Mechanical Properties of Electrolytically Produced Copper Coatings Reinforced with Pigment Particles
Abstract
1. Introduction
2. Experimental
2.1. Materials and Method
2.2. Characterization of the Pigment Particles
2.3. Characterization of Copper Deposits Obtained without and with Added Pigment Particles (CuMC-PigP)
3. Results
3.1. Characterization of Pigment Powder Particles
3.1.1. Morphology of the Pigment Particles
3.1.2. XRD Analysis of the Pigment Particles
3.2. Characterization of Cu and CuMC-PigP Coatings
3.2.1. Morphological Analysis of the Copper Coatings–SEM Analysis
3.2.2. Topographical Analysis of the Cu Deposits—AFM Analysis
3.2.3. Textural Analysis of the Copper Deposits—XRD
3.2.4. Mechanical Features of Cu and CuMC-PigP Coatings—The Vickers Microhardness Test
Determination of the Microhardness of Pigment-Free Cu and CuMC-PigP Coatings by Use of C–L CHM
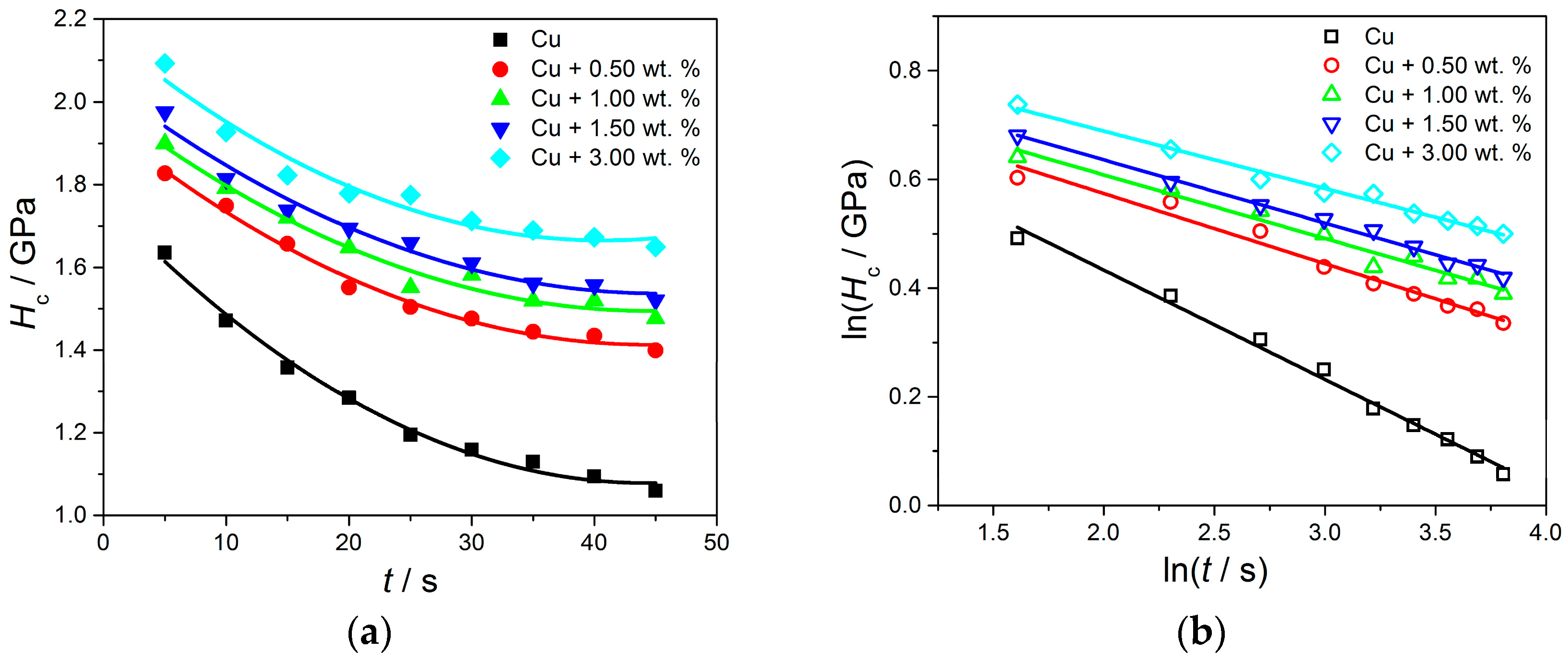
The Creep Resistance of Cu and CuMC-PigP Coatings—Indentation Creep Test
3.3. Wettability of Cu and CuMC-PigP Coatings
4. Discussion
5. Conclusions
- ○
- The addition of the pigment particles to the electrolyte did not affect significantly the morphology or the preferred orientation of the CuMC-PigP coatings.
