Research Progress of Titanium Sponge Production: A Review
Abstract
1. Introduction
2. Thermal Reduction
2.1. Thermal Reduction of the Precursor TiCl4
2.1.1. Hunter Process
2.1.2. Kroll Process
2.1.3. Armstrong Process
2.1.4. ARC (Albany Research Center) Process
2.1.5. Vapor-Phase Reduction Process
2.1.6. CSIR-Ti (Council for Scientific and Industrial Research) Process
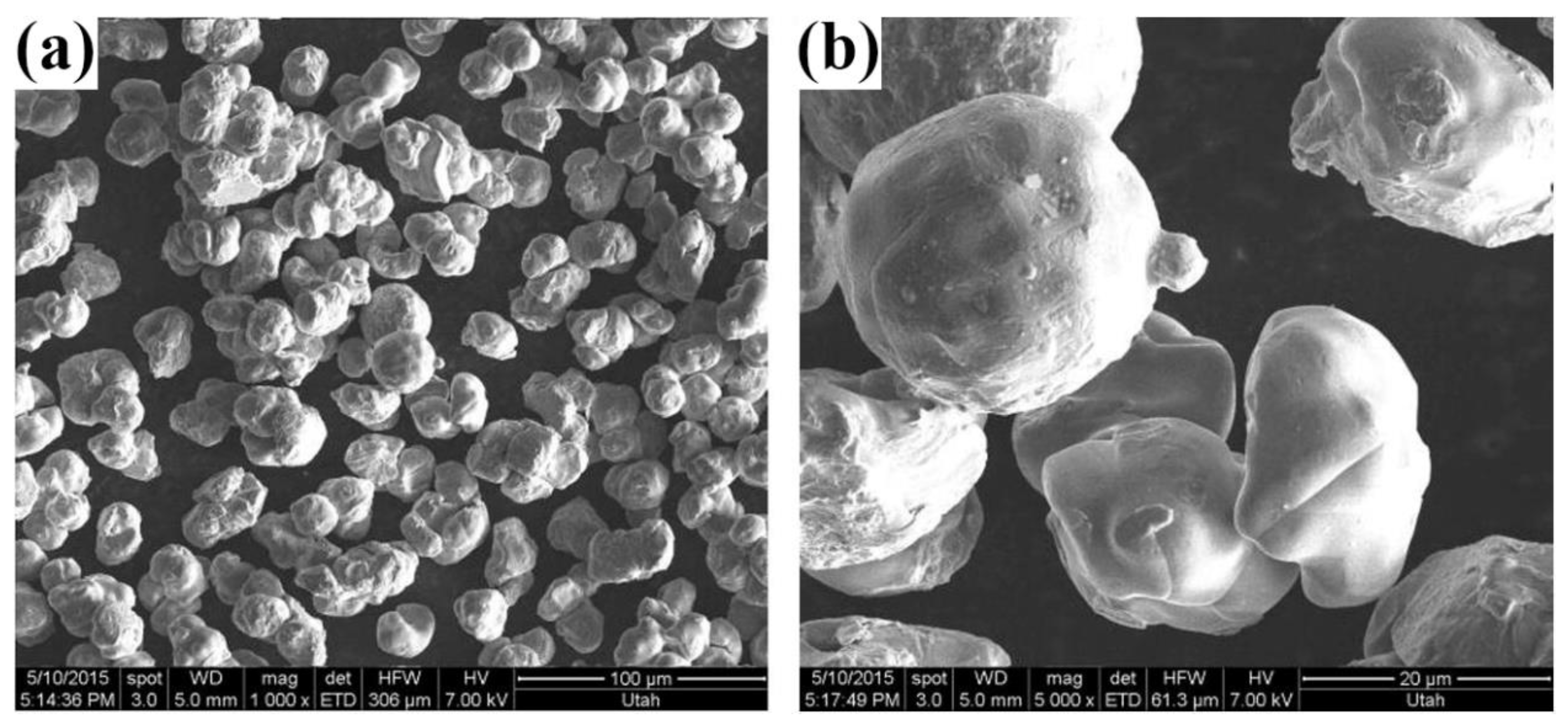
2.1.7. TiRO Process
2.1.8. Other Processes
2.2. Thermal Reduction of the Precursor TiO2
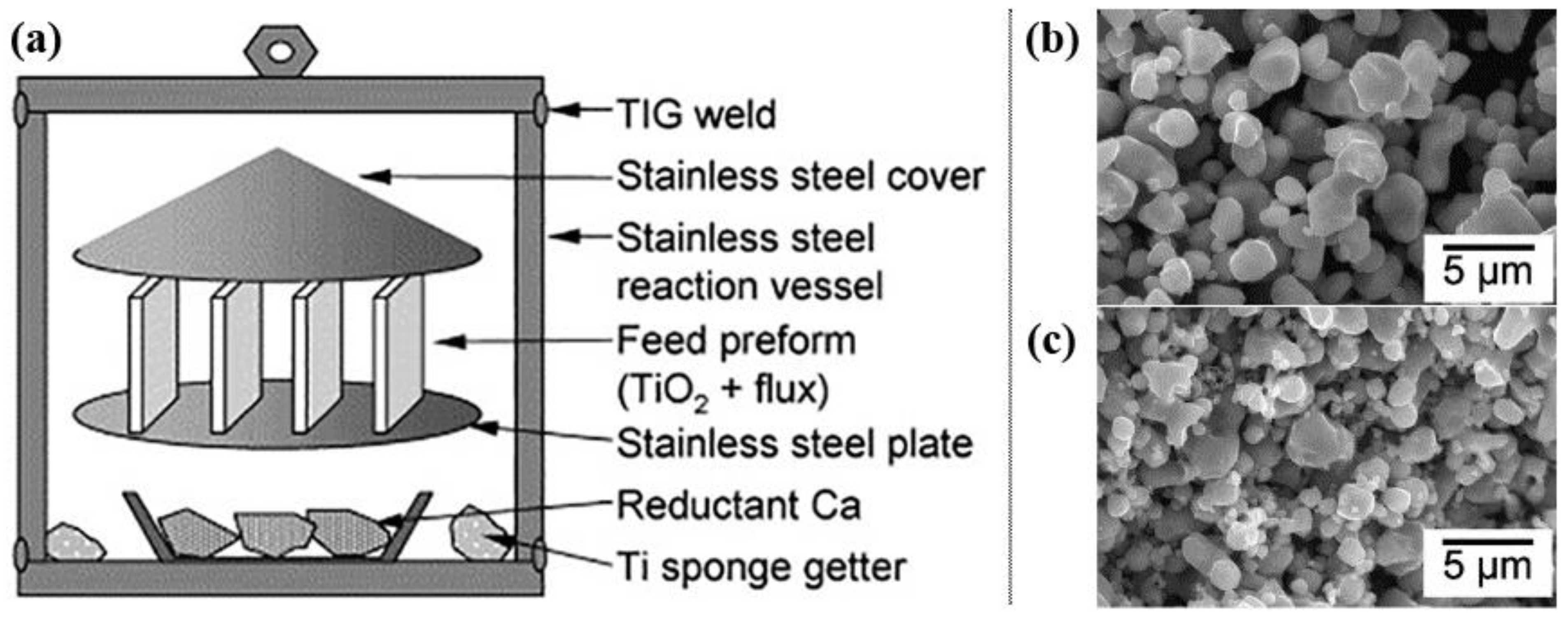
2.2.1. PRP (Preform Reduction Process) Process
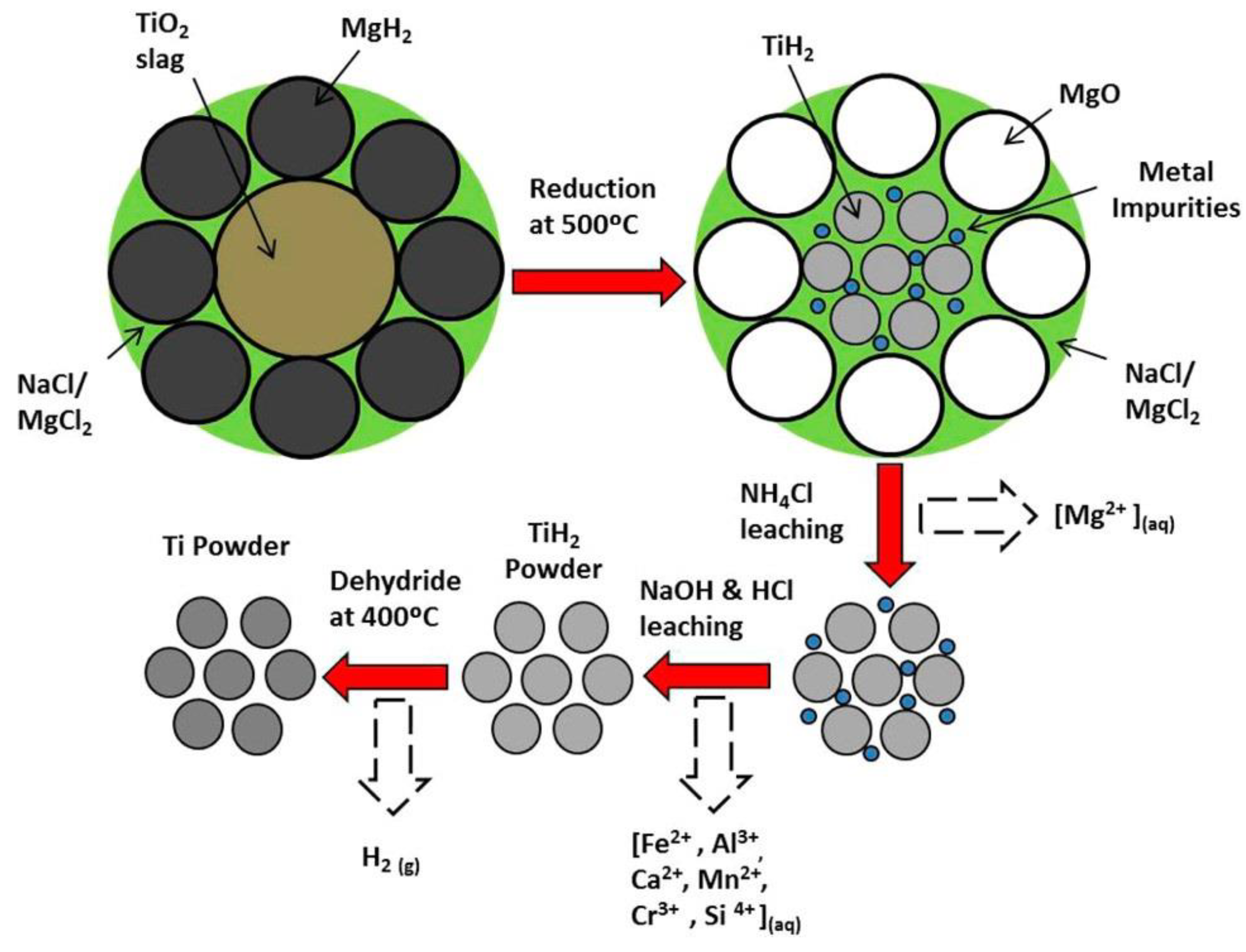
2.2.2. MHR (Metal Hydride Reduction) Process
2.2.3. EMR (Electronically Mediated Reaction) Process
2.2.4. HAMR (Hydrogen-Assisted Magnesiothermic Reduction) Process
2.2.5. SHS (Self-Propagating High-Temperature Synthesis) Process
2.2.6. Other Processes
2.3. Thermal Reduction of Other Precursors
3. Electrolysis
3.1. Electrolysis with Titanium-Containing Materials as the Cathode
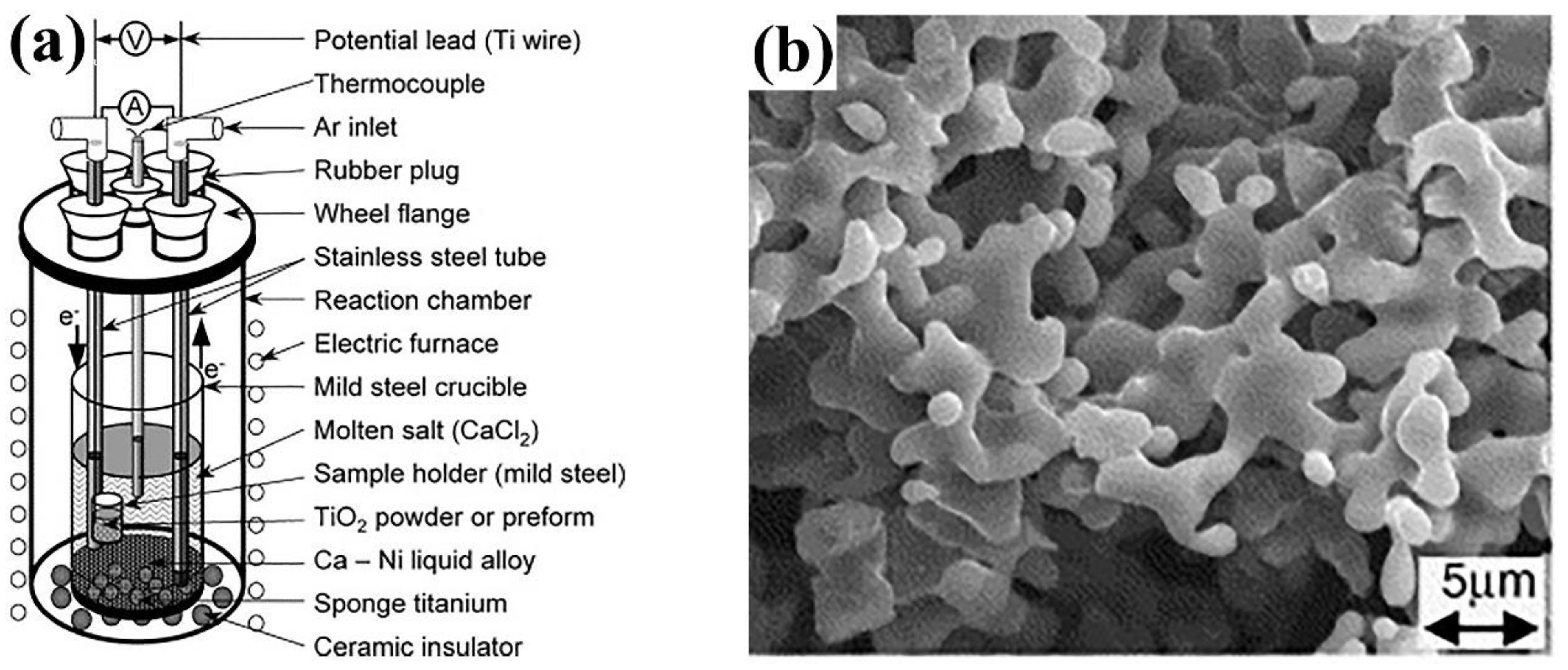
3.1.1. FFC (Fray–Farthing–Chen) Process
3.1.2. OS (Ono–Suzuki) Process
3.1.3. QIT (Quebec Iron and Titane) Process
3.2. Electrolysis with Titanium-Containing Materials as the Anode
3.2.1. USTB (University of Science and Technology Beijing) Process
3.2.2. MER (Materials and Electrochemical Research) Process and Chinuka Process
3.3. Electrolysis with Titanium-Containing Materials in Electrolyte
SOM Process
4. Comprehensive Analysis of Various Processes
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fashu, S.; Lototskyy, M.; Davids, M.W.; Pickering, L.; Linkov, V.; Sun, T.; Tang, R.; Xiao, F.; Fursikov, P.V.; Tarasov, B.P. A review on crucibles for induction melting of titanium alloys. Mater. Des. 2020, 186, 108295. [Google Scholar] [CrossRef]
- Qiu, G.; Guo, Y. Current situation and development trend of titanium metal industry in China. Int. J. Miner. Metall. Mater. 2022, 4, 599–610. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J.C. Perspectives on titanium science and technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Boyer, R.R. Titanium for aerospace: Rationale and applications. Adv. Perform. Mater. 1995, 2, 349–368. [Google Scholar] [CrossRef]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Hartman, A.D.; Gerdemann, S.J.; Hansen, J.S. Producing lower-cost titanium for automotive applications. JOM 1998, 50, 16–19. [Google Scholar] [CrossRef]
