Electrodeposition of Tin-Reduced Graphene Oxide Composite from Deep Eutectic Solvents Based on Choline Chloride and Ethylene Glycol
Abstract
1. Introduction
2. Materials and Methods
3. Results
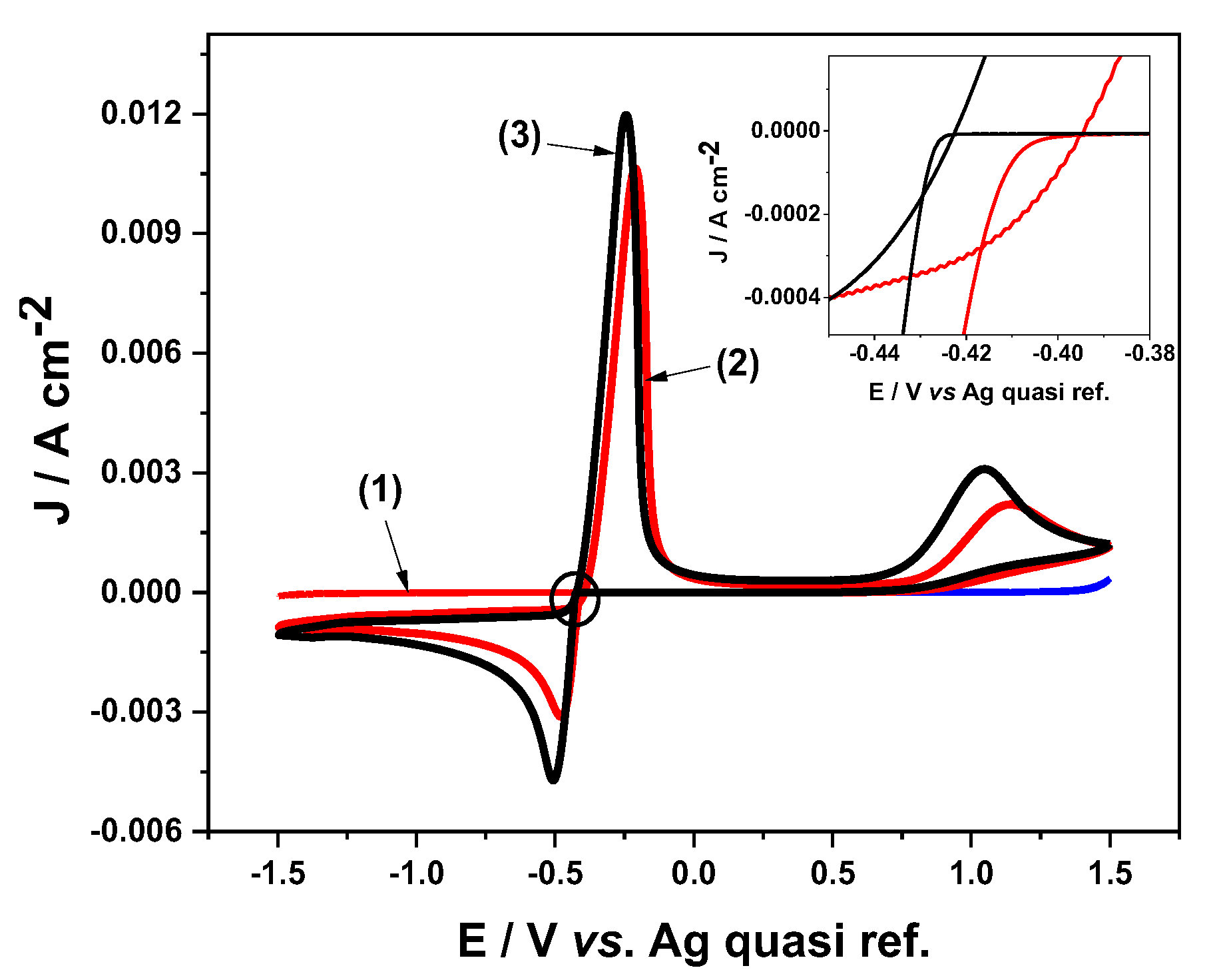
3.1. Cyclic Voltammetry
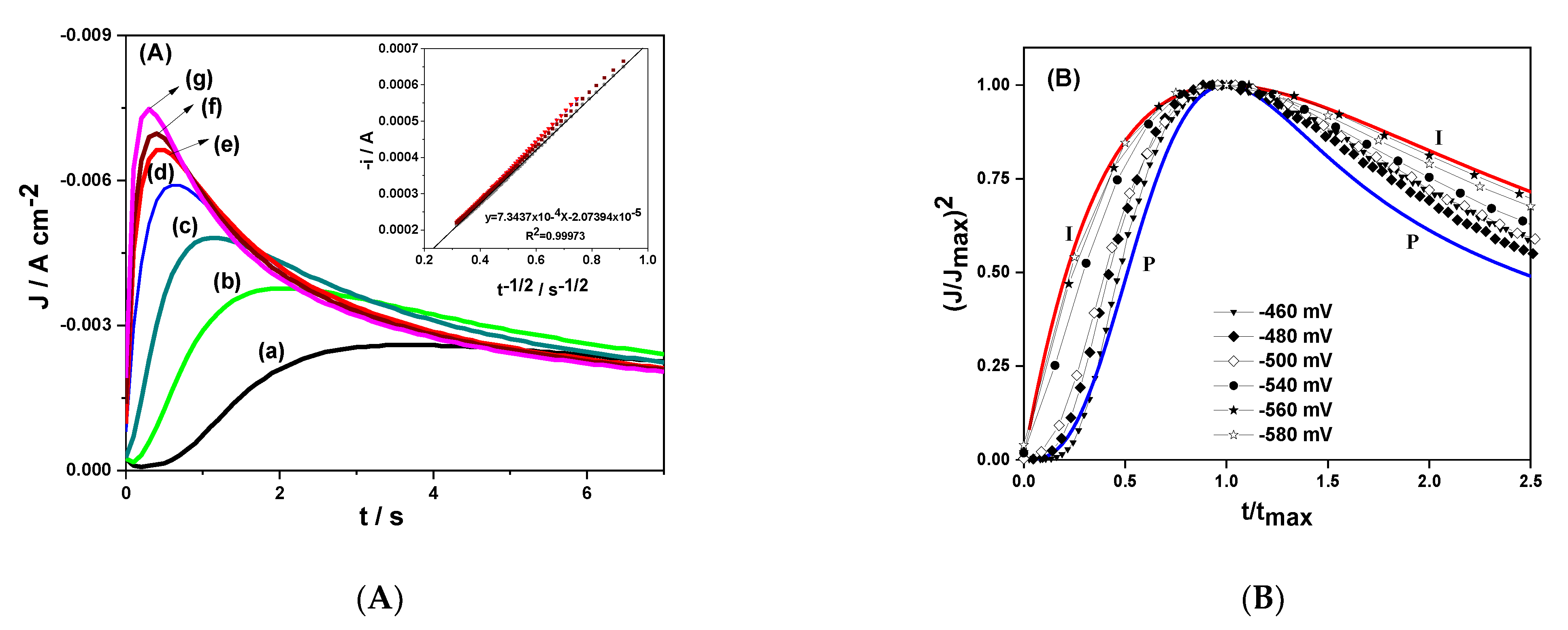
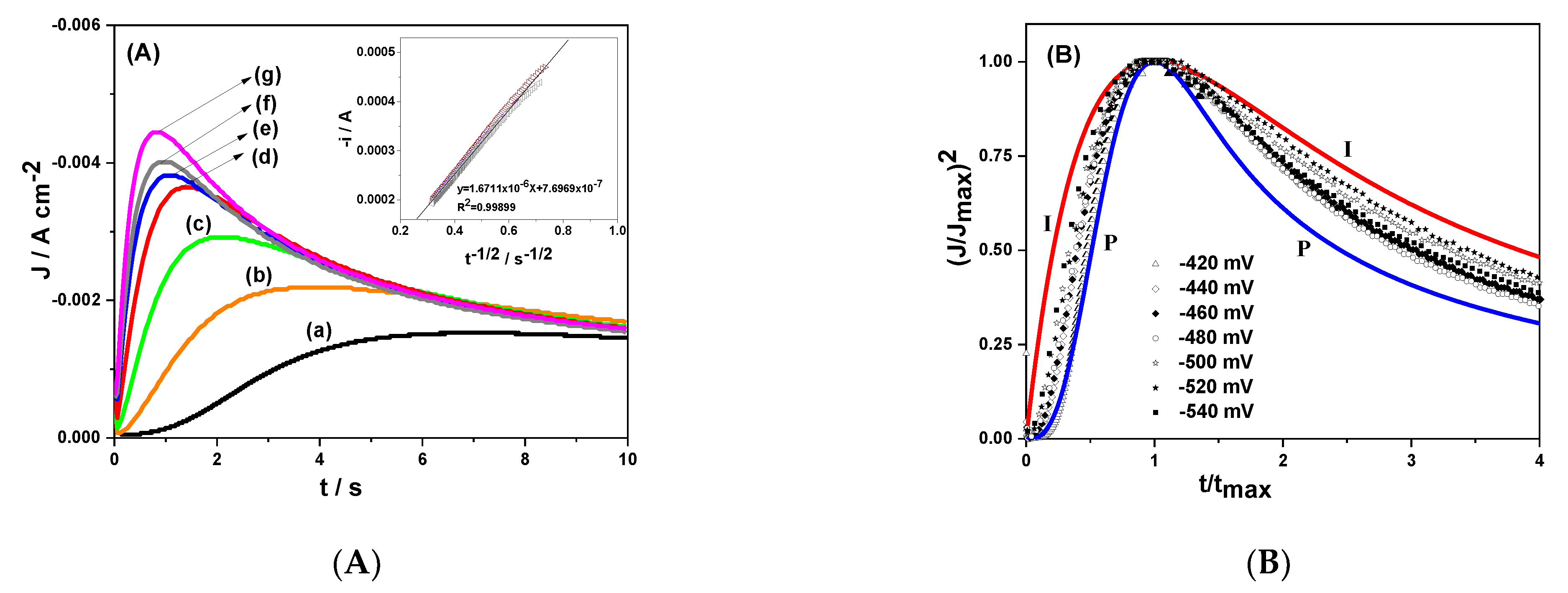
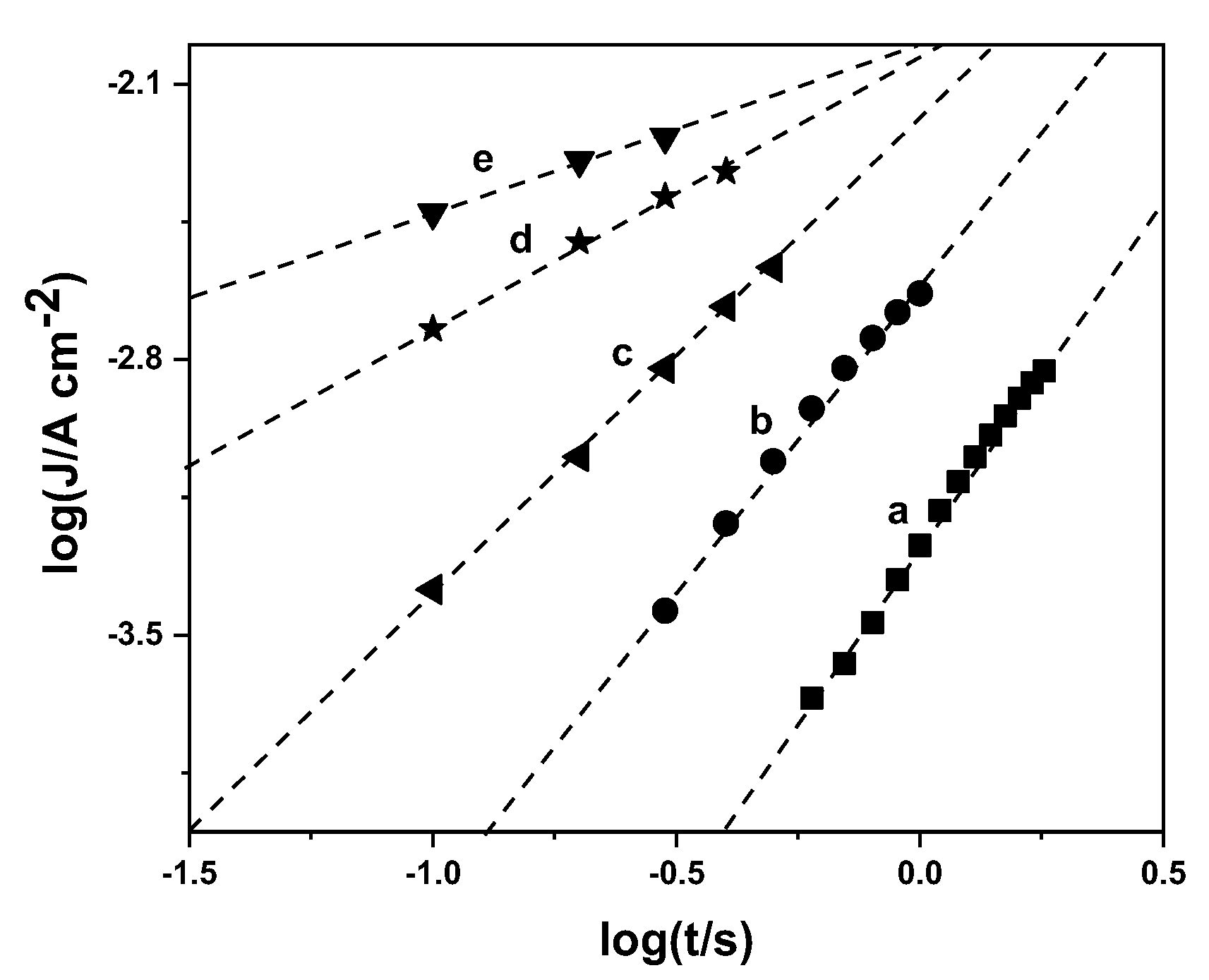
3.2. Chronoamperometry
3.3. Physical Characterization of Sn-rGO Composite Coatings
