Experimental Investigation on Ignition Effects of Fuel Tank Impacted by Bi2O3-Reinforced PTFE/Al Reactive Material Projectile
Abstract
1. Introduction
2. Experimental Section
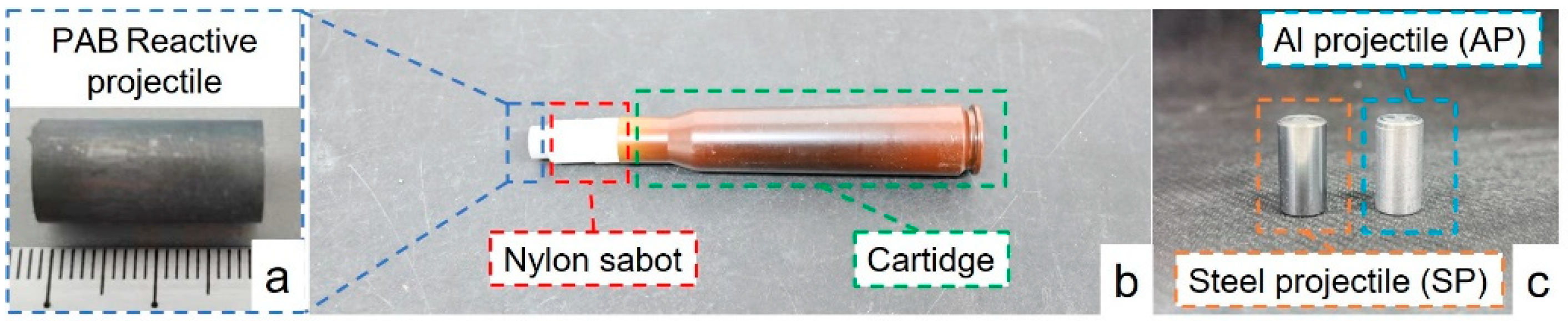
2.1. Specimens’ Preparation
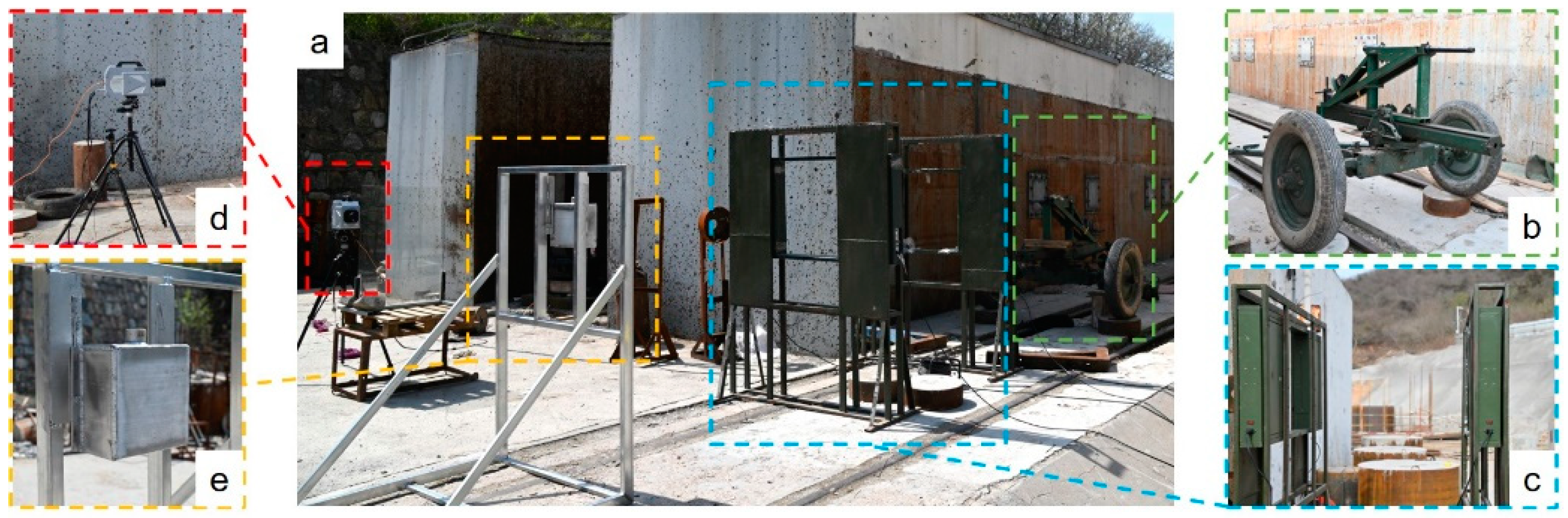
2.2. Test System Setup
3. Experimental Results and Phenomenon Description
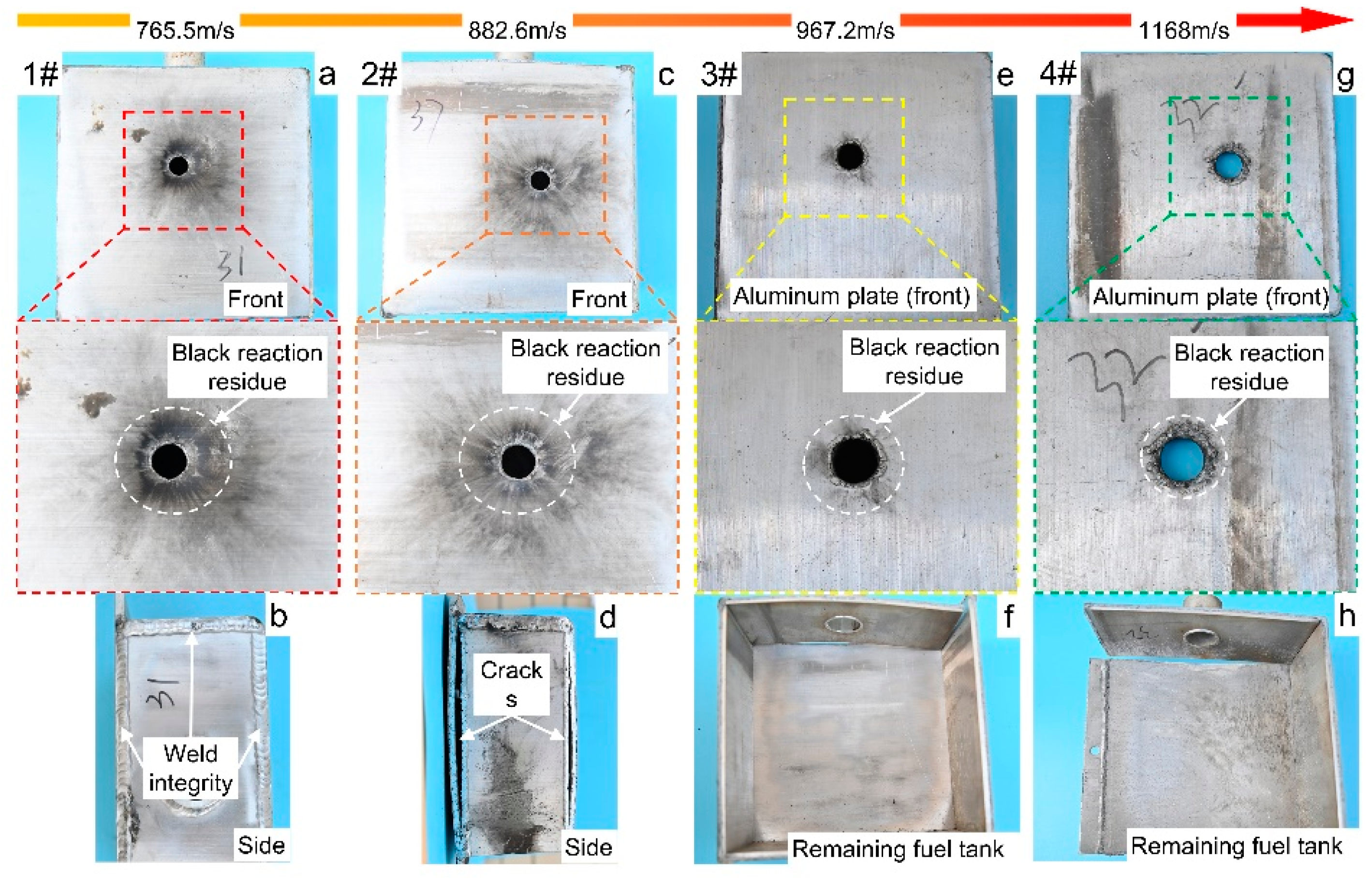