- Takahashi, K.; Mori, K.; Takebe, H. Application of titanium and its alloys for automobile parts. MATEC Web Conf. 2020, 321, 02003. [Google Scholar] [CrossRef]
- Froes, F.H.; Friedrich, H.; Kiese, J.; Bergoint, D. Titanium in the family automobile: The cost challenge. JOM 2004, 56, 40–44. [Google Scholar] [CrossRef]
- Gorynin, I.V. Titanium alloys for marine application. Mater. Sci. Eng. A 1999, 263, 112–116. [Google Scholar] [CrossRef]
- Rohith, K.; Shreyas, S.; Vishnu, A.K.B.; Sheshank, R.V.; Ganesha, B.B.; Vinod, B. Recent material advancement for marine application. Mater. Today Proc. 2019, 18, 4854–4859. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A. A review on alloy design, biological response, and strengthening of β-titanium alloys as biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef] [PubMed]
- Gepreel, M.A.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Horie, T. Development of titanium consumer products: From titanium art to tumblers. In Titanium for Consumer Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–195. [Google Scholar]
- Froes, F.H. Is it ethical to use advanced materials such as titanium in sports equipment? In Titanium for Consumer Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–129. [Google Scholar]
- Global Titanium Metal Market Research Report. 2020. Available online: https://www.360researchreports.com/global-titanium-metal-market-15056371 (accessed on 2 November 2022).
- Titanium Metal Market Size—Growth Rate, Trend Analysis & Industry Outlook. 2023. Available online: https://www.marketresearchfuture.com/reports/titanium-metal-market-7482 (accessed on 2 November 2022).
- Titanium Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/titanium-statistics-and-information (accessed on 2 November 2022).
- Froes, F.H.S.; Gungor, M.N.; Imam, M.A. Cost-affordable titanium: The component fabrication perspective. JOM 2007, 59, 28–31. [Google Scholar] [CrossRef]
- Titanium—Industrial Base, Price Trends, and Technology Initiatives. Available online: https://www.rand.org/pubs/monographs/MG789.html (accessed on 2 November 2022).
