Abstract
In this work, an innovative process involving directional sulfurization–vacuum distillation is proposed to effectively remove trace levels of mercury impurities from crude selenium. First, a reaction between sulfur and mercury is used to break the strong chemical Se-Hg bond to achieve the sulfide mineralization of mercury. Second, selenium and mercury are separated by vacuum distillation based on a difference in volatility. Thermodynamic analysis confirms the feasibility of this method. The experimental results show that the sulfidation reaction potential energy of different sulfidizing agents is in the order S > Na2S > FeS, and the optimum conditions are determined to be the following: a sulfidation temperature of 473 K and time of 30 min, and vacuum distillation experimental parameters of 503 K, 60 min, and 10–20 Pa system pressure. The overall experimental results show that the maximum removal of mercury is 97.49%. The content of mercury in the refined selenium was reduced from 0.32% to 0.0088% in the volatile matter. The results have practical value for the separation of selenium and mercury from hazardous wastes.
1. Introduction
The demand for selenium is increasing in the fields of electronic semiconductors, atomic energy, solar energy, and healthcare [1,2]. Selenium with a purity of over 99.99% has become a key material to support the development of advanced technology and new products because of its excellent metallic properties [3,4]. However, the process of selenium recovery and purification often presents environmental hazards, especially when crude selenium contains harmful elements such as mercury [5,6]. Generally, 90% of crude selenium comes from smelting byproducts produced from copper anode slime [7,8]. Mercury, which is highly toxic, is enriched during the processing of metallurgical grade crude selenium. Therefore, achieving hazard-free selenium production is the goal for selenium metallurgy. In the Chinese non-ferrous metal industry standards for 99.99% selenium, it is specified that the content of mercury impurities must be less than 3 ppm.
The hazard-free separation of selenium and mercury is a common research problem. At present, the main metallurgical methods for removing mercury from crude selenium include pyrocalcination and wet precipitation [9,10,11]. Pyrocalcination mercury removal involves the addition of calcium oxide to fix selenium and volatilize mercury at a high temperature of approximately 700 K [12]. The wet precipitation-based separation technology for selenium and mercury involves a complete oxidation of the selenium and mercury present in crude selenium into ions that enter the leaching solution. Then, through pH adjustment or the addition of a precipitant, mercury is selectively precipitated, while selenium remains in solution, thereby achieving mercury removal. The removal of mercury from selenium by pyrometallurgical methods results in the consumption of large amounts of CaO and inevitably produces secondary toxic mercury vapors [13]. It also leads to both high energy consumption and costs. Due to the high toxicity of mercury vapors and the strict requirements for flue gas management and equipment sealing, the absorption devices in Chinese mercury production industries cannot meet the atmospheric emissions standard of 0.015 mg/m3. The above processes can achieve mercury removal and crude selenium purification, but the waste liquid containing selenium needs to be disposed of in an environmentally friendly manner, which complicates the process. In recent years, some physical methods such as fractional crystallization and controlling potential oxidation–vacuum distillation have been used to remove Hg from crude selenium [14,15].
In this work, a selective sulfurization–vacuum distillation method was applied for the removal of mercury from crude selenium. The main process involves the introduction of sulfur groups into the Se-Hg system to modify the phase of mercury, and then mercury can be selectively mineralized [16,17]. Se and Hg are separated, which is convenient for subsequent recovery, and then a physical vacuum distillation method is used to volatilize selenium. Through this process, the targeted removal of mercury is achieved, while elemental selenium remains unchanged, thereby achieving directional removal of mercury and purification of crude selenium. The process mainly includes (1) adding sulfur to form a new phase with Hg to achieve mineralization and (2) purifying the crude selenium by using the altered volatilization characteristics of mercury after mineralization. Unlike traditional methods, this method for the separation of selenium and mercury is hazard-free, generates no waste liquids, is a short process, and meets the requirements for clean production. This study provides a practical basis for hazard-free selenium and mercury separation.
2. Experimental Section
2.1. Experimental Materials
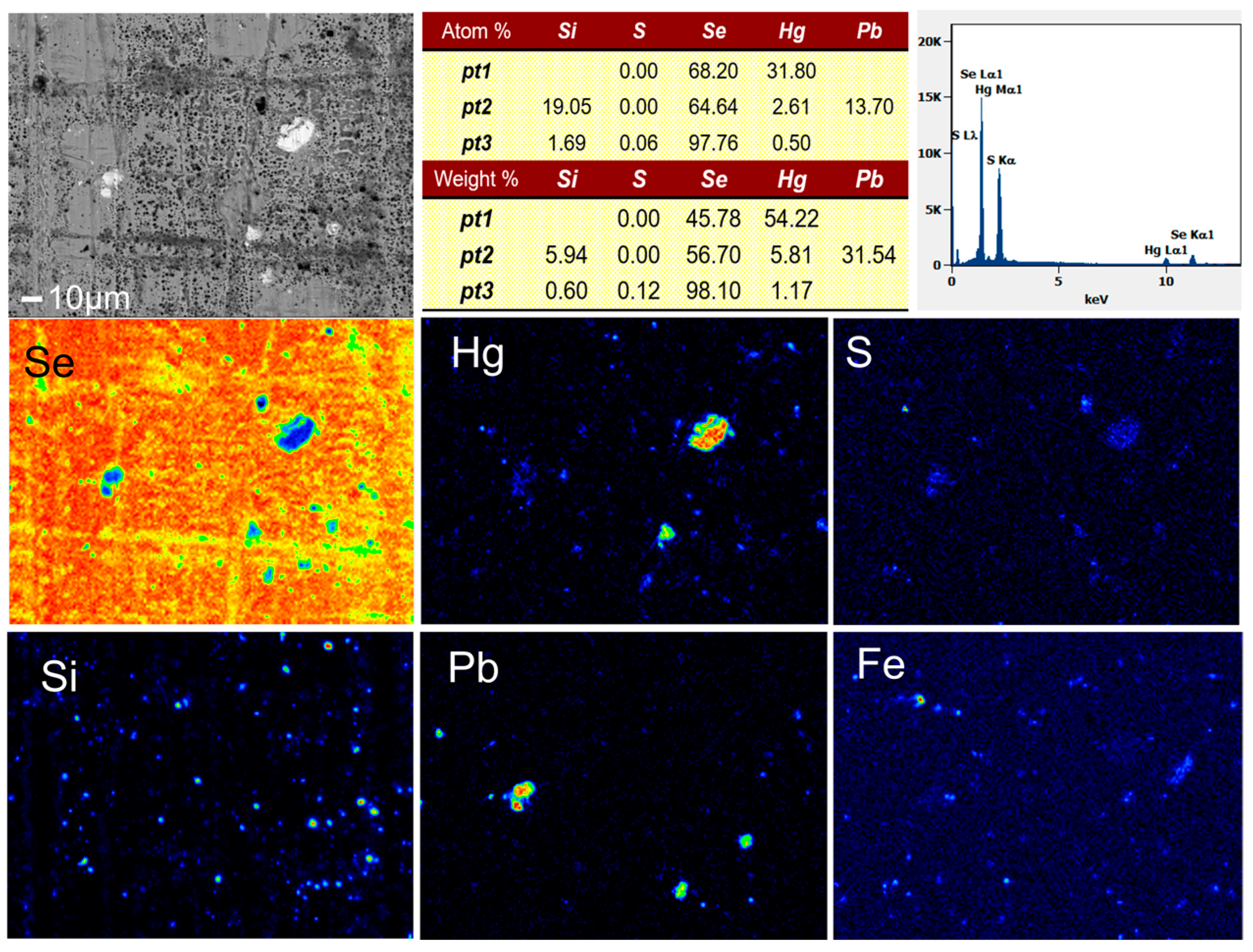
The crude selenium used in this study was produced by the sulfation roasting of copper anode slime from a smelter in Shandong, China. The sulfurizing agents used in the experiment were sulfur powder, sodium sulfide, and ferrous sulfide chemical reagents. The purity of the samples was more than 99%, and the Hg content was less than 0.0001%. The content of metal impurities in the crude selenium sample was determined by inductively coupled plasma–atomic emission spectrometry (ICP-AES) and chemical titration (Table 1). The raw material contained 96.50% Se and 0.32% Hg. An EPMA image and the energy spectral analysis results of crude selenium samples are shown in Figure 1. The elemental impurities Fe, Hg, Pb, S, and Si are roughly located in the same positions, indicating that these impurities in the crude selenium show a certain degree of aggregation. The energy spectrum of each point is obtained by EPMA scanning and shows that the mercury mainly exists in the form of HgSe.

