Evolution of the Interelectrode Gap during Co-Rotating Electrochemical Machining
Abstract
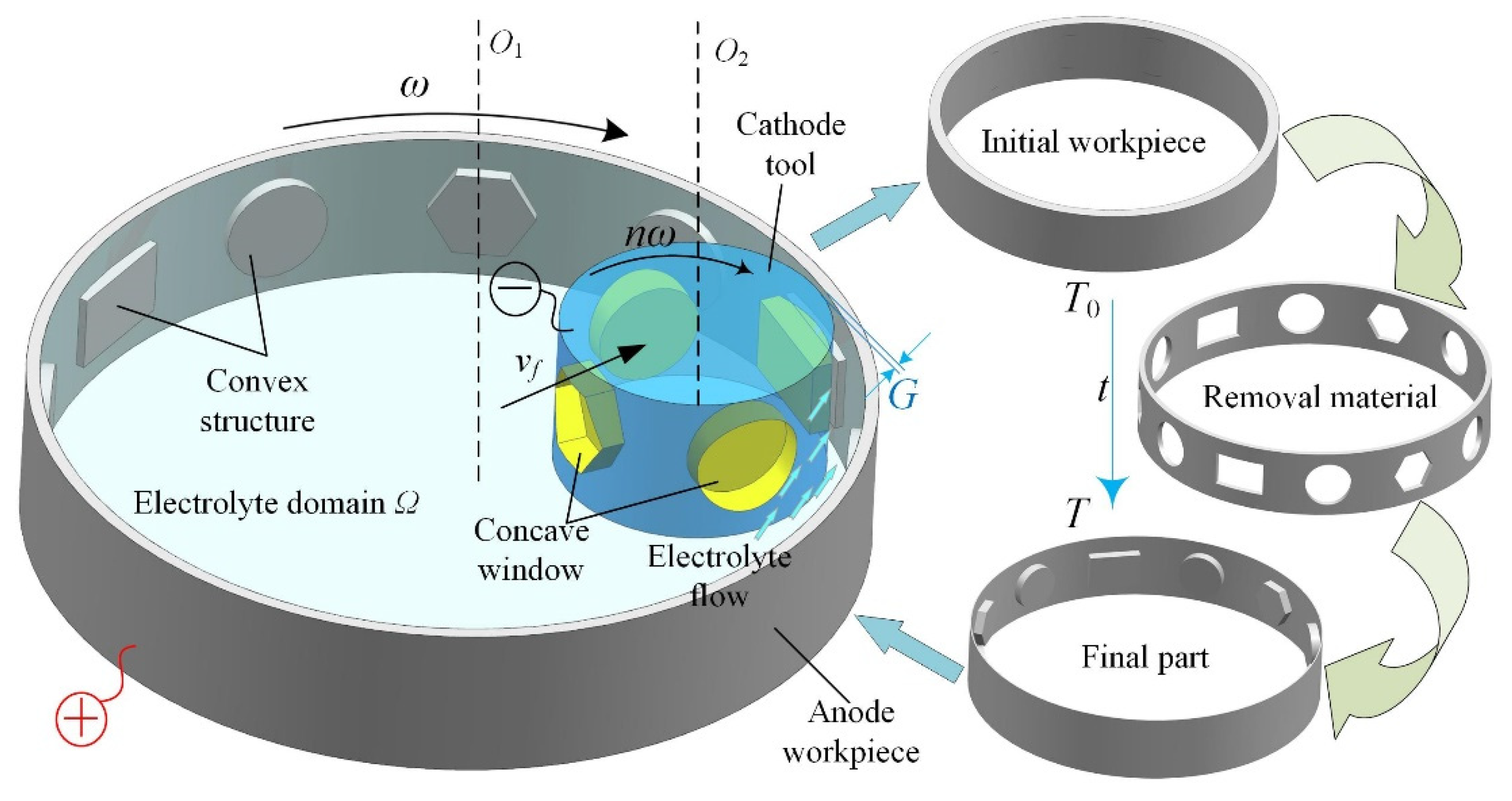
:1. Introduction
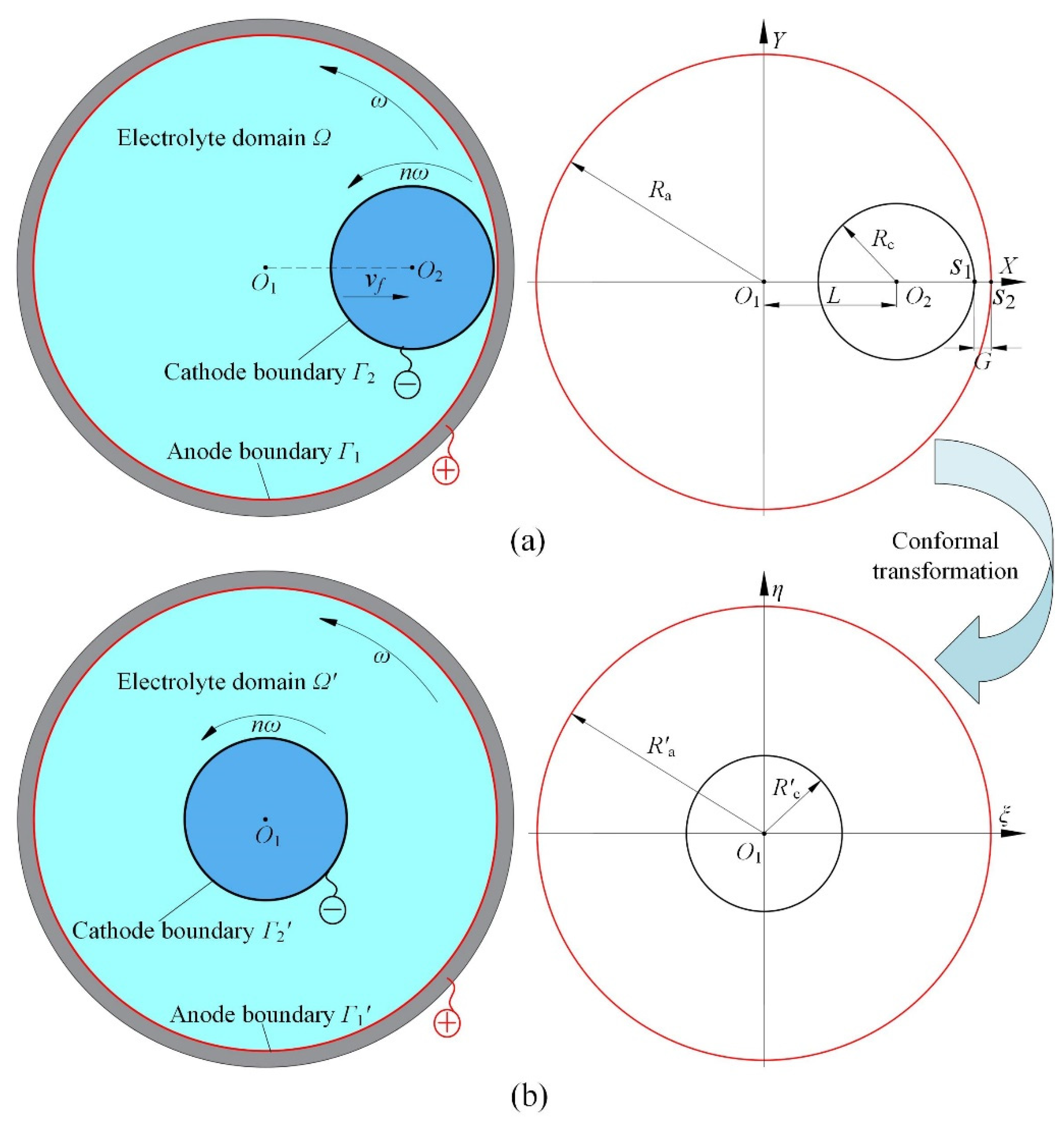
2. Mathematical Model
2.1. Electric Field Model
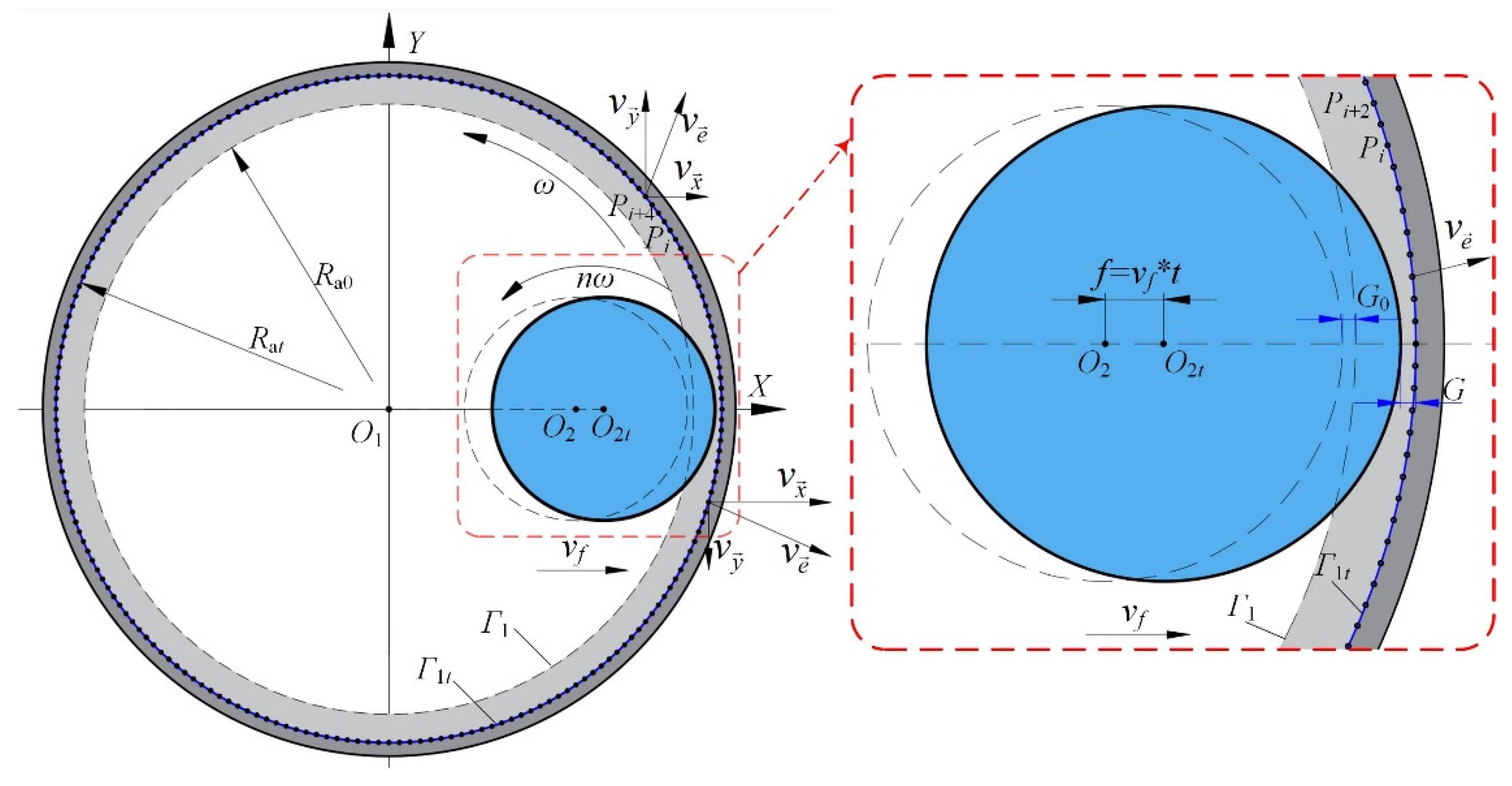
2.2. Material Removal Model
3. Simulation of the Material Removal Process