- ○
- Due to an incorporation of pigment particles in the coatings, a rise in coating thickness was observed with the rise in the concentration of particles.
- ○
- The roughness of the CuMC-PigP coatings rose with the rise in the concentration of particles.
- ○
- There is a critical concentration of pigment particles (c = 1.00 wt %) after which there is a significant effect on coating hardness. Starting from this concentration, the Cu co-deposition with pigment particles led to a change in the composite hardness system from a “soft deposit on hard cathode” type to a “hard deposit on soft cathode” type.
- ○
- The dominant creep mechanism in the pigment-free and CuMC-PigP coatings comprised dislocation creep and dislocation climb.
- ○
- With an incorporation of pigment particles, the wettability of the Cu coatings changed from hydrophilic (for the pigment-free coating) to hydrophobic coatings obtained with various concentrations of pigment particles.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bite, I.; Krieke, G.; Zolotarjovs, A.; Laganovska, K.; Liepina, V.; Smits, K.; Auzins, K.; Grigorjeva, L.; Millers, D.; Skuja, L. Novel Method of Phosphorescent Strontium Aluminate Coating Preparation on Aluminum. Mater. Des. 2018, 160, 794–802. [Google Scholar] [CrossRef]
- Tayebi, M.; Ostad Movahed, S.; Ahmadpour, A. The Effect of the Surface Coating of a Strontium Mono-Aluminate Europium Dysprosium-Based (SrAl2O4:Eu2+,Dy3+) Phosphor by Polyethylene (PE), Polystyrene (PS) and Their Dual System on the Photoluminescence Properties of the Pigment. RSC Adv. 2019, 9, 38703–38712. [Google Scholar] [CrossRef]
- Sharma, V.; Das, A.; Kumar, V. Eu2+,Dy3+ Codoped SrAl2O4 Nanocrystalline Phosphor for Latent Fingerprint Detection in Forensic Applications. Mater. Res. Express 2016, 3, 015004. [Google Scholar] [CrossRef]
- Van der Heggen, D.; Joos, J.J.; Feng, A.; Fritz, V.; Delgado, T.; Gartmann, N.; Walfort, B.; Rytz, D.; Hagemann, H.; Poelman, D.; et al. Persistent Luminescence in Strontium Aluminate: A Roadmap to a Brighter Future. Adv. Funct. Mater. 2022, 32, 2208809. [Google Scholar] [CrossRef]
- Nance, J.; Sparks, T.D. Comparison of Coatings for SrAl2O4:Eu2+, Dy3+ Powder in Waterborne Road Striping Paint under Wet Conditions. Prog. Org. Coat. 2020, 144, 105637. [Google Scholar] [CrossRef]
- Lyu, L.; Chen, Y.; Yu, L.; Li, R.; Zhang, L.; Pei, J. The Improvement of Moisture Resistance and Organic Compatibility of SrAl2O4: Eu2+, Dy3+ Persistent Phosphors Coated with Silica–Polymer Hybrid Shell. Materials 2020, 13, 426. [Google Scholar] [CrossRef]
- Van der Heggen, D.; Zilenaite, R.; Ezerskyte, E.; Fritz, V.; Korthout, K.; Vandenberghe, D.; De Grave, J.; Garrevoet, J.; Vincze, L.; Poelman, D.; et al. A Standalone, Battery-Free Light Dosimeter for Ultraviolet to Infrared Light. Adv. Funct. Mater. 2022, 32, 2109635. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, J.; Wu, J.; Zhang, S.; Zhou, P.; Chen, X.; Xu, R. Preparation of Long Persistent Phosphor SrAl2O4:Eu2+, Dy3+ and Its Application in Dye-Sensitized Solar Cells. J. Mater. Sci. Mater. Electron. 2016, 27, 1350–1356. [Google Scholar] [CrossRef]
- Xiao, Y.; Luo, B.; Cheng, B.; Huang, Q.; Ye, Y.; Fang, L.; Zhou, L.; Lei, S. Enhanced Visible Light Catalysis Activity of CdS-Sheathed SrAl2O4:Eu2+,Dy3+ Nanocomposites. Dalton Trans. 2018, 47, 7941–7948. [Google Scholar] [CrossRef]
