Removal of Reactive Black 5 Azo Dye from Aqueous Solutions by a Combination of Reduction and Natural Adsorbents Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Fe Based Alloy
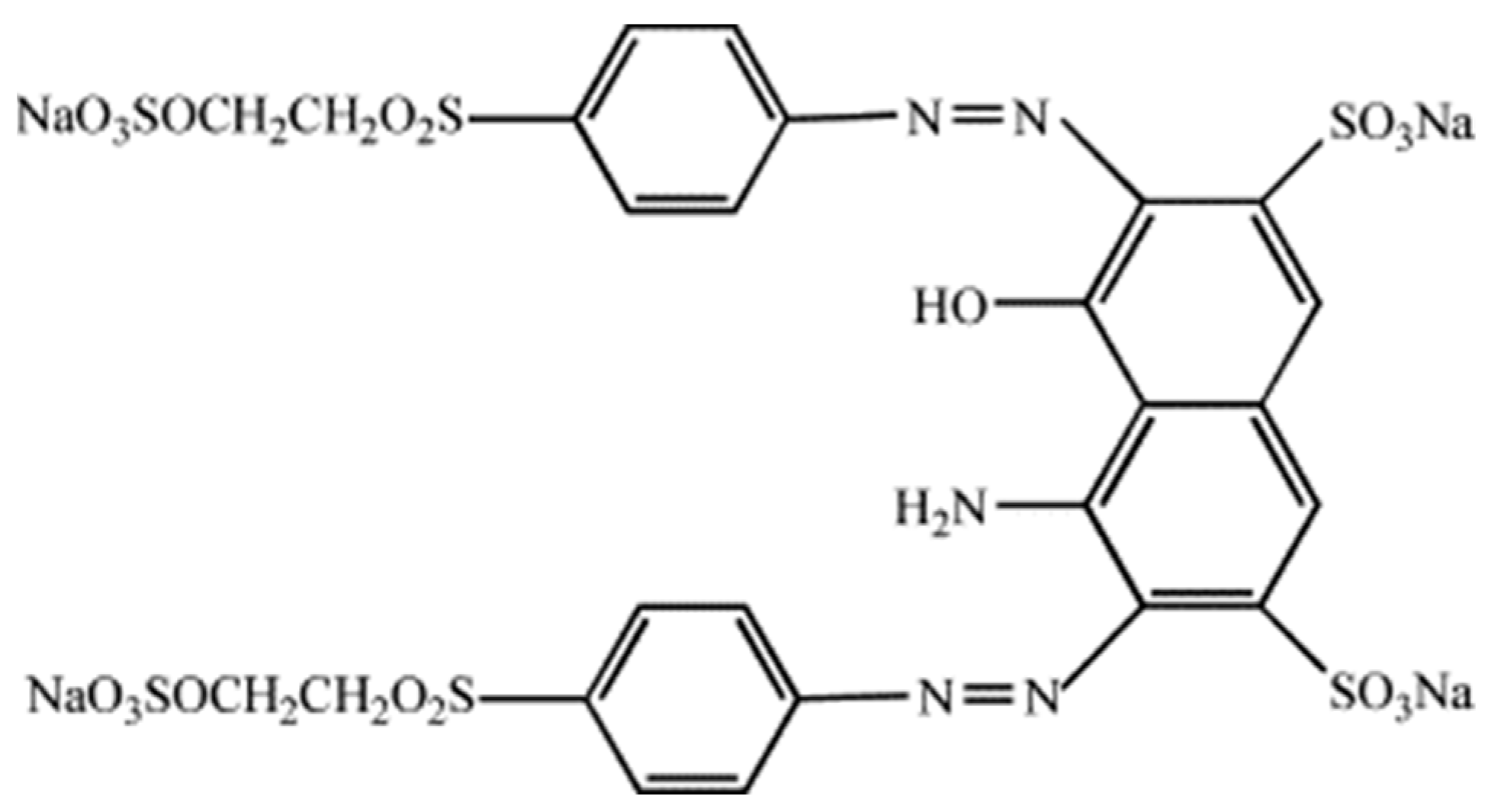
2.3. Preparation of Reactive Black 5 Solution
2.4. Experimental Analysis Methods
3. Results and Discussion
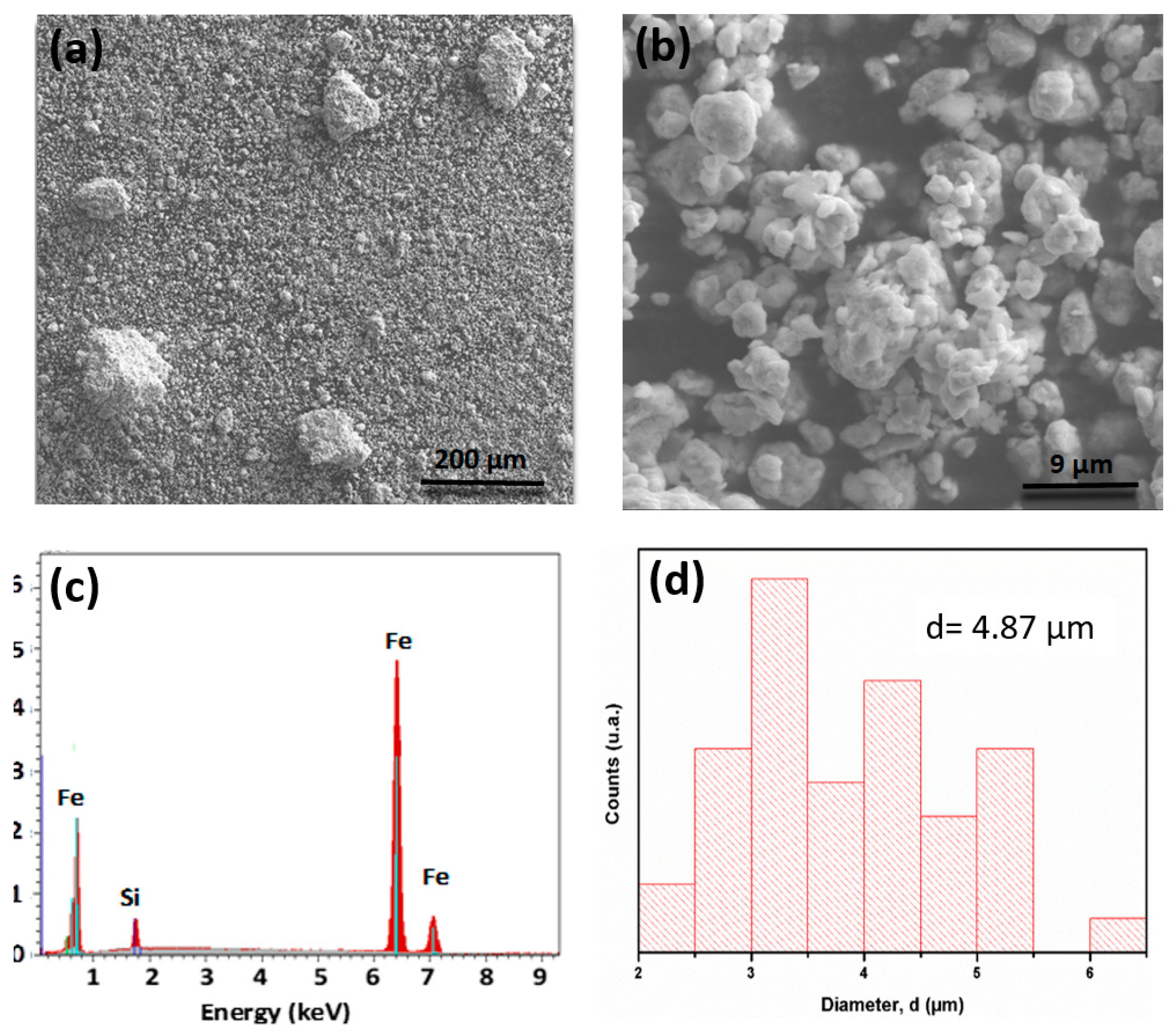
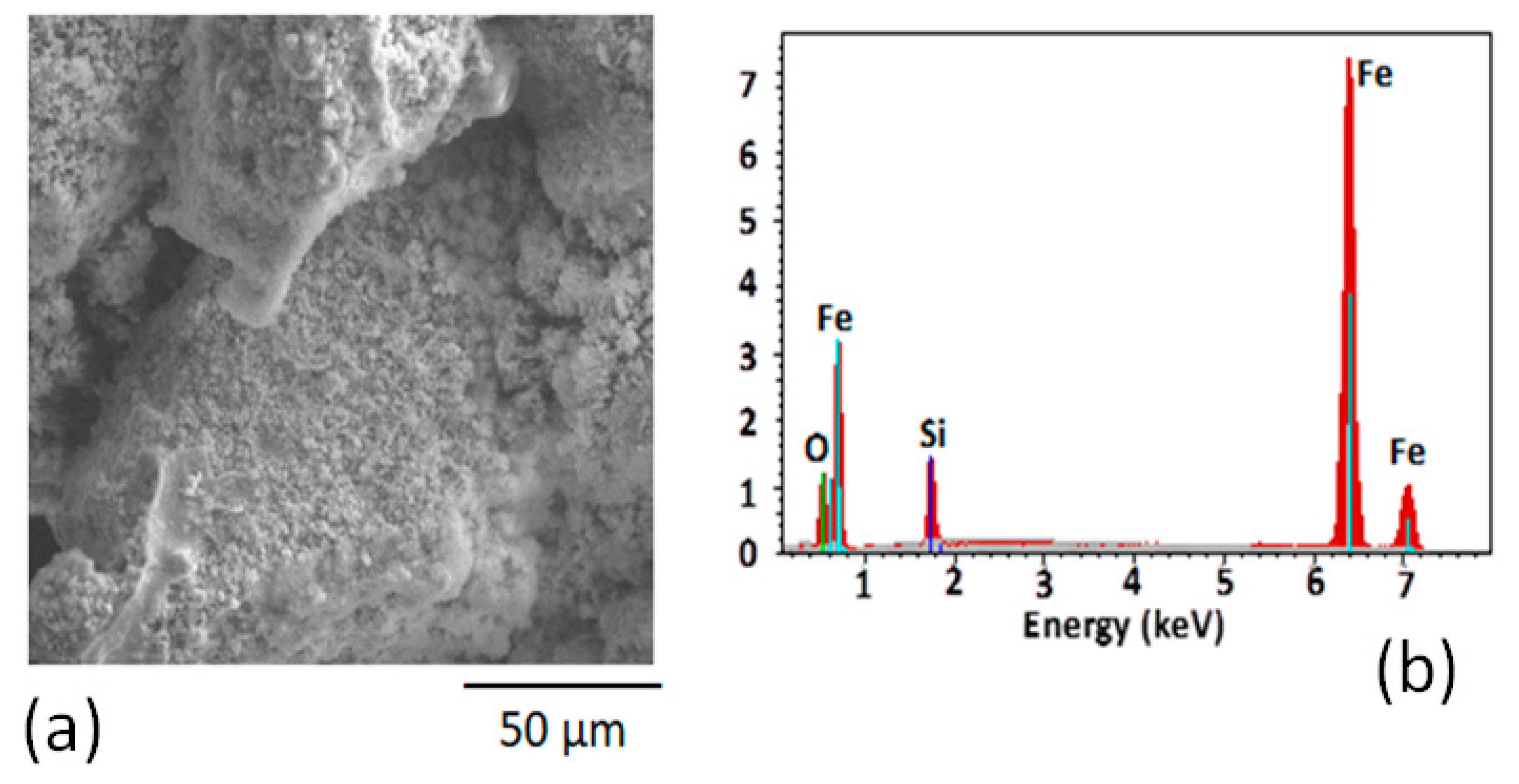
3.1. Characterization of Nanocrystalline FeSiB Powders
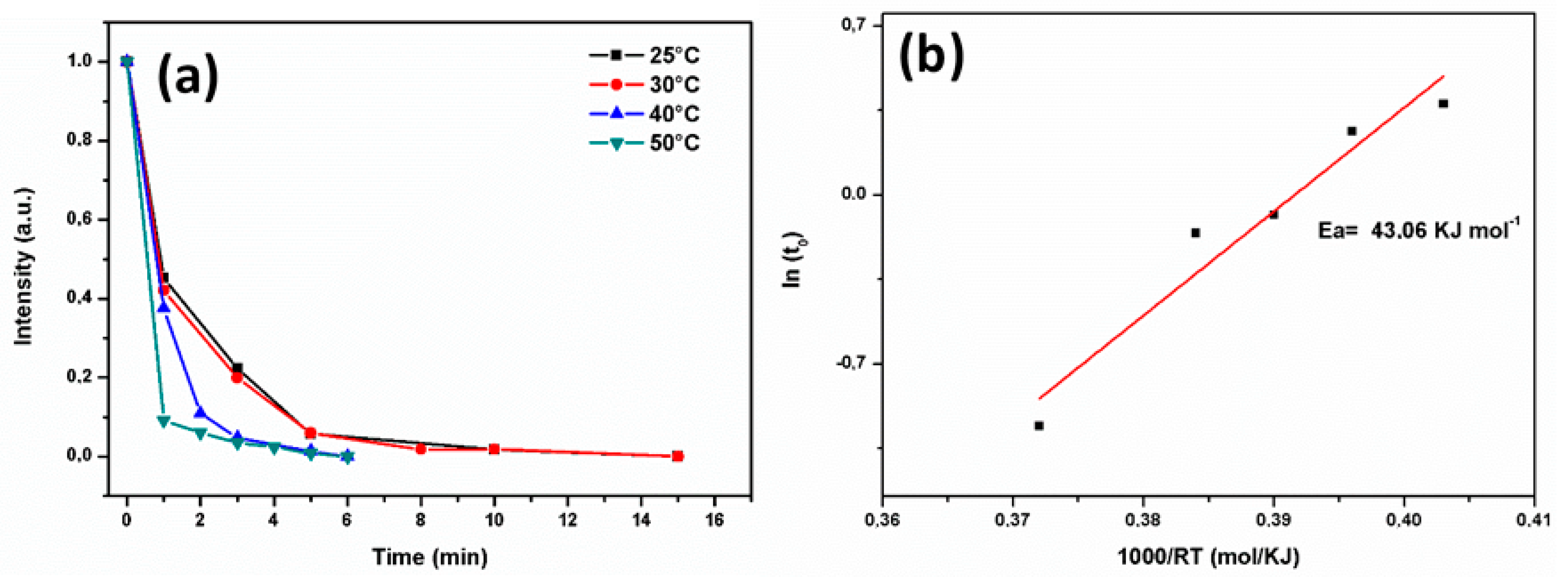
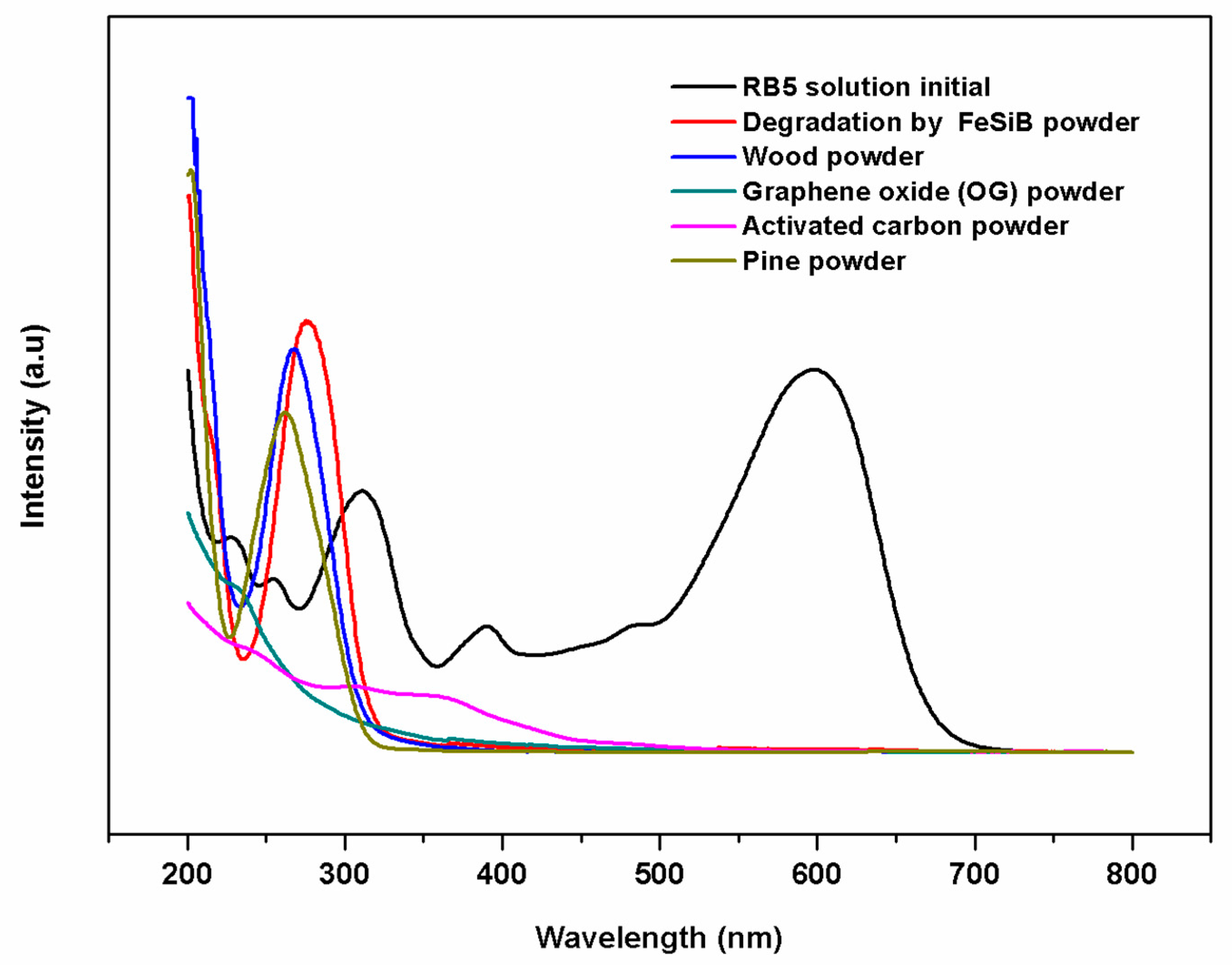
3.2. Decolorization of RB5 Aqueous Solution by Using Nanocrystalline FeSiB Powders
Fe → Fe3+ + 3e− (anode)
H2O → H3O+ + OH−
2H3O+ + 2e−→ [H2] + 2H2O
R–N=N–R’ + [H2] → R–NH–NH–R′
R–NH–NH–R′ + H2→ R–NH2 + R′–NH2
3.3. Secondary Treatment of Decolorized RB5 Aqueous Solution by Using Natural Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mbarek, W.B.; Escoda, L.; Saurina, J.; Pineda, E.; Alminderej, F.M.; Khitouni, M.; Suñol, J.J. Nanomaterials as a Sustainable Choice for Treating Wastewater: A Review. Materials 2022, 15, 8576. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Gupta, V.K.; Mosquera, E.; Gracia, F.; Narayanan, V.; Stephen, A. Visible Light Induced Degradation of Methyl Orange Using β-Ag0.333V2O5 Nanorod Catalysts by Facile Thermal Decomposition Method. J. Saudi Chem. Soc. 2015, 19, 521–527. [Google Scholar] [CrossRef]
