Thermodynamic Study on Initial Oxidation Behavior of TiAl-Nb Alloys at High Temperature
Abstract
1. Introduction
2. Method
3. Results and Discussion
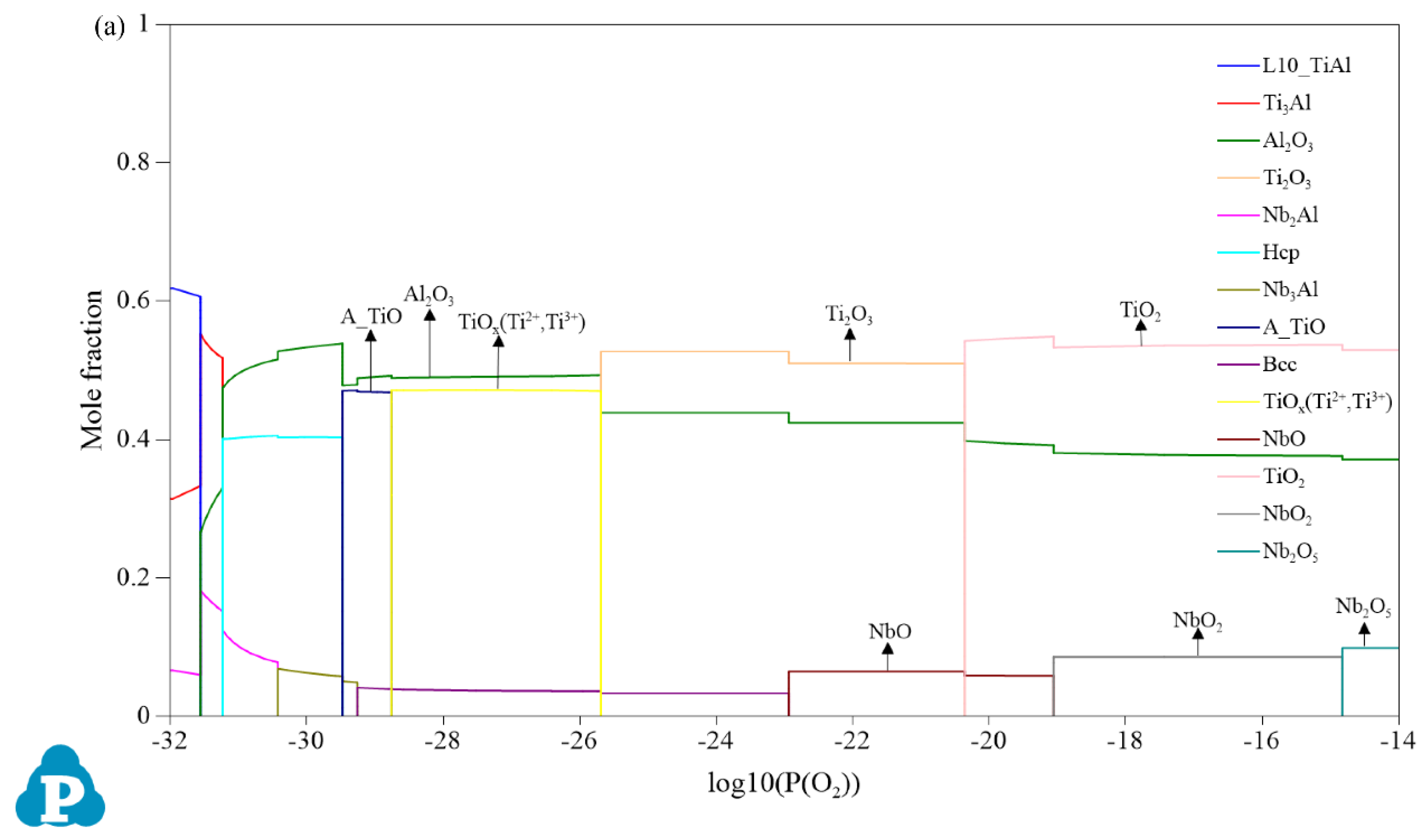
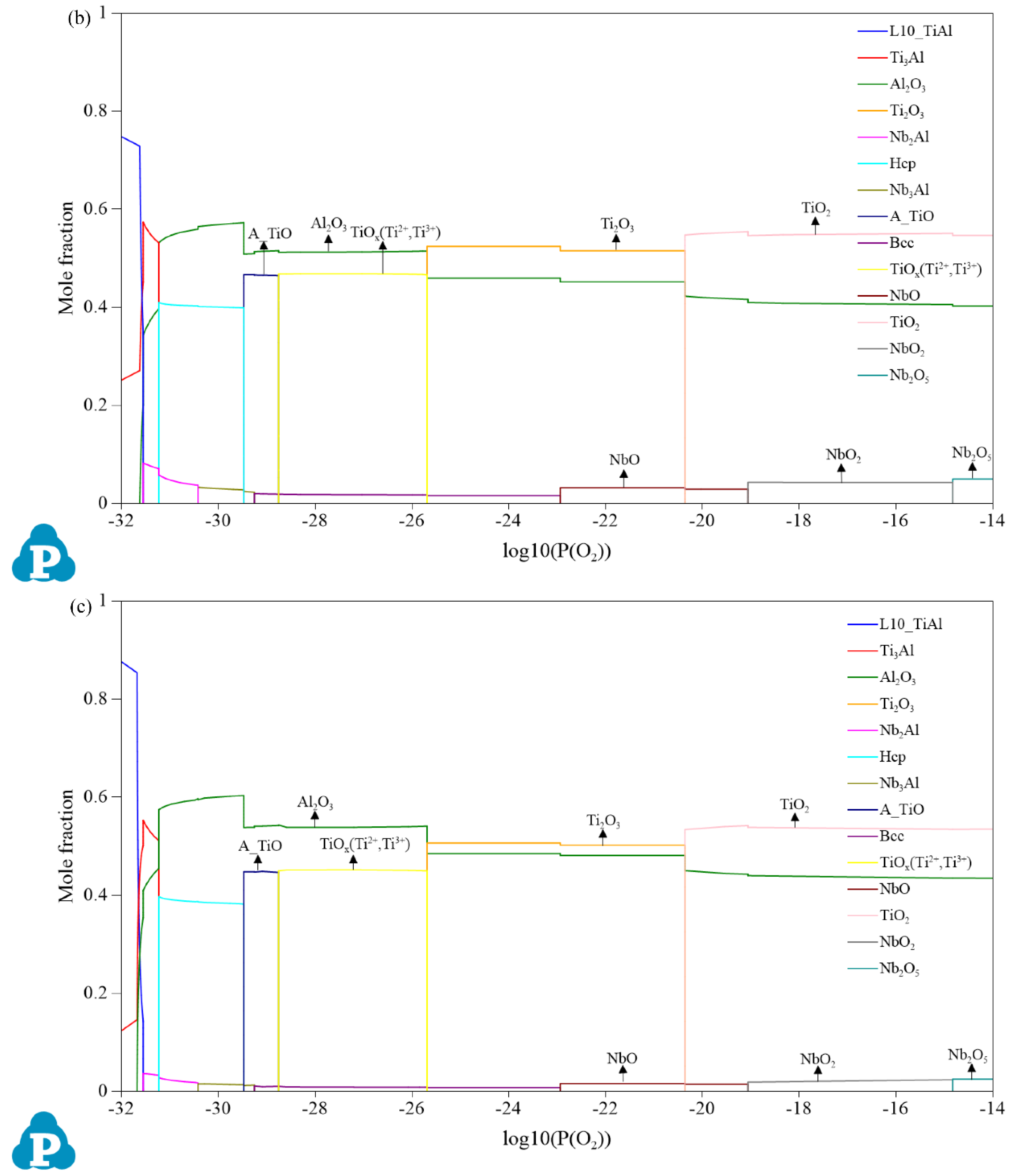
3.1. Oxidation Products
3.2. Mole Fractions of the Oxides
3.3. Relevance with the Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duan, Z.; Han, Y.; Song, X.; Chen, H. Creep behaviour of equiaxed fine-grain γ-TiAl-based alloy prepared by powder metallurgy. Mater. Sci. Technol. 2020, 36, 1457–1464. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Intermetallic titanium aluminides in aerospace applications—Processing, microstructure and properties. Mater. High Temp. 2016, 33, 560–570. [Google Scholar] [CrossRef]
- Cai, J.M.; Mi, G.B.; Gao, F.; Huang, H.; Cao, J.X.; Huang, X.; Cao, C.X. Research and Development of Some Advanced High Temperature Titanium Alloys for Aero-engine. J. Mater. Eng. 2016, 44, 1–10. [Google Scholar]
- Wu, G.D.; Cui, G.R.; Qu, S.J.; Feng, A.H.; Cao, G.J.; Ge, B.H.; Xiang, H.P.; Shen, J.; Chen, D.L. High-temperature oxidation mechanisms of nano-/submicro-scale lamellar structures in an intermetallic alloy. Scr. Mater. 2019, 171, 102–107. [Google Scholar] [CrossRef]