- Arellano-Tánori, O.; Meléndrez, R.; Pedroza-Montero, M.; Castañeda, B.; Chernov, V.; Yen, W.M.; Barboza-Flores, M. Persistent Luminescence Dosimetric Properties of UV-Irradiated SrAl2O4:Eu2+, Dy3+ Phosphor. J. Lumin. 2008, 128, 173–184. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Rodriguez, M.Á.; Fernandez, J.F. Long Lasting Phosphors: SrAl2O4:Eu,Dy as the Most Studied Material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Kim, D.Y. Co-Deposition of Pigment Particles with Nickel through Electrodeposition. Ph.D. thesis, University of Toronto, Toronto, ON, Canada, 2015. [Google Scholar]
- Pingale, A.D.; Owhal, A.; Katarkar, A.S.; Belgamwar, S.U. Fabrication and Tribo-Mechanical Performance of Cu@Al2O3 Composite. Mater. Today Proc. 2022, 64, 1175–1181. [Google Scholar] [CrossRef]
- Sadoun, A.M.; Mohammed, M.M.; Fathy, A.; El-Kady, O.A. Effect of Al2O3 Addition on Hardness and Wear Behavior of Cu–Al2O3 Electro-Less Coated Ag Nanocomposite. J. Mater. Res. Technol. 2020, 9, 5024–5033. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Haigh, J. Strengths and Advantages of Electrodeposition as a Semiconductor Growth Technique for Applications in Macroelectronic Devices. J. Electrochem. Soc. 2006, 153, G47. [Google Scholar] [CrossRef]
- Gupta, S.K.; Misra, R.D. Experimental Study of Pool Boiling Heat Transfer on Copper Surfaces with Cu-Al2O3 Nanocomposite Coatings. Int. Commun. Heat Mass Transf. 2018, 97, 47–55. [Google Scholar] [CrossRef]
- Hayashi, H.; Izumi, S.; Tari, I. Codeposition of α-Alumina Particles from Acid Copper Sulfate Bath. J. Electrochem. Soc. 1993, 140, 362–365. [Google Scholar] [CrossRef][Green Version]
- Bengoa, L.N.; Ispas, A.; Bengoa, J.F.; Bund, A.; Egli, W.A. Ultrasound Assisted Electrodeposition of Cu-SiO2 Composite Coatings: Effect of Particle Surface Chemistry. J. Electrochem. Soc. 2019, 166, D244–D251. [Google Scholar] [CrossRef]
- Maharana, H.S.; Lakra, S.; Pal, S.; Basu, A. Electrophoretic Deposition of Cu-SiO2 Coatings by DC and Pulsed DC for Enhanced Surface-Mechanical Properties. J. Mater. Eng. Perform. 2016, 25, 327–337. [Google Scholar] [CrossRef]
- Zamblau, I.; Varvara, S.; Muresan, L.M. Corrosion Behavior of Cu–SiO2 Nanocomposite Coatings Obtained by Electrodeposition in the Presence of Cetyl Trimethyl Ammonium Bromide. J. Mater. Sci. 2011, 46, 6484–6490. [Google Scholar] [CrossRef]
- Gupta, S.K.; Misra, R.D. Effect of Two-Step Electrodeposited Cu–TiO2 Nanocomposite Coating on Pool Boiling Heat Transfer Performance. J. Therm. Anal. Calorim. 2019, 136, 1781–1793. [Google Scholar] [CrossRef]
- Gupta, S.K.; Misra, R.D. An Experimental Investigation on Flow Boiling Heat Transfer Enhancement Using Cu-TiO2 Nanocomposite Coating on Copper Substrate. Exp. Therm. Fluid Sci. 2018, 98, 406–419. [Google Scholar] [CrossRef]
- Ning, D.; Zhang, A.; Murtaza, M.; Wu, H. Effect of Surfactants on the Electrodeposition of Cu-TiO2 Composite Coatings Prepared by Jet Electrodeposition. J. Alloys Compd. 2019, 777, 1245–1250. [Google Scholar] [CrossRef]
- Mangam, V.; Bhattacharya, S.; Das, K.; Das, S. Friction and Wear Behavior of Cu–CeO2 Nanocomposite Coatings Synthesized by Pulsed Electrodeposition. Surf. Coat. Technol. 2010, 205, 801–805. [Google Scholar] [CrossRef]
- Akhtar, K.; Hira, U.; Khalid, H.; Zubair, N. Uniform Fine Particles of ZrO2 as Reinforcement Filler in the Electrodeposited Cu-ZrO2 Nanocomposite Coating on Steel Substrate. J. Alloys Compd. 2019, 772, 15–24. [Google Scholar] [CrossRef]
- Maharana, H.S.; Panda, S.; Basu, A. Effect of Texture and Microstructure on Properties of Electrodeposited Cu-SiO2 and Cu-Y2O3 Coatings. Surf. Coat. Technol. 2017, 315, 558–566. [Google Scholar] [CrossRef]