- Mahmood, F.; Shahid, M.; Hussain, S.; Shahzad, T.; Tahir, M.; Ijaz, M.; Hussain, A.; Mahmood, K.; Imran, M.; Babar, S.A.K. Potential Plant Growth-Promoting Strain Bacillus Sp. SR-2-1/1 Decolorized Azo Dyes through NADH-Ubiquinone:Oxidoreductase Activity. Bioresour. Technol. 2017, 235, 176–184. [Google Scholar] [CrossRef]
- Abramian, L.; El-Rassy, H. Adsorption Kinetics and Thermodynamics of Azo-Dye Orange II onto Highly Porous Titania Aerogel. Chem. Eng. J. 2009, 150, 403–410. [Google Scholar] [CrossRef]
- Goudarzi, M.; Bazarganipour, M.; Salavati-Niasari, M. Synthesis, Characterization and Degradation of Organic Dye over Co3O4 Nanoparticles Prepared from New Binuclear Complex Precursors. RSC Adv. 2014, 4, 46517–46520. [Google Scholar] [CrossRef]
- Roy, A.; Adhikari, B.; Majumder, S.B. Equilibrium, Kinetic, and Thermodynamic Studies of Azo Dye Adsorption from Aqueous Solution by Chemically Modified Lignocellulosic Jute Fiber. Ind. Eng. Chem. Res. 2013, 52, 6502–6512. [Google Scholar] [CrossRef]
- Manivel, A.; Lee, G.J.; Chen, C.Y.; Chen, J.H.; Ma, S.H.; Horng, T.L.; Wu, J.J. Synthesis of MoO3 Nanoparticles for Azo Dye Degradation by Catalytic Ozonation. Mater. Res. Bull. 2015, 62, 184–191. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of Dyes from Aqueous Solution by Fenton Processes: A Review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef]
- Singla, P.; Sharma, M.; Pandey, O.P.; Singh, K. Photocatalytic Degradation of Azo Dyes Using Zn-Doped and Undoped TiO2 Nanoparticles. Appl. Phys. A 2013, 116, 371–378. [Google Scholar] [CrossRef]
