In Situ Observation of Solidification and Crystallization of Low-Alloy Steels: A Review
Abstract
1. Introduction
2. Brief Description of Experimental Apparatus for In Situ Characterization
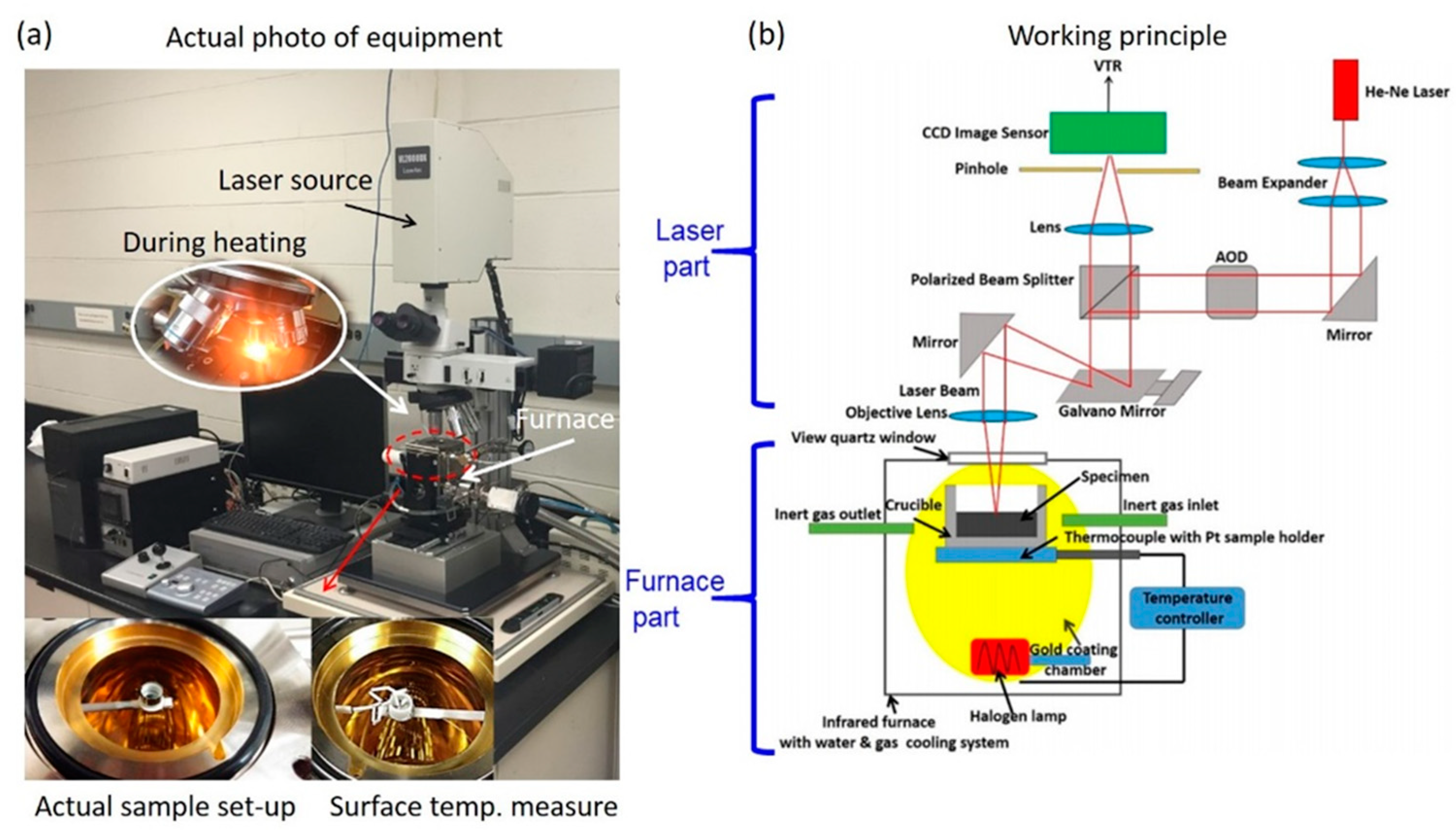
2.1. Instrumentation of a High-Temperature Confocal Laser Scanning Microscope
2.2. Special Design of Instrumentation for Solidification Research
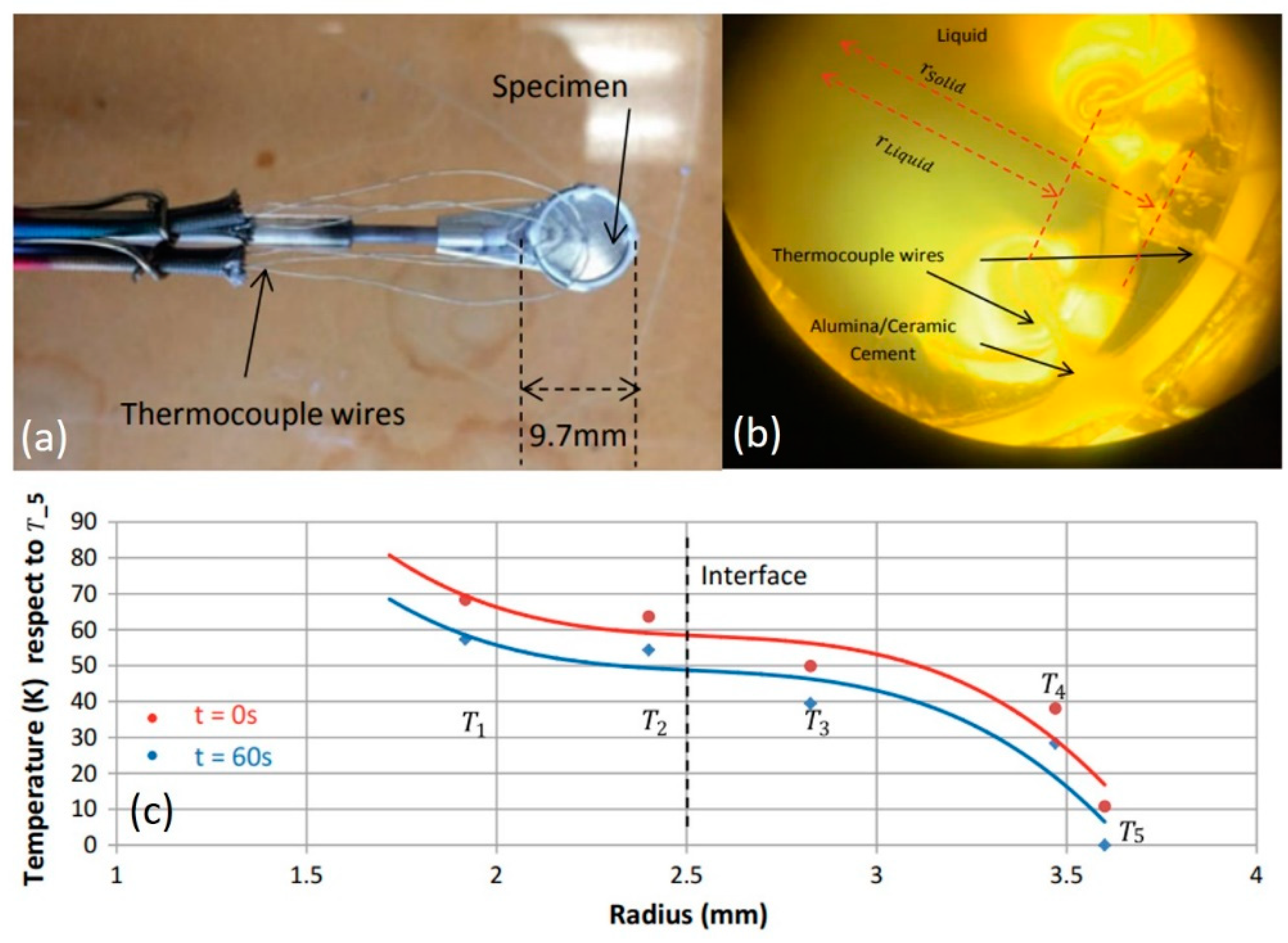
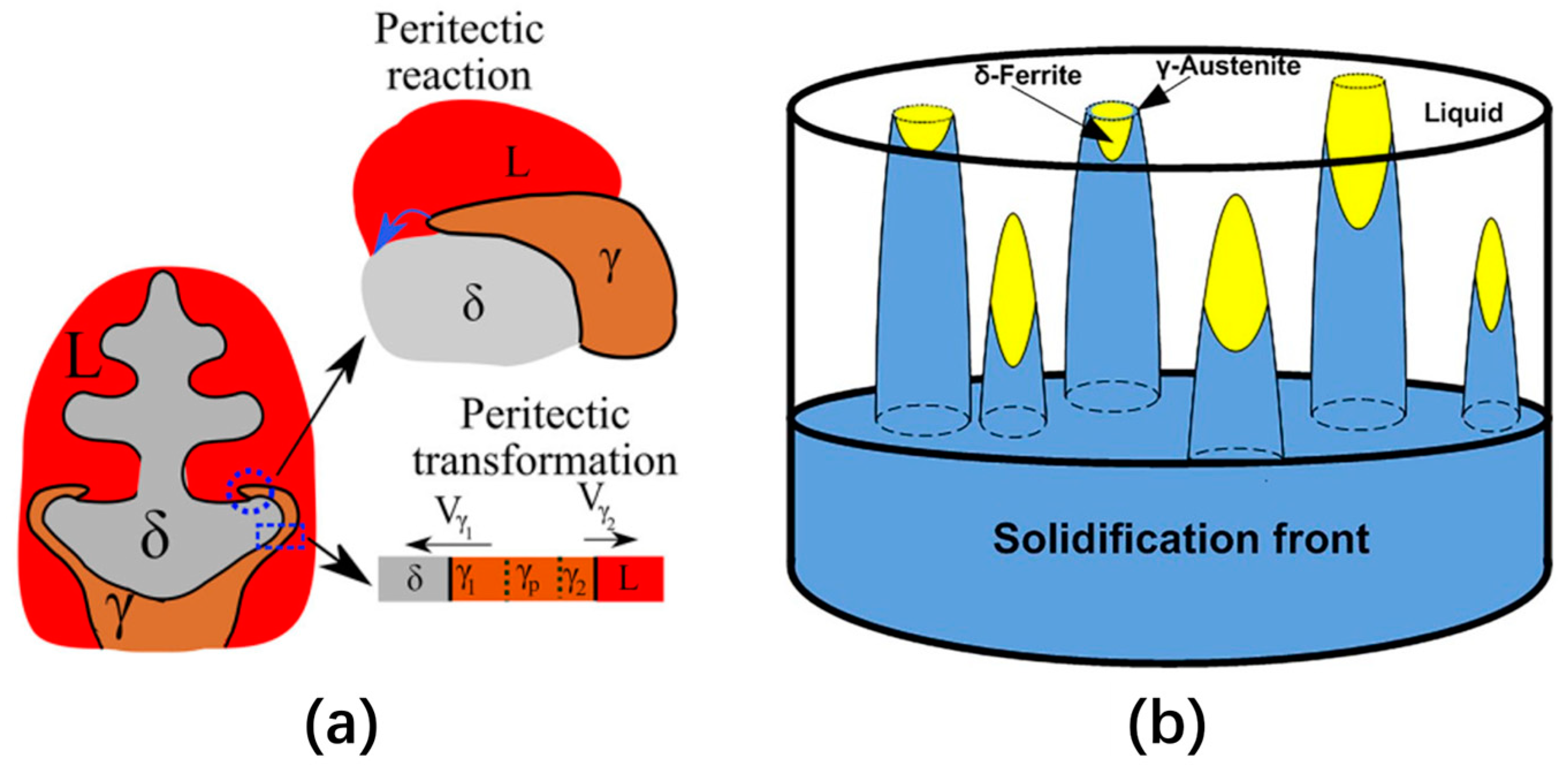
2.2.1. Concentric Solidification Technique
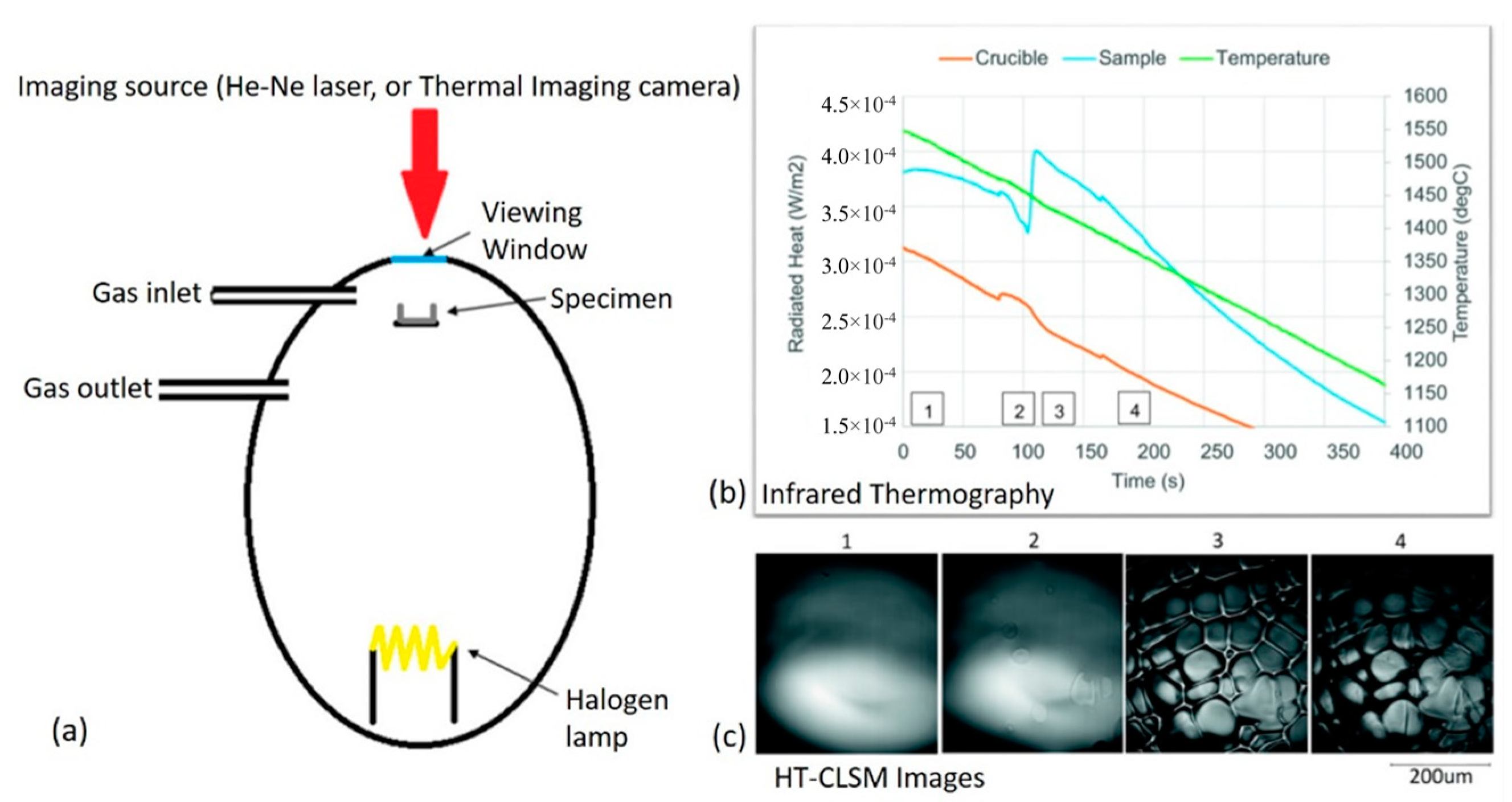
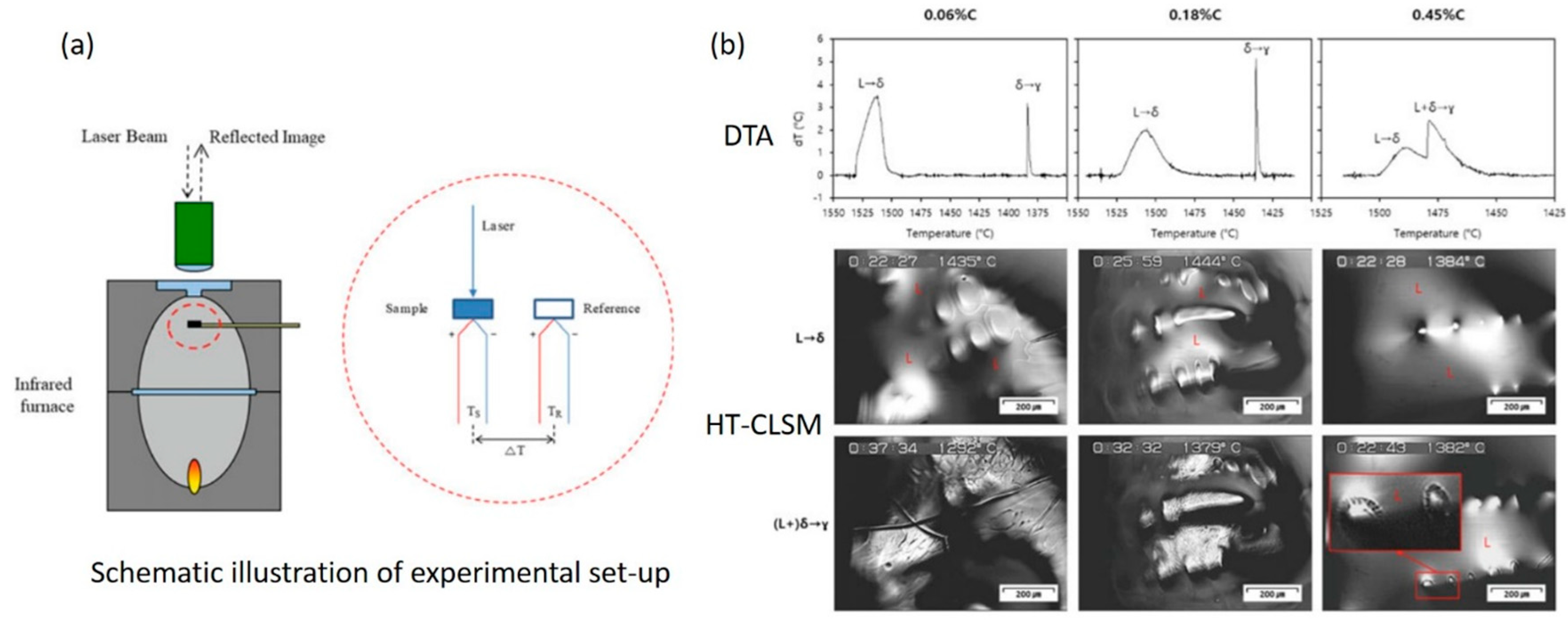
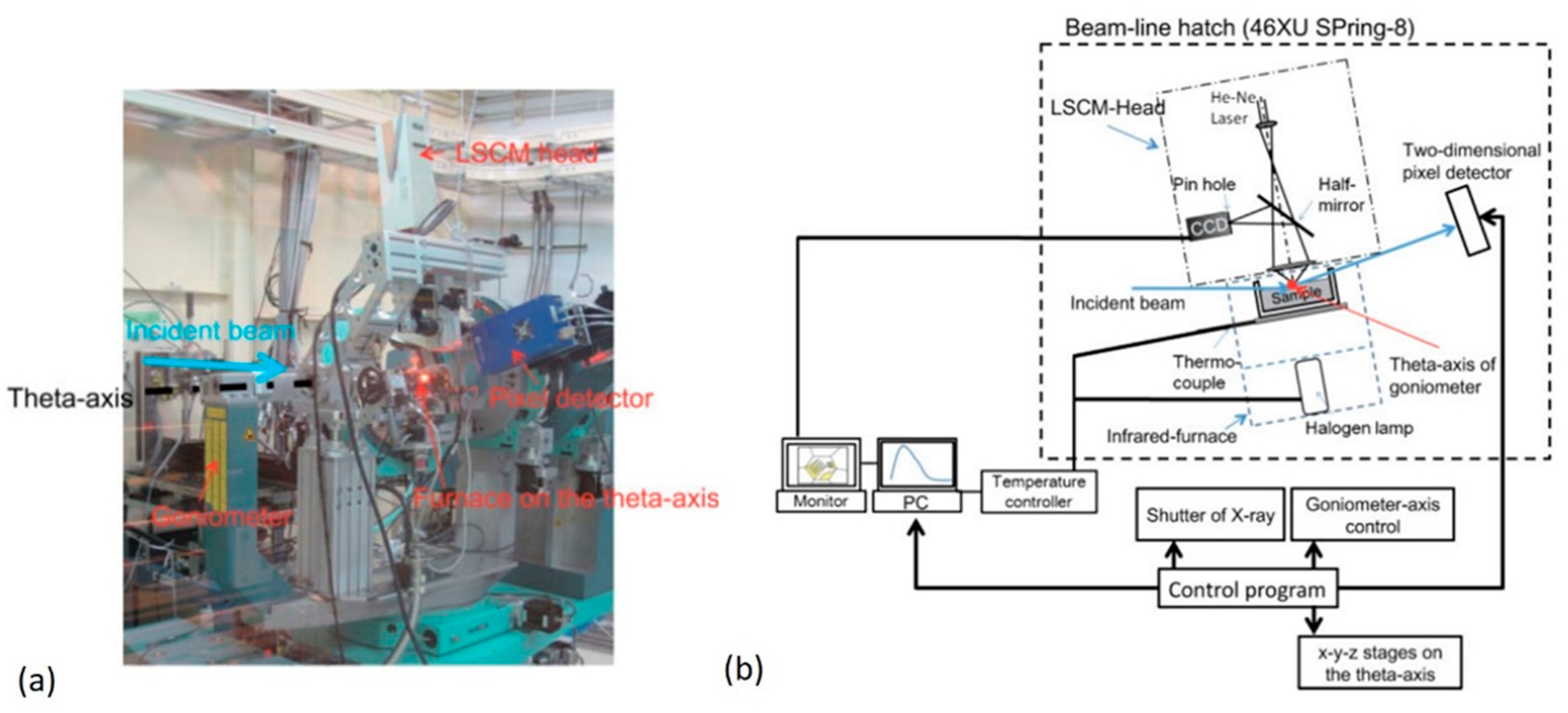
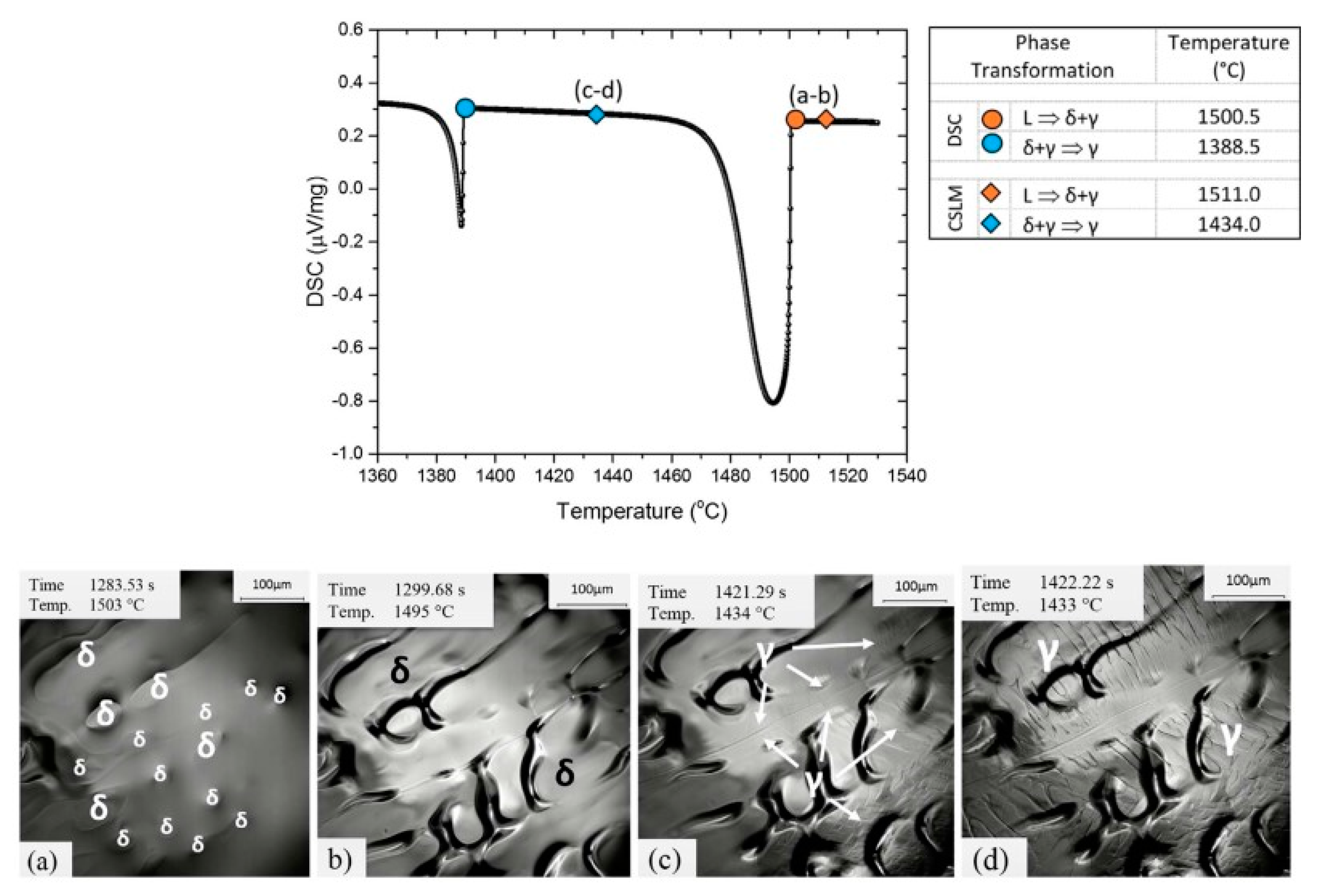
2.2.2. Combinational Approach
3. Crystallization and Microstructure Evolution during Solidification by HT-CLSM
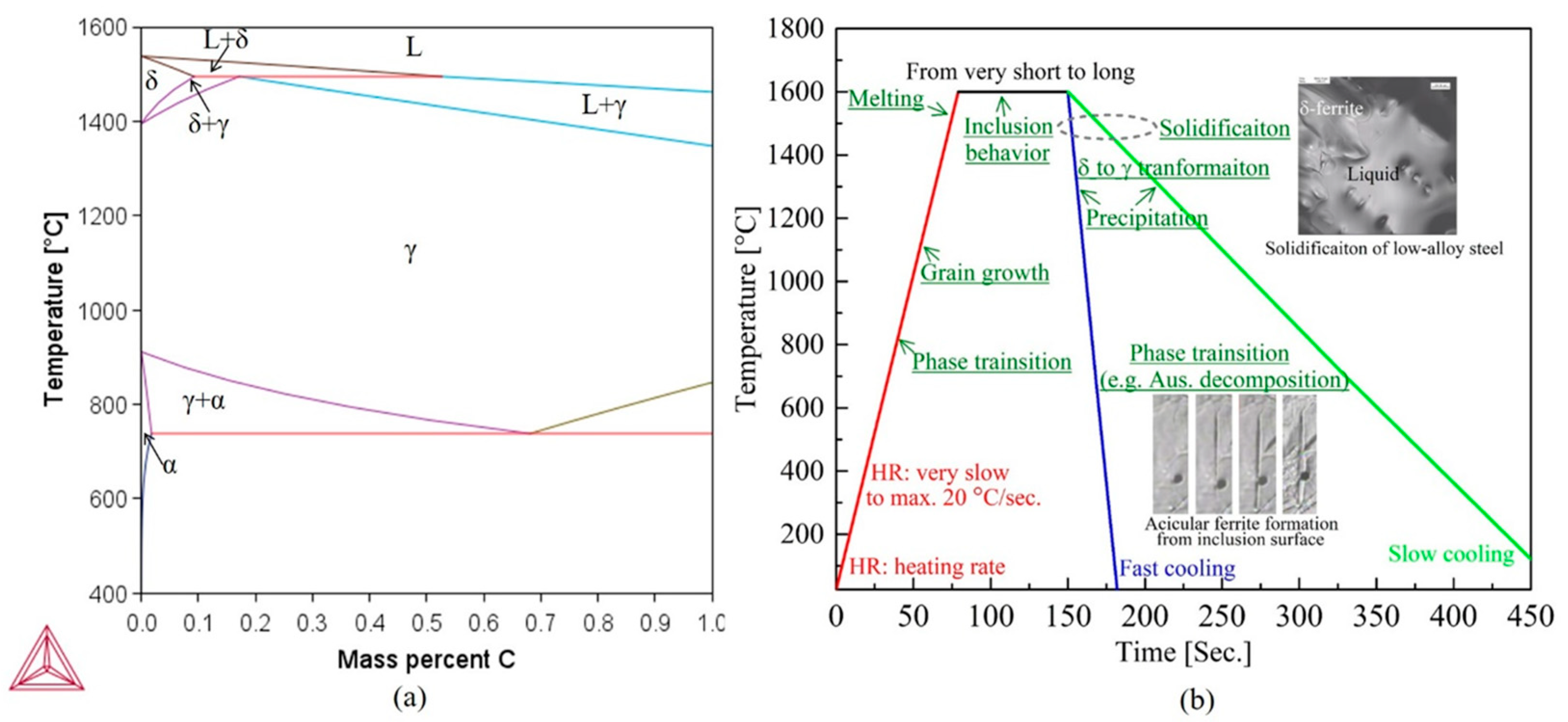
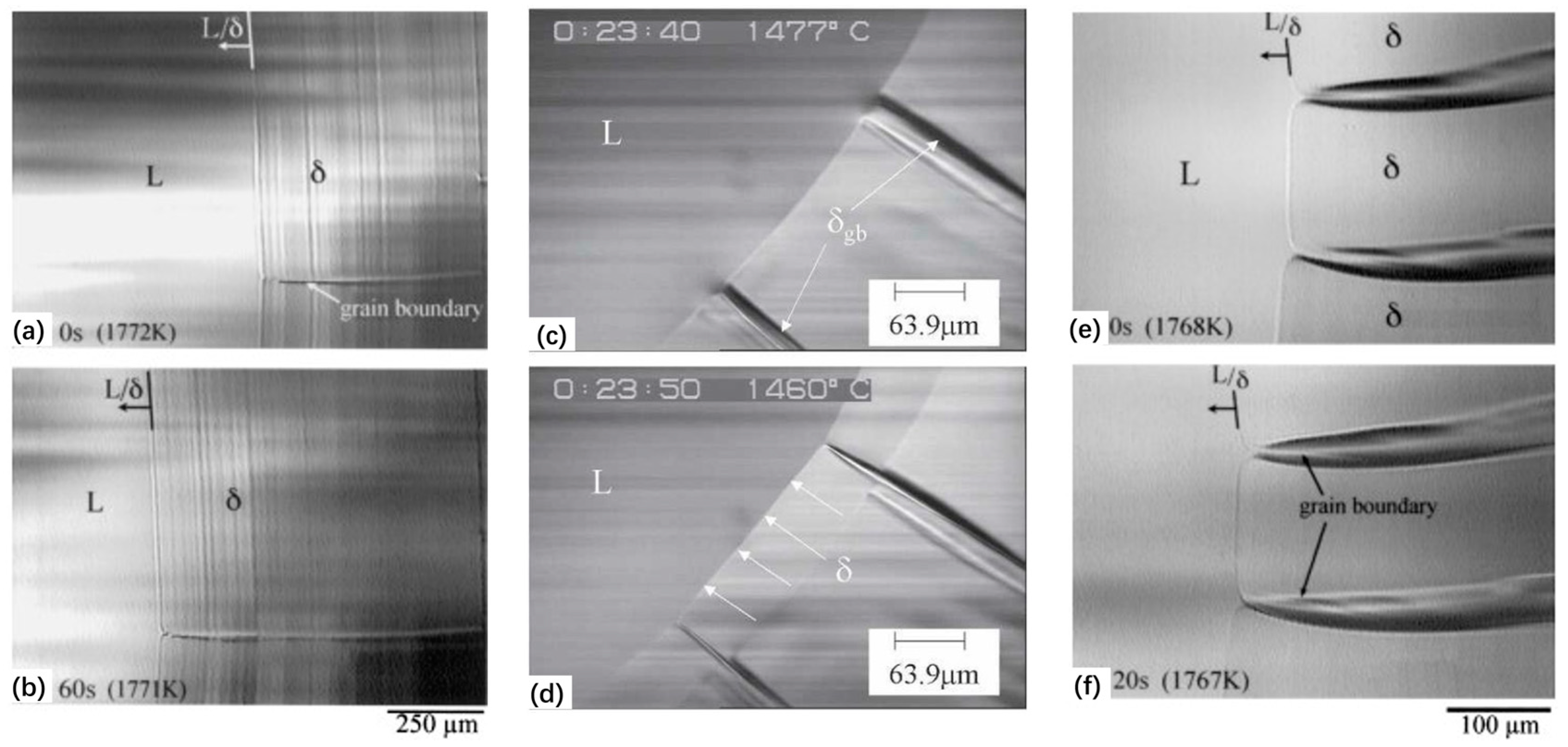
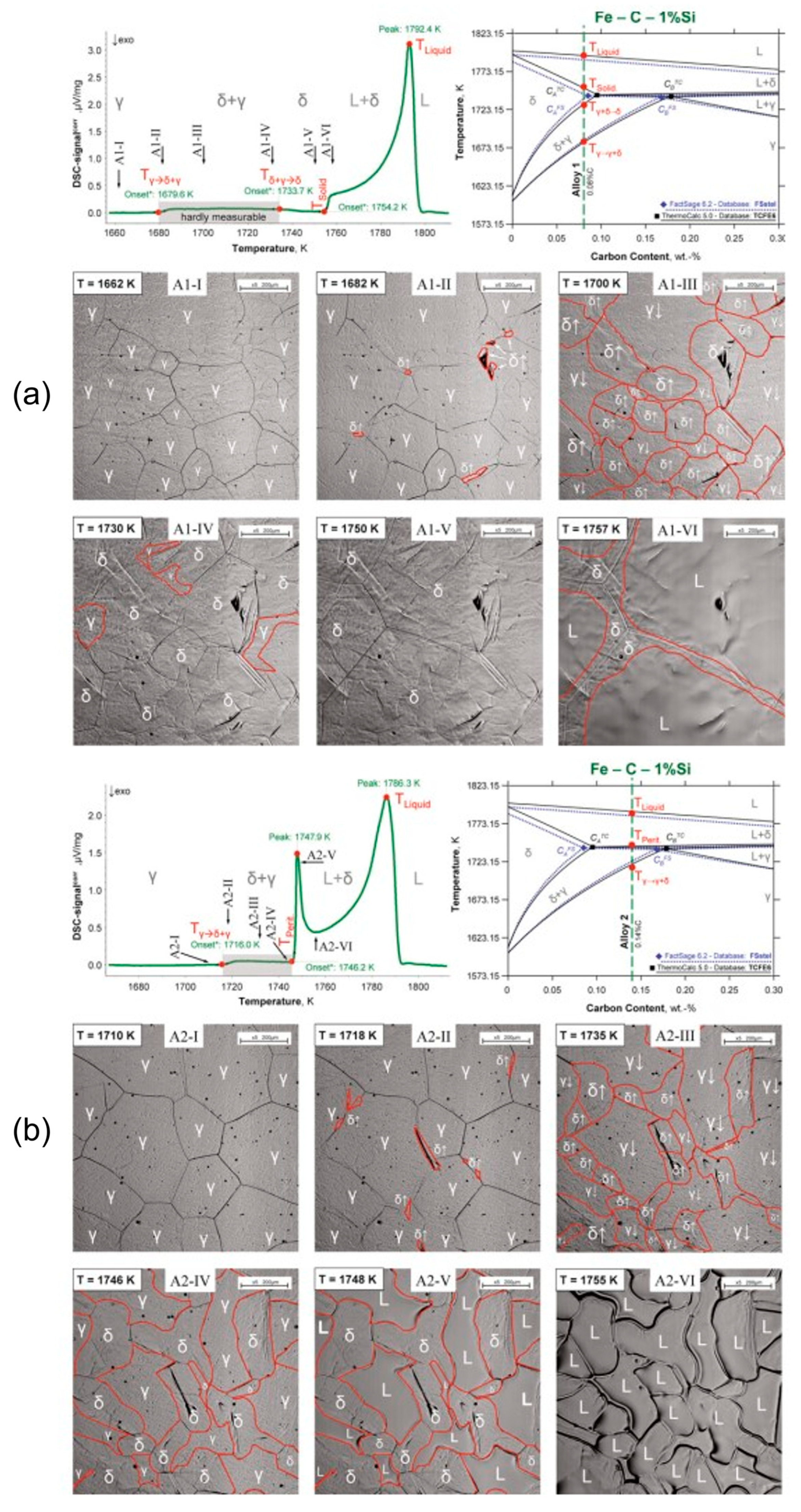
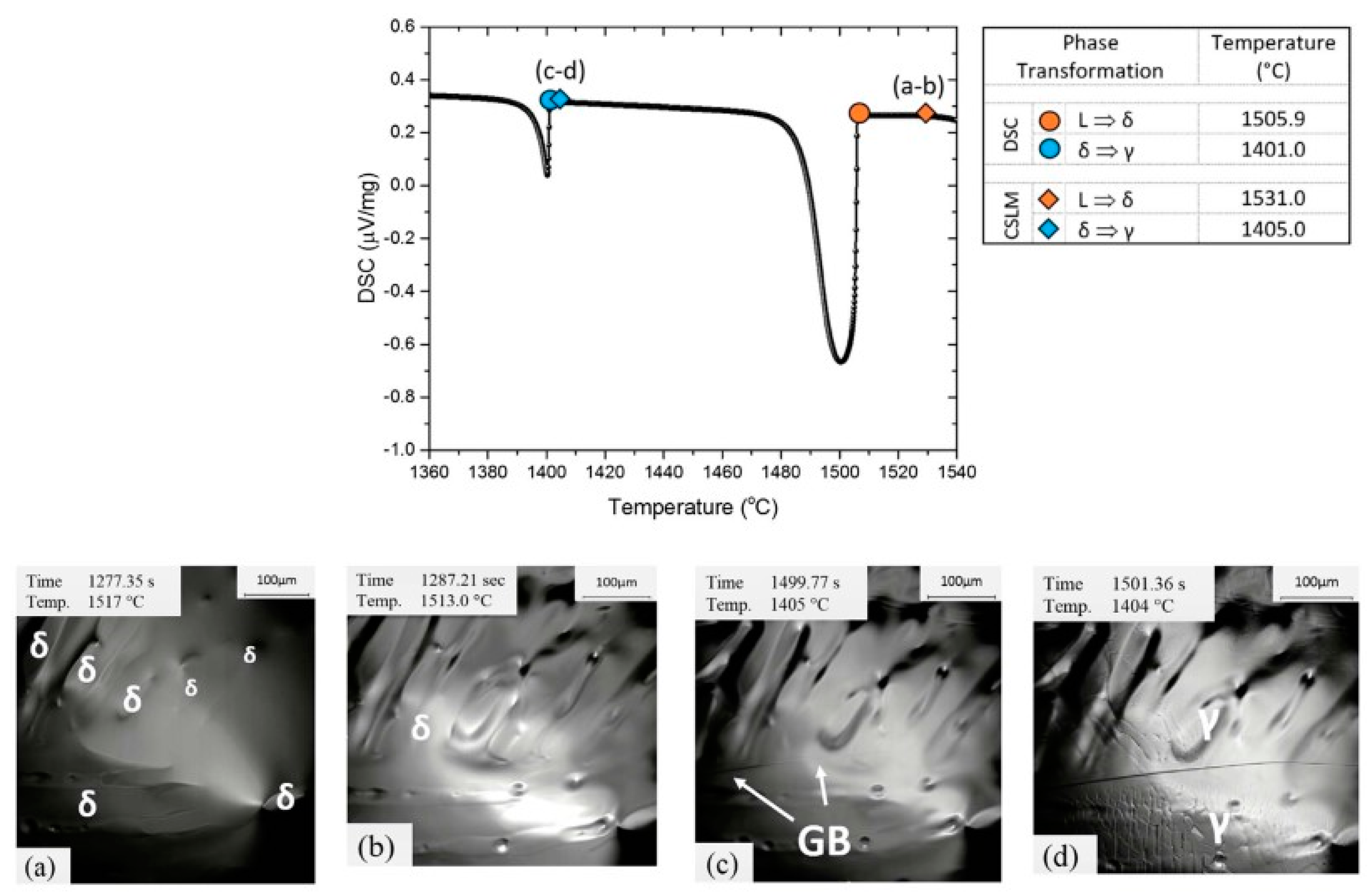
3.1. In Situ Observation of Crystallization during Solidification of Low-Alloy Steels
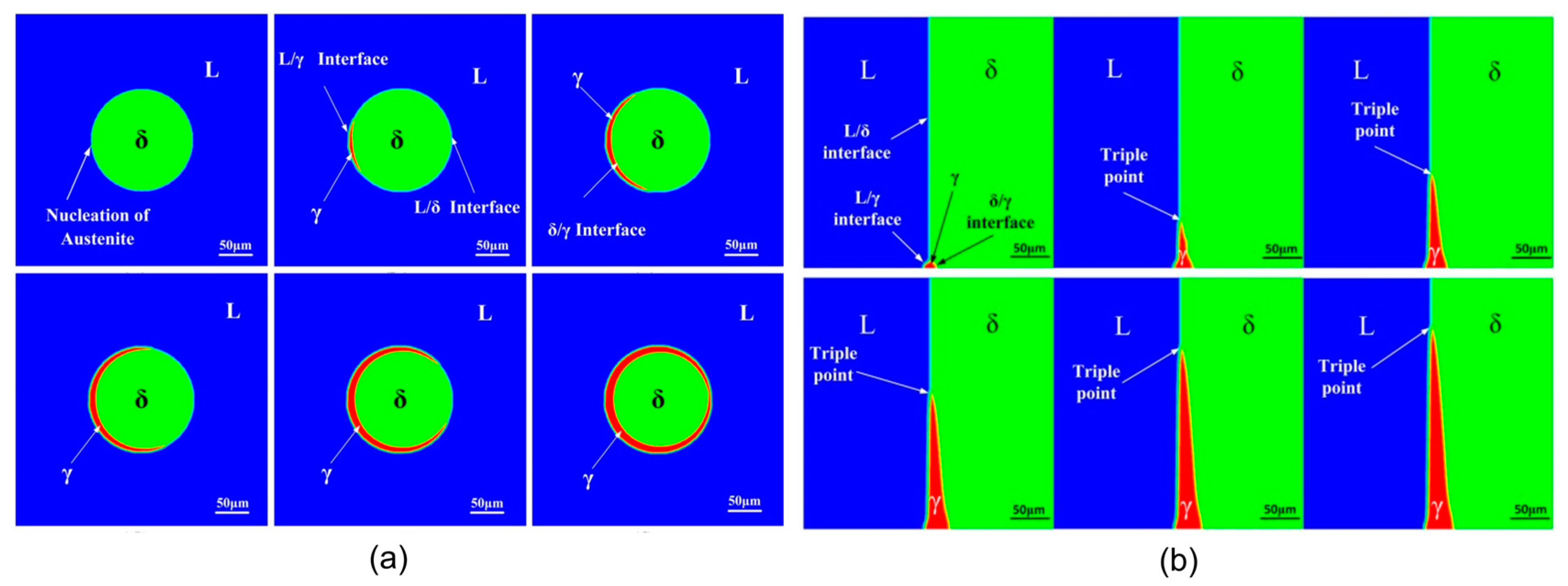
3.2. Microstructure Evolution from Delta-Ferrite to Austenite in Low-Alloy Steels
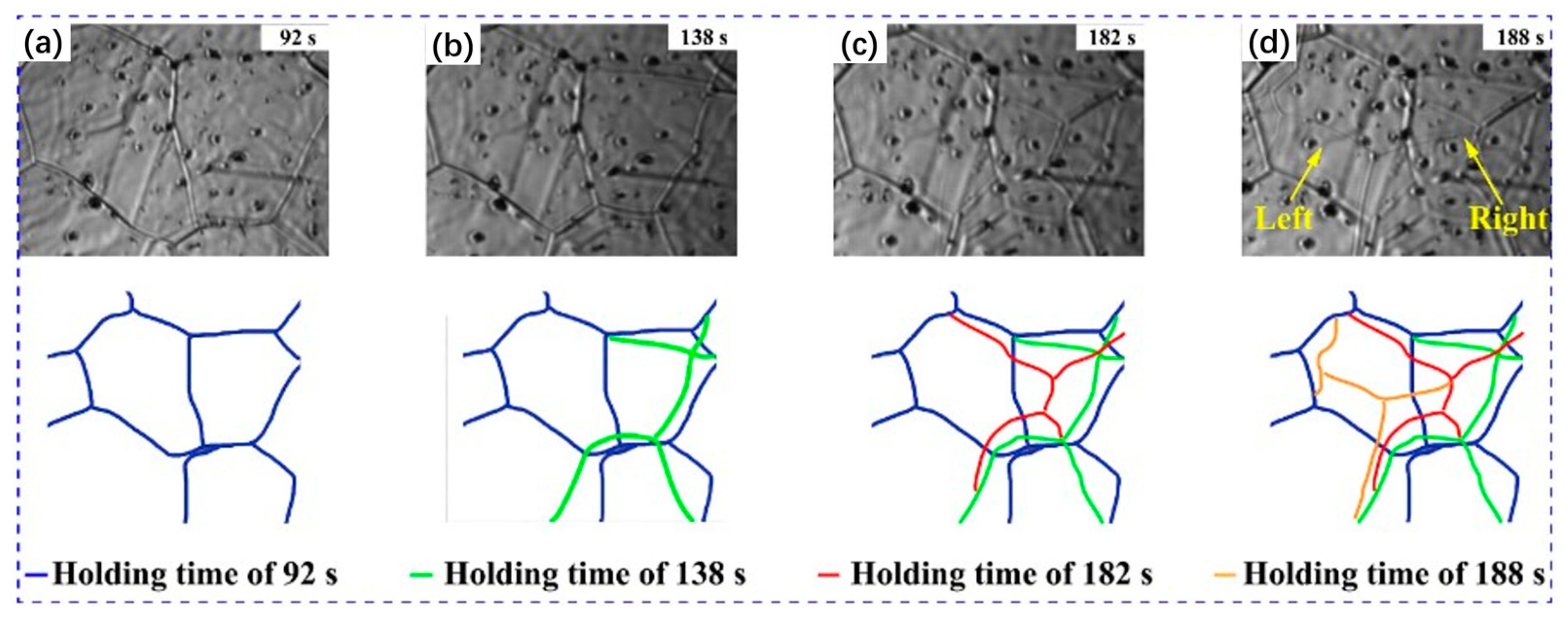
3.3. Subsequent Microstructure Evolution during Austenite Decomposition in Low-Alloy Steels
4. Other In Situ Characterization Methodologies Used for the Crystallization of Low-Alloy Steels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fredriksson, H. Solidification mechanisms. A study of the crystallization process in metal alloys. Scand. J. Metallurgy 1991, 20, 43–49. [Google Scholar]
- Fredriksson, H.; Åkerlind, U. Materials Processing during Casting; Wiley Online Library: Hoboken, NJ, USA, 2006; Volume 210. [Google Scholar]
- Jernkontoret (The Swedish Steel Producers’ Association). A Guide to the Solidification of Steels; Ljungberg Truckeri AB: Södertälje, Sweden, 1977; pp. 5–54. [Google Scholar]
- Bernhard, M.; Presoly, P.; Bernhard, C.; Hahn, S.; Ilie, S. An Assessment of Analytical Liquidus Equations for Fe-C-Si-Mn-Al-P-Alloyed Steels Using DSC/DTA Techniques. Metall. Mater. Trans. B 2021, 52, 2821–2830. [Google Scholar] [CrossRef]
- Presoly, P.; Pierer, R.; Bernhard, C. Identification of Defect Prone Peritectic Steel Grades by Analyzing High-Temperature Phase Transformations. Metall. Mater. Trans. A 2013, 44, 5377–5388. [Google Scholar] [CrossRef]
- Hechu, K.; Slater, C.; Santillana, B.; Clark, S.; Sridhar, S. A novel approach for interpreting the solidification behaviour of peritectic steels by combining CSLM and DSC. Mater. Charact. 2017, 133, 25–32. [Google Scholar] [CrossRef]
- Wielgosz, E.; Kargul, T. Differential scanning calorimetry study of peritectic steel grades. J. Therm. Anal. Calorim. 2015, 119, 1547–1553. [Google Scholar] [CrossRef]
- Shibata, H.; Emi, T. Confocal scanning laser microscope technique to in-situ observe phase transformations and behaviors of nonmetallic inclusions and precipitates in metals at elevated temperatures. Materia 1997, 36, 809–813. [Google Scholar]
- Chikama, H.; Shibata, H.; Emi, T.; Suzuki, M. “In-situ” real time observation of planar to cellular and cellular to dendritic transition of crystals growing in Fe–C alloy melts. Mater. Trans. JIM 1996, 37, 620–626. [Google Scholar] [CrossRef]
