Relation between Viscosity and Conductivity of CaO-MgO-FeO-Al2O3-SiO2 System for Copper Smelting Slags
Abstract
1. Introduction
2. Experiment
2.1. Preparation of Slag Samples
2.2. Determination of Slag Viscosity
2.3. Determination of Slag Conductivity
3. Results and Discussion
3.1. Chemical Composition of Slag Samples
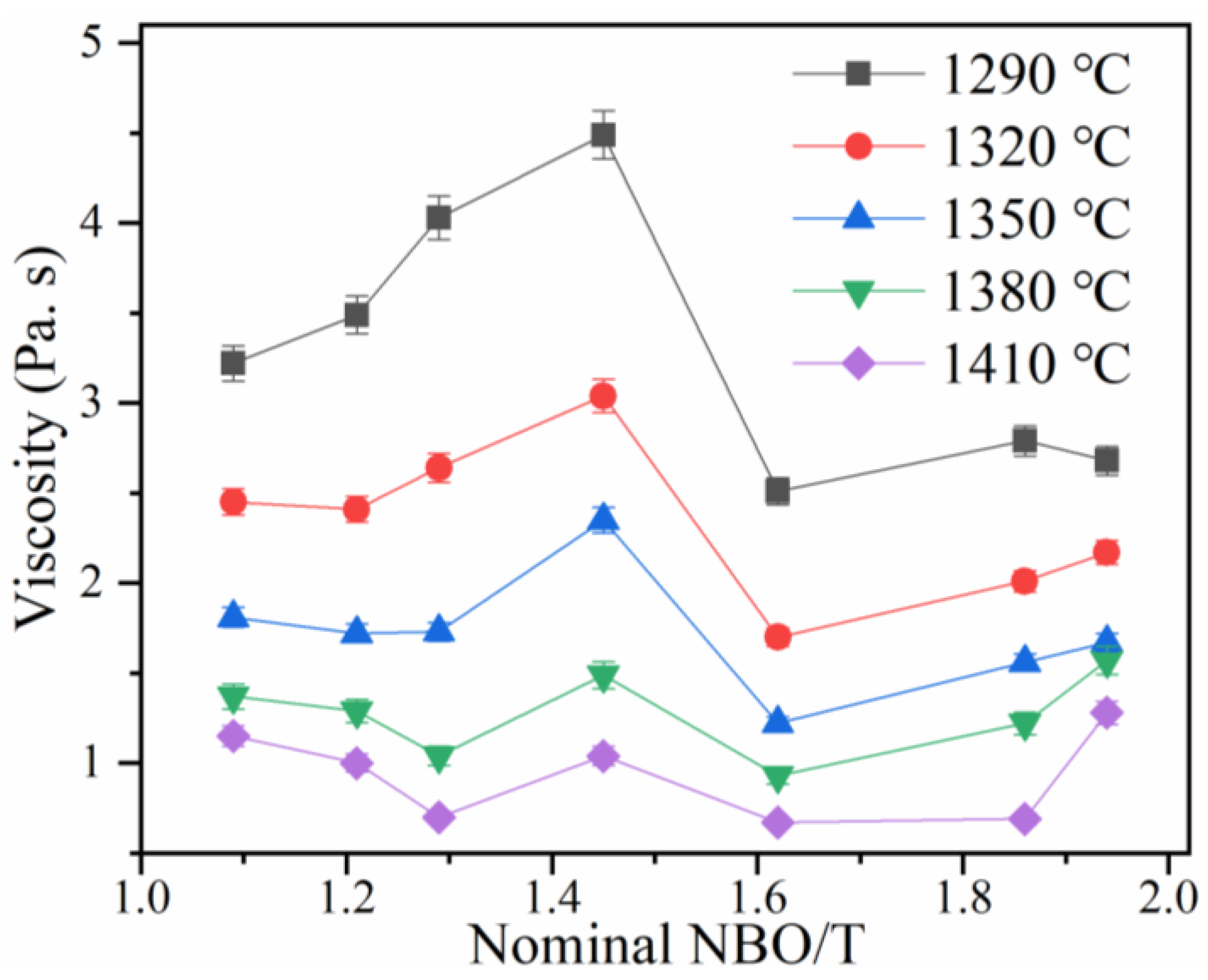
3.2. Effect of NBO/T and Temperature on Viscosity
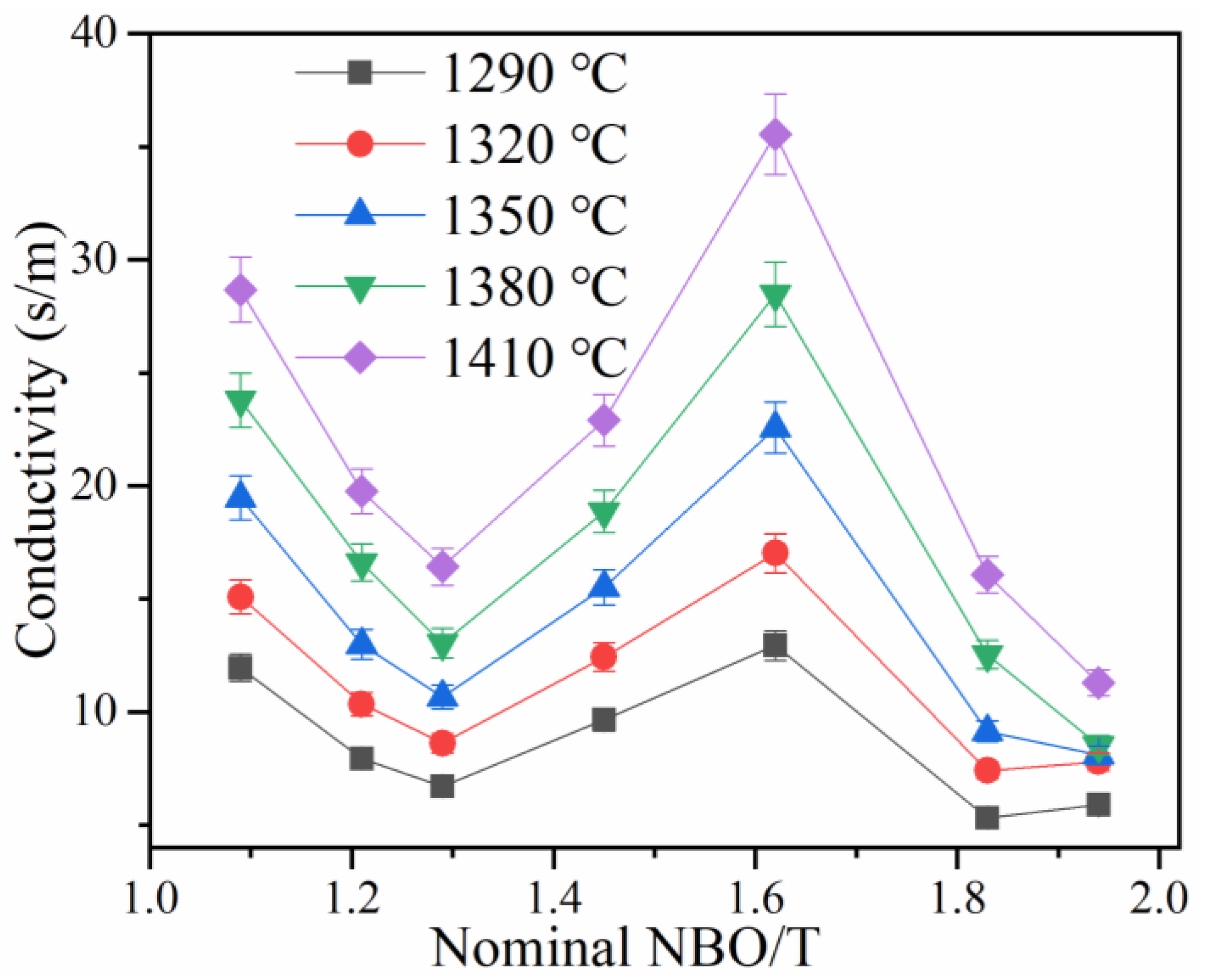
3.3. Effect of NBO/T and Temperature on Conductivity
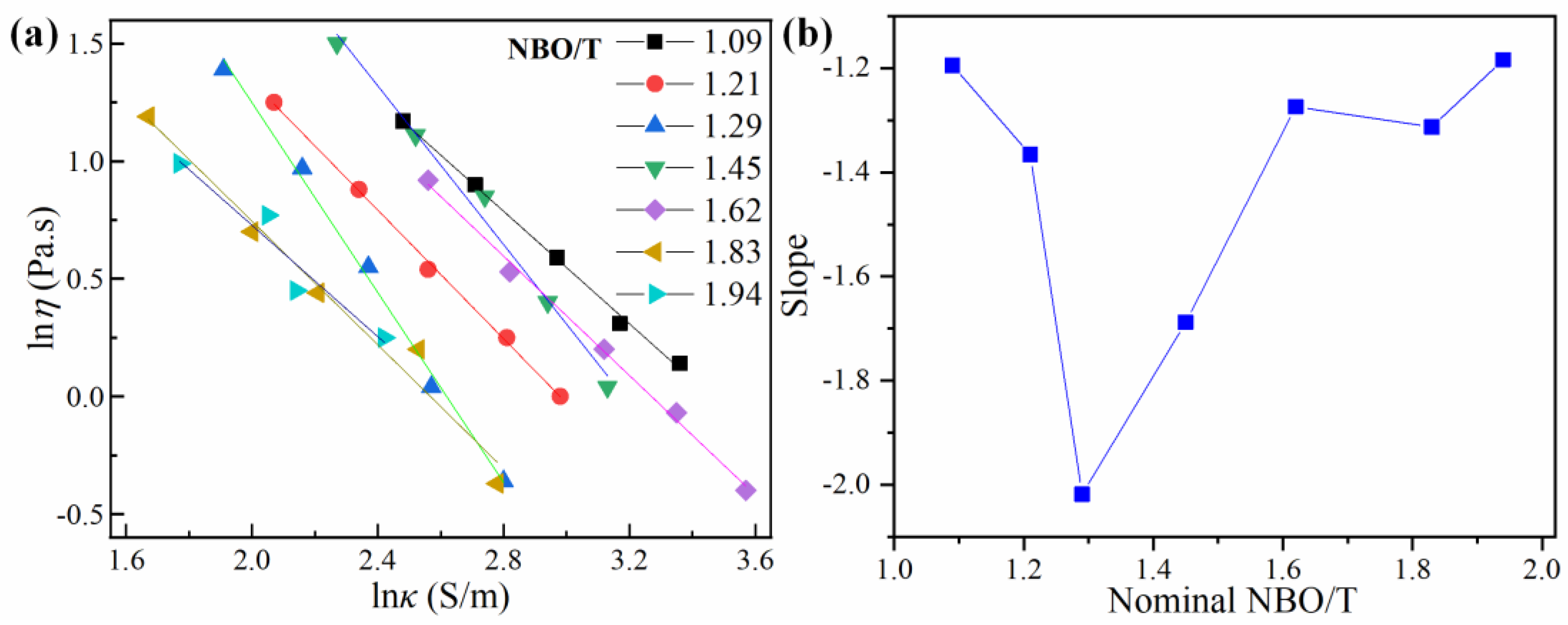
3.4. Relationship between Conductivity and Viscosity
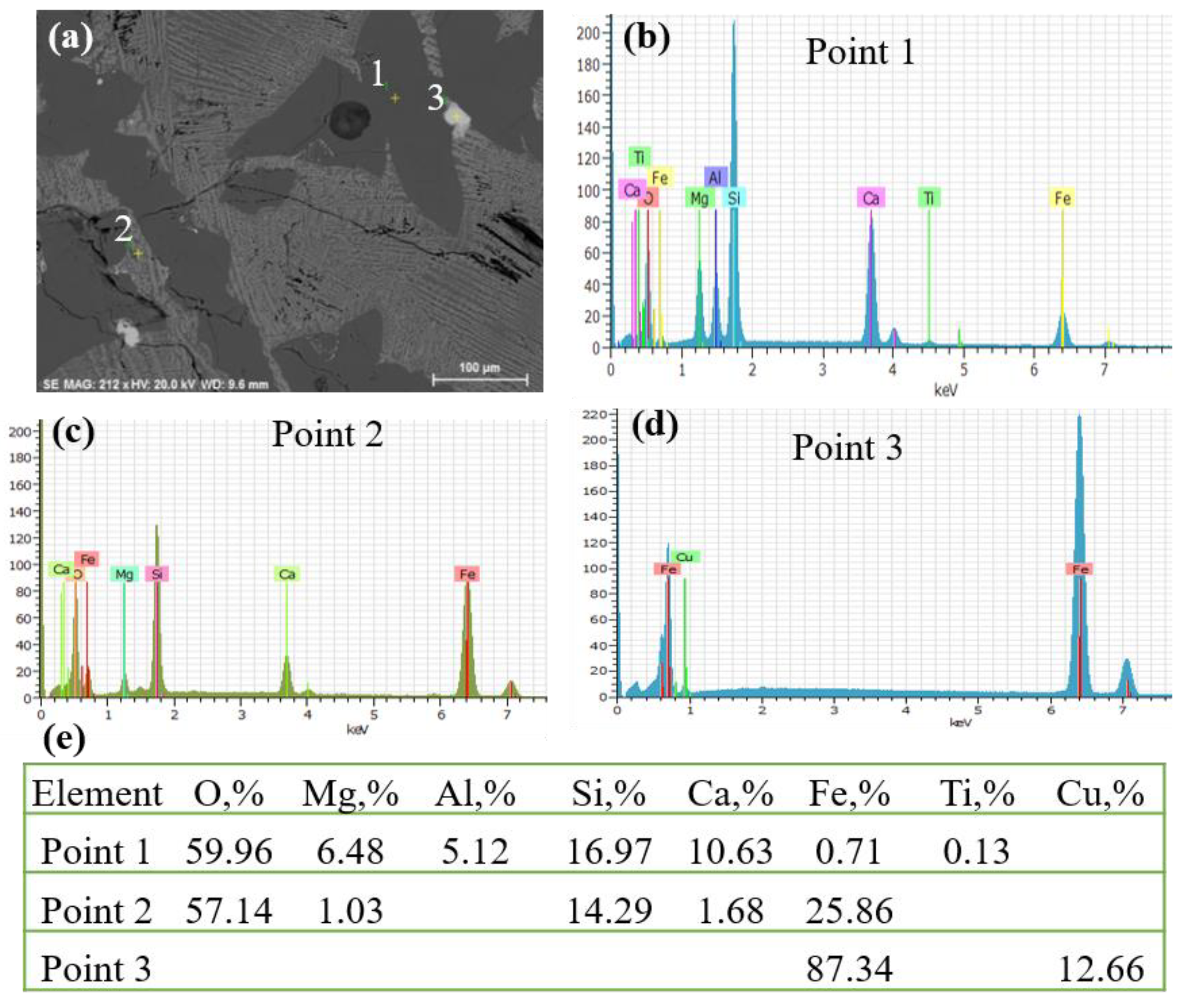
3.5. SEM Analysis of Z3 Slag
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mambwe, P.; Swennen, R.; Cailteux, J.; Mumba, C.; Dewaele, S.; Muchez, P. Review of the origin of breccias and their resource potential in the Central Africa Copperbelt. Ore Geol. Rev. 2023, 156, 105389. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, X.; Lyu, X.; Gao, W.; Su, H.; Li, C. Extraction and separation of copper and iron from copper smelting slag: A review. J. Clean. Prod. 2022, 368, 133095. [Google Scholar] [CrossRef]
- Souza, R.; Queiroz, C.; Brant, J.; Brocchi, E. Pyrometallurgical processing of a low copper content concentrate based on a thermodynamic assessment. Miner. Eng. 2019, 130, 156–164. [Google Scholar] [CrossRef]
