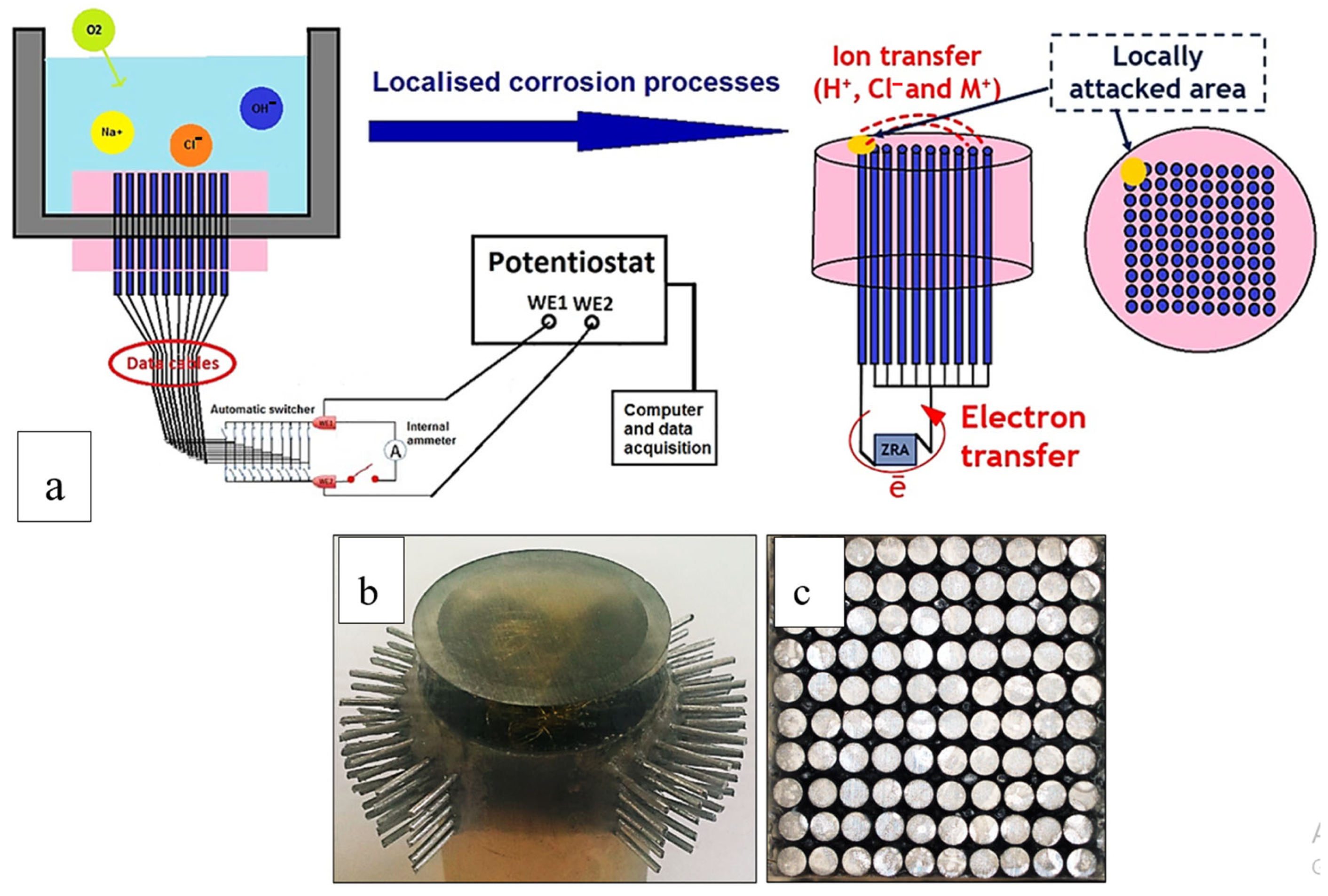
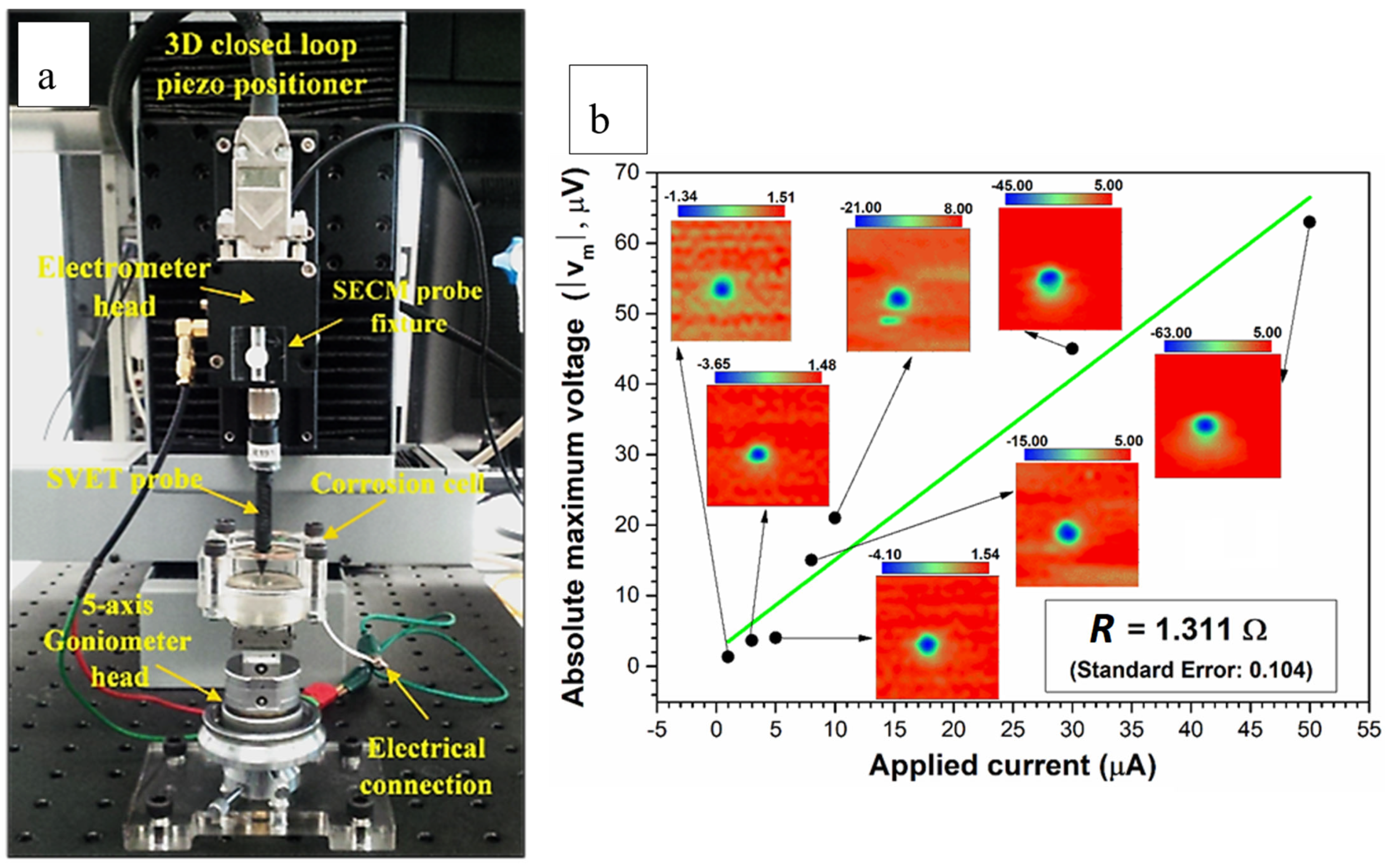
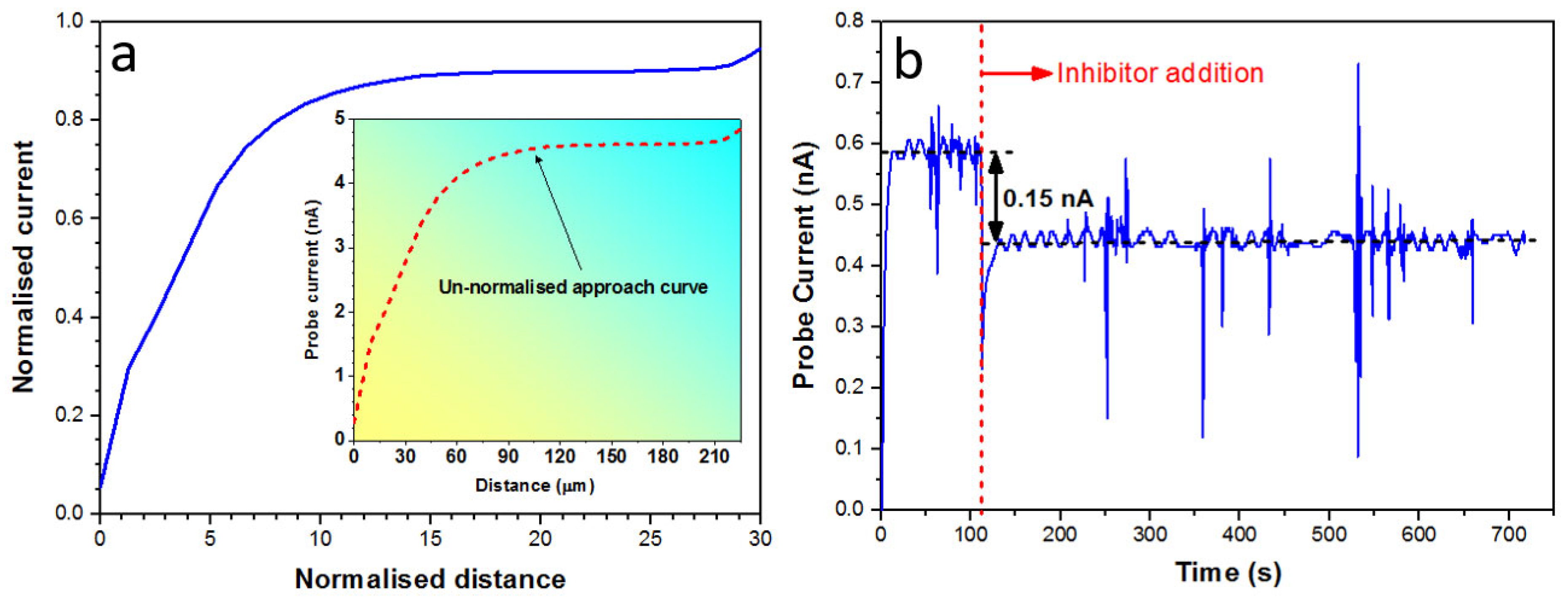
3.1. WBE Investigations on the Millimetre Scale
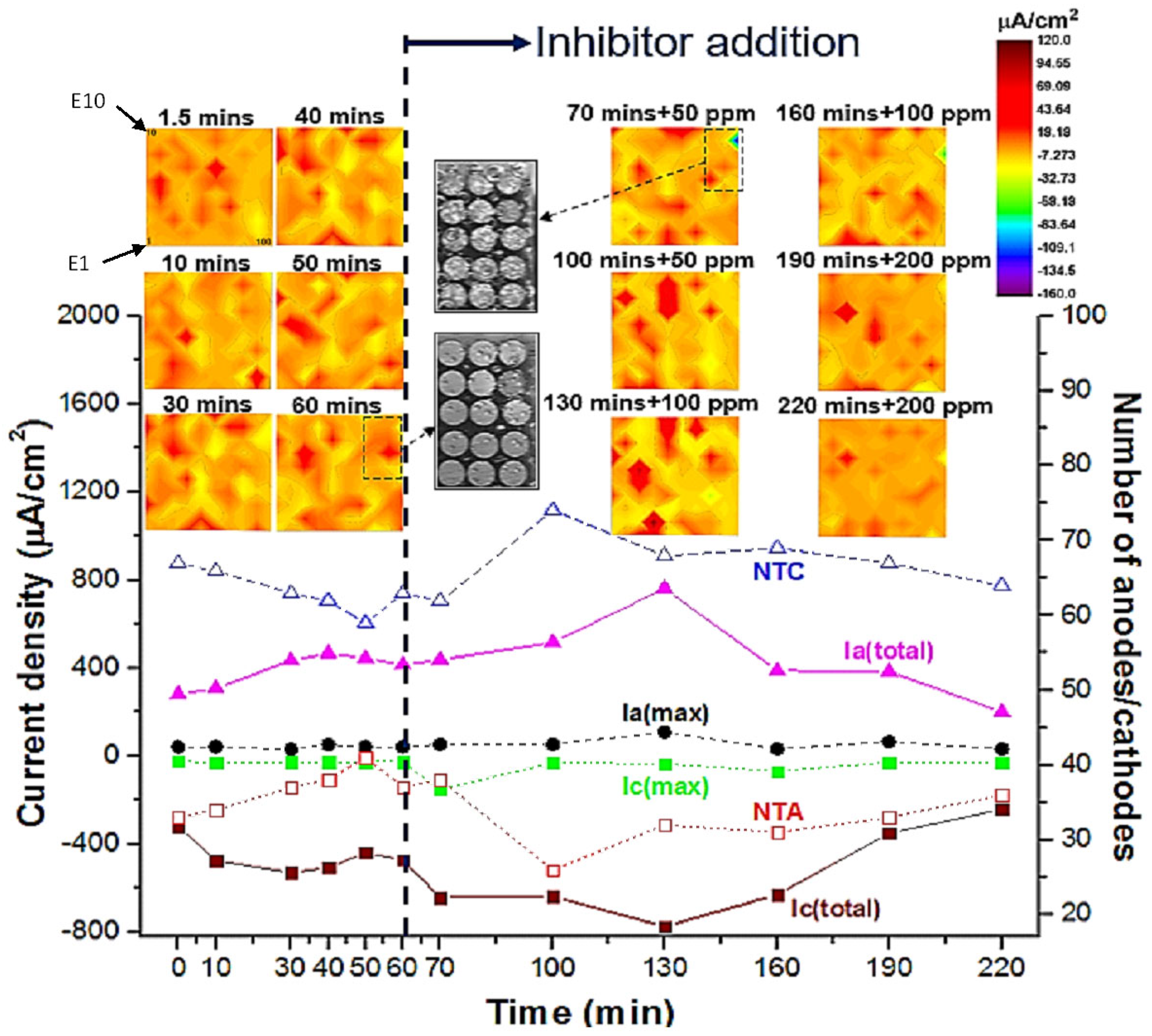
Figure 5 shows galvanic current density maps (net values) over the WBE surface that were recorded after 1.5, 10, 30, 40, 50, and 60 min exposure in 0.05 M NaCl solution without inhibitor and after 70, 100, 130, 160, 190 and 220 min with inhibitor addition. From these maps, it can be seen that anodic and cathodic areas initiated and terminated dynamically after inhibition addition at 70 min, suggesting localised corrosion inhibition took place over a range of time and length scales. As can be seen from the current density maps, prior to the addition of the inhibitor, the overall cathodic activity of the WBE is more widespread during the first 1.5 min than for longer exposure times with nearly 70% of the electrodes behaving as net cathodes. After 10 min of immersion, a random distribution of anodes and cathodes among wires becomes apparent. After 30 min of immersion, strong anodes are observed some of which become stable since they persist to 40, 50, and 60 min of immersion times. Some of these anodes comprise four or more electrodes and are surrounded by multiple wires acting as net cathodes. Such distribution of net anodes and cathodes is on a much larger scale (several mm) than the scale at which localised corrosion initiation occurs.
