Influence of Mo Content on the Precipitation Behavior of 13Ni Maraging Ultra-High Strength Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
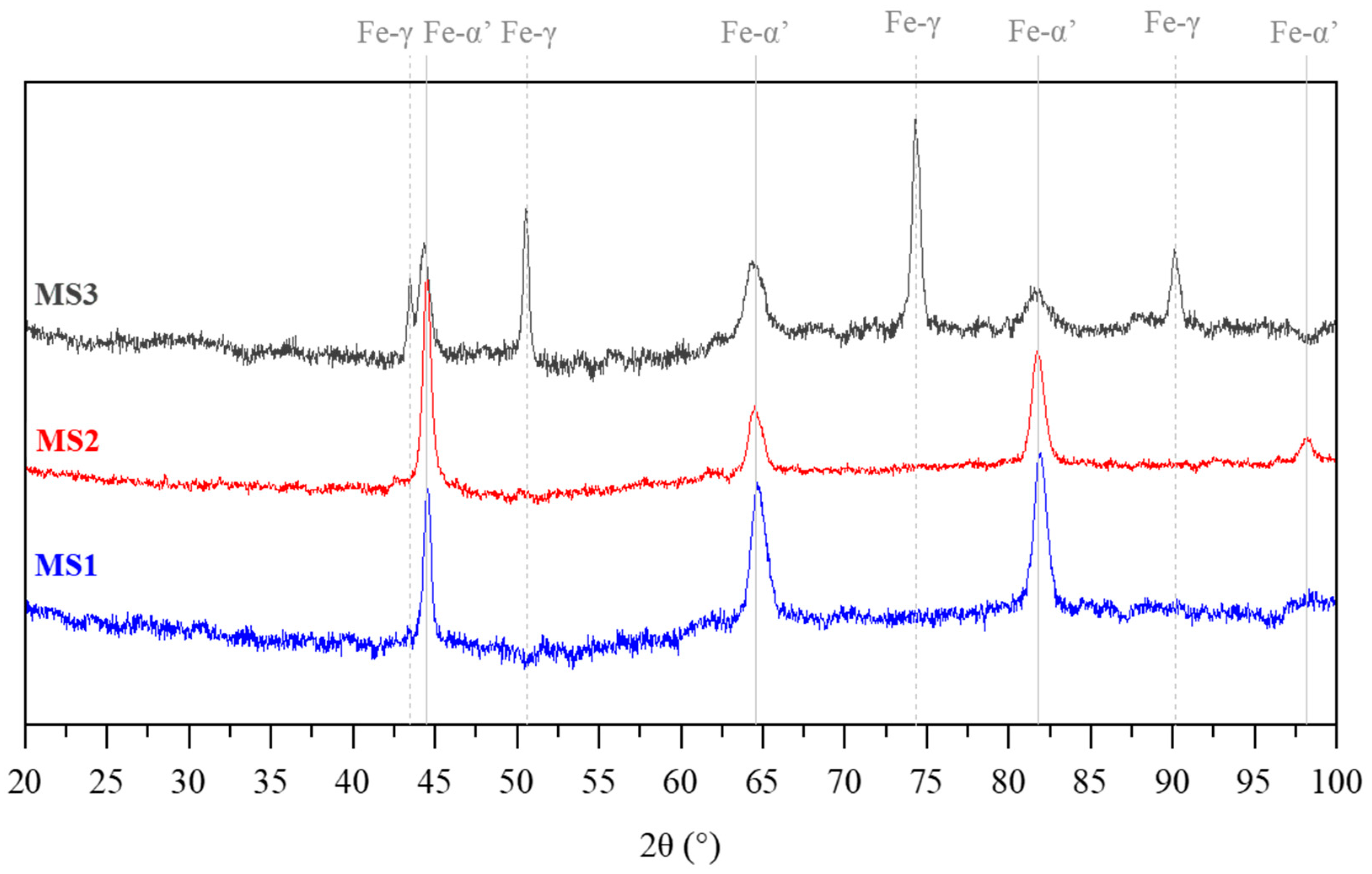
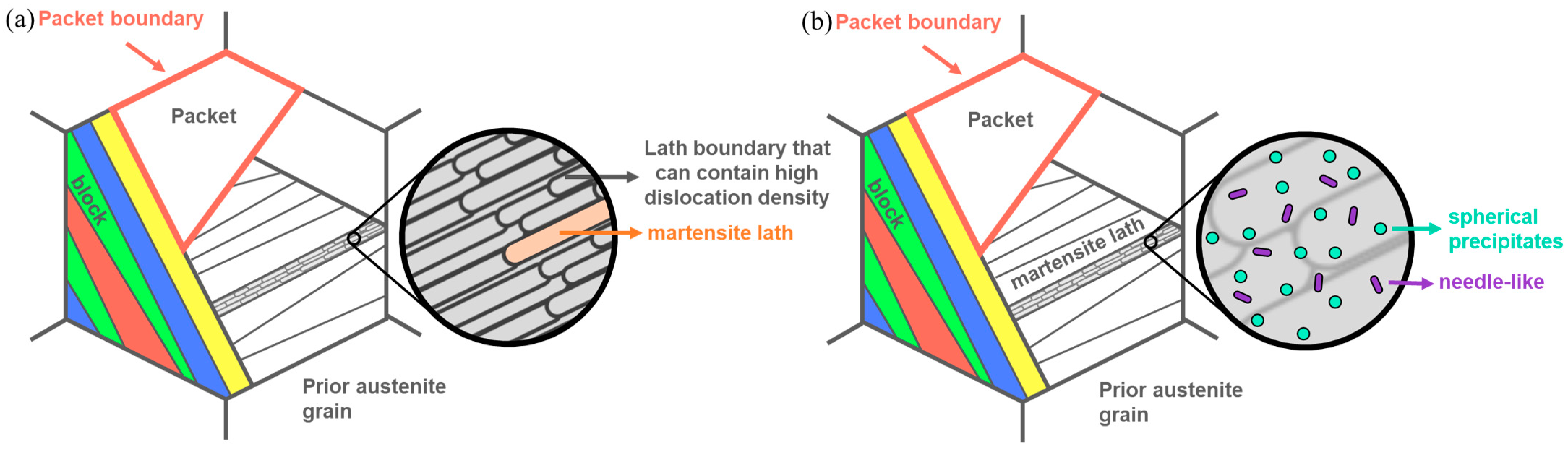
3.1. Microstructure
3.2. Phase Transformation
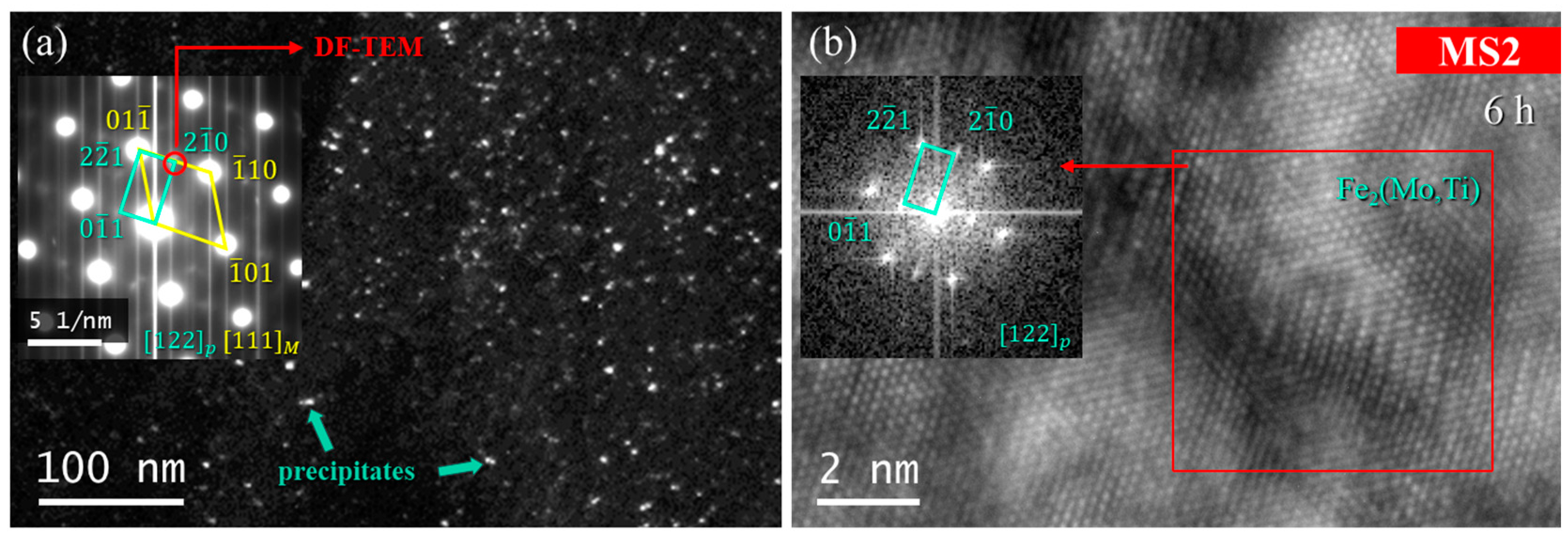
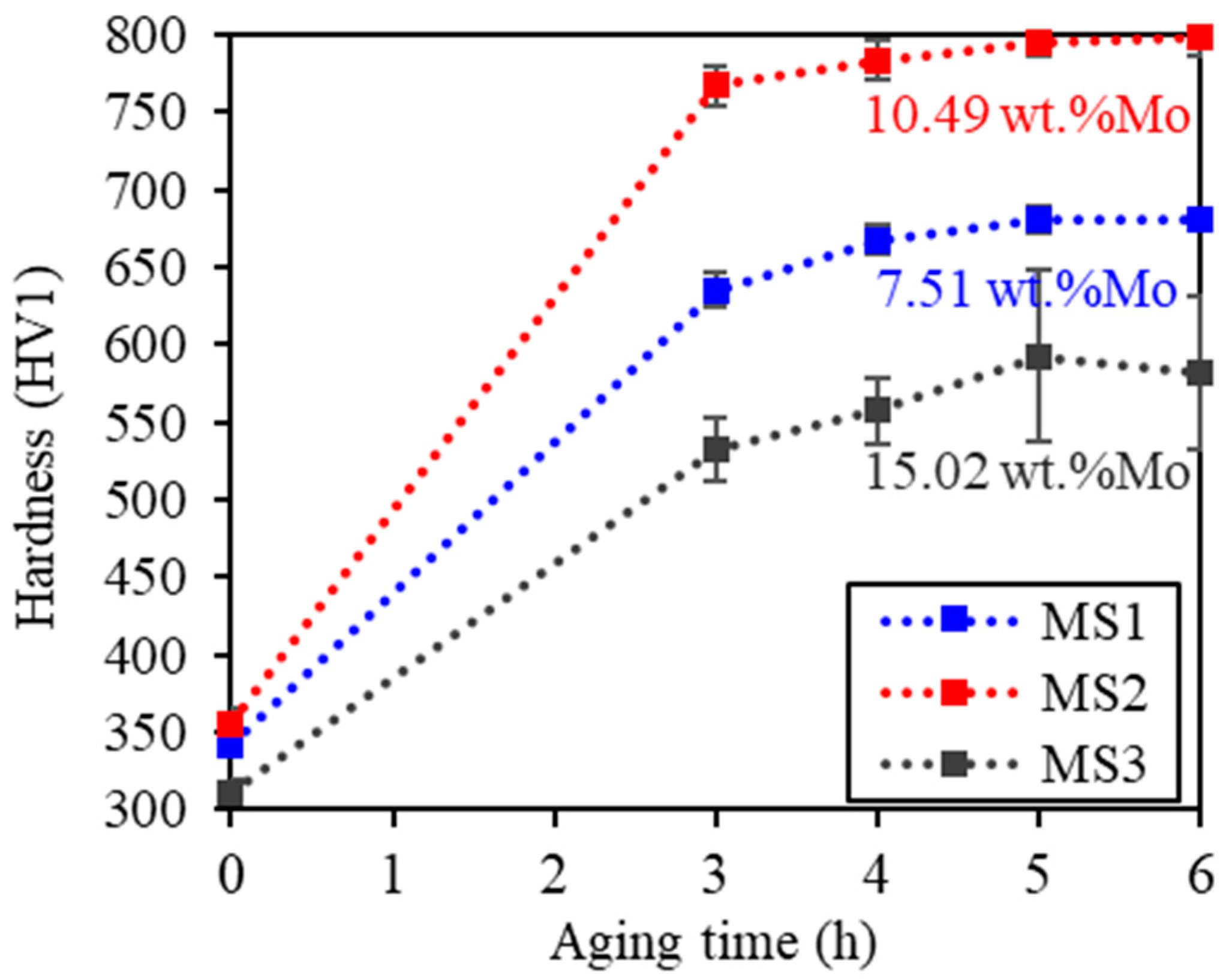
3.3. Precipitation Hardening
4. Conclusions
- 13Ni maraging steels exhibit a martensitic microstructure with pack, block, lath, and a high density of dislocations and dislocation tangles. During aging, martensite recovery occurs, involving the rearrangement of dislocations and the formation of subgrains, while precipitation took place in areas previously occupied by the dislocation tangles.
- 13Ni maraging steels containing high levels of molybdenum (15.02 wt.%Mo) may also exhibit retained austenite (RA), inside the martensite laths and at the lath boundaries.
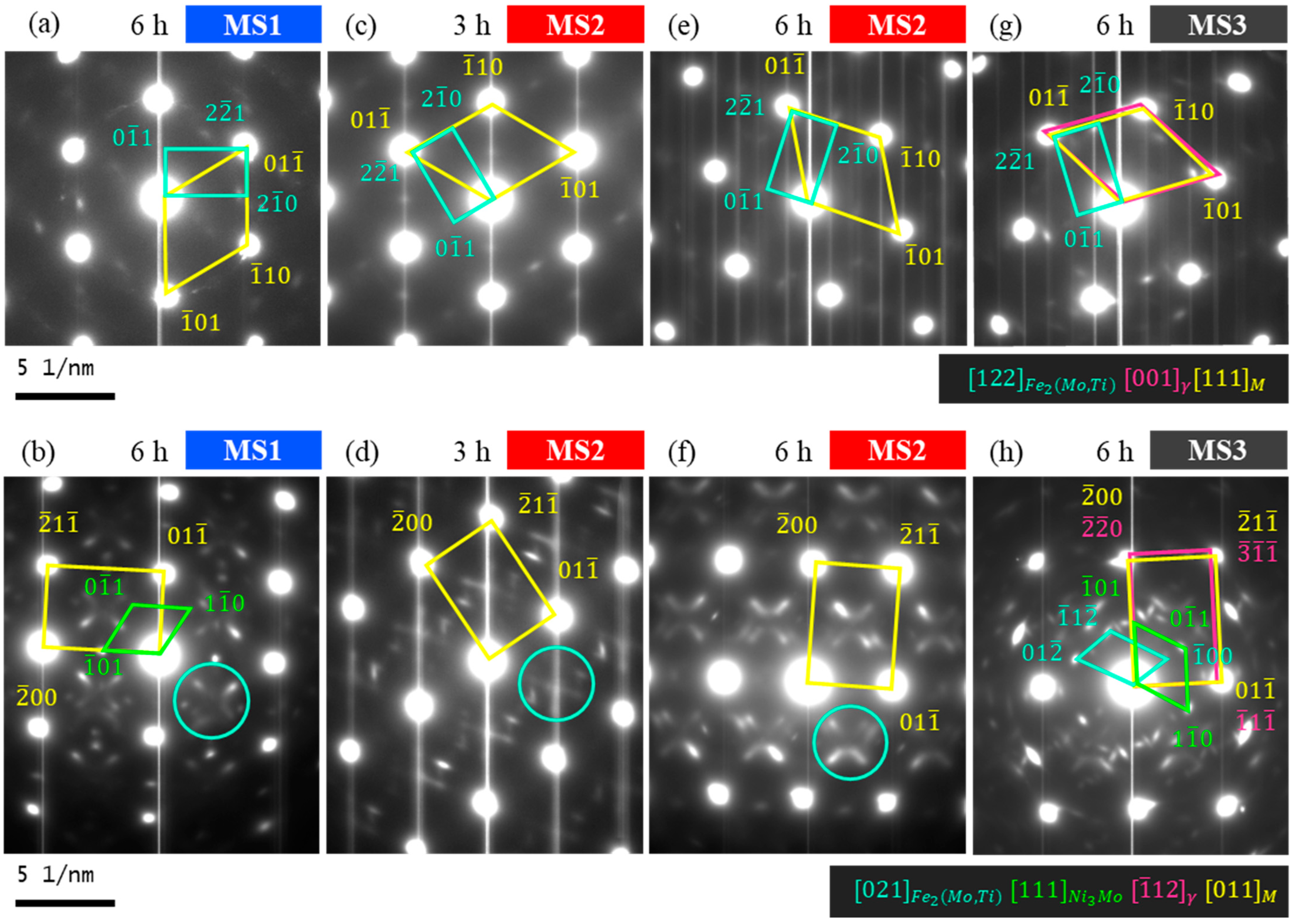
- The type of the precipitate formed in the early stages depends on the molybdenum content and also on the nickel and titanium content. The preference is to form Ni3Ti; however, when there are more molybdenum atoms available, it can form Ni3Mo.
- Later than Ni3Ti and Ni3Mo formation, the Fe2(Mo,Ti) Laves precipitates form, which are more stable. This phase is uniformly distributed throughout the matrix as single-crystals particles with a spherical morphology.