- Shibata, H.; Arai, Y.; Suzuki, M.; Emi, T. Kinetics of peritectic reaction and transformation in Fe-C alloys. Metall. Mater. Trans. B 2000, 31, 981–991. [Google Scholar] [CrossRef]
- Arai, Y.; Emi, T.; Fredriksson, H.; Shibata, H. In-situ observed dynamics of peritectic solidification and δ/γ transformation of Fe-3 to 5 at. pct Ni alloys. Metall. Mater. Trans. A 2005, 36, 3065–3074. [Google Scholar] [CrossRef]
- Yin, H.; Emi, T.; Shibata, H. Determination of free energy of δ-ferrite/γ-austenite interphase boundary of low carbon steels by in-situ observation. ISIJ Int. 1998, 38, 794–801. [Google Scholar] [CrossRef]
- Yin, H.; Shibata, H.; Emi, T.; Suzuki, M. “In-situ” observation of collision, agglomeration and cluster formation of alumina inclusion particles on steel melts. ISIJ Int. 1997, 37, 936–945. [Google Scholar] [CrossRef]
- Shibata, H.; Yin, H.; Emi, T. The capillary effect promoting collision and agglomeration of inclusion particles at the inert gas–steel interface. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1998, 356, 957–966. [Google Scholar] [CrossRef]
- Shibata, H.; Yin, H.; Yoshinaga, S.; Emi, T.; Suzuki, M. In-situ Observation of Engulfment and Pushing of Nonmetallic Inclusions in Steel Melt by Advancing Melt/Solid Interface. ISIJ Int. 1998, 38, 149–156. [Google Scholar] [CrossRef]
- Cho, J.W.; Emi, T.; Shibata, H.; Suzuki, M. Heat Transfer across Mold Flux Film in Mold during Initial Solidification in Continuous Casting of Steel. ISIJ Int. 1998, 38, 834–842. [Google Scholar] [CrossRef]
- Cho, J.W.; Shibata, H. Effect of solidification of mold fluxes on the heat transfer in casting mold. J. Non-Crystalline Solids 2001, 282, 110–117. [Google Scholar] [CrossRef]
- Dippenaar, R.J. Continuous Casting of Advanced Steels of Near-Peritectic Composition. Mater. Sci. Forum 2010, 654–656, 17–22. [Google Scholar] [CrossRef]
- Reid, M.; Phelan, D.; Dippenaar, R. Concentric Solidification for High Temperature Laser Scanning Confocal Microscopy. ISIJ Int. 2004, 44, 565–572. [Google Scholar] [CrossRef]
- Phelan, D.; Stanford, N.; Dippenaar, R. In situ observations of Widmanstätten ferrite formation in a low-carbon steel. Mater. Sci. Eng. A 2005, 407, 127–134. [Google Scholar] [CrossRef]
- Phelan, D.; Reid, M.; Dippenaar, R. Kinetics of the peritectic phase transformation: In-situ measurements and phase field modeling. Metall. Mater. Trans. A 2006, 37, 985–994. [Google Scholar] [CrossRef]
- Phelan, D.; Dippenaar, R. Instability of the Delta-ferrite/austenite Interface in Low Carbon Steels: The Influence of Delta-ferrite Recovery Sub-structures. ISIJ Int. 2004, 44, 414–421. [Google Scholar] [CrossRef]
- Griesser, S.; Reid, M.; Bernhard, C.; Dippenaar, R. Diffusional constrained crystal nucleation during peritectic phase transitions. Acta Mater. 2014, 67, 335–341. [Google Scholar] [CrossRef]
- Griesser, S.; Bernhard, C.; Dippenaar, R. Effect of nucleation undercooling on the kinetics and mechanism of the peritectic phase transition in steel. Acta Mater. 2014, 81, 111–120. [Google Scholar] [CrossRef]
- Niknafs, S.; Phelan, D.; Dippenaar, R. High-temperature laser-scanning confocal microscopy as a tool to study the interface instability during unsteady-state solidification of low-carbon steel. J. Microsc. 2013, 249, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Dippenaar, R.; Kim, S. The peritectic phase transition of steel during the initial stages of solidification in the mold. In Proceedings of the AISTech Conference, Cleveland, OH, USA, 4–7 May 2015. [Google Scholar]