In order to quantify the time and spatial dependent variations in the degree of localisation of corrosion reactions, parameters including maximum anodic current (
Ia(max)), maximum cathodic current (
Ic(max)), total anodic current (
Ia(total)), total cathodic current (
Ic(total)), number of total anodes (
NTA) and number of total cathodes (
NTC) have been calculated from current density maps before and after the inhibitor addition and recorded as a function of time, based on a previously reported method [
27]. During the OCP corrosion period (before inhibitor addition), as shown in
Figure 5, the number of cathodes
NTC decreases while the
NTA increases revealing the de-activation of cathodic sites likely due to the deposition of a corrosion product layer which is thought to be mainly composed of mixed oxides and hydroxides (e.g., Al-O-H-Cl). Moreover, the overall anodic activity of the WBE surface (
Ia(total)) increases while the total cathodic activity of the surface (
Ic(total)) also increases by the same amount to balance electrode reactions between coupled wires. By 60 min immersion, the number ratio of cathodes to anode is around 1.5. Hydrogen bubbles (100–200 µm in diameter) were found to evolve over the corroding WBE surface following 60 min of immersion. The generation of hydrogen indicates that active anodes have been driven into the surface from the base of trenches, active pits, or IGC network where they spread laterally and in-depth and remerge elsewhere on the surface, as described in other works [
1,
34]. As a consequence of these processes, an acidic anolyte solution forms in sub-surface occluded areas, and therefore hydrogen reduction reaction takes place in sub-surface active cathodes such as Cu build-up on pit/IGC walls behind the active attack. These are in agreement with a more detailed analysis of multiscale galvanic interactions using various-sized WBEs reported in the authors’ recent work [
27].
After 60 min initial OCP corrosion immersion, inhibitor Ce(dpp)
3 was added to the base NaCl electrolyte to make up inhibitor concentrations of 50, 100, and 200 ppm. Inhibitor dosing was conducted at 65, 125, and 185 min with a 5-min delay in recordings from the addition of inhibitor to attain good electrolyte mixing and to achieve a degree of stabilisation in surface electrochemical reactions. WBE measurements in inhibited solutions were performed at 30 min intervals with the first data recorded after 70 min of immersion. By comparing the WBE current density distribution maps in
Figure 5, localised electrode activities (both anodic and cathodic) increased after adding 50 and 100 ppm concentrations of the Ce(dpp)
3, suggesting corrosion acceleration immediately upon the addition of the corrosion inhibitor. This is not unusual, similar corrosion acceleration behaviour has been observed previously in different metal systems with RE salts as inhibitors [
35]. This has been explained as the initial high corrosion rates required for generating a protective/passive film for later anodic inhibition. Corrosion acceleration occurred over local areas, for example, wire 39 showed the highest
Ia(max) value (about 109 μA/cm
2) after the addition of the inhibitor (130 min + 100 ppm). At the same time, both
Ia(total) and
Ic(total) increased, indicating overall activation reactions of the surface also occurred due to the inhibitor addition. After 160 min immersion both anodic and cathodic activities decreased in 100 ppm inhibitor solution, but this concentration was not enough to suppress surface activities completely. By increasing the inhibitor concentration to 200 ppm, localised anodes and cathodes reduced significantly as can be observed by comparing
NTA,
NTC,
Ia(total), and
Ic(total) values in
Figure 5. As a result, only a few low-current-generating anodes (
Ia(max) of about 33 μA/cm
2) remained active after 220 min of immersion. Moreover, most of the anodes were confined to individual wires whereas at earlier periods the anodes comprised two or more wires. Clearly, inhibitor addition at 200 ppm suppressed the large-scale interaction of anodes and cathodes, confining activity to individual electrodes. These observations suggest a multi-time and length scale nature of localised corrosion inhibition mechanism.