Table 1.
Content of elements in crude selenium raw material.
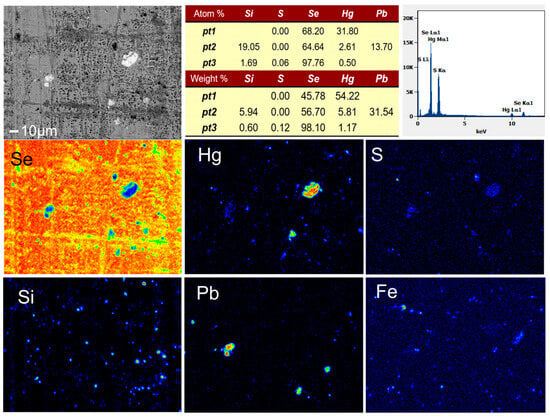
Figure 1.
EPMA image and EDS analysis of crude selenium.
2.2. Experimental Procedure
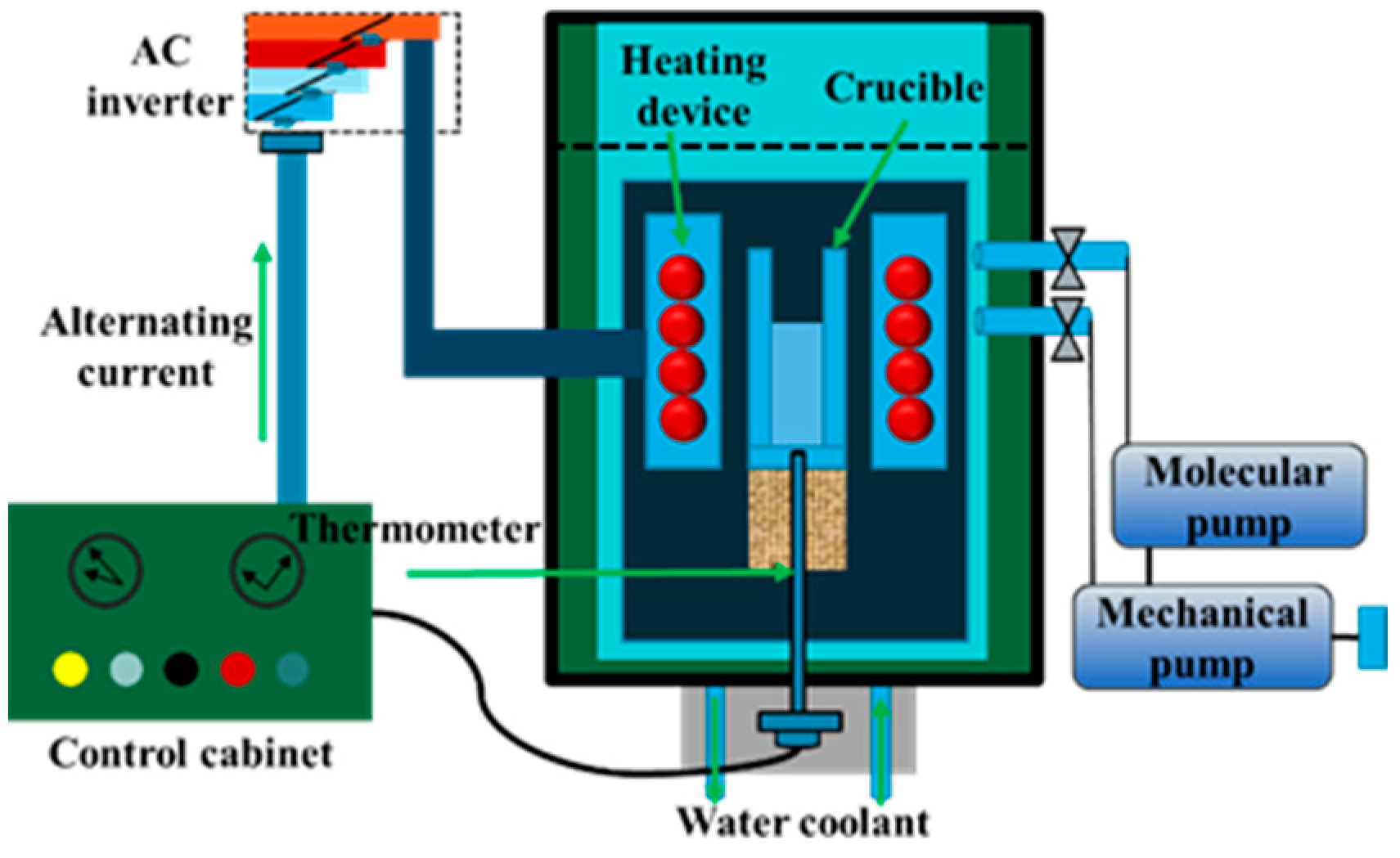
The experimental process is divided into a sulfidation process and vacuum distillation process, both of which occur in internally heated vacuum distillation furnaces, and a schematic for the equipment is shown in Figure 2. The equipment mainly includes a heating device, a vacuum atmosphere control system, a temperature and pressure measurement system, and a water circulation cooling device. During the experiment, the heating element monitors the temperature, the vacuum pump controls the pressure in the cavity, the crucible is used for the placement of materials, and the thermocouple and vacuum gauge measure temperature and pressure. The maximum capacity of the equipment is 300 g, the maximum heating temperature is 1573 K, and the heating rate can be controlled at 10–50 K/min.