- AboliGhasemabadi, M.; Ben Mbarek, W.; Casabella, O.; Roca-Bisbe, H.; Pineda, E.; Escoda, L.; Suñol, J.J. Application of Mechanically Alloyed MnAl Particles to De-Colorization of Azo Dyes. J. Alloys Compd. 2018, 741, 240–245. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Pineda, E.; Escoda, L.; Suñol, J.J.; Khitouni, M. High Efficiency Decolorization of Azo Dye Reactive Black 5 by Ca-Al Particles. J. Environ. Chem. Eng. 2017, 5, 6107–6113. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Saurina, J.; Escoda, L.; Pineda, E.; Khitouni, M.; Suñol, J.-J. Effects of the Addition of Fe, Co on the Azo Dye Degradation Ability of Mn-Al Mechanically Alloyed Powders. Metals 2020, 10, 1578. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.; Marchant, R.; Smyth, W. Microbial Decolourisation and Degradation of Textile Dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Peternel, I.T.; Koprivanac, N.; Božić, A.M.L.; Kušić, H.M. Comparative Study of UV/TiO2, UV/ZnO and Photo-Fenton Processes for the Organic Reactive Dye Degradation in Aqueous Solution. J. Hazard. Mater. 2007, 148, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Tso, C.-P.; Tung, L.-Y. Rapid Degradation of Methyl Orange with Nanoscale Zerovalent Iron Particles. J. Environ. Eng. Manag. 2010, 20, 137–143. [Google Scholar]
- Bokare, A.D.; Chikate, R.C.; Rode, C.V.; Paknikar, K.M. Iron-Nickel Bimetallic Nanoparticles for Reductive Degradation of Azo Dye Orange G in Aqueous Solution. Appl. Catal. B Environ. 2008, 79, 270–278. [Google Scholar] [CrossRef]
- Jia, Z.; Duan, X.; Zhang, W.; Wang, W.; Sun, H.; Wang, S.; Zhang, L.C. Ultra-Sustainable Fe78Si9B13 Metallic Glass as a Catalyst for Activation of Persulfate on Methylene Blue Degradation under UV-Vis Light. Sci. Rep. 2016, 6, 38520. [Google Scholar] [CrossRef]
