The Formation Mechanism of a Multilayer-Structure Oxide Film during the Oxidation of FeCrAl in Air at 700 °C
Abstract
1. Introduction
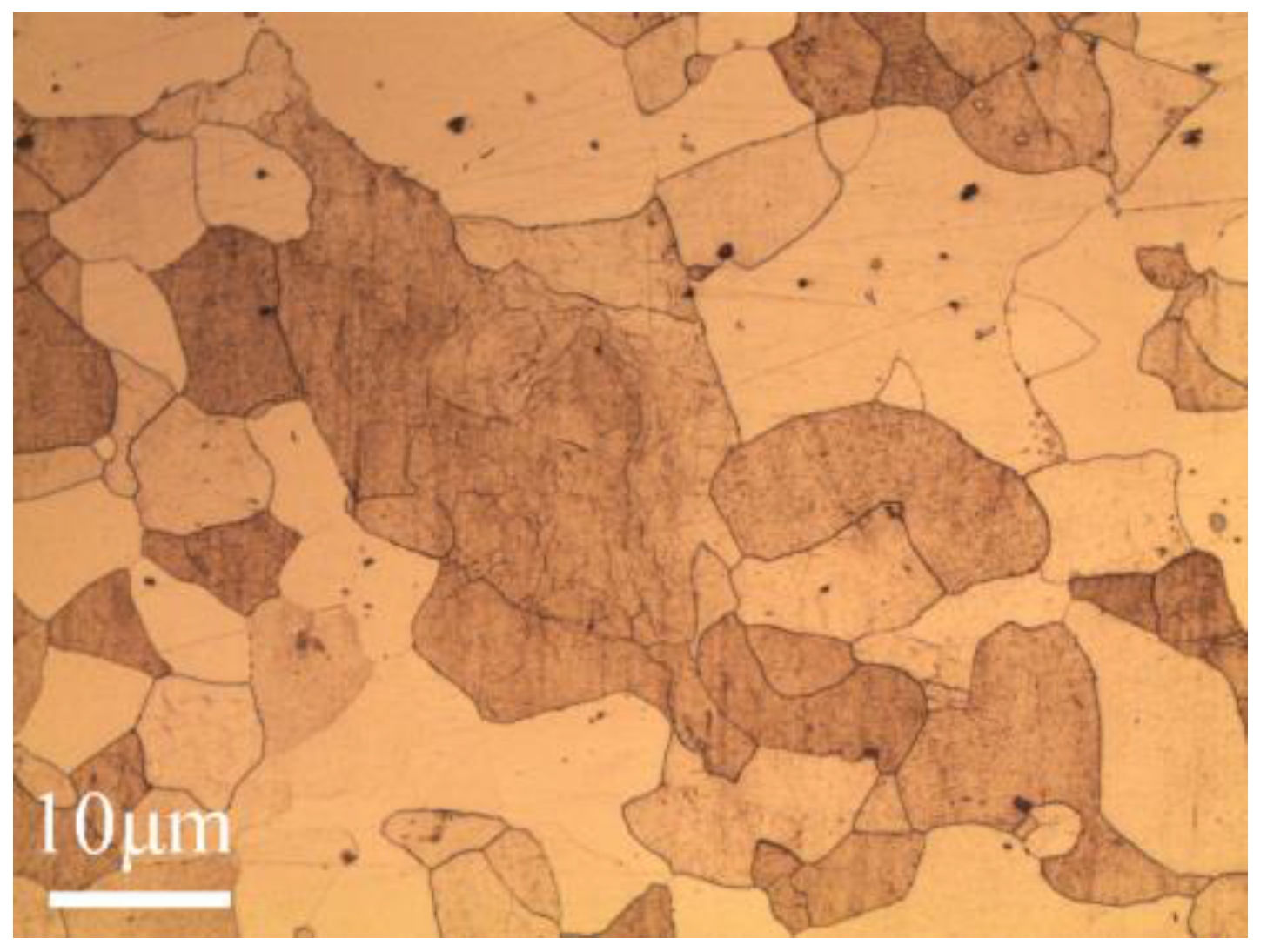
2. Materials and Methods
3. Results
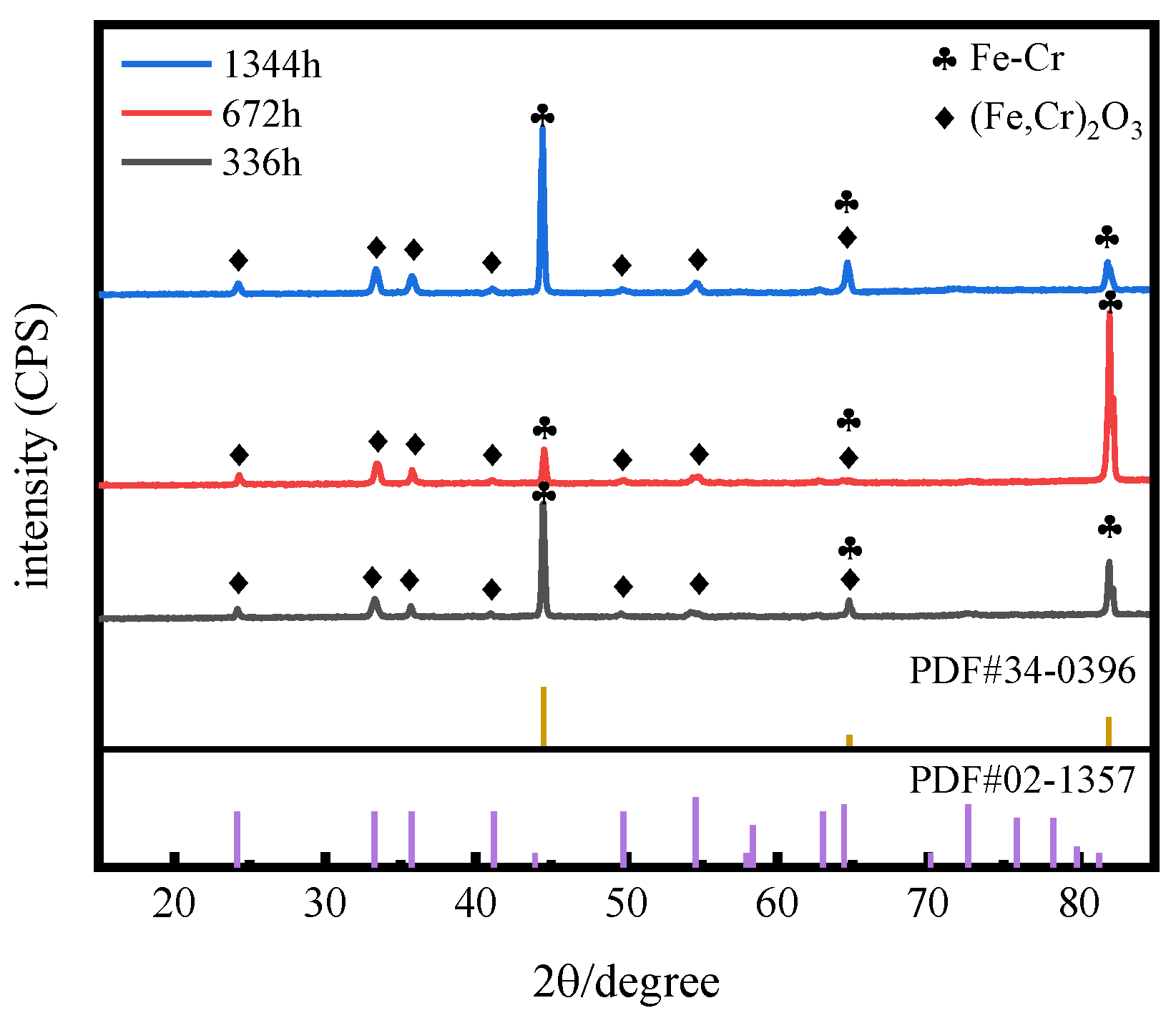
3.1. Characterization of Oxide Layers
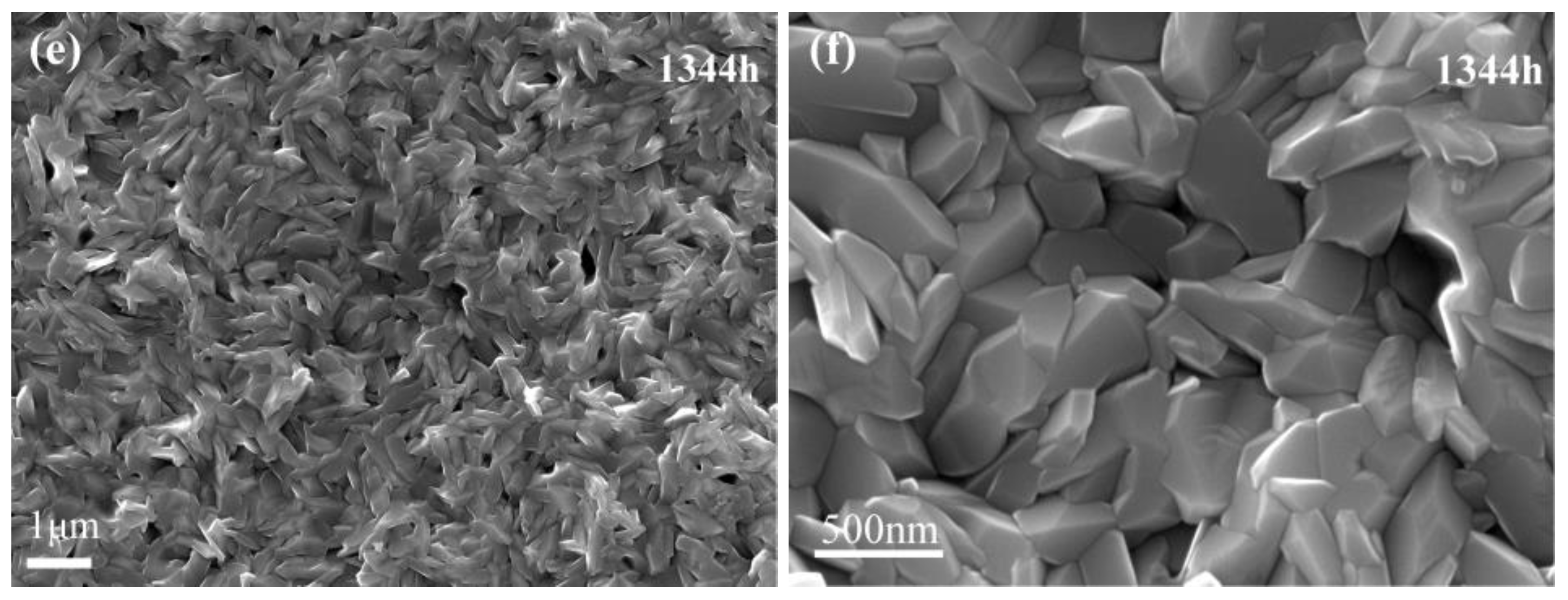
3.1.1. Morphology of the Oxide Film Surface
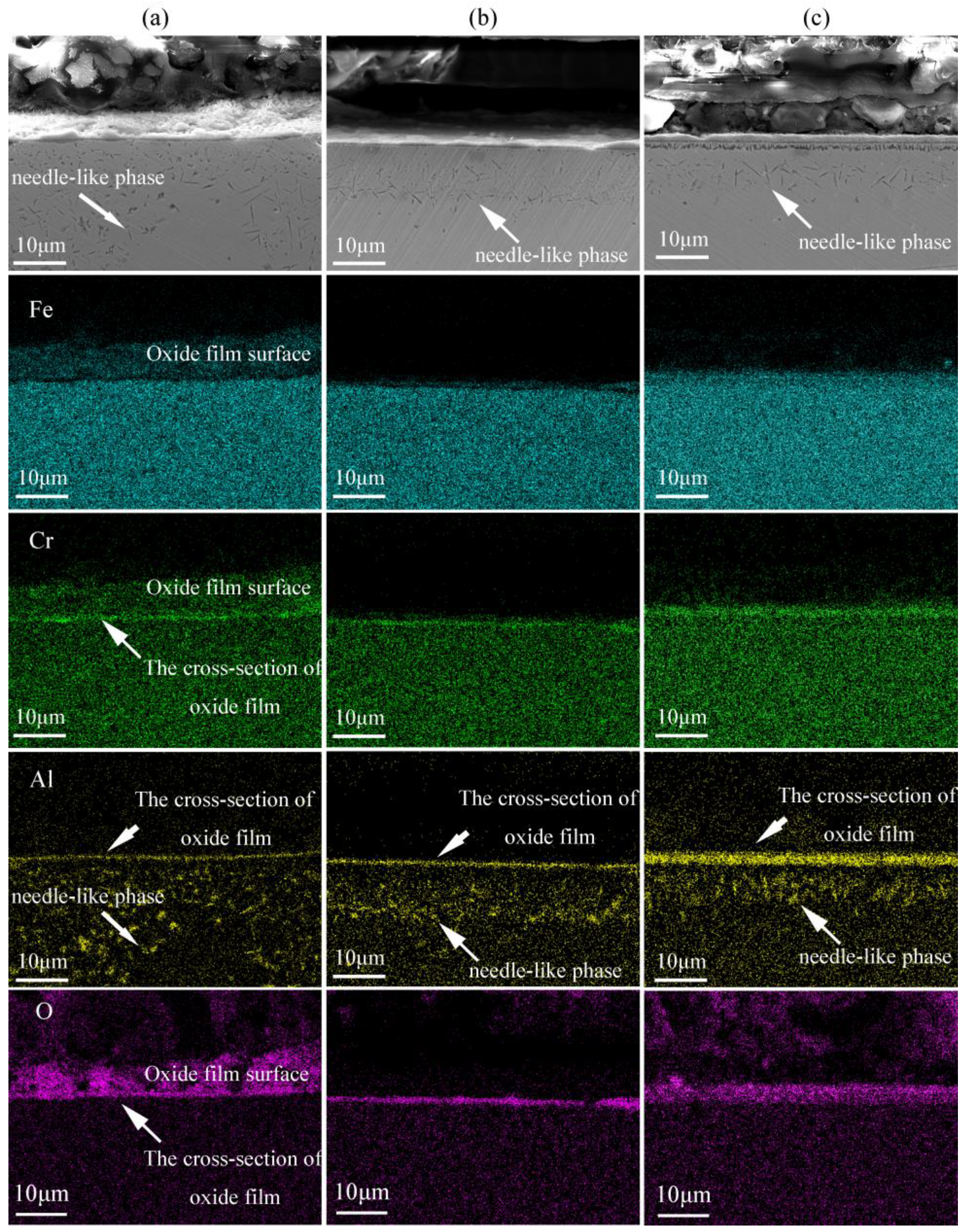
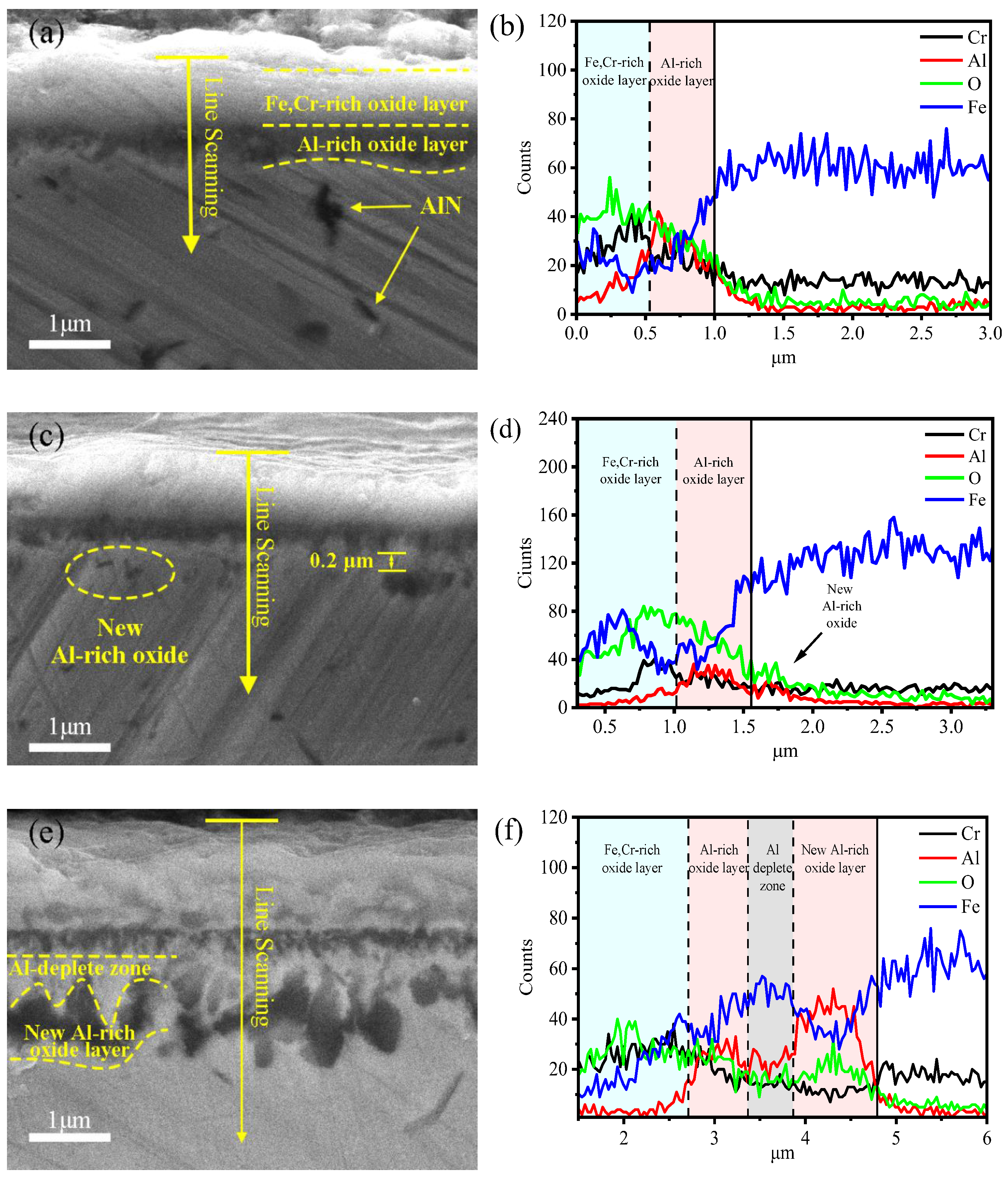
3.1.2. The Cross-Section Morphology and Composition of the Oxide Film
3.2. Morphology of AlN Precipitation in the Matrix
4. Discussion
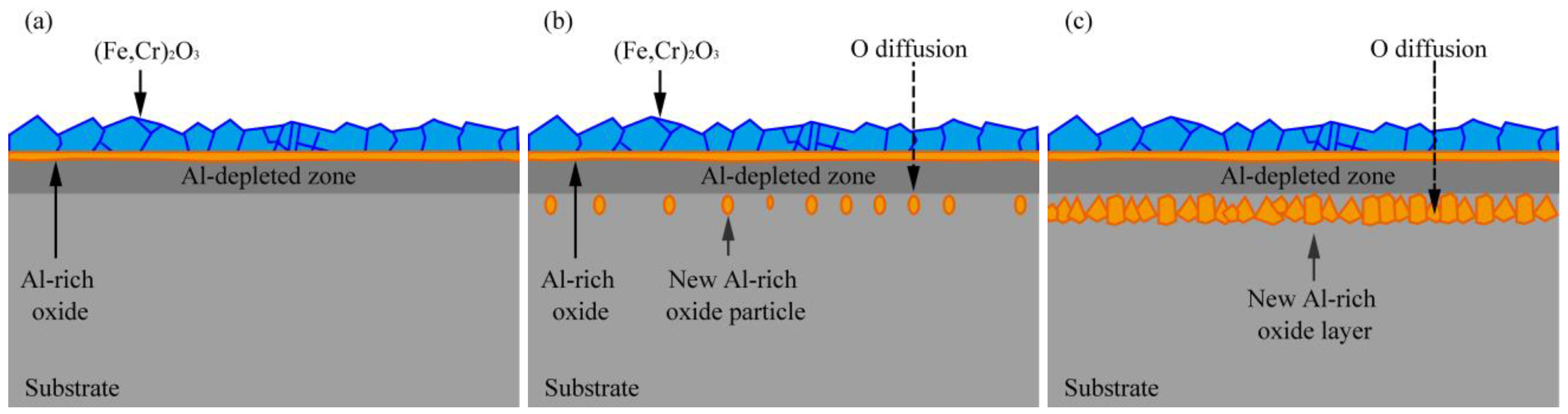
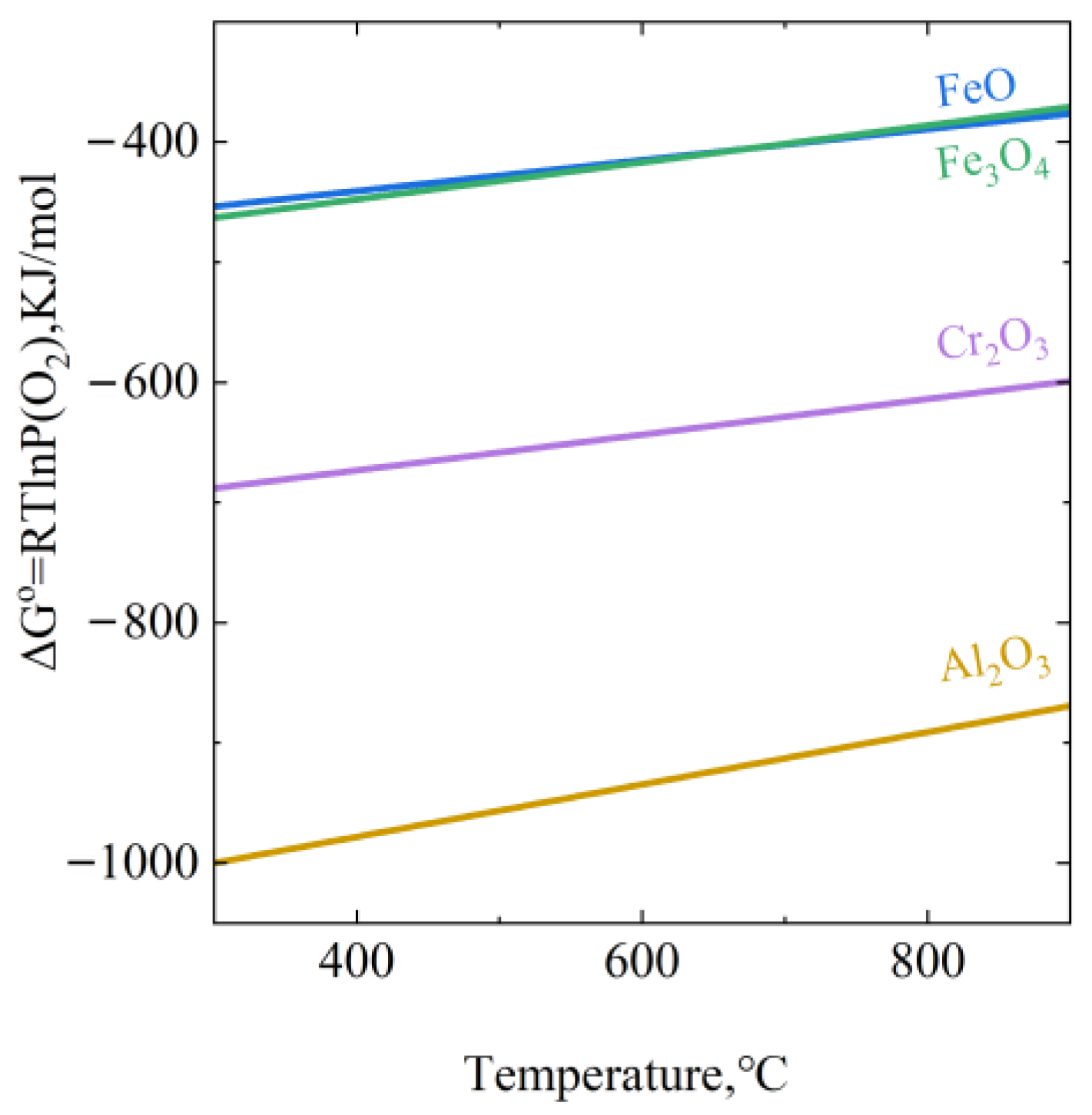
4.1. Mechanism of Oxide Film Growth
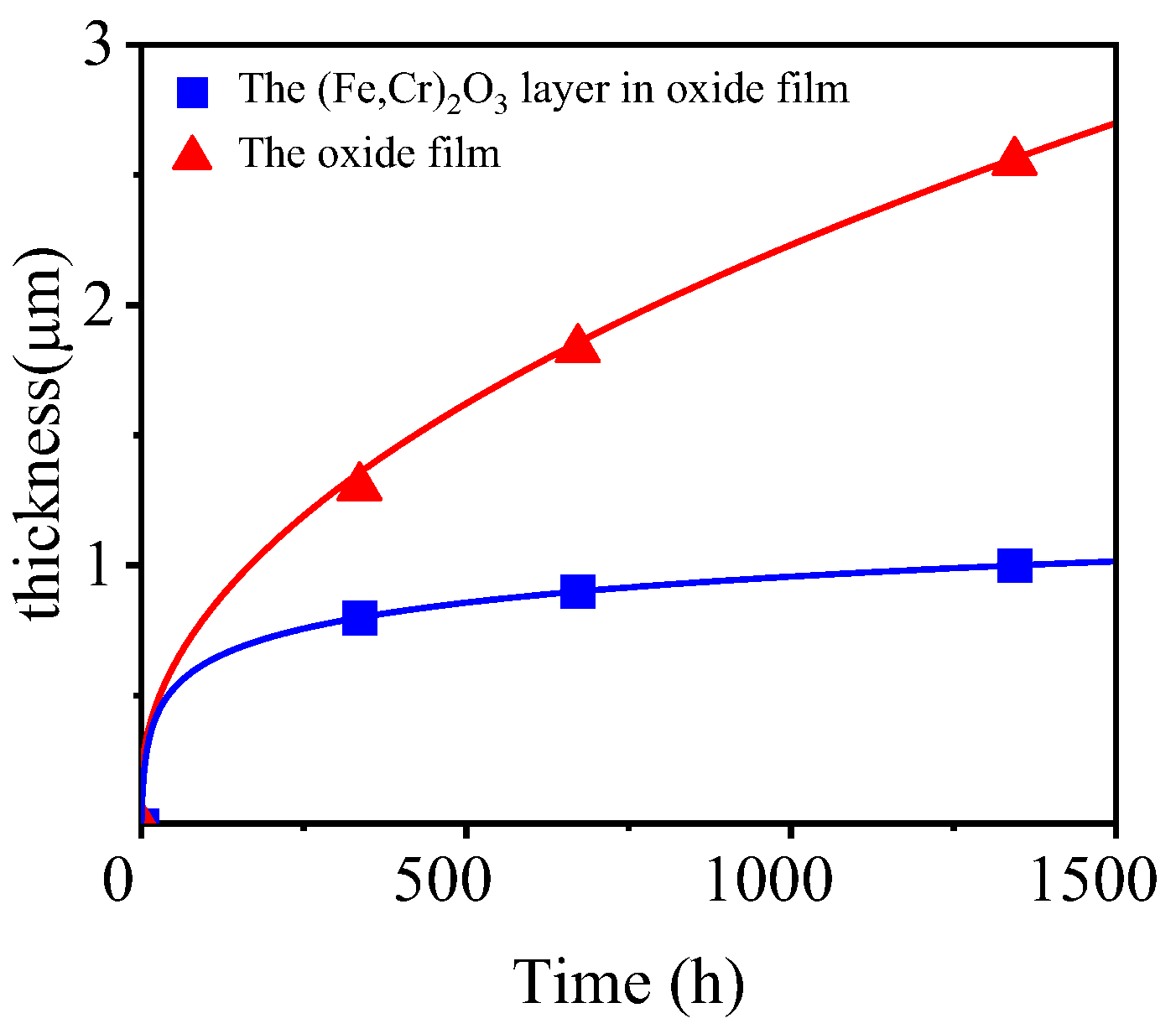
4.2. Oxidation Kinetics
4.3. Interaction between AlN and Oxide Film
5. Conclusions
- At the early stage of oxidation, a double-layer oxide film was formed on the alloy surface. The external layer of the oxide film is (Fe,Cr)2O3 and the internal layer is Al-rich oxides.
- With the extension of the oxidation time, the structure of the oxide film evolved from being double-layered to four-layered. The four-layered oxide film consists of a (Fe,Cr)2O3 layer, an Al-rich oxide layer, an Al-depleted zone, and an Al-rich oxide layer. The main components of the Al-depleted zone are Fe and Cr, which have not been completely oxidized.