- Higher molybdenum contents accelerated the precipitation and coarsening kinetics of the Laves Fe2(Mo,Ti) phase.
- The ultra-high strength of 13Ni maraging steels is achieved by the combination of the precipitate type and size distribution. The base composition of 13Ni maraging steels achieves the peak hardness by the precipitation of the Laves Fe2(Mo,Ti) phase with a size distribution in the range of 3–14 nm.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hornbogen, E.; Rittner, K. Development of Thermo-Mechanical Treatments of a Maraging Steel for Yield Strengths above 3 GPa. Steel Res. 1987, 58, 172–177. [Google Scholar] [CrossRef]
- Schmidt, M.; Rohrbach, K. Heat Treatment of Maraging Steels. In ASM Handbook—VOLUME 4: Heat Treating; ASM International: Phoenix, AZ, USA, 1991; pp. 528–548. [Google Scholar]
- Sha, W.; Guo, Z. Maraging Steels: Modelling of Microstructure, Properties and Applications; Woodhead Publishing Limited: New York, NY, USA, 2009; ISBN 9781845693756. [Google Scholar]
- Maganée, A.; Drapier, J.M.; Dumont, J.; Coutsouradis, D.; Habraken, L. Cobalt Monograph Series—Cobalt-Containing High-Strength Steels: A Critical Review of the Physical Metallurgy of Cobaltcontaining High-Strength Steels, and a Survey of Their Processing, Properties and Uses; Centre D’Information du Cobalt: Brussels, Belgium, 1974. [Google Scholar]
- Pereloma, E.; Edmonds, D.V. Phase Transformations in Steels-Volume 2: Diffusionless Transformations, High Strength Steels, Modelling and Advanced Analytical Techniques; Woodhead Publishing Limited: Cambridge, UK, 2012; ISBN 978-0-85709-611-1. (online). [Google Scholar]