- Lutterotti, L. MAUD CPD Newsletter (IUCr), No. 24, 2000.-References-Scientific Research Publishing. Available online: https://www.scirp.org/%28S%28czeh2tfqyw2orz553k1w0r45%29%29/reference/referencespapers.aspx?referenceid=782192 (accessed on 26 April 2022).
- Gnanamoorthy, G.; Ali, D.; Yadav, V.K.; Dhinagaran, G.; Venkatachalam, K.; Narayanan, V. New Construction of Fe3O4/RGO/ZnSnO3 Nanocomposites Enhanced Photoelectro Chemical Properties. Opt. Mater. 2020, 109, 110353. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Muthamizh, S.; Sureshbabu, K.; Munusamy, S.; Padmanaban, A.; Kaaviya, A.; Nagarajan, R.; Stephen, A.; Narayanan, V. Photocatalytic Properties of Amine Functionalized Bi2Sn2O7/RGO Nanocomposites. J. Phys. Chem. Solids 2018, 118, 21–31. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Azabou, M.; Pineda, E.; Fiol, N.; Escoda, L.; Suñol, J.J.; Khitouni, M. Rapid Degradation of Azo-Dye Using Mn-Al Powders Produced by Ball-Milling. RSC Adv. 2017, 7, 12620–12628. [Google Scholar] [CrossRef]
- Sarsamb, L.A.; Mohammeda, D.H. Spectrophotometric Determination of Benzocaine by Azo-Dye Formation Reaction. J. Univ. Anbar Pure Sci. 2011, 5, 24–30. [Google Scholar] [CrossRef]
- Al-Safar, R.S.; Othman, N.S. Spectrophotometric Determination of Sulphacetamide Sodium via Diazotization and Coupling Reaction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 052017. [Google Scholar] [CrossRef]