- The occurrence of the Al-depleted zone is mainly due to the depletion of Al in the matrix by an Al-rich oxide layer formed at 336 h. The formation of the new Al-rich oxide layer is mainly because of the internal oxidation of the alloy.
- The growth of the oxide film follows a parabolic law.
- The AlN phase was formed due to the diffusion of N into the alloy matrix in the early stage of oxidation. The precipitation of the AlN phase might be one of the reasons for the appearance of the Al-depleted zone.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agüero, A. Progress in the Development of Coatings for Protection of New Generation Steam Plant Components. Energy Mater. 2008, 3, 35–44. [Google Scholar] [CrossRef]
- Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T.; Massey, C.P.; Pint, B.A. Development of Low-Cr ODS FeCrAl Alloys for Accident-Tolerant Fuel Cladding. J. Nucl. Mater. 2018, 501, 59–71. [Google Scholar] [CrossRef]
- Terrani, K.A.; Pint, B.A.; Kim, Y.-J.; Unocic, K.A.; Yang, Y.; Silva, C.M.; Meyer, H.M.; Rebak, R.B. Uniform Corrosion of FeCrAl Alloys in LWR Coolant Environments. J. Nucl. Mater. 2016, 479, 36–47. [Google Scholar] [CrossRef]
- Xu, Z.; Song, L.; Zhao, Y.; Liu, S. The Formation Mechanism and Effect of Amorphous SiO2 on the Corrosion Behaviour of Fe-Cr-Si ODS Alloy in LBE at 550 °C. Corros. Sci. 2021, 190, 109634. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Song, L.; Yang, X.; Zhao, Y.; Mao, X. Effect of Silicon on Oxidation Behavior of 9Cr-ODS Steel at 650 °C. Fusion Eng. Des. 2021, 167, 112384. [Google Scholar] [CrossRef]
- Eklund, J.; Paz, M.D.; Jönsson, B.; Liske, J.; Svensson, J.; Jonsson, T. Field Exposure of FeCrAl Model Alloys in a Waste-fired Boiler at 600 °C: The Influence of Cr and Si on the Corrosion Behaviour. Mater. Corros. 2019, 70, 1476–1485. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Zeng, D.; Zhu, C.Y.; Rose, S.R.; Datta, P.K. Steam Oxidation Resistance of Ni-Aluminide/Fe-Aluminide Duplex Coatings Formed on Creep Resistant Ferritic Steels by Low Temperature Pack Cementation Process. Corros. Sci. 2011, 53, 496–502. [Google Scholar] [CrossRef]
- Rebak, R.B.; Gupta, V.K.; Larsen, M. Oxidation Characteristics of Two FeCrAl Alloys in Air and Steam from 800 °C to 1300 °C. JOM 2018, 70, 1484–1492. [Google Scholar] [CrossRef]
- Chen, G.; Yang, H.; Sun, H.; Wang, F.; Wang, H.; Kong, Q.; An, X.; Zhang, Y.; Wang, J. Exploring the High-Temperature Steam Oxidation Behaviors of the Lean-Cr (7–10 Wt%) FeCrAl Alloys. Corros. Sci. 2022, 194, 109927. [Google Scholar] [CrossRef]
- Josefsson, H.; Liu, F.; Svensson, J.-E.; Halvarsson, M.; Johansson, L.-G. Oxidation of FeCrAl Alloys at 500–900 °C in Dry O2. Mater. Corros. 2005, 56, 801–805. [Google Scholar] [CrossRef]