- He, Y.; Yang, K.; Sha, W. Transmission Electron Microscopy of New High-Strength Grade Maraging Steels. Microsc. Anal. 2007, 21, 5–7. [Google Scholar]
- Wang, W.; Yan, W.; Duan, Q.; Shan, Y.; Zhang, Z.; Yang, K. Study on Fatigue Property of a New 2.8GPa Grade Maraging Steel. Mater. Sci. Eng. A 2010, 527, 3057–3063. [Google Scholar] [CrossRef]
- Niu, M.; Zhou, G.; Wang, W.; Shahzad, M.B.; Shan, Y.; Yang, K. Precipitate Evolution and Strengthening Behavior during Aging Process in a 2.5 GPa Grade Maraging Steel. Acta Mater. 2019, 179, 296–307. [Google Scholar] [CrossRef]
- Menzel, J.; Klaar, H.-J. Systematische Gefiigeuntersuchungen Am Martensitaushirtenden Stahl X 2 NiCoMo 13 15 10. Steel Res. 1990, 61, 30–38. [Google Scholar] [CrossRef]
- De Carvalho, L.G.; Plaut, R.L.; Padilha, A.F. Precipitation Kinetic Analysis in a Maraging 350 Steel Using KJMA and Austin-Rickett Equations. Defect Diffus. Forum 2022, 420, 118–128. [Google Scholar] [CrossRef]
- De Carvalho, L.G.; Plaut, R.L.; de Lima, N.B.; Padilha, A.F. Kinetics of Martensite Reversion to Austenite during Overaging in a Maraging 350 Steel. ISIJ Int. 2019, 59, 1119–1127. [Google Scholar] [CrossRef]
- Tian, J.; Wang, W.; Li, H.; Shahzad, M.B.; Shan, Y.; Jiang, Z.; Yang, K. Effect of Deformation on Precipitation Hardening Behavior of a Maraging Steel in the Aging Process. Mater. Charact. 2019, 155, 109827. [Google Scholar] [CrossRef]