- Nie, X.; Dong, L.; Bai, C.; Chen, D.; Qiu, G. Preparation of Ti by direct electrochemical reduction of solid TiO2 and its reaction mechanism. Trans. Nonferr. Met. Soc. China 2006, 16, s723–s727. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, J.; Tian, Q.; Guo, X. Review of the effect of oxygen on titanium and deoxygenation technologies for recycling of titanium metal. JOM 2019, 71, 3209–3220. [Google Scholar] [CrossRef]
- Hunter, M.A. Metallic titanium. J. Am. Chem. Soc. 1910, 32, 330–336. [Google Scholar] [CrossRef]
- Kroll, W. The production of ductile titanium. Trans. Electrochem. Soc. 1940, 78, 35–47. [Google Scholar] [CrossRef]
- Reddy, R.G.; Shinde, P.S.; Liu, A. Review—The emerging technologies for producing low-cost titanium. J. Electrochem. Soc. 2021, 168, 042502. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Paramore, J.D.; Sun, P.; Chandran, K.S.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M.; Free, M. Powder metallurgy of titanium—Past, present, and future. Int. Mater. Rev. 2018, 63, 407–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.Z.; Sun, P.; Zheng, S.; Xia, Y.; Free, M. A perspective on thermochemical and electrochemical processes for titanium metal production. JOM 2017, 69, 1861–1868. [Google Scholar] [CrossRef]
- Song, Y.; Dou, Z.; Zhang, T.; Liu, Y. Research progress on the extractive metallurgy of titanium and its alloys. Miner. Process Extr. Metall. Rev. 2021, 42, 535–551. [Google Scholar] [CrossRef]
- Jena, K.D.; Xu, S.; Hayat, M.D.; Zhang, W.; Cao, P. Aiming at low-oxygen titanium powder: A review. Powder Technol. 2021, 394, 1195–1217. [Google Scholar] [CrossRef]
- Jackson, M.; Dring, K. A review of advances in processing and metallurgy of titanium alloys. Mater. Sci. Technol. 2006, 22, 881–887. [Google Scholar] [CrossRef]
- Takeda, O.; Ouchi, T.; Okabe, T. Recent Progress in Titanium Extraction and Recycling. Metall. Mater. Trans. B 2020, 51, 1315–1328. [Google Scholar] [CrossRef]
- Sohn, H. Production technology of titanium by Kroll process. J. Korean Inst. Resour. Recycl. 2020, 29, 3–14. [Google Scholar] [CrossRef]
- Mo, W. Titanium Metallurgy; Metallurgical Industry Press: Beijing, China, 1998. [Google Scholar]
- Barbis, D.P.; Gasior, R.M.; Walker, G.P.; Capone, J.A.; Schaeffer, T.S. Titanium powders from the hydride-dehydride process. In Titanium Powder Metallurgy; Butterworth-Heinemann: Waltham, MA, USA, 2015; pp. 101–116. [Google Scholar]
- Withers, J.; Laughlin, J.; Elkadi, Y.; DeSilva, J.; Loutfy, R. The production of Ti alloy powder from chloride precursors. Key Eng. Mater. 2010, 436, 35–39. [Google Scholar] [CrossRef]
- Kohli, R. Production of Titanium from Ilmenite: A Review; Lawrence Berkeley Lab.: Berkeley, CA, USA, 1981; pp. 1–93. [Google Scholar]
- Kosemura, S.; Ampo, S.; Fukasawa, E.; Hatta, Y. Production of titanium metal at Toho Titanium Co., Ltd. Min. Mater. Process. Inst. Jpn. 2007, 123, 693–697. [Google Scholar]
- Growley, G. How to extract low-cost titanium. Adv. Mater. Process. 2003, 161, 25–27. [Google Scholar]
- MacDonald, D.; Fernandez, R.; Delloro, F.; Jodoin, B. Cold spraying of Armstrong process titanium powder for additive manufacturing. J. Therm. Spray Technol. 2017, 26, 598–609. [Google Scholar] [CrossRef]
- Chen, W.; Yamamoto, Y.; Peter, W.H. Investigation of pressing and sintering processes of CP-Ti powder made by Armstrong process. Key Eng. Mater. 2010, 436, 123–130. [Google Scholar] [CrossRef]
- Chen, W.; Yamamoto, Y.; Peter, W.H.; Gorti, S.B.; Sabau, A.S.; Clark, M.B.; Nunn, S.D.; Kiggans, J.O.; Blue, C.A.; Williams, J.C.; et al. Cold compaction study of Armstrong process® Ti-6Al-4V powders. Powder Technol. 2011, 214, 194–199. [Google Scholar] [CrossRef]
- Xu, X.; Nash, P. Sintering mechanisms of Armstrong prealloyed Ti-6Al-4V powders. Mater. Sci. Eng. A 2014, 607, 409–416. [Google Scholar] [CrossRef]
- Araci, K.; Mangabhai, D.; Akhtar, K. Production of titanium by the Armstrong process. In Titanium Powder Metallurgy; Butterworth-Heinemann: Waltham, MA, USA, 2015; pp. 149–162. [Google Scholar]
- Gerdemann, S.J.; Oden, L.L.; White, J.C. Continuous production of titanium powder. In Proceedings of the Titanium Extraction and Processing, Indianapolis, IN, USA, 14–18 September 1997; pp. 14–18. [Google Scholar]
- Hansen, D.A.; Gerdemann, S.J. Producing titanium powder by continuous vapor-phase reduction. JOM 1998, 50, 56–58. [Google Scholar] [CrossRef]
- Elger, G.W.; Traut, D.E.; Slavens, G.J.; Gerdemann, S.J. Preparation of submicron titanium nitride powder by vapor-phase reactions. Metall. Trans. B 1989, 20, 493–497. [Google Scholar] [CrossRef]
- Linards, G.; Anatolijs, Z.; Juris, S.; Valts, L.; Dainis, G.; Pauls, S. Numerical and experimental study of a niobium reactor preheating for titanium production by a vapor-phase reduction. Int. J. Appl. Electromagn. Mech. 2020, 63, s93–s100. [Google Scholar]