- Tsisar, V.; Stergar, E.; Gavrilov, S.; Van Renterghem, W.; Louette, P.; Lucas, S. Effect of Variation in Oxygen Concentration in Static Pb–Bi Eutectic on Long-Term Corrosion Performance of Al-Alloyed Austenitic Steels at 500 °C. Corros. Sci. 2022, 195, 109963. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Engkvist, J.; Bexell, U.; Grehk, M.; Olsson, M. High Temperature Oxidation of FeCrAl-Alloys–Influence of Al-Concentration on Oxide Layer Characteristics: High Temperature Oxidation of FeCrAl Alloys. Mater. Corros. 2009, 60, 876–881. [Google Scholar] [CrossRef]
- Gurrappa, I.; Weinbruch, S.; Naumenko, D.; Quadakkers, W.J. Factors Governing Breakaway Oxidation of FeCrAl-Based Alloys. Mater. Corros. 2000, 51, 224–235. [Google Scholar] [CrossRef]
- Magnusson, H.; Sandström, R. Influence of Aluminium on Creep Strength of 9–12% Cr Steels. J. Mater. Sci. Eng. A 2009, 527, 118–125. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, S.; Tang, X.; Pan, H.; Chen, X.; Xiang, Z.D. Influence of Al on Scale Formation and Growth Kinetics of 10wt.% Cr Creep Resistant Ferritic Steels at 650 °C in Air. Corros. Sci. 2014, 80, 374–382. [Google Scholar] [CrossRef]
- Li, N.; Parker, S.S.; Saleh, T.A.; Maloy, S.A.; Nelson, A.T. Intermediate Temperature Corrosion Behaviour of Fe-12Cr-6Al-2Mo-0.2Si-0.03Y Alloy (C26M) at 300–600 °C. Corros. Sci. 2019, 157, 274–283. [Google Scholar] [CrossRef]