3.1. The PABs Impact the Full Fuel Tank
3.2. The APs and SP Impact the Full Fuel Tank
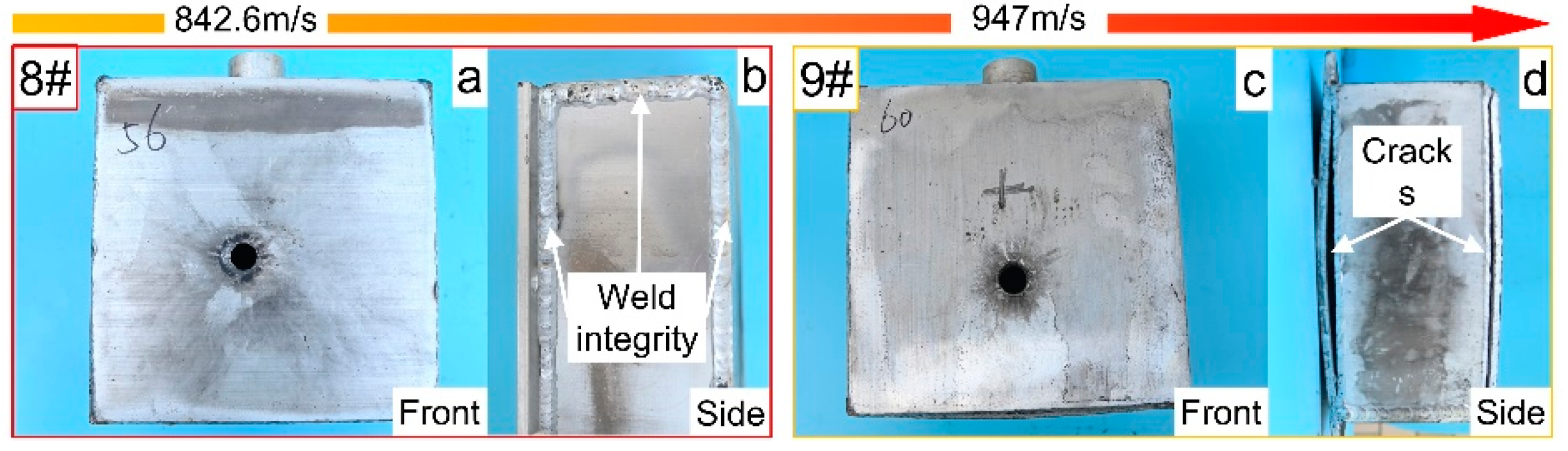
3.3. The PABs Impact the Fuel Tank Filled with 50% Kerosene
4. Discussion
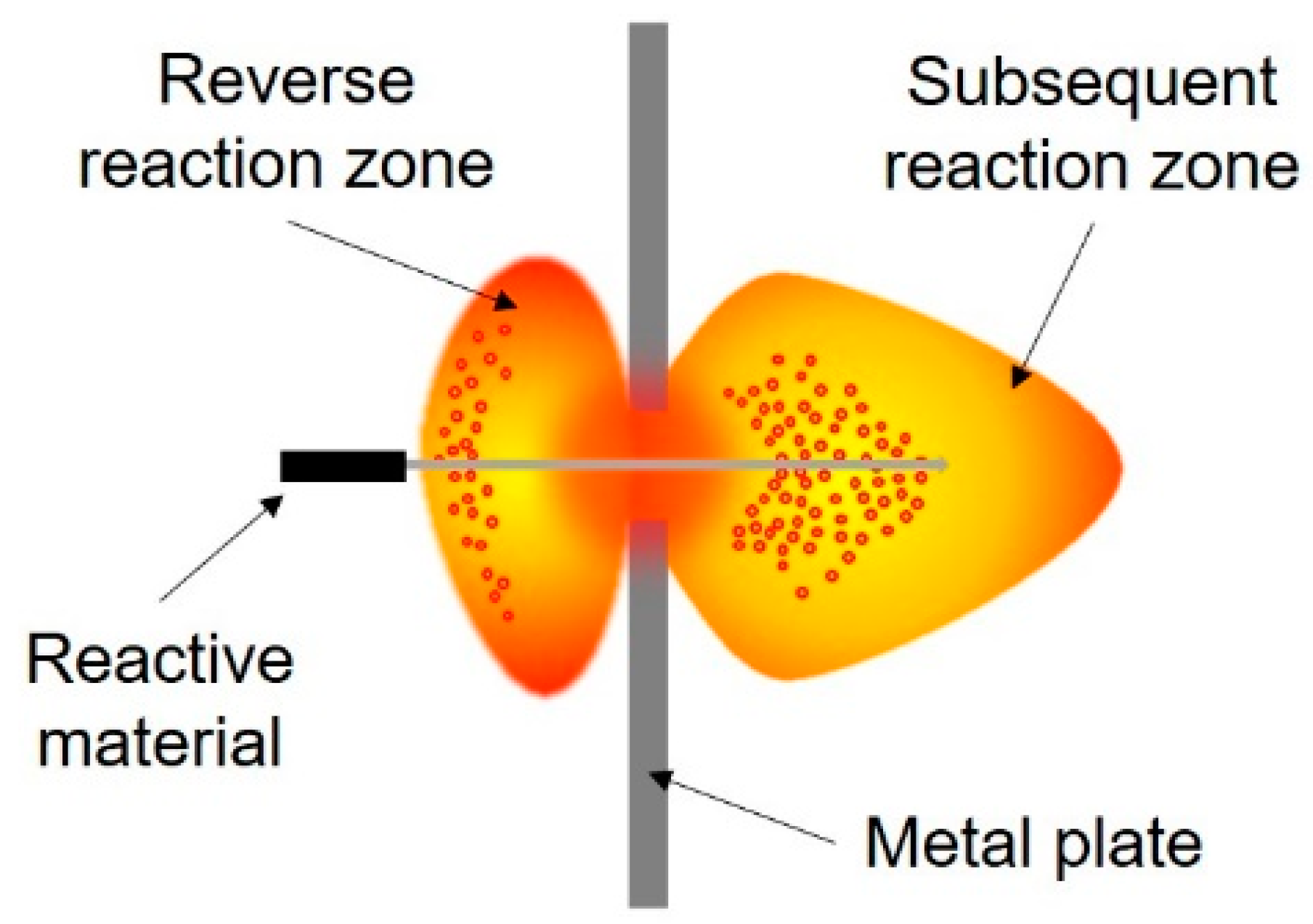
4.1. Influence of Energy Release Reaction to Ignition Effect of the Projectile Impacting the Fuel Tank