- Vmiren, D.S.V.; Oosthuizen, S.J.; Heydenrych, M.D. Titanium production via metallothermic reduction of TiCl4 in molten salt: Problems and products. J. S. Afr. Inst. Min. Metall. 2011, 111, 141–148. [Google Scholar]
- Oosthuizen, S.J.; Swanepoel, J.J. Development status of the CSIR-Ti process. IOP Conf. Ser. Mater. Sci. Eng. 2018, 430, 012008. [Google Scholar] [CrossRef]
- Serwale, M.R.; Coetsee, T.; Fazluddin, S. Purification of crude titanium powder produced by metallothermic reduction by acid leaching. J. S. Afr. Inst. Min. Metall. 2020, 120, 349–354. [Google Scholar] [CrossRef]
- Doblin, C.; Chryss, A.; Monch, A. Titanium Powder from the TiRO™ Process. Key Eng. Mater. 2012, 520, 95–100. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, Y.; Fang, Z.Z. Selected processes for Ti production—A cursory review. In Extractive Metallurgy of Titanium; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–362. [Google Scholar]
- Doblin, C.; Freeman, D.; Richards, M. The TiRO™ process for the continuous direct production of titanium powder. Key Eng. Mater. 2013, 551, 37–43. [Google Scholar] [CrossRef]
- Doblin, C.; Cantin, G.M.D.; Gulizia, S. Processing TiRO™ powder for strip production and other powder consolidation applications. Key Eng. Mater. 2016, 704, 293–301. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Matsunaga, T.; Ono, K.; Harada, T.N.; Deura, T.N. Titanium powder prepared by magnesiothermic reduction of Ti2+ in molten salt. Metall. Mater. Trans. B 1999, 30, 403–410. [Google Scholar] [CrossRef]
- Ardani, M.R.; Hamid, S.; Lee, H.L.; Mohamed, A.R.; Ibrahim, I. Low temperature synthesis method of TiH2 powder from TiCl4 reduction with MgH2. In TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1669–1679. [Google Scholar]
- Ardani, M.R.; Hamid, S.; Lee, H.L.; Mohamed, A.R.; Ibrahim, I. Characterization of TiH2 powders produced from TiCl4-MgH2 reactions under hydrogen atmosphere. J. Mater. Eng. Perform. 2021, 30, 3243–3257. [Google Scholar] [CrossRef]
- Ardani, M.R.; Hamid, S.; Foo, D.C.Y.; Mohamed, A.R. Synthesis of Ti powder from the reduction of TiCl4 with metal hydrides in the H2 atmosphere: Thermodynamic and techno-economic analyses. Processes 2021, 9, 1567. [Google Scholar] [CrossRef]
- Kado, Y.; Kishimoto, A.; Uda, T. New smelting process for titanium: Magnesiothermic reduction of TiCl4 into liquid Bi and subsequent refining by vacuum distillation. Metall. Mater. Trans. B 2015, 46, 57–61. [Google Scholar] [CrossRef]
- Tanaka, T.; Ouchi, T.; Okabe, T.H. Lanthanothermic Reduction of TiO2. Metall. Mater. Trans. B 2020, 51, 1485–1494. [Google Scholar] [CrossRef]
- Okabe, T.H.; Oda, T.; Mitsuda, Y. Titanium powder production by preform reduction process (PRP). J. Alloys Compd. 2004, 364, 156–163. [Google Scholar] [CrossRef]
- Wan, H.; Xu, B.; Dai, Y.; Yang, B.; Liu, D.; Sen, W. Preparation of titanium powders by calciothermic reduction of titanium dioxide. J. Cent. South Univ. 2012, 19, 2434–2439. [Google Scholar] [CrossRef]
- Wan, H.; Xu, B.; Yang, B.; Sen, W.; Dai, Y. A novel process for preparing titanium powder by calcium vapor. In Proceedings of the Ti-2011: Proceedings of the 12th World Conference on Titanium, Beijing, China, 19–24 June 2011; Volume I, pp. 101–105. [Google Scholar]
- Xu, B.; Yang, B.; Jia, J.; Liu, D.; Xiong, H.; Deng, Y. Behavior of calcium chloride in reduction process of titanium dioxide by calcium vapor. J. Alloys Compd. 2013, 576, 208–214. [Google Scholar] [CrossRef]
- Jia, J.; Xu, B.; Yang, B.; Wang, D.; Liu, D. Preparation of titanium powders from TiO2 by calcium vapor reduction. JOM 2013, 65, 630–635. [Google Scholar] [CrossRef]
- Jia, J.; Xu, B.; Yang, B.; Wang, D.; Xiong, H.; Liu, D. Behavior of intermediate CaTiO3 in reduction process of TiO2 by calcium vapor. Key Eng. Mater. 2013, 551, 25–31. [Google Scholar] [CrossRef]
- Borok, B.A. Obtaining powders of alloys and steels by a complex reduction of oxide mixtures by CaH2. Trans. Cent. Res. Inst. Ferr. Met. 1965, 43, 69–80. [Google Scholar]
- Froes, F.H. The production of low-cost titanium powders. JOM 1998, 50, 41–43. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Middlemas, S.; Guo, J.; Fan, P. A new, energy-efficient chemical pathway for extracting Ti metal from Ti minerals. J. Am. Chem. Soc. 2013, 135, 18248–18251. [Google Scholar] [CrossRef]
- Okabe, T.H.; Sadoway, D.R. Metallothermic reduction as an electronically mediated reaction. J. Mater. Res. 1998, 13, 3372–3377. [Google Scholar] [CrossRef]
- Okabe, T.H.; Waseda, Y. Producing titanium through an electronically mediated reaction. JOM 1997, 49, 28–32. [Google Scholar] [CrossRef]