- Li, N.; Parker, S.S.; Wood, E.S.; Nelson, A.T. Oxide Morphology of a FeCrAl Alloy, Kanthal APMT, Following Extended Aging in Air at 300 °C to 600 °C. Metall. Mat. Trans. A 2018, 49, 2940–2950. [Google Scholar] [CrossRef]
- Tomaszewicz, P.; Wallwork, G.R. The Oxidation of High-Purity Iron-Chromium-Aluminum Alloys at 800~. Oxid. Met. 1983, 20, 75–109. [Google Scholar] [CrossRef]
- Götlind, H.; Liu, F.; Svensson, J.-E.; Halvarsson, M.; Johansson, L.-G. The Effect of Water Vapor on the Initial Stages of Oxidation of the FeCrAl Alloy Kanthal AF at 900 °C. Oxid. Met. 2007, 67, 251–266. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Naumenko, D.; Wessel, E.; Kochubey, V.; Singheiser, L. Growth Rates of Alumina Scales on Fe–Cr–Al Alloys. Oxid. Met. 2004, 61, 17–37. [Google Scholar] [CrossRef]
- Dömstedt, P. Corrosion Studies of Low-Alloyed FeCrAl Steels in Liquid Lead at 750 °C. Oxid. Met. 2019, 91, 511–524. [Google Scholar] [CrossRef]
- Popovic, M.P.; Bolind, A.M.; Aussat, Y.; Gubser, A.J.; Hosemann, P. Oxidative Passivation of Fe–Cr–Al Steels in Lead-Bismuth Eutectic under Oxygen-Controlled Static Conditions at 700° and 800 °C. J. Nucl. Mater. 2019, 523, 172–181. [Google Scholar] [CrossRef]