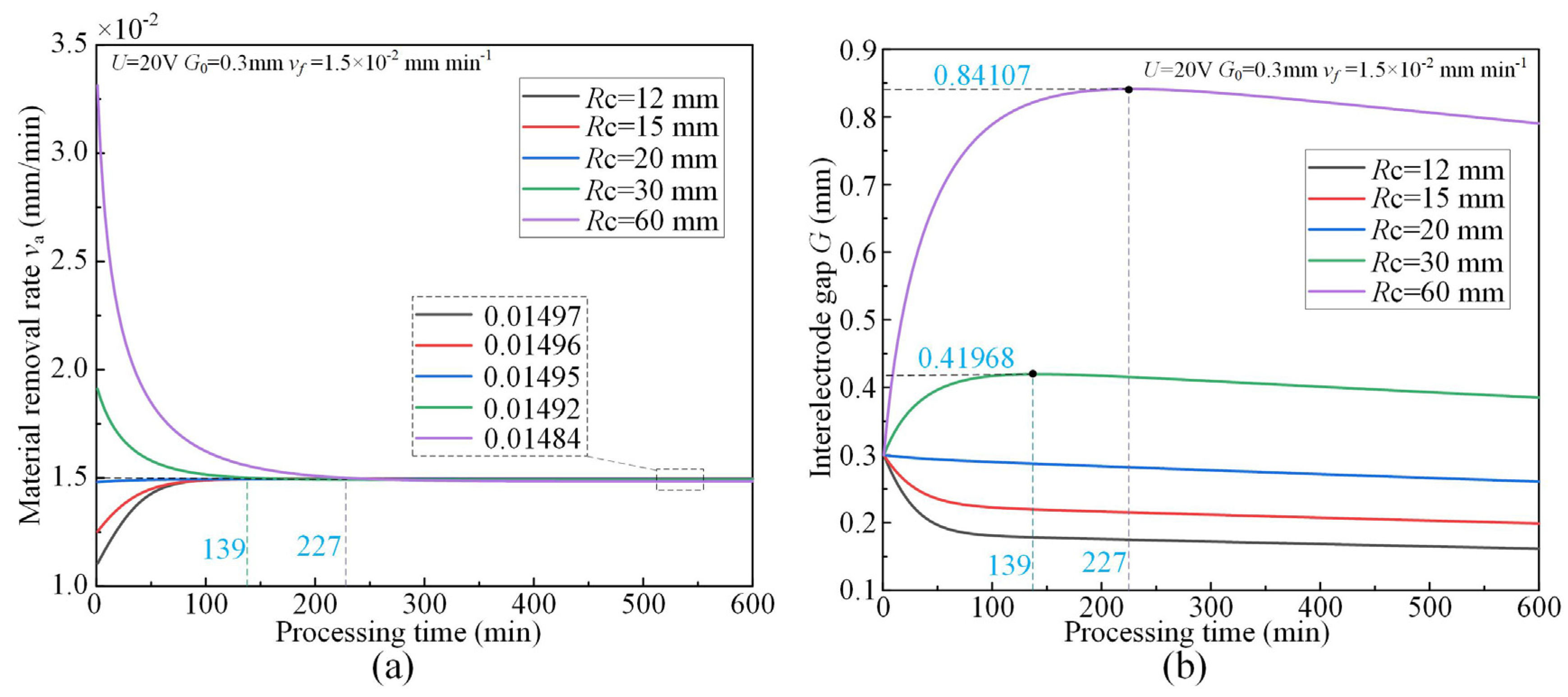
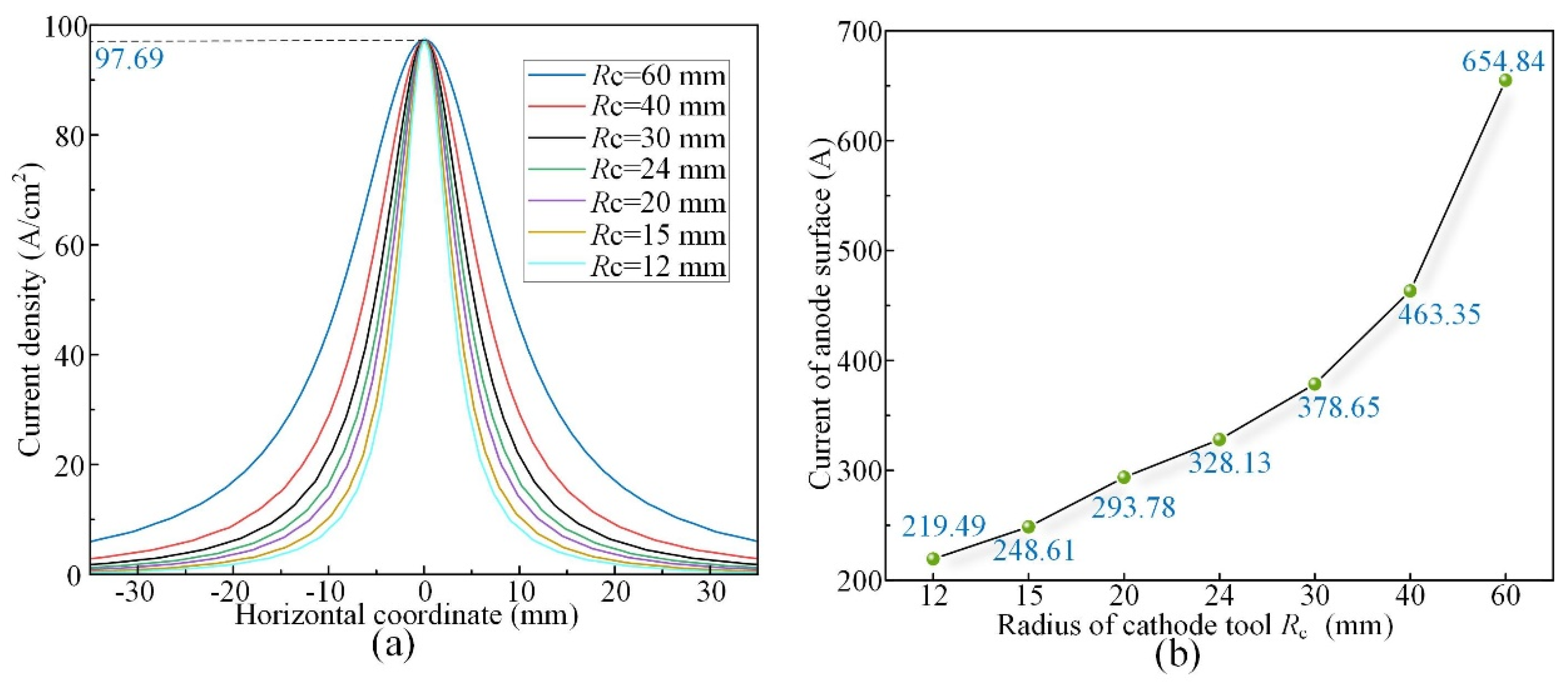
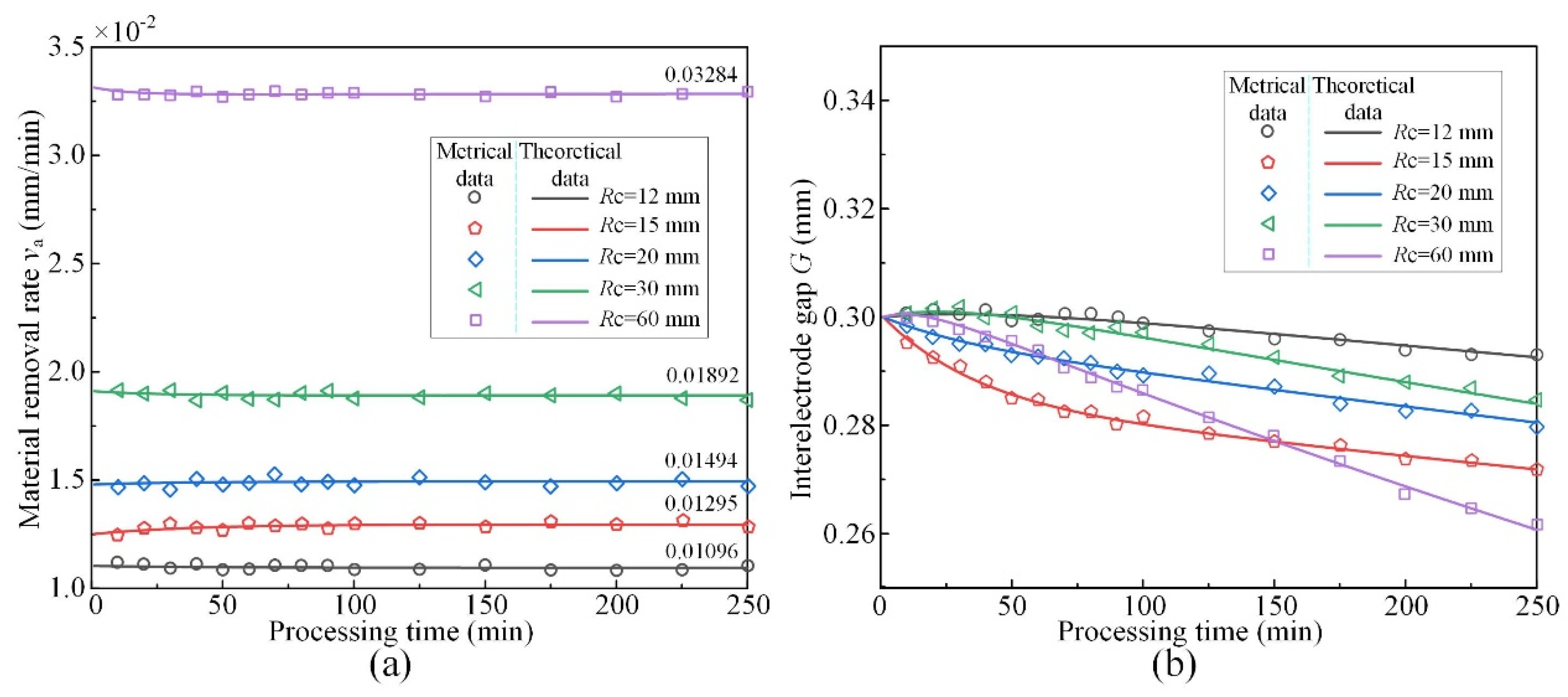
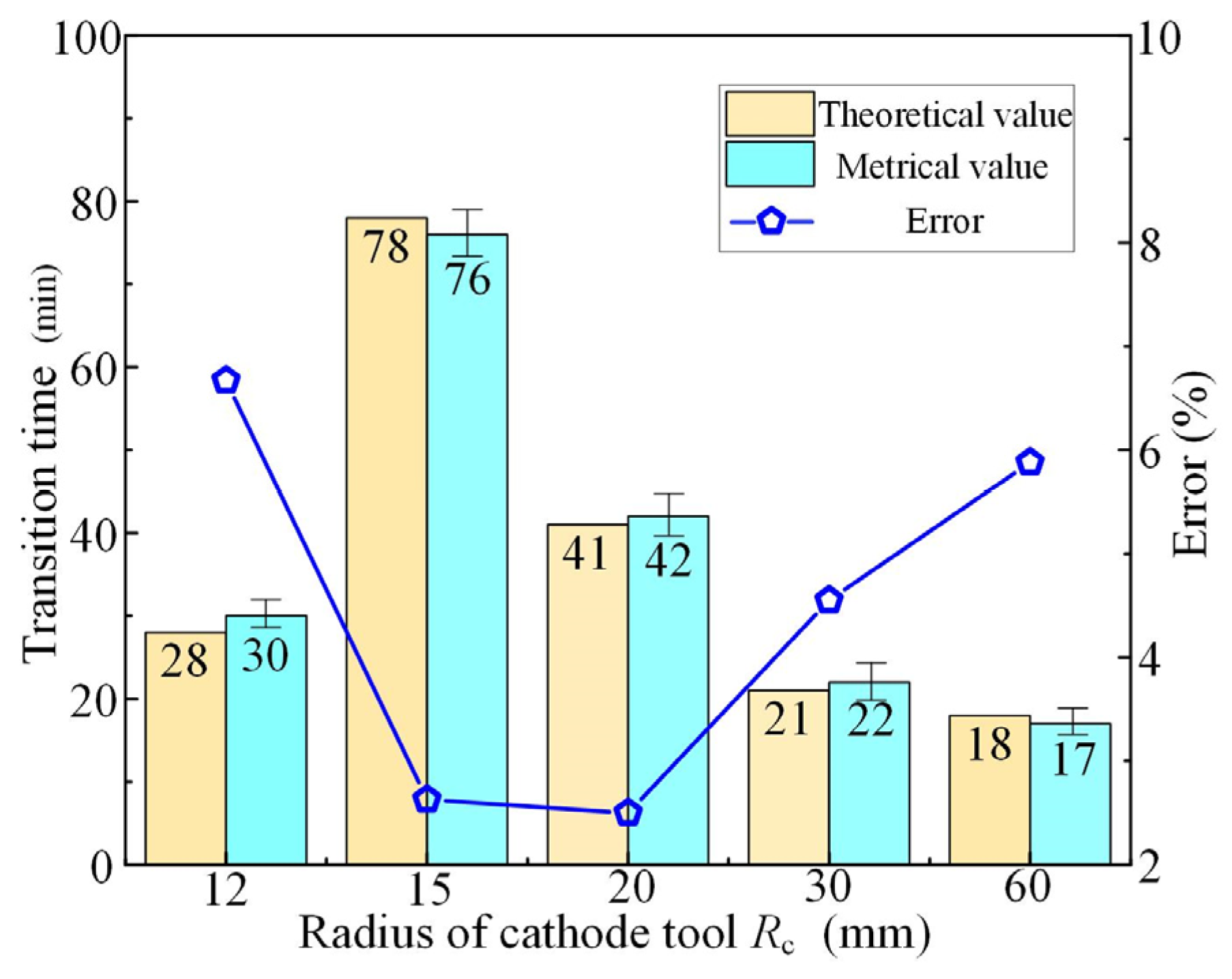
3.1. Effect of Cathode Tool Radii
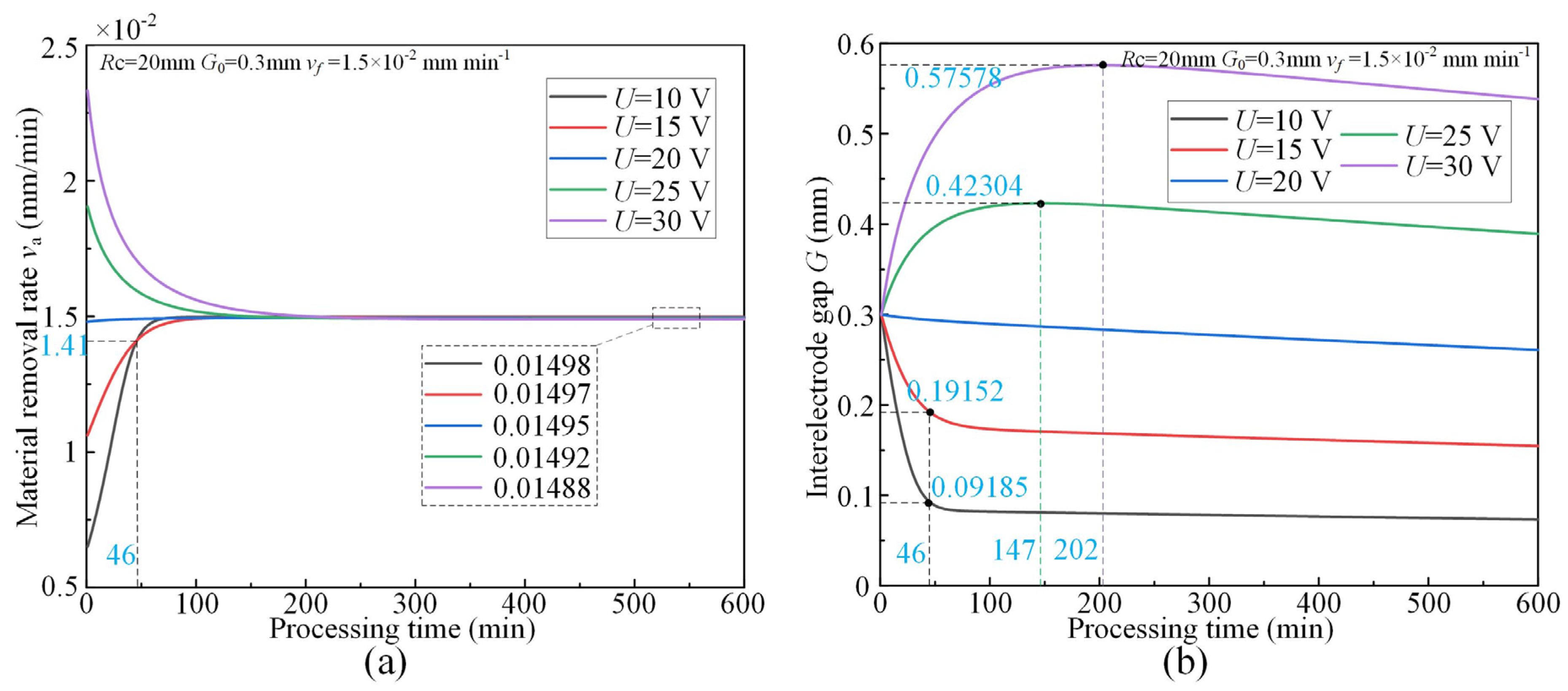
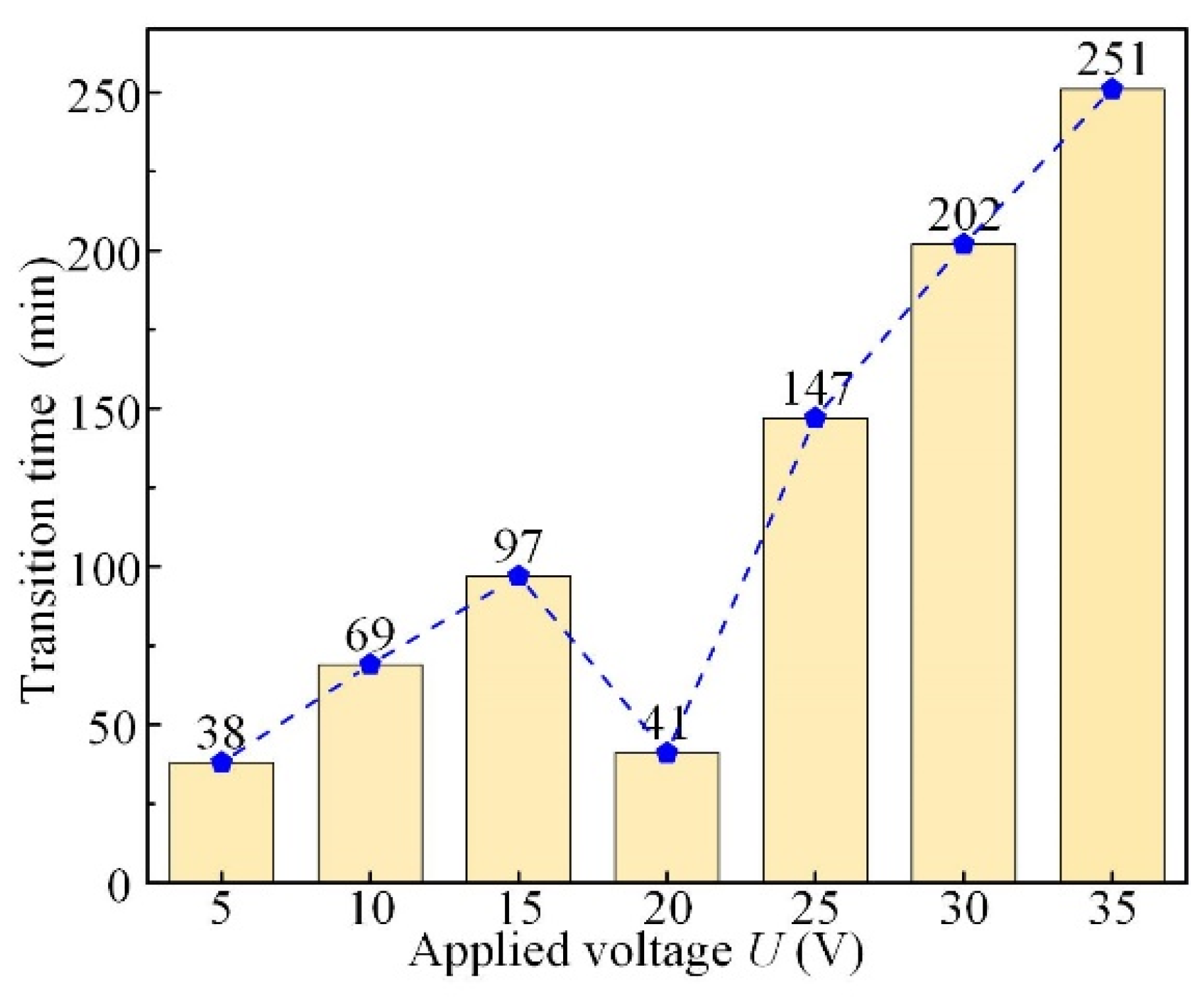
3.2. Effect of Applied Voltage
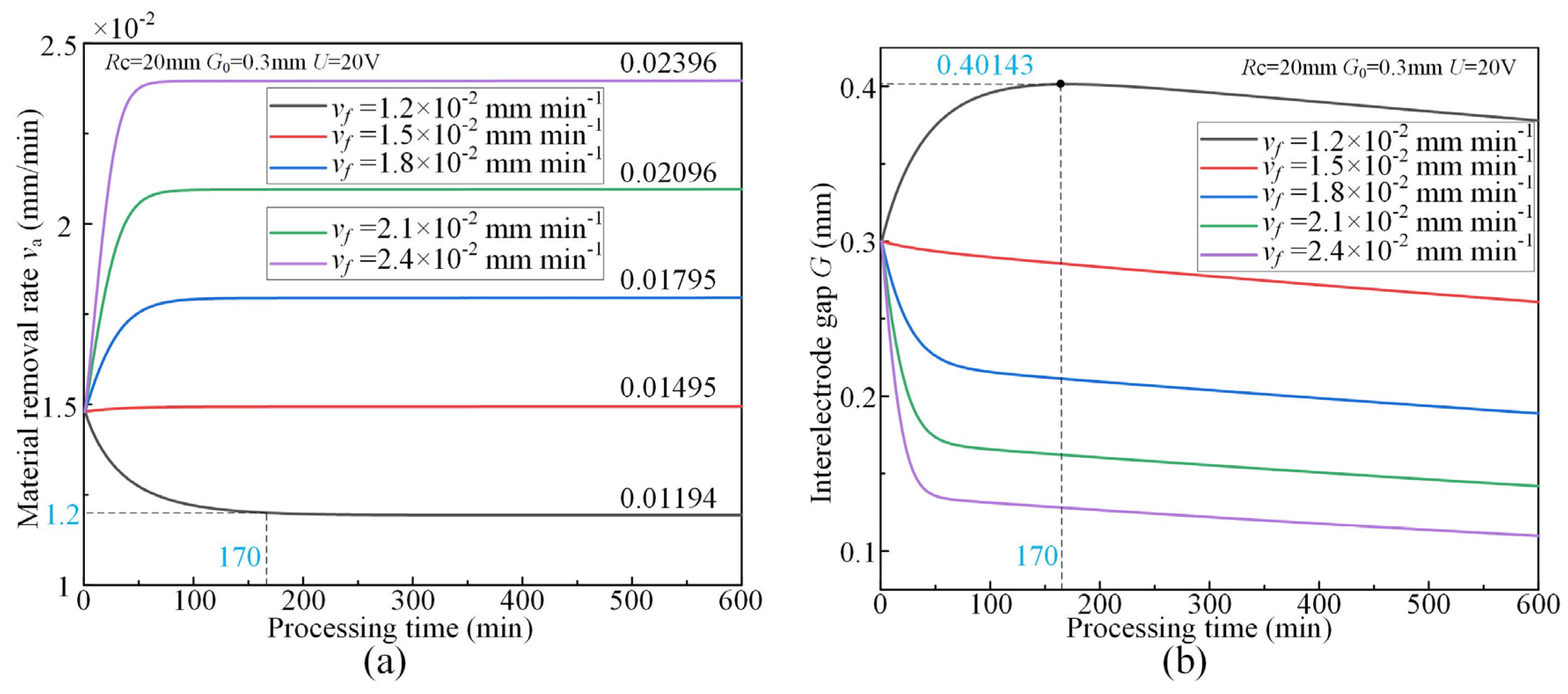
3.3. Effect of Feed Rate