- Ouyang, P.X.; Mi, G.B.; Cao, J.X.; Huang, X.; He, L.J.; Li, P.J. Microstructure Characteristics after combustion and fireproof mechanism of TiAl-based alloys. Mater. Today Commun. 2018, 16, 364–373. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Yang, R. Advances and challenges of TiAl base alloys. Acta Metall. Sin. 2015, 51, 129–147. [Google Scholar]
- Rakowski, J.M.; Meier, G.H.; Pettit, F.S.; Dettenwanger, F.; Schumann, E.; Rühle, M. The effect of surface preparation on the oxidation behavior of gamma TiAl-base intermetallic alloys. Scr. Mater. 1996, 35, 1417–1422. [Google Scholar] [CrossRef]
- Rahmel, A.; Schütze, M.; Quadakkers, W.J. Fundamentals of TiAl oxidation—A critical review. Mater. Corros. 1995, 46, 271–285. [Google Scholar] [CrossRef]
- Kim, D.; Seo, D.; Saari, H.; Sawatzky, T.; Kim, Y.-W. Isothermal oxidation behavior of powder metallurgy beta gamma TiAl–2Nb–2Mo alloy. Intermetallics 2011, 19, 1509–1516. [Google Scholar] [CrossRef]
- Schmitz-Niederau, M.; Schutze, M. The oxidation behavior of several Ti-Al alloys at 900 degrees C in air. Oxid. Met. 1999, 52, 225–240. [Google Scholar] [CrossRef]
- Shanabarger, M.R. Comparative study of the initial oxidation behavior of a series of titanium–aluminum alloys. Appl. Surf. Sci. 1998, 134, 179–186. [Google Scholar] [CrossRef]
- Kim, S.W.; Hong, J.K.; Na, Y.S.; Yeom, J.T.; Kim, S.E. Development of TiAl alloys with excellent mechanical properties and oxidation resistance. Mater. Des. 2014, 54, 814–819. [Google Scholar] [CrossRef]
