Investigation of Mechanical Properties and Wear Resistance of A2/B2 Type Medium-Entropy Alloy Matrix Reinforced with Tungsten Particles by In-Situ Reaction
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
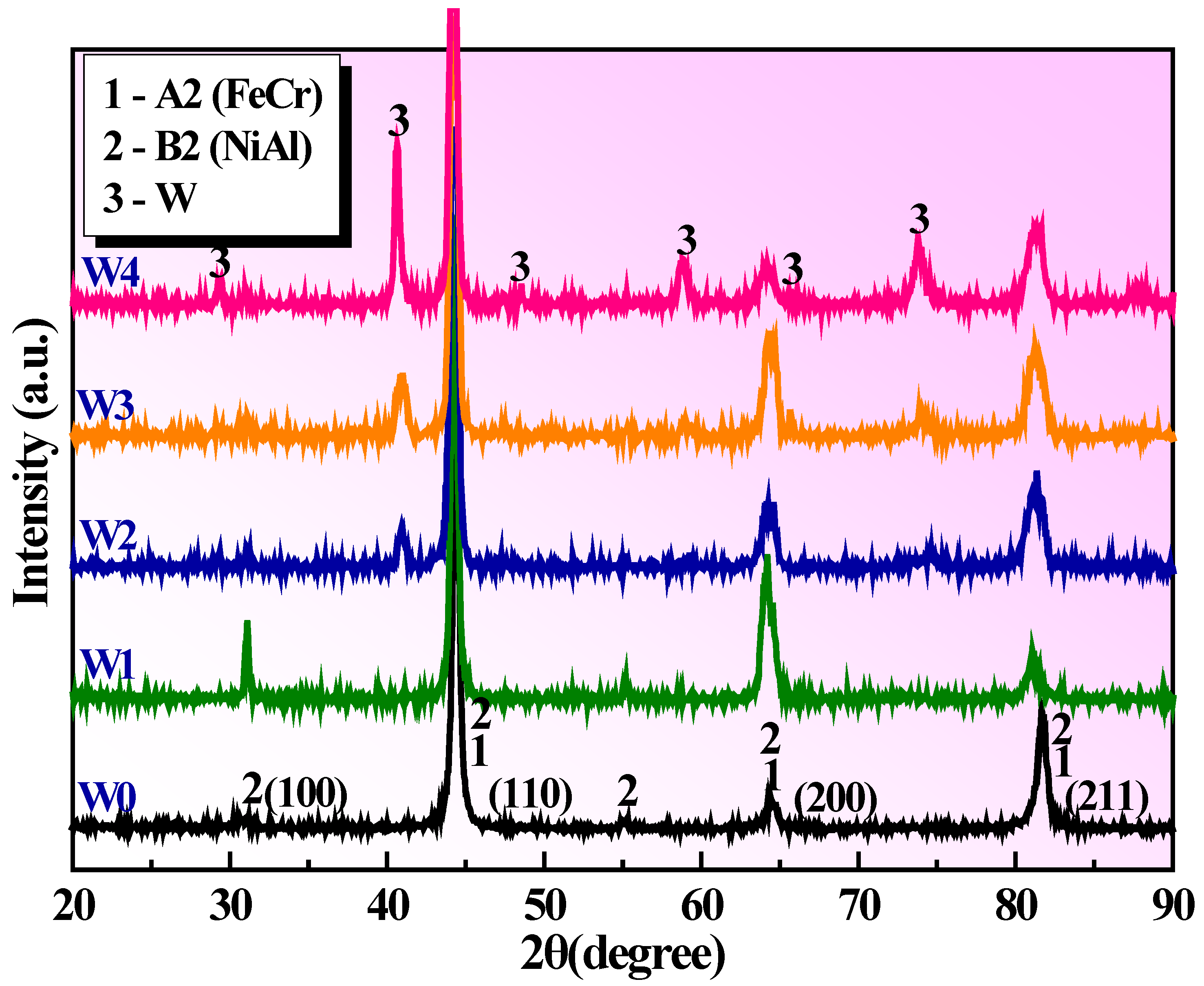
3.1. Phase Characterization
3.2. Phase Formation Prediction
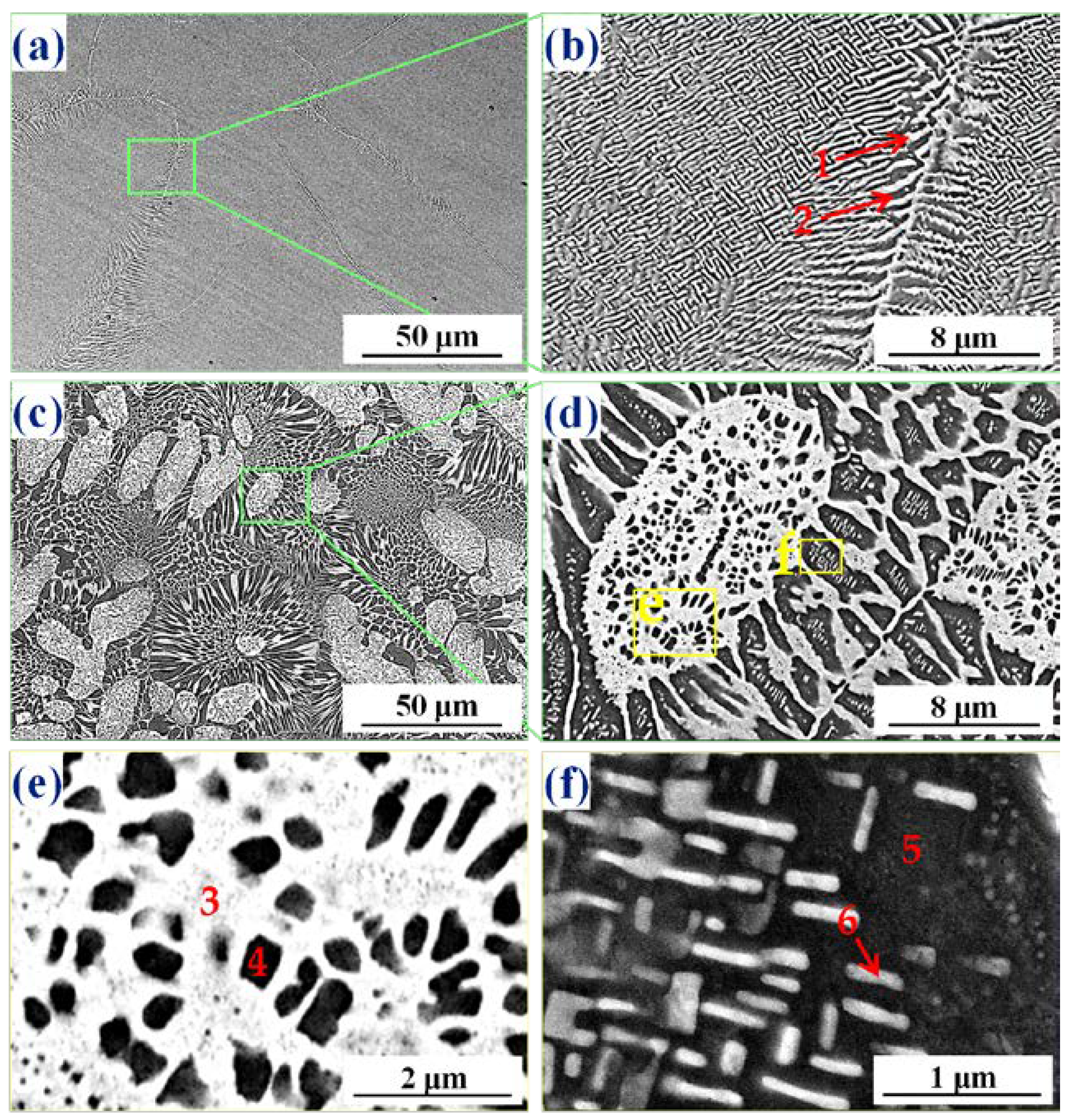
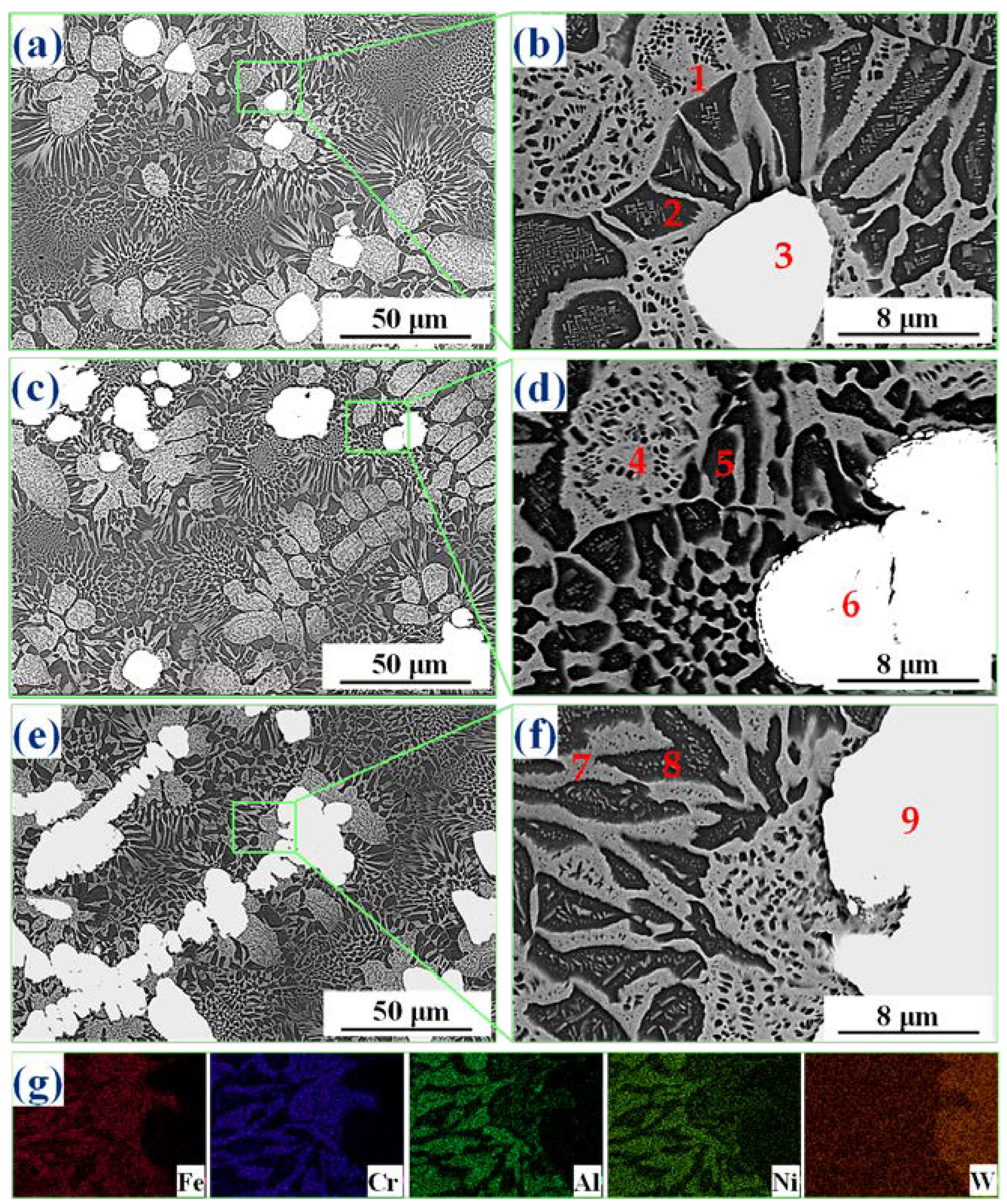
3.3. Microstructure
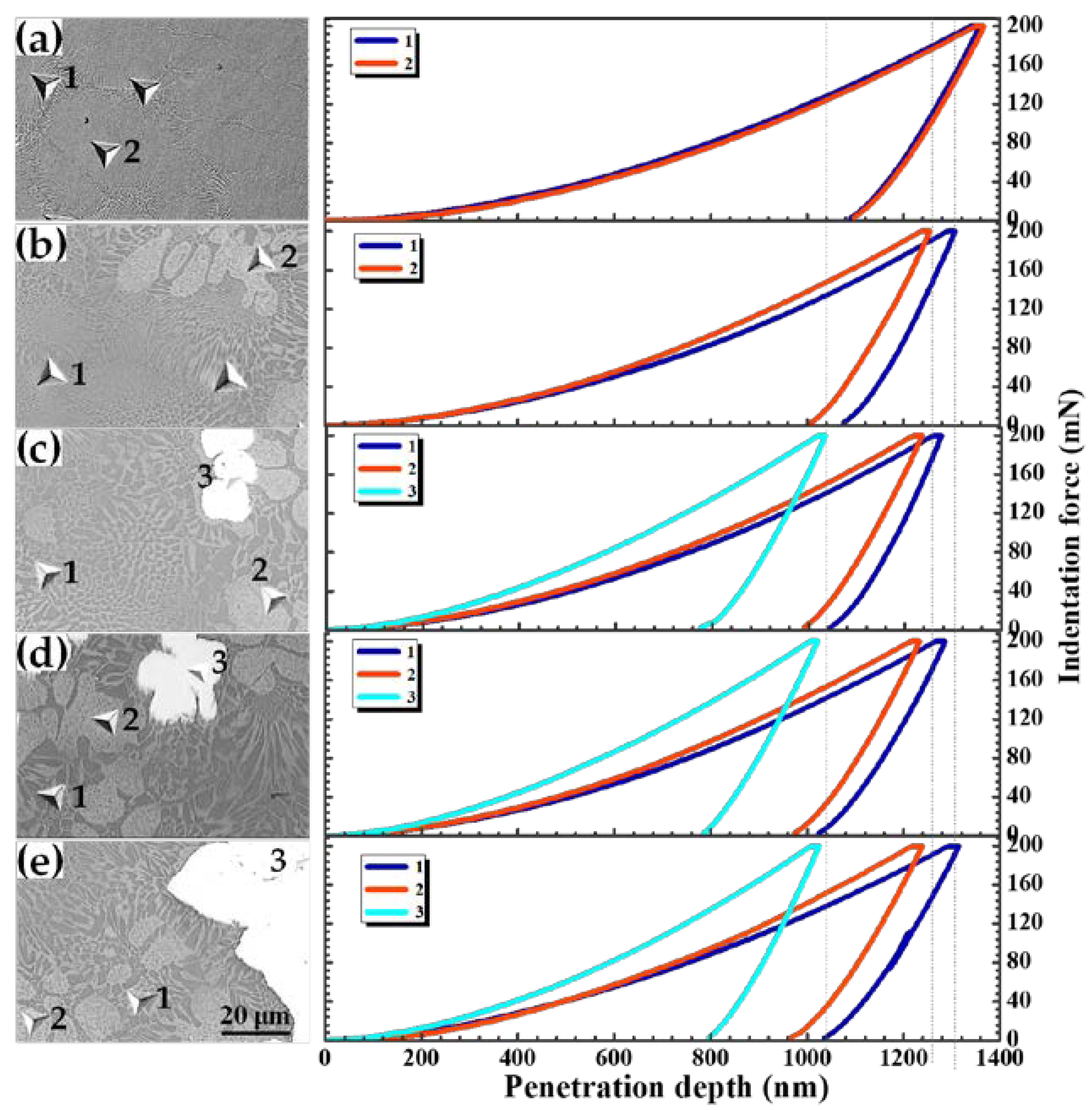
3.4. Mechanical Properties
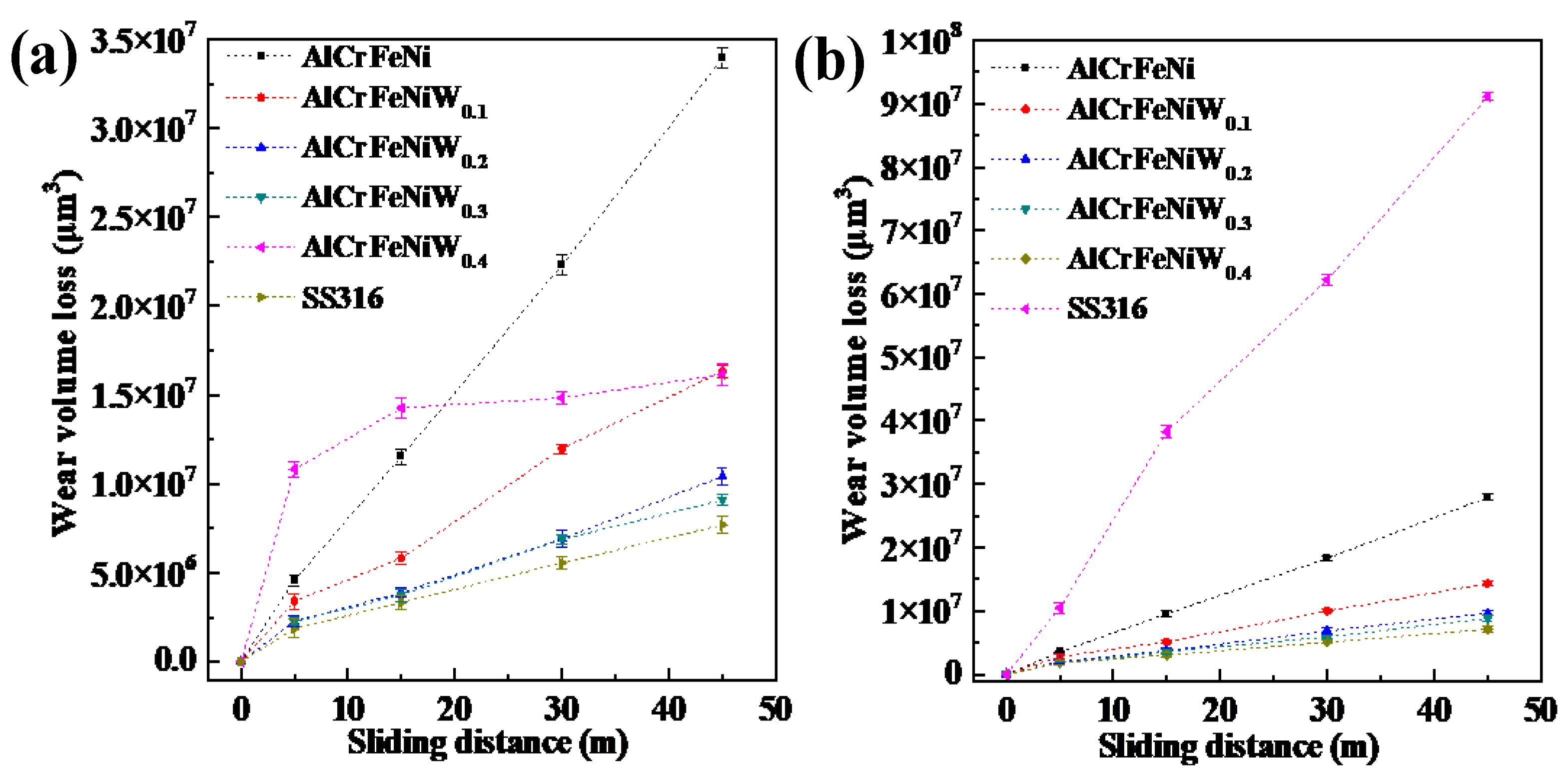
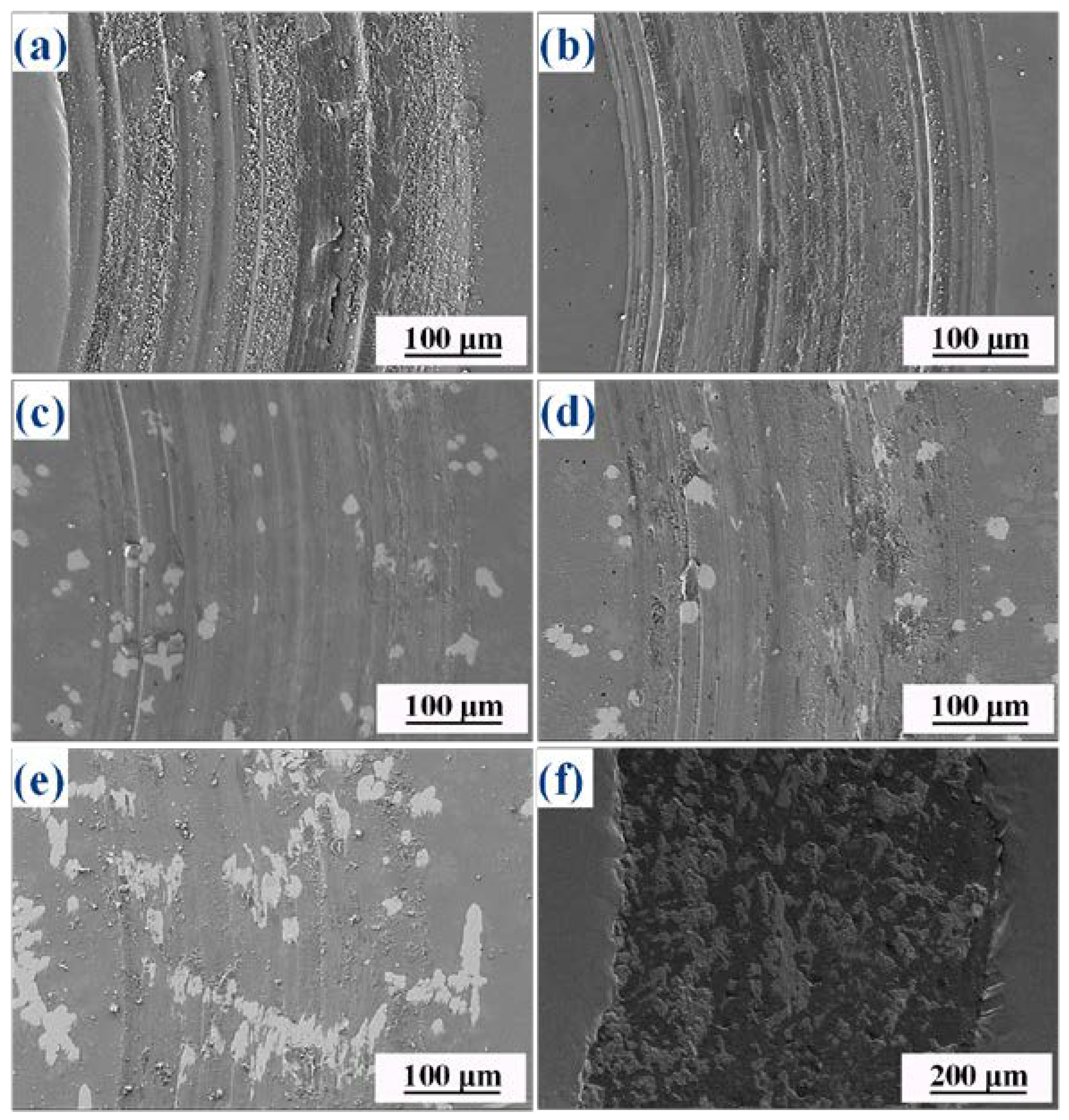
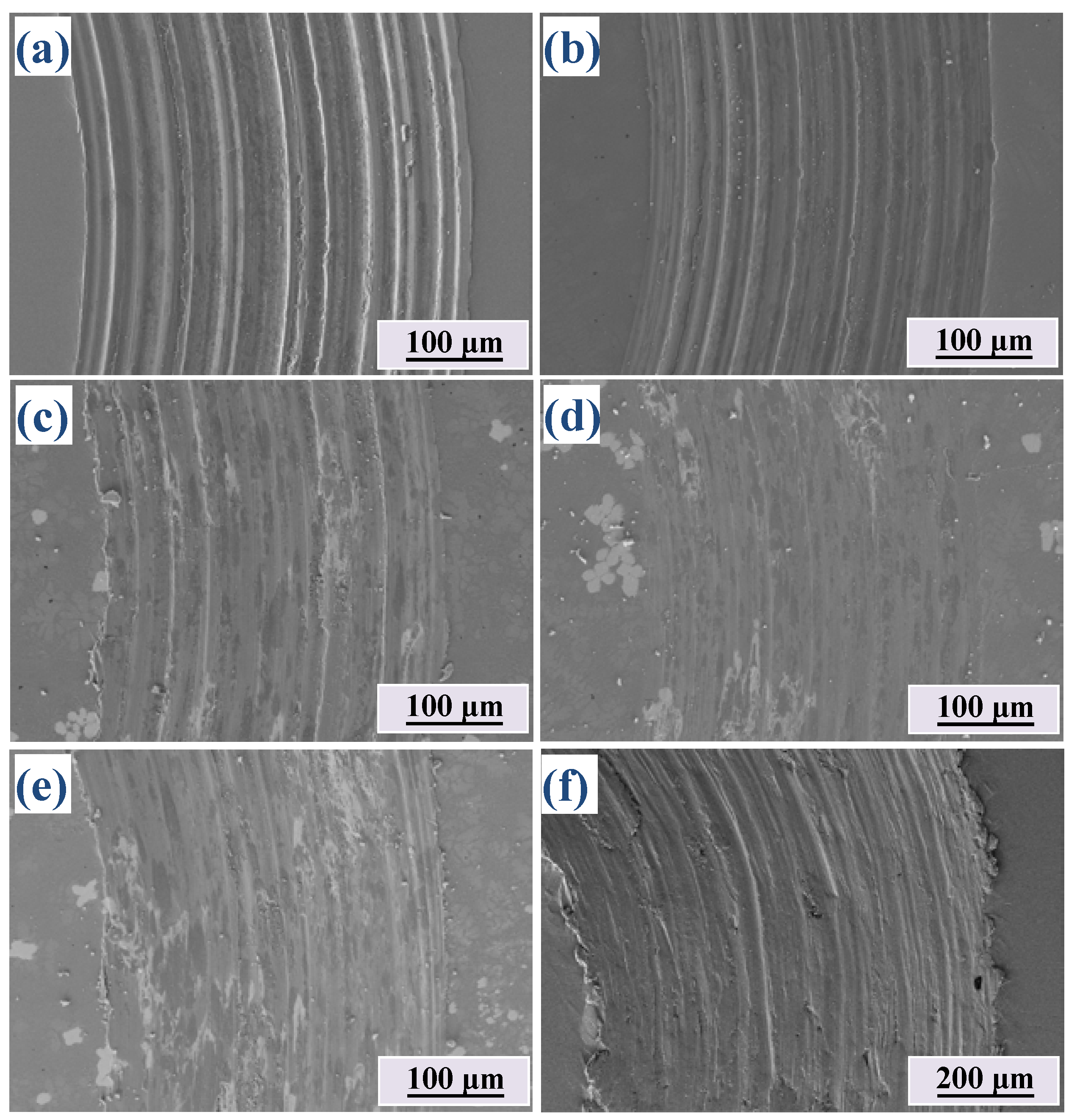
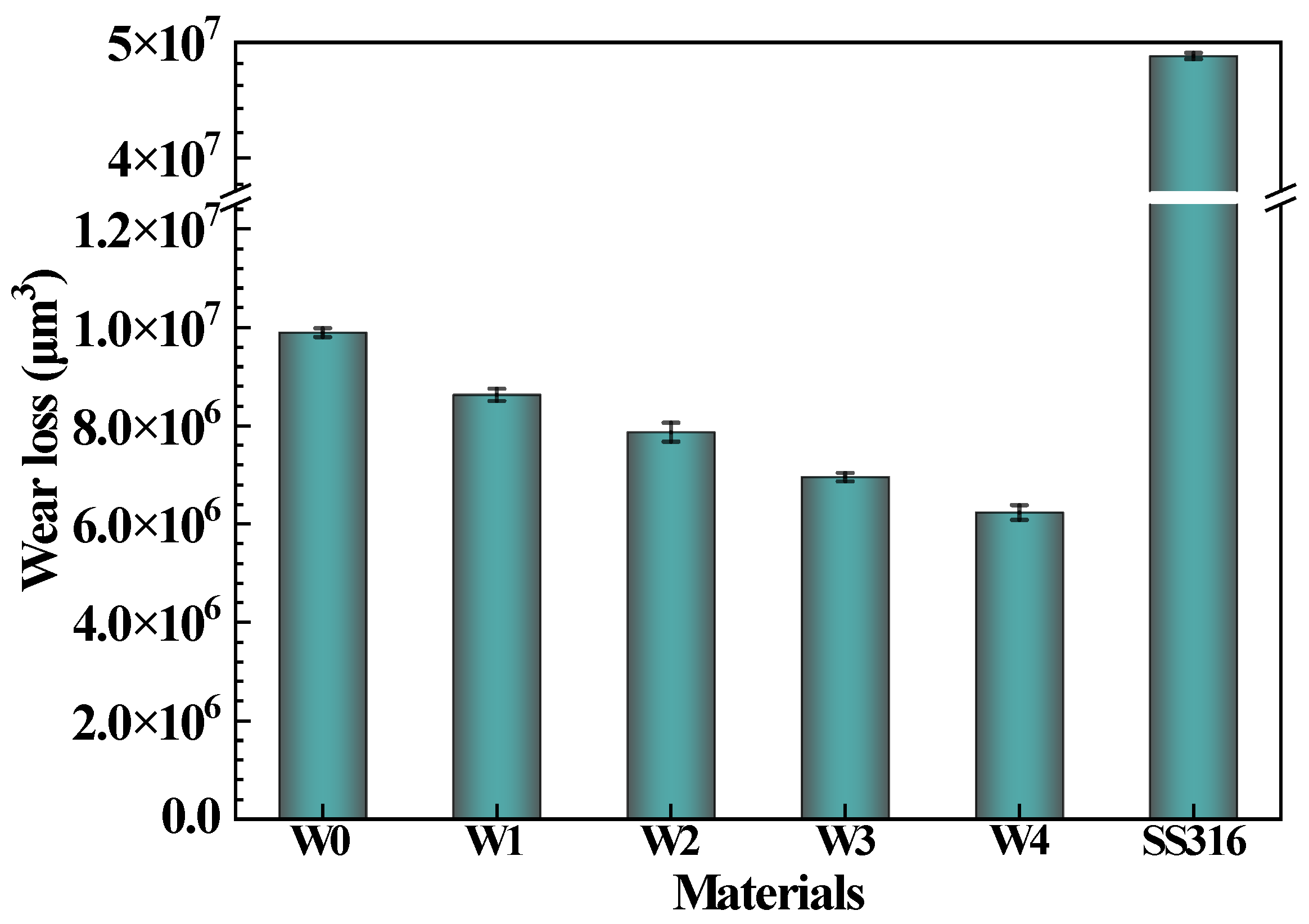
3.5. Wear Resistance
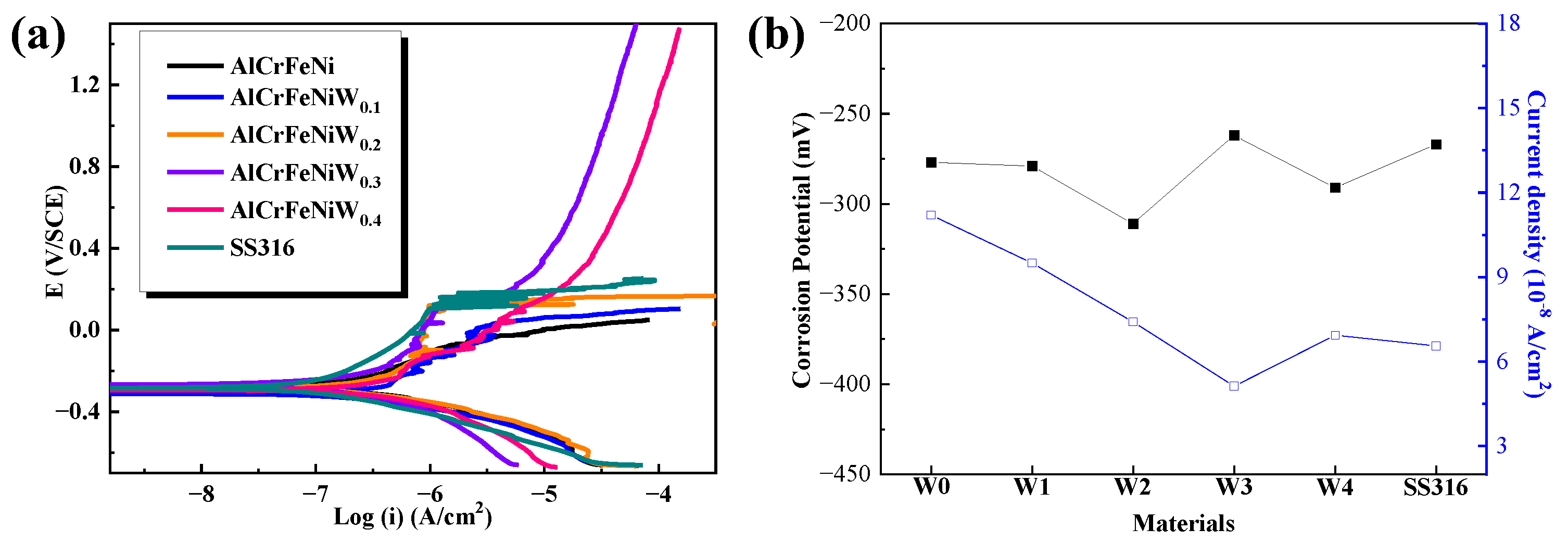
3.6. Corrosion and Corrosive Wear
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-H. Three Strategies for the Design of Advanced High-Entropy Alloys. Entropy 2016, 18, 252. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.; Huang, Z.; Zhou, Q.; Ren, Y.; Du, Y.; Wang, H. Mechanical and dry sliding tribological properties of CoCrNiNbx medium-entropy alloys at room temperature. Tribol. Int. 2021, 163, 107160. [Google Scholar] [CrossRef]
- Owen, L.R.; Jones, N.G. Lattice distortions in high-entropy alloys. J. Mater. Res. 2018, 33, 2954–2969. [Google Scholar] [CrossRef]
- Peighambardoust, N.S.; Alamdari, A.A.; Unal, U.; Motallebzadeh, A. In Vitro biocompatibility evaluation of Ti1.5ZrTa0.5Nb0.5Hf0.5 refractory high-entropy alloy film for orthopedic implants: Microstructural, mechanical properties and corrosion behavior. J. Alloys Compd. 2021, 883, 160786. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, Y.J.; Taylor, A.; Styles, M.J.; Marceau, R.K.W.; Ceguerra, A.V.; Gibson, M.A.; Liu, Z.K.; Fraser, H.L.; Birbilis, N. A lightweight single-phase AlTiVCr compositionally complex alloy. Acta Mater. 2017, 123, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.J.; Tian, Y.Z.; Lin, H.R.; Lu, S.; Yang, H.J.; Zhang, Z.F. Modulating the prestrain history to optimize strength and ductility in CoCrFeMnNi high-entropy alloy. Scr. Mater. 2019, 163, 111–115. [Google Scholar] [CrossRef]