- Liyanage, D.; Moon, S.C.; Du Toit, M.; Dippenaar, R. Quantitative Thermal Analysis of Solidification in a High-Temperature Laser-Scanning Confocal Microscope. In Advanced Real Time Imaging II; Springer: Berlin/Heidelberg, Germany, 2019; pp. 131–141. [Google Scholar] [CrossRef]
- Moon, S.-C.; Phelan, D.; Reid, M.; Griesser, S.; Liyanage, D.; Dippenaar, R. Development of HT-LSCM Techniques for the In Situ Study of the Peritectic Phase Transition. In TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Berlin/Heidelberg, Germany, 2020; pp. 25–37. [Google Scholar] [CrossRef]
- Mu, W.; Dogan, N.; Coley, K.S. In Situ Observations of Agglomeration of Non-metallic Inclusions at Steel/Ar and Steel/Slag Interfaces by High-Temperature Confocal Laser Scanning Microscope: A Review. JOM 2018, 70, 1199–1209. [Google Scholar] [CrossRef]
- Mu, W.; Dogan, N.; Coley, K.S. In situ observation of deformation behavior of chain aggregate inclusions: A case study for Al2O3 at a liquid steel/argon interface. J. Mater. Sci. 2018, 53, 13203–13215. [Google Scholar] [CrossRef]
- Mu, W.; Xuan, C. Agglomeration Mechanism of Complex Ti-Al Oxides in Liquid Ferrous Alloys Considering High-Temperature Interfacial Phenomenon. Metall. Mater. Trans. B 2019, 50, 2694–2705. [Google Scholar] [CrossRef]
- Qiu, Z.; Malfliet, A.; Blanpain, B.; Guo, M. Capillary Interaction Between Micron-Sized Ce2O3 Inclusions at the Ar Gas/Liquid Steel Interface. Metall. Mater. Trans. B 2022, 53, 1775–1791. [Google Scholar] [CrossRef]
- Miao, K.; Haas, A.; Sharma, M.; Mu, W.; Dogan, N. In Situ Observation of Calcium Aluminate Inclusions Dissolution into Steelmaking Slag. Metall. Mater. Trans. B 2018, 49, 1612–1623. [Google Scholar] [CrossRef]
- Sharma, M.; Mu, W.; Dogan, N. In Situ Observation of Dissolution of Oxide Inclusions in Steelmaking Slags. JOM 2018, 70, 1220–1224. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, L.; Zhang, J.; Wu, S.; Zhu, P.; Ren, Y. In situ observation of the dissolution of Al2O3 particles in CaO-Al2O3-SiO2 slags. Metall. Mater. Trans. B 2021, 52, 3288–3301. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, P.; Ren, C.; Liu, N.; Zhang, L. Dissolution of SiO2 inclusions in CaO-SiO2-based slags in situ observed using high-temperature confocal scanning laser microscopy. Metall. Mater. Trans. B 2022, 53, 682–692. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. A Review of In Situ Observations of Crystallization and Growth in High Temperature Oxide Melts. JOM 2018, 70, 1210–1219. [Google Scholar] [CrossRef]
- Yu, X.; Wen, G.H.; Tang, P.; Ma, F.J.; Wang, H. Behavior of Mold Slag Used for 20Mn23Al Nonmagnetic Steel During Casting. J. Iron Steel Res. Int. 2011, 18, 20–25. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Wen, G.H.; Zhang, Y.Y. Crystallization behavior of F-free mold fluxes. Int. J. Miner. Metall. 2011, 18, 150–158. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, J.W.; Sohn, I. In-situ Crystallization of Highly Volatile Commercial Mold Flux Using an Isolated Observation System in the Confocal Laser Scanning Microscope. Metall. Mater. Trans. B 2014, 45, 1186–1191. [Google Scholar] [CrossRef]