Based on total anodic and cathodic current densities (
Ia(total) and
Ic(total)) data in
Figure 5, the time-dependent inhibitor efficiency,
η (%), can be calculated by means of the following equation [
8]:
where “
x” represents anodic (“a”) and cathodic (“c”) states of current density values used in Equation (2). Note that negative inhibitor efficiency values are an indication of electrochemical activation in the presence of inhibitors.
Table 1 shows the time-dependent measurement of anodic and cathodic inhibition efficiencies for Ce(dpp)
3 at 50, 100, and 200 ppm concentrations. The anodic activity increased to maximum
ηa = −83.5% at 130 min in the presence of 100 ppm, showing an obvious anodic activation by the inhibitor. After another 30 min of immersion (160 min in total), anodic activities declined over the WBE surface, suggesting effective corrosion inhibition (i.e., improved inhibition efficiency). On the cathodic side, 100 ppm inhibitor addition increased the cathodic activity of WBE to
ηc = −33.9% up to 160 min of immersion. Since this is not as high as the anodic activity it means the inhibitor interaction over the cathodic sites is lower than the anodic sites. The maximum activation of both anodic and cathodic reactions was detected after 130 min of immersion in the presence of 100 ppm Ce(dpp)
3 while both reactions were considerably de-activated after 220 min in the presence of a 200 ppm inhibitor. Both the macro scale net anodic galvanic reactions and the short-ranged anodic-cathodic activities over the most anodically active wire with the greatest number of active sites (surrounded by corrosion rings) are considerably decreased after increasing the inhibitor concentration to 200 ppm.
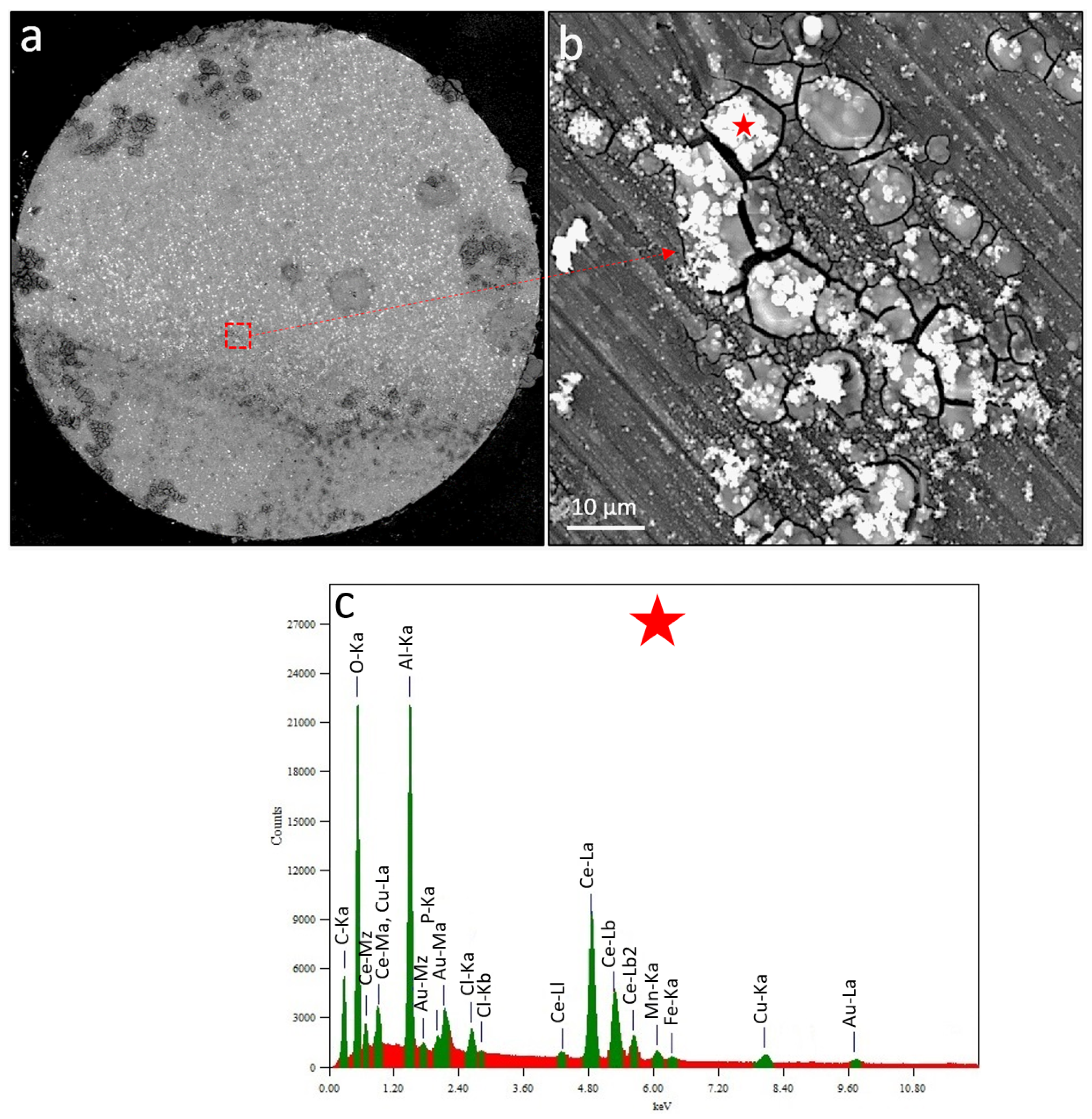
Figure 6a shows the BSE image of the corrosion morphology of the most anodic wire after test termination. Dark grey corrosion rings and bright precipitates are present on the electrode surface. Obviously, the anodic regions within large corrosion rings are less covered by inhibitor deposits as opposed to the surrounding microstructure maintaining the local cathodic currents on the wire surface.
