The Hot Ductility, Microstructures, Mechanical Properties and Corrosion Resistance in an Advanced Boron-Containing Complex Phase Steel Heat-Treated Using the Quenching and Partitioning (Q&P) Process
Abstract
1. Introduction
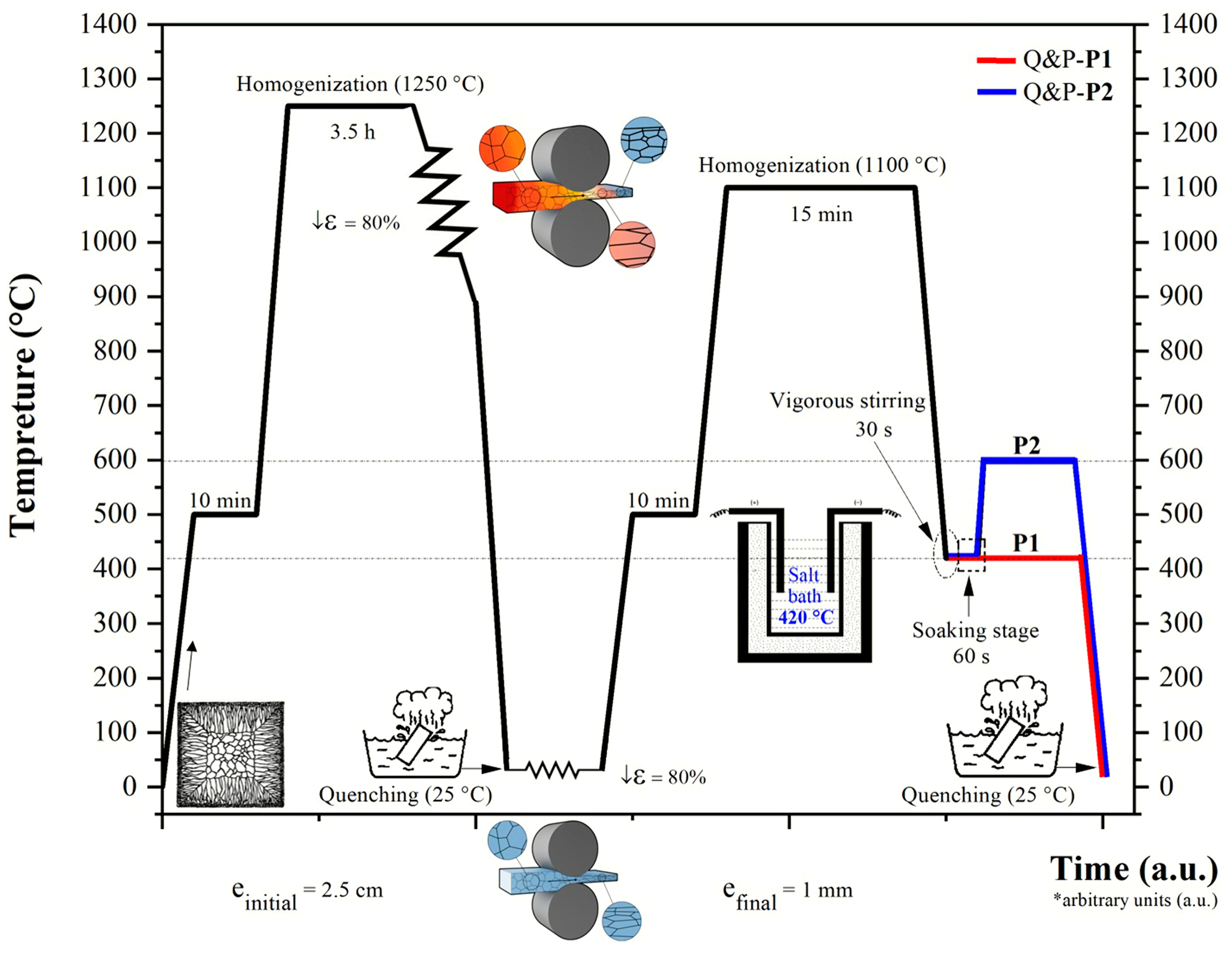
2. Materials and Methods
3. Results and Discussion
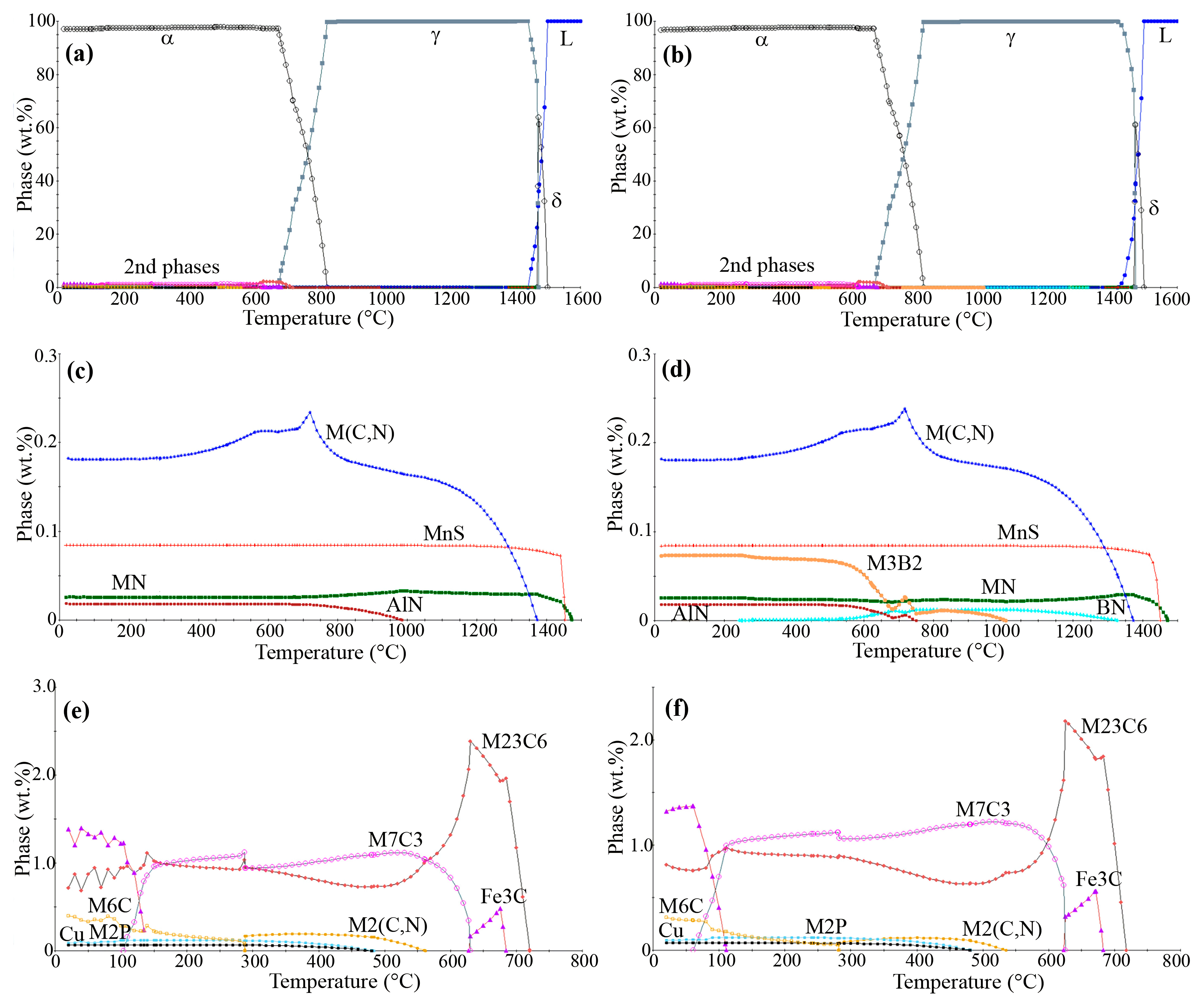
3.1. Thermodynamical Equilibrium Calculations
3.2. X-ray Diffraction Study in the As-Cast Condition
3.3. Grain Size Measurements in the As-Cast Condition
3.4. Hot Ductility Behavior
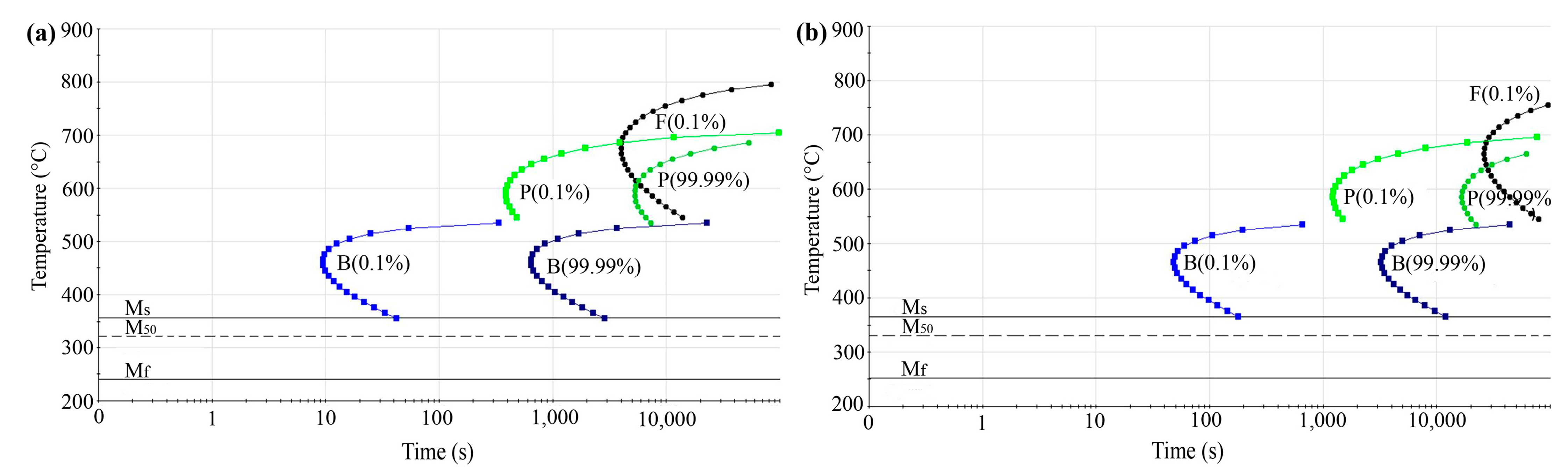
3.5. Analysis of the Martensitic Transformation
3.6. X-ray Diffraction Study of the Q&P Conditions
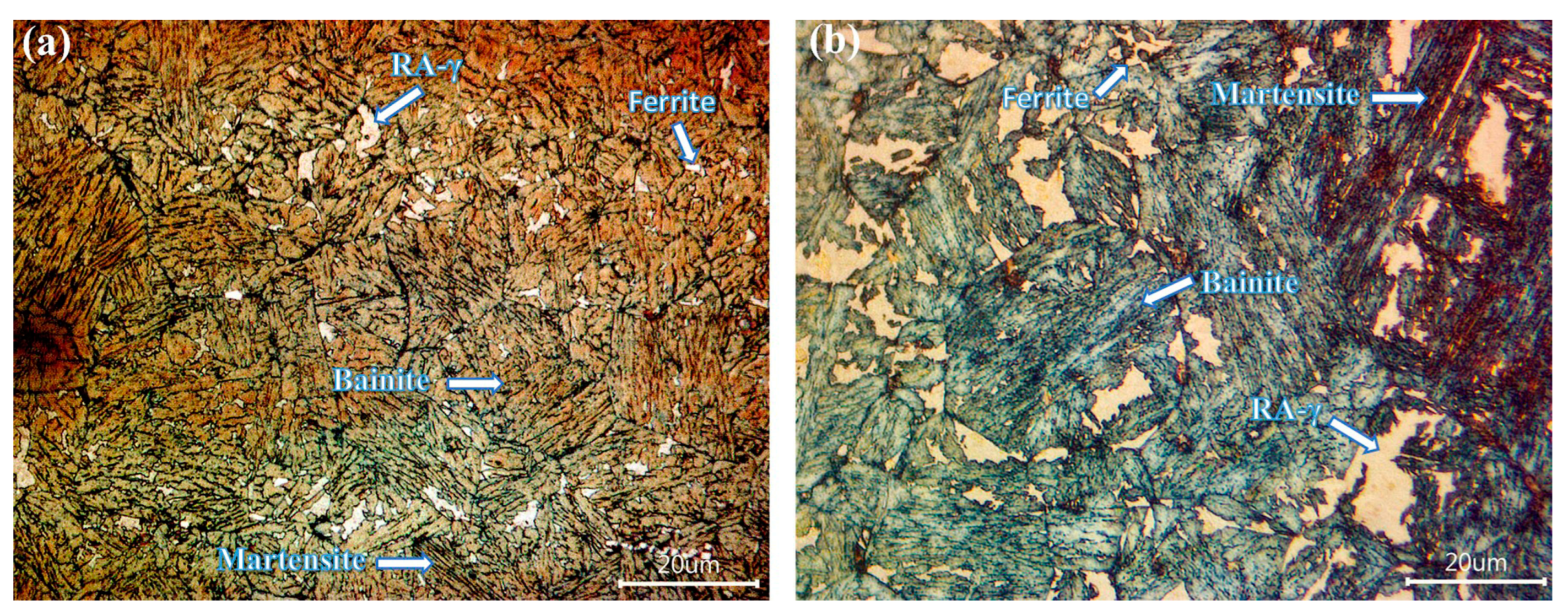
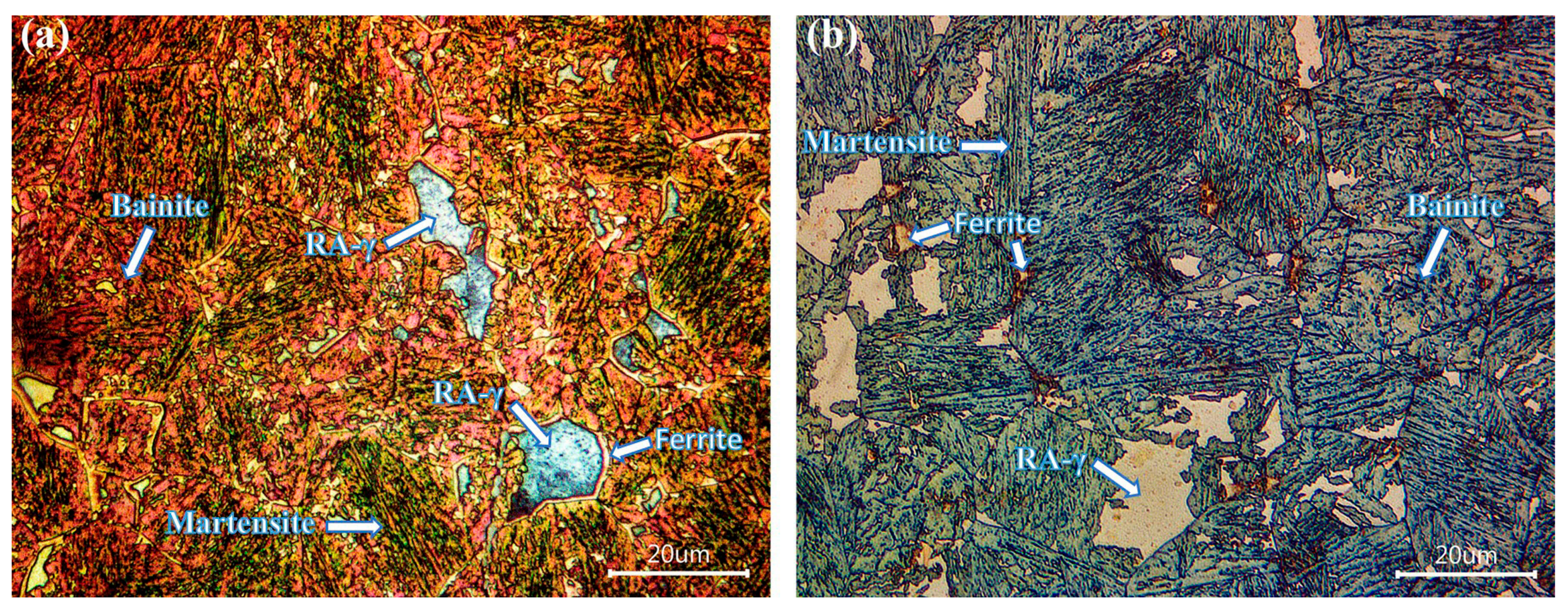
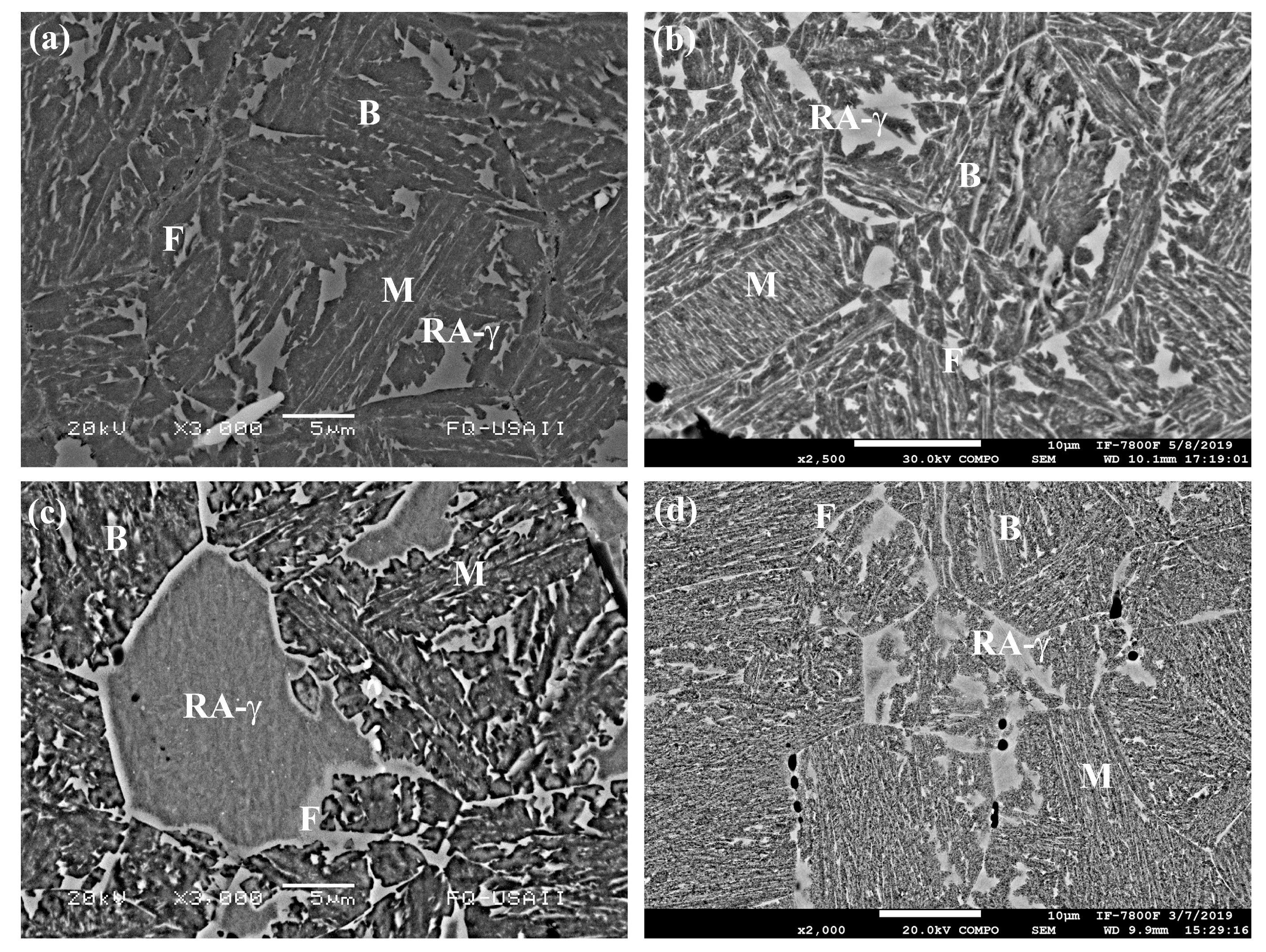
3.7. Microstructural Characterization of CP Steels in the Q&P Conditions
3.8. Mechanical Characterization of CP Steels in the Q&P Conditions
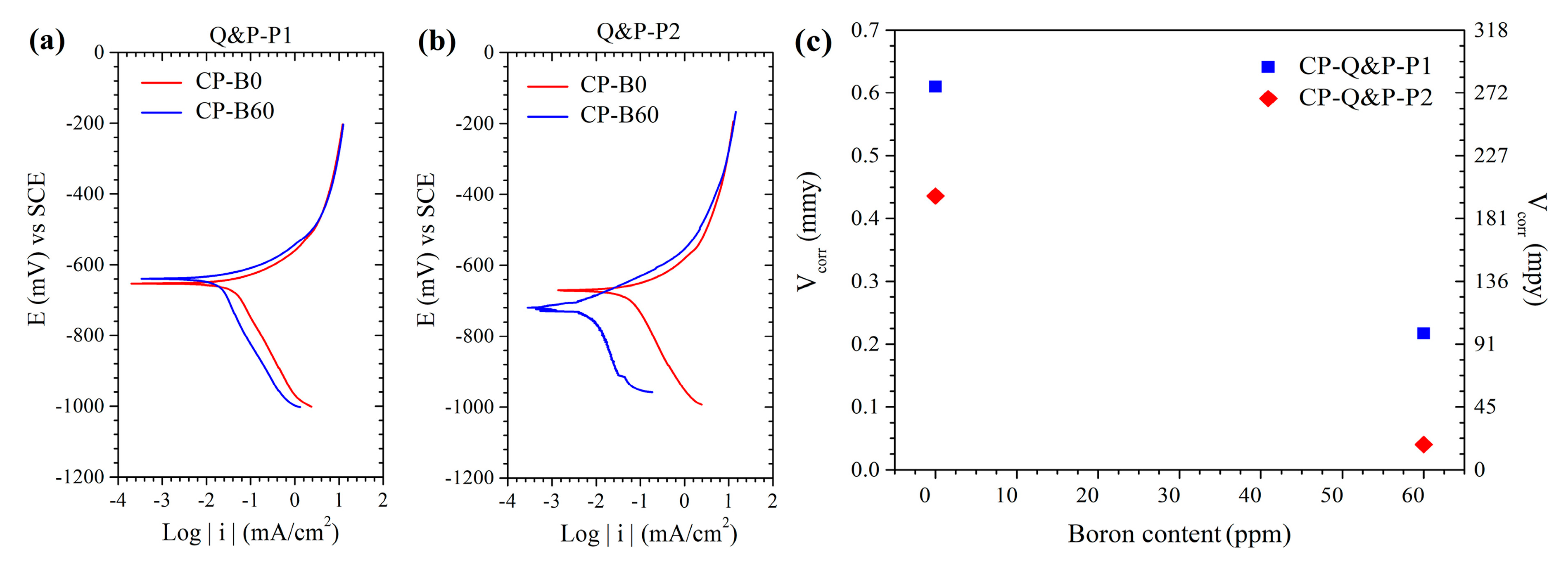
3.9. Corrosion Characterization Behavior of CP Steels Produced under the Q&P Conditions
4. Conclusions
- (1)
- Although the experimental advanced complex phase (CP) steel was fabricated from scrap steel, the fabricated boron-free and boron-containing steels gave no processing problems (i.e., on solidification or during thermo-mechanical, mechanical and thermal treatment);
- (2)
- The solidification grain size of the CP steel is refined by the boron addition from an average value of 207 μm for the boron-free steel to 140 μm for the boron-containing steel. The refining effect of B is attributed to an inoculation mechanism during the solidification process;
- (3)
- The B addition to CP steel by refining the grain size improved the hot ductility, increasing the %RA value from 40 to 60 in the trough. However, the hot ductility for both the boron-free and boron-containing steels met the recommended RA values (i.e., >40%) needed to avoid cracking during the straightening operation in continuous casting of the steels although this was marginally so for the boron-free steel. Fracture surface features generally show ductile behavior. This hot ductility behavior indicates that the CP steel can probably be processed satisfactorily along the entire path of mechanical forming, with the addition of boron giving it added assurance;
