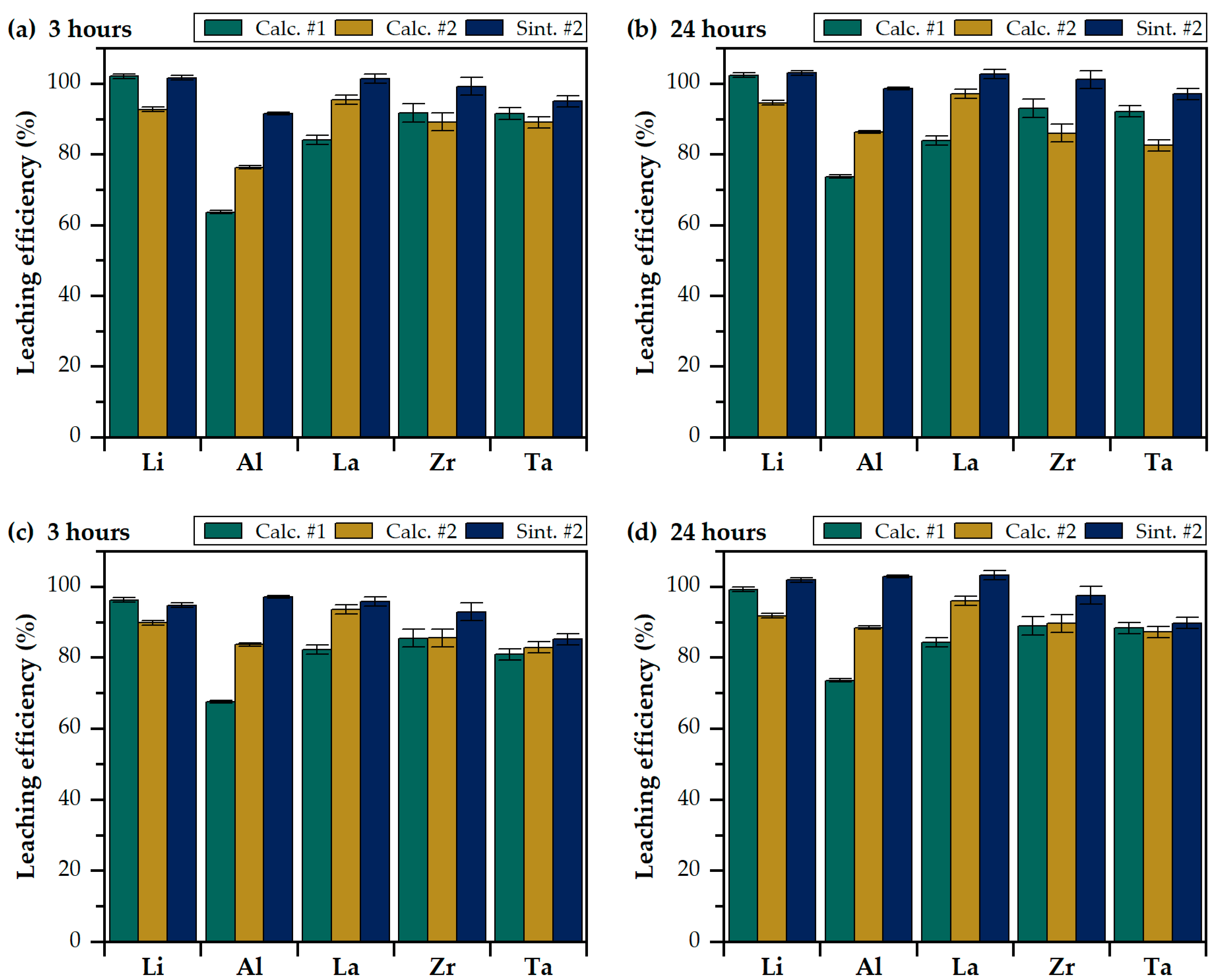
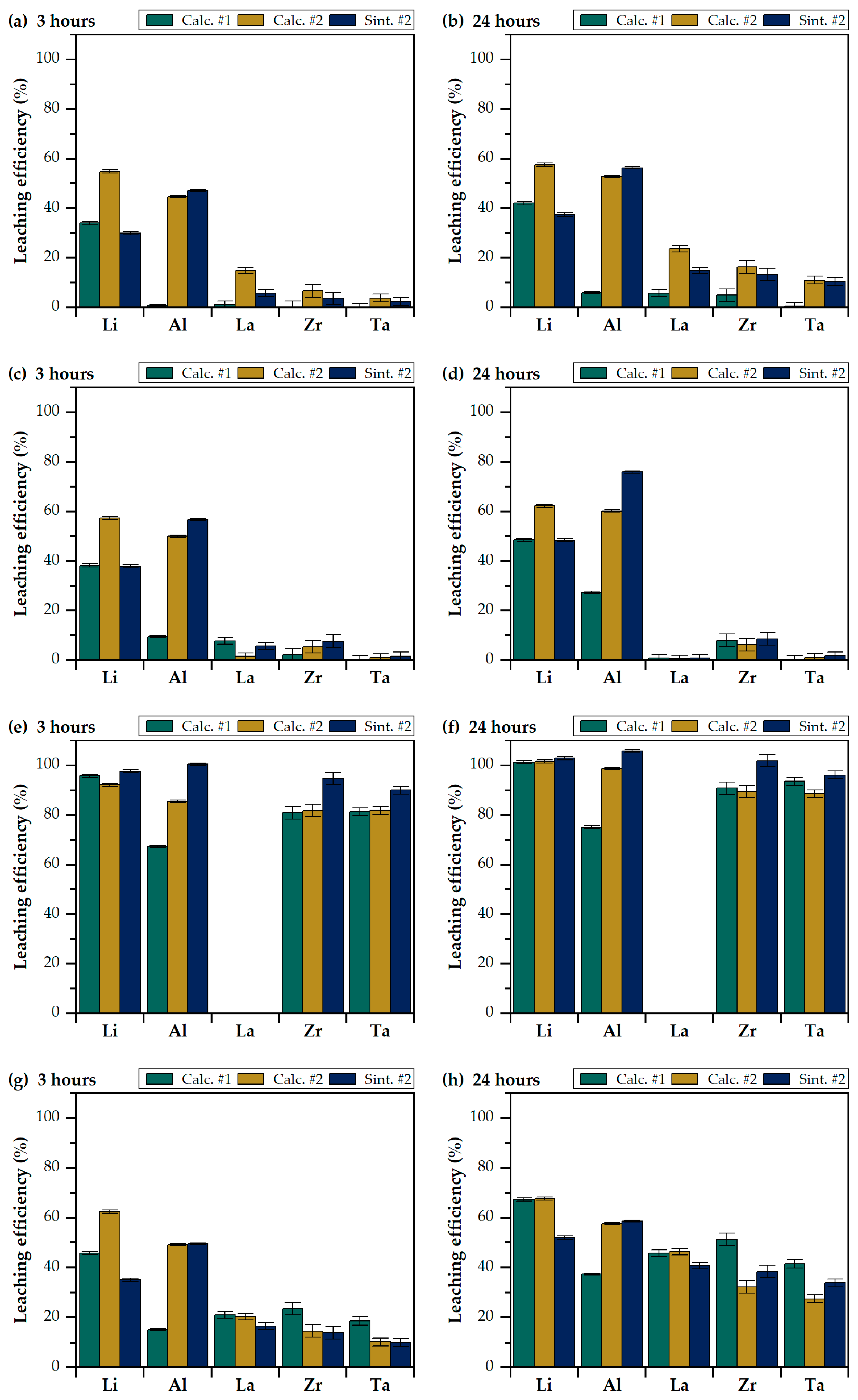
3.3. Leaching with Mineral Acids
3.4. Leaching with Organic Acids
3.5. Assessment of Lixiviants
- CRM leaching efficiency YCRM: a measure of Li, La, and Ta leaching efficiencies after 24 h normalized to 100%;
- Li selectivity SLi: a measure of how selectively a lixiviant transfers Li into the leach solution after 24 h of leaching (≤1 unselective, >1 selective);
- La selectivity SLa: a measure of how selectively a lixiviant transfers La into the leach solution—or the residue if La is not leached—after 24 h of leaching (≤1 unselective, >1 selective);
- Increase in leaching efficiency ∆Y: average increase in leaching efficiencies of all elements analyzed from 3 to 24 h, weighted according to the chemical composition of the feed (<1—decrease in leaching efficiency, 1—no change in leaching efficiency, and >1—increase in leaching efficiency);
- Share of residue after leaching wresidue: a measure of the proportion of the solid residue in relation to the initial sample weight.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smalley, R.E. Future Global Energy Prosperity: The Terawatt Challenge. MRS Bull. 2005, 30, 412–417. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef]
- Nagaura, T. Lithium Ion Rechargeable Battery. Prog. Batter. Sol. Cells 1990, 9, 209–217. [Google Scholar]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Pillot, C. EU Battery Demand and Supply (2019–2030) in a Global Context. Available online: https://www.eurobat.org/wp-content/uploads/2021/05/Avicenne_EU_Market_-_summary_110321.pdf (accessed on 28 January 2023).