- Wang, Y.; Sridhar, S. Reoxidation on the Surface of Molten Low-Carbon Aluminum-Killed Steel. Steel Res. Int. 2005, 76, 355–361. [Google Scholar] [CrossRef]
- Wang, Y.; Sridhar, S. The effect of gas flow rate on the evolution of the surface oxide on a molten low carbon Al killed steel. J. Mater. Sci. 2005, 40, 2179–2184. [Google Scholar] [CrossRef]
- Takamura, J.I.; Mizoguchi, S. Roles of oxides in steels performance. In Proceedings of the Sixth International Iron and Steel Congress, 21–26 October 1990; Iron and Steel Institute of Japan: Tokyo, Japan, 1990. [Google Scholar]
- Koseki, T.; Thewlis, G. Overview inclusion assisted microstructure control in C–Mn and low alloy steel welds. Mater. Sci. Technol. 2005, 21, 867–879. [Google Scholar] [CrossRef]
- Mu, W.; Jönsson, P.G.; Nakajima, K. Recent Aspects on the Effect of Inclusion Characteristics on the Intragranular Ferrite Formation in Low Alloy Steels: A Review. High Temp. Mater. Process. 2017, 36, 309–325. [Google Scholar] [CrossRef]
- Mu, W.; Mao, H.; Jönsson, P.G.; Nakajima, K. Effect of Carbon Content on the Potency of the Intragranular Ferrite Formation. Steel Res. Int. 2016, 87, 311–319. [Google Scholar] [CrossRef]
- Zhang, D.; Terasaki, H.; Komizo, Y.-I. In situ observation of the formation of intragranular acicular ferrite at non-metallic inclusions in C–Mn steel. Acta Mater. 2010, 58, 1369–1378. [Google Scholar] [CrossRef]
- Mu, W.; Shibata, H.; Hedström, P.; Jönsson, P.G.; Nakajima, K. Ferrite Formation Dynamics and Microstructure Due to Inclusion Engineering in Low-Alloy Steels by Ti2O3 and TiN Addition. Metall. Mater. Trans. B 2016, 47, 2133–2147. [Google Scholar] [CrossRef]
- Zou, X.-D.; Sun, J.-C.; Zhao, D.-P.; Matsuura, H.; Wang, C. Effects of Zr addition on evolution behavior of inclusions in EH36 shipbuilding steel: From casting to welding. J. Iron Steel Res. Int. 2018, 25, 164–172. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K.; Mayerhofer, A.; Bernhard, C. On the Capability of Nonmetallic Inclusions to Act as Nuclei for Acicular Ferrite in Different Steel Grades. Metall. Mater. Trans. B 2017, 48, 1992–2006. [Google Scholar] [CrossRef]
- Wan, X.-L.; Wu, K.-M.; Huang, G.; Wei, R.; Cheng, L. In situ observation of austenite grain growth behavior in the simulated coarse-grained heat-affected zone of Ti-microalloyed steels. Int. J. Miner. Metall. Mater. 2014, 21, 878–885. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, X.; Li, G.; Wang, Y.; Zheng, W.; Hou, Y. Grain refinement in coarse-grained heat-affected zone of Al–Ti–Mg complex deoxidised steel. Sci. Technol. Weld. Join. 2019, 24, 43–51. [Google Scholar] [CrossRef]
- Yao, H.; Ren, Q.; Yang, W.; Zhang, L. In Situ Observation and Prediction of the Transformation of Acicular Ferrites in Ti-Containing HLSA Steel. Metall. Mater. Trans. B 2022, 53, 1827–1840. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K.; Mayerhofer, A.; Bernhard, C.; Dippenaar, R.J. In situ observation of acicular ferrite formation using HT-LSCM: Possibilities, challenges and influencing factors. In Proceedings of the Materials Science and Technology Conference and Exhibition, Columbus, OH, USA, 4–8 October 2015. [Google Scholar]