- Tang, K.; van der Eijk, C.; Gouttebroze, S.; Du, Q.; Safarian, J.; Tranell, G. Rheological properties of Al2O3–CaO–SiO2 slags. Calphad 2022, 77, 102421. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, J.H. Viscosity-Structure Relationship of CaO–Al2O3–FetO–SiO2–MgO Ruhrstahl-Heraeus (RH) Refining Slags. ISIJ Int. 2021, 61, 724–733. [Google Scholar] [CrossRef]
- Weymann, H.D. On the hole theory of viscosity, compressibility, and expansivity of liquids. Kolloid-Z. Und Z. Für Polym. 1962, 181, 131–137. [Google Scholar] [CrossRef]
- Suzuki, M.; Jak, E. Development of a Quasi-chemical Viscosity Model for Fully Liquid Slags in the Al2O3–CaO–‘FeO’–MgO–SiO2 System: The Revised Model to Incorporate Ferric Oxide. ISIJ Int. 2014, 54, 2134–2143. [Google Scholar] [CrossRef]
- Urbain, G. Viscosity estimation of slags. Steel Res. 1987, 58, 111–116. [Google Scholar] [CrossRef]
- Dai, B.; Wu, X.; Zhang, L. Establishing a novel and yet simple methodology based on the use of modified inclined plane (M-IP) for high-temperature slag viscosity measurement. Fuel 2018, 233, 299–308. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Chou, K.-C.; Xue, Q.-G.; Mills, K.C. Modeling Viscosities of CaO-MgO-FeO-MnO-SiO2 Molten Slags. Metall. Mater. Trans. B 2012, 43, 64–72. [Google Scholar] [CrossRef]