- Wang, Z.; Baker, I.; Guo, W.; Poplawsky, J.D. The effect of carbon on the microstructures, mechanical properties, and deformation mechanisms of thermo-mechanically treated Fe40.4Ni11.3Mn34.8Al7.5Cr6 high entropy alloys. Acta Mater. 2017, 126, 346–360. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Jiao, Z.; Yan, S.; Guo, L.; Feng, L.; Yu, J. Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 2020, 174, 109178. [Google Scholar] [CrossRef]
- Zhang, Z.; Armstrong, D.E.J.; Grant, P.S. The effects of irradiation on CrMnFeCoNi high-entropy alloy and its derivatives. Prog. Mater. Sci. 2022, 123, 100807. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Liu, Y.; Fang, Q.; Wu, Y.; Chen, S.; Liu, C.T. Microstructure and mechanical properties of equimolar FeCoCrNi high entropy alloy prepared via powder extrusion. Intermetallics 2016, 75, 25–30. [Google Scholar] [CrossRef]
- Tsau, C.-H.; Lin, S.-X.; Fang, C.-H. Microstructures and corrosion behaviors of FeCoNi and CrFeCoNi equimolar alloys. Mater. Chem. Phys. 2017, 186, 534–540. [Google Scholar] [CrossRef]
- Chen, S.; Oh, H.S.; Gludovatz, B.; Kim, S.J.; Park, E.S.; Zhang, Z.; Ritchie, R.O.; Yu, Q. Real-time observations of TRIP-induced ultrahigh strain hardening in a dual-phase CrMnFeCoNi high-entropy alloy. Nat. Commun. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantor, B. Multicomponent high-entropy Cantor alloys. Prog. Mater. Sci. 2021, 120, 100754. [Google Scholar] [CrossRef]
- Zeng, Z.; Xiang, M.; Zhang, D.; Shi, J.; Wang, W.; Tang, X.; Tang, W.; Wang, Y.; Ma, X.; Chen, Z.; et al. Mechanical properties of Cantor alloys driven by additional elements: A review. J. Mater. Res. Technol. 2021, 15, 1920–1934. [Google Scholar] [CrossRef]
- Bhalla, S.; Melnekoff, D.T.; Aleman, A.; Leshchenko, V.; Restrepo, P.; Keats, J.; Onel, K.; Sawyer, J.R.; Madduri, D.; Richter, J.; et al. Patient similarity network of newly diagnosed multiple myeloma identifies patient subgroups with distinct genetic features and clinical implications. Sci. Adv. 2021, 7, eabg9551. [Google Scholar] [CrossRef]