- Mu, W.; Shibata, H.; Hedström, P.; Jönsson, P.G.; Nakajima, K. Combination of in situ microscopy and calorimetry to study austenite decomposition in inclusion engineered steels. Steel Res. Int. 2016, 87, 10–14. [Google Scholar] [CrossRef]
- Komizo, Y.I. In-situ Observation Techniques of Solidification and Phase Transformation during Welding. Trans. JWRI 2011, 40, 7–20. [Google Scholar]
- Komizo, Y.; Terasaki, H.; Yonemura, M.; Osuki, T. Development of in-Situ Microstructure Observation Techniques in Welding. Weld. World 2008, 52, 56–63. [Google Scholar] [CrossRef]
- Piva, S.P.T.; Tang, D.; Kumar, D.; Pistorius, P.C. Mass Transfer in High-Temperature Laser Confocal Microscopy. In TMS Annual Meeting & Exhibition; Springer: Berlin/Heidelberg, Germany, 2018; pp. 193–200. [Google Scholar] [CrossRef]
- Britt, S.T.; Pistorius, P.C. Investigation into the Temperature of Metallic High-Temperature Confocal Scanning Laser Microscope Samples. Metall. Mater. Trans. B 2022, 53, 2153–2165. [Google Scholar] [CrossRef]
- Lombardi, A.; Mu, W.; Ravindran, C.; Dogan, N.; Barati, M. In-situ investigation of incipient melting in a 319 type Al alloy using laser scanning confocal microscopy. Mater. Charact. 2018, 141, 328–337. [Google Scholar] [CrossRef]
- Lombardi, A.; Mu, W.; Ravindran, C.; Dogan, N.; Barati, M. Influence of Al2Cu morphology on the incipient melting characteristics in B206 Al alloy. J. Alloys Compd. 2018, 747, 131–139. [Google Scholar] [CrossRef]
- Andilab, B.; Ravindran, C.; Dogan, N.; Lombardi, A.; Byczynski, G. In-situ analysis of incipient melting of Al2Cu in a novel high strength Al-Cu casting alloy using laser scanning confocal microscopy. Mater. Charact. 2020, 159, 110064. [Google Scholar] [CrossRef]
- Wang, W.; Mu, W.; Hou, Z.; Sukenaga, S.; Shibata, H.; Larsson, H.; Mao, H. In-situ real time observation of martensite transformation in duplex fcc+hcp cobalt based entropic alloys. Materialia 2020, 14, 100928. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.G.; Inoue, A. In situ observation of solidification behavior in undercooled Pd–Cu–Ni–P alloy by using a confocal scanning laser microscope. Acta Mater. 2001, 49, 615–622. [Google Scholar] [CrossRef]
- Yamashita, F.; Wakoh, M.; Ishikawa, K.; Shibata, H. In Situ Observation of Nonmetallic Inclusion Formation in NiTi Alloys. Mater. Trans. 2017, 58, 1729–1734. [Google Scholar] [CrossRef]
- Nakano, J.; Sridhar, S.; Moss, T.; Bennett, J.; Kwong, K.-S. Crystallization of Synthetic Coal−Petcoke Slag Mixtures Simulating Those Encountered in Entrained Bed Slagging Gasifiers. Energy Fuels 2009, 23, 4723–4733. [Google Scholar] [CrossRef]
- Sohn, I.; Dippenaar, R. In-Situ Observation of Crystallization and Growth in High-Temperature Melts Using the Confocal Laser Microscope. Metall. Mater. Trans. B 2016, 47, 2083–2094. [Google Scholar] [CrossRef]
- Mu, W.; Hedström, P.; Shibata, H.; Jönsson, P.G.; Nakajima, K. High-Temperature Confocal Laser Scanning Microscopy Studies of Ferrite Formation in Inclusion-Engineered Steels: A Review. JOM 2018, 70, 2283–2295. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- TCS Steels/Fe-Alloys Database Version 12.0, (TCFE12), Stockholm, Sweden, December 2021. Available online: http://www.thermocalc.com/products-services/databases/ (accessed on 1 March 2023).