4.2. Influence of Kerosene Content on the Ignition Effect of PAB Impacting the Fuel Tank
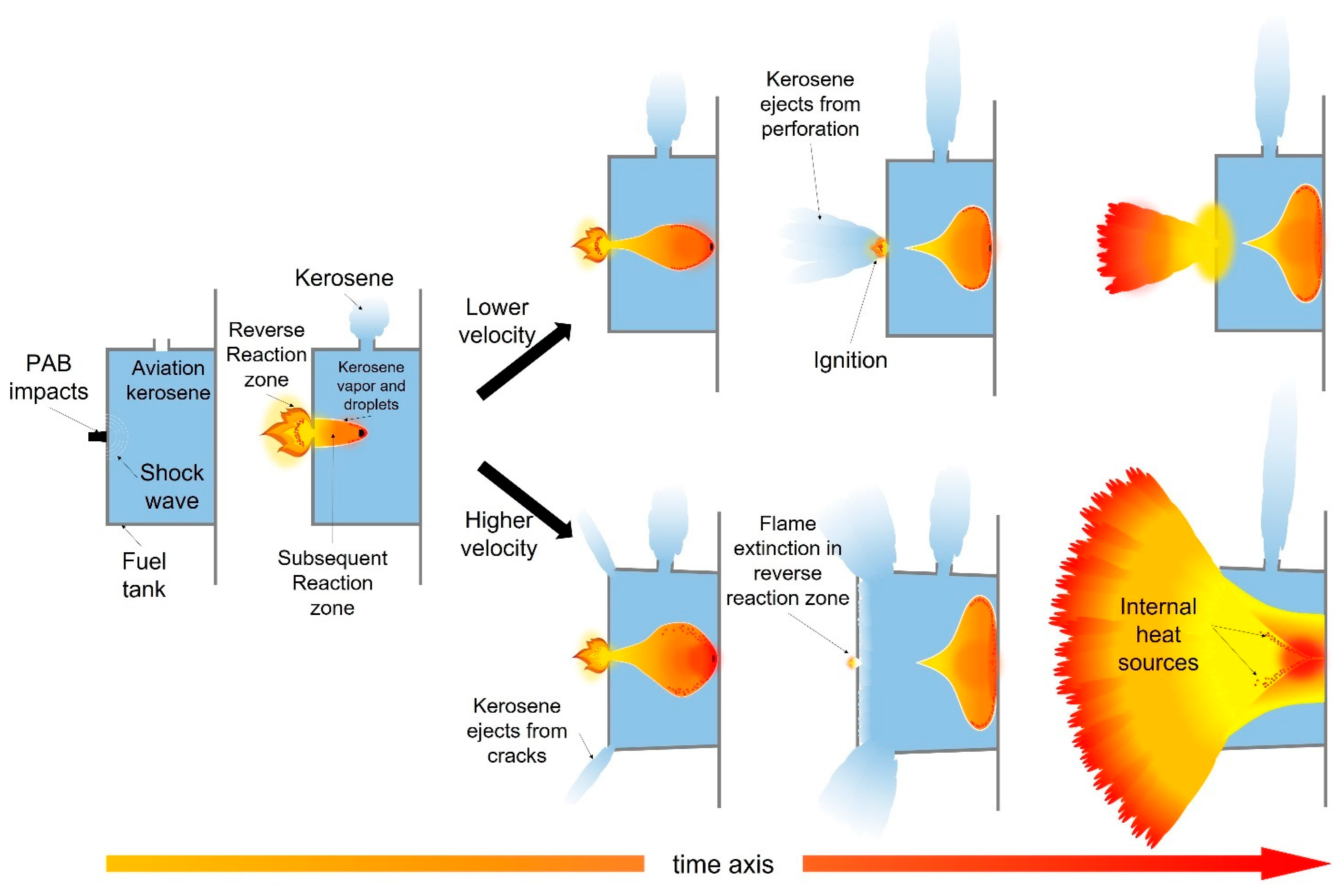
4.3. Influence of Velocity on Ignition Effect of PAB Impacting the Fuel Tank
5. Conclusions
- Under different impact velocities, PAB reactive materials have different damage behaviors and ignition modes for the fuel tank and kerosene, respectively. With the increase of the impact velocity, the fuel tank changed from structural integrity to weld fracture, until it was finally damaged. The change of the energy release characteristics of reactive materials and the damage behavior of the fuel tank promoted the conversion of aviation kerosene ignition mode: aviation kerosene was ignited by the flame in the reverse reaction zone at a low velocity, while the high-temperature-activated reaction fragments were the ignition heat source at a high speed.
- PAB completely ignited kerosene at the velocity of 882.6 m/s. AP ignited kerosene at the velocity of 1254 m/s, but not at the velocity of 1099.7 m/s. SP could not ignite kerosene even if it reached the velocity of 1244 m/s. The order of ignition ability of these three materials is: PAB > AP > SP.