- Truong, T.D.; Asala, G.; Ola, O.T.; Ojo, O.A.; Odeshi, A.G. Texture and Damage Evolution in Additively Manufactured 18%Ni-M350 Maraging Steel under Dynamic Impact Loading: Processing Parameters and Heat Treatment Effects. Materialia 2023, 32, 101961. [Google Scholar] [CrossRef]
- Zeisl, S.; Van Steenberge, N.; Schnitzer, R. Strengthening Effect of NiAl and Ni3Ti Precipitates in Co-Free Maraging Steels. J. Mater. Sci. 2023, 58, 7149–7160. [Google Scholar] [CrossRef]
- Patil, V.V.; Mohanty, C.P.; Prashanth, K.G. Selective Laser Melting of a Novel 13Ni400 Maraging Steel: Material Characterization and Process Optimization. J. Mater. Res. Technol. 2023, 27, 3979–3995. [Google Scholar] [CrossRef]
- Niu, M.C.; Yin, L.C.; Yang, K.; Luan, J.H.; Wang, W.; Jiao, Z.B. Synergistic Alloying Effects on Nanoscale Precipitation and Mechanical Properties of Ultrahigh-Strength Steels Strengthened by Ni3Ti, Mo-Enriched, and Cr-Rich Co-Precipitates. Acta Mater. 2021, 209, 116788. [Google Scholar] [CrossRef]
- Tian, J.; Wang, W.; Babar Shahzad, M.; Yan, W.; Shan, Y.; Jiang, Z.; Yang, K. A New Maraging Stainless Steel with Excellent Strength–Toughness–Corrosion Synergy. Materials 2017, 10, 1293. [Google Scholar] [CrossRef]
- Bourgeot, J.; Maitrepierre, P.; Manenc, J.; Thomas, B. Hardening Fe-Ni-Mo and Fe-Ni-Co-Mo Martensitic Alloys by Tempering. IRSID Int. Rep. No 116 1971. [Google Scholar]