- Qu, S.J.; Tang, S.Q.; Feng, A.H.; Feng, C.; Shen, J.; Chen, D.L. Microstructural evolution and high-temperature oxidation mechanisms of a titanium aluminide based alloy. Acta Mater. 2018, 148, 300–310. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Qu, S.J.; Xiang, H.P.; Wang, C.; Gao, J.B.; Feng, A.H.; Chen, D.L. Oxidation mechanisms of an intermetallic alloy at high temperatures. Scr. Mater. 2021, 199, 113852. [Google Scholar] [CrossRef]
- Tang, S.Q.; Qu, S.J.; Feng, A.H.; Feng, C.; Shen, J.; Chen, D.L. Core-multishell globular oxidation in a new TiAlNbCr alloy at high temperatures. Sci. Rep. 2017, 7, 3483. [Google Scholar] [CrossRef] [PubMed]
- Małecka, J. Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys. Materials 2022, 15, 2137. [Google Scholar] [CrossRef] [PubMed]
- Mitoraj-Królikowska, M.; Drożdż, E. Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems. Materials 2022, 15, 1640. [Google Scholar] [CrossRef] [PubMed]
- Šulhánek, P.; Ďuriška, L.; Palcut, M.; Babincová, P.; Sahul, M.; Čaplovič, Ľ.; Kusý, M.; Orovčík, Ľ.; Nagy, Š.; Satrapinskyy, L.; et al. Influence of Isothermal Annealing on Microstructure, Morphology and Oxidation Behavior of AlTiSiN/TiSiN Nanocomposite Coatings. Nanomaterials 2023, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.K. First-Principles Calculations and CALPHAD Modeling of Thermodynamics. J. Phase Equilib. Diffus. 