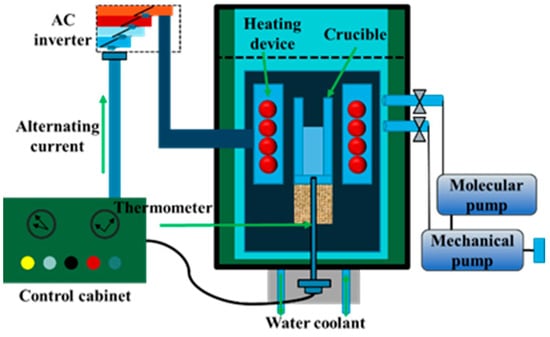
Figure 2.
Internally heated vacuum furnace structure.
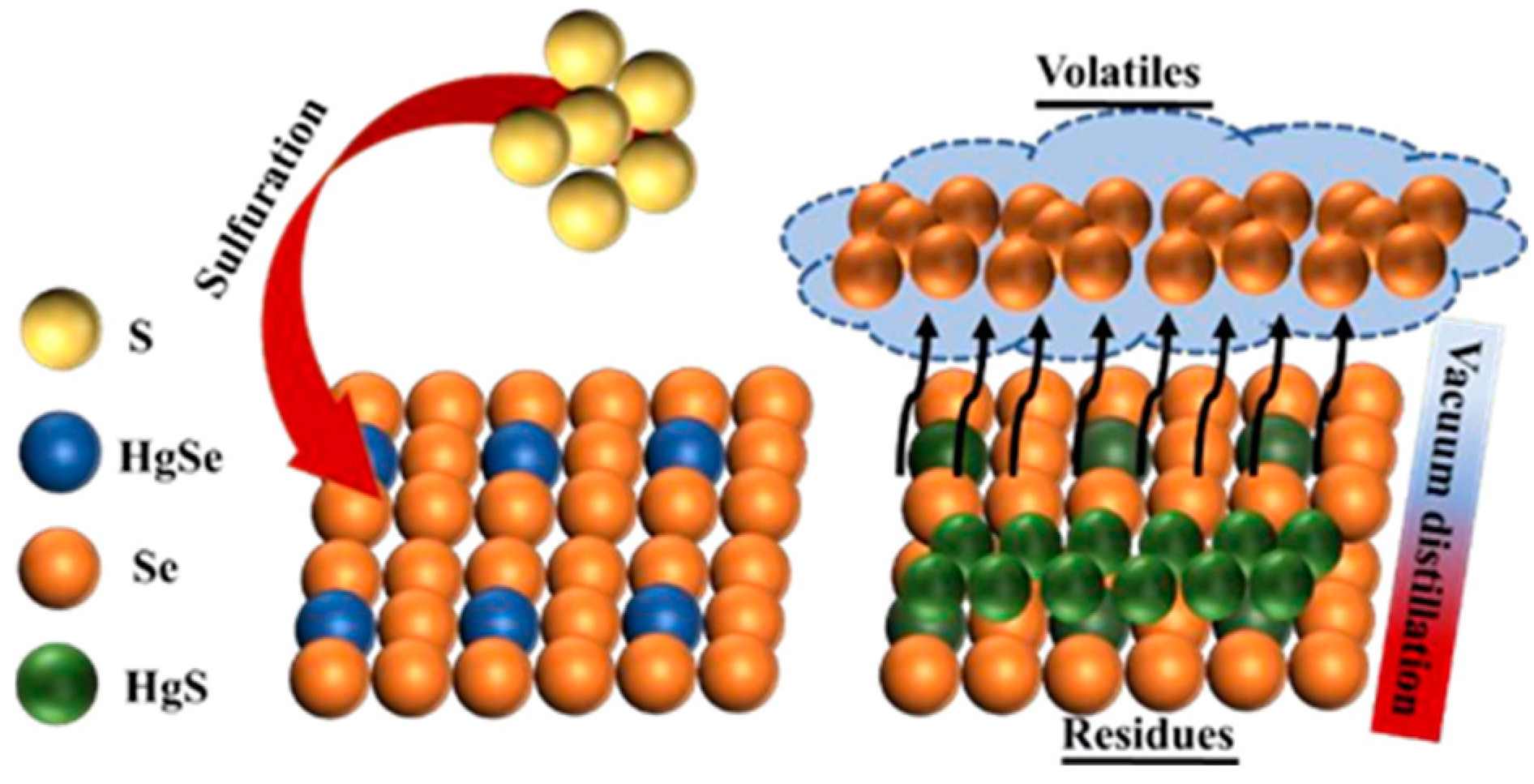
In the sulfidation stage, the curing temperature is mainly controlled by the heating device. The heating rate is 10 K/min, and the temperature is maintained for a certain amount of time. Under normal pressure, maintaining an argon atmosphere can effectively avoid oxidation of samples, sulfidizing agents, and air during sulfidation. In the vacuum distillation stage, the obtained sulfide products are crushed and placed in the crucible. The pressure in the equipment furnace is controlled at 15–30 Pa by a vacuum pump. Then, the heating device is turned on to achieve the set temperature. The heating rate is set to 25 K/min, and the furnace is allowed to cool down after the high temperature is maintained for a certain period of time. The volatiles produced during vacuum distillation are collected in the condenser, and the residue is stored at the bottom of the crucible for collection. Figure 3 shows the mechanism for the sulfuration and vacuum distillation of crude selenium.
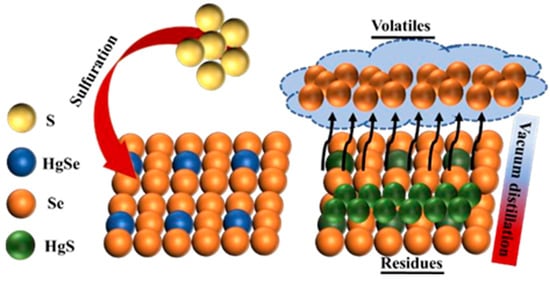
Figure 3.
Schematic of the reaction mechanism of crude selenium sulfurization–vacuum distillation.