- Bonicamp, J.M.; Martin, K.L.; McBride, G.R.; Clark, R.W. Beer’s Law Is Not a Straight Line: Amplification of Errors by Transformation. Chem. Educ. 1999, 4, 81–88. [Google Scholar] [CrossRef]
- Valcarcel, M.I. Principles of Analytical Chemistry; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Fan, J.; Guo, Y.; Wang, J.; Fan, M. Rapid Decolorization of Azo Dye Methyl Orange in Aqueous Solution by Nanoscale Zerovalent Iron Particles. J. Hazard. Mater. 2009, 166, 904–910. [Google Scholar] [CrossRef]
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A Highly Efficient Degradation Mechanism of Methyl Orange Using Fe-Based Metallic Glass Powders. Sci. Rep. 2016, 6, 21947. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, L. Heterogeneous UV-Fenton Catalytic Degradation of Dyestuff in Water with Hydroxyl-Fe Pillared Bentonite. Catal. Today 2007, 126, 463–470. [Google Scholar] [CrossRef]
- Xie, S.H.; Peng, G.Q.; Tu, X.M.; Qian, H.X.; Zeng, X.R. Fe-Based Powders Prepared by Ball-Milling with Considerable Degradation Efficiency to Methyl Orange Compared with Fe-Based Metallic Glasses. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 1207–1214. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.Q.; Li, H.; Yang, H.; Huo, J.; Wang, J.; Chang, C.; Wang, X.; Li, R.W.; Wang, G. Fast Decolorization of Azo Dyes in Both Alkaline and Acidic Solutions by Al-Based Metallic Glasses. J. Alloys Compd. 2017, 701, 759–767. [Google Scholar] [CrossRef]