2009, 30, 517–534. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, X.; Zhang, R.; Wang, X.; Yin, H.; Qu, X.; Liu, Z.-K. High-throughput thermodynamic calculations of phase equilibria in solidified 6016 Al-alloys. Comp. Mater. Sci. 2019, 167, 19–24. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Snead, L.; Zinkle, S.J. Development of novel Cu-Cr-Nb-Zr alloys with the aid of computational thermodynamics. Mater. Des. 2018, 156, 370–380. [Google Scholar] [CrossRef]
- Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. Alloy Phase Diagrams Study and Its Application for New Alloy Development. J. Jpn. Inst. Met. 2008, 72, 545–556. [Google Scholar] [CrossRef]
- Hickel, T.; Kattner, U.R.; Fries, S.G. Computational thermodynamics: Recent developments and future potential and prospects Preface. Phys. Status Solidi B-Basic Solid State Phys. 2014, 251, 9–13. [Google Scholar] [CrossRef]
- Gorr, B.; Christ, H.J.; Mukherji, D.; Rosler, J. Thermodynamic calculations in the development of high-temperature Co–Re-based alloys. J. Alloys Compd. 2014, 582, 50–58. [Google Scholar] [CrossRef]
- Xia, S.; Lousada, C.M.; Mao, H.; Maier, A.C.; Korzhavyi, P.A.; Sandström, R.; Wang, Y.; Zhang, Y. Nonlinear Oxidation Behavior in Pure Ni and Ni-Containing Entropic Alloys. Front. Mater. 2018, 5, 53. [Google Scholar] [CrossRef]