2.3. Characterization
The contents of the major elements in random samples were determined by chemical titration. The concentrations of Hg impurities in the experimental products were measured by using inductively coupled plasma-mass spectrometry (ICP-MS, 7700x; Agilent, Santa Clara, CA, USA) according to the Standards for the Nonferrous Metals Industry of the People’s Republic of China (YS/T 223-2007 and YS/T 226.13-2009) issued by the National Development and Reform Commission. The elemental distributions of the products were determined by electron probe microanalysis (EPMA, JXA8230; JEOL, Tokyo, Japan). Phases were determined by using X-ray diffraction (XRD, Mini Flex 600; Rigaku, Tokyo, Japan) with a scanning angle of 10 to 80°.
3. Results and Discussion
3.1. Theoretical Analysis
3.1.1. Sulfidation Process
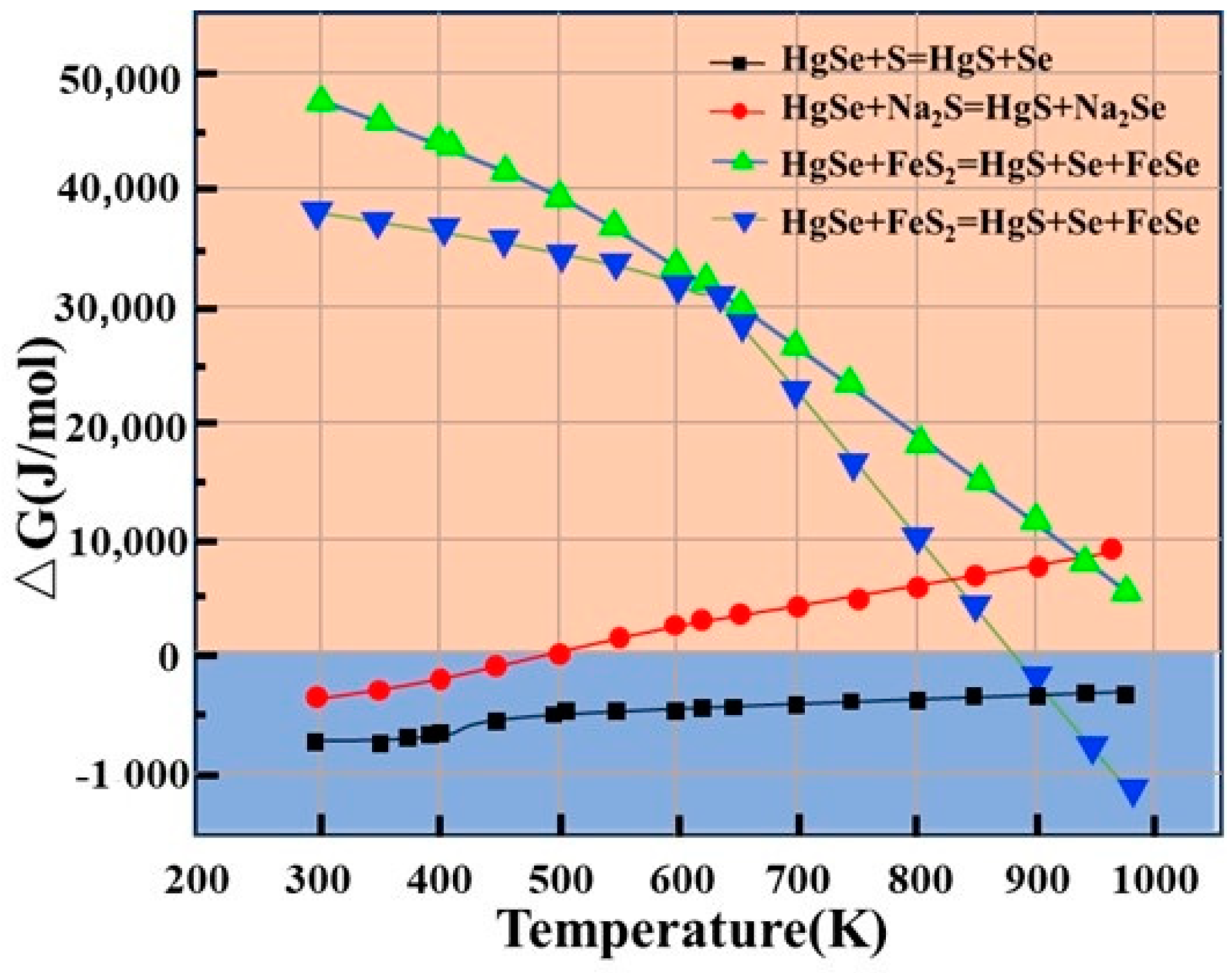
Three common curing agents, sulfur powder (S), sodium sulfide (Na2S), and ferrous sulfide (FeS2), are used in the curing process. The change in Gibbs free energy (△G) is an important basis for determining whether the substitution reaction occurs. When △G is negative, the reaction is spontaneous at constant temperature and pressure. In contrast, the reaction is nonspontaneous if the △G value is positive. △G can be expressed by Formula (1). The reactions of the three sulfidizing agents with HgSe are shown in Table 2. At the same time, the feasibility of decomposition of HgSe was considered, and the calculation results are shown in Figure 4.
△G = △G0 + RTInQ

Table 2.
Thermodynamic analysis of chemical reactions during the sulfuration process of crude selenium.
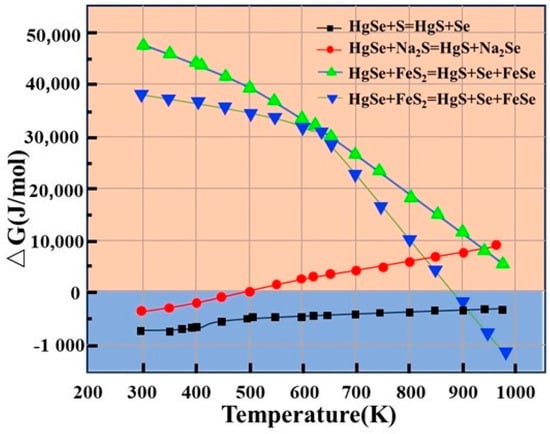
Figure 4.
Relationship between change in Gibbs free energy and temperature of different sulfidizing agents (S, Na2S and FeS2).