- Park, I.; Abiko, T.; Okabe, T.H. Production of titanium powder directly from TiO2 in CaCl2 through an electronically mediated reaction (EMR). J. Phys. Chem. Solids 2005, 66, 410–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.Z.; Xia, Y.; Huang, Z.; Lefler, H.; Zhang, T.; Sun, P.; Free, M.L.; Guo, J. A novel chemical pathway for energy efficient production of Ti metal from upgraded titanium slag. Chem. Eng. J. 2016, 286, 517–527. [Google Scholar] [CrossRef]
- Xia, Y.; Fang, Z.Z.; Zhang, Y.; Lefler, H.; Zhang, T.; Sun, P.; Huang, Z. Hydrogen assisted magnesiothermic reduction (HAMR) of commercial TiO2 to produce titanium powder with controlled morphology and particle size. Mater. Trans. 2017, 58, 355–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.Z.; Sun, P.; Zhang, T.; Xia, Y.; Zhou, C.; Huang, Z. Thermodynamic destabilization of Ti-O solid solution by H2 and deoxygenation of Ti using Mg. J. Am. Chem. Soc. 2016, 138, 6916–6919. [Google Scholar] [CrossRef] [PubMed]
- Lefler, H.; Fang, Z.Z.; Zhang, Y.; Sun, P.; Xia, Y. Mechanisms of hydrogen-assisted magnesiothermic reduction of TiO2. Metall. Mater. Trans. B 2018, 49, 2998–3006. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Zhang, Y.; Fang, Z.Z.; Zheng, S.; Sun, P.; Xia, Y.; Li, P.; Zhang, Y.; Zou, X. An investigation of the reduction of TiO2 by Mg in H2 atmosphere. Chem. Eng. Sci. 2019, 195, 484–493. [Google Scholar] [CrossRef]
- Subrahmanyam, J.; Vijayakumar, M. Self-propagating high-temperature synthesis. J. Mater. Sci. 1992, 27, 6249–6273. [Google Scholar] [CrossRef]
- Merzhanov, A.G. The chemistry of self-propagating high-temperature synthesis. J. Mater. Chem. 2004, 14, 1779–1786. [Google Scholar] [CrossRef]
- Fan, S.; Dou, Z.; Zhang, T.; Liu, Y.; Niu, L. Deoxidation mechanism in reduced titanium powder prepared by multistage deep reduction of TiO2. Metall. Mater. Trans. B 2019, 50, 282–290. [Google Scholar] [CrossRef]
- Fan, S.; Dou, Z.; Zhang, T.; Yan, J.; Niu, L. Effect of sample preparation pressure on transformation law of low-valent titanium oxide in a multi-stage reduction process. Metals 2020, 10, 1259. [Google Scholar] [CrossRef]
- Fan, S.; Dou, Z.; Zhang, T.; Yan, J. Self-propagating reaction mechanism of Mg-TiO2 system in preparation process of titanium powder by multi-stage reduction. Rare Metals 2021, 40, 2645–2656. [Google Scholar] [CrossRef]
- Lazarev, P.A.; Sytschev, A.E.; Boyarchenko, O.D.; Aborkin, A.V. Self-propagating high-temperature synthesis in the Ti-Al-Si system. Inorg. Mater. 2021, 57, 1201–1207. [Google Scholar] [CrossRef]
- Lazarev, P.A.; Sychev, A.E.; Kochetov, N.A.; Sachkova, N.V. Preparation of an Al-Ti-Mg composite by self-propagating high-temperature synthesis. Inorg. Mater. 2021, 57, 324–329. [Google Scholar] [CrossRef]
- Cheng, C.; Dou, Z.; Zhang, T.; Liu, Y.; Niu, L. Distribution and control mechanism of Al and O residuals in ferrotitanium prepared by aluminothermic reduction with insufficient Al. JOM 2019, 71, 809–814. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, T.; Dou, Z. Formation mechanism and distribution of Al and O in the ferrotitanium with different Ti contents prepared by thermite method. JOM 2019, 71, 3584–3589. [Google Scholar] [CrossRef]
- Cheng, C.; Dou, Z.; Zhang, T.; Su, J.; Zhang, H.; Liu, Y.; Niu, L. Sulfur distribution in preparation of high titanium ferroalloy by thermite method with different CaO additions. Rare Metals 2019, 38, 793–799. [Google Scholar] [CrossRef]
- Cheng, C.; Dou, Z.; Zhang, T.; Su, J.; Zhang, H.; Liu, Y.; Niu, L. Oxygen content of high ferrotitanium prepared by thermite method with different melt separation temperatures. Rare Metals 2019, 38, 892–898. [Google Scholar] [CrossRef]
- Zhou, X.; Dou, Z.; Zhang, T.; Yan, J.; Yan, J. Preparation of low-oxygen Ti powder from TiO2 through combining self-propagating high temperature synthesis and electrodeoxidation. Trans. Nonferr. Met. Soc. China 2022, 32, 3469–3477. [Google Scholar] [CrossRef]
- Nersisyan, H.; Kwon, S.C.; Ri, V.; Lee, Y.J.; Yoo, B.U.; Lee, J.H. Shape-controlled synthesis of titanium microparticles using calciothermic reduction concept. J. Solid State Chem. 2018, 267, 13–21. [Google Scholar] [CrossRef]
- Wan, H.; Xu, B.; Yang, B.; Liu, D.; Dai, Y. Ti powder preparation by calciothermic reduction of TiO2 in vacuum. Chin. J. Vac. Sci. Technol. 2012, 32, 539–544. [Google Scholar]
- Wan, H.; Xu, B.; Dai, Y.; Yang, B.; Liu, D. Research progress on preparation of titanium powders by calciothermic reduction. Funct. Mater. 2012, 43, 700–703. [Google Scholar]
- Suzuki, R.O.; Inoue, S. Calciothermic reduction of titanium oxide in molten CaCl2. Metall. Mater. Trans. B 2003, 34, 277–285. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yoshida, M.; Matsuura, S.; Natsui, S.; Tsuji, E.; Habazaki, H.; Suzuki, R.O. Rapid reduction of titanium dioxide nano-particles by reduction with a calcium reductant. J. Phys. Chem. Solids 2014, 75, 1041–1048. [Google Scholar] [CrossRef]