- Popovic, M.P.; Chen, K.; Shen, H.; Stan, C.V.; Olmsted, D.L.; Tamura, N.; Asta, M.; Abad, M.D.; Hosemann, P. A Study of Deformation and Strain Induced in Bulk by the Oxide Layers Formation on a Fe-Cr-Al Alloy in High-Temperature Liquid Pb-Bi Eutectic. Acta Mater. 2018, 151, 301–309. [Google Scholar] [CrossRef]
- Smialek, J.L. Invited Review Paper in Commemoration of Over 50 Years of Oxidation of Metals: Alumina Scale Adhesion Mechanisms: A Retrospective Assessment. Oxid. Met. 2022, 97, 1–50. [Google Scholar] [CrossRef]
- Liu, Z.W.; Sun, C.; Gauvin, R.; Wu, W.; Zeng, Y.; Demers, H. High Spatial Resolution EDS Mapping of Nanoparticles at Low Accelerating Voltage. J. Test. Eval. 2016, 44, 20140457. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The Oxidation Behaviour of NiAl-I. Phase Transformations in the Alumina Scale during Oxidation of NiAl and NiAl-Cr Alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Zeng, C.; Ling, Y.; Li, S.; Rao, Y.; Chen, Y. The Effect of Chromium on the Gamma to Alpha Phase Transition of Alumina Coating Formed on 316L SS by a Cathodic Micro Arc Deposition (CMAD) Process. Surf. Coat. Technol. 2015, 263, 15–20. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, S.; Gao, F.; Zhang, Z.G.; Gesmundo, F. The Nature of the Third-Element Effect in the Oxidation of Fe–XCr–3at.% Al Alloys in 1atm O2 at 1000 °C. Corros. Sci. 2008, 50, 345–356. [Google Scholar] [CrossRef]