Figure 6b shows, at higher magnification, the morphology of the area which is highlighted by a red-dashed rectangle in
Figure 6a. The region in
Figure 6b contains a cracked cap of corrosion products (including surface oxides) partially covered by bright precipitates. EDS of the white precipitate (
Figure 6c) highlighted by a red star in
Figure 6b revealed Fe, Mn, Cu (probably from an underlying IMP), and O, P, Ce, and Cl-containing inhibitor precipitates formed during the inhibition process. Various researchers reported similar inhibition morphologies of corrosion products at cathodic IMPs while investigating RE mercaptoacetate [
36] and diphenyl phosphate [
24] inhibition on AA2024-T3. The precipitation is mainly known to be attributed to a rise in local pH causing the condensation of both aluminium and cerium products.
A combination of the WBE maps in
Figure 5 and surface analytical images obviously is able to reveal many aspects of the localised corrosion mechanism. However, the mm-sized WBE method is able to probe electrochemical processes occurring only on each mm-sized wire surface, processes and mechanisms occurring over a sub-millimeter scale are not measurable. In order to investigate electrochemical processes occurring over individual wires, scanning probe techniques including SVET and SECM have been employed to probe the localised corrosion inhibition of AA2024-T3 in the presence and absence of Ce(dpp)
3 inhibitor molecules.
3.2. SVET and SECM Investigations on the Micro-Meter Scale
Both SVET and SECM could provide data for exploring corrosion and inhibition reactions at selected local areas at minuscule scales on each individual wire, however, each will mechanistically reveal specific information from concurrent corrosion processes.