- (4)
- A bainitic matrix was always generated after the quenching and partitioning (Q&P) process, and within this matrix there were retained austenite (RA-γ) islands, martensite (M), bainite (B) and ferrite (F). In the case of the Q&P-P1 treatment, in both the boron-free and boron-containing steels, a RA-γ block morphology was observed, whereas for Q&P-P2, an acicular morphology of large islands of RA-γ were present. A surrounding layer of ferrite was also observed for the RA-γ of the boron treated steel given the P1 partitioning treatment;
- (5)
- B improves the room temperature strength and ductility of these CP-Q&P steels. With only a small addition of 60 ppm of B, there is an equivalence of up to 10 times more carbon in the retained austenite, producing a more stable retained austenite and leading to better ductility and strength. With this boron addition to the CP steel, the UTS increases by 200 MPa for both the P1 and P2 partitioning routes. Changing the partitioning route from one to two steps results in an approx. 100 MPa increase in UTS for both the boron-free and boron-containing steels. Hence, the greatest benefit comes with a boron addition and the P2 partitioning route so that a YS of 1212 MPa and UTS of 1594 MPa were achieved;
- (6)
- The B addition improves the corrosion resistance in the advanced complex phase (CP) steel when heat-treated using the quenching and partitioning (Q&P) process. The B addition gives a better distribution of the phases and carbon contents lead to less galvanic reaction. The P2 route by going to a higher partitioning temperature of 600 °C relieves the internal stresses and so improves the corrosion resistance. Again, the boron-containing CP steel given the two-step partitioning process (P2) has the greatest corrosion resistance of all the examined conditions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mintz, B.; Crowther, D.N. Hot ductility of steels and its relationship to the problem of transverse cracking in continuous casting. Inter. Mater. Rev. 2010, 55, 168–196. [Google Scholar] [CrossRef]