- Liu, W.H.; Yang, T.; Liu, C.T. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11. [Google Scholar] [CrossRef]
- Salishchev, G.; Tikhonovsky, M.; Shaysultanov, D.G.; Stepanov, N.; Kuznetsov, A.V.; Kolodiy, I.; Tortika, A.S.; Senkov, O. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructures and mechanical properties of multiprincipal component CoCrFeNiTix alloys. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, H.; Fang, F.; Xie, Z.; Jiang, J. Microstructure and mechanical properties of CoCrFeNiZrx eutectic high-entropy alloys. Mater. Des. 2017, 134, 226–233. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.; Qiao, D.; Lu, Y.; Cao, Z.; Li, T. Effects of Ta addition on the microstructures and mechanical properties of CoCrFeNi high entropy alloy. Mater. Chem. Phys. 2018, 210, 43–48. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, M.D.; Zhou, Z.; Fan, J.T.; Cui, P.; Yu, P.F.; Jing, Q.; Ma, M.Z.; Liaw, P.K.; Li, G.; et al. Effects of rare-earth element, Y, additions on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2018, 725, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Xu, J.; Wang, T.; Wang, N.; Han, Z.; Wang, Y. Microstructure, mechanical properties and corrosion resistance of CoCrFeNiWx (x = 0, 0.2, 0.5) high entropy alloys. Intermetallics 2019, 112, 106550. [Google Scholar] [CrossRef]
- Ma, H.; Shao, Y.; Shek, C.H. CoCuFeNi high entropy alloy reinforced by in-situ W particles. Mater. Sci. Eng. A 2020, 797, 140218. [Google Scholar] [CrossRef]
- Ma, H.; Shek, C.H. Effects of Hf on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. J. Alloys Compd. 2020, 827, 154159. [Google Scholar] [CrossRef]