- The damage degree and ignition effect of PAB impacting the fuel tank filled with 50% kerosene were weaker than those of impacting the full fuel tank. If the same effect needs to be achieved, the speed of impacting the fuel tank filled with 50% kerosene needs to be increased (about 60–70 m/s).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, H.L.; Song, B.F.; Pei, Y.; Shi, S. Fuel Leaking Analysis of Fuel Tank by Projectiles Impact with Mechanical Properties of Projectiles. Adv. Mater. Res. 2012, 644, 203–206. [Google Scholar] [CrossRef]
- Varas, D.; Zaera, R.; Lopez-Puente, J. Numerical modelling of partially filled aircraft fuel tanks submitted to Hydrodynamic Ram. Aerosp. Sci. Technol. 2012, 16, 19–28. [Google Scholar] [CrossRef]
- Bing, C. Experimental Study on the Impact Inflame Damage to Simulative Fuel Tank of Cruise Missile by Fragments. Chin. J. Explos. Propellants 2008, 31, 45–49. [Google Scholar]
- Wang, H.F.; Zheng, Y.F.; Yu, Q.B.; Liu, Z.W.; Yu, W.M. Impact-induced initiation and energy release behavior of reactive materials. J. Appl. Phys. 2011, 110, 074904. [Google Scholar] [CrossRef]
- Xu, F.Y.; Zheng, Y.F.; Yu, Q.B.; Zhang, X.P.; Wang, H.F. Damage effects of aluminum plate by reactive material projectile impact. Int. J. Impact Eng. 2017, 104, 38–44. [Google Scholar] [CrossRef]
- Xu, F.Y.; Zheng, Y.F.; Yu, Q.B.; Wang, Y.Z.; Wang, H.F. Experimental study on penetration behavior of reactive material projectile impacting aluminum plate. Int. J. Impact Eng. 2016, 95, 125–132. [Google Scholar] [CrossRef]
- Wang, H.F.; Geng, B.Q.; Guo, H.G.; Zheng, Y.F.; Yu, Q.B.; Ge, C. The effect of sintering and cooling process on geometry distortion and mechanical properties transition of PTFE/Al reactive materials. Def. Technol. 2020, 16, 720–730. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Wang, H.X.; Dong, W.; Li, Y.C. The Effect of Crystallinity on Compressive Properties of Al-PTFE. Polymers 2016, 8, 356. [Google Scholar] [CrossRef]
- Liu, S.B.; Yuan, Y.; Zheng, Y.F.; Ge, C.; Wang, H.F. Enhanced ignition behavior of reactive material projectiles impacting fuel-filled tank. Def. Technol. 2019, 15, 533–540. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Ge, C.; Guo, H.; Zheng, Y. Experimental investigation on enhanced damage to fuel tanks by reactive projectiles impact. Def. Technol. 2021, 17, 599–608. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.X.; Li, S.K.; Zhang, X.B. Investigation on reaction energy, mechanical behavior and impact insensitivity of W-PTFE-Al composites with different W percentage. Mater. Des. 2016, 92, 397–404. [Google Scholar] [CrossRef]
- Zhou, L.; Piekiel, N.; Chowdhury, S.; Zachariah, M.R. Time-Resolved Mass Spectrometry of the Exothermic Reaction between Nanoaluminum and Metal Oxides: The Role of Oxygen Release. J. Phys. Chem. C. 2010, 114, 14269–14275. [Google Scholar] [CrossRef]
- Wu, J.X.; Feng, B.; Gao, Z.R.; Li, Y.C.; Wu, S.Z.; Yin, Q.; Huang, J.Y.; Ren, X.X. Investigation on the thermal decomposition and thermal reaction process of PTFE/Al/MoO3 fluorine-containing thermite. J. Fluorine Chem. 2021, 241, 109676. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Fang, X.; Li, Y.; Yu, Z.; Gao, Z.; Wu, S.; Yang, L.; Wu, J.; Kui, J. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials 2018, 11, 2451. [Google Scholar] [CrossRef]
- Huang, J.Y.; Fang, X.; Li, Y.C.; Feng, B.; Wang, H.X.; Du, K. The Mechanical and Reaction Behavior of PTFE/Al/Fe2O3 under Impact and Quasi-Static Compression. Adv. Mater. Sci. Eng. 2017, 2017, 3540320. [Google Scholar] [CrossRef]