- Fincham, C.J.B.; Richardson, F.D. The behavior of sulphur in silicate and aluminate melts. Philos. Trans. R. Soc. A 1954, 223, 40–62. [Google Scholar]
- Jong, B.D.; Schramm, C.M.; Parziale, V.E. Silicon-29 magic angle spinning NMR study on local silicon environments in amorphous and crystalline lithium silicates. J. Am. Chem. Soc. 1984, 106, 4396–4402. [Google Scholar] [CrossRef]
- Schramm, S.; Oldfield, E. High-resolution oxygen-17 NMR of solids. J. Am. Chem. Soc. 1984, 106, 2502–2506. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Scarfe, C.M. Relations between the anionic structure and viscosity of silicate melts—A Raman spectroscopicstudy. Am. Mineral. 1980, 65, 290–301. [Google Scholar]
- Min, D.J.; Tsukihashi, F. Recent Advances in Understanding Physical Properties of Metallurgical Slags. Met. Mater. Int. 2017, 23, 1. [Google Scholar] [CrossRef]
- Engelhardt, L.G.; Zeigan, D.; Jancke, H.; Wieker, W.; Hoebbel, D. 29Si-NMR-Spektroskopie an Silicatlösungen. II. Zur Abhängigkeit der Struktur der Silicatanionen in wäßrigen Natriumsilicatlösungen vom Na: Si-Verhältnis. Z. Für Anorg. Und Allg. Chem. 1975, 418, 17–28. [Google Scholar] [CrossRef]
- De Jong, B.; Keefer, K.D.; Brown, G.E., Jr.; Taylor, C.M. Polymerization of silicate and aluminate tetrahedra in glasses, melts, and aqueous solutions—III. Local silicon environments and internal nucleation in silicate glasses. Geochim. Cosmochim. Acta 1981, 45, 1291–1308. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chou, K.C.; Mills, K. A Structurally Based Viscosity Model for Oxide Melts. Metall. Mater. Trans. B 2014, 45, 698–706. [Google Scholar] [CrossRef]
- Avramov, I.; CRüssel Keding, R. Effect of chemical composition on viscosity of oxide glasses. J. Non-Cryst. Solids 2003, 324, 29–35. [Google Scholar] [CrossRef]
- Wu, G.; Yazhenskikh, E.; Hack, K.; Wosch, E.; Müller, M. Viscosity model for oxide melts relevant to fuel slags. Part 1: Pure oxides and binary systems in the system SiO2–Al2O3–CaO–MgO–Na2O–K2O. Fuel Process. Technol. 2015, 137, 93–103. [Google Scholar] [CrossRef]
- Wu, G.; Seebold, S.; Yazhenskikh, E.; Hack, K.; Müller, M. Viscosity model for oxide melts relevant to fuel slags. Part 3: The iron oxide containing low order systems in the system SiO2–Al2O3–CaO–MgO–Na2O–K2O–FeO–Fe2O3. Fuel Process. Technol. 2018, 171, 339–349. [Google Scholar] [CrossRef]
- Mills, K.; Yuan, L.; Li, Z.; Zhang, G.; Chou, K. A review of the factors affecting the thermophysical properties of silicate slags. High Temp. Mater. Process. 2012, 31, 301–321. [Google Scholar] [CrossRef]
- Shen, X.; Chen, M.; Wang, N.; Wang, D. Viscosity Property and Melt Structure of CaO–MgO–SiO2–Al2O3–FeO Slag System. ISIJ Int. 2019, 59, 9–15. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chou, K.C. Correlation Between Viscosity and Electrical Conductivity of Aluminosilicate Melts. Metall. Mater. Trans. B 2012, 43, 849–855. [Google Scholar] [CrossRef]