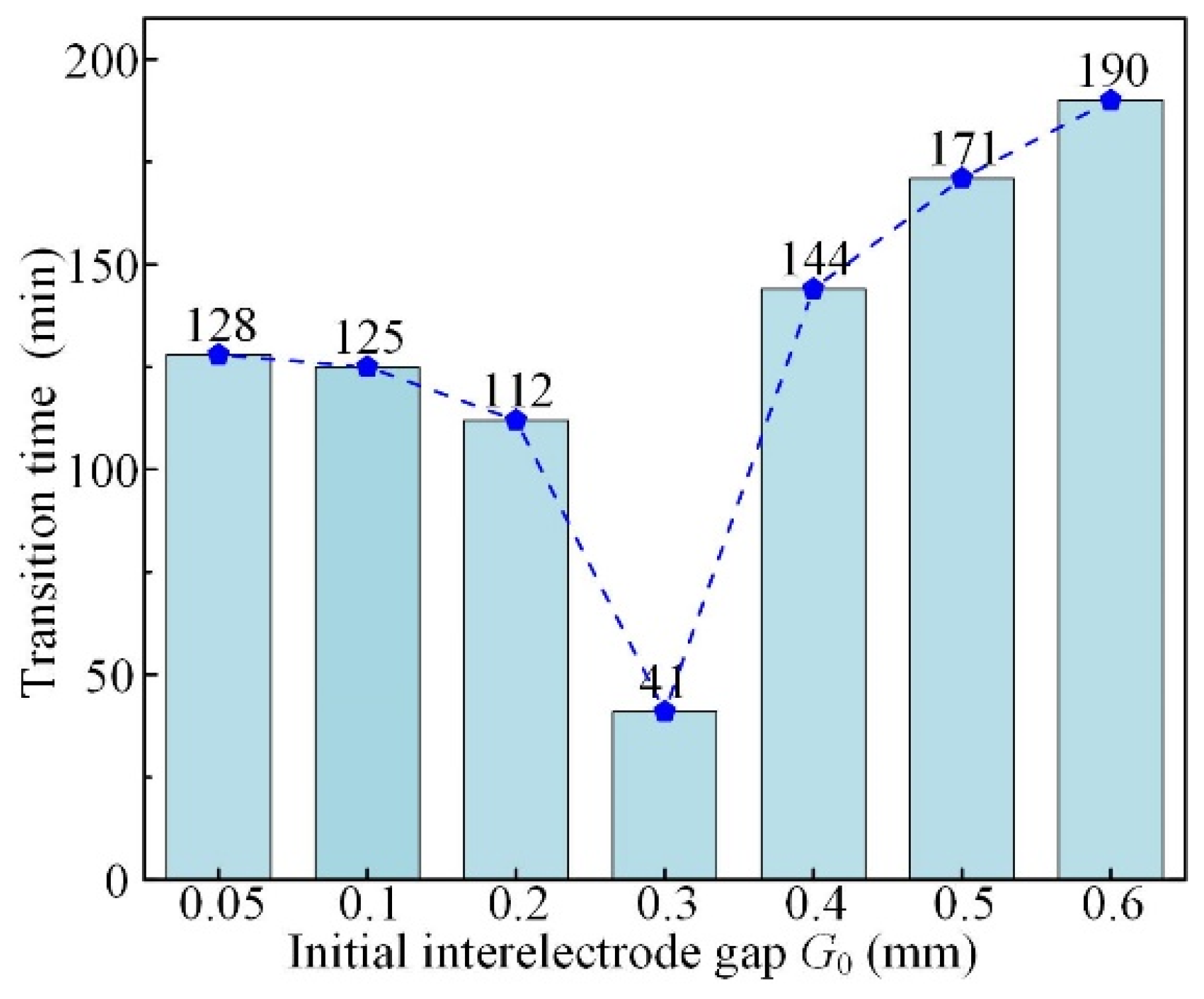
3.4. Effect of Initial Interelectrode Gap
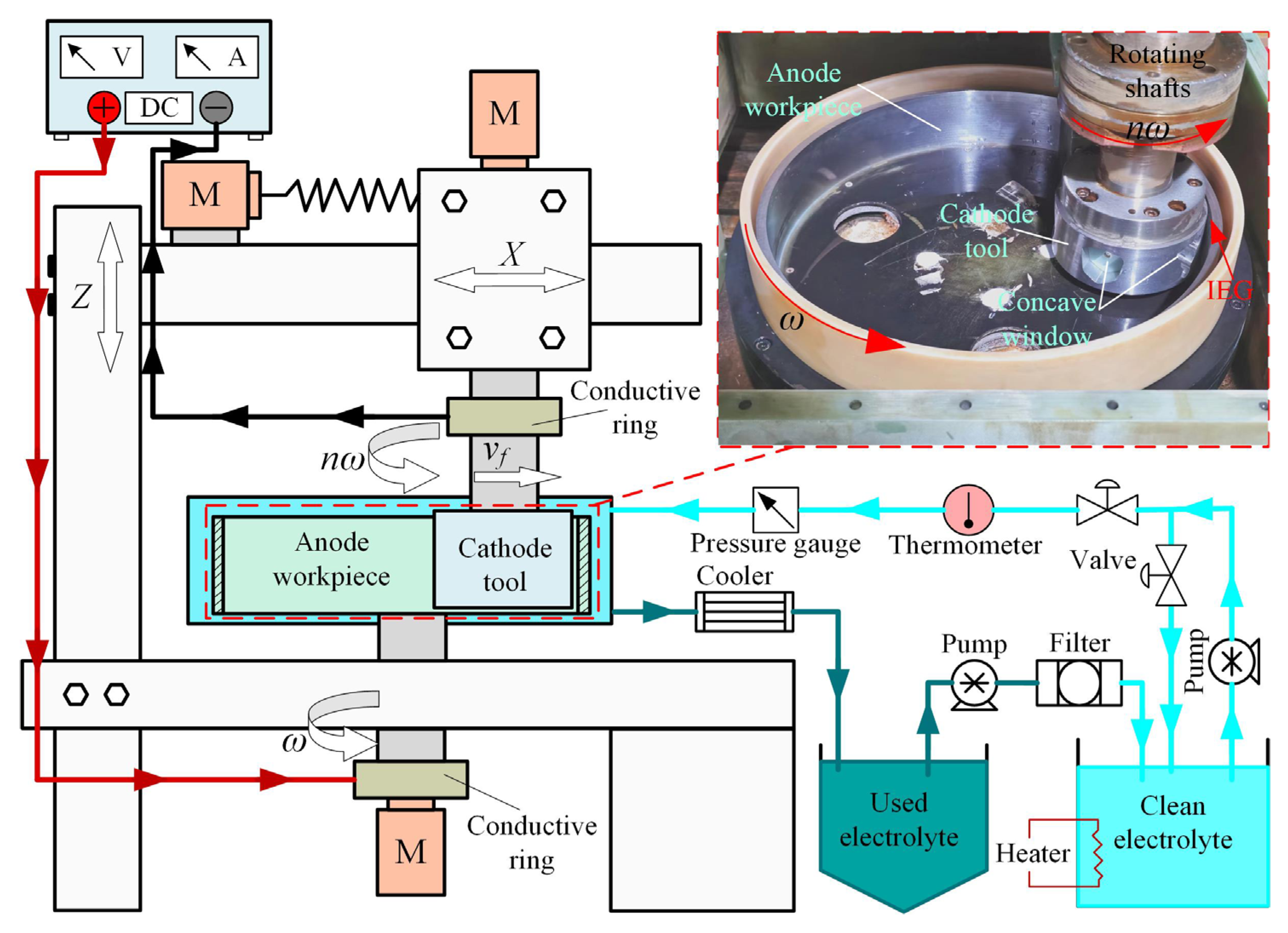
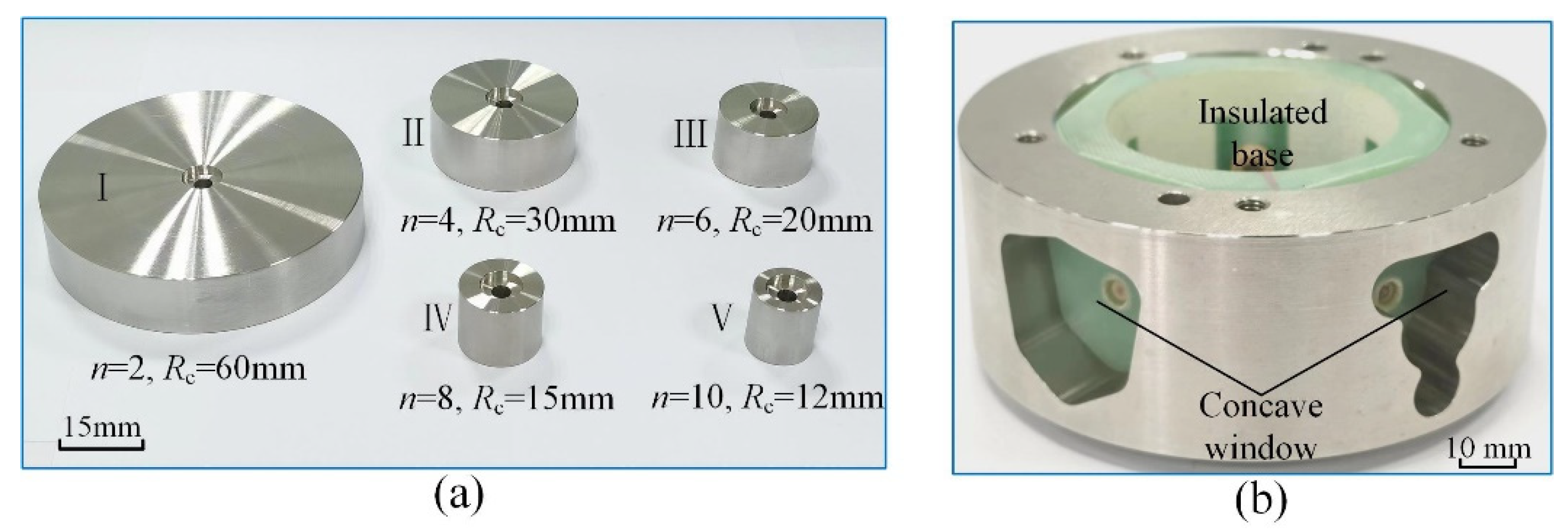
4. Experimental Validation
4.1. Experimental System
4.2. Experimental Results
5. Conclusions
- (1)
- The complex electric field model of ICRECM is simplified by conformal transformation, and then the analytical solution of electric field intensity is exactly calculated. A material removal model is built on the basis of the electric field model, and the dynamic simulation of the material removal process is realized.
- (2)
- The simulative results indicate that the quasi-equilibrium state is accompanied by the gradual reduction in IEG. The initial MRR and final IEG increase with the increase in cathode radius. The MRR in the quasi-equilibrium state is determined by the feed rate. The initial IEG does not affect the final machining state, but it has a strong effect on the transition time.
- (3)
- The experimental results show that the transition time can be greatly shortened using the optimized feed rate (Minimum transition time 17 min). The convex structures are successfully machined inside the annular workpiece with the optimized feed rate (0.014 mm/min) and voltage (16 V). There is almost no fluctuation in MRR during the manufacturing of convex structures, indicating that the machining has reached a quasi-equilibrium state in a very short time.