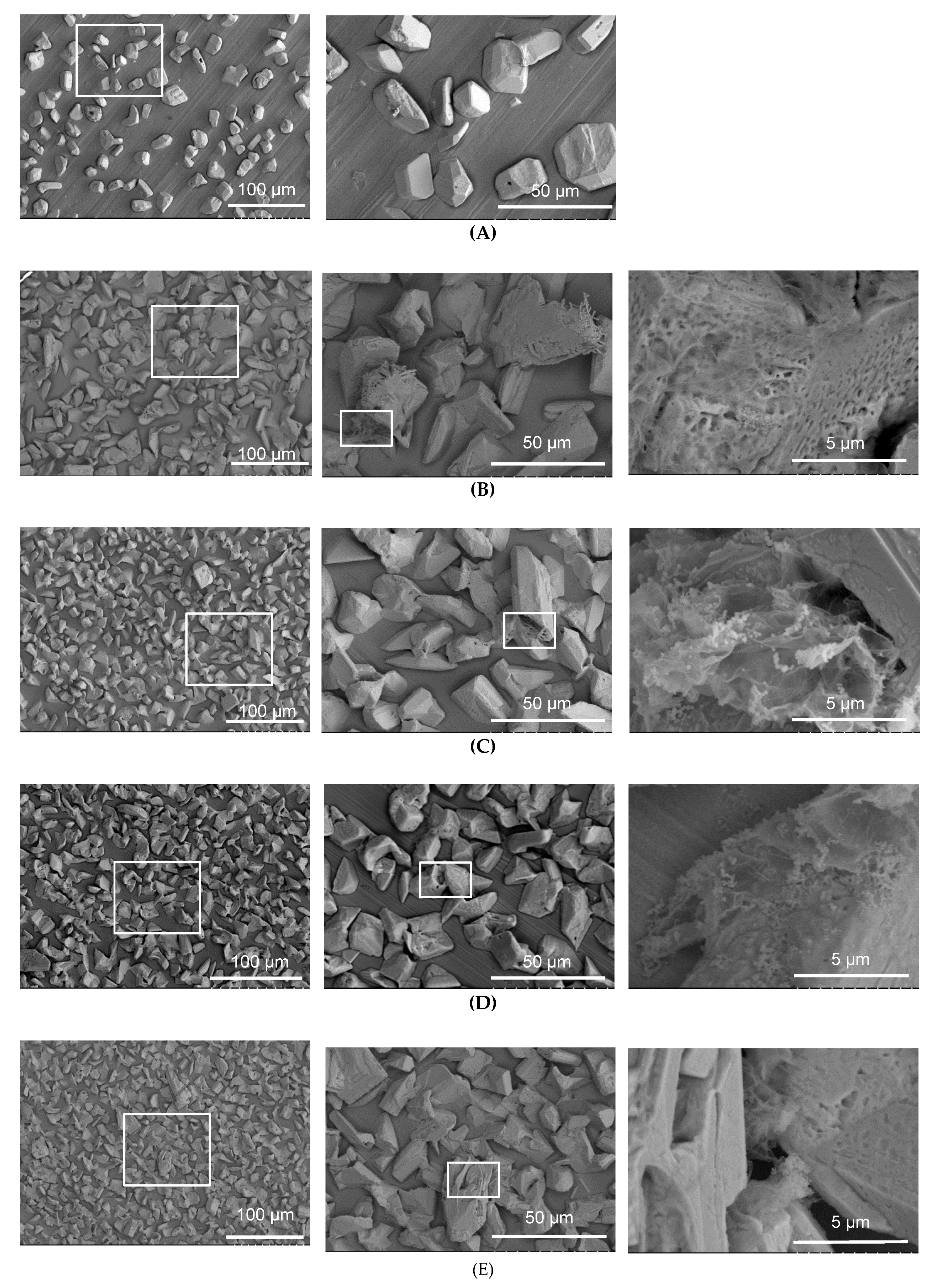
3.3.1. Surface Morphology
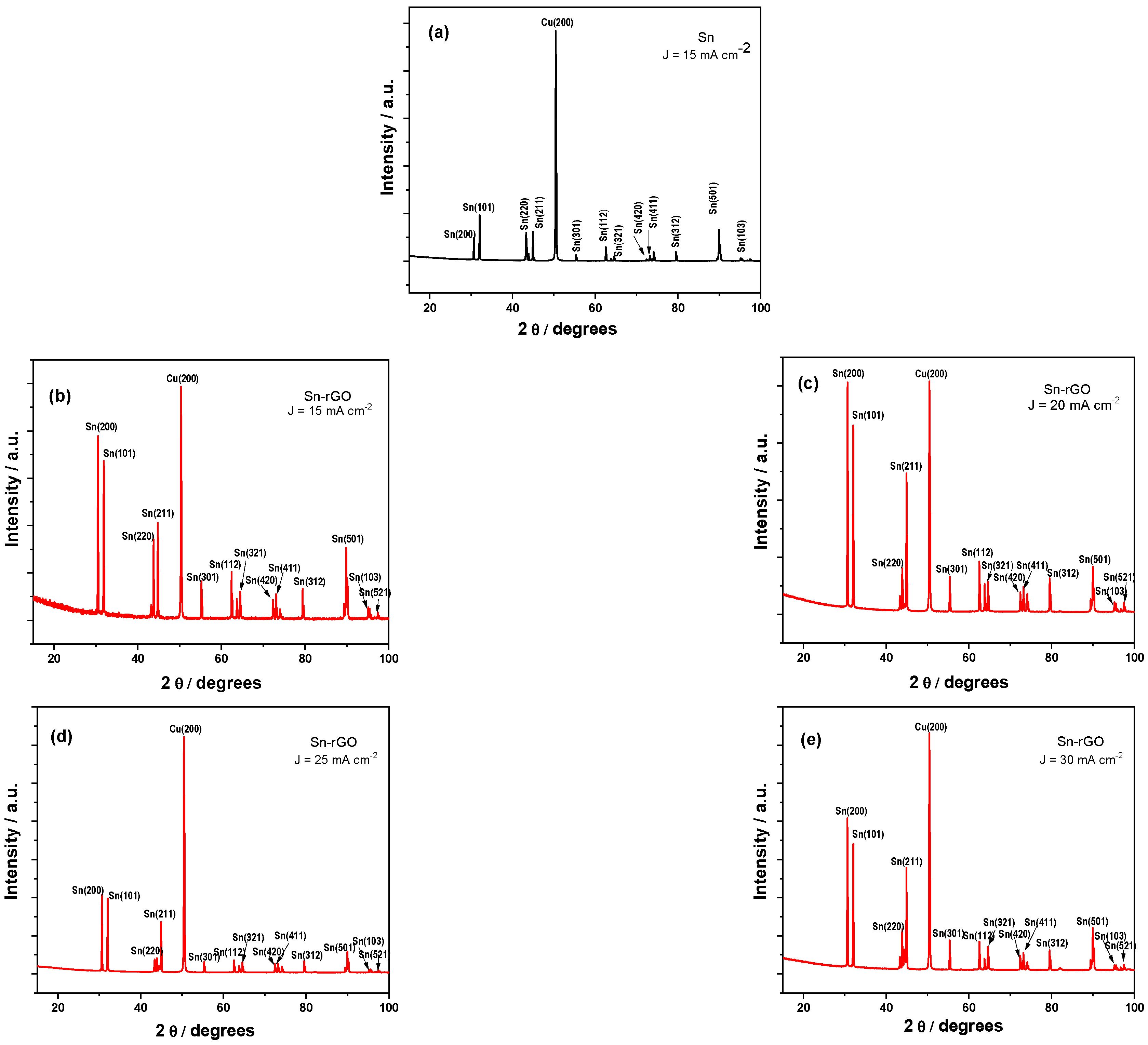
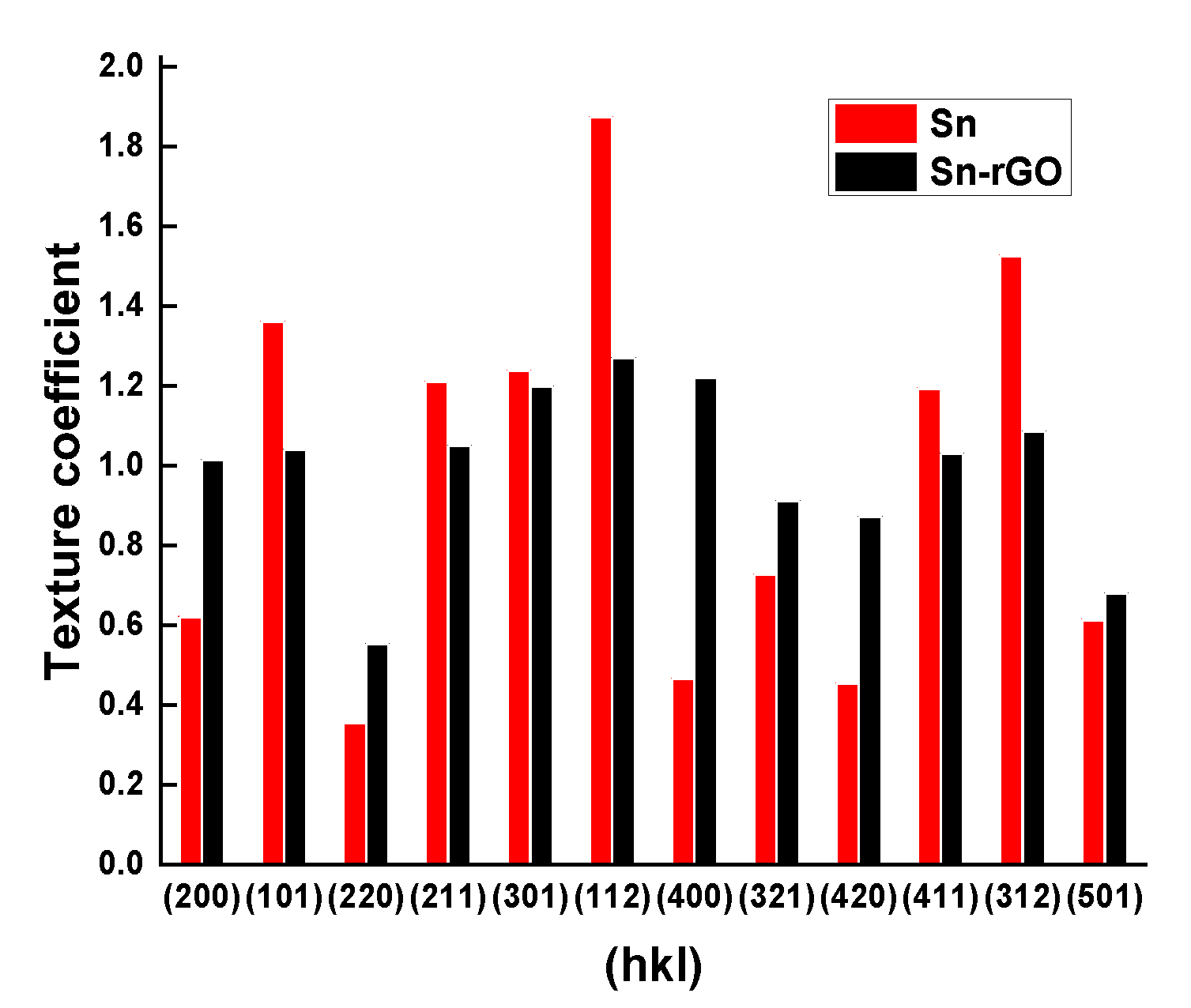
3.3.2. XRD Analysis
3.3.3. Raman Spectroscopy
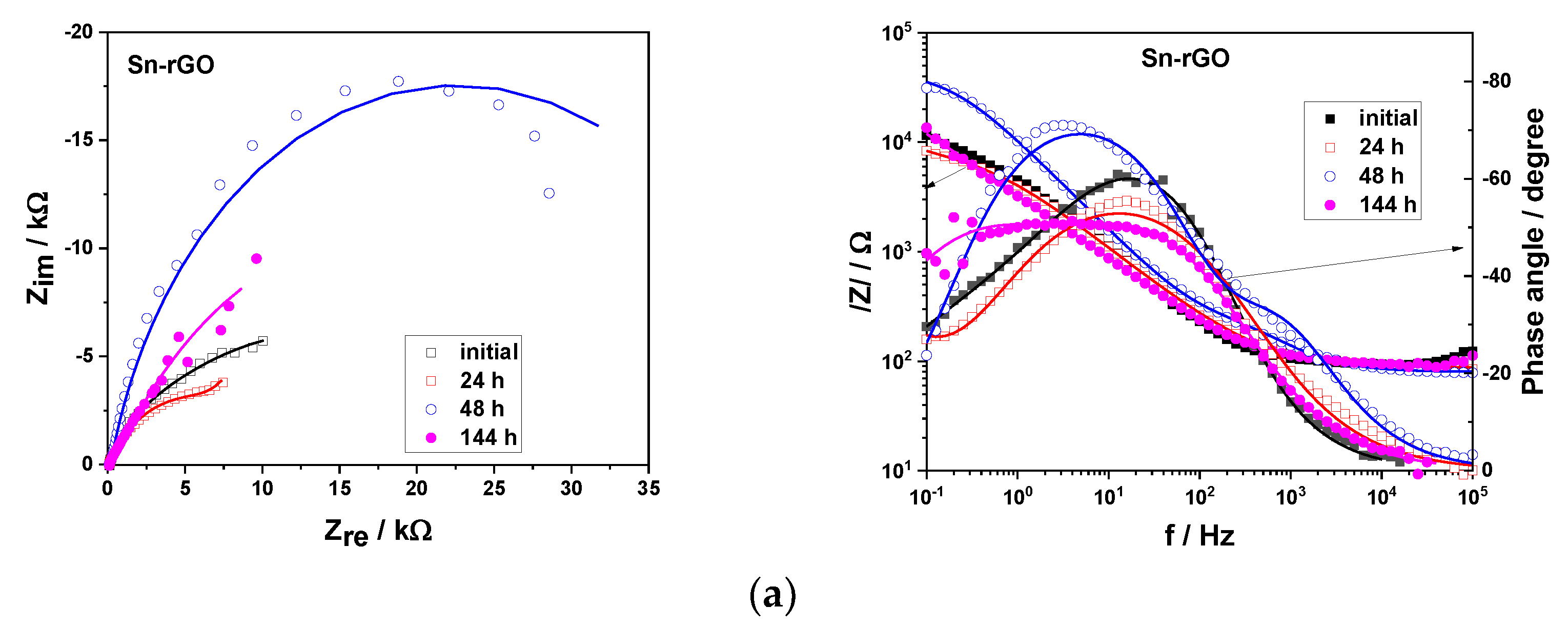
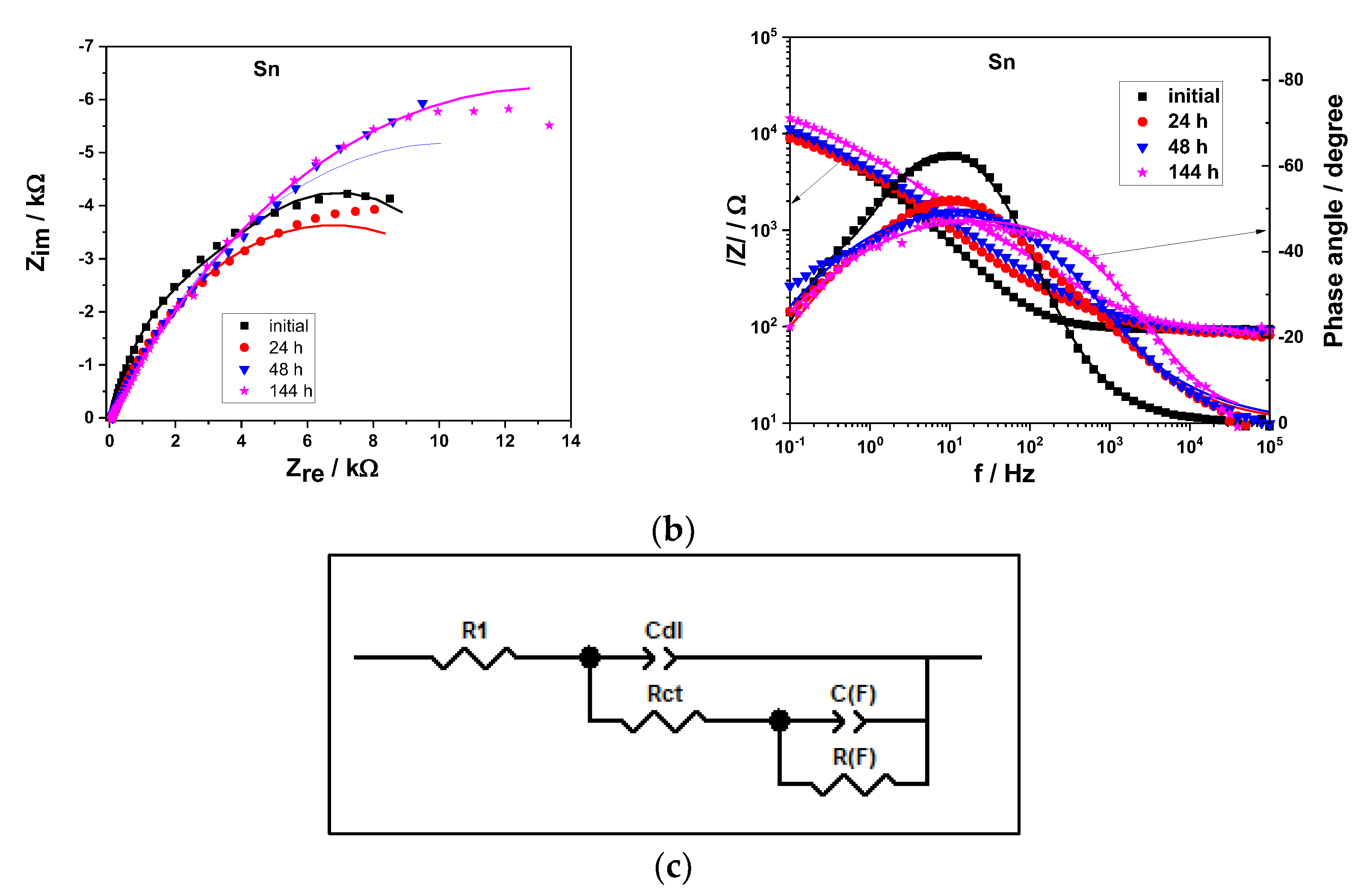
3.4. Corrosion Behavior of the Sn and Sn-rGO Composite Coatings
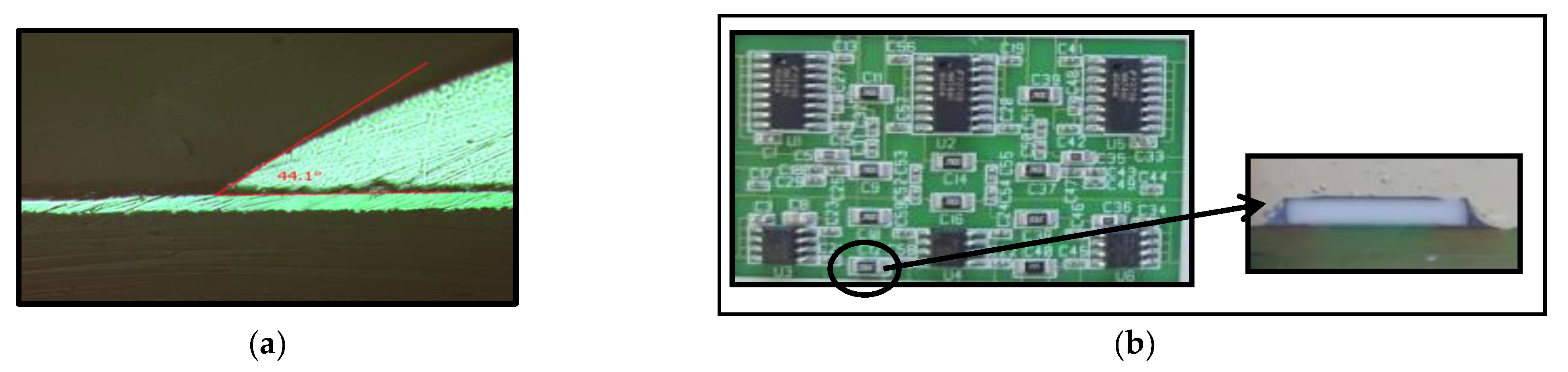
3.5. Solderability Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano. Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.C. Applications of Graphene and Graphene-Oxide Based Nanomaterials, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; William Andrew Publishing: Waltham, MA, USA, 2015; pp. 1–38. [Google Scholar]
- Sadabadi, H.; Ghaderi, O.; Kordijazi, A.; Rohatgi, P.K. Graphene derivatives reinforced metal matrix nanocomposite coatings: A review. J. Met. Mater. Miner. 2022, 32, 1–14. [Google Scholar] [CrossRef]
- Biswal, H.J.; Vundavilli, P.R.; Gupta, A. Perspective—Electrodeposition of graphene reinforced metal matrix composites for enhanced mechanical and physical properties: A review. J. Electrochem. Soc. 2022, 167, 146501. [Google Scholar] [CrossRef]
- Hu, Z.; Tong, G.; Lin, D.; Chen, C.; Guo, H.; Xu, J.; Zhou, L. Graphene-reinforced metal matrix nanocomposites—A review. Mater. Sci. Technol. 2016, 32, 930–953. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, D.; Hu, X.; Han, H.; Lin, M.; Wang, C. An electrochemical sensor based on reduced graphene oxide/gold nanoparticles modified electrode for determination of iron in coastal waters. Sens. Actuators B Chem. 2017, 243, 1–7. [Google Scholar] [CrossRef]
- Hussain, A.K.; Al Naib, U.M.B. Recent developments in graphene based metal matrix composite coatings for corrosion protection application: A review. J. Met. Mater. Miner. 2019, 29, 1–9. Available online: https://jmmm.material.chula.ac.th/index.php/jmmm/article/view/540 (accessed on 17 December 2022).