- Samiee, S.; Goharshadi, E.K.; Nancarrow, P. Successful Degradation of Reactive Black 5 by Engineered Fe/Pd Nanoparticles: Mechanism and Kinetics Aspects. J. Taiwan Inst. Chem. Eng. 2016, 67, 406–417. [Google Scholar] [CrossRef]
- Feng, W.; Nansheng, D.; Helin, H. Degradation Mechanism of Azo Dye C. I. Reactive Red 2 by Iron Powder Reduction and Photooxidation in Aqueous Solutions. Chemosphere 2000, 41, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-B.; Wen, F.-R.; Chen, H.-X.; Liu, J.-J.; Tao, G.-Y.; Xu, N.-J.; Xue, J.-Q. Corrosion Characteristics of Fe3Si Intermetallic Coatings Prepared by Molten Salt Infiltration in Sulfuric Acid Solution. J. Alloys Compd. 2019, 778, 972–981. [Google Scholar] [CrossRef]
- Neoh, C.H.; Lam, C.Y.; Lim, C.K.; Yahya, A.; Bay, H.H.; Ibrahim, Z.; Noor, Z.Z. Biodecolorization of Recalcitrant Dye as the Sole Sourceof Nutrition Using Curvularia Clavata NZ2 and Decolorization Ability of Its Crude Enzymes. Environ. Sci. Pollut. Res. 2015, 22, 11669–11678. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Toxicological Assessment and UV/TiO2-Based Induced Degradation Profile of Reactive Black 5 Dye. Environ. Manag. 2018, 61, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Vernekar, D.; Jagadeesan, D. Tunable Acid–Base Bifunctional Catalytic Activity of FeOOH in an Orthogonal Tandem Reaction. Catal. Sci. Technol. 2015, 5, 4029–4038. [Google Scholar] [CrossRef]
- Liu, X.; Liang, M.; Liu, M.; Su, R.; Wang, M.; Qi, W.; He, Z. Highly Efficient Catalysis of Azo Dyes Using Recyclable Silver Nanoparticles Immobilized on Tannic Acid-Grafted Eggshell Membrane. Nanoscale Res. Lett. 2016, 11, 440. [Google Scholar] [CrossRef]