- Esmaily, M.; Qiu, Y.; Bigdeli, S.; Venkataraman, M.B.; Allanore, A.; Birbilis, N. High-temperature oxidation behaviour of AlxFeCrCoNi and AlTiVCr compositionally complex alloys. npj Mater. Degrad. 2020, 4, 25. [Google Scholar] [CrossRef]
- Serena, S.; Moreno, B.; Chinarro, E.; Jurado, J.R.; Caballero, A. Application of the thermodynamic calculation of the Pt-Ni-Ru-(O2) system to the development of Pt-based catalyst. J. Alloys Compd. 2014, 583, 481–487. [Google Scholar] [CrossRef]
- Xiang, J.M.; Mi, G.B.; Qu, S.J.; Huang, X.; Chen, Z.; Feng, A.H.; Shen, J.; Chen, D.L. Thermodynamic and microstructural study of Ti2AlNb oxides at 800 °C. Sci. Rep. 2018, 8, 12761. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Z.; Zhou, X. Oxidation of Intermetallic Alloys in Ti-Al-Nb Ternary System. Corrosion 1992, 48, 939–946. [Google Scholar] [CrossRef]
- Varma, S.K.; Chan, A.; Mahapatra, B.N. Static and Cyclic Oxidation of Ti–44Al and Ti–44Al–xNb Alloys. Oxid. Met. 2001, 55, 423–435. [Google Scholar] [CrossRef]
- Jiang, H.; Hirohasi, M.; Lu, Y.; Imanari, H. Effect of Nb on the high temperature oxidation of Ti–(0–50 at.%)Al. Scr. Mater. 2002, 46, 639–643. [Google Scholar] [CrossRef]
- Sundman, B.; Kattner, U.R.; Palumbo, M.; Fries, S.G. OpenCalphad—A free thermodynamic software. Integr. Mater. Manuf. Innov. 2015, 4, 1–15. [Google Scholar] [CrossRef]