- Griesser, S.; Dippenaar, R. Enhanced Concentric Solidification Technique for High-Temperature Laser-Scanning Confocal Microscopy. ISIJ Int. 2014, 54, 533–535. [Google Scholar] [CrossRef]
- Griesser, S.; Pierer, R.; Reid, M.; Dippenaar, R. SolTrack: An automatic video processing software for in situ interface tracking. J. Microsc. 2012, 248, 42–48. [Google Scholar] [CrossRef]
- Yan, P.; Guo, M.; Blanpain, B. In Situ Observation of the Formation and Interaction Behavior of the Oxide/Oxysulfide Inclusions on a Liquid Iron Surface. Metall. Mater. Trans. B 2014, 45, 903–913. [Google Scholar] [CrossRef]
- Presoly, P.; Pierer, R.; Bernhard, C. Linking up of HT-LSCM and DSC measurements to characterize phase diagrams of steels. IOP Conf. Series: Mater. Sci. Eng. 2012, 33, 012064. [Google Scholar] [CrossRef]
- Slater, C.; Hechu, K.; Sridhar, S. Characterisation of solidification using combined confocal scanning laser microscopy with infrared thermography. Mater. Charact. 2017, 126, 144–148. [Google Scholar] [CrossRef]
- Phelan, D.; Moon, S.C.; Cuiuri, D.; Li, H.; Dippenaar, R. The development of HTCM-DTA for the study of solidification. In Proceedings of the 5th Decennial International Conference on Solidification Processing, Old Windsor, UK, 25–27 July 2017. [Google Scholar]
- Withers, P.J. Synchrotron X-ray diffraction. Pract. Residual Stress Meas. Methods 2013, 163–194. [Google Scholar] [CrossRef]
- Komizo, Y.; Terasaki, H. In situ time resolved X-ray diffraction using synchrotron. Sci. Technol. Weld. Join. 2011, 16, 79–86. [Google Scholar] [CrossRef]
- Lin, S.; Borggren, U.; Stark, A.; Borgenstam, A.; Mu, W.; Hedström, P. In-Situ High-Energy X-ray Diffraction Study of Austenite Decomposition During Rapid Cooling and Isothermal Holding in Two HSLA Steels. Metall. Mater. Trans. A 2021, 52, 1812–1825. [Google Scholar] [CrossRef]
- Ioannidou, C.; König, H.-H.; Semjatov, N.; Ackelid, U.; Staron, P.; Körner, C.; Hedström, P.; Lindwall, G. In-situ synchrotron X-ray analysis of metal Additive Manufacturing: Current state, opportunities and challenges. Mater. Des. 2022, 219, 110790. [Google Scholar] [CrossRef]
- Kerr, H.W.; Kurz, W. Solidification of peritectic alloys. Int. Mater. Rev. 1996, 41, 129–164. [Google Scholar] [CrossRef]
- Stefanescu, D.M. Microstructure Evolution during the Solidification of Steel. ISIJ Int. 2006, 46, 786–794. [Google Scholar] [CrossRef]
- Azizi, G.; Thomas, B.G.; Zaeem, M.A. Review of Peritectic Solidification Mechanisms and Effects in Steel Casting. Metall. Mater. Trans. B 2020, 51, 1875–1903. [Google Scholar] [CrossRef]
- Grill, A.; JK, B. Influence of Carbon Content on Rate of Heat Extraction in the Mould of a Continous-Casting Machine. In Ironmakg and Steelmakg; Maney Publishing: Leeds, UK, 1976; Volume 3, pp. 76–79. [Google Scholar]
- Emi, T.; Fredriksson, H. High-speed continuous casting of peritectic carbon steels. Mater. Sci. Eng. A 2005, 413–414, 2–9. [Google Scholar] [CrossRef]
- Luo, S.; Liu, G.; Wang, P.; Wang, X.; Wang, W.; Zhu, M. In Situ Observation and Phase-Field Modeling of Peritectic Solidification of Low-Carbon Steel. Metall. Mater. Trans. A 2020, 51, 767–777. [Google Scholar] [CrossRef]
- Yin, H.; Emi, T.; Shibata, H. Morphological instability of δ-ferrite/γ-austenite interphase boundary in low carbon steels. Acta Mater. 1999, 47, 1523–1535. [Google Scholar] [CrossRef]
- McDonald, N.J.; Sridhar, S. Peritectic reaction and solidification in iron-nickel alloys. Metall. Mater. Trans. A 2003, 34, 1931–1940. [Google Scholar] [CrossRef]
- Liu, Z.; Kobayashi, Y.; Yang, J.; Nagai, K.; Kuwabara, M. “In-situ” observation of the δ/γ phase transformation on the surface of low carbon steel containing phosphorus at various cooling rates. ISIJ Int. 2006, 46, 847–853. [Google Scholar] [CrossRef]
- Phelan, D.; Reid, M.; Dippenaar, R. Kinetics of the peritectic reaction in an Fe–C alloy. Mater. Sci. Eng. A 2008, 477, 226–232. [Google Scholar] [CrossRef]
- Griesser, S.; Bernhard, C.; Dippenaar, R. Mechanism of the Peritectic Phase Transition in Fe–C and Fe–Ni Alloys under Conditions Close to Chemical and Thermal Equilibrium. ISIJ Int. 2014, 54, 466–473. [Google Scholar] [CrossRef]
- Moon, S.-C.; Phelan, D.; Dippenaar, R. New insights of the peritectic phase transition in steel through in-situ measurement of thermal response in a high-temperature confocal microscope. Mater. Charact. 2021, 172, 110841. [Google Scholar] [CrossRef]
- Ohno, M.; Matsuura, K. Diffusion-controlled peritectic reaction process in carbon steel analyzed by quantitative phase-field simulation. Acta Mater. 2010, 58, 6134–6141. [Google Scholar] [CrossRef]
- Matsuura, K.; Itoh, Y.; Narita, T. A Solid-Liquid Diffusion Couple Study of a Peritectic Reaction in Iron-Carbon System. ISIJ Int. 1993, 33, 583–587. [Google Scholar] [CrossRef]