- Brune, T.; Senk, D.; Walpot, R.; Steenken, B. Hot ductility behavior of boron containing microalloyed steels with varying manganese content. Metal. Mater. Trans. B 2015, 46, 1400–1408. [Google Scholar] [CrossRef]
- Komenda, J.; Martin, D.; Lönnqvist, J. The effect of boron addition on precipitation and hot ductility of 1.5Mn-0.1Nb-Ti carbon steels in as-cast condition. Mater. Sci. Forum 2016, 879, 990–995. [Google Scholar] [CrossRef]
- Komenda, J.; Luo, C.; Lönnqvist, J. Interaction of carbon, titanium, and boron in micro-alloy steels and its effect on hot ductility. Alloys 2022, 1, 133–148. [Google Scholar] [CrossRef]
- Li, Q.; Liu, W. Effect of boron on hot ductility and room-temperature tensile properties of microalloyed steels with titanium and niobium. Materials 2019, 12, 2290. [Google Scholar] [CrossRef]
- Mejía, I.; Altamirano, G.; Bedolla-Jacuinde, A.; Cabrera, J.M. Effect of boron on the hot ductility behavior of a low carbon advanced ultra-high strength steel (A-UHSS). Metall. Mater. Trans. A 2013, 44, 5165–5176. [Google Scholar] [CrossRef]
- Kang, S.E.; Banerjee, J.R.; Maina, E.M.; Mintz, B. Influence of B and Ti on the hot ductility of high Al and high Al, Nb containing TWIP steels. Mater. Sci. Technol. 2013, 29, 1225–1232. [Google Scholar] [CrossRef]
- Kang, S.E.; Banerjee, J.R.; Tulin, A.S.; Mintz, B. Influence of B on hot ductility of high Al, TWIP steels. Mater. Sci. Technol. 2014, 30, 486–494. [Google Scholar] [CrossRef]
- Mejía, I.; Salas-Reyes, A.E.; Calvo, J.; Cabrera, J.M. Effect of Ti and B microadditions on the hot ductility behavior of a high-Mn austenitic Fe-23Mn-1.5Al-1.3Si-0.5C TWIP steel. Mater. Sci. Eng. A 2015, 648, 311–329. [Google Scholar] [CrossRef]