- Mané, U.; Gurav, P.; Deshmukh, A.; Govindwar, S. Degradation of Textile Dye Reactive Navy-Blue Rx (Reactive Blue–9) by an Isolated Actinomycete Streptomyces Krainskii SUK-5. Malays. J. Microbiol. 2008, 4, 1–5. [Google Scholar] [CrossRef]
- Chatterjeea, S.; Limb, S.R.; Woo, S.H. Removal of Reactive Black 5 by zero-valent iron modified with various surfactants. Chem. Eng. J. 2010, 160, 27–32. [Google Scholar] [CrossRef]
- Chen, S.Q.; Li, M.; Ma, X.Y.; Zhou, M.J.; Wang, D.; Yan, M.Y.; Li, Z.; Yao, K.F. Influence of Inorganic Ions on Degradation Capability of Fe-Based Metallic Glass towards Dyeing Wastewater Remediation. Chemosphere 2021, 264, 128392. [Google Scholar] [CrossRef]
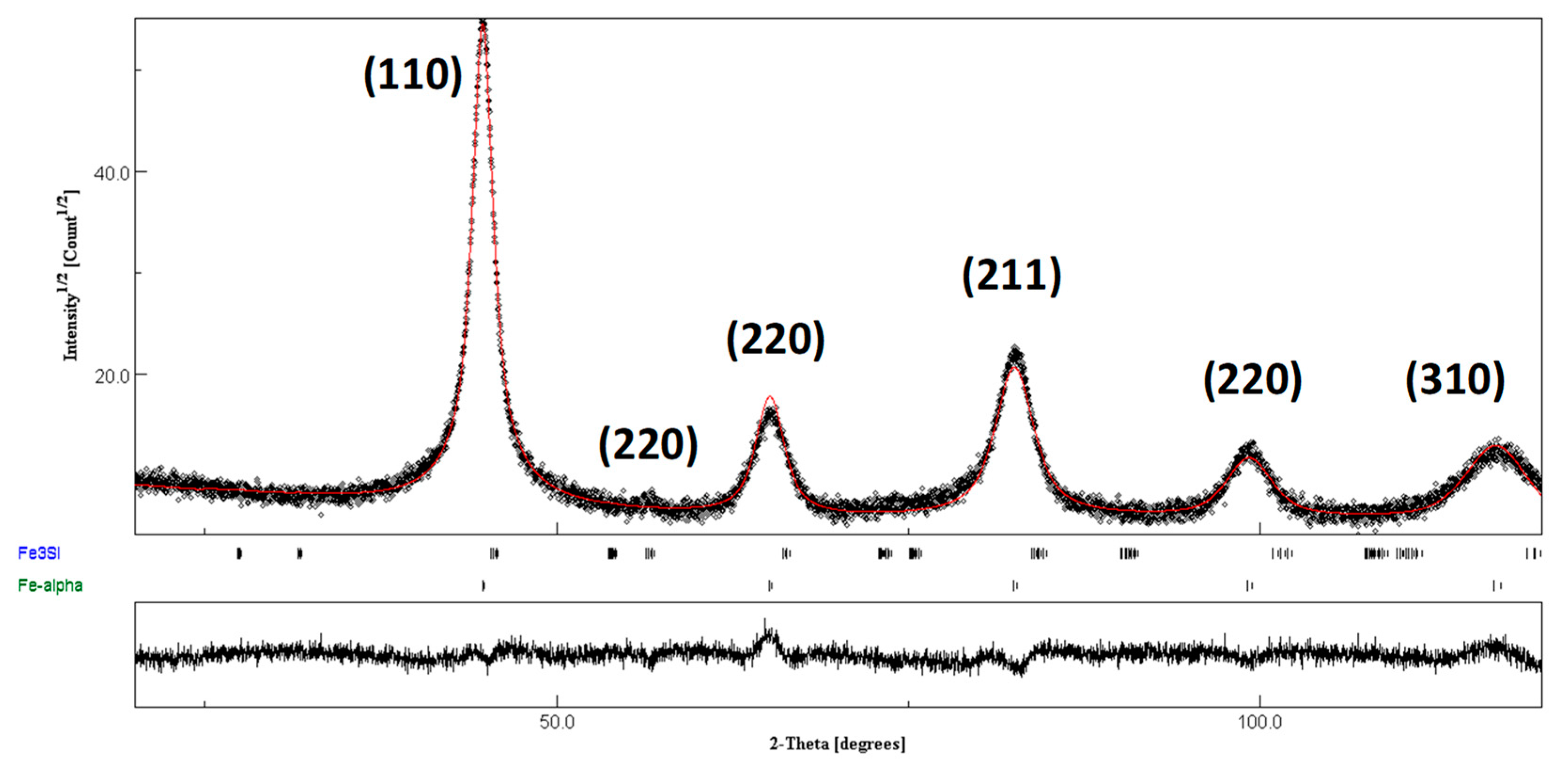

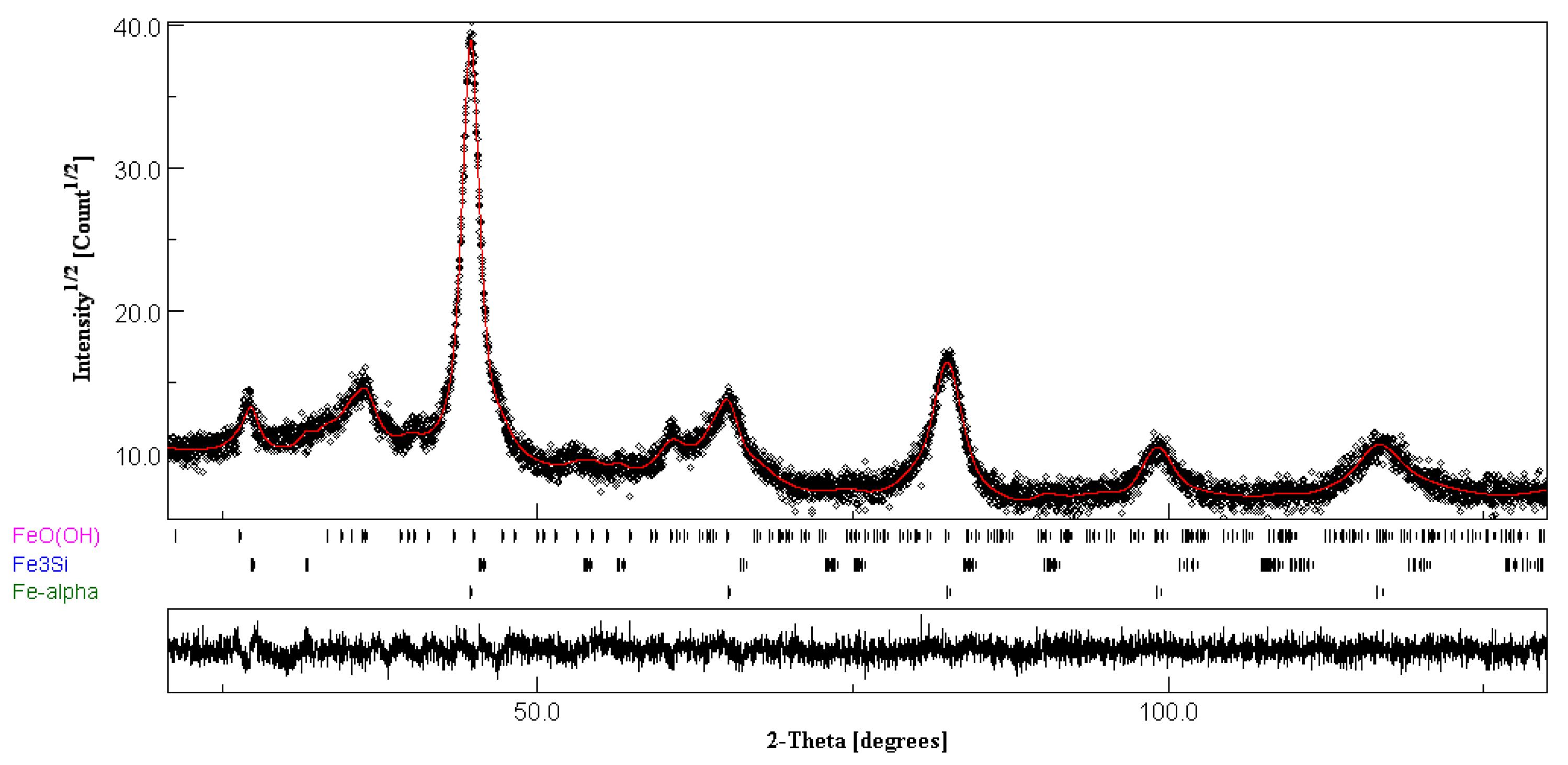




| Sample | Phase | % | a (Å) | Crystalline Size (nm) | Microstrain % | Rwp | Rexp | GoF |
|---|---|---|---|---|---|---|---|---|
| Fe80Si10B10 | α-Fe | 88.8 | 2.862 (3) | 10.2 (1) | 0.52 (2) | 11.481 | 8.730 | 1.315 |
| Fe3Si | 11.2 | 3.986 (4) | 13.3 (11) | 0.60 (1) |
| Sample | Phase | % | a (Å) | Crystalline Size (Å) | Microstrain % | Rwp | Rexp | GoF |
|---|---|---|---|---|---|---|---|---|
| Fe80Si10B10 | α-Fe | 77.7 | 2.864 (2) | 106 (1) | 0.053 (2) | 11.783 | 9.897 | 1.191 |
| Fe3Si | 9.9 | 3.987 (3) | 76 (6) | 0.058 (4) | ||||
| FeO (OH) | 12.4 | 3.055 (7) 4.601 (2) 9.927 (3) | 179 (1) | 0.032 (3) |
| +MS | |
|---|---|
| Molecular Weight | Molecular Compound |
| HPLC-MS of Reactive Black 5 solution after degradation by FeSiB powder | |