- RECHARGE Battery Industry Association. Battery Market and Technologies. Available online: https://rechargebatteries.org/battery-market-and-technologies/ (accessed on 28 January 2023).
- Zhang, Y.S.; Schneider, K.; Qiu, H.; Zhu, H.L. A perspective of low carbon lithium-ion battery recycling technology. Carbon Capture Sci. Technol. 2022, 5, 100074. [Google Scholar] [CrossRef]
- Nigl, T.; Baldauf, M.; Hohenberger, M.; Pomberger, R. Lithium-Ion Batteries as Ignition Sources in Waste Treatment Processes-A Semi-Quantitate Risk Analysis and Assessment of Battery-Caused Waste Fires. Processes 2021, 9, 49. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energ. Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Gonzalez Puente, P.M.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.; Hood, Z.D.; Rupp, J.L.M. Processing thin but robust electrolytes for solid-state batteries. Nat. Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Takada, K. Progress in solid electrolytes toward realizing solid-state lithium batteries. J. Power Source 2018, 394, 74–85. [Google Scholar] [CrossRef]
- Schneider, K.; Goldmann, D. Oxide-based lithium solid-state batteries from a recycling perspective. In Proceedings of the 16. Recy & DepoTech-Konferenz, Leoben, Austria, 9–11 November 2022; Pomberger, R., Ed.; Abfallverwertungstechnik & Abfallwirtschaft Eigenverlag. pp. 381–386. [Google Scholar]
- Blengini, G.A.; El Latunussa, C.; Eynard, U.; Torres de Matos, C.; Wittmer, D.M.A.G.; Georgitzikis, K.; Pavel, C.C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials (2020): Final Report; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-21049-8. [Google Scholar]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Doose, S.; Mayer, J.K.; Michalowski, P.; Kwade, A. Challenges in Ecofriendly Battery Recycling and Closed Material Cycles: A Perspective on Future Lithium Battery Generations. Metals 2021, 11, 291. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Nasser, O.A.; Petranikova, M. Review of Achieved Purities after Li-ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance. Batteries 2021, 7, 60. [Google Scholar] [CrossRef]
- Gupta, C.K.; Mukherjee, T.K. Hydrometallurgy in Extraction Processes; CRC Press: Boca Raton, FL, USA, 1990; ISBN 0849368049. [Google Scholar]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Source 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Okonkwo, E.G.; Wheatley, G.; He, Y. The role of organic compounds in the recovery of valuable metals from primary and secondary sources: A mini-review. Resour. Conserv. Recycl. 2021, 174, 105813. [Google Scholar] [CrossRef]
- Schwich, L.; Küpers, M.; Finsterbusch, M.; Schreiber, A.; Fattakhova-Rohlfing, D.; Guillon, O.; Friedrich, B. Recycling Strategies for Ceramic All-Solid-State Batteries-Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling. Metals 2020, 10, 1523. [Google Scholar] [CrossRef]
- Ali Nowroozi, M.; Iqbal Waidha, A.; Jacob, M.; van Aken, P.A.; Predel, F.; Ensinger, W.; Clemens, O. Towards Recycling of LLZO Solid Electrolyte Exemplarily Performed on LFP/LLZO/LTO Cells. ChemistryOpen 2022, 11, e202100274. [Google Scholar] [CrossRef]