- Qiao, Z.Y.; Hao, S.M. New progress of CALPHAD approach. J. Mater. Metall. 2005, 4, 9. [Google Scholar]
- Yang, J.J.; Liu, L.B.; Zhao, Y.; Zhang, L.G. Thermodynamic analysis of Ti2AlNb-based alloy in the initial stage of high temperature oxidation. Mater. Sci. Eng. Powder Metall. 2020, 25, 6. [Google Scholar]
- Liu, X.J.; Wang, C.P.; Gan, S.X.; Ohnuma, I.; Kainuma, R.; Ishida, K. Development of thermodynamic database for copper base alloy systems and its application in material design. Chin. J. Nonferr. Met. 2011, 21, 2511–2522. [Google Scholar]
- Cao, W.; Chen, S.L.; Zhang, F.; Wu, K.; Yang, Y.; Chang, Y.A.; Schmid-Fetzer, R.; Oates, W.A. PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation. Calphad 2009, 33, 328–342. [Google Scholar] [CrossRef]
- Kao, C.R. Recent progress in phase diagram calculation and application. JOM-J. Miner. Met. Mater. Soc. 2003, 55, 47. [Google Scholar] [CrossRef]
- Chen, S.L.; Daniel, S.; Zhang, F.; Chang, Y.A.; Yan, X.Y.; Xie, F.Y.; Schmid-Fetzer, R.; Oates, W.A. The PANDAT software package and its applications. Calphad 2002, 26, 175–188. [Google Scholar] [CrossRef]
- Leyens, C. Oxidation and Protection of Titanium Alloys and Titanium Aluminides. In Titanium and Titanium Alloys; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 187–230. [Google Scholar]