- Zhao, J.; Pei, S.; Ren, W.; Gao, L.; Cheng, H. Efficient preparation of large-area graphene oxide sheets for transparent conductive films. ACS Nano 2010, 4, 5245–5252. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Fernandez-Merino, M.J.; Guardia, L.; Martinez-Alonso, A.; Tascon, J.M.D. Environmentally friendly approaches toward the mass production of processable graphene from graphite oxide. J. Mater. Chem. 2011, 21, 298–306. [Google Scholar] [CrossRef]
- Wang, G.X.; Yang, J.; Park, J.; Guo, X.L.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.Q.; Wu, N.Q.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2–graphene nanocomposites. UV assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Photocatalytic reduction of graphene oxides hybridized by ZnO nanoparticles in ethanol. Carbon 2011, 49, 11–18. [Google Scholar] [CrossRef]
- Liu, X.; Kim, H.; Guo, L.J. Optimization of thermally reduced graphene oxide for an efficient hole transport layer in polymer solar cells. Org. Electron. 2013, 14, 591–598. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Hung, Y.-F.; Cheng, C.; Huang, C.-K.; Yang, C.-R. A facile method for batch preparation of electrochemically reduced graphene oxide. Nanomaterials 2019, 9, 376. [Google Scholar] [CrossRef]
- Gao, F.; Qi, X.; Cai, X.; Wang, Q.; Gao, F.; Sun, W. Electrochemically reduced graphene modified carbon ionic electrode for sensitive sensing of rutin. Thin Solid Films 2012, 520, 5064–5069. [Google Scholar] [CrossRef]
- Hung, Y.F.; Cheng, C.; Huang, C.K.; Yang, C.R.; Tseng, S.F. Investigation of electrochemical reduction effects on graphene oxide powders for high-performance supercapacitors. Int. J. Adv. Manuf. Technol. 2021, 113, 1203–1213. [Google Scholar] [CrossRef]
- Quezada Renteria, J.A.; Ruiz-Garcia, C.; Sauvage, T.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R.; Ania, C.O. Photochemical and electrochemical reduction of graphene oxide thin films: Tuning the nature of surface defects. Phys. Chem. Chem. Phys. 2020, 22, 20732–20743. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct electrodeposition of graphene enabling the one-step synthesis of graphene–metal nanocomposite films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Chen, J.J.; Wang, F.B.; Sheng, Z.H.; Xia, X.H. A facile approach to the synthesis of highly electroactive Pt nanoparticles on graphene as an anode catalyst for direct methanol fuel cells. Chem. Commun. 2010, 46, 5951–5953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, Y.; Li, F.; Wu, T.; Niu, L.; Chen, W. Facile electrochemical codeposition of ‘‘clean’’ graphene–Pd nanocomposite as an anode catalyst for formic acid electrooxidation. Electrochem. Commun. 2012, 19, 21–24. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, O.; Winther-Jensen, B.; MacFarlane, D.R. Graphene/zinc nano-composites by electrochemical co-deposition. Phys. Chem. Chem. Phys. 2012, 14, 14034–14040. [Google Scholar] [CrossRef]
- Kuang, D.; Xu, L.; Liu, L.; Hu, W.; Wu, Y. Graphene–nickel composites. Appl. Surf. Sci. 2013, 273, 484–490. [Google Scholar] [CrossRef]
- Xie, G.; Forslund, M.; Pan, J. Direct electrochemical synthesis of reduced graphene oxide (rGO)/copper composite films and their electrical/electroactive properties. ACS Appl. Mater. Interfaces 2014, 6, 7444–7455. [Google Scholar] [CrossRef]
- Toosinezhad, A.; Alinezhadfar, M.; Mahdavi, S. Cobalt/graphene electrodeposits: Characteristics, tribological behavior, and corrosion properties. Surf. Coat.Technol. 2020, 385, 125418. [Google Scholar] [CrossRef]
- Berlia, R.; Punith Kumar, M.K.; Srivastava, C. Electrochemical behavior of Sn-graphene composite coating. RSC Adv. 2015, 5, 71413–71418. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, C. Optimum amount of graphene oxide for enhanced corrosion resistance by tin-graphene oxide composite coatings. Thin Solid Films 2018, 661, 98–107. [Google Scholar] [CrossRef]
- Hari Mohan, E.; Sarada, B.V.; Venkata Ram Naidu, R.; Salian, G.; Haridas, A.K.; Appa Rao, B.V.; Rao, T.N. Graphene-modified electrodeposited dendritic porous tin structures as binder free anode for high performance lithium-sulfur batteries. Electrochim. Acta 2016, 219, 701–710. [Google Scholar] [CrossRef]
- Sharma, A.; Sohn, H.-R.; Jung, J.P. Effect of Graphene Nanoplatelets on Wetting, Microstructure, and Tensile Characteristics of Sn-3.0Ag-0.5Cu (SAC) Alloy. Metall. Mater. Trans. A 2015, 47, 494–503. [Google Scholar] [CrossRef]
- Xu, L.Y.; Zhang, Z.K.; Jing, H.Y.; Wei, J.; Han, Y.D. Effect of graphene nanosheets on the corrosion behavior of Sn–Ag–Cu solders. J. Mater. Sci. Mater. Electron. 2015, 26, 5625–5634. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, S.; Wang, J.; Xue, P.; Zhong, S.; Long, W. Effect of Nanoparticles Addition on the Microstructure and Properties of Lead-Free Solders: A Review. Appl. Sci. 2019, 9, 2044. [Google Scholar] [CrossRef]
- Li, M.l.; Gao, L.l.; Zhang, L.; Jiang, N.; Zhong, S.; Zhang, L. Interfacial reaction and properties of Sn/Cu solder reinforced with graphene nanosheets during solid–liquid diffusion and reflowing. J. Mater. Sci. Mater. Electron. 2021, 32, 26666–26675. [Google Scholar] [CrossRef]
- Rekha, M.Y.; Kamboj, A.; Srivastava, C. Electrochemical behavior of SnNi-graphene oxide composite coatings. Thin Solid Films 2018, 653, 82–92. [Google Scholar] [CrossRef]
- Rekha, M.Y.; Kamboj, A.; Srivastava, C. Electrochemical behaviour of SnZn-graphene oxide composite coatings. Thin Solid Films 2017, 636, 593–601. [Google Scholar] [CrossRef]
- Singh, S.K.; Samanta, S.; Das, A.K.; Sahoo, R.R. Tribological investigation of Ni-graphene oxide composite coating produced by pulsed electrodeposition. Surf. Interfaces 2018, 12, 61–70. [Google Scholar] [CrossRef]
- Szeptycka, B.; Gajewska-Midzialek, A.; Babul, T. Electrodeposition and corrosion resistance of Ni-graphene composite coatings. J. Mater. Eng. Perform. 2016, 25, 3134–3138. [Google Scholar] [CrossRef]
- Polo-Luque, M.L.; Simonet, B.M.; Valcárcel, M. Functionalization and dispersion of carbon nanotubes in ionic liquids. Trends Anal. Chem. 2013, 47, 99–110. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, W.; Zhang, J.; Qiao, X.; Zhang, J.; He, J.; Liu, C.-Y. Stable dispersions of reduced graphene oxide in ionic liquids. J. Mater. Chem. 2010, 20, 5401–5403. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; Al-Saadi, M.A.; Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Endres, F.; Abbott, A.P.; MacFarlane, D.R. Electrodeposition from Ionic Liquids, 2nd ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017; ISBN 978-3-527-33602-9. [Google Scholar]
