MoSe2 Complex with N and B Dual-Doped 3D Carbon Nanofibers for Sodium Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
2.2. Materials Syntheses
2.2.1. Preparation of BCM
2.2.2. Preparation of MoSe2/BCM
2.2.3. Preparation of N&B-BCM
2.2.4. Preparation of MoSe2/N&B-BCM
2.3. Electrochemical Measurements
- (1)
- The slurry preparation: The active material (the composite material detailed above), conductive material (acetylene black), and binder (the solute was PVDF, the solvent was NMP, and the mass fraction was 8 wt%) were weighed. The active material and the conductive material were ground in an agate mortar for 40 min, and then the mixed powder was poured into a 5 mL glass bottle with a cover. The binder was added and then stirred at a constant speed for 3 h.
- (2)
- The electrode preparation: A 10 × 15 cm piece of copper foil was wiped with an alcohol cotton ball to remove surface dust; the evenly stirred slurry was coated onto the surface of the copper foil; the foil was kept in a vacuum drying oven for 12 h, and the temperature was set at 80 °C; the dried copper foil was further impacted with a punch to obtain an electrode sheet with a diameter of 14 mm; the obtained electrode sheet was placed in a tablet press with a 10 MPa pressure and was pressed down and kept as such for 3 s; and the quality of the electrode sheet was weighed. A card machine was used to make the active material copper foil into several Ø = 14 mm copper foils, and they were weighed, and the average mass of the Ø = 14 mm copper foils was calculated. Combining the average mass of the electrode sheet and copper foil of Ø = 14 mm with the slurry ratio, the mass of the active material on the surface of the electrode sheet was calculated. The weight of the electrode was 2 mg/cm2.
- (3)