- Salas-Reyes, A.E.; Altamirano-Guerrero, G.; Chávez-Alcalá, J.F.; Barba-Pingarrón, A.; Figueroa, I.A.; Bolarín-Miró, A.M.; Sánchez-De Jesús, F.; Deaquino-Lara, R.; Salinas, A. Influence of boron content on the solidification structure, magnetic properties and hot mechanical behavior in an advanced as-cast TWIP steel. Metals 2020, 10, 1230. [Google Scholar] [CrossRef]
- Raabe, D.; Sun, B.; Kwiatkowski Da Silva, A.; Gault, B.; Yen, H.W.; Sedighiani, K.; Thouden-Sukumar, P.; Souza-Filho, I.R.; Katnagallu, S.; Jägle, E.; et al. Current challenges and opportunities in microstructure-related properties of advanced high-strength steels. Metall. Mater. Trans. A 2020, 51, 5517–5586. [Google Scholar] [CrossRef]
- Speer, J.G.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. Sci. 2004, 51, 2611–2622. [Google Scholar] [CrossRef]
- Li, Y.J.; Liu, D.; Zhang, W.N.; Kang, J.; Chen, D.; Yuan, G.; Wang, G.D. Quenching above martensite start temperature in quenching and partitioning (Q&P) steel through control of partial phase transformation. Mater. Lett. 2018, 230, 36–39. [Google Scholar]
- Pashangeh, S.; Somani, M.; Ghasemi-Banadkouki, S.S. Structure-property correlations of a medium C steel following quenching and isothermal holding above and below the Ms temperature. ISIJ Int. 2021, 61, 442–451. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Guo, Z.; Gu, J.; Gu, C. Corrosion behavior of a quenched and partitioned medium carbon steel in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 130, 64–75. [Google Scholar] [CrossRef]
- Miettinen, J.; Vassilev, G. Thermodynamic description of ternary Fe-B-X systems. Part 2: FeB-Ni. Arch. Metall. Mater. 2014, 59, 609–614. [Google Scholar] [CrossRef]
- Werner, D.W. Boron and Boron Containing Steels; Verlag Stahleisen mbH: Dusselfdorf, Germany, 1995. [Google Scholar]
- Watanabe, S.; Ohtani, H.; Kunitake, T. The influence of hot rolling and heat treatment on the distribution of boron in steels. Trans. ISIJ 1983, 23, 31–37. [Google Scholar] [CrossRef]
- Sharma, M.; Ortlepp, I.; Bleck, W. Boron in heat-treatable steels: A review. Steel Res. Int. 2019, 90, 1900133. [Google Scholar] [CrossRef]