- Malkowski, T.F.; Boeding, E.D.; Fattakhova-Rohlfing, D.; Wettengl, N.; Finsterbusch, M.; Veith, G.M. Digestion processes and elemental analysis of oxide and sulfide solid electrolytes. Ionics 2022, 28, 3223–3231. [Google Scholar] [CrossRef]
- Geiger, C.A.; Alekseev, E.; Lazic, B.; Fisch, M.; Armbruster, T.; Langner, R.; Fechtelkord, M.; Kim, N.; Pettke, T.; Weppner, W. Crystal chemistry and stability of “Li7La3Zr2O12” garnet: A fast lithium-ion conductor. Inorg. Chem. 2011, 50, 1089–1097. [Google Scholar] [CrossRef]
- Buschmann, H.; Dölle, J.; Berendts, S.; Kuhn, A.; Bottke, P.; Wilkening, M.; Heitjans, P.; Senyshyn, A.; Ehrenberg, H.; Lotnyk, A.; et al. Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”. Phys. Chem. Chem. Phys. 2011, 13, 19378–19392. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 97th ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017; ISBN 9781498754293. [Google Scholar]
- Rosen, M.; Ye, R.; Mann, M.; Lobe, S.; Finsterbusch, M.; Guillon, O.; Fattakhova-Rohlfing, D. Controlling the lithium proton exchange of LLZO to enable reproducible processing and performance optimization. J. Mater. Chem. A 2021, 9, 4831–4840. [Google Scholar] [CrossRef]
- Ye, R.; Ihrig, M.; Imanishi, N.; Finsterbusch, M.; Figgemeier, E. A Review on Li+/H+ Exchange in Garnet Solid Electrolytes: From Instability against Humidity to Sustainable Processing in Water. ChemSusChem 2021, 14, 4397–4407. [Google Scholar] [CrossRef]
- Cheng, L.; Crumlin, E.J.; Chen, W.; Qiao, R.; Hou, H.; Franz Lux, S.; Zorba, V.; Russo, R.; Kostecki, R.; Liu, Z.; et al. The origin of high electrolyte-electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 18294–18300. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Li7La3Zr2O12 electrolyte stability in air and fabrication of a Li/Li7La3Zr2O12/Cu0.1V2O5 solid-state battery. J. Power Source 2013, 239, 326–331. [Google Scholar] [CrossRef]
- Sharafi, A.; Yu, S.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H.M.; Nanda, J.; Chi, M.; Siegel, D.J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 2017, 5, 13475–13487. [Google Scholar] [CrossRef]
- Truong, L.; Thangadurai, V. Soft-Chemistry of Garnet-Type Li5+xBaxLa3–xNb2O12 (x = 0, 0.5, 1): Reversible H+ ↔ Li+ Ion-Exchange Reaction and Their X-ray, 7Li MAS NMR, IR, and AC Impedance Spectroscopy Characterization. Chem. Mater. 2011, 23, 3970–3977. [Google Scholar] [CrossRef]
- Liu, C.; Rui, K.; Shen, C.; Badding, M.E.; Zhang, G.; Wen, Z. Reversible ion exchange and structural stability of garnet-type Nb-doped Li7La3Zr2O12 in water for applications in lithium batteries. J. Power Source 2015, 282, 286–293. [Google Scholar] [CrossRef]
- Yow, Z.F.; Oh, Y.L.; Gu, W.; Rao, R.P.; Adams, S. Effect of Li+/H+ exchange in water treated Ta-doped Li7La3Zr2O12. Solid State Ion. 2016, 292, 122–129. [Google Scholar] [CrossRef]
- Cao, S.; Song, S.; Xiang, X.; Hu, Q.; Zhang, C.; Xia, Z.; Xu, Y.; Zha, W.; Li, J.; Gonzale, P.M.; et al. Modeling, Preparation, and Elemental Doping of Li7La3Zr2O12 Garnet-Type Solid Electrolytes: A Review. J. Korean Ceram. Soc 2019, 56, 111–129. [Google Scholar] [CrossRef]