- (4)
- There are optimum parameters to make the machining enter the quasi-equilibrium state at the beginning. The metrical data are in good agreement with the theoretical data (Maximum error 1.975%), indicating that the established model can effectively predict the evolution process of MRR and IEG.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.Y.; Xu, Q.; Zhu, D.; Gong, T. A high efficiency electrochemical machining method of blisk channels. CIRP Ann. 2013, 62, 187–190. [Google Scholar] [CrossRef]
- Klocke, F.; Zeis, M.; Klink, A.; Veselovac, D. Experimental Research on the Electrochemical Machining of Modern Titanium- and Nickel-based Alloys for Aero Engine Components. Procedia CIRP 2013, 6, 368–372. [Google Scholar] [CrossRef]
- Poursaeidi, E.; Kavandi, A.; Vaezi, K.; Kalbasi, M.R.; Mohammadi Arhani, M.R. Fatigue crack growth prediction in a gas turbine casing. Eng. Fail. Anal. 2014, 44, 371–381. [Google Scholar] [CrossRef]
- Wang, D.; Yu, J.; Le, H.; Zhu, D. Localized sinking electrochemical machining of pre-shaped convex structure on revolution surface using specifically designed cathode tool. J. Manuf. Process. 2023, 99, 605–617. [Google Scholar] [CrossRef]
- Scippa, A.; Grossi, N.; Campatelli, G. FEM based Cutting Velocity Selection for Thin Walled Part Machining. Procedia CIRP 2014, 14, 287–292. [Google Scholar] [CrossRef]
- Kundu, P.; Luo, X.; Qin, Y.; Chang, W.; Kumar, A. A novel current sensor indicator enabled WAFTR model for tool wear prediction under variable operating conditions. J. Manuf. Process. 2022, 82, 777–791. [Google Scholar] [CrossRef]
- Bolsunovskiy, S.; Vermel, V.; Gubanov, G.; Kacharava, I.; Kudryashov, A. Thin-walled part machining process parameters optimization based on finite-element modeling of workpiece vibrations. Procedia CIRP 2013, 8, 276–280. [Google Scholar] [CrossRef]
- Zadafiya, K.; Kumari, S.; Chattarjee, S.; Abhishek, K. Recent trends in non-traditional machining of shape memory alloys (SMAs): A review. CIRP J. Manuf. Sci. Technol. 2021, 32, 217–227. [Google Scholar] [CrossRef]
- Singh Patel, D.; Agrawal, V.; Ramkumar, J.; Jain, V.K.; Singh, G. Micro-texturing on free-form surfaces using flexible-electrode through-mask electrochemical micromachining. J. Mater. Process. Technol. 2020, 282, 116644. [Google Scholar] [CrossRef]
- Thangamani, G.; Thangaraj, M.; Moiduddin, K.; Mian, S.H.; Alkhalefah, H.; Umer, U. Performance Analysis of Electrochemical Micro Machining of Titanium (Ti-6Al-4V) Alloy under Different Electrolytes Concentrations. Metals 2021, 11, 247. [Google Scholar] [CrossRef]
- Hotoiu, L.; Deconinck, J.; Diver, C.; Tormey, D. Ultra-short pulse simulation for characterising oxide layer formation on stainless steel during μECM. CIRP J. Manuf. Sci. Technol. 2020, 31, 370–376. [Google Scholar] [CrossRef]
- Liu, J.; Duan, S.; Zhou, Q.; Xu, Z. Optimization of tool nozzle structure for electrochemical boring of inner cavity in engine spindles. CIRP J. Manuf. Sci. Technol. 2023, 44, 1–15. [Google Scholar] [CrossRef]
- Rajurkar, K.P.; Sundaram, M.M.; Malshe, A.P. Review of Electrochemical and Electrodischarge Machining. Procedia CIRP 2013, 6, 13–26. [Google Scholar] [CrossRef]
- Sharma, V.; Patel, D.S.; Jain, V.K.; Ramkumar, J. Wire electrochemical micromachining: An overview. Int. J. Mach. Tools Manuf. 2020, 155, 103579. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Le, H.; Fu, T.; Zhu, D. Effect of carbides on the electrochemical dissolution behavior of solid-solution strengthened cobalt-based superalloy Haynes 188 in NaNO3 solution. Corros. Sci. 2023, 220, 111270. [Google Scholar] [CrossRef]
- Wang, D.; He, B.; Zhu, Z.; Zhu, D. Investigation of the Material Removal Process in Counter-rotating Electrochemical Machining. Procedia CIRP 2018, 68, 704–708. [Google Scholar] [CrossRef]
- De Silva, A.K.M.; Altena, H.S.J.; McGeough, J.A. Influence of electrolyte concentration on copying accuracy of precision-ECM. CIRP Ann.—Manuf. Technol. 2003, 52, 165–168. [Google Scholar] [CrossRef]
- Béjar, M.A.; Gutiérrez, F. On the determination of current efficiency in electrochemical machining with a variable gap. J. Mater. Process. Technol. 1993, 37, 691–699. [Google Scholar] [CrossRef]
- Clifton, D.; Mount, A.R.; Alder, G.M.; Jardine, D. Ultrasonic measurement of the inter-electrode gap in electrochemical machining. Int. J. Mach. Tools Manuf. 2002, 42, 1259–1267. [Google Scholar] [CrossRef]
- Hewidy, M.S.; Ebeid, S.J.; El-Taweel, T.A.; Youssef, A.H. Modelling the performance of ECM assisted by low frequency vibrations. J. Mater. Process. Technol. 2007, 189, 466–472. [Google Scholar] [CrossRef]
- Hewidy, M.S. Controlling of metal removal thickness in ECM process. J. Mater. Process. Technol. 2005, 160, 348–353. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, K.; Zhao, D. Experimental investigation on monitoring interelectrode gap of ECM with six-axis force sensor. Int. J. Adv. Manuf. Technol. 2011, 55, 565–572. [Google Scholar] [CrossRef]
- Rajurkar, K.P.; Wei, B.; Kozak, J.; McGeough, J.A. Modelling and Monitoring Interelectrode Gap in Pulse Electrochemical Machining. CIRP Ann. 1995, 44, 177–180. [Google Scholar] [CrossRef]
- Mount, A.R.; Clifton, D.; Howarth, P.; Sherlock, A. An integrated strategy for materials characterisation and process simulation in electrochemical machining. J. Mater. Process. Technol. 2003, 138, 449–454. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; He, B.; Zhu, D. Analysis and control of inter-electrode gap during leveling process in counter-rotating electrochemical machining. Chin. J. Aeronaut. 2019, 32, 2557–2565. [Google Scholar] [CrossRef]
- Cao, W.; Wang, D.; Zhu, D. Modeling and experimental validation of interelectrode gap in counter-rotating electrochemical machining. Int. J. Mech. Sci. 2020, 187, 105920. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Chen, P.; Wang, Z. Electrochemical machining gap prediction with multi-physics coupling model based on two-phase turbulence flow. Chin. J. Aeronaut. 2020, 33, 1057–1063. [Google Scholar] [CrossRef]