- López-Chipres, E.; Mejía, I.; Maldonado, C.; Bedolla-Jacuinde, A.; Cabrera, J.M. Hot ductility behavior of boron microalloyed steels. Mater. Sci. Eng. A 2007, 460–461, 464–470. [Google Scholar] [CrossRef]
- Yamanaca, K.; Ohmori, Y. Effect of boron on transformation of low-carbon low-alloy steels. J. Iron Steel Inst. Jpn. 1976, 62, 895–904. [Google Scholar] [CrossRef]
- Kurtz, R.A. Grain Refinement in Steel Castings. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1960. [Google Scholar]
- Mintz, B.; Yue, S.; Jonas, J.J. Hot ductility of steels and its relationship to the problem of transverse cracking during continuous-casting. Inter. Mater. Rev. 1991, 36, 187–217. [Google Scholar] [CrossRef]
- Mintz, B. The influence of composition on the hot ductility of steels and to the problem of transverse cracking. ISIJ Int. 1999, 39, 833–855. [Google Scholar] [CrossRef]
- Mintz, B.; Jonas, J.J. Influence of strain-rate on production of deformation-induced ferrite and hot ductility of steels. Mater. Sci. Technol. 1994, 10, 721–727. [Google Scholar] [CrossRef]
- Mintz, B. Importance of Ar3 temperature in controlling ductility and width of hot ductility trough in steels, and its relationship to transverse cracking. Mater. Sci. Technol. 1996, 12, 132–138. [Google Scholar] [CrossRef]
- Miller, M.K.; Pareige, P.J.; Russell, K.F. Seeing and Catching Atoms. In An Oak Ridge National Laboratory Report; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2001. [Google Scholar]
- Laha, K.; Kyono, J.; Sakai, T.; Kishimoto, S.; Shinya, N. Austenitic stainless steel through the self-healing effect of boron for creep cavitation. Metall. Mater. Trans. A 2005, 36, 399–409. [Google Scholar] [CrossRef]
- Liu, Y.; Du, L.X.; Wu, H.Y.; Misra, R.D.K. Hot ductility and fracture phenomena of low-carbon V-N-Cr microalloyed steels. Steel Res. Int. 2019, 91, 1900265. [Google Scholar] [CrossRef]
- Salas-Reyes, A.E.; Mejía, I.; Bedolla-Jacuinde, A.; Boulaajaj, A.; Calvo, J.; Cabrera, J.M. Hot ductility behavior of high-Mn austenitic Fe-22Mn-1.5Al-1.5Si-0.45C TWIP steels microalloyed with TI and V. Mater. Sci. Eng. A 2014, 611, 77–89. [Google Scholar] [CrossRef]