The calculation results for the change in Gibbs free energy for the reaction of the three sulfidizing agents, S, Na2S, and FeS2, with HgSe in crude selenium are shown in Figure 4. The change in Gibbs free energy of R-1 at 298––1000 K is less than 0 and gradually decreases with the increasing temperature, indicating that reaction 1 can be spontaneous. The change in Gibbs free energy of R-4 is greater than 0; therefore, the reaction cannot occur. Thus, it is demonstrated that HgSe in the crude selenium slag will not undergo double decomposition and will be replaced by sulfur atoms in sulfur to generate HgS. For the change in Gibbs free energy of R-2 at 298–1000 K, when the temperature reaches 521 K, the change in Gibbs free energy gradually increases to more than 0, indicating that the increase in reaction temperature is not conducive to a gradual reduction in the sulfidation ability of NaS. For R-3, the change in Gibbs free energy of the reaction is less than 0 at 398–1000 K. The change in Gibbs free energy decreases with increasing temperature, indicating that the occurrence of R-3 requires a higher temperature. Comprehensive analysis shows that the reaction potential energy of sulfuration of HgSe in crude selenium is the order S > Na2S > FeS2.
3.1.2. Vacuum Distillation Process
The saturated vapor pressure of different metal gases varies greatly, which forms the basis of metal vacuum distillation. The saturated vapor pressure p usually has a nonlinear relationship with temperature T, as shown in Formula (2) [18].
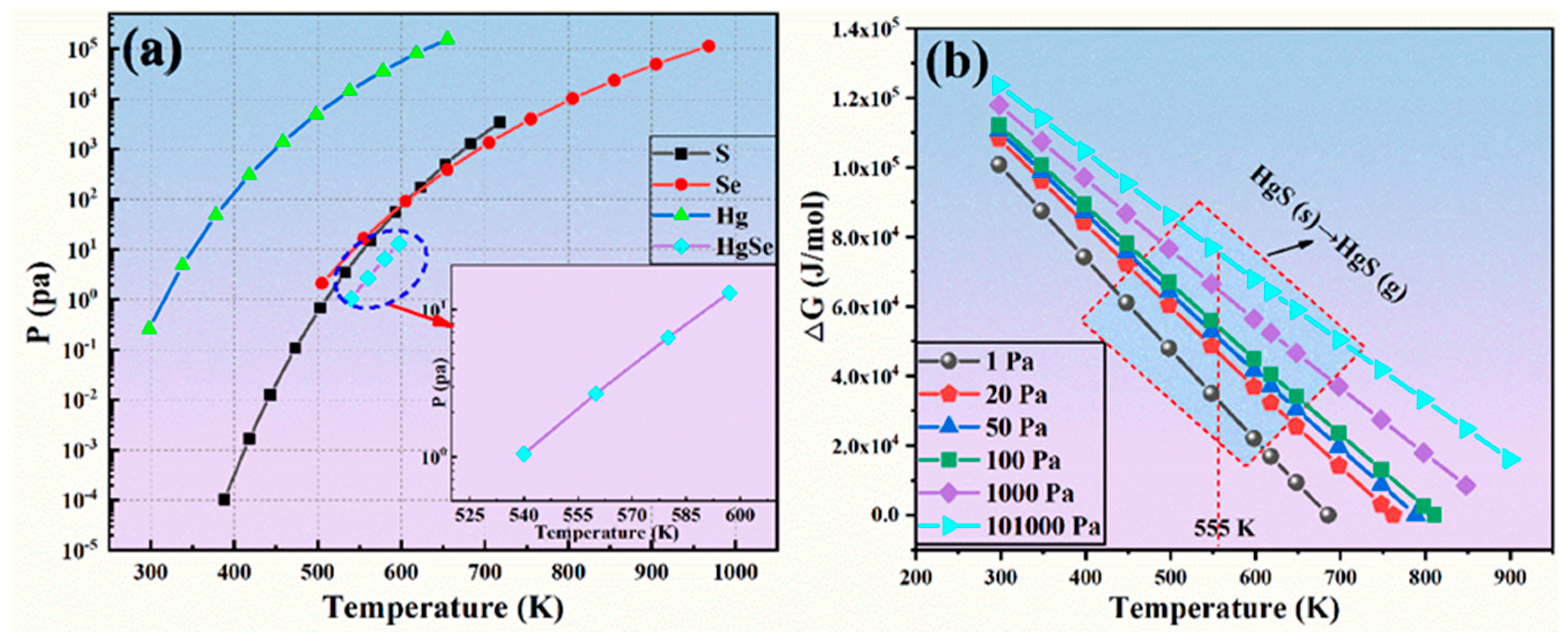
The volatilization characteristics of HgS produced in the sulfidation process and HgSe in the crude selenium were analyzed. Hg is obviously interdependent with Se and S, and S and Hg are combined for synthesis. This finding is consistent with the above thermodynamic calculations. The calculation results for the saturated vapor pressure of HgSe, Hg, Se and S are shown in Figure 5a. According to the relationship between the saturated vapor pressure and temperature of a material, the saturated vapor pressure of Se in the system is highest after sulfidation, and it volatilizes. When the temperature is 555 K, the saturated vapor pressure of Se is 16.56 Pa, and the saturated vapor pressure of the raw material HgSe is 2.7 Pa, which proves that the volatility of Se is far greater than that of HgSe. In other words, when the pressure is between 2.7 Pa and 16.56 Pa at 555 K, Se is in an unsaturated state, while HgSe is in a supersaturated state. At a similar saturated vapor pressure during the actual process, HgSe volatilized with Se in the vacuum distillation process. Therefore, HgSe changed to HgS and demonstrated the volatilization characteristics of HgS due to sulfidation. The conversion of HgS (s) into HgS (g) is shown in Figure 5b, and the change in Gibbs free energy for the reaction is greater than 0, which proves that it exists in a solid-state at 555 K and is not converted to gaseous molecules. It can be seen from the above analysis that a change in the vacuum conditions can induce Hg to remain in the residue of the vacuum distillation process as the sulfide product (HgS) and Se volatilizes in the molecular state to achieve crude selenium purification and the removal of Hg.
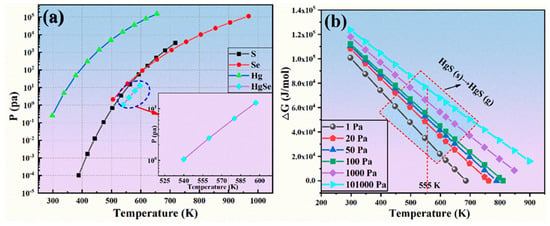
Figure 5.
(a) Vapor pressure of sulfuration products versus temperature; (b) change in Gibbs free energy versus temperature for HgS(s) to HgS(g) conversion at different pressures.