- Dai, J.J.; Zhu, J.Y.; Chen, C.Z.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Kong, Y.; Chen, L.; Du, Y. Influence of oxygen addition on the oxidation resistance of TiAlN. Scr. Mater. 2023, 224, 115148. [Google Scholar] [CrossRef]
- Maurice, V.; Despert, G.; Zanna, S.; Josso, P.; Bacos, M.P.; Marcus, P. XPS study of the initial stages of oxidation of α2-Ti3Al and γ-TiAl intermetallic alloys. Acta Mater. 2007, 55, 3315–3325. [Google Scholar] [CrossRef]
- Schmiedgen, M.; Graat, P.C.J.; Baretzky, B.; Mittemeijer, E.J. The initial stages of oxidation of γ-TiAl: An X-ray photoelectron study. Thin Solid Film. 2002, 415, 114–122. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation Mechanism of Biomedical Titanium Alloy Surface and Experiment. Int. J. Corros. 2020, 2020, 1678615. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhao, L.L.; Li, G.Y.; Zhang, L.Q.; Song, X.P.; Ye, F.; Chen, G.L. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 19, 131–136. [Google Scholar] [CrossRef]
- Yoshihara, M.; Miura, K. Effects of Nb addition on oxidation behavior of TiAl. Intermetallics 1995, 3, 357–363. [Google Scholar] [CrossRef]
- Xue, X.Y. XPS Study on Oxidation Scale of Ti-42Al-8Nb TiAl Alloys. Rare Met. Mater. Eng. 2016, 45, 2635–2641. [Google Scholar]