- Wang, W.; Dai, S.; Zhou, L.; Zhang, J.; Tian, W.; Xu, J. Viscosity and structure of MgO–SiO2-based slag melt with varying B2O3 content. Ceram. Int. 2020, 46, 3631–3636. [Google Scholar] [CrossRef]
- Winterhager, H.; Greiner, L.; Kammel, R. Investigation of the Density and the Electrical Conductivity of Metals of the Systems CaO-Al2O3-SiO2 and CaO-MgO-Al2O3-SiO2; Westdeutscher Verlag: Cologne, Germany, 1966. [Google Scholar]
- Talapaneni, T.; Yedla, N.; Sarkar, S.; Pal, S. Effect of Basicity. Al2O3 and MgO content on the softening and melting properties of the CaO-MgO-SiO2-Al2O3 high alumina quaternary slag system. Metall. Res. Technol. 2016, 113, 501. [Google Scholar] [CrossRef]
- Li, W.; Cao, X.; Jiang, T.; Yang, H.; Xue, X. Relation between Electrical Conductivity and Viscosity of CaO–SiO2–Al2O3–MgO System. ISIJ Int. 2016, 56, 205–209. [Google Scholar] [CrossRef]
| Slag | FeO/SiO2 | Xi (Mole Fraction) | Cu (wt.%) | ||||
|---|---|---|---|---|---|---|---|
| CaO | MgO | Al2O3 | FeO | SiO2 | |||
| Z1 | 0.41 | 0.22 | 0.13 | 0.050 | 0.050 | 0.55 | 0.23 |
| Z2 | 0.56 | 0.23 | 0.13 | 0.050 | 0.068 | 0.52 | 0.39 |
| Z3 | 0.71 | 0.22 | 0.13 | 0.055 | 0.097 | 0.50 | 0.27 |
| Z4 | 0.79 | 0.21 | 0.12 | 0.066 | 0.16 | 0.45 | 0.13 |