3.2. Experimental Analysis
3.2.1. Selection of Sulfidizing Agent
Three different sulfurizers, S, Na2S, and FeS2, were used to determine their potential for the removal of mercury from crude selenium. Four groups of experiments were designed, including one containing no sulfidizing agent (control) and three separate groups with S, Na2S, and FeS2. Approximately 20 g of crude selenium was placed into a crucible, a sulfidizing agent was added at a 5:1 mass ratio, and the sample was mixed evenly and placed in a vacuum furnace. According to the theoretical analysis of sulfidation, the heating temperatures are 473 K, 473 K, and 973 K for S, Na2S, and FeS2, respectively, and the samples were heated for 30 min under atmospheric pressure. After the completion of the three groups of experiments containing sulfidizing agents, the distillation temperature was set to 523 K, the vacuum pressure was set to 15–30 Pa, and the distillation time was set to 90 min. The sample without sulfidizing agent was directly distilled at 523 K and 15–30 Pa for 90 min, during which the other conditions were kept the same to avoid influences from altered experimental conditions. The above experimental results can be seen in Table 3.

Table 3.
Experimental results with the addition of different sulfidizing agents.
The calculation of the evaporation rate and removal efficiency of Hg is shown in Formulas (3) and (4).
where is the evaporation rate, %; is the volatile mass, g; and is the total mass of the input material, g. is the removal efficiency of Hg, %; is the mass of crude selenium, g; is the content of Hg in crude selenium, ppm; and is the content of Hg in the volatiles, ppm.
Based on comparisons with the blank experiment, the effect of elemental sulfur on mercury removal was the best among the different curing agents. The mercury content in the volatile matter was 523 ppm, and the removal efficiency of Hg reached 81.55%. The three curing agents had a good effect on removing elemental mercury. When FeS2 was used as a sulfurizing agent in the experiment, the reaction temperature was high (973 K) and exceeded the boiling point of selenium. Due to the large amount of selenium volatilization that took place during the sulfurizing process, a small amount of selenium volatilized to the condensing plate during the distillation process, resulting in a very high mercury content. Therefore, it was concluded that FeS2 was not suitable as a sulfurizing agent in the experiment. The content of Hg in the crude selenium raw material was 3200 ppm, and the content of Hg in the volatile matter in the four groups of experiments was 1939 ppm. Therefore, the efficiency of elemental sulfur for Hg removal and purification was better than that of direct distillation, while the efficiency of Na2S and FeS2 for Hg removal was lower than that of direct vacuum distillation. Therefore, Na2S and FeS2 cannot be used as sulfurizing agents for the sulfur-driven removal of mercury.
3.2.2. Analysis of Hg Removal Efficiency
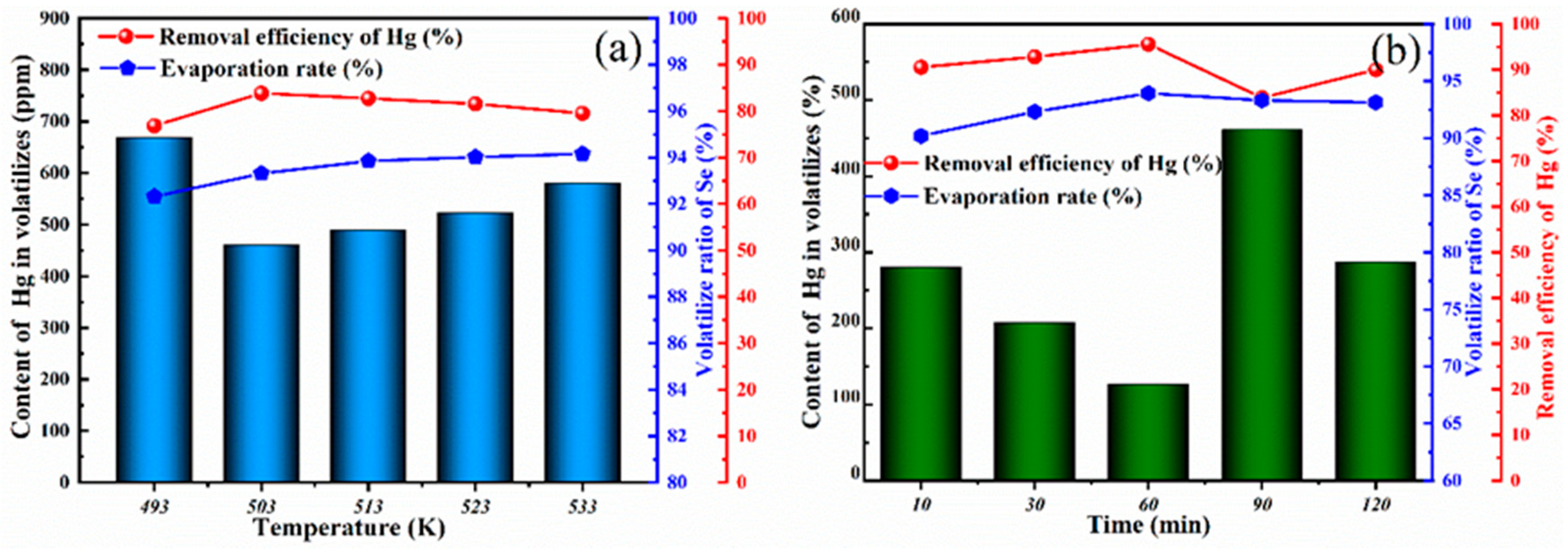
With elemental S as the sulfurizing agent, a vacuum distillation experiment was carried out on the sulfurized product at 473 K to explore the effect of time and temperature on mercury removal efficiency and the purification of crude selenium. Distillation temperature and time were used as variables, and the other conditions were consistent with the above process. The distillation temperature was 493–533 K, and the distillation time was 10–120 min. The experimental results are shown in Figure 6.
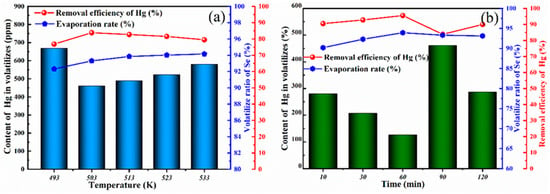
Figure 6.
(a) The effect of distillation temperature on the content of Hg in volatiles and the removal efficiency of Hg; (b) the effect of distillation time on the content of Hg in volatiles and the removal efficiency of Hg.
To obtain the best conditions for mercury removal, the effects of time and temperature on the mercury content in the volatile matter were evaluated. In the process of vacuum distillation, the effect of temperature on the removal efficiency of Hg was first considered, and the time was set to 90 min. The relationship between the distillation temperature and removal efficiency of Hg is shown in Figure 6a. The removal efficiency of Hg increased when the temperature increased from 493 K to 503 K. The highest Hg removal efficiency was reached at 503 K. At this time, the content of Hg in the volatile fraction was 461 ppm, and the removal rate was 83.85%. Notably, when the temperature reached 503 K, the content of Hg in the volatiles gradually increased, resulting in a decrease in mercury removal efficiency. The reason this occurred is that the temperature changed the volatilization characteristics of the trace levels of incompletely vulcanized HgSe gas molecules. The relationship between the holding time and removal efficiency of Hg is shown in Figure 6b. When the holding time was 60 min or greater, the evaporation rate was 93.93%. It can be inferred that when the holding time is 60 min or greater, the distillation time has little effect on the evaporation rate. The volatile substances in the raw materials are essentially completely volatilized, and the removal efficiency of Hg does not change with time. At 60 min, the removal efficiency of Hg was 95.56%, and the mercury content in the volatile matter was 126 ppm. Combined with the above experimental results, it can be concluded that mercury removal is best when the holding time is changed to 60 min at 503 K. Thus, mercury removal via a vacuum distillation process involving the directional sulfurization of crude selenium residue was verified.