- Wu, J.X.; Feng, B.; Gao, Z.R.; Yin, Q.; Huang, J.Y.; Wu, S.Z.; Li, Y.C. Effect of multi-oxidants on mechanical response and reactive characteristics of PTFE/Al reactive material under dynamic impact. Mater. Lett. 2021, 283, 128886. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducere, J.M.; Baijot, V.; Pinon, S.; Calais, T.; Esteve, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame. 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Martirosyan, K.S.; Wang, L.; Vicent, A.; Luss, D. Synthesis and performance of bismuth trioxide nanoparticles for high energy gas generator use. Nanotechnology 2009, 20, 405609. [Google Scholar] [CrossRef]
- Martirosyan, K.S.; Wang, L.; Luss, D. Tuning the Gas Pressure Discharge of Nanoenergetic Materials by Boron and Carbon Nanotubes Additives. In Proceedings of the NSTI Nanotechnology Conference and Expo, Boston, MA, USA, 13–16 June 2011; pp. 323–326. [Google Scholar]
- Yuan, Y.; Geng, B.Q.; Sun, T.; Yu, Q.B.; Wang, H.F. Impact-Induced Reaction Characteristic and the Enhanced Sensitivity of PTFE/Al/Bi2O3 Composites. Polymers 2019, 11, 2049. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Z.Y.; He, S.; Ge, C.; Yu, Q.B.; Zheng, Y.F.; Wang, H.F. Shock-induced reaction behaviors of functionally graded reactive material. Def. Technol. 2021, 17, 1687–1698. [Google Scholar] [CrossRef]
- Lan, J.; Liu, J.X.; Zhang, S.; Xue, X.Y.; He, C.; Wu, Z.Y.; Yang, M.; Li, S.K. Influence of multi-oxidants on reaction characteristics of PTFE-Al-XmOY reactive material. Mater. Des. 2020, 186, 108325. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.C.; Huang, J.Y.; Wu, J.X.; Liu, Q.; Wu, S.Z.; Gao, Z.R.; Zhang, S.; Yang, L. The effect of al particle size on thermal decomposition, mechanical strength and sensitivity of Al/ZrH2/PTFE composite. Def. Technol. 2021, 17, 829–835. [Google Scholar] [CrossRef]
- Feng, S.S.; Wang, C.L.; Huang, G.Y. Experimental Study on the Reaction Zone Distribution of Impact-Induced Reactive Materials. Propellants Explos. Pyrotech. 2017, 42, 896–905. [Google Scholar] [CrossRef]



| Test Number | Projectile Type | Target | Impact Velocity (m/s) | Ignition Time (ms) | Damage Degree of Fuel Tank | Ignition Status |
|---|---|---|---|---|---|---|
| 1# | PAB | Impletion | 765.5 | 5.75 | Intactness | Part |
| 2# | PAB | Impletion | 882.6 | 7.75 | Weld cracking | Whole |
| 3# | PAB | Impletion | 967.2 | 22 | Tank ruptured | Whole |
| 4# | PAB | Impletion | 1168.0 | 25 | Tank ruptured | Whole |
| 5# | AP | Impletion | 1099.7 | - | Tank ruptured | - |
| 6# | AP | Impletion | 1254.0 | 10.25 | Tank ruptured | Whole |
| 7# | SP | Impletion | 1244.0 | - | Tank ruptured | - |
| 8# | PAB | Half | 842.6 | 8.25 | Intactness | Part |
| 9# | PAB | Half | 947.0 | 11.5 | Weld cracking | Whole |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yin, Q.; Yao, M.; Huang, J.; Li, R.; Gao, Z.; Wu, S.; Li, Y.; Wu, J. Experimental Investigation on Ignition Effects of Fuel Tank Impacted by Bi2O3-Reinforced PTFE/Al Reactive Material Projectile. Metals 2023, 13, 399. https://doi.org/10.3390/met13020399
Wang R, Yin Q, Yao M, Huang J, Li R, Gao Z, Wu S, Li Y, Wu J. Experimental Investigation on Ignition Effects of Fuel Tank Impacted by Bi2O3-Reinforced PTFE/Al Reactive Material Projectile. Metals. 2023; 13(2):399. https://doi.org/10.3390/met13020399
Chicago/Turabian StyleWang, Ruiqi, Qin Yin, Miao Yao, Junyi Huang, Rongxin Li, Zhenru Gao, Shuangzhang Wu, Yuchun Li, and Jiaxiang Wu. 2023. "Experimental Investigation on Ignition Effects of Fuel Tank Impacted by Bi2O3-Reinforced PTFE/Al Reactive Material Projectile" Metals 13, no. 2: 399. https://doi.org/10.3390/met13020399
APA StyleWang, R., Yin, Q., Yao, M., Huang, J., Li, R., Gao, Z., Wu, S., Li, Y., & Wu, J. (2023). Experimental Investigation on Ignition Effects of Fuel Tank Impacted by Bi2O3-Reinforced PTFE/Al Reactive Material Projectile. Metals, 13(2), 399. https://doi.org/10.3390/met13020399