- Peng, F.; Mu, D.; Li, R.; Liu, Y.; Ji, Y.; Dai, C.; Ding, F. Impurity removal with highly selective and efficient methods and the recycling of transition metals from spent lithium-ion batteries. RSC Adv. 2019, 9, 21922–21930. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants: Other Organic Ligands; Springer: Boston, MA, USA, 1977; ISBN 978-1-4757-1570-5. [Google Scholar]
- Martell, A.E. Critical Stability Constants: Second Supplement; Springer: New York, NY, USA, 1989; ISBN 9781461567646. [Google Scholar]
- Martell, A.E. Critical Stability Constants: First Supplement; Springer: New York, NY, USA, 1982; ISBN 9781461567615. [Google Scholar]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Kolthoff, I.M.; Elmquist, R. The solubilities of lanthanum oxalate and of lanthanum hydroxide in water. The mobility of the lanthanum ion at 25°. J. Am. Chem. Soc. 1931, 53, 1217–1225. [Google Scholar] [CrossRef]
- Balboul, B.A.A.; El-Roudi, A.M.; Samir, E.; Othman, A.G. Non-isothermal studies of the decomposition course of lanthanum oxalate decahydrate. Thermochim. Acta 2002, 387, 109–114. [Google Scholar] [CrossRef]
- Schwich, L.; Schubert, T.; Friedrich, B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals 2021, 11, 177. [Google Scholar] [CrossRef]

| Element | Calcined LLZO #1 | Calcined LLZO #2 | Sintered LLZO #2 | Mean Value | Sample Standard Deviation | Theoretical Composition |
|---|---|---|---|---|---|---|
| Li | 5.76 | 5.19 | 5.42 | 5.46 | 0.29 | 5.13 |
| Al | 0.66 | 0.51 | 0.66 | 0.61 | 0.09 | 0.15 |
| La | 42.72 | 40.17 | 42.95 | 41.95 | 1.54 | 47.72 |
| Zr | 15.35 | 14.56 | 14.40 | 14.77 | 0.51 | 16.72 |
| Ta | 6.69 | 6.57 | 7.10 | 6.79 | 0.28 | 8.29 |
| Lixiviant | CRM Leaching Efficiency (%) | Li Selectivity (-) | La Selectivity (-) | Increase of Leaching Efficiency (-) | Share of Residue after Leaching (wt%) |
|---|---|---|---|---|---|
| Water | 13.9 | 2.5 | 1.2 1 | 1.0 | 86.4 |
| Sulfuric acid | 95.1 | 1.1 | 1.0 | 1.0 | 3.4 |
| Hydrochloric acid | 93.6 | 1.0 | 1.0 | 1.0 | 4.7 |
| Acetic acid | 22.6 | 1.7 | 0.6 | 5.7 | 76.7 |
| Formic acid | 18.3 | 2.0 | 1.3 1 | 0.8 | 93.5 |
| Oxalic acid | 64.9 | 1.3 | 4.4 1 | 1.0 | 90.8 |
| Citric acid | 47.0 | 1.3 | 0.9 | 2.3 | 51.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, K.; Kiyek, V.; Finsterbusch, M.; Yagmurlu, B.; Goldmann, D. Acid Leaching of Al- and Ta-Substituted Li7La3Zr2O12 (LLZO) Solid Electrolyte. Metals 2023, 13, 834. https://doi.org/10.3390/met13050834
Schneider K, Kiyek V, Finsterbusch M, Yagmurlu B, Goldmann D. Acid Leaching of Al- and Ta-Substituted Li7La3Zr2O12 (LLZO) Solid Electrolyte. Metals. 2023; 13(5):834. https://doi.org/10.3390/met13050834
Chicago/Turabian StyleSchneider, Kirstin, Vivien Kiyek, Martin Finsterbusch, Bengi Yagmurlu, and Daniel Goldmann. 2023. "Acid Leaching of Al- and Ta-Substituted Li7La3Zr2O12 (LLZO) Solid Electrolyte" Metals 13, no. 5: 834. https://doi.org/10.3390/met13050834
APA StyleSchneider, K., Kiyek, V., Finsterbusch, M., Yagmurlu, B., & Goldmann, D. (2023). Acid Leaching of Al- and Ta-Substituted Li7La3Zr2O12 (LLZO) Solid Electrolyte. Metals, 13(5), 834. https://doi.org/10.3390/met13050834