- Eslami, M.; Saghafian, H.; Golestani-fard, F.; Robin, A. Effect of Electrodeposition Conditions on the Properties of Cu–Si3N4 Composite Coatings. Appl. Surf. Sci. 2014, 300, 129–140. [Google Scholar] [CrossRef]
- Robin, A.; de Santana, J.C.P.; Sartori, A.F. Co-Electrodeposition and Characterization of Cu–Si3N4 Composite Coatings. Surf. Coat. Technol. 2011, 205, 4596–4601. [Google Scholar] [CrossRef]
- Banthia, S.; Sengupta, S.; Das, S.; Das, K. Cu, Cu-SiC Functionally Graded Coating for Protection against Corrosion and Wear. Surf. Coat. Technol. 2019, 374, 833–844. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, L.; Zhao, H.; Shen, B.; Hu, W. Microstructure and Performance of Electroformed Cu/Nano-SiC Composite. Mater. Des. 2007, 28, 1958–1962. [Google Scholar] [CrossRef]
- Wang, C.; Gan, X.; Tao, J.; Xie, M.; Yi, J.; Liu, Y. Compression and Electromagnetic Shielding Properties of CNTs Reinforced Copper Foams Prepared through Electrodeposition. Vacuum 2019, 167, 159–162. [Google Scholar] [CrossRef]
- Bengoa, L.N.; Seré, P.R.; Pary, P.; Conconi, M.S.; Folgueiras, J.M.; Morel, E.N.; Torga, J.; Egli, W.A. Self-Lubricating Cu-MWCNT Coatings Deposited from an Ecofriendly Glutamate-Based Electrolyte. Surf. Coat. Technol. 2020, 388, 125590. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, R.; Zhan, K.; Bao, L.; Zhang, Y.; Yang, Z.; Yan, Y.; Zhao, B.; Yang, J. Preparation of Electro-Reduced Graphene Oxide/Copper Composite Foils with Simultaneously Enhanced Thermal and Mechanical Properties by DC Electro-Deposition Method. Mater. Sci. Eng. A 2021, 805, 140574. [Google Scholar] [CrossRef]
- Mathew, R.T.; Singam, S.; Kollu, P.; Bohm, S.; Prasad, M.J.N.V. Achieving Exceptional Tensile Strength in Electrodeposited Copper through Grain Refinement and Reinforcement Effect by Co-Deposition of Few Layered Graphene. J. Alloys Compd. 2020, 840, 155725. [Google Scholar] [CrossRef]
- Joseph, A.; Kirubasankar, B.; Mathew, A.M.; Narayanasamy, M.; Yan, C.; Angaiah, S. Influence of Pulse Reverse Current Parameters on Electrodeposition of Copper-Graphene Nanocomposite Coating. Appl. Surf. Sci. Adv. 2021, 5, 100116. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, J.; Zhang, S.; Li, N.; Liu, C.; Yu, X.; Song, Y.; Han, S.; Chen, L.; Xi, M.; et al. Copper Matrix Composites Reinforced by Three-Dimensional Netlike Graphene towards Enhanced Mechanical Property and Wear Resistance. Compos. Commun. 2022, 32, 101187. [Google Scholar] [CrossRef]
- Park, J.-H.; Fujita, Y.; Hagio, T.; Phouthavong, V.; Kamimoto, Y.; Bessho, T.; Ichino, R. Preliminary Study on Electrodeposition of Copper Platings and Codeposition of Carbon Nanotubes from Organic Solvent. Coatings 2023, 13, 802. [Google Scholar] [CrossRef]
- Hagio, T.; Park, J.-H.; Naruse, Y.; Goto, Y.; Kamimoto, Y.; Ichino, R.; Bessho, T. Electrodeposition of Nano-Diamond/Copper Composite Platings: Improved Interfacial Adhesion between Diamond and Copper via Formation of Silicon Carbide on Diamond Surface. Surf. Coat. Technol. 2020, 403, 126322. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Luo, J.; Cheng, P.; Wang, Y.; Wang, H.; Ding, G. Microstructure of Cu-Diamond Composites with near-Perfect Interfaces Prepared via Electroplating and Its Thermal Properties. Mater. Charact. 2019, 150, 199–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Lu, S. Preparation and Application of Layered Double Hydroxide Nanosheets. RSC Adv. 2021, 11, 24254–24281. [Google Scholar] [CrossRef]
- Copper Plating Service. Available online: https://www.sharrettsplating.com/coatings/copper (accessed on 9 November 2023).