- Bolivar, R.; Friedrich, B. Magnesiothermic reduction from titanium dioxide to produce titanium powder. J. Sust. Metall. 2019, 5, 219–229. [Google Scholar] [CrossRef]
- Tanaka, T.; Ouchi, T.; Okabe, T.H. Magnesiothermic reduction of TiO2 assisted by LaCl3. J. Sust. Metall. 2020, 6, 667–679. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, J.; Yang, B.; Chen, X.; Wang, D.; Kong, L. Simulation on calciothermic reduction process of titanium dioxide. In Proceedings of the 5th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 16–20 February 2014; pp. 483–490. [Google Scholar]
- Bayat, O.; Khavandi, A.R.; Ghasemzadeh, R. Effect of blend granulometry on calciothermic reduction of TiO2. Int. J. Self-Propag. High-Temp. Synth. 2012, 21, 151–155. [Google Scholar] [CrossRef]
- Lee, J.H.; Nersisyan, H.; Huynh, T.; Lim, K.; Kim, W.; Choi, W. Combustion-alumino-magnesiothermic reduction of TiO2 to produce a Ti-rich ingot. Metall. Mater. Trans. B 2022, 53, 3147–3158. [Google Scholar] [CrossRef]
- Vershinnikov, V.I.; Ignat’eva, T.I.; Aleshin, V.V.; Mikhailov, Y.M. Fine Ti powders through metallothermic reduction in TiO2-Mg-Ca mixtures. Int. J. Self-Propag. High-Temp. Synth. 2018, 27, 55–59. [Google Scholar] [CrossRef]
- Yang, G.; Xu, B.; Wan, H.; Wang, F.; Yang, B.; Wang, Z. Effect of CaCl2 on microstructure of calciothermic reduction products of Ti2O3 to prepare porous titanium. Metals 2018, 8, 698. [Google Scholar] [CrossRef]
- Takeda, O.; Okabe, T.H. Fundamental study on magnesiothermic reduction of titanium dichloride. Metall. Mater. Trans. B 2006, 37, 823–830. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Y.; Feng, N. Cleaner production of Ti powder by a two-stage aluminothermic reduction process. JOM 2017, 69, 1795–1800. [Google Scholar] [CrossRef]
- Song, M.; Yang, Y.; Zhao, H.; Xiang, M.; Zhu, Q.; Jia, J.; Hu, C.; Yue, F. Synthesis of TiCl2 powders through reactive gas phase infiltration in a fluidized bed reactor. Particuology 2021, 57, 95–103. [Google Scholar] [CrossRef]
- Takeda, O.; Okabe, T.H. Fundamental study on synthesis and enrichment of titanium subchloride. J. Alloys Compd. 2008, 457, 376–383. [Google Scholar] [CrossRef]
- Zhao, K.; Feng, N.; Wang, Y. Fabrication of Ti-Al intermetallics by a two-stage aluminothermic reduction process using Na2TiF6. Intermetallics 2017, 85, 156–162. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y. Preparation of Ti-6Al-4V alloy powder by aluminothermic reduction. In Nanoscience and Nanotechnology in Advanced Composites Symposium; Springer: Cham, Switzerland, 2020; pp. 1681–1689. [Google Scholar]
- Oki, T.; Inoue, H. Reduction of titanium dioxide by calcium in hot cathode spot. Mem. Fac. Eng. Nagoya Univ. 1968, 19, 164–166. [Google Scholar]
- Chen, G.Z.; Fray, D.J.; Farthing, T. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 2000, 407, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Fray, D.J.; Chen, G.Z. Reduction of titanium and other metal oxides using electrodeoxidation. Mater. Sci. Technol. 2004, 20, 295–300. [Google Scholar] [CrossRef]
- Gruber, H.; Krautz, E. Magnetoresistance and conductivity in the binary system titanium-oxygen. II. Semiconductive Titanium Oxides. Phys. Status Solidi (A) 1983, 75, 511–518. [Google Scholar] [CrossRef]
- Schwandt, C.; Fray, D.J. Determination of the kinetic pathway in the electrochemical reduction of titanium dioxide in molten calcium chloride. Electrochim. Acta 2005, 51, 66–76. [Google Scholar] [CrossRef]
- Schwandt, C.; Doughty, G.R.; Fray, D.J. The FFC-Cambridge process for titanium metal winning. Key Eng. Mater. 2010, 436, 13–25. [Google Scholar] [CrossRef]
- Schwandt, C. Understanding the electro-deoxidation of titanium dioxide to titanium metal via the FFC-Cambridge process. Trans. Inst. Min. Metall. Sect. C-Miner. Process. Extr. Metall. 2013, 122, 213–218. [Google Scholar] [CrossRef]
- Ma, M.; Wang, D.; Wang, W.; Hu, X.; Jin, X.; Chen, G.Z. Extraction of titanium from different titania precursors by the FFC Cambridge process. J. Alloys Compd. 2006, 420, 37–45. [Google Scholar] [CrossRef]
- Benson, L.L.; Mellor, I.; Jackson, M. Direct reduction of synthetic rutile using the FFC process to produce low-cost novel titanium alloys. J. Mater. Sci. 2016, 51, 4250–4261. [Google Scholar] [CrossRef]
- Bhagat, R.; Jackson, M.; Inman, D.; Dashwood, R. The production of Ti-Mo alloys from mixed oxide precursors via the FFC Cambridge process. J. Electrochem. Soc. 2008, 155, e63–e69. [Google Scholar] [CrossRef]
- Yan, X.; Fray, D.J. Electrosynthesis of NbTi and Nb3Sn superconductors from oxide precursors in CaCl2-based melts. Adv. Funct. Mater. 2005, 15, 1757–1761. [Google Scholar] [CrossRef]
- Jackson, B.; Jackson, M.; Dye, D.; Inman, D.; Dashwood, R. Production of NiTi via the FFC Cambridge process. J. Electrochem. Soc. 2008, 155, e171–e177. [Google Scholar] [CrossRef]