- Wang, X.M.; He, X.L. Effect of boron addition on structure and properties of low carbon bainitic steels. ISIJ Inter. 2002, 42, S38–S46. [Google Scholar] [CrossRef]
- Zurutuza, I.; Isasti, N.; Detemple, E.; Schwinn, V.; Mohrbacher, H.; Uranga, P. Effect of Nb and Mo additions in the micro-structure/tensile property relationship in high strength quenched and quenched and tempered boron steels. Metals 2021, 11, 29. [Google Scholar] [CrossRef]
- Yoshida, S.; Ushioda, K.; Agren, J. Knetic modelo f the γ to α phase transformation at grain boundaries on boron-bearing low-alloy steel. ISIJ Int. 2014, 54, 685–692. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.K. Effect of austenite grain size on martensitic transformation of a low alloy steel. Mater. Sci. Forum 2005, 475-479, 3169–3172. [Google Scholar] [CrossRef]
- Celada-Casero, C.; Sietsma, J.; Santofimia, M.J. The role of the austenitic grain size in the martensitic transformation in low carbon steels. Mater. Des. 2019, 167, 107625. [Google Scholar] [CrossRef]
- Aoued, S.; Danoix, F.; Allain, S.; Gaudez, S.; Geandier, G.; Hell, J.C.; Soler, M.; Gouné, M. Microstructure evolution and competitive reactions during quenching and partitioning of a model Fe-C-Mn-Si alloy. Metals 2020, 10, 137. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Kang, P.; Duan, X.; Song, C. Study of microstructure, mechanical properties and impact-abrasive wear behavior of medium-carbon steel treated by quenching and partitioning (Q&P) process. Wear 2018, 414–415, 21–30. [Google Scholar]
- Bigg, T.D. Quenching and Partitioning: A New Steel Heat Treatment Concept. Ph.D. Thesis, The University of Leeds, Leeds, UK, 2011. [Google Scholar]
- Entezari, E.; Mousalou, H.; Yazdani, S.; González-Velázquez, J.L.; Szpunar, J.A. The evaluation of quenching temperature effect on microstructural and mechanical properties of advanced high strength low carbon steel after quenching partitioning treatment. Procedia Struct. Integr. 2022, 37, 145–152. [Google Scholar] [CrossRef]