- Shao, Y.; Ma, H.; Wang, Y. Effect of Mo Addition on The Microstructure and Mechanical Properties of CoCuFeNi High Entropy Alloy. Metals 2020, 10, 1017. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Li, Q.; Wang, S.; Wu, M.; Yang, S.; Xiang, D. Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy. Metals 2022, 12, 191. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, B.; Li, C.; Wang, Q.; Dong, C.; Liaw, P.K.; Xu, F.; Sun, L. The BCC/B2 Morphologies in AlxNiCoFeCr High-Entropy Alloys. Metals 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, Y.A.; Kunwar, A.; Moelans, N. Phase-field approach to simulate BCC-B2 phase separation in the AlnCrFe2Ni2 medium-entropy alloy. J. Mater. Sci. 2022, 57, 10600–10612. [Google Scholar] [CrossRef]
- Jin, D.-M.; Wang, Z.-H.; Li, J.-F.; Niu, B.; Wang, Q. Formation of coherent BCC/B2 microstructure and mechanical properties of Al–Ti–Zr–Nb–Ta–Cr/Mo light-weight refractory high-entropy alloys. Rare Met. 2022, 41, 2886–2893. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Gao, P.; Xiang, Q.; Qu, Y.; Cheng, J.; Ren, Y.; Yu, B.; Qiu, K. AlxCrFeNi medium entropy alloys with high damping capacity. J. Alloys Compd. 2021, 876, 159991. [Google Scholar] [CrossRef]
- Jiao, W.; Li, T.; Chang, X.; Lu, Y.; Yin, G.; Cao, Z.; Li, T. A novel Co-free Al0.75CrFeNi eutectic high entropy alloy with superior mechanical properties. J. Alloys Compd. 2022, 902, 163814. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, W.; Xia, Z.; Xiong, W.; Fu, Z. Influence of synthesis method on microstructure and mechanical behavior of Co-free AlCrFeNi medium-entropy alloy. Intermetallics 2019, 108, 45–54. [Google Scholar] [CrossRef]
- Wu, M.; Yuan, J.; Diao, G.; Li, D. Achieving a Combination of Higher Strength and Higher Ductility for Enhanced Wear Resistance of AlCrFeNiTi0.5 High-Entropy Alloy by Mo Addition. Metals 2022, 12, 1910. [Google Scholar] [CrossRef]
- Ossiansson, M.; Gupta, M.; Löbel, M.; Lindner, T.; Lampke, T.; Joshi, S. Assessment of CrFeCoNi and AlCrFeCoNi High-Entropy Alloys as Bond Coats for Thermal Barrier Coatings. J. Therm. Spray Technol. 2022, 31, 1404–1422. [Google Scholar] [CrossRef]