- Fukamachi, M.; Kawabe, Y.; Nakazawa, K.; Muneki, S. Transmission Electron Microscopy Studies of Structural Changes in 13Ni-15Co-10Mo Maraging Steel as a Result of Aging. J. Japan Inst. Metals 1983, 47, 237–242. [Google Scholar] [CrossRef][Green Version]
- Floreen, S. The Physical Metallurgy of Maraging Steels. Metall. Rev. 1968, 13, 115–128. [Google Scholar] [CrossRef]
- Niu, M.C.; Chen, C.J.; Li, W.; Yang, K.; Luan, J.H.; Wang, W.; Jiao, Z.B. Atomic-Scale Understanding of Solute Interaction Effects on Grain Boundary Segregation, Precipitation, and Fracture of Ultrahigh-Strength Maraging Steels. Acta Mater. 2023, 253, 118972. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Dey, G.K.; Asundi, M.K. Precipitation Hardening in 350 Grade Maraging Steel. Metall. Trans. A 1993, 24, 2429–2442. [Google Scholar] [CrossRef]
- Vasudevan, V.K.; Kim, S.J.; Wayman, C.M. Precipitation Reactions and Strengthening Behavior in 18 Wt Pct Nickel Maraging Steels. Metall. Trans. A 1990, 21A, 2655–2668. [Google Scholar] [CrossRef]
- Da Fonseca, D.P.M.; de Carvalho, L.G.; de Lima, N.B.; Padilha, A.F. Austenite Formation in the Oxidized Layer of Ultra-High-Strength 13Ni15Co10Mo Maraging Steel. Metals 2022, 12, 2115. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume 59, ISBN 9781439883570. [Google Scholar]
- Krauss, G. Solidification, Segregation, and Banding in Carbon and Alloy Steels. Metall. Mater. Trans. B 2003, 34, 781–792. [Google Scholar] [CrossRef]