- Bai, B.; Gao, G.; Gui, X.; Tan, Z.; Weng, Y. Enhanced mechanical properties of ultrahigh strength Mn-Si-Cr-C steels treated by a novel bainitic transformation plus quenching and partitioning process. Heat Treat. Surf. Eng. 2019, 1, 63–71. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Centeno, D.; Tressia, G.; Avila, J.A.; Cezario, F.E.M.; Márquez-Rossy, A.; Ariza, E.A.; Masouni, M. Development of a complex microstructure on commercial carbon-silicon grade steel by governing the phase transformation mechanisms to design novel quenching and partitioning processing. J. Mater. Res. Technol. 2022, 18, 4590–4603. [Google Scholar] [CrossRef]
- Mesplont, C. Phase Transformations and Microstructure-Mechanical Properties Relations in Complex Phase High Strength Steels. Ph.D. Thesis, Universitaire de Lille, Lille, France, 2002. [Google Scholar]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corr. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Mehner, T.; Morgenstern, R.; Frint, P.; Scharf, I.; Wagner, M.F.X. Corrosion characteristics of a quenching and partitioning steel determined by electrochemical impedance spectroscopy. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 373, 012003. [Google Scholar] [CrossRef]
- Yang, L. Techniques for Corrosion Monitoring, 2nd ed.; Elservier: Cambridge, MA, USA, 2021. [Google Scholar]
- Cetin, M. Effect of boron added corrosion behavior of cast 304 stainless steel. Prot. Met. Phys. Chem. 2019, 55, 1217–1225. [Google Scholar]










| Element | CP-B0 Steel | CP-B60 Steel |
|---|---|---|
| C | 0.16 | 0.16 |
| Mn | 1.94 | 1.93 |
| Si | 0.591 | 0.59 |
| Cr | 0.44 | 0.44 |
| Ni | 0.12 | 0.12 |
| Mo | 0.401 | 0.397 |
| Cu | 0.068 | 0.068 |
| Nb | 0.151 | 0.156 |
| Ti | 0.018 | 0.014 |
| V | 0.01 | 0.01 |
| Al | 0.012 | 0.01 |
| S | 0.031 | 0.03 |
| P | 0.022 | 0.023 |
| N | 0.012 | 0.012 |
| B | 0 | 0.006 |
| Fe | Bal. | Bal. |
| Steel | Thickness (mm) | Width (mm) | Elongation (%) | YS (MPa) | UTS (MPa) |
|---|---|---|---|---|---|
| CP-Q&P-B0-P1 | 1.43 | 4.88 | 6.9 | 1090.4 | 1300.7 |
| CP-Q&P-B0-P2 | 1.3 | 5.22 | 8.2 | 1074.1 | 1399.5 |