| 184 | 4-(vinylsulfonyl) phenol |
| 201 | 4-(2-hydroxyethyl)sulfonyl) benzenoamina |
| 202 | 4-(2-hydroxyethyl)sulfonyl) phenol |
| 200 | 4-(2-aminoethyl)sulfonyl) benzenoamina |
| 226 | 5-((4-hydroxyfenyl)sulfonyl)-2 penteno |
| HPLC-Ms of Reactive Black 5 solution degraded by FeSiB powder after the adsorption process by OG powder | |
| 202 | 4-(2-hydroxyethyl)sulfonyl) phenol. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Mbarek, W.; Daza, J.; Escoda, L.; Fiol, N.; Pineda, E.; Khitouni, M.; Suñol, J.-J. Removal of Reactive Black 5 Azo Dye from Aqueous Solutions by a Combination of Reduction and Natural Adsorbents Processes. Metals 2023, 13, 474. https://doi.org/10.3390/met13030474
Ben Mbarek W, Daza J, Escoda L, Fiol N, Pineda E, Khitouni M, Suñol J-J. Removal of Reactive Black 5 Azo Dye from Aqueous Solutions by a Combination of Reduction and Natural Adsorbents Processes. Metals. 2023; 13(3):474. https://doi.org/10.3390/met13030474
Chicago/Turabian StyleBen Mbarek, Wael, Jason Daza, Lluisa Escoda, Núria Fiol, Eloi Pineda, Mohamed Khitouni, and Joan-Josep Suñol. 2023. "Removal of Reactive Black 5 Azo Dye from Aqueous Solutions by a Combination of Reduction and Natural Adsorbents Processes" Metals 13, no. 3: 474. https://doi.org/10.3390/met13030474
APA StyleBen Mbarek, W., Daza, J., Escoda, L., Fiol, N., Pineda, E., Khitouni, M., & Suñol, J.-J. (2023). Removal of Reactive Black 5 Azo Dye from Aqueous Solutions by a Combination of Reduction and Natural Adsorbents Processes. Metals, 13(3), 474. https://doi.org/10.3390/met13030474