- Fang, J.Y.C.; Liu, W.H.; Yang, T.; Wu, Y.; Jiao, Z.B. Multicomponent Precipitation and Strengthening in Intermetallic-Strengthened Alloys. Front. Mater. 2022, 9, 2–7. [Google Scholar] [CrossRef]
- Feitosa, A.L.M.; Escobar, J.; Ribamar, G.G.; Avila, J.A.; Padilha, A.F. Direct Observation of Austenite Reversion During Aging of 18Ni (350 Grade) Maraging Steel Through In-Situ Synchrotron X-ray Diffraction. Metall. Mater. Trans. A 2022, 53, 420–431. [Google Scholar] [CrossRef]
- Morito, S.; Huang, X.; Furuhara, T.; Maki, T.; Hansen, N. The Morphology and Crystallography of Lath Martensite in Alloy Steels. Acta Mater. 2006, 54, 5323–5331. [Google Scholar] [CrossRef]
- Morito, S.; Tanaka, H.; Konishi, R.; Furuhara, T.; Maki, T. The Morphology and Crystallography of Lath Martensite in Fe-C Alloys. Acta Mater. 2003, 51, 1789–1799. [Google Scholar] [CrossRef]
- Morito, S.; Saito, H.; Ogawa, T.; Furuhara, T.; Maki, T. Effect of Austenite Grain Size on the Morphology and Crystallog- Raphy of Lath Martensite in Low Carbon Steels. ISIJ Int. 2005, 45, 91–94. [Google Scholar] [CrossRef]
- Raghavan, V. Fe-Mo-Ti (Iron-Molybdenum-Titanium). J. Phase Equilibria 2003, 24, 182–183. [Google Scholar] [CrossRef]
- Argon, A.S. Strengthening Mechanisms in Crystal Plasticity; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Maki, T.; Tsuzaki, K.; Tamura, I. The Morphology of Microstructure Composed of Lath Martensites in Steels. Trans. Iron Steel Inst. Jpn. 1980, 20, 207–214. [Google Scholar] [CrossRef]