- Chevalier, S.; Lesage, B.; Legros, C.; Borchardt, G.; Strehl, G.; Kilo, M. Oxygen Diffusion in Alumina. Application to Synthetic and Thermally Grown Al2O3. DDF 2005, 237–240, 899–910. [Google Scholar] [CrossRef]
- Gaskell, D.R. Introduction to the Thermodynamics of Materials, 6th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-11903-8. [Google Scholar]
- Lim, J. Effects of Chromium and Silicon on Corrosion of Iron Alloys in Leadbismuth Eutectic. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2006. [Google Scholar]
- Hagel, W.C. Oxygen-ion diffusion in hematite. Trans. Metall. Soc. AIME 1966, 236, 179–184. [Google Scholar]
- Frank, W.; Engell, H.J.; Seeger, A. Solubility and Interstitial Migration of Oxygen in Bcc Iron. Trans. Met. Soc. AIME 1968, 242, 749–750. [Google Scholar]
- Heuer, A.H. Oxygen and Aluminum Diffusion in α-Al2O3: How Much Do We Really Understand? J. Eur. Ceram. Soc. 2008, 28, 1495–1507. [Google Scholar] [CrossRef]
- Veskovic-Bukudura, S.; Kovac, J.; Karpe, B.; Umek, P.; Nagode, A.; Cerc-Korosec, R.; Bizjak, M. High Temperature Corrosion of Commercial FeCrAl Alloys (Kanthal AF) in Nitrogen Gas. J. Min. Metall. Sect. B 2019, 55, 55–63. [Google Scholar] [CrossRef]
- Israelsson, N.; Unocic, K.A.; Hellström, K.; Jonsson, T.; Norell, M.; Svensson, J.-E.; Johansson, L.-G. A Microstructural and Kinetic Investigation of the KCl-Induced Corrosion of an FeCrAl Alloy at 600 °C. Oxid. Met. 2015, 84, 105–127. [Google Scholar] [CrossRef]