- Günen, A.; Lindner, T.; Karakaş, M.S.; Kanca, E.; Töberling, G.; Vogt, S.; Gök, M.S.; Lampke, T. Effect of the boriding environment on the wear response of laser-clad AlCoCrFeNi high entropy alloy coatings. Surf. Coat. Technol. 2022, 447, 128830. [Google Scholar] [CrossRef]
- Ghadami, F.; Ghadami, S.; Davoudabadi, M.A. Sliding wear behavior of the nanoceria-doped AlCrFeCoNi high-entropy alloy coatings deposited by air plasma spraying technique. J. Therm. Spray Technol. 2022, 31, 1263–1275. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462, 203493. [Google Scholar] [CrossRef]
- Terajima, T.; Nakata, K.; Kimura, H.; Inoue, A. Development of W-Reinforced Zr-Based Metallic Glass. J. Jpn. Inst. Met. Mater. 2010, 74, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Bauri, R.; Yadav, D.; Shyam Kumar, C.N.; Balaji, B. Tungsten particle reinforced Al 5083 composite with high strength and ductility. Mater. Sci. Eng. A 2015, 620, 67–75. [Google Scholar] [CrossRef]
- Mazilkin, A.; Abramova, M.M.; Enikeev, N.A.; Lomakin, I.V.; Valiev, R.Z.; Ivanisenko, Y.; Kübel, C.; Etienne, A.; Sauvage, X.; Radiguet, B. The effect of tungsten on microstructure and mechanical performance of an ultrafine Fe-Cr steel. Mater. Lett. 2018, 227, 292–295. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Han, K.; Lu, Y.; Wang, T.; Cao, Z.; Lu, Y. Effects of Tungsten on Microstructure and Mechanical Properties of CrFeNiV0.5Wx and CrFeNi2V0.5Wx High-Entropy Alloys. J. Mater. Eng. Perform. 2015, 24, 4594–4600. [Google Scholar] [CrossRef]
- Chang, R.; Fang, W.; Bai, X.; Xia, C.; Zhang, X.; Yu, H.; Liu, B.; Yin, F. Effects of tungsten additions on the microstructure and mechanical properties of CoCrNi medium entropy alloys. J. Alloys Compd. 2019, 790, 732–743. [Google Scholar] [CrossRef]