- Singh, K.; Khanna, V.; Sonu; Singh, S.; Bansal, S.A.; Chaudhary, V.; Khosla, A. Paradigm of state-of-the-art CNT reinforced copper metal matrix composites: Processing, characterizations, and applications. J. Mater. Res. Technol. 2023, 24, 8572–8605. [Google Scholar] [CrossRef]
- Bharadishettar, N.; Bhat, U.K.; Panemangalore, D.B. Coating technologies for copper based antimicrobial active surfaces: A perspective review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Yao, K.-M.; Xu, M.; Huang, X.-Z.; Mo, D.-C.; Lyu, S.-S. Electrochemical deposition of copper films to develop the latent sebaceous fingerprints on metal substrates. J. Electroanal. Chem. 2023, 941, 117526. [Google Scholar] [CrossRef]
- Cruz-Matías, I.; Ayala, D.; Hiller, D.; Gutsch, S.; Zacharias, M.; Estradé, S.; Peiró, F. Sphericity and Roundness Computation for Particles Using the Extreme Vertices Model. J. Comput. Sci. 2019, 30, 28–40. [Google Scholar] [CrossRef]
- Mladenović, I.; Jakšić, Z.; Obradov, M.; Vuković, S.; Isić, G.; Tanasković, D.; Lamovec, J. Subwavelength Nickel-Copper Multilayers as an Alternative Plasmonic Material. Opt. Quantum Electron. 2018, 50, 203. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.D.; Maksimović, V.M.; Avramović, L. Correlation of Morphology and Crystal Structure of Metal Powders Produced by Electrolysis Processes. Metals 2021, 11, 859. [Google Scholar] [CrossRef]
- Bérubé, L.P.; L’Espérance, G. A Quantitative Method of Determining the Degree of Texture of Zinc Electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Broitman, E. Indentation Hardness Measurements at Macro-, Micro-, and Nanoscale: A Critical Overview. Tribol. Lett. 2017, 65, 23. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Nikolić, N.D.; Lamovec, J.S.; Vasiljević-Radović, D.; Radojević, V. Application of the Composite Hardness Models in the Analysis of Mechanical Characteristics of Electrolytically Deposited Copper Coatings: The Effect of the Type of Substrate. Metals 2021, 11, 111. [Google Scholar] [CrossRef]
- Chicot, D.; Lesage, J. Absolute Hardness of Films and Coatings. Thin Solid Film. 1995, 254, 123–130. [Google Scholar] [CrossRef]
- Lesage, J.; Chicot, D.; Pertuz, A.; Jouan, P.-Y.; Horny, N.; Soom, A. A Model for Hardness Determination of Thin Coatings from Standard Micro-Indentation Tests. Surf. Coat. Technol. 2005, 200, 886–889. [Google Scholar] [CrossRef]