- Ono, K.; Miyazaki, S. Study on the limit of deoxidation of titanium and the reduction of titanium dioxide by saturated calcium vapors. J. Jpn. Inst. Met. Mater. 1985, 49, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Suzuki, R.O. A new concept for producing Ti sponge: Calciothermic reduction. JOM 2002, 54, 59–61. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Fukui, S. Reduction of TiO2 in molten CaCl2 by Ca deposited during CaO electrolysis. Mater. Trans. 2004, 45, 1665–1671. [Google Scholar] [CrossRef]
- Suzuki, R.O. Calciothermic reduction of TiO2 and in situ electrolysis of CaO in the molten CaCl2. J. Phys. Chem. Solids 2005, 66, 461–465. [Google Scholar] [CrossRef]
- Kobayashi, K.; Oka, Y.; Suzuki, R.O. Influence of current density on the reduction of TiO2 in molten salt (CaCl2 + CaO). Mater. Trans. 2009, 50, 2704–2708. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Yashima, Y.; Suzuki, N.; Ahmadi, E.; Natsui, S.; Kikuchi, T. Titanium production via titanium sulfide. MATEC Web Conf. 2020, 321, 07003. [Google Scholar] [CrossRef]
- Francois, G. Method for Electrowinning of Titanium Metal or Alloy from Titanium Oxide Containing Compound in the Liquid State. U.S. Patent 7504017B2, 17 March 2009. [Google Scholar]
- Zhao, K.; Wang, Y.; Gao, F. Electrochemical extraction of titanium from carbon-doped titanium dioxide precursors by electrolysis in chloride molten salt. Ionics 2019, 25, 6107–6114. [Google Scholar] [CrossRef]
- Mohanty, J.; Behera, P.K. Use of pre-treated TiO2 as cathode material to produce Ti metal through molten salt electrolysis. Trans. Indian Inst. Met. 2019, 72, 859–865. [Google Scholar] [CrossRef]
- Eugene, W. Cell Feed Material for the Production of Titanium. U.S. Patent 2868703A, 13 January 1959. [Google Scholar]
- Popov, B.N.; Kimble, M.C.; White, R.E.; Wendt, H. Electrochemical behaviour of titanium (II) and titanium (III) compounds in molten lithium chloride/potassium chloride eutectic melts. J. Appl. Electrochem. 1991, 21, 351–357. [Google Scholar] [CrossRef]
- Jiao, S.; Zhu, H. Novel metallurgical process for titanium production. J. Mater. Res. 2006, 21, 2172–2175. [Google Scholar] [CrossRef]
- Zhu, H.; Jiao, S.; Xiao, J.; Zhu, J. Titanium production through electrolysis of titanium oxycarbide consumable anode—The USTB process. In Extractive Metallurgy of Titanium; Elsevier: Amsterdam, The Netherlands, 2020; pp. 315–329. [Google Scholar]
- Jiao, S.; Ning, X.; Huang, K.; Zhu, H. Electrochemical dissolution behavior of conductive TiCxO1-x solid solutions. Pure Appl. Chem. 2010, 82, 1691–1699. [Google Scholar] [CrossRef]
- Jiao, S.; Zhu, H. Electrolysis of Ti2CO solid solution prepared by TiC and TiO2. J. Alloys Compd. 2007, 438, 243–246. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Jiao, S.; Zhu, H. Producing metallic titanium through electro-refining of titanium nitride anode. Electrochem. Commun. 2013, 35, 135–138. [Google Scholar] [CrossRef]
- Wang, Q.; Song, J.; Wu, J. A new consumable anode material of titanium oxycarbonitride for the USTB titanium process. Phys. Chem. Chem. Phys. 2014, 16, 8086–8091. [Google Scholar] [CrossRef]
- Withers, J.C.; Loutfy, R.O. Thermal and Electrochemical Process for Metal Production. European Patent 1957683B1, 12 August 2008. [Google Scholar]
- Fray, D.J.; Jiao, S. Treatment of Titanium Ores. U.S. Patent 9181604B2, 10 November 2015. [Google Scholar]
- Withers, J.C. Production of titanium powder by an electrolytic method and compaction of the powder. In Titanium Powder Metallurgy; Butterworth-Heinemann: Waltham, MA, USA, 2015; pp. 33–49. [Google Scholar]
- Pal, U.B.; Woolley, D.E.; Kenney, G.B. Emerging SOM technology for the green synthesis of metals from oxides. JOM 2001, 53, 32–35. [Google Scholar] [CrossRef]
- Pal, U.B.; Powell, A.C. The use of solid-oxide-membrane technology for electrometallurgy. JOM 2007, 59, 44–49. [Google Scholar] [CrossRef]
- Suput, M.; Delucas, R.; Pati, S.; Ye, G.S.; Pal, U.; Powell, A.C. Solid oxide membrane (SOM) technology for environmentally sound production of titanium. In Proceedings of the Sohn International Symposium on Advanced Processing of Metals and Materials, San Diego, CA, USA, 27–31 August 2006; pp. 273–283. [Google Scholar]
- Zou, X.; Lu, X.; Li, C.; Zhou, Z. A direct electrochemical route from oxides to Ti-Si intermetallics. Electrochim. Acta 2010, 55, 5173–5179. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Lu, X. Extraction of Ti and Ti alloy from titaniferous residue using SOM process. Adv. Mater. Res. 2012, 550–553, 1811–1816. [Google Scholar] [CrossRef]
- Ye, X.; Lu, X.; Li, C.; Ding, W.; Zou, X.; Gao, Y.; Zhong, Q. Preparation of Ti-Fe based hydrogen storage alloy by SOM method. Int. J. Hydrogen Energy 2011, 36, 4573–4579. [Google Scholar] [CrossRef]
- Li, S.; Zou, X.; Zheng, K.; Lu, X.; Xu, Q.; Chen, C.; Zhou, Z. Direct production of TiAl3 from Ti/Al-containing oxides precursors by solid oxide membrane (SOM) process. J. Alloys Compd. 2017, 727, 1243–1252. [Google Scholar] [CrossRef]