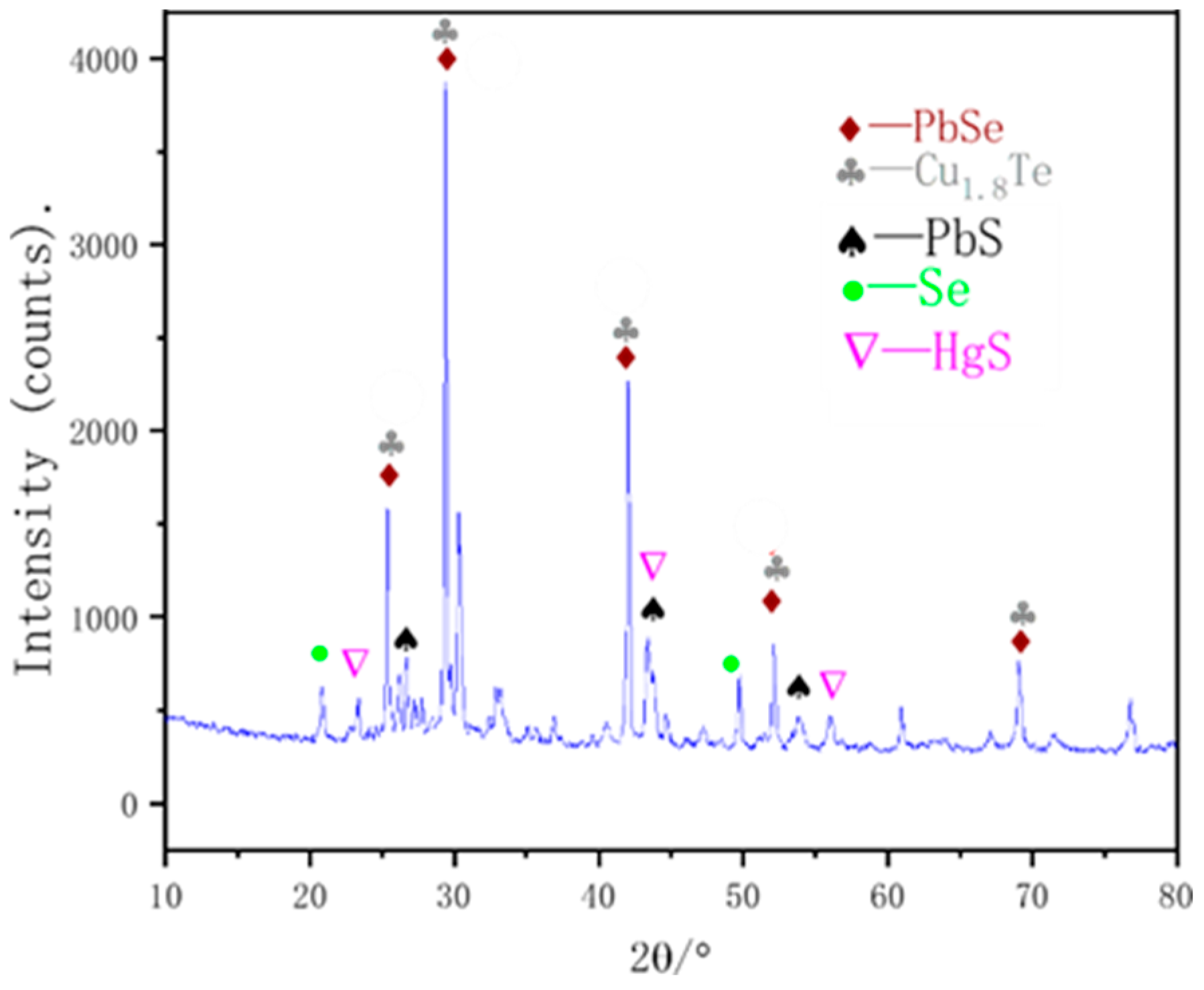
To determine the phase of mercury present in the vacuum distillation residue, XRD was performed, and the results are shown in Figure 7. The results indicate the presence of difficult-to-volatilize PbSe, PbS, and Cu1.8Te in the residue, as well as incomplete volatilization of elemental selenium. The presence of HgS and a decrease in the mercury content in the volatile matter indicates that HgSe does in fact react with elemental sulfur to generate HgS during the sulfurization reaction.
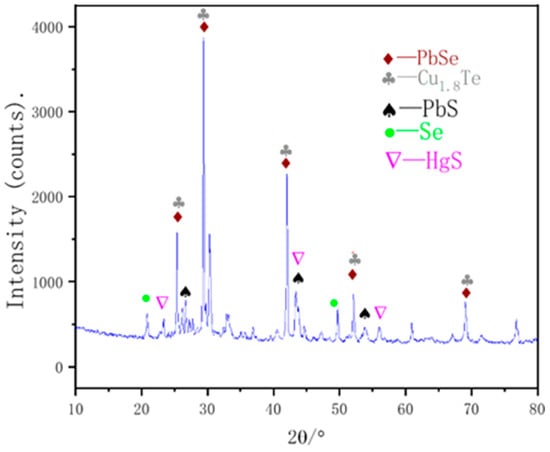
Figure 7.
XRD analysis of vacuum distillation residues.
3.2.3. Secondary Vacuum Distillation Purification
The flow of selenium vapor during the vacuum distillation process of sulfide products is viscous, resulting in the incomplete removal of Hg in the distillation process. To further purify selenium, secondary vacuum distillation was carried out. An amount of 18.41 g of distillation volatiles was added under optimized conditions (503 K, 60 min) into a crucible, the pressure was set to 10–20 Pa, and the distillation experiment was conducted again. The results are shown in Table 4.
where is the removal efficiency of Hg during secondary distillation, %; is the volatile mass under optimized conditions for the sulfurization–vacuum distillation process; is the content of Hg in volatiles under optimized conditions for the sulfurization–vacuum process, ppm; is the mass of secondary volatiles; and is the content of Hg in the secondary volatiles.
where is the total removal efficiency of Hg, %; is the removal efficiency of Hg under optimized conditions for the sulfurization–vacuum distillation process; and is the secondary removal efficiency of Hg, %.