- Blankenship, C.P.; Hornbogen, E.; Starke, E.A. Predicting Slip Behavior in Alloys Containing Shearable and Strong Particles. Mater. Sci. Eng. A 1993, 169, 33–41. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials, 2nd ed.; Cambridge University Press: New York, NY, USA, 2008; ISBN 9780521866750. [Google Scholar]
- Peng, S.; Wang, Z.; Li, J.; Fang, Q.; Wei, Y. Beyond Orowan Hardening: Mapping the Four Distinct Mechanisms Associated with Dislocation-Precipitate Interaction. Int. J. Plast. 2023, 169, 103710. [Google Scholar] [CrossRef]
- Berns, H.; Theisen, W. Ferrous Materials: Steel and Cast Iron; Springer: Berlin/Heidelberg, Germany, 2008; Volume 53, ISBN 9783540718475. [Google Scholar]
- Hossain, R.; Pahlevani, F.; Quadir, M.Z.; Sahajwalla, V. Stability of Retained Austenite in High Carbon Steel under Compressive Stress: An Investigation from Macro to Nano Scale. Sci. Rep. 2016, 6, 34958. [Google Scholar] [CrossRef]

| Fe | Ni | Co | Mo | Ti | Si | Mn | |
|---|---|---|---|---|---|---|---|
| MS1 | Bal. | 13.40 | 14.96 | 7.51 | 0.250 | 0.150 | 0.160 |
| MS2 | Bal. | 12.85 | 15.64 | 10.49 | 0.721 | 0.040 | 0.040 |
| MS3 | Bal. | 14.06 | 15.21 | 15.02 | 0.240 | 0.060 | 0.040 |
| Sample | Aging Time (h) | Indexed Precipitates | Crystal Structure | Orientation Relationship p-M | Morphology 1 | Size 2 |
|---|---|---|---|---|---|---|
| MS1 | 6 | Ni3Mo | Orthorhombic | )M [011]p//[012]M | Spherical | Small |
| Ni3Mo | Orthorhombic | )M [111]p//[001]M | – | – | ||