- Fredriksson, H.; Nylén, T. Mechanism of peritectic reactions and transformations. Met. Sci. 1982, 16, 283–294. [Google Scholar] [CrossRef]
- Mullins, W.W.; Sekerka, R.F. Stability of a Planar Interface During Solidification of a Dilute Binary Alloy. J. Appl. Phys. 1964, 35, 444–451. [Google Scholar] [CrossRef]
- Dippenaar, R.J.; Phelan, D.J. Delta-ferrite recovery structures in low-carbon steels. Metall. Mater. Trans. B 2003, 34, 495–501. [Google Scholar] [CrossRef]
- Fredriksson, H.; Stjerndahl, J. Solidification of iron-base alloys. Met. Sci. 1982, 16, 575–586. [Google Scholar] [CrossRef]
- Arai, Y. Master’s Thesis. Tohoku University, Sendai, Japan, 1998. [Google Scholar]
- Phelan, D.; Reid, M.; Dippenaar, R. Experimental and modeling studies into high temperature phase transformations. Comput. Mater. Sci. 2005, 34, 282–289. [Google Scholar] [CrossRef]
- Hillert, M. Solidification and Casting of Metals; The Metals Society: London, UK, 1979; p. 81. [Google Scholar]
- Wei, Z.-J.; Ji, C.; Chen, T.-C.; Zhu, M.-Y. In Situ Observations and Microstructure Evolution Behavior of Static Recrystallization of Microalloyed Continuous Casting Slabs in a Solidification End Reduction Process. Steel Res. Int. 2022, 93, 2100348. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Kattner, U.R.; Moon, K.-W.; Perepezko, J.H. DTA and heat-flux DSC measurements of alloy melting and freezing. In Methods for Phase Diagram Determination; Elsevier: Amsterdam, The Netherlands, 2007; pp. 151–221. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Goswami, R. Melting and superheating of metals and alloys. Prog. Mater. Sci. 1997, 42, 287–300. [Google Scholar] [CrossRef]
- Bernhard, M.; Fuchs, N.; Presoly, P.; Angerer, P.; Friessnegger, B.; Bernhard, C. Characterization of the γ-loop in the Fe-P system by coupling DSC and HT-LSCM with complementary in-situ experimental techniques. Mater. Charact. 2021, 174, 111030. [Google Scholar] [CrossRef]
- Bernhard, M.; Presoly, P.; Fuchs, N.; Bernhard, C.; Kang, Y.-B. Experimental study of high temperature phase Equilibria in the Iron-rich part of the Fe-P and Fe-CP systems. Metall. Mater. Trans. A 2020, 51, 5351–5364. [Google Scholar] [CrossRef]
- Liu, T.; Long, M.; Chen, D.; Wu, S.; Tang, P.; Liu, S.; Duan, H.; Yang, J. Investigations of the peritectic reaction and transformation in a hypo-peritectic steel: Using high-temperature confocal scanning laser microscopy and differential scanning calorimetry. Mater. Charact. 2019, 156, 109870. [Google Scholar] [CrossRef]
- Egry, I. Structure and properties of levitated liquid metals. J. Non-Crystalline Solids 1999, 250–252, 63–69. [Google Scholar] [CrossRef]
- Cummings, D.L.; Blackburn, D.A. Oscillations of magnetically levitated aspherical droplets. J. Fluid Mech. 1991, 224, 395–416. [Google Scholar] [CrossRef]
- Yu, J.; Koshikawa, N.; Arai, Y.; Yoda, S.; Saitou, H. Containerless solidification of oxide material using an electrostatic levitation furnace in microgravity. J. Cryst. Growth 2001, 231, 568–576. [Google Scholar] [CrossRef]
- Watanabe, M.; Ozawa, S.; Fukuyama, H.; Tsukada, T.; Hibiya, T. Levitation Research in Japan. In Metallurgy in Space: Recent Results from ISS; Springer International Publishing: New York, NY, USA, 2022; pp. 235–260. [Google Scholar]
- Yasuda, H.; Yoshimoto, T.; Mizuguchi, T.; Tamura, Y.; Nagira, T.; Yoshiya, M. Effect of the Melt Flow on the Solidified Structure of Middle Carbon Steel by Means of the Levitation Method Using Alternating and Static Magnetic Fields. ISIJ Int. 2007, 47, 612–618. [Google Scholar] [CrossRef]
- Fukuyama, H.; Higashi, H.; Yamano, H. Normal spectral emissivity, specific heat capacity, and thermal conductivity of type 316 austenitic stainless steel containing up to 10 mass% B4C in a liquid state. J. Nucl. Mater. 2022, 568, 153865. [Google Scholar] [CrossRef]
- Yasuda, H.; Ohnaka, I.; Ishii, R.; Fujita, S.; Tamura, Y. Investigation of the Melt Flow on Solidified Structure by a Levitation Technique Using Alternative and Static Magnetic Fields. ISIJ Int. 2005, 45, 991–996. [Google Scholar] [CrossRef]
- Fukuyama, H.; Sawada, R.; Nakashima, H.; Ohtsuka, M.; Yoshimi, K. Study of solidification pathway of a MoSiBTiC alloy by optical thermal analysis and in-situ observation with electromagnetic levitation. Sci. Rep. 2019, 9, 15049. [Google Scholar] [CrossRef]
- Shoji, E.; Isogai, S.; Suzuki, R.; Kubo, M.; Tsukada, T.; Kai, T.; Shinohara, T.; Matsumoto, Y.; Fukuyama, H. Neutron computed tomography of phase separation structures in solidified CuCo alloys and investigation of relationship between the structures and melt convection during solidification. Scr. Mater. 2020, 175, 29–32. [Google Scholar] [CrossRef]
- Kobayashi, A.; Nagayama, K. Microstructure and Solidification Process of Fe-Cu Immiscible Alloy by Using Containerless Process. J. Jpn. Inst. Met. Mater. 2017, 81, 251–256. [Google Scholar] [CrossRef]