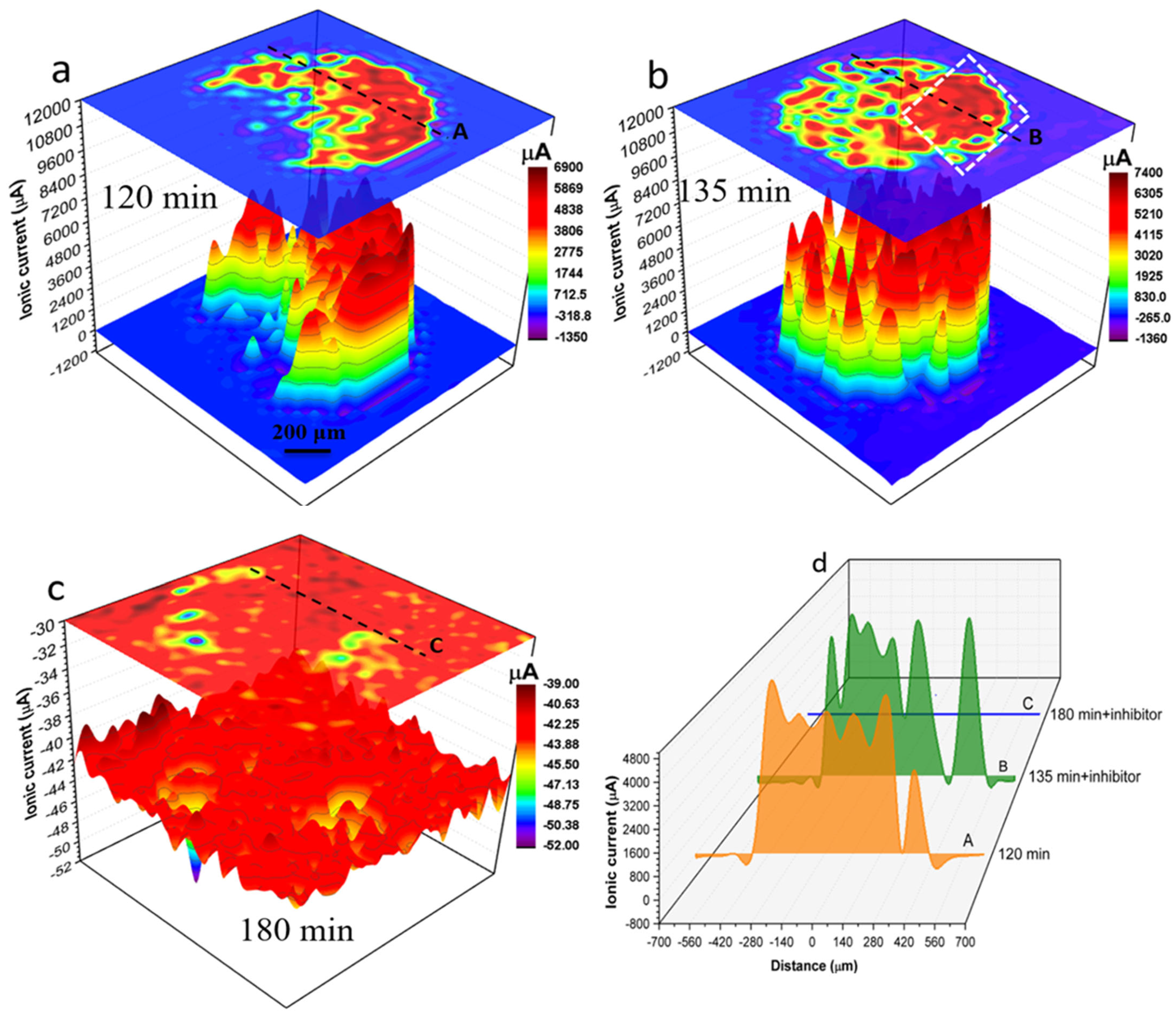