- Zou, X.; Lu, X.; Li, C.; Zhao, B. Electrochemical extraction of Fe-Ti-Si alloys direct from Ti bearing compound ores. Trans. Inst. Min. Metall. Sect. C-Miner. Process. Extr. Metall. 2011, 120, 118–124. [Google Scholar] [CrossRef]
- Xia, Y.; Lefler, H.D.; Fang, Z.Z.; Zhang, Y.; Sun, P. Energy consumption of the Kroll and HAMR processes for titanium production. In Extractive Metallurgy of Titanium; Elsevier: Amsterdam, The Netherlands, 2020; pp. 389–410. [Google Scholar]
- Norgate, T.E.; Jahanshahi, S.; Rankin, W.J. Assessing the environmental impact of metal production processes. J. Clean Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
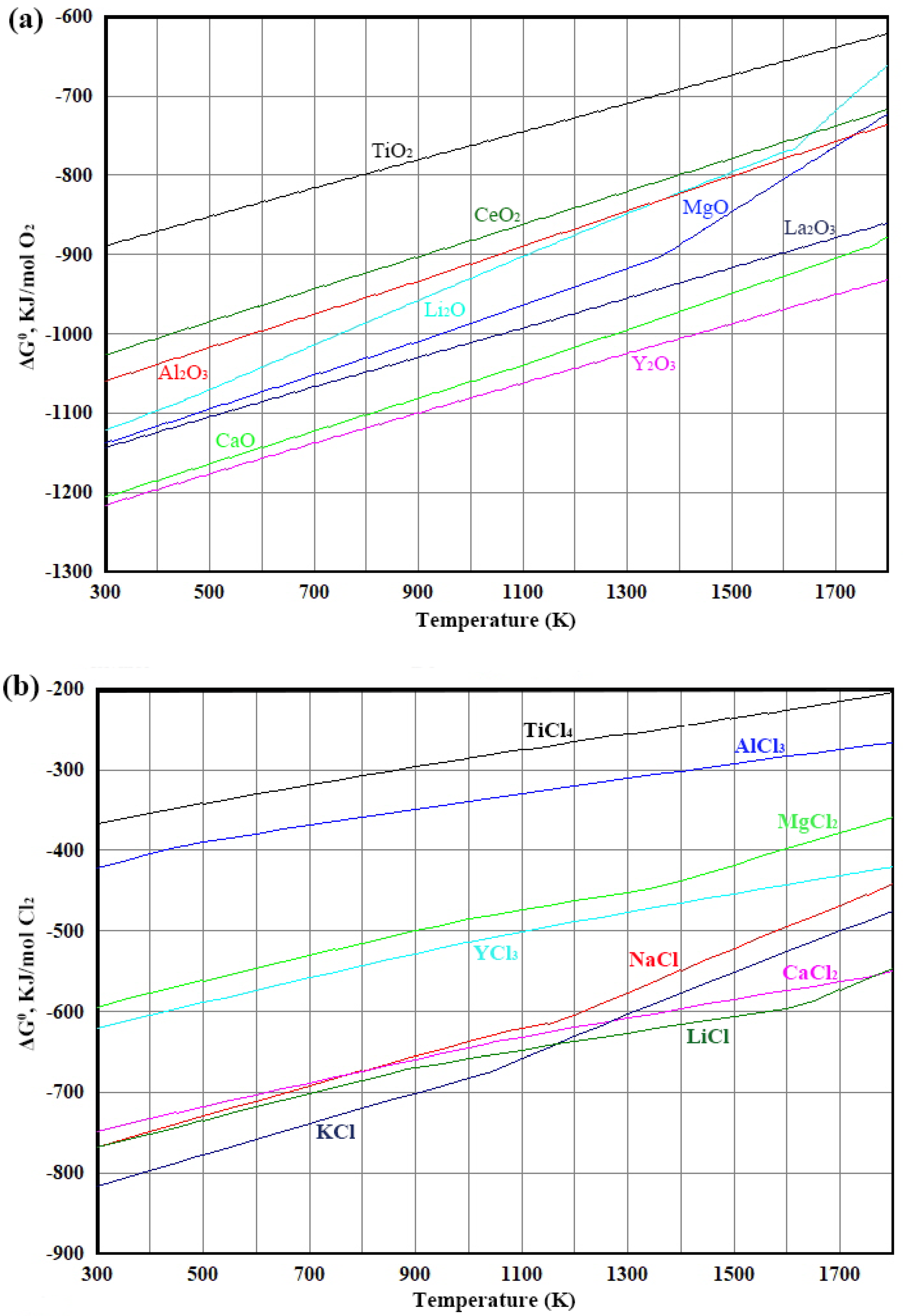
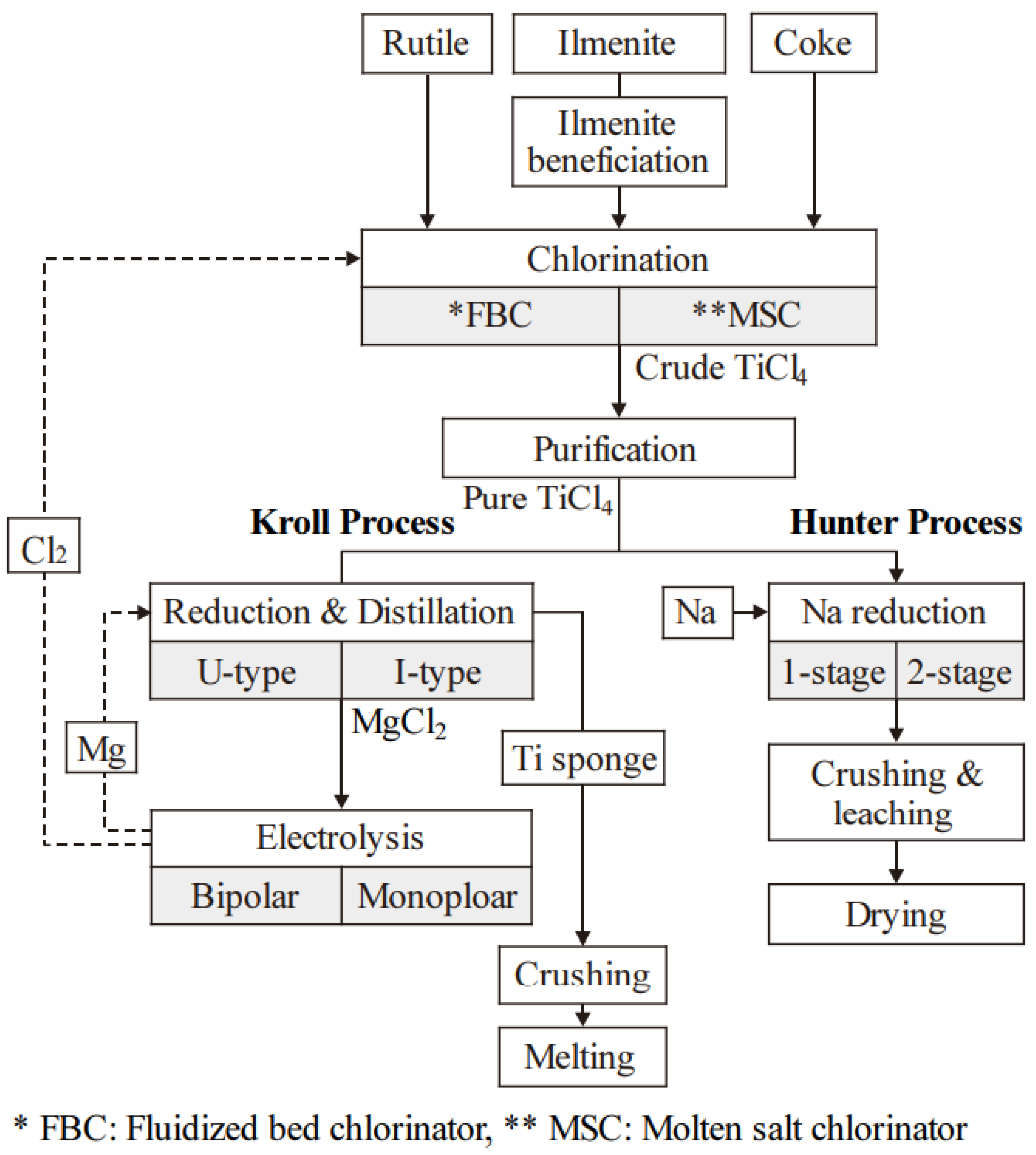
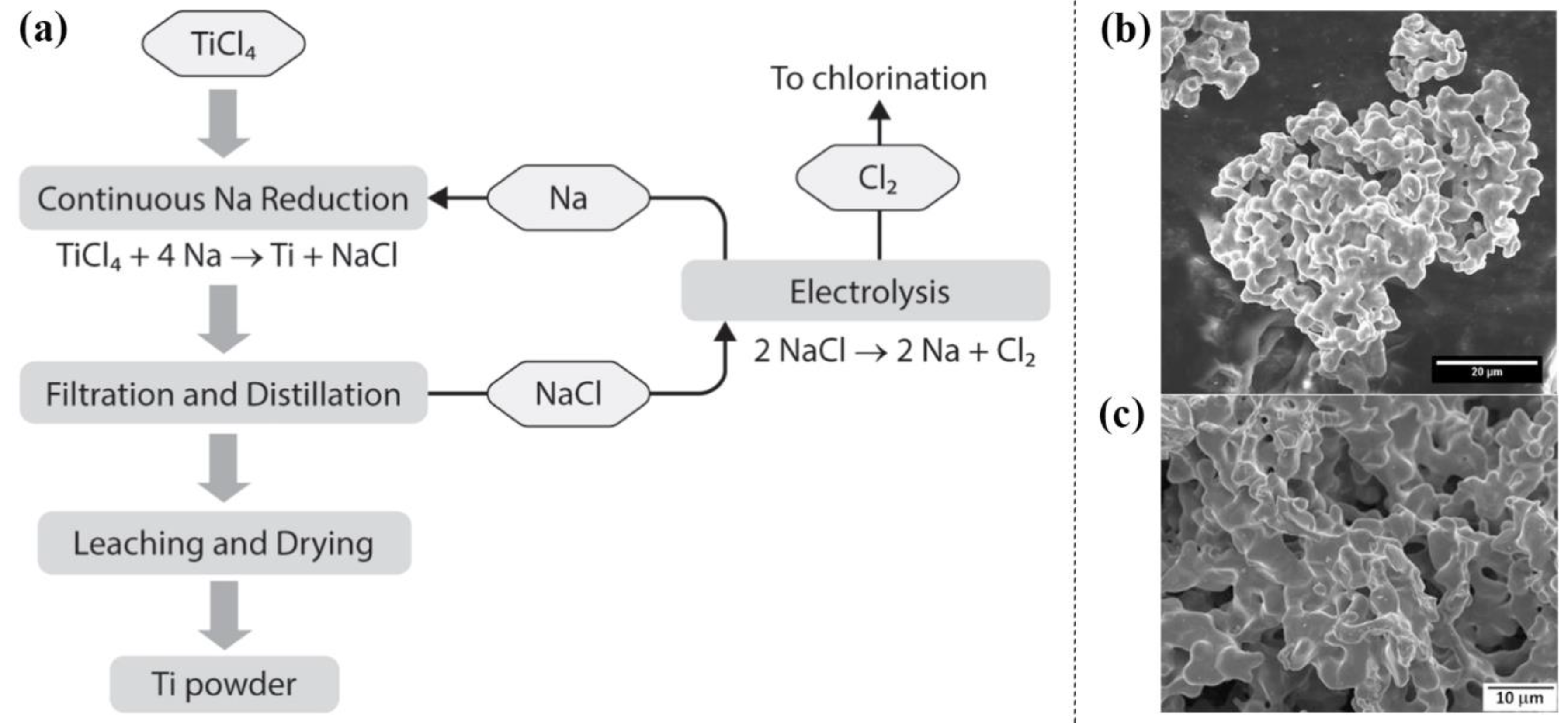

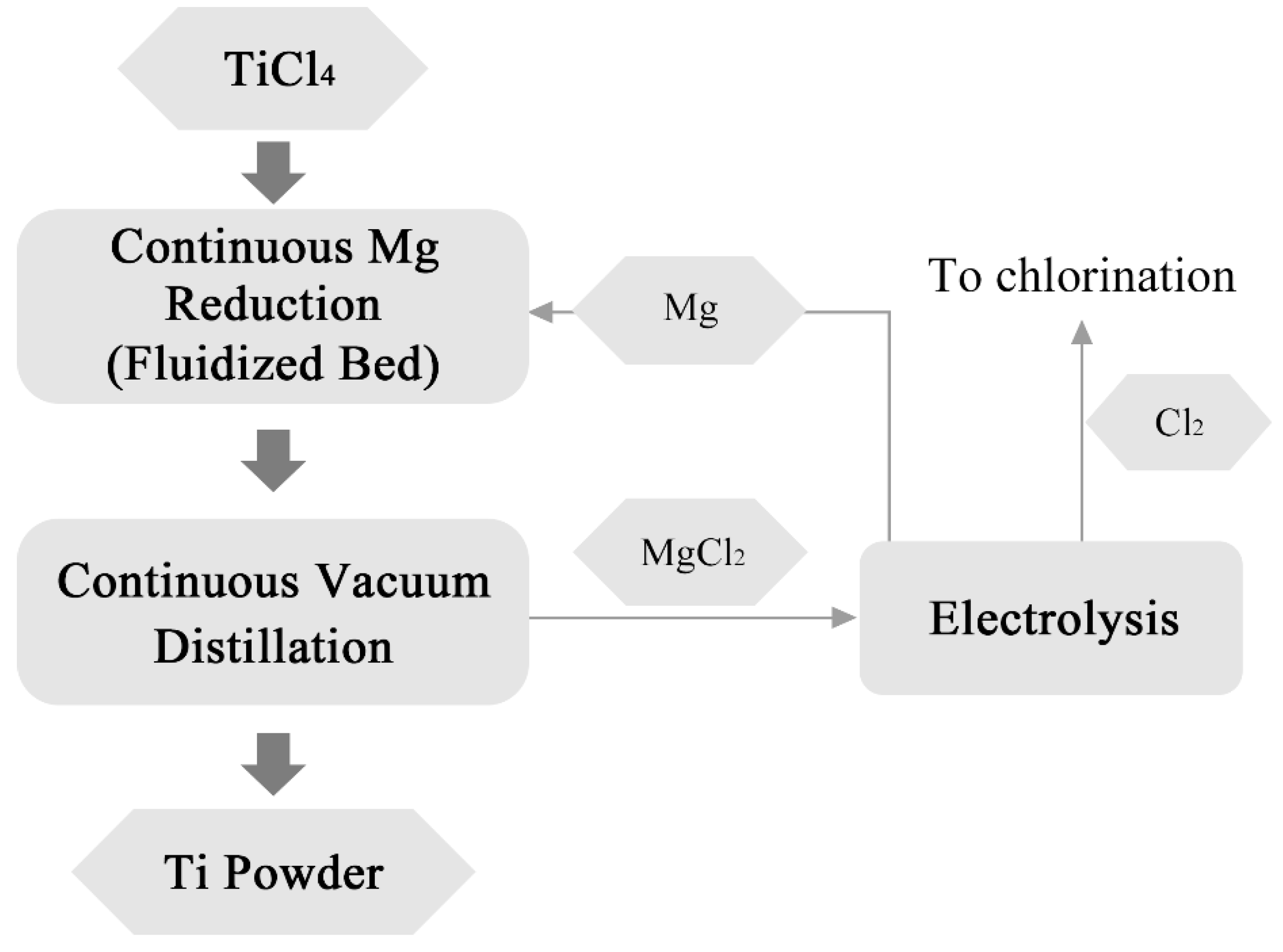
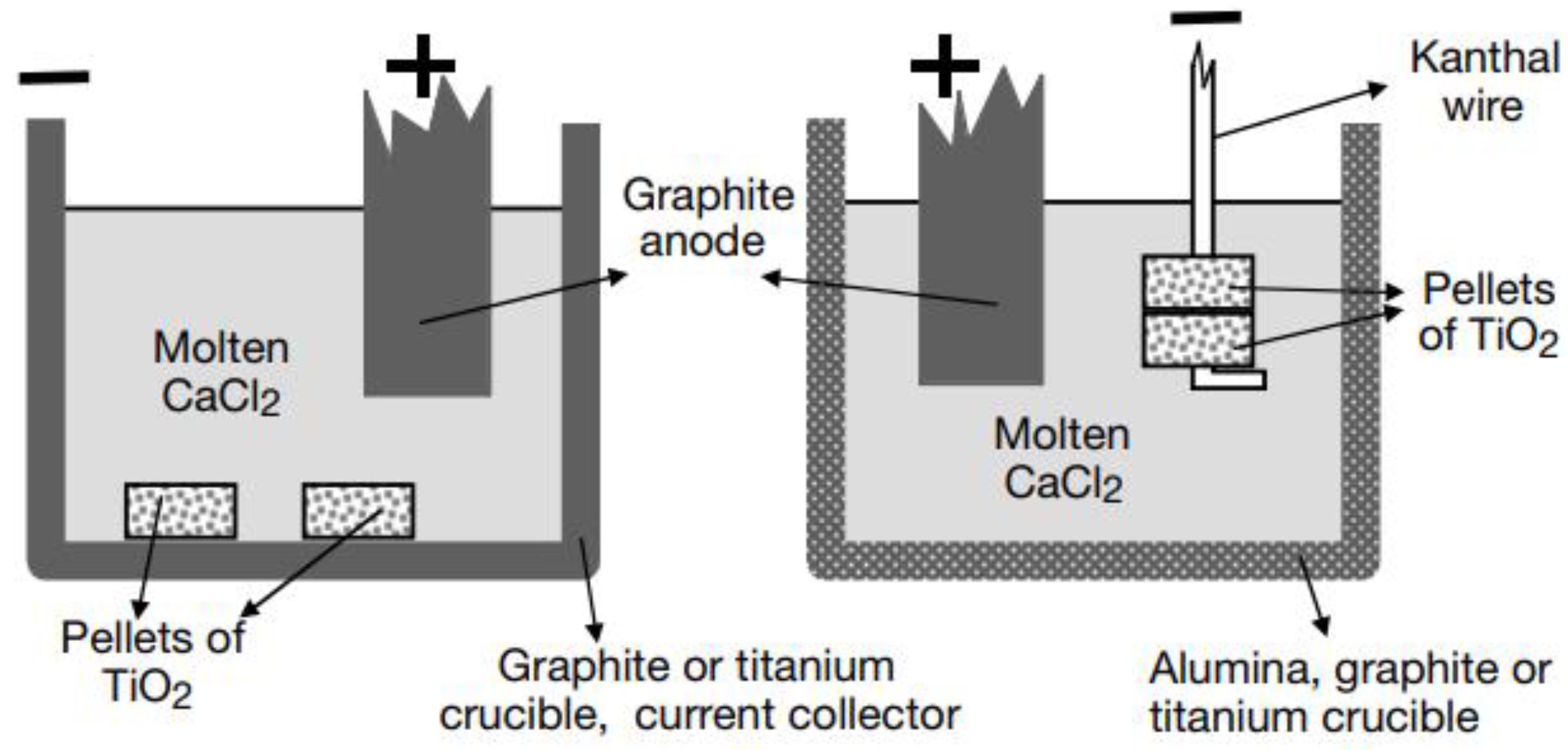
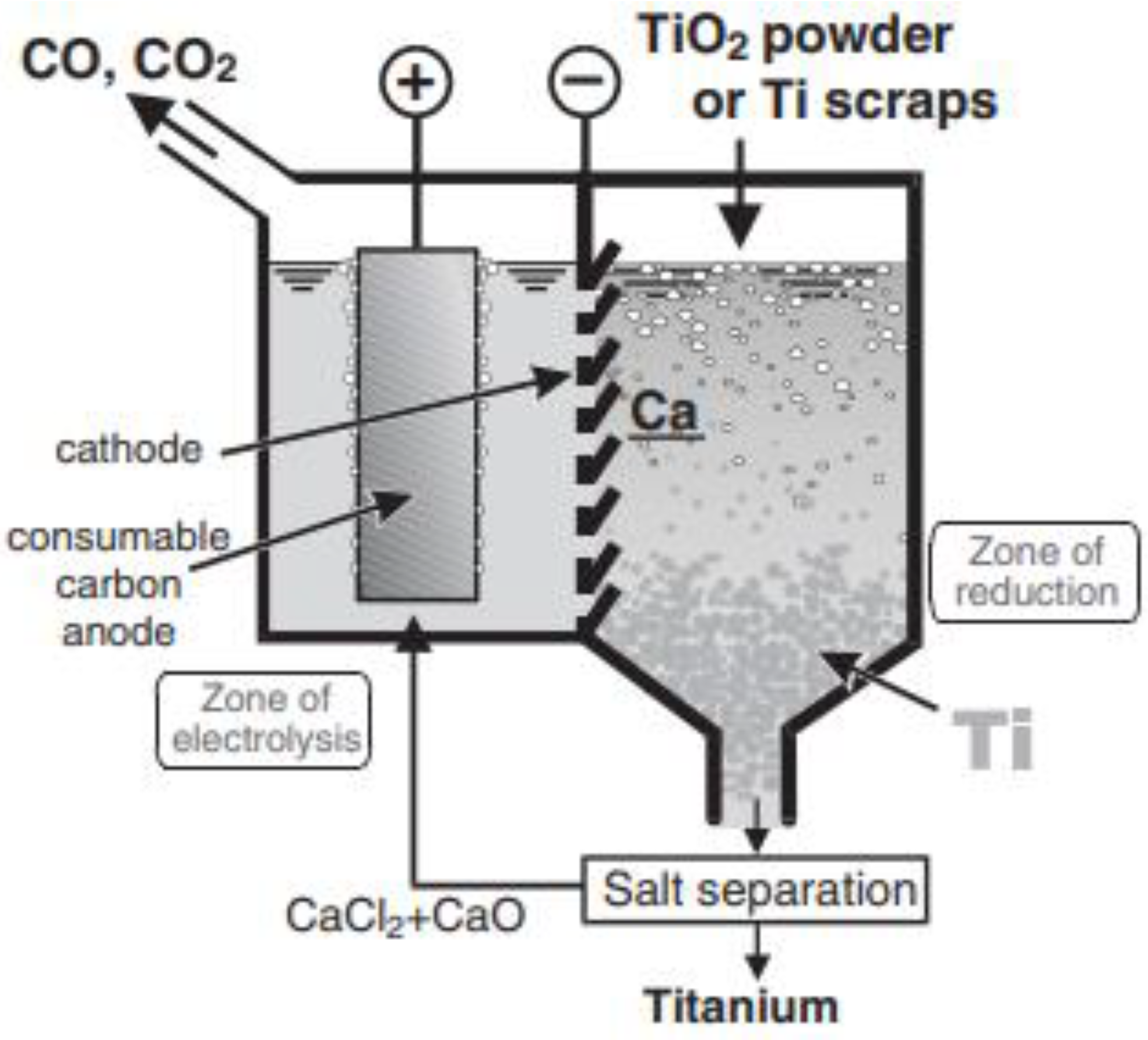
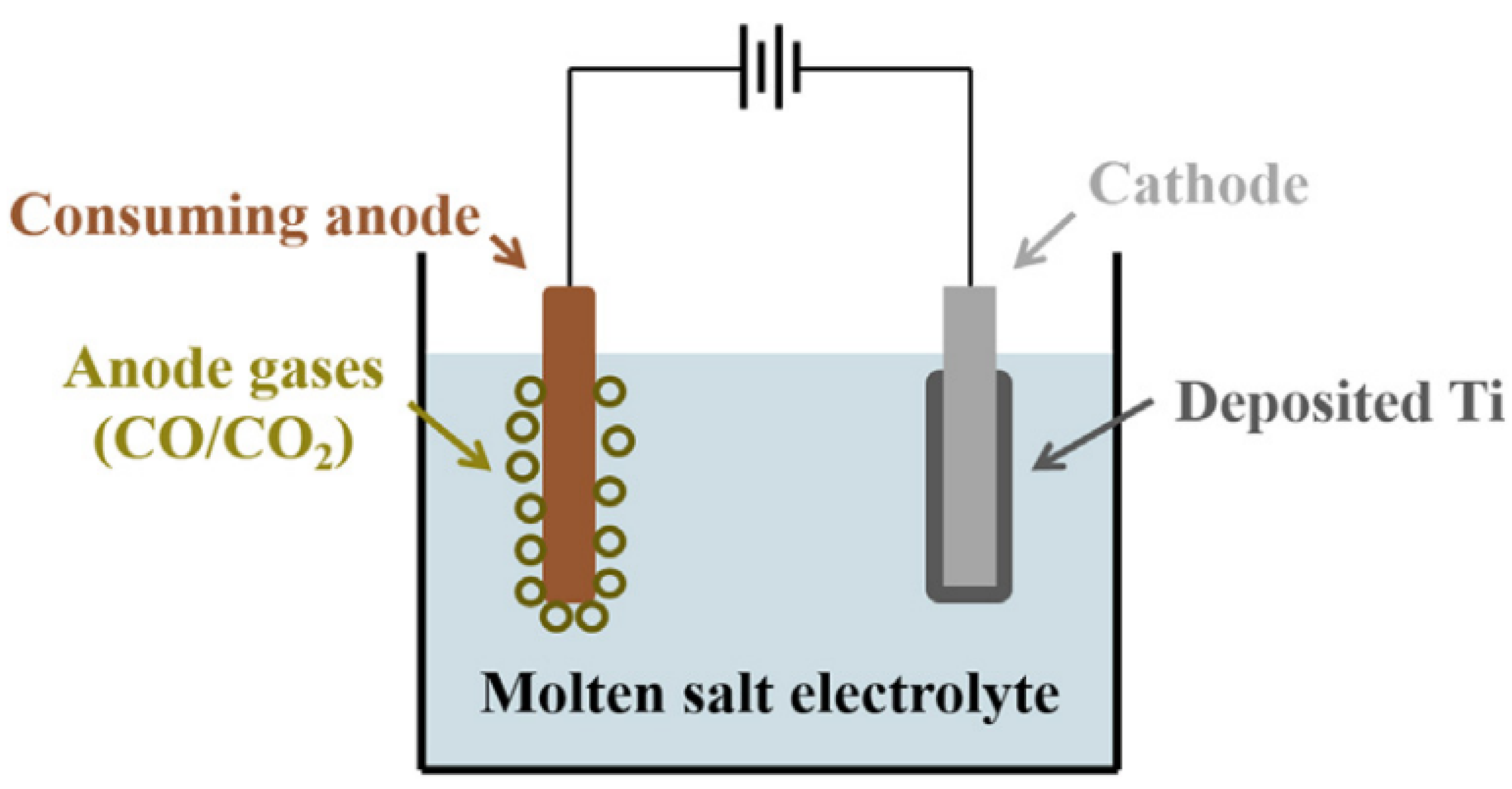
| Classification | Processes | Precursors | Core Reactions | Advantages | Disadvantages | Development Date | Refs. |
|---|---|---|---|---|---|---|---|
| Thermal reduction | Hunter process | TiCl4 | Titanium powder with low oxygen content and metallic impurities | Low productivity; expensive reductant; high energy consumption | 1910 | [23] | |
| Kroll process | TiCl4 | Titanium powder with low oxygen content and metallic impurities | Low productivity; high energy consumption | 1940 | [24] | ||
| ARC process | TiCl4 | | Continuous production; controllable reaction speed | Expensive reductant | 1997 | [44] | |
| Vapor-phase reduction process | TiCl4 | Continuous production | High temperature; titanium powder with high oxygen content or high magnesium and chlorine content | 1998 | [45] | ||
| Armstrong process | TiCl4 | Continuous production; titanium powder with excellent compressibility and denseness | Expensive reductant; residual impurities | 2003 | [38] | ||
| CSIR-Ti process | TiCl4 | | Continuous production; CP 4 Grade titanium powder | Oxygen content is difficult to control | 2011 | [48] | |
| TiRO process | TiCl4 | Continuous production; CP 2 Grade titanium powder | Titanium powder with high oxygen content | 2011 | [51] | ||
| EMR process | TiO2 | | Continuous production; titanium powder with high purity | Complicated process; difficult separation of metal and salt | 1997 | [71] | |
| MHR process | TiO2 | Single-step reaction | High energy consumption and pollution | 1998 | [68] | ||
| PRP process | TiO2 | High reduction efficiency; titanium powder with high purity | Expensive reductant | 2004 | [61] | ||
| HAMR process | TiO2 | | Titanium powder with low oxygen content | High temperature; high energy consumption | 2016 | [73] | |
| SHS process | TiO2 | Low demand for raw materials; high efficiency | Uncontrollable process | 2019 | [80] | ||
| Electrolysis | FFC process | TiO2 | | Semi-continuous production; titanium powder with low oxygen content | Low current efficiency; slow oxygen diffusion; difficult separation of metal and salt | 2000 | [109] |
| SOM process | TiO2-containing flux | | Oxygen or water vapor as the major by-product | Low production efficiency | 2001 | [142] | |
| OS process | TiO2 | | Semi-continuous production; titanium powder with low oxygen content | Low current efficiency; titanium powder is easily contaminated | 2002 | [121] | |
| USTB process | TiCxO1−x (0 < x < 1) | | Semi-continuous production; titanium powder with high purity | Low current efficiency | 2006 | [131] | |
| MER process | TixOyC | | Semi-continuous production; titanium powder with high purity | Carbon contamination; low current efficiency | 2008 | [137] | |
| QIT process | TiO2 | Titanite can be used as raw materials | High impurities content | 2009 | [126] | ||
| Chinuka process | Ti2CO | | Titanite can be used as raw materials | High impurities content | 2015 | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Lv, M.; Mao, L.; Duan, B.; Yang, Y.; Chen, G.; Lu, X.; Li, C. Research Progress of Titanium Sponge Production: A Review. Metals 2023, 13, 408. https://doi.org/10.3390/met13020408
Feng Q, Lv M, Mao L, Duan B, Yang Y, Chen G, Lu X, Li C. Research Progress of Titanium Sponge Production: A Review. Metals. 2023; 13(2):408. https://doi.org/10.3390/met13020408
Chicago/Turabian StyleFeng, Qisheng, Mingrui Lv, Lu Mao, Baohua Duan, Yuchen Yang, Guangyao Chen, Xionggang Lu, and Chonghe Li. 2023. "Research Progress of Titanium Sponge Production: A Review" Metals 13, no. 2: 408. https://doi.org/10.3390/met13020408
APA StyleFeng, Q., Lv, M., Mao, L., Duan, B., Yang, Y., Chen, G., Lu, X., & Li, C. (2023). Research Progress of Titanium Sponge Production: A Review. Metals, 13(2), 408. https://doi.org/10.3390/met13020408