- Soni, V.K.; Sanyal, S.; Sinha, S.K. Influence of tungsten on microstructure evolution and mechanical properties of selected novel FeCoCrMnWx high entropy alloys. Intermetallics 2021, 132, 107161. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, N.; Dwivedi, A.; Subramaniam, A. A geometrical parameter for the formation of disordered solid solutions in multi-component alloys. Intermetallics 2014, 53, 112–119. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Yang, Y.; Wang, J.; Liu, C.T. Atomic-size effect and solid solubility of multicomponent alloys. Scr. Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- Rao, Z.; Dutta, B.; Körmann, F.; Lu, W.; Zhou, X.; Liu, C.; da Silva, A.K.; Wiedwald, U.; Spasova, M.; Farle, M.; et al. Beyond Solid Solution High-Entropy Alloys: Tailoring Magnetic Properties via Spinodal Decomposition. Adv. Funct. Mater. 2021, 31, 2007668. [Google Scholar] [CrossRef]
- Findik, F. Modulated (Spinodal) Alloys. Period. Eng. Nat. Sci. (PEN) 2013, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.H.; Pouraliakbar, H.; Lee, B.J.; Kim, Y.K.; Rizi, M.S.; Hong, S.I. Strengthening and deformation behavior of as-cast CoCrCu1.5MnNi high entropy alloy with micro-/nanoscale precipitation. Mater. Sci. Eng. A 2022, 853, 143729. [Google Scholar] [CrossRef]
- Saboktakin Rizi, M.; Minouei, H.; Lee, B.J.; Toroghinejad, M.R.; Hong, S.I. Effects of carbon and molybdenum on the nanostructural evolution and strength/ductility trade-off in Fe40Mn40Co10Cr10 high-entropy alloys. J. Alloys Compd. 2022, 911, 165108. [Google Scholar] [CrossRef]
- Wang, S.D.; Wu, M.Y.; Xu, D.K.; Han, E.-h. Improving corrosive wear resistance of Mg-Zn-Y-Zr alloys through heat treatment. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Sheng, L.Y.; Qiao, Y.X.; Han, E.-H.; Dong, C. Influence of phase dissolution and hydrogen absorption on the stress corrosion cracking behavior of Mg-7%Gd-5%Y-1%Nd-0.5%Zr alloy in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 142, 185–200. [Google Scholar] [CrossRef]
- Brigham, R.J. Pitting of Molybdenum Bearing Austenitic Stainless Steel. Corrosion 2013, 28, 177–179. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, L.; Xiao, S.; Zhang, H.; Zhang, F.; Li, N.; Guo, S. Effect of W on the thermal stability, mechanical properties and corrosion resistance of Fe-based bulk metallic glass. Intermetallics 2022, 143, 107485. [Google Scholar] [CrossRef]