Table 4.
Experimental results of secondary distillation.
According to the above experimental data, the evaporation rate of secondary distillation is 98.91%. The second distillation process was carried out with 95.54% Hg removal efficiency in the first vacuum distillation process and with a 126 ppm mercury content in the volatiles. The removal efficiency of Hg in secondary distillation was 43.46% on the basis of the initial mass. Based on both distillation processes, the total removal efficiency of Hg was 97.49%, all of the mercury in the crude selenium was sulfurized, and secondary distillation resulted in a final amount of 88.52 ppm mercury in the volatile matter. Thus, selenium products with a low mercury content were obtained. This shows that a combination of selective sulfidation and vacuum distillation processes can effectively reduce the concentration of highly toxic mercury in crude selenium and achieve the purification of Se.
The above discussion demonstrates that it is reasonable to combine sulfidation with vacuum distillation for the removal of mercury from crude selenium to purify selenium. In addition, no selenium-containing aqueous solutions are used in this method for removing mercury from crude selenium, and the vacuum distillation method for removing mercury circumvents the accumulation of harmful substances (Hg, Se) in a closed environment. Although mercury removal via combined sulfidation and vacuum distillation has been studied in the laboratory environment, this process needs to be further studied as a continuous and automated process to produce corresponding cost-effective equipment for practical applications.
4. Conclusions
In this study of mercury removal from crude selenium by sulfuration and vacuum treatment, the following three conclusions were reached:
- (1)
- Theoretical analysis shows that the sulfuration reaction potential energy of S, Na2S, and FeS2 for mercury (HgSe) in crude selenium is in the order S > Na2S > FeS2, and the Hg removal after sulfuration that is used to change the phase of mercury is far greater than only vacuum distillation.
- (2)
- Through the experiments, it was found that S was the best curing agent, and vacuum distillation of the curing product could effectively reduce the mercury content of Se to 523 ppm.
- (3)
- A comprehensive analysis shows that the maximum removal rate of mercury was 97.49%, 0.32% of the mercury in the crude selenium was sulfurized, and secondary distillation resulted in a final amount of 88.52 ppm mercury in the volatile matter. The research results have practical value for the separation and purification of selenium and mercury from hazardous wastes.
Funding
This research was funded by the Yunnan Science and Technology Plan Project (202201BE070001-056), the Analysis and Testing Foundation of Kunming University of Science and Technology (2022T20210172), and the Construction of High-Level Talents of Kunming University of Science and Technology (grant No. 20210172).
Data Availability Statement
Not applicable.
Acknowledgments
The authors sincerely acknowledge the anonymous reviewers for their insights and comments, which further improved the quality of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, D.; Wang, D.; Hong, T.; Wang, Z.; Wang, Y.; Qin, Y.; Su, L.; Yang, T.; Gao, X.; Ge, Z.; et al. Lattice plainification advances highly effective SnSe crystalline thermoelectrics. Science 2023, 380, 841–846. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Lin, X.-M.; Minella, C.B.; Wang, D.; Diemant, T.; Behm, R.J.; Fichtner, M. Selenium and selenium-sulfur cathode materials for high-energy rechargeable magnesium batteries. J. Power Sources 2016, 323, 213–219. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Mehta, S. Selenium nanomaterials: An overview of recent developments in synthesis, properties and potential applications. Prog. Mater. Sci. 2016, 83, 270–329. [Google Scholar] [CrossRef]
- Staicu, L.C.; Van Hullebusch, E.D.; Lens, P.N. Lens, Production, recovery and reuse of biogenic elemental selenium. Environ. Chem. Lett. 2015, 13, 89–96. [Google Scholar] [CrossRef]
- Yu, J.G.; Yue, B.Y.; Wu, X.W.; Liu, Q.; Jiao, F.P.; Jiang, X.Y.; Chen, X.Q. Removal of mercury by adsorption: A review. Environ. Sci. Pollut. Res. 2016, 23, 5056–5076. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Eom, Y.; Lee, T.G. Mercury recovery from mercury-containing wastes using a vacuum thermal desorption system. Waste Manag. 2017, 60, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Microbial recovery of critical metals from secondary sources. Bioresour. Technol. 2022, 344, 126208. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, Y.; Tang, A.; Pan, D.; Li, B. Recovery of scattered and precious metals from copper anode slime by hydrometallurgy: A review. Hydrometallurgy 2020, 197, 105460. [Google Scholar] [CrossRef]
- Feng, Q.; Li, A.J.; An, Y.F.; Zhang, B.; Zhao, Y.Q. Research Development on Separation and Extraction of Selenium and Mercury. Solid State Phenom. 2022, 330, 161–168. [Google Scholar] [CrossRef]
- Xing, P.; Ma, B.; Wang, C.; Chen, Y. Cleaning of lead smelting flue gas scrubber sludge and recovery of lead, selenium and mercury by the hydrometallurgical route. Environ. Technol. 2018, 39, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Bai, C.; Chen, G. Research on acidic Se-Hg slime treatment with POX alkaline leaching. Chin. Nonferr. Metall. 2022, 5, 51. (In Chinese) [Google Scholar]
- Yang, W.; Dai, L.; Yang, K. Research on Microwave Enhancement of Zinc Smelting Acid Sludge by Calcium Addition and Selenium Fixing. Yunnan Metall. 2021, 5, 50. (In Chinese) [Google Scholar]
- Vereecken, J.; D’Hondt, D.; Gérain, N.; Van Lierde, A. Selective recovery by precipitation of selenium and mercury from acid leaching solutions. In EMC’91: Non-Ferrous Metallurgy—Present and Future; Springer: Berlin/Heidelberg, Germany, 1991; pp. 89–95. [Google Scholar]
- He, B.; Wang, W.; Jiang, W.; Xu, B.; Yang, H. Removing of Fe, Pb and Hg from Crude Selenium by Fractional Crystallization. Metals 2023, 13, 739. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, W.; Zha, G.; Liu, L.; Zhen, T.; Yang, B.; Xu, B. Removal of impurity Pb during crude selenium purification by controlling potential oxidation and vacuum distillation. Vacuum 2022, 195, 110674. [Google Scholar] [CrossRef]
- Li, Z.; Deng, J.; Liu, D.; Jiang, W.; Zha, G.; Huang, D. Waste-free separation and recovery of copper telluride slag by directional sulfidation-vacuum distillation. J. Clean. Prod. 2022, 335, 130356. [Google Scholar] [CrossRef]
- Stinn, C.; Allanore, A. Selective sulfidation of metal compounds. Nature 2022, 602, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Yongnian, D.; Bin, Y. Vacuum Metallurgy of Nonferrous Metal Materials; Metallurgical Industry Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).