- Lesage, J.; Pertuz, A.; Puchi-Cabrera, E.S.; Chicot, D. A Model to Determine the Surface Hardness of Thin Films from Standard Micro-Indentation Tests. Thin Solid Film. 2006, 497, 232–238. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Lamovec, J.S.; Vasiljević Radović, D.G.; Vasilić, R.; Radojević, V.J.; Nikolić, N.D. Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111). Metals 2020, 10, 488. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Lamovec, J.S.; Vasiljević-Radović, D.G.; Radojević, V.J.; Nikolić, N.D. Mechanical features of copper coatings electrodeposited by the pulsating current (PC) regime on Si(111) substrate. Int. J. Electrochem. Sci. 2020, 15, 12173–12191. [Google Scholar] [CrossRef]
- Sargent, P.M.; Ashby, M.F. Indentation Creep. Mater. Sci. Technol. 1992, 8, 594–601. [Google Scholar] [CrossRef]
- Farhat, S.; Rekaby, M.; Awad, R. Vickers Microhardness and Indentation Creep Studies for Erbium-Doped ZnO Nanoparticles. SN Appl. Sci. 2019, 1, 546. [Google Scholar] [CrossRef]
- Vuksanovic, M.; Mladenovic, I.; Tomic, N.; Petrovic, M.; Radojevic, V.; Marinkovic, A.; Jancic-Heinemann, R. Mechanical Properties of Biomass-Derived Silica Nanoparticles Reinforced PMMA Composite Material. Sci. Sinter. 2022, 54, 211–221. [Google Scholar] [CrossRef]
- Parau, A.C.; Juravlea, G.A.; Raczkowska, J.; Vitelaru, C.; Dinu, M.; Awsiuk, K.; Vranceanu, D.M.; Ungureanu, E.; Cotrut, C.M.; Vladescu, A. Comparison of 316L and Ti6Al4V Biomaterial Coated by ZrCu-Based Thin Films Metallic Glasses: Structure, Morphology, Wettability, Protein Adsorption, Corrosion Resistance, Biomineralization. Appl. Surf. Sci. 2023, 612, 155800. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of Composite Coatings Containing Nanoparticles in a Metal Deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Zhu, Y.; Pang, Z.; Wang, J.; Ge, M.; Ju, A. Research on the Afterglow Properties of Red-Emitting Phosphor: SrAl2O4:Eu2+, Dy3+/Light Conversion Agent for Red Luminous Fiber. J. Mater. Sci. Mater. Electron. 2016, 27, 7554–7559. [Google Scholar] [CrossRef]
- Avramović, L.; Maksimović, V.M.; Baščarević, Z.; Ignjatović, N.; Bugarin, M.; Marković, R.; Nikolić, N.D. Influence of the Shape of Copper Powder Particles on the Crystal Structure and Some Decisive Characteristics of the Metal Powders. Metals 2019, 9, 56. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Lamovec, J.S.; Vasiljević-Radović, D.G.; Vasilić, R.; Radojević, V.J.; Nikolić, N.D. Implementation of the Chicot–Lesage Composite Hardness Model in a Determination of Absolute Hardness of Copper Coatings Obtained by the Electrodeposition Processes. Metals 2021, 11, 1807. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Nikolić, N.D. Influence of Parameters and Regimes of the Electrodeposition on Hardness of Copper Coatings. Metals 2023, 13, 683. [Google Scholar] [CrossRef]