- The battery assembly: The entire battery assembly process was carried out in a vacuum glove box filled with Ar. All the batteries used in this paper were CR2025 button cells. From bottom to top, they have a positive electrode shell, an electrode sheet (the side coated with the active material facing up), a separator, a sodium metal sheet, nickel foam, and a negative electrode shell. Two~three drops of electrolyte were added. After assembly was complete, a paper towel was used to wipe off the excess electrolyte that spilled out of the battery to prevent the battery from short-circuiting.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Fu, Q.; Li, C.; Li, H.; Tang, H. Nitrogen and phosphorus dual-doped graphene aerogel confined monodisperse iron phosphide nanodots as an ultrafast and long-term cycling anode material for sodium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 15083–15091. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, K.; Liu, J.; Xia, S. Nitrogen-doped mesoporous carbon as an anode material for high performance potassium-ion batteries. Electrochim. Acta 2020, 340, 135947. [Google Scholar] [CrossRef]
- Lu, W.; Zhu, J.; He, S.A.; Cui, Z.; Wang, H.; Xu, C.; Liu, Q.; Zou, R. Enhanced reversible sodium storage by thin carbon layer encapsulated MoS2 nanospheres on interwoven carbon nanotubes. Solid State Ion. 2021, 359, 115522. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Shao, S.; Ma, Y.; Zhang, J.; Kang, W.; Xu, J. Transformation of Two-Dimensional Iron Sulfide Nanosheets from FeS2 to FeS as High-Rate Anodes for Pseudocapacitive Sodium Storage. ACS Appl. Energy Mater. 2020, 3, 12672–12681. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, X. Hierarchical Scalelike Yolk–Shell Construction Assembled via Ultrathin MoSe2 Nanoplates Incorporated into Metal–Organic Frameworks Derived Porous Carbon Spheres as Highly Durable Anode for Enhanced Sodium Storage. ACS Sustain. Chem. Eng. 2020, 8, 19040–19050. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, Y.; Chu, P.; Wang, S.; Li, Y.; Ren, X.; Zhang, P.; Sun, L. Heterostructure enhanced sodium storage performance for SnS2 in hierarchical SnS2/Co3S4 nanosheet array composite. J. Mater. Chem. A 2021, 9, 1630–1642. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Huang, B.; Cai, W.; Sui, J.; Yang, Z.; Wang, H.E. MoSe2 Nanoplatelets with Enriched Active Edge Sites for Superior Sodium-ion Storage and Enhanced Alkaline Hydrogen Evolution Activity. Chem. Eng. J. 2019, 382, 123047. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Lao, M.; Zhang, Y.; Luo, W.; Yan, Q.; Sun, W.; Dou, S.X. Alloy-Based Anode Materials toward Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700622. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2019, 10, 1902485. [Google Scholar] [CrossRef]
- Luo, M.; Yu, H.; Hu, F.; Liu, T.; Cheng, X.; Zheng, R.; Bai, Y.; Shui, M.; Shu, J. Metal Selenides for High Performance Sodium Ion Batteries. Chem. Eng. J. 2019, 380, 122557. [Google Scholar] [CrossRef]
- Niu, F.; Yang, J.; Wang, N.; Zhang, D.; Fan, W.; Yang, J.; Qian, Y. MoSe2-Covered N,P-Doped Carbon Nanosheets as a Long-Life and High-Rate Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1700522. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, K.; Shen, B.; Zhen, M. Synergetic Effect of Nitrogen/Sulfur Dual-Doped Hierarchically Porous Carbon Networks for Li–S Batteries. ACS Sustain. Chem. Eng. 2019, 8, 749–758. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Lee, S.J.; Theerthagiri, J.; Nithyadharseni, P.; Arunachalam, P.; Balaji, D.; Kumar, A.M.; Madhavan, J.; Mittal, V.; Choi, M.Y. Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renew. Sustain. Energy Rev. 2021, 143, 110849. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and sodium-ion batteries: 50 years of research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Hueso, K.B.; Armand, M.; Rojo, T. High temperature sodium batteries: Status, challenges and future trends. Energy Environ. Sci. 2013, 6, 734–749. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Chen, J.; Yang, B.; Liu, B.; Lang, J.; Yan, X. Recent advances in anode materials for sodium-and potassium-ion hybrid capacitors. Curr. Opin. Electrochem. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Tong, Y.; Liu, X.; Zheng, J.; Han, D.; Li, H.; Niu, L. Recent advances in potassium-ion hybrid capacitors: Electrode materials, storage mechanisms and performance evaluation. Energy Storage Mater. 2021, 41, 108–132. [Google Scholar] [CrossRef]
- Okoshi, T.; Yamada, K.; Hirasawa, T.; Emori, A. Battery condition monitoring (BCM) technologies about lead–acid batteries. J. Power Sources 2006, 158, 874–878. [Google Scholar] [CrossRef]
- Vahid, S.; Abarzadeh, M.; Weise, N.; El-Refaie, A. A Novel Three-Port dc-dc Power Converter with Adaptive Boundary Current Mode Controller for a Residential PV-Battery System. In Proceedings of the 46th Annual Conference of the IEEE Industrial Electronics Society, Singapore, 18–21 October 2020; pp. 4263–4268. [Google Scholar]
- Jiang, F.; Yin, L.; Yu, Q.; Zhong, C.; Zhang, J. Bacterial cellulose nanofibrous membrane as thermal stable separator for lithium-ion batteries. J. Power Sources 2015, 279, 21–27. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, P.; Lu, X.; Hou, X.; Zhao, M.; Huang, J.; Xia, X.; Wei, Q. Fibrous network of c@ MoS2 nanocapsule-decorated cotton linters interconnected by bacterial cellulose for lithium-and sodium-ion batteries. ChemSusChem 2019, 12, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Zhao, X.; Yang, F. Core-sheath structured porous carbon nanofiber composite anode material derived from bacterial cellulose/polypyrrole as an anode for sodium-ion batteries. Carbon 2015, 95, 552–559. [Google Scholar] [CrossRef]
- Ma, L.; Bi, Z.; Xue, Y.; Zhang, W.; Huang, Q.; Zhang, L.; Huang, Y. Bacterial cellulose: An encouraging eco-friendly nano-candidate for energy storage and energy conversion. J. Mater. Chem. A 2020, 8, 5812–5842. [Google Scholar] [CrossRef]
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef]
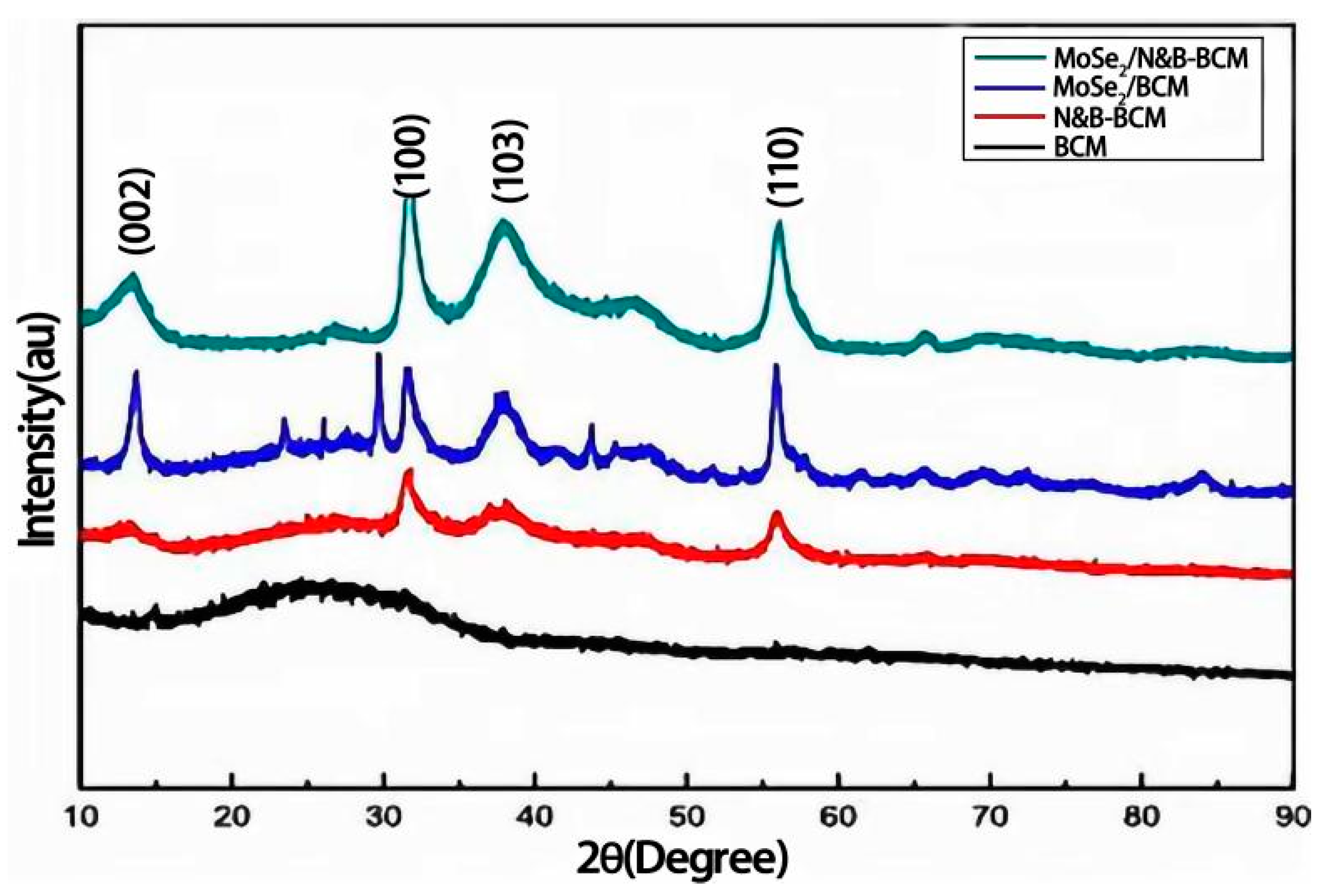
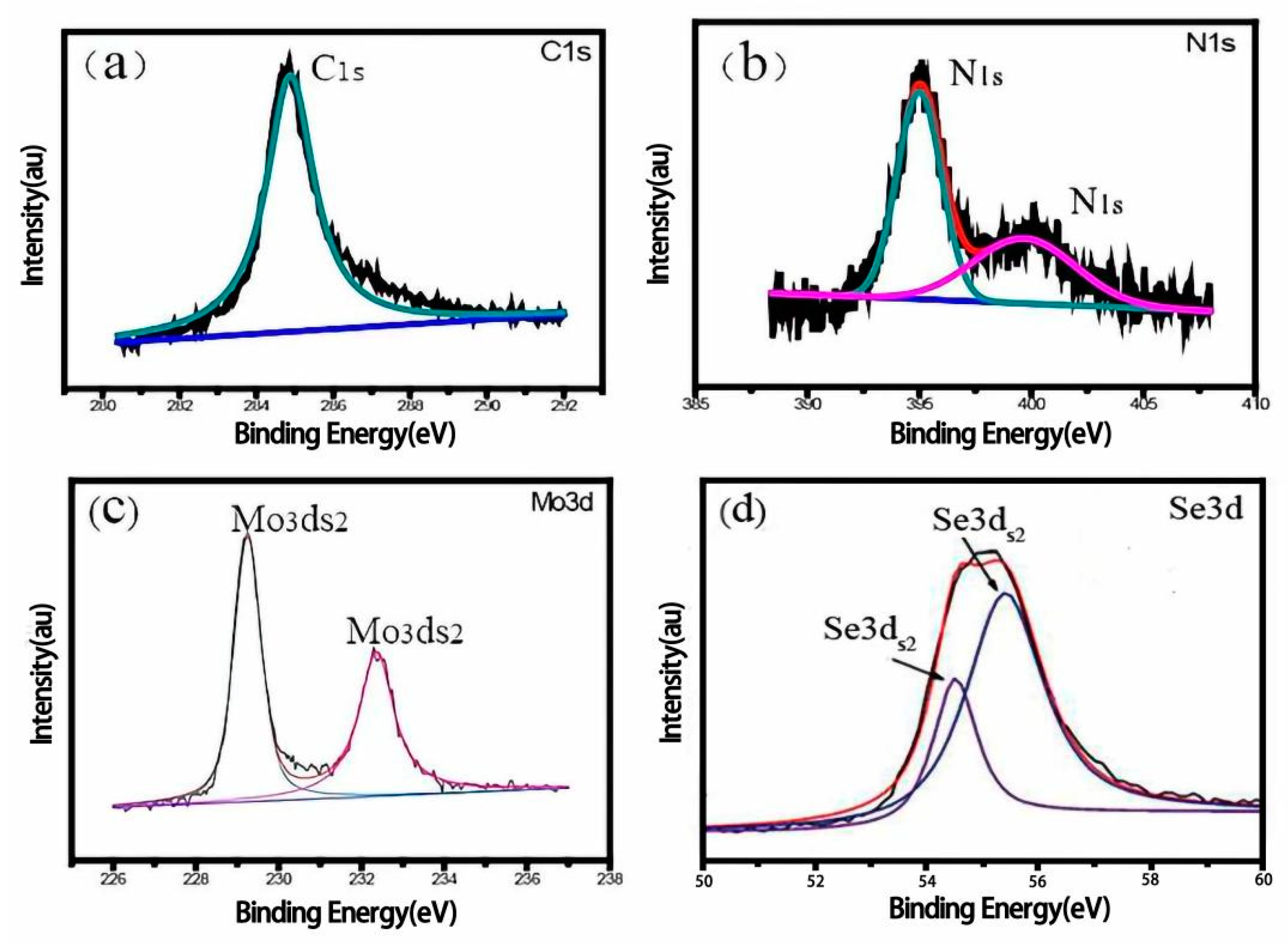

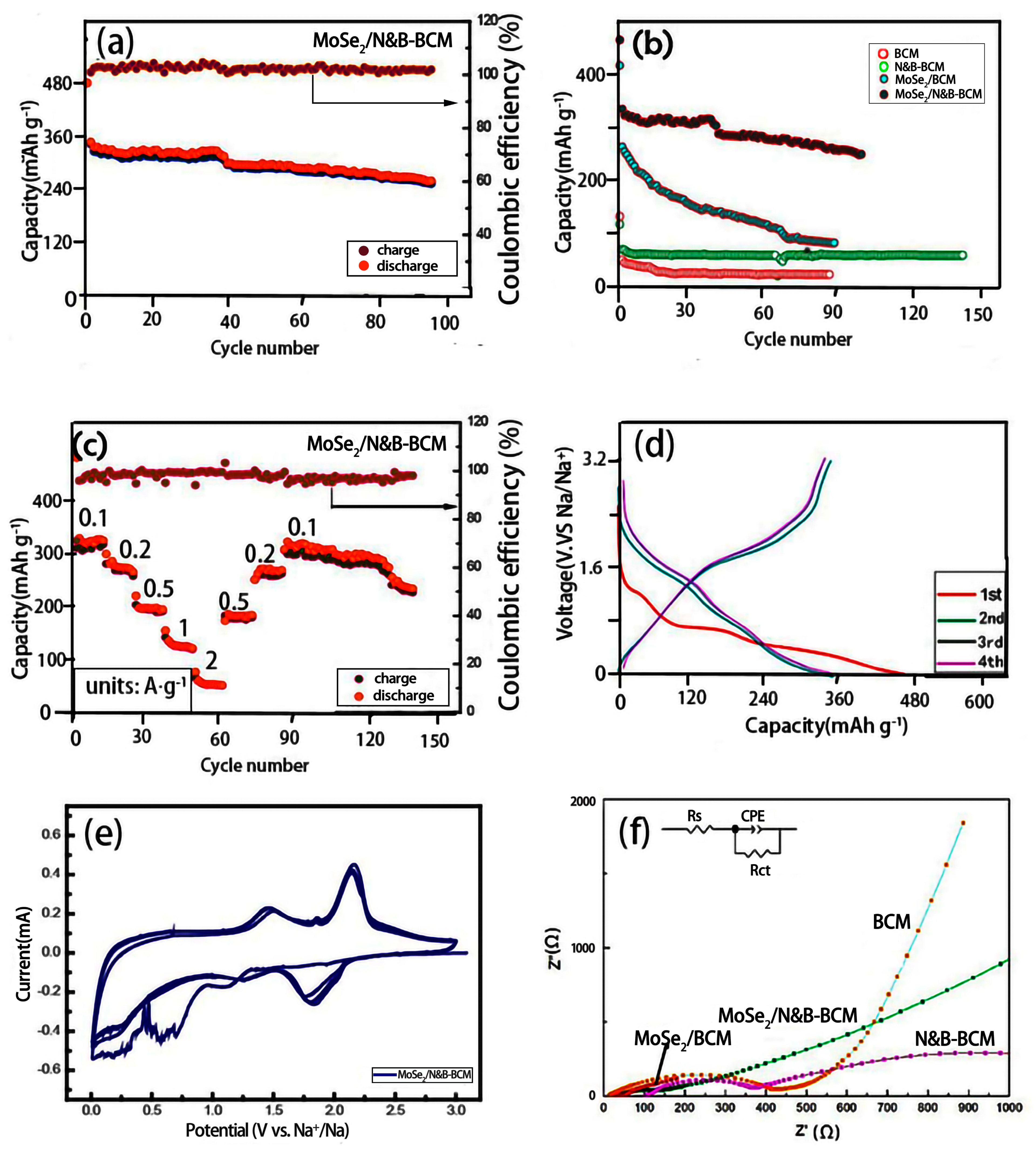
| Resistance Value | BCM | N&B-BCM | MoSe2/BCM | MoSe2/N&B-BCM |
|---|---|---|---|---|
| Rs | 28.5 Ω | 20.82 Ω | 28.46 Ω | 11.96 Ω |
| Rct | 1719 Ω | 604 Ω | 453 Ω | 182.4 Ω |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, C.; Yin, X. MoSe2 Complex with N and B Dual-Doped 3D Carbon Nanofibers for Sodium Batteries. Metals 2023, 13, 518. https://doi.org/10.3390/met13030518
Zhao W, Liu C, Yin X. MoSe2 Complex with N and B Dual-Doped 3D Carbon Nanofibers for Sodium Batteries. Metals. 2023; 13(3):518. https://doi.org/10.3390/met13030518
Chicago/Turabian StyleZhao, Weigang, Cuirong Liu, and Xu Yin. 2023. "MoSe2 Complex with N and B Dual-Doped 3D Carbon Nanofibers for Sodium Batteries" Metals 13, no. 3: 518. https://doi.org/10.3390/met13030518
APA StyleZhao, W., Liu, C., & Yin, X. (2023). MoSe2 Complex with N and B Dual-Doped 3D Carbon Nanofibers for Sodium Batteries. Metals, 13(3), 518. https://doi.org/10.3390/met13030518