| CP-Q&P-B60-P1 | 1.15 | 4.69 | 9.7 | 1055.7 | 1515.8 |
| CP-Q&P-B60-P2 | 1.35 | 4.69 | 11.3 | 1212.2 | 1593.9 |
| CP-Q&P-P1 | CP-Q&P-P2 | |||
|---|---|---|---|---|
| B Content (ppm) | Ecorr (mV vs. SCE) | Icorr (mA/cm2) | Ecorr (mV vs. SCE) | Icorr (mA/cm2) |
| 0 | −652 | 42.1 | −670 | 45.3 |
| 60 | −637.6 | 17.7 | −717 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Reyes, A.E.; Altamirano-Guerrero, G.; Deaquino, R.; Salinas, A.; Lara-Rodriguez, G.; Figueroa, I.A.; González-Parra, J.R.; Mintz, B. The Hot Ductility, Microstructures, Mechanical Properties and Corrosion Resistance in an Advanced Boron-Containing Complex Phase Steel Heat-Treated Using the Quenching and Partitioning (Q&P) Process. Metals 2023, 13, 257. https://doi.org/10.3390/met13020257
Salas-Reyes AE, Altamirano-Guerrero G, Deaquino R, Salinas A, Lara-Rodriguez G, Figueroa IA, González-Parra JR, Mintz B. The Hot Ductility, Microstructures, Mechanical Properties and Corrosion Resistance in an Advanced Boron-Containing Complex Phase Steel Heat-Treated Using the Quenching and Partitioning (Q&P) Process. Metals. 2023; 13(2):257. https://doi.org/10.3390/met13020257
Chicago/Turabian StyleSalas-Reyes, Antonio Enrique, Gerardo Altamirano-Guerrero, Rogelio Deaquino, Armando Salinas, Gabriel Lara-Rodriguez, Ignacio Alejandro Figueroa, Jesús Rafael González-Parra, and Barrie Mintz. 2023. "The Hot Ductility, Microstructures, Mechanical Properties and Corrosion Resistance in an Advanced Boron-Containing Complex Phase Steel Heat-Treated Using the Quenching and Partitioning (Q&P) Process" Metals 13, no. 2: 257. https://doi.org/10.3390/met13020257
APA StyleSalas-Reyes, A. E., Altamirano-Guerrero, G., Deaquino, R., Salinas, A., Lara-Rodriguez, G., Figueroa, I. A., González-Parra, J. R., & Mintz, B. (2023). The Hot Ductility, Microstructures, Mechanical Properties and Corrosion Resistance in an Advanced Boron-Containing Complex Phase Steel Heat-Treated Using the Quenching and Partitioning (Q&P) Process. Metals, 13(2), 257. https://doi.org/10.3390/met13020257