- Lamovec, J.; Jović, V.; Randjelović, D.; Aleksić, R.; Radojević, V. Analysis of the Composite and Film Hardness of Electrodeposited Nickel Coatings on Different Substrates. Thin Solid Film. 2008, 516, 8646–8654. [Google Scholar] [CrossRef]
- Mladenovic, I.; Lamovec, J.; Vasiljevic-Radovic, D.; Radojevic, V.; Nikolic, N. Determination of the Absolute Hardness of Electrolytically Produced Copper Coatings by Application of the Chicot-Lesage Composite Hardness Model. J. Serb. Chem. Soc. 2022, 87, 899–910. [Google Scholar] [CrossRef]
- Marmur, A. Hydro- Hygro- Oleo- Omni-Phobic? Terminology of Wettability Classification. Soft Matter 2012, 8, 6867. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Wang, S.; Feng, L.; Liu, H.; Sun, T.; Zhang, X.; Jiang, L.; Zhu, D. Manipulation of Surface Wettability between Superhydrophobicity and Superhydrophilicity on Copper Films. Chem. Phys. Chem. 2005, 6, 1475–1478. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W. Superhydrophobic, Superamphiphobic and SLIPS Materials as Anti-Corrosion and Anti-Biofouling Barriers. New J. Chem. 2021, 45, 15170–15179. [Google Scholar] [CrossRef]
- Celis, J.P.; Roos, J.R.; Buelens, C. A Mathematical Model for the Electrolytic Codeposition of Particles with a Metallic Matrix. J. Electrochem. Soc. 1987, 134, 1402–1408. [Google Scholar] [CrossRef]
- Gupta, S.K.; Misra, R.D. Experimental Pool Boiling Heat Transfer Analysis with Copper–Alumina Micro/Nanostructured Surfaces Developed by a Novel Electrochemical Deposition Technique. Int. J. Thermophys. 2023, 44, 112. [Google Scholar] [CrossRef]
- Jones, B.J.; McHale, J.P.; Garimella, S.V. The Influence of Surface Roughness on Nucleate Pool Boiling Heat Transfer. J. Heat Transfer. 2009, 131, 121009. [Google Scholar] [CrossRef]
- Triantou, K.I.; Pantelis, D.I.; Guipont, V.; Jeandin, M. Microstructure and tribological behavior of copper and composite copper+alumina cold sprayed coatings for various alumina contents. Wear 2015, 336–337, 96–107. [Google Scholar] [CrossRef]
- Requena, G.C.; Degischer, H.P. Effects of Particle Reinforcement on Creep Behaviour of AlSi1MgCu. Int. J. Mater. Res. 2022, 96, 807–813. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, B.; Ren, B.; Tu, D.; Wang, Z.; Wang, C.; Zheng, Y.; Li, X.; Wang, D.; Ren, Z.; et al. Smart Mechanoluminescent Phosphors: A Review of Strontium-Aluminate-Based Materials, Properties, and Their Advanced Application Technologies. Adv. Sci. 2023, 10, 2204925. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]