- Fang, Y.K.; Osama, M.; Rashmi, W.; Shahbaz, K.; Khalid, M.; Mjalli, F.S.; Farid, M.M. Synthesis and thermo-physical properties of deep eutectic solvent-based graphene nanofluids. Nanotechnology 2016, 27, 075702. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, J.; Hou, Y.; Chu, Q. Enhanced corrosion performance of Zn coating by incorporating graphene oxide electrodeposited from deep eutectic solvent. RSC Adv. 2015, 5, 60698–60707. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, C.M.; Pecht, M. A review of lead-free solders for electronic applications. Microelectron. Reliab. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Anicai, L.; Petica, A.; Costovici, S.; Prioteasa, P.; Visan, T. Electrodeposition of Sn and NiSn alloys coatings using choline chloride based ionic liquids: Evaluation of corrosion behavior. Electrochim. Acta 2013, 114, 868–877. [Google Scholar] [CrossRef]
- Gu, C.D.; Mai, Y.J.; Zhou, J.P.; You, Y.H.; Tu, J.P. Non-aqueous electrodeposition of porous tin-based film as an anode for lithium-ion battery. J. Power Sources 2012, 214, 200–207. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; McKenzie, K.J.; Ryder, K.S. Electrodeposition of zinc-tin alloys from deep eutectic solvents based on choline chloride. J. Electroanal. Chem. 2007, 599, 288–294. [Google Scholar] [CrossRef]
- Salomé, S.; Pereira, N.M.; Ferreira, E.S.; Pereira, C.M.; Silva, A.F. Tin electrodeposition from choline chloride based solvent: Influence of the hydrogen bond donors. J. Electroanal. Chem. 2013, 703, 80–87. [Google Scholar] [CrossRef]
- Vieira, L.; Burt, J.; Richardson, P.; Schloffer, D.; Fuchs, D.; Moser, A.; Bartlett, P.; Reid, G.; Gollas, B. Tin, bismuth, and tin-bismuth alloy electrodeposition from chlorometalate salts in deep eutectic solvents. Chemistryopen 2017, 6, 393–401. [Google Scholar] [CrossRef]
- Yingxin, G.; Haseeb, A.S.M.A.; Sabri, M.F.M. Electrodeposition of lead-free solder alloys. Solder. Surf. Mt. Technol. 2013, 25, 76–90. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, S. Characterization of tin films synthesized from ethaline deep eutectic solvent. Mater. Sci. Eng. B 2014, 190, 104–110. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, W.; Gao, X.; Duan, B. Electrodeposition of SnBi coatings based on deep eutectic solvent. Surf. Eng. 2014, 30, 59–63. [Google Scholar] [CrossRef]
- Anicai, L.; Petica, A.; Costovici, S.; Moise, C.; Brincoveanu, O.; Visan, T. Electrodeposition of Sn–In alloys involving deep eutectic solvents. Coatings 2019, 9, 800. [Google Scholar] [CrossRef]
- Shaban, M.; Kholidy, I.; Ahmed, G.M.; Negem, M.; Abd El-Salam, H.M. Cyclic voltammetry growth and characterization of Sn–Ag alloys of different nanomorphologies and compositions for efficient hydrogen evolution in alkaline solutions. RSC Adv. 2019, 9, 22389–22400. [Google Scholar] [CrossRef] [PubMed]
- Rosoiu, S.P.; Costovici, S.; Moise, C.; Petica, A.; Anicai, L.; Visan, T.; Enachescu, M. Electrodeposition of ternary Sn-Cu-Ni alloys as lead-free solders using deep eutectic solvents. Electrochim. Acta 2021, 398, 139339. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Anicai, L.; Lazar, O.A.; Rosoiu, S.; Pantazi, A.; Costa, R.; Enachescu, M.; Pereira, C.M.; Silva, A.F. Electrodeposition of Sn and Sn composites with carbon materials using choline chloride-based ionic liquids. Coatings 2019, 9, 798. [Google Scholar] [CrossRef]
- Rosoiu, S.P.; Pantazi, A.G.; Petica, A.; Cojocaru, A.; Costovici, S.; Zanella, C.; Visan, T.; Anicai, L.; Enachescu, M. Electrodeposition of NiSn-rGO composite coatings from deep eutectic solvents and their physicochemical characterization. Metals 2020, 10, 1455. [Google Scholar] [CrossRef]
- IPC-TM-650 Procedure 2.1.1 rev.F- Microsectioning, Manual and Semi or Automatic Method. Available online: https://www.ipc.org/sites/default/files/test_methods_docs/2–1-01f.pdf (accessed on 10 December 2022).
- IPC-610E- Acceptability of Electronic Assemblies. Available online: http://www.ipc.org/TOC/IPC-A-610E.pdf (accessed on 10 December 2022).
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Venkatesha, T.V.; Shabadi, R. Preparation and corrosion behavior of Ni and Ni–graphene composite coatings. Mater. Res. Bull. 2013, 48, 1477–1483. [Google Scholar] [CrossRef]
- Ghosh, S.; Ryder, K.; Roy, S. Electrochemical and transport properties of ethaline containing copper and tin chloride. Trans. IMF 2013, 92, 41–46. [Google Scholar] [CrossRef]
- Tseluikin, V.; Dzhumieva, A.; Yakovlev, A.; Mostovoy, A.; Lopukhova, M. Electrodeposition of graphene oxide modified composite coatings based on nickel-chromium alloy. Crystals 2021, 11, 415. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Tachikawa, N.; Serizawa, N.; Katayama, Y.; Miura, T. Electrochemistry of Sn(II)/Sn in a hydrophobic room-temperature ionic liquid. Electrochim. Acta 2008, 53, 6530–6534. [Google Scholar] [CrossRef]
- Leong, T.-I.; Hsieh, Y.-T.; Sun, I.-W. Electrochemistry of tin in the 1-ethyl-3-methylimidazolium dicyanamide room temperature ionic liquid. Electrochim. Acta 2011, 56, 3941–3946. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001; p. 163. [Google Scholar]
- Gunawardena, G.; Hills, G.; Montenegro, I.; Sharifker, B. Electrochemical nucleation. Part I. General considerations. J. Electroanal. Chem. 1982, 138, 225–239. [Google Scholar] [CrossRef]
- Alanyalioglu, M.; Segura, J.J.; Oro-Sole, J.; Casan-Pastor, N. The synthesis of graphene sheets with controlled thickness and order using surfactant-assisted electrochemical processes. Carbon 2012, 50, 142–152. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterization. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, B.; Li, D.; Forsyth, M.; MacFarlane, D.R. Direct electro-deposition of graphene from aqueous suspensions. Phys. Chem. Chem. Phys. 2011, 13, 9187–9193. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Sharma, A.; Jang, Y.J.; Jung, J.P. Effect of current density on morphology of electroplated tin. Surf. Eng. 2014, 31, 458–464. [Google Scholar] [CrossRef]