| Z5 | 0.87 | 0.19 | 0.11 | 0.050 | 0.20 | 0.45 | 0.24 |
| Z6 | 1.10 | 0.16 | 0.088 | 0.069 | 0.30 | 0.38 | 0.23 |
| Z7 | 1.17 | 0.15 | 0.085 | 0.059 | 0.32 | 0.39 | 0.42 |
| Slag | 1290 °C | 1320 °C | 1350 °C | 1380 °C | 1410 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z1 | 11.96 | 3.22 | 15.09 | 2.45 | 19.48 | 1.81 | 23.79 | 1.37 | 28.67 | 1.15 |
| Z2 | 7.94 | 3.49 | 10.35 | 2.41 | 12.99 | 1.72 | 16.61 | 1.29 | 19.76 | 1.02 |
| Z3 | 6.72 | 4.03 | 8.63 | 2.64 | 10.66 | 1.73 | 13.04 | 1.04 | 16.43 | 0.71 |
| Z4 | 9.65 | 4.49 | 12.43 | 3.04 | 15.52 | 2.35 | 18.87 | 1.49 | 22.92 | 1.04 |
| Z5 | 12.94 | 2.51 | 17.02 | 1.73 | 22.57 | 1.22 | 28.48 | 0.93 | 35.55 | 0.67 |
| Z6 | 5.33 | 3.28 | 7.42 | 2.01 | 9.14 | 1.56 | 12.54 | 1.22 | 16.08 | 0.69 |
| Z7 | 5.89 | 2.68 | 7.81 | 2.17 | 7.79 | 1.67 | 8.53 | 1.57 | 11.29 | 1.28 |
| Slag | Basicity | Basicity | NBO/Si | NBO/Si | NBO/T |
|---|---|---|---|---|---|
| (Al2O3 Ignored) | (Al2O3 as Base) | (Al2O3 as Base) | (Al2O3 Ignored) | ||
| Z1 | 0.74 | 1.01 | 2.03 | 1.48 | 1.09 |
| Z2 | 0.81 | 1.10 | 2.20 | 1.63 | 1.21 |
| Z3 | 0.90 | 1.23 | 2.46 | 1.80 | 1.29 |
| Z4 | 1.09 | 1.53 | 3.06 | 2.18 | 1.45 |
| Z5 | 1.10 | 1.43 | 2.86 | 2.20 | 1.62 |
| Z6 | 1.42 | 1.96 | 3.92 | 2.84 | 1.83 |
| Z7 | 1.41 | 1.87 | 3.74 | 2.83 | 1.94 |
| Slag | Equation | R2 | ||||
|---|---|---|---|---|---|---|