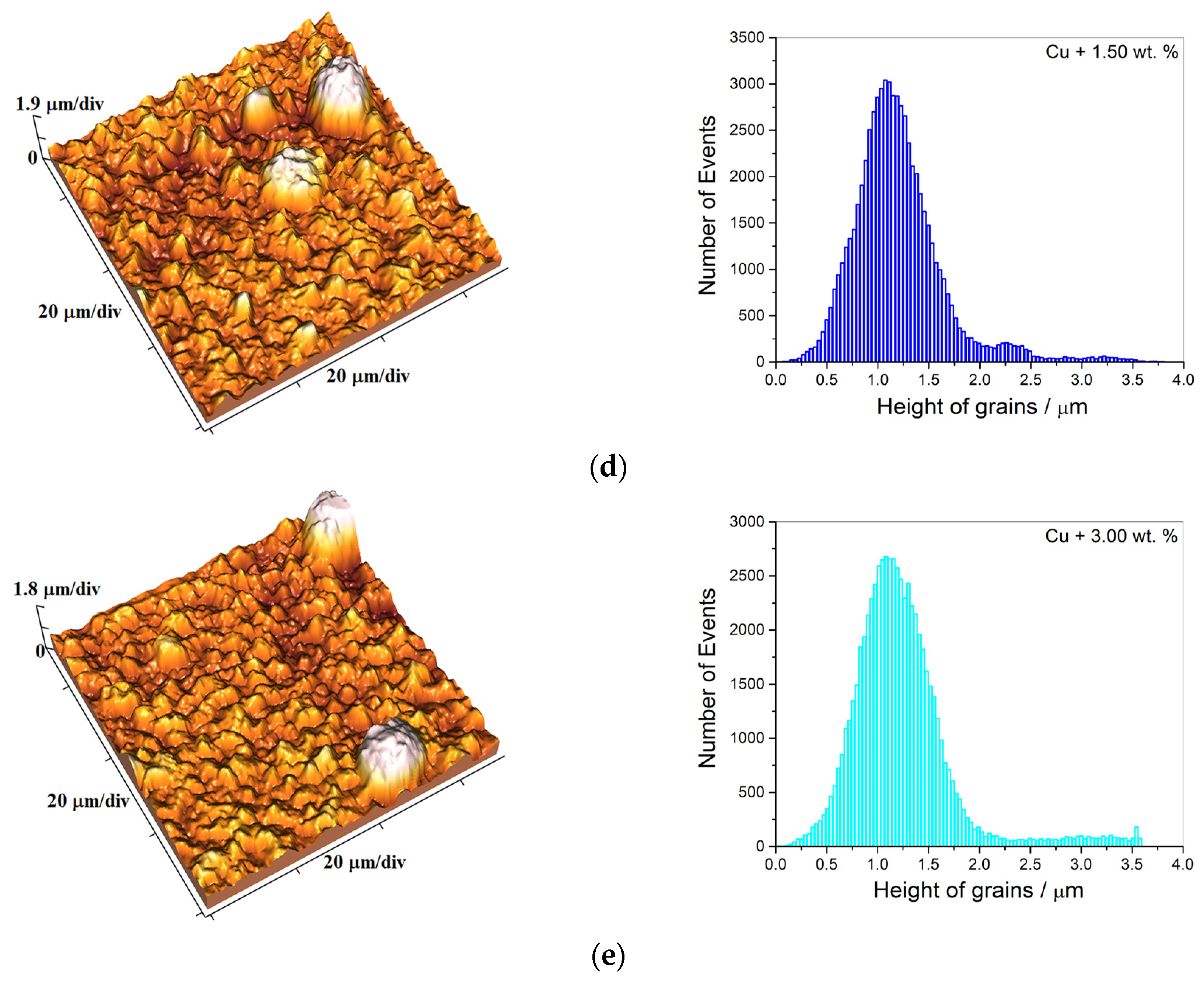



| c/wt % | 0 | 0.50 | 1.00 | 1.50 | 3.00 |
|---|---|---|---|---|---|
| δ/μm | 13.8 ± 0.11 | 17.0 ± 0.41 | 21.4 ± 0.67 | 22.5 ± 1.11 | 22.6 ± 2.17 |
| c/wt % | 0 | 0.50 | 1.00 | 1.50 | 3.00 |
|---|---|---|---|---|---|
| Ra/nm | 197.1 | 239.9 | 252.4 | 313.5 | 349.7 |
| TC(hkl) | RTC(hkl)/% | |||||||
|---|---|---|---|---|---|---|---|---|
| c/wt | TC(111) | TC(200) | TC(220) | TC(311) | RTC(111) | RTC(200) | RTC(220) | RTC(311) |
| 0 | 0.29 | 0.33 | 6.1 | 0.96 | 3.8 | 4.3 | 79.4 | 12.5 |
| 0.50 | 0.24 | 0.31 | 6.5 | 0.86 | 3.0 | 3.9 | 82.2 | 10.9 |
| 1.00 | 0.29 | 0.32 | 6.1 | 1.00 | 3.8 | 4.1 | 79.1 | 13.0 |
| 1.50 | 0.27 | 0.33 | 6.3 | 0.86 | 3.5 | 4.3 | 81.2 | 11.0 |
| 3.00 | 0.21 | 0.34 | 6.6 | 0.78 | 2.6 | 4.3 | 83.2 | 9.90 |
| c/wt % | 0 | 0.50 | 1.00 | 1.50 | 3.00 |
|---|---|---|---|---|---|
| m | 0.4221 | 0.4323 | 0.4314 | 0.4351 | 0.4439 |
| c/wt % | Slope (k) | Intercept (n) | Stress Exponent (µ) | R2 |
|---|---|---|---|---|
| 0 | −0.2015 ± 0.00694 | 0.8363 ± 0.02156 | 4.96 | 0.99 |
| 0.50 | −0.1171 ± 0.00764 | 0.8429 ± 0.02373 | 8.54 | 0.97 |
| 1.00 | −0.1293 ± 0.00775 | 0.8329 ± 0.02408 | 7.73 | 0.98 |
| 1.50 | −0.1164 ± 0.00367 | 0.8692 ± 0.01141 | 8.59 | 0.99 |
| 3.00 | −0.1063 ± 0.00427 | 0.9022 ± 0.01325 | 9.41 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, I.O.; Vuksanović, M.M.; Dimitrijević, S.P.; Vasilić, R.; Radojević, V.J.; Vasiljević-Radović, D.G.; Nikolić, N.D. Mechanical Properties of Electrolytically Produced Copper Coatings Reinforced with Pigment Particles. Metals 2023, 13, 1979. https://doi.org/10.3390/met13121979
Mladenović IO, Vuksanović MM, Dimitrijević SP, Vasilić R, Radojević VJ, Vasiljević-Radović DG, Nikolić ND. Mechanical Properties of Electrolytically Produced Copper Coatings Reinforced with Pigment Particles. Metals. 2023; 13(12):1979. https://doi.org/10.3390/met13121979
Chicago/Turabian StyleMladenović, Ivana O., Marija M. Vuksanović, Stevan P. Dimitrijević, Rastko Vasilić, Vesna J. Radojević, Dana G. Vasiljević-Radović, and Nebojša D. Nikolić. 2023. "Mechanical Properties of Electrolytically Produced Copper Coatings Reinforced with Pigment Particles" Metals 13, no. 12: 1979. https://doi.org/10.3390/met13121979
APA StyleMladenović, I. O., Vuksanović, M. M., Dimitrijević, S. P., Vasilić, R., Radojević, V. J., Vasiljević-Radović, D. G., & Nikolić, N. D. (2023). Mechanical Properties of Electrolytically Produced Copper Coatings Reinforced with Pigment Particles. Metals, 13(12), 1979. https://doi.org/10.3390/met13121979