- Matson, D.M.; Fair, D.J.; Hyers, R.W.; Rogers, J.R. Contrasting Electrostatic and Electromagnetic Levitation Experimental Results for Transformation Kinetics of Steel Alloys. Ann. N. Y. Acad. Sci. 2004, 1027, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.F.; Shuleshova, O.; Witusiewicz, V.T.; Wu, Y.; Yang, Y.; Ivashko, O.; Dippel, A.C.; Zimmermann, M.V.; Nielsch, K.; Kaban, I. In situ study of non-equilibrium solidification of CoCrFeNi high-entropy alloy and CrFeNi and CoCrNi ternary suballoys. Acta Mater. 2021, 212, 116880. [Google Scholar] [CrossRef]
- Yan, P.; Chang, J.; Wang, W.; Zhu, X.; Lin, M.; Wei, B. Eutectic growth kinetics and microscopic mechanical properties of rapidly solidified CoCrFeNiMo0.8 high entropy alloy. Acta Mater. 2022, 237, 118149. [Google Scholar] [CrossRef]
- Andreoli, A.F.; Han, X.; Kaban, I. In situ studies of non-equilibrium crystallization of AlxCoCrFeNi (x = 0.3, 1) high-entropy alloys. J. Alloys Compd. 2022, 922, 166209. [Google Scholar] [CrossRef]

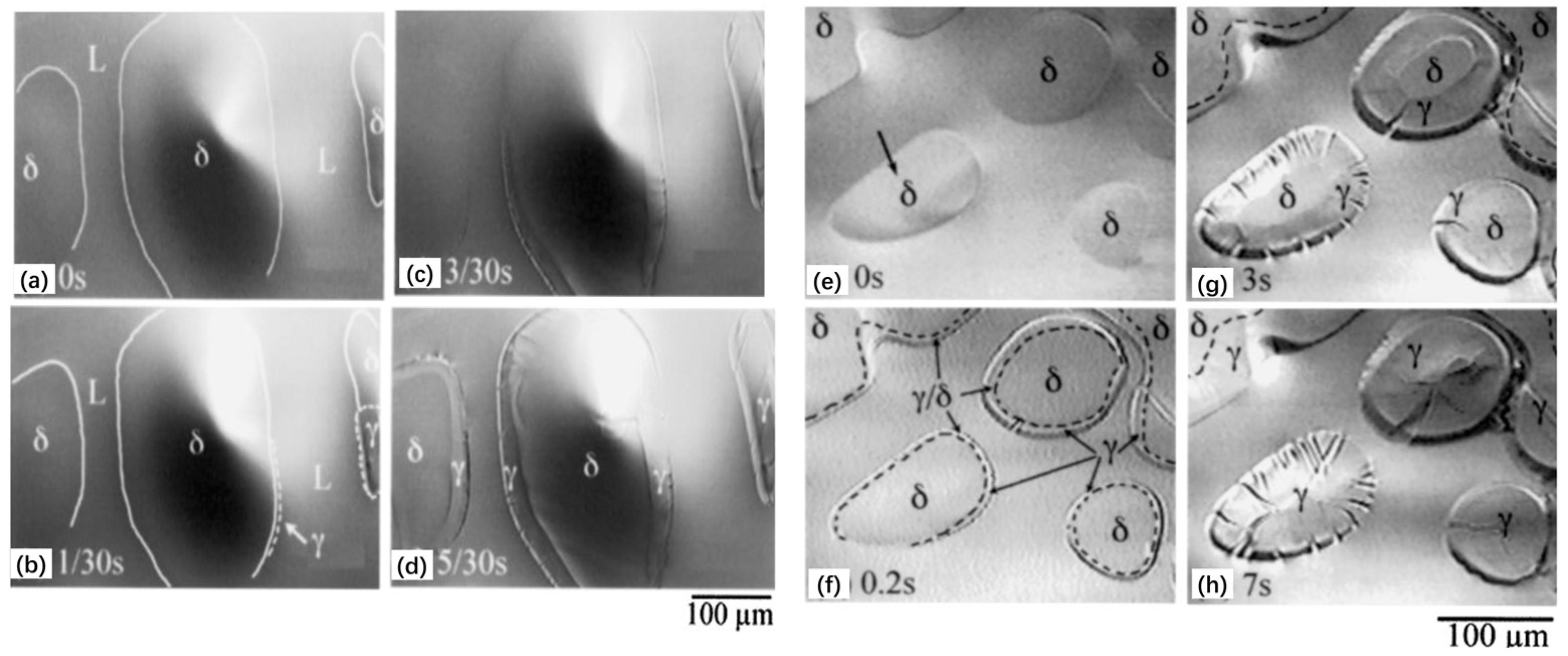
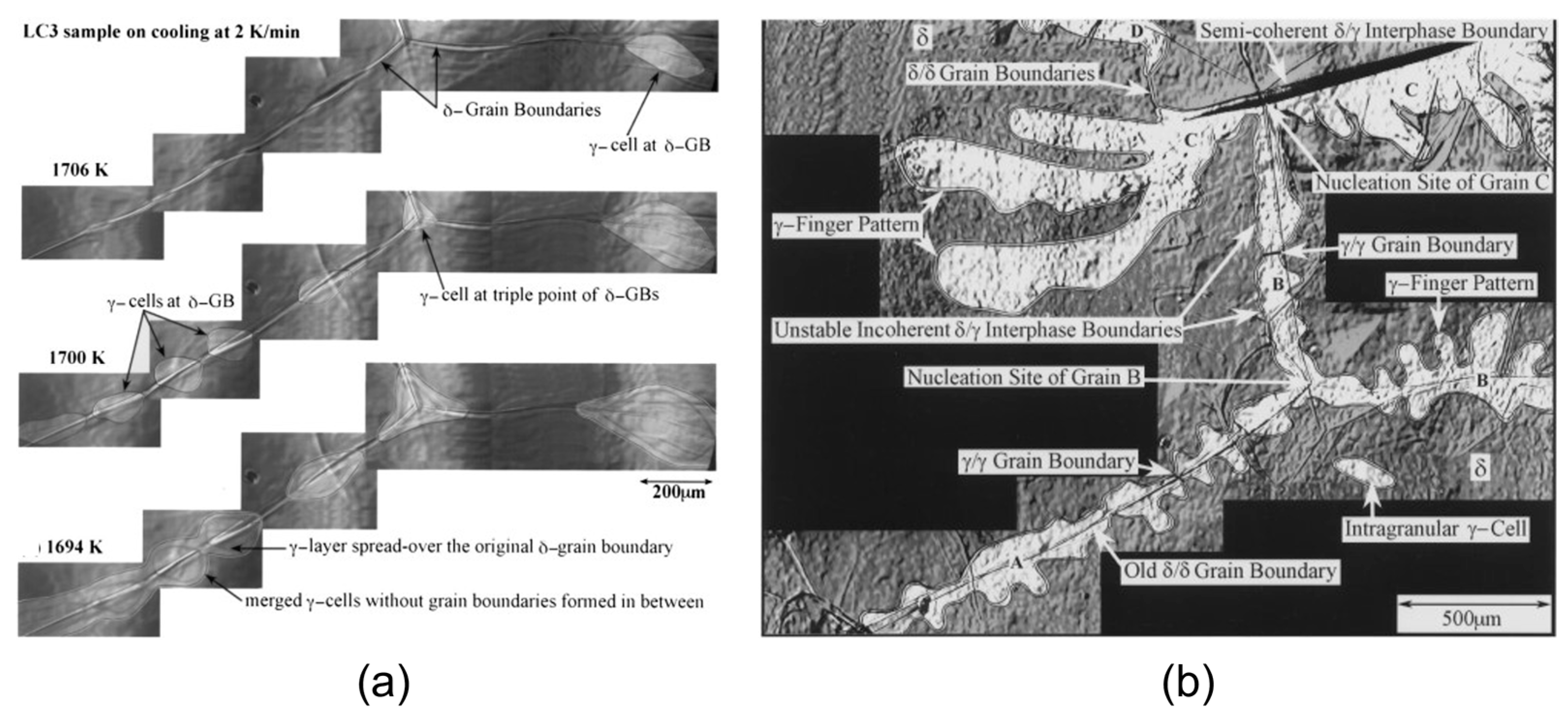
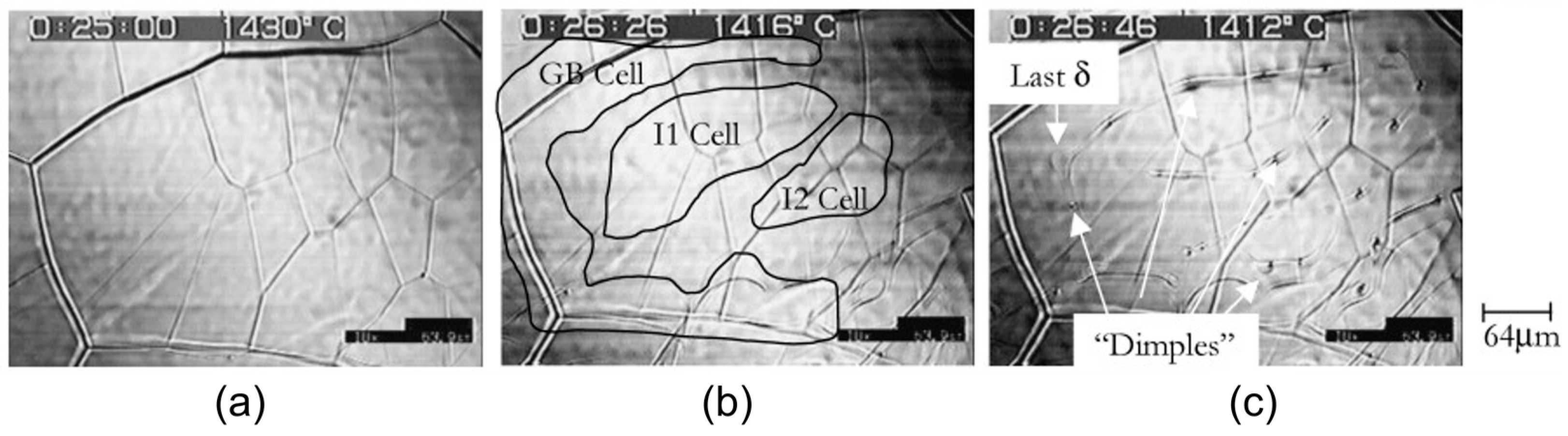
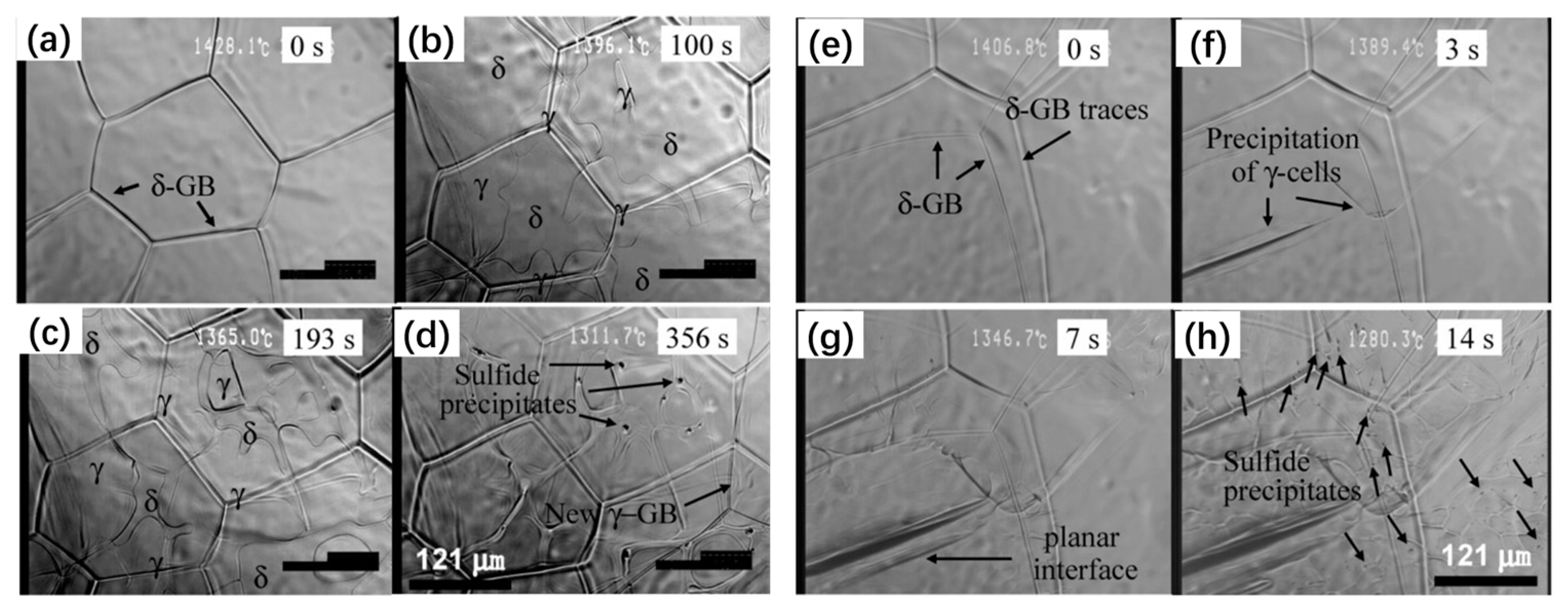
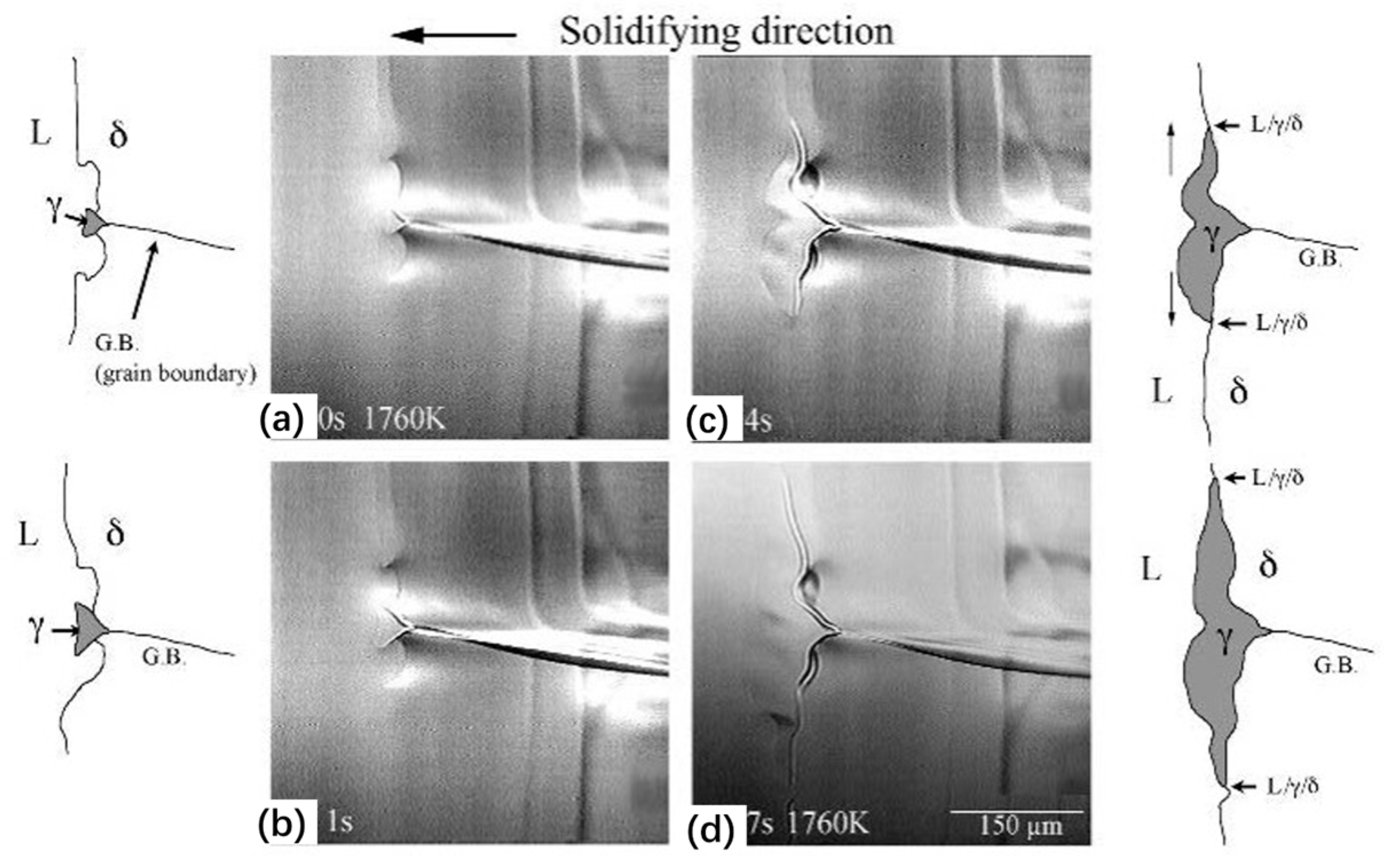
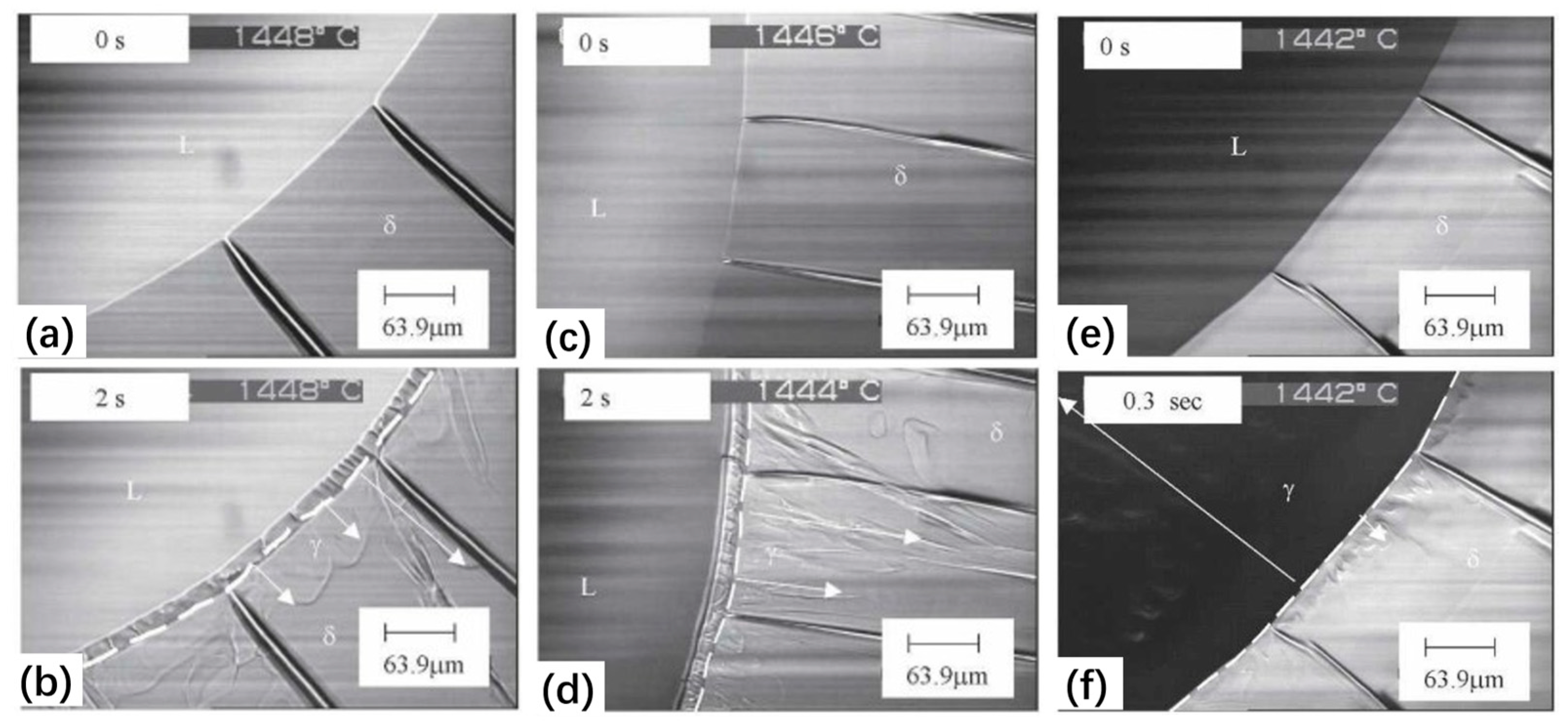

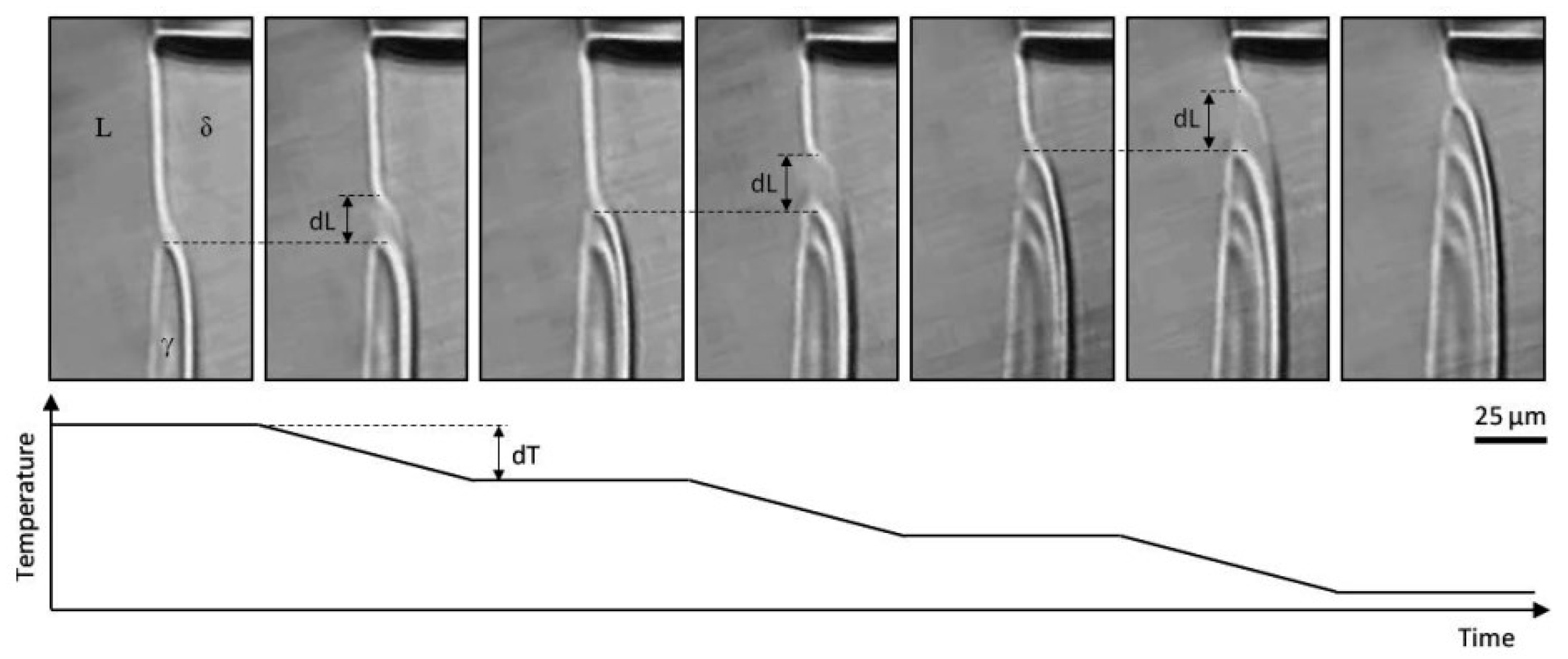
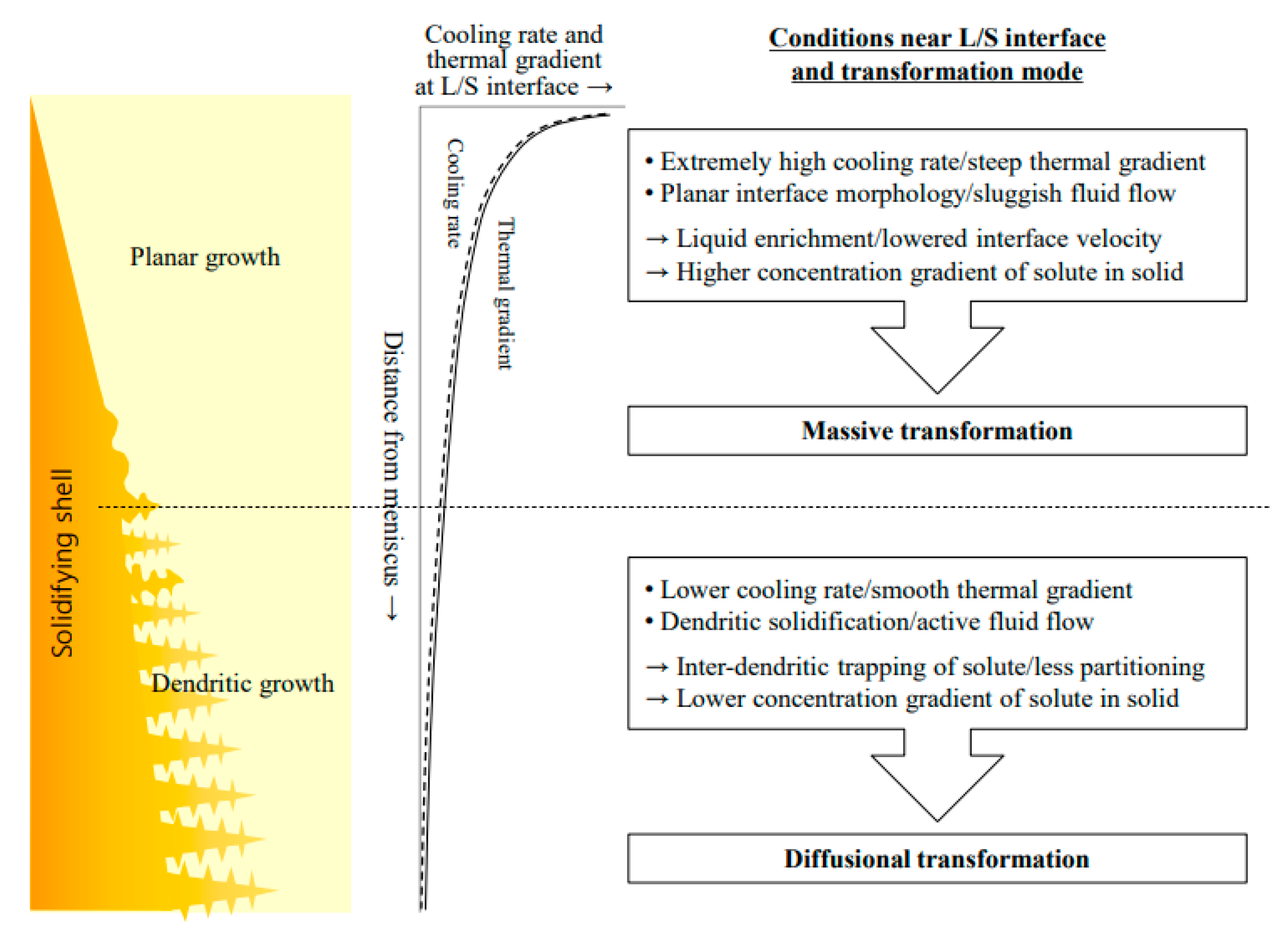

| Authors (Year) | Alloy System | Highlighted Key Findings | Ref. |
|---|---|---|---|
| Chikama et al. (1996) | Fe-C (0.2, 0.83) | growth of planner/cellular/dendritic transition | [9] |
| Yin et al. (1998) | Fe-C (0.04) | free energy of γ/δ can be obtained by measuring the dihedral angle | [12] |
| Yin et al. (1999) | Fe-C (0.04) | morphological instability of δ/γ interphase boundary was observed | [87] |
| Shibata et al. (2000) | Fe-C (0.14, 0.42) | peritectic reaction and transformation of steels were firstly analyzed using HT-CLSM | [10] |
| McDonald et al. (2003) | Fe-Ni (4.3, 4.7) | peritectic reaction increased with increased undercooling | [88] |
| Phelan et al. (2004) | Fe-C (0.06) | γ grew preferentially along δ sub-boundaries | [22] |
| Reid et al. (2004) | Fe-C (0.17, 0.42) | concentric solidification technique has been firstly reported | [19] |
| Arai et al. (2005) | Fe-Ni (3.7, 5.1, 5.3) | initial stages of the peritectic transformation have been clearly observed | [11] |
| Liu et al. (2006) | Fe-C | the effect of phosphorus and cooling rates on the δ→γ transformation was reported | [89] |
| Phelan et al. (2006) | Fe-C (0.18) | the L/δ interface propagated at a higher velocity than the γ/δ interface at a higher cooling rate | [21] |
| Phelan et al. (2008) | Fe-C (0.18) | a new mechanism that the peritectic reaction was controlled by the rate of heat dissipation released by the growing γ along the L/δ interface | [90] |
| Presoly et al. (2013) | Fe-C (0.08–0.25) | phase transformation of steels during the heating process observed by DSC and HT-SLCM | [5] |
| Griesser et al. (2014) | Fe-C and Fe-Ni | the dependency of the early nucleation process on the presence of solute diffusion fields of the newly forming cluster was firstly clarified | [23] |
| Griesser et al. (2014) | Fe-C (0.1, 0.18, 0.43) | three different modes of peritectic transformation were reported based on different undercooling conditions | [24] |
| Griesser et al. (2014) | Fe-C and Fe-Ni | re-melting of δ during the peritectic reaction has been clearly observed | [91] |
| Moon et al. (2015) | Fe-C (0.05–0.44) | potential countermeasures to avoid surface defects during steel casting were proposed | [26] |
| Hechu et al. (2017) | Fe-C (0.07, 0.11) | solidification of peritectic steels was observed by DSC and HT-SLCM | [6] |
| Luo et al. (2020) | Fe-C (0.83) | peritectic solidification has been clearly investigated by the phase modeling method | [86] |
| Moon et al. (2021) | Fe-C (0.06, 0.18, 0.45) | a new technique was developed through a spatial combination of a DTA and an HT-CLSM | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Q.; Mu, W. In Situ Observation of Solidification and Crystallization of Low-Alloy Steels: A Review. Metals 2023, 13, 517. https://doi.org/10.3390/met13030517
Wang Y, Wang Q, Mu W. In Situ Observation of Solidification and Crystallization of Low-Alloy Steels: A Review. Metals. 2023; 13(3):517. https://doi.org/10.3390/met13030517
Chicago/Turabian StyleWang, Yong, Qiang Wang, and Wangzhong Mu. 2023. "In Situ Observation of Solidification and Crystallization of Low-Alloy Steels: A Review" Metals 13, no. 3: 517. https://doi.org/10.3390/met13030517
APA StyleWang, Y., Wang, Q., & Mu, W. (2023). In Situ Observation of Solidification and Crystallization of Low-Alloy Steels: A Review. Metals, 13(3), 517. https://doi.org/10.3390/met13030517