- Praveen, B.M.; Venkatesha, T.V. Electrodeposition and properties of Zn-nanosized TiO2 composite coatings. Appl. Surf. Sci. 2008, 254, 2418–2424. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Producing cobalt–graphene composite coating by pulse electrodeposition with excellent wear and corrosion resistance. Appl. Surf. Sci. 2015, 351, 889–896. [Google Scholar] [CrossRef]
- Bérubé, L.P.; L’Espérance, G. A quantitative method of determining the degree of texture of zinc electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Wang, X.; Park, J.; Dou, S.; Ahn, H.; Kim, K. Sn/graphene nanocomposite with 3D architecture for enhanced reversible lithium storage in lithium ion batteries. J. Mater. Chem. 2009, 19, 8378–8384. [Google Scholar] [CrossRef]
- Feng, B.; Xie, J.; Cao, G.; Zhu, T.; Zhao, X. Facile synthesis of ultrafine CoSn nanocrystals anchored on graphene by one-pot route and the improved electrochemical Li-storage properties. New J. Chem. 2013, 37, 474–480. [Google Scholar] [CrossRef]





| Coating Type | Metallic Substrate | Electrolyte Composition | Main Operation Parameters | Applications | Reference |
|---|---|---|---|---|---|
| Sn Sn + G composite | Mild steel | 9.2 g L−1 SnCl2 | 6.5 mA cm−2 for 20 min. RT pH 3.5 mild stirring | Corrosion protection | [28] |
| 26.7 g L−1 NH4Cl | |||||
| 30.9 g L−1 H3BO3 | |||||
| 43.6 g L−1 Na gluconate | |||||
| 0.05 g L−1 G | |||||
| Sn Sn GO composite | Mild steel | 20 g L−1 SnSO4 | 6.25 mA cm−2 for 20 min. RT pH 4.0 mild stirring | Corrosion protection | [29] |
| 140 g L−1 C6H11NaO7 | |||||
| 20 g L−1 C2H3NaO2 | |||||
| 0.5 g L−1 SLS | |||||
| 0.125–2.5 g L−1 GO | |||||
| Sn Sn/rGO and Sn/G/rGO composites | Carbon paper | 21.44 g L−1 SnSO4 | 2 A cm−2 for 10 s RT mild stirring | Binder free anode for high performance Li-S batteries | [30] |
| 54 mL L−1 conc.H2SO4(67–70%) | |||||
| 0.8 g L−1 GO | |||||
| 96.5Sn–3Ag–0.5Cu (denoted SAC) SAC/GNS composites | Individual rod specimens (ϕ 6.5 × 3 mm) | Powder metallurgy method | The increase of the solder corrosion resistance for electronic packaging applications | [32] | |
| Sn-Ni alloy Sn-Ni/GO composite | Mild steel | 50 g L−1 SnCl2 • 2 H2O | 3 mA cm−2 for 20 min. 45–50 °C pH 2.5 mild stirring | Corrosion protection | [35] |
| 300 g L−1 NiCl2 • 6 H2O | |||||
| 85 g L−1 NH4HF2 | |||||
| 2 g L−1 CTAB | |||||
| 0.125–0.5 g L−1 GO | |||||
| Sn-Zn alloy Sn-Zn/GO composite | Mild steel | 20 g L−1 SnSO4 | 6.25 mA cm−2 for 20 min. RT pH 4.5 mild stirring | Corrosion protection | [36] |
| 20 g L−1 ZnSO4 • 7 H2O | |||||
| 140 g L−1 C6H11NaO7 | |||||
| 20 g L−1 C2H3NaO2 | |||||
| 0.5 g L−1 SLS | |||||
| 0.125–0.5 g L−1 GO | |||||
| Coating Type | Metallic Substrate | Type of DES | Precursor of Sn2+ Ions | Main Operating Parameters | Application | Reference |
|---|---|---|---|---|---|---|
| Sn | Low carbon steel | Choline chloride-ethylene glycol (1:2 molar ratio) | 0.05 M SnCl2 • 2H2O | 1.57 mA cm−2 for 3600 s 25–45 °C Stirring (700–1300 rpm) | Protective coatings | [52] |
| Sn | Cu Mild steel | Choline chloride-ethylene glycol (1:2 molar ratio); Choline chloride-malonic acid (1:1 molar ratio) | 0.5 M SnCl2 • 2H2O | 2–10 mA cm−2 for 20 min. 40–80 °C 1–10 mA cm−2 for 15 min. 90 °C | Protective coatings | [46] |
| Sn | Cu foil | Choline chloride-ethylene glycol (1:2 molar ratio) | 0.1 M SnCl2 • 2H2O | Constant voltages between 0.5–0.7 V for 2–15 min. RT | Anode for lithium-ion battery | [47] |
| SnBi alloy | Cu foil | Choline chloride-ethylene glycol (1:2 molar ratio) | 0.05 mol L−1 SnCl2 | Constant potentials between −1.1 and −1.5 V vs. Ag wire ref. for 1 C cm−2 90 °C | Solder alloy showing composition around the eutectic point | [53] |
| 0.05 mol L−1 BiCl3 | ||||||
| 0.1 mol L−1 H3BO3 | ||||||
| SnIn alloy | Cu | Choline chloride-ethylene glycol (1:2 molar ratio) | 0.05–0.1 M InCl3 | 2–10 mA cm−2 for 30 min. 60 °C | Solder alloy showing composition around the eutectic point | [54] |
| 0.03–0.05 M SnCl2 • 2 H2O | ||||||
| SnAg alloy | Pt Cu | Choline chloride-ethylene glycol (1:2 molar ratio) | 0.03–0.075 M SnCl2 | 10–20 mA cm−2 for 20–25 min. 40 °C | Solder alloy Decorative coatings Electrocatalyst for HER in alkaline solutions | [55] |
| 0.05–0.15 M AgCl | ||||||
| 0.003–0.075 M C5H11NO2S (methionine) | ||||||
| SnCuNi | Cu | Choline chloride-ethylene glycol (1:2 molar ratio) | 500 mM SnCl2 • 2 H2O | 8 mA cm−2 for 30 min. 60 °C stirring | Solder alloy showing Sn-0.65Cu-0.06Ni stoichiometry close to the commercial one | [56] |
| 0.055 mM NiCl2 • 6 H2O | ||||||
| 0.345 mM CuCl2 • 2 H2O |
| Immersion Period | Sn-rGO | Sn | ||
|---|---|---|---|---|
| Ecorr, V/Ag/AgCl | jcorr, μA cm−2 | Ecorr, V/Ag/AgCl | jcorr, μA cm−2 | |
| Initial | −0.707 | 8.48 ± 0.24 | −0.661 | 15.84 ± 1.22 |
| 144 h | −0.243 | 18.96 ± 0.58 | −0.51 | 15.92 ± 2.17 |
| Immersion Period | Sn-rGO | Sn | ||
|---|---|---|---|---|
| Rct/Ω | RF/Ω | Rct/Ω | RF/Ω | |
| Initial | 4121 | 24,941 | 7279 | 4631 |
| 24 h | 9783 | 16,304 | 1173 | 12,306 |
| 48 h | 300 | 44,757 | 253 | 20,165 |
| 144 h | 3890 | 32,541 | 25 | 26,259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costovici, S.; Pantazi, A.; Balan, D.; Cojocaru, A.; Visan, T.; Enachescu, M.; Anicai, L. Electrodeposition of Tin-Reduced Graphene Oxide Composite from Deep Eutectic Solvents Based on Choline Chloride and Ethylene Glycol. Metals 2023, 13, 203. https://doi.org/10.3390/met13020203
Costovici S, Pantazi A, Balan D, Cojocaru A, Visan T, Enachescu M, Anicai L. Electrodeposition of Tin-Reduced Graphene Oxide Composite from Deep Eutectic Solvents Based on Choline Chloride and Ethylene Glycol. Metals. 2023; 13(2):203. https://doi.org/10.3390/met13020203
Chicago/Turabian StyleCostovici, Stefania, Aida Pantazi, Danut Balan, Anca Cojocaru, Teodor Visan, Marius Enachescu, and Liana Anicai. 2023. "Electrodeposition of Tin-Reduced Graphene Oxide Composite from Deep Eutectic Solvents Based on Choline Chloride and Ethylene Glycol" Metals 13, no. 2: 203. https://doi.org/10.3390/met13020203
APA StyleCostovici, S., Pantazi, A., Balan, D., Cojocaru, A., Visan, T., Enachescu, M., & Anicai, L. (2023). Electrodeposition of Tin-Reduced Graphene Oxide Composite from Deep Eutectic Solvents Based on Choline Chloride and Ethylene Glycol. Metals, 13(2), 203. https://doi.org/10.3390/met13020203