- Kumar, D.; Maulik, O.; Sharma, V.K.; Prasad, Y.V.S.S.; Kumar, V. Understanding the Effect of Tungsten on Corrosion Behavior of AlCuCrFeMnWx High-Entropy Alloys in 3.5 wt.% NaCl Solution. J. Mater. Eng. Perform. 2018, 27, 4481–4488. [Google Scholar] [CrossRef]

| Elements | Enthalpy of Mixing (KJ/mol) | VECi | Atomic Radius (Å) | ||||
|---|---|---|---|---|---|---|---|
| Al | Cr | Fe | Ni | W | |||
| Al | 0 | 3 | 1.432 | ||||
| Cr | −10 | 0 | 6 | 1.249 | |||
| Fe | −11 | −1 | 0 | 8 | 1.241 | ||
| Ni | −22 | −7 | −2 | 0 | 10 | 1.246 | |
| W | −2 | 1 | 0 | −3 | 0 | 6 | 1.367 |
| MEAs | ΔHmix(kJ/mol) | δ (%) | VEC | ΔSmix(J/mol/K) | Ω | Λ |
|---|---|---|---|---|---|---|
| W0 | −13.25 | 6.26 | 6.75 | 11.53 | 1.43 | 0.29 |
| W1 | −12.71 | 6.24 | 6.73 | 12.19 | 1.63 | 0.31 |
| W2 | −12.19 | 6.22 | 6.71 | 12.57 | 1.8 | 0.32 |
| W3 | −11.72 | 6.19 | 6.69 | 12.83 | 1.96 | 0.33 |
| W4 | −11.28 | 6.17 | 6.68 | 13.01 | 2.11 | 0.34 |
| Alloys | Areas | Al | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|
| AlCrFeNi | 1 | 17.3 | 38.6 | 33.8 | 10.3 | |
| 2 | 38.2 | 13.0 | 15.2 | 33.7 | ||
| AlCrFeNiW0.1 | 3 | 9.1 | 43.5 | 36.6 | 6.6 | 4.2 |
| 4 | 12.5 | 36.1 | 34.1 | 15.7 | 1.7 | |
| 5 | 32.5 | 15.1 | 18.3 | 33.6 | 0.5 | |
| 6 | 30.5 | 17.3 | 20.2 | 30.8 | 1.3 |
| Alloys | Areas | Al | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|
| AlCrFeNiW0.2 | 1 | 15.2 | 37.0 | 30.1 | 12.8 | 4.9 |
| 2 | 33.7 | 14.8 | 18.4 | 32.3 | 0.9 | |
| 3 | 2.6 | 18.6 | 5.7 | 2.7 | 70.4 | |
| AlCrFeNiW0.3 | 4 | 14.9 | 35.9 | 30.9 | 13.3 | 4.9 |
| 5 | 41.6 | 8.7 | 13.1 | 36.0 | 0.5 | |
| 6 | 3.9 | 19.7 | 3.2 | 2.4 | 70.8 | |
| AlCrFeNiW0.4 | 7 | 11.4 | 39.0 | 34.6 | 10.3 | 4.8 |
| 8 | 32.6 | 15.2 | 19.1 | 32.3 | 0.7 | |
| 9 | 2.9 | 8.9 | 3.6 | 1.2 | 83.4 |
| Alloys | Regions | Micro-Hardness (MPa) | Overall Hardness (HV0.5) |
|---|---|---|---|