| Elements | Fe | Cr | Al | Mo |
|---|---|---|---|---|
| Content (wt.%) | balance | 12.7 | 2.0 | 1.5 |
| Time (h) | 336 | 672 | 1344 |
|---|---|---|---|
| Thickness (μm) | 1.3 | 1.8 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Wang, X.; Zhou, D.; Wu, Q.; Li, C.; Song, L.; Liu, S. The Formation Mechanism of a Multilayer-Structure Oxide Film during the Oxidation of FeCrAl in Air at 700 °C. Metals 2023, 13, 305. https://doi.org/10.3390/met13020305
Gao Z, Wang X, Zhou D, Wu Q, Li C, Song L, Liu S. The Formation Mechanism of a Multilayer-Structure Oxide Film during the Oxidation of FeCrAl in Air at 700 °C. Metals. 2023; 13(2):305. https://doi.org/10.3390/met13020305
Chicago/Turabian StyleGao, Zichen, Xinyu Wang, Danna Zhou, Qingsheng Wu, Chunjing Li, Liangliang Song, and Shaojun Liu. 2023. "The Formation Mechanism of a Multilayer-Structure Oxide Film during the Oxidation of FeCrAl in Air at 700 °C" Metals 13, no. 2: 305. https://doi.org/10.3390/met13020305
APA StyleGao, Z., Wang, X., Zhou, D., Wu, Q., Li, C., Song, L., & Liu, S. (2023). The Formation Mechanism of a Multilayer-Structure Oxide Film during the Oxidation of FeCrAl in Air at 700 °C. Metals, 13(2), 305. https://doi.org/10.3390/met13020305