- Grafov, B.M. The general approach to the theory of the first passage problem for electrochemical stochastic diffusion in equilibrium. Russ. J. Electrochem. 2017, 53, 897–902. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Meng, D.; Liu, L.; Fang, Z. Multi-Physical Field Coupling Simulation and Experiments with Pulse Electrochemical Machining of Large Size TiAl Intermetallic Blade. Metals 2023, 13, 985. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, Z.; Zhu, D.; He, B.; Ge, Y. Reduction of stray currents in counter-rotating electrochemical machining by using a flexible auxiliary electrode mechanism. J. Mater. Process. Technol. 2017, 239, 66–74. [Google Scholar] [CrossRef]
- Cao, W.; Wang, D.; Cui, G.; Le, H. Analysis of the roundness error elimination in counter-rotating electrochemical machining. J. Manuf. Process. 2022, 76, 57–66. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, D.; Cao, W.; Zhu, D. Investigation of anode shaping process during co-rotating electrochemical machining of convex structure on inner surface. Chin. J. Aeronaut. 2022. [CrossRef]
- Islam, S.M.R.; Akbulut, A.; Arafat, S.M.Y. Exact solutions of the different dimensional CBS equations in mathematical physics. Partial Differ. Equ. Appl. Math. 2022, 5, 100320. [Google Scholar] [CrossRef]
- Galeriu, C. Electric charge in hyperbolic motion: The special conformal transformation solution. Eur. J. Phys. 2019, 40, 065203. [Google Scholar] [CrossRef]
- Chantry, L.; Cayatte, V.; Sauty, C. Conformal representation of Kerr space-time poloidal sub-manifolds. Class. Quantum Gravity 2020, 37, 105003. [Google Scholar] [CrossRef]
- Wang, Z.G.; Liao, T.; Wang, Y.N. Modeling electric field of power metal-oxide-semiconductor field-effect transistor with dielectric trench based on Schwarz-Christoffel transformation. Chin. Phys. B 2019, 28, 058503. [Google Scholar] [CrossRef]
- Eyal, O.; Goldstein, A. Gauss’ law for moving charges from first principles. Results Phys. 2019, 14, 102454. [Google Scholar] [CrossRef]
- Bermúdez Manjarres, A.D.; Nowakowski, M.; Batic, D. Coulomb law in the nonuniform Euler-Heisenberg theory. Int. J. Mod. Phys. A 2020, 35, 2050211. [Google Scholar] [CrossRef]
- Gow, R.; McGuire, G. Invariant rational functions, linear fractional transformations and irreducible polynomials over finite fields. Finite Fields Their Appl. 2022, 79, 101991. [Google Scholar] [CrossRef]
- Ter Horst, S. A pre-order on operators with positive real part and its invariance under linear fractional transformations. J. Math. Anal. Appl. 2014, 420, 1376–1390. [Google Scholar] [CrossRef]
- Jain, V.K.; Gehlot, D. Anode shape prediction in through-mask-ECMM using FEM. Mach. Sci. Technol. 2015, 19, 286–312. [Google Scholar] [CrossRef]
- Pattavanitch, J.; Hinduja, S.; Atkinson, J. Modelling of the electrochemical machining process by the boundary element method. CIRP Ann.—Manuf. Technol. 2010, 59, 243–246. [Google Scholar] [CrossRef]
- Jia, X. Electric potential and electric field between two confocal parabolic conductor plates. J. Zhejiang Univ. Sci. Ed. 2018, 45, 427–428. [Google Scholar]
- Mithu, M.A.H.; Fantoni, G.; Ciampi, J.; Santochi, M. On how tool geometry, applied frequency and machining parameters influence electrochemical microdrilling. CIRP J. Manuf. Sci. Technol. 2012, 5, 202–213. [Google Scholar] [CrossRef]
- Cao, W.; Wang, D.; Ren, Z.; Zhu, D. Evolution of convex structure during counter-rotating electrochemical machining based on kinematic modeling. Chin. J. Aeronaut. 2021, 34, 39–49. [Google Scholar] [CrossRef]



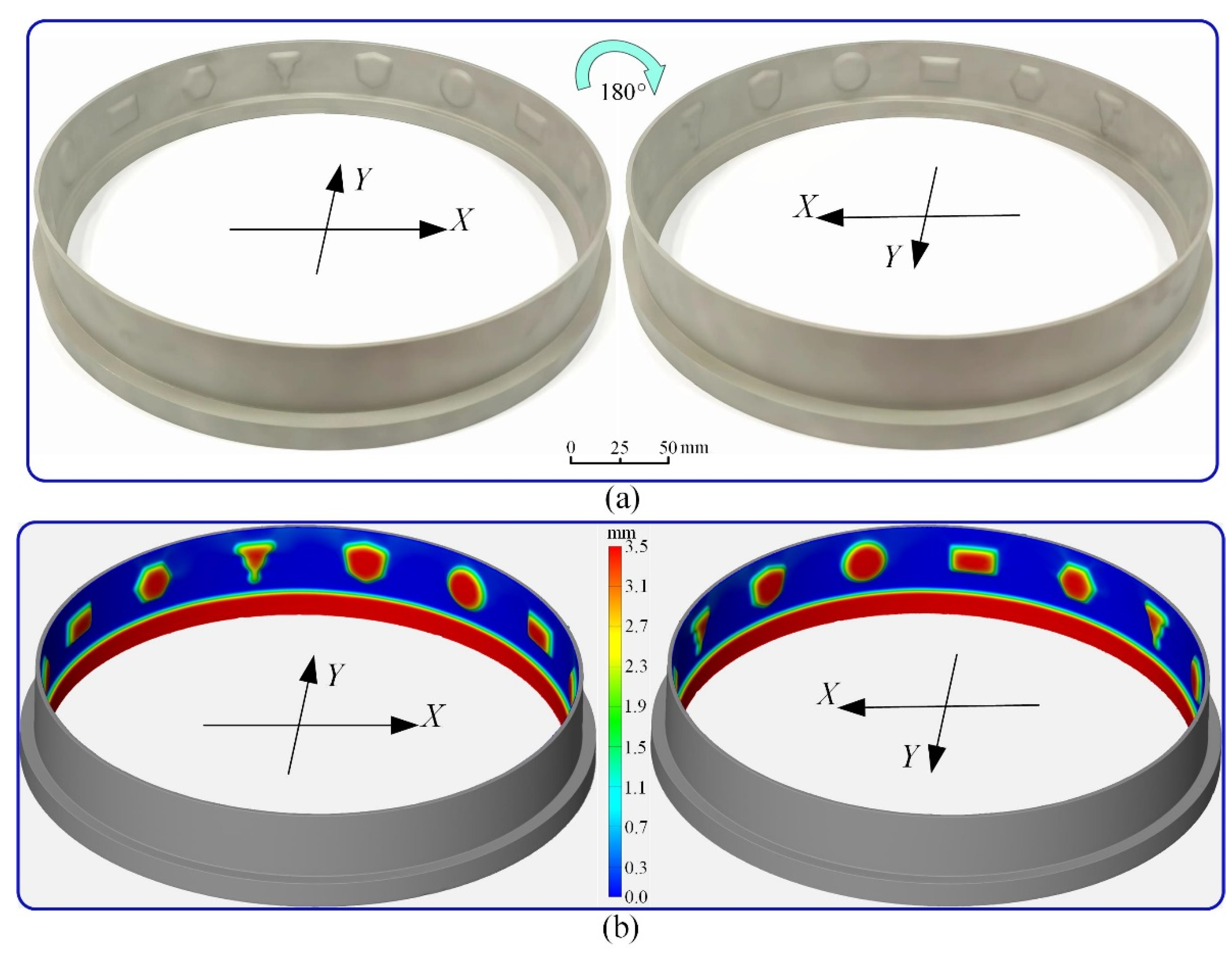
| Parameters | Value |
|---|---|
| Electrolyte conditions | 30 °C 0.5 Mpa |
| Electrolyte type | 20% NaNO3 |
| Anode material | 304 SS |
| Applied voltage, U (V) | 16, 20 |
| Initial IEG, G0 (mm) | 0.3 |
| Radius of the anode, Ra (mm) | 120, 150 |
| Angular speed of anode, ω (r/min) | 2 |
| Processing time (min) | 250 |
| Machining Parameter | Value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Radius of the cathode, Rc (mm) | 12 | 15 | 20 | 30 | 60 | 50 |
| Radius of the anode, Ra (mm) | 120 | 120 | 120 | 120 | 120 | 150 |
| Angular velocity ratio, n | 10 | 8 | 6 | 4 | 2 | 3 |
| Feed rate, vf (mm/min) | 0.011 | 0.013 | 0.015 | 0.019 | 0.033 | 0.014 |
| Applied voltage, U (V) | 20 | 20 | 20 | 20 | 20 | 16 |
| Processing Time (min) | MRR (mm/min) | IEG (mm) | ||||
|---|---|---|---|---|---|---|
| Metrical Data | Theoretical Data | Error (%) | Metrical Data | Theoretical Data | Error (%) | |
| 10 | 0.01369 | 0.01386 | 1.242 | 0.3009 | 0.2986 | 0.764 |
| 20 | 0.01382 | 0.01389 | 0.507 | 0.2989 | 0.2974 | 0.502 |
| 30 | 0.01374 | 0.01391 | 1.237 | 0.2976 | 0.2964 | 0.403 |
| 40 | 0.01384 | 0.01392 | 0.578 | 0.2964 | 0.2955 | 0.304 |
| 50 | 0.01389 | 0.01393 | 0.288 | 0.2955 | 0.2947 | 0.271 |
| 60 | 0.01385 | 0.01393 | 0.578 | 0.2943 | 0.294 | 0.102 |
| 70 | 0.01367 | 0.01394 | 1.975 | 0.2931 | 0.2933 | 0.068 |
| 80 | 0.01392 | 0.01394 | 0.172 | 0.2923 | 0.2927 | 0.137 |
| 90 | 0.01386 | 0.01394 | 0.577 | 0.2915 | 0.2922 | 0.24 |
| 100 | 0.01372 | 0.01395 | 1.676 | 0.2909 | 0.2916 | 0.241 |
| 125 | 0.01385 | 0.01395 | 0.722 | 0.2894 | 0.2903 | 0.311 |
| 150 | 0.01399 | 0.01395 | 0.286 | 0.2885 | 0.289 | 0.173 |
| 175 | 0.01388 | 0.01396 | 0.598 | 0.2874 | 0.2878 | 0.139 |
| 200 | 0.01392 | 0.01396 | 0.287 | 0.2869 | 0.2866 | 0.105 |
| 225 | 0.01389 | 0.01396 | 0.504 | 0.286 | 0.2853 | 0.245 |
| 250 | 0.01391 | 0.01396 | 0.359 | 0.2847 | 0.2842 | 0.176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Wang, D.; Fu, T.; Zhu, D. Evolution of the Interelectrode Gap during Co-Rotating Electrochemical Machining. Metals 2023, 13, 1771. https://doi.org/10.3390/met13101771
Zhou S, Wang D, Fu T, Zhu D. Evolution of the Interelectrode Gap during Co-Rotating Electrochemical Machining. Metals. 2023; 13(10):1771. https://doi.org/10.3390/met13101771
Chicago/Turabian StyleZhou, Shuofang, Dengyong Wang, Tianyu Fu, and Di Zhu. 2023. "Evolution of the Interelectrode Gap during Co-Rotating Electrochemical Machining" Metals 13, no. 10: 1771. https://doi.org/10.3390/met13101771
APA StyleZhou, S., Wang, D., Fu, T., & Zhu, D. (2023). Evolution of the Interelectrode Gap during Co-Rotating Electrochemical Machining. Metals, 13(10), 1771. https://doi.org/10.3390/met13101771