| Fe2(Mo,Ti) | Hexagonal | 1)M [122]p//[111]M | – | – | ||
| MS2 | 3 | Fe2(Mo,Ti) | Hexagonal | 1)M [122]p//[111]M | Spherical | Small |
| 6 | Fe2(Mo,Ti) | Hexagonal | 1)M [122]p//[111]M | Spherical | Small and large | |
| MS3 | 6 | Fe2(Mo,Ti) | Hexagonal | )M [021]p//[011]M | Spherical | Small |
| Ni3Mo | Orthorhombic | )M [111]p//[001]M | Spherical | Small and large | ||
| Fe2(Mo,Ti) | Hexagonal | 1)M [122]p//[111]M | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonseca, D.P.M.; Altoé, M.V.P.; Archanjo, B.S.; Annese, E.; Padilha, A.F. Influence of Mo Content on the Precipitation Behavior of 13Ni Maraging Ultra-High Strength Steels. Metals 2023, 13, 1929. https://doi.org/10.3390/met13121929
da Fonseca DPM, Altoé MVP, Archanjo BS, Annese E, Padilha AF. Influence of Mo Content on the Precipitation Behavior of 13Ni Maraging Ultra-High Strength Steels. Metals. 2023; 13(12):1929. https://doi.org/10.3390/met13121929
Chicago/Turabian Styleda Fonseca, Daniela P. M., Maria Virginia P. Altoé, Braulio S. Archanjo, Emilia Annese, and Angelo F. Padilha. 2023. "Influence of Mo Content on the Precipitation Behavior of 13Ni Maraging Ultra-High Strength Steels" Metals 13, no. 12: 1929. https://doi.org/10.3390/met13121929
APA Styleda Fonseca, D. P. M., Altoé, M. V. P., Archanjo, B. S., Annese, E., & Padilha, A. F. (2023). Influence of Mo Content on the Precipitation Behavior of 13Ni Maraging Ultra-High Strength Steels. Metals, 13(12), 1929. https://doi.org/10.3390/met13121929