| AlCrFeNi | 1 Matrix (BCC + B2) | 5622.9 | 596.8 |
| 2 Matrix (BCC + B2) | 5633.8 | ||
| AlCrFeNiW0.1 | 1 Matrix (BCC + B2) | 6046.5 | 669.2 |
| 2 Block BCC | 6553.1 | ||
| AlCrFeNiW0.2 | 1 Matrix (BCC + B2) | 6185.5 | 736.9 |
| 2 Block BCC | 6889.8 | ||
| 3 W-particle | 10,326 | ||
| AlCrFeNiW0.3 | 1 Matrix (BCC + B2) | 6274.1 | 802.5 |
| 2 Block BCC | 6955.3 | ||
| 3 W-particle | 10,298.3 | ||
| AlCrFeNiW0.4 | 1 Matrix (BCC + B2) | 6201.9 | 856.1 |
| 2 Block BCC | 6966.9 | ||
| 3 W-particle | 10,491 |
| Alloys | AlCrFeNi | AlCrFeNiW0.1 | AlCrFeNiW0.2 | AlCrFeNiW0.3 | AlCrFeNiW0.4 |
|---|---|---|---|---|---|
| Yield strength (MPa) | 1222 | 1347 | 1463 | 1543 | 1642 |
| Fracture strength (MPa) | 2636 | 2744 | 2986 | 2894 | 2294 |
| Fracture strain (%) | 41.2 | 40.8 | 40.7 | 33.8 | 23.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Diao, G.; Xu, Z.; Sim, R.; Chen, W.; Chen, D.; Li, D. Investigation of Mechanical Properties and Wear Resistance of A2/B2 Type Medium-Entropy Alloy Matrix Reinforced with Tungsten Particles by In-Situ Reaction. Metals 2023, 13, 656. https://doi.org/10.3390/met13040656
Wu M, Diao G, Xu Z, Sim R, Chen W, Chen D, Li D. Investigation of Mechanical Properties and Wear Resistance of A2/B2 Type Medium-Entropy Alloy Matrix Reinforced with Tungsten Particles by In-Situ Reaction. Metals. 2023; 13(4):656. https://doi.org/10.3390/met13040656
Chicago/Turabian StyleWu, Mingyu, Guijiang Diao, Zhen Xu, Ruiken Sim, Wengang Chen, Daolun Chen, and Dongyang Li. 2023. "Investigation of Mechanical Properties and Wear Resistance of A2/B2 Type Medium-Entropy Alloy Matrix Reinforced with Tungsten Particles by In-Situ Reaction" Metals 13, no. 4: 656. https://doi.org/10.3390/met13040656
APA StyleWu, M., Diao, G., Xu, Z., Sim, R., Chen, W., Chen, D., & Li, D. (2023). Investigation of Mechanical Properties and Wear Resistance of A2/B2 Type Medium-Entropy Alloy Matrix Reinforced with Tungsten Particles by In-Situ Reaction. Metals, 13(4), 656. https://doi.org/10.3390/met13040656