| Z1 | 4.133 − 1.195 | 0.997 | 186.35 | 156.23 | −13.60 | 14.84 |
| Z2 | 4.071 − 1.366 | 0.998 | 227.18 | 166.41 | 16.22 | −8.89 |
| Z3 | 5.284 − 2.018 | 0.994 | 320.22 | 158.94 | −23.21 | 14.12 |
| Z4 | 5.372 − 1.688 | 0.985 | 261.97 | 155.32 | −18.55 | 14.17 |
| Z5 | 4.168 − 1.274 | 0.997 | 234.03 | 184.18 | −17.15 | 16.73 |
| Z6 | 3.371 − 1.313 | 0.966 | 261.38 | 198.91 | −18.96 | 17.01 |
| Z7 | 3.095 − 1.184 | 0.892 | 130.14 | 110.36 | −9.08 | 10.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, K.; Xie, F.; Lu, D. Relation between Viscosity and Conductivity of CaO-MgO-FeO-Al2O3-SiO2 System for Copper Smelting Slags. Metals 2023, 13, 786. https://doi.org/10.3390/met13040786
Zhang L, Jiang K, Xie F, Lu D. Relation between Viscosity and Conductivity of CaO-MgO-FeO-Al2O3-SiO2 System for Copper Smelting Slags. Metals. 2023; 13(4):786. https://doi.org/10.3390/met13040786
Chicago/Turabian StyleZhang, Lei, Kaixi Jiang, Feng Xie, and Diankun Lu. 2023. "Relation between Viscosity and Conductivity of CaO-MgO-FeO-Al2O3-SiO2 System for Copper Smelting Slags" Metals 13, no. 4: 786. https://doi.org/10.3390/met13040786
APA StyleZhang, L., Jiang, K., Xie, F., & Lu, D. (2023). Relation between Viscosity and Conductivity of CaO-MgO-FeO-Al2O3-SiO2 System for Copper Smelting Slags. Metals, 13(4), 786. https://doi.org/10.3390/met13040786