- Chen, J. The High Temperature Oxidation Behaviour of Ti-Al-Nb System Alloys and its Mechanism. Master’s Thesis, Tongji University, Shanghai, China, 2022. [Google Scholar]
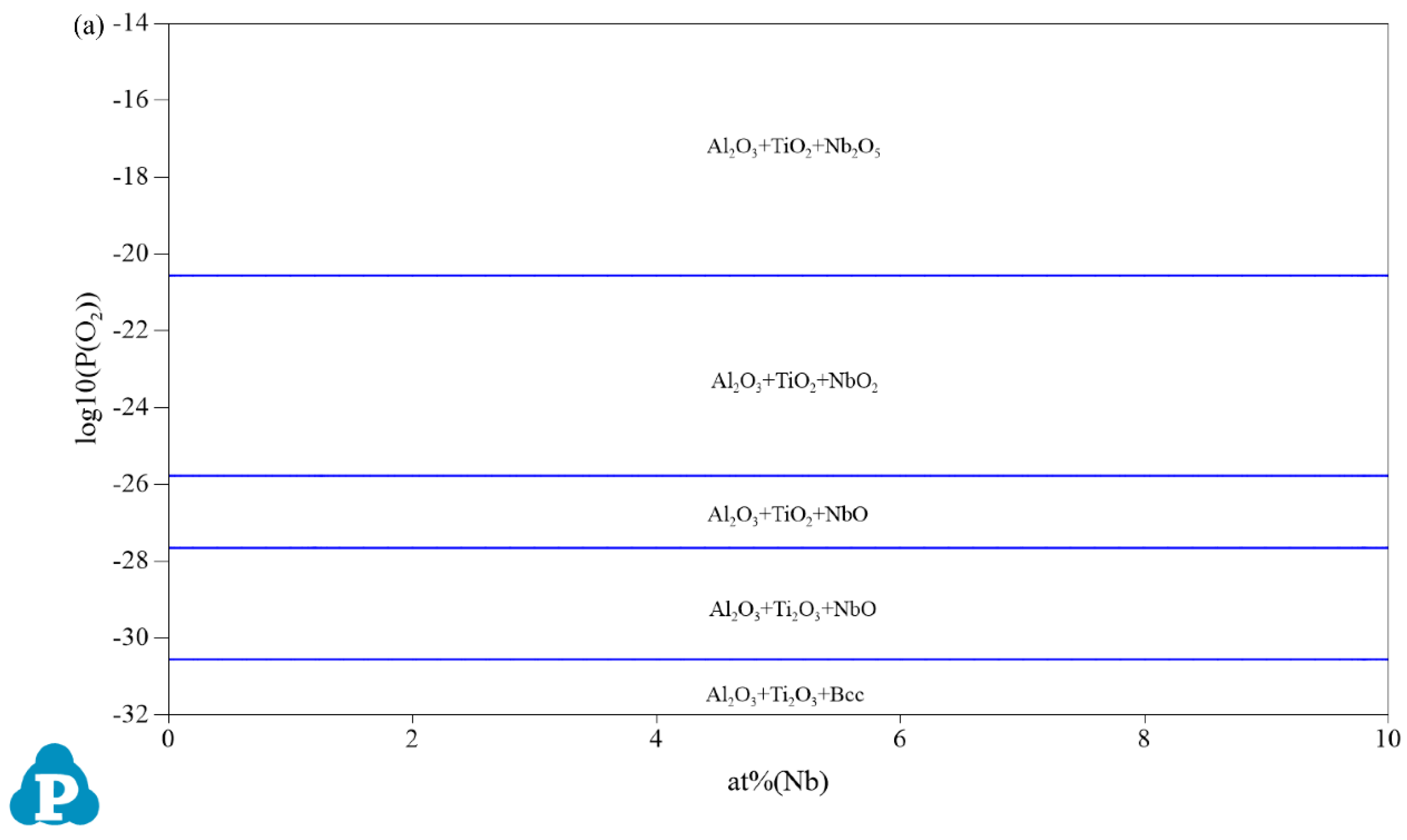
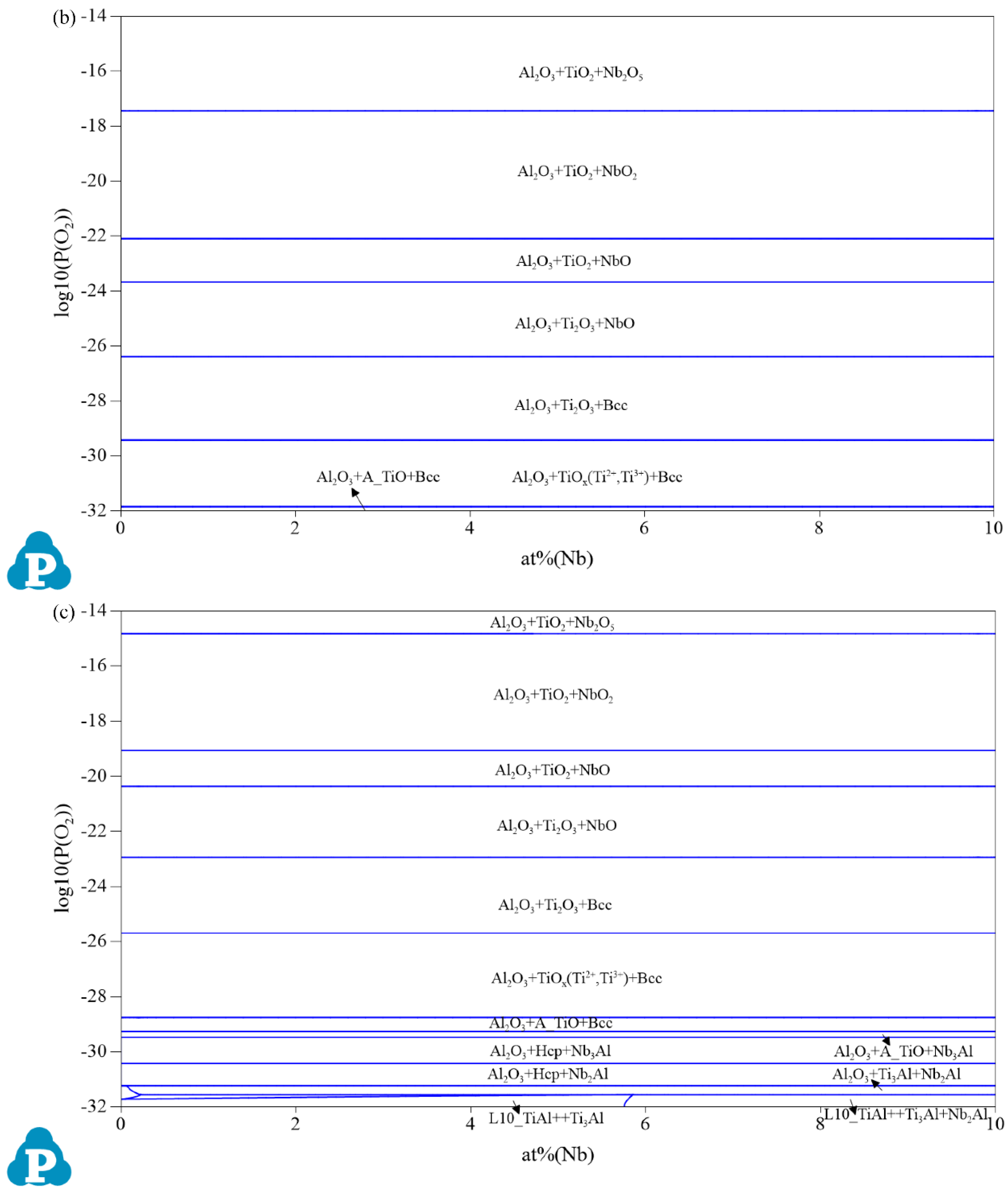

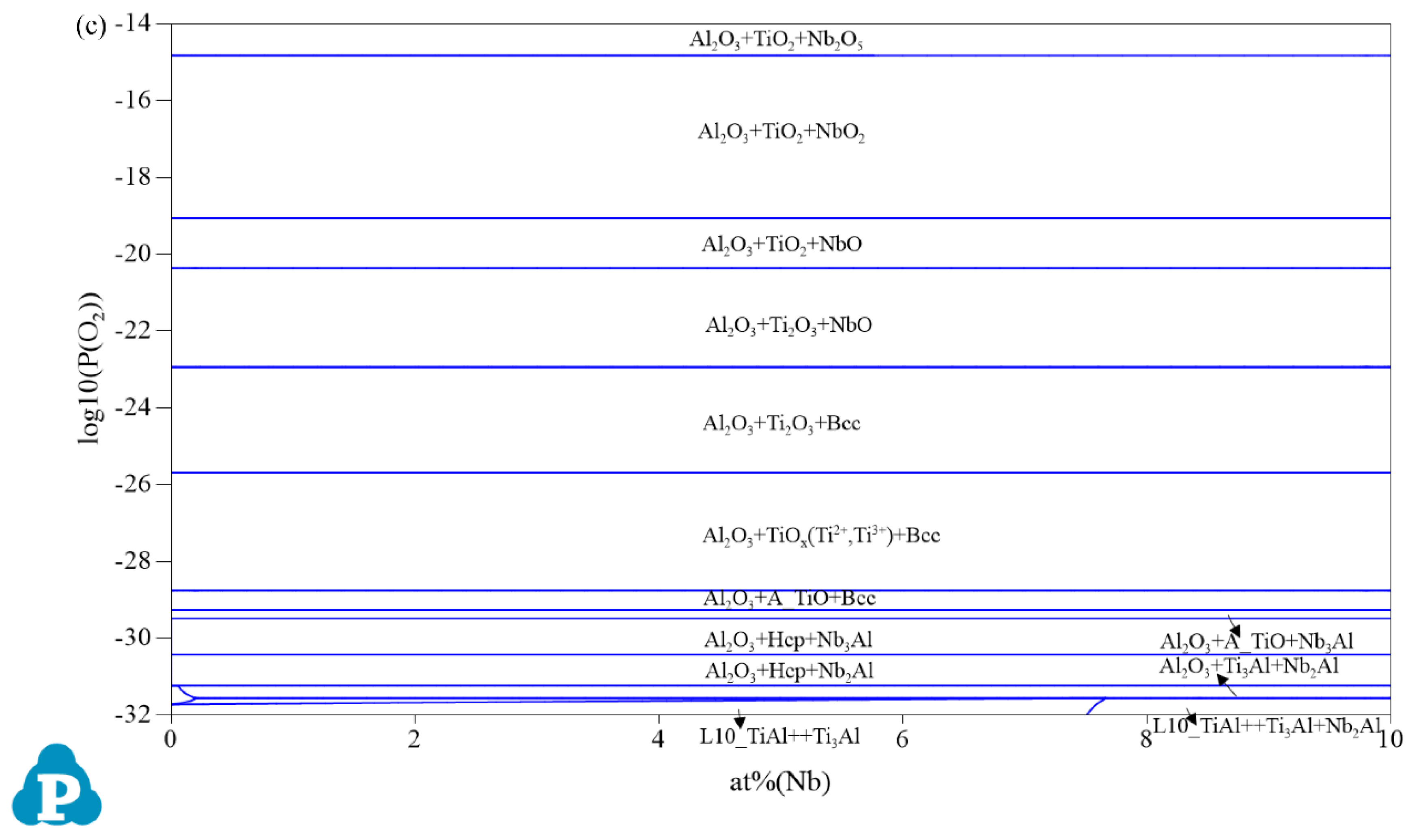

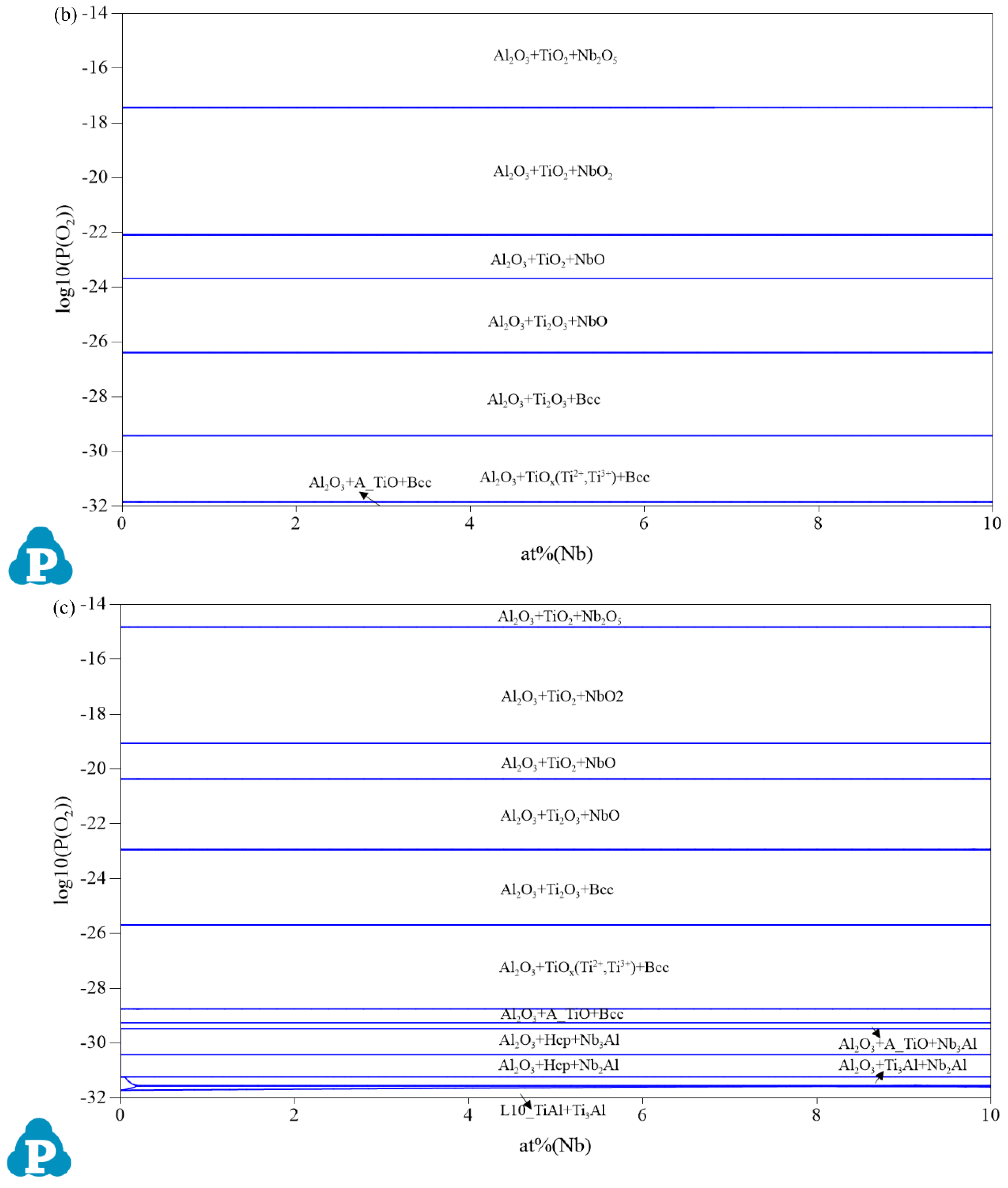
| Composition | Pressure (P/MPa) | Temperature (T/°C) | Partial Pressure of O₂ (P(O2)/MPa) |
|---|---|---|---|
| Ti-42Al-xNb | 0.1 | 700~900 | 10−38~10−20 |
| Ti-45Al-xNb | |||
| Ti-48Al-xNb | |||
| (x = 0–10 at.%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Feng, A.; Wang, H.; Qu, S.; Wang, H. Thermodynamic Study on Initial Oxidation Behavior of TiAl-Nb Alloys at High Temperature. Metals 2023, 13, 485. https://doi.org/10.3390/met13030485
Dong Z, Feng A, Wang H, Qu S, Wang H. Thermodynamic Study on Initial Oxidation Behavior of TiAl-Nb Alloys at High Temperature. Metals. 2023; 13(3):485. https://doi.org/10.3390/met13030485
Chicago/Turabian StyleDong, Zicheng, Aihan Feng, Hao Wang, Shoujiang Qu, and Hao Wang. 2023. "Thermodynamic Study on Initial Oxidation Behavior of TiAl-Nb Alloys at High Temperature" Metals 13, no. 3: 485. https://doi.org/10.3390/met13030485
APA StyleDong, Z., Feng, A., Wang, H., Qu, S., & Wang, H. (2023). Thermodynamic Study on Initial Oxidation Behavior of TiAl-Nb Alloys at High Temperature. Metals, 13(3), 485. https://doi.org/10.3390/met13030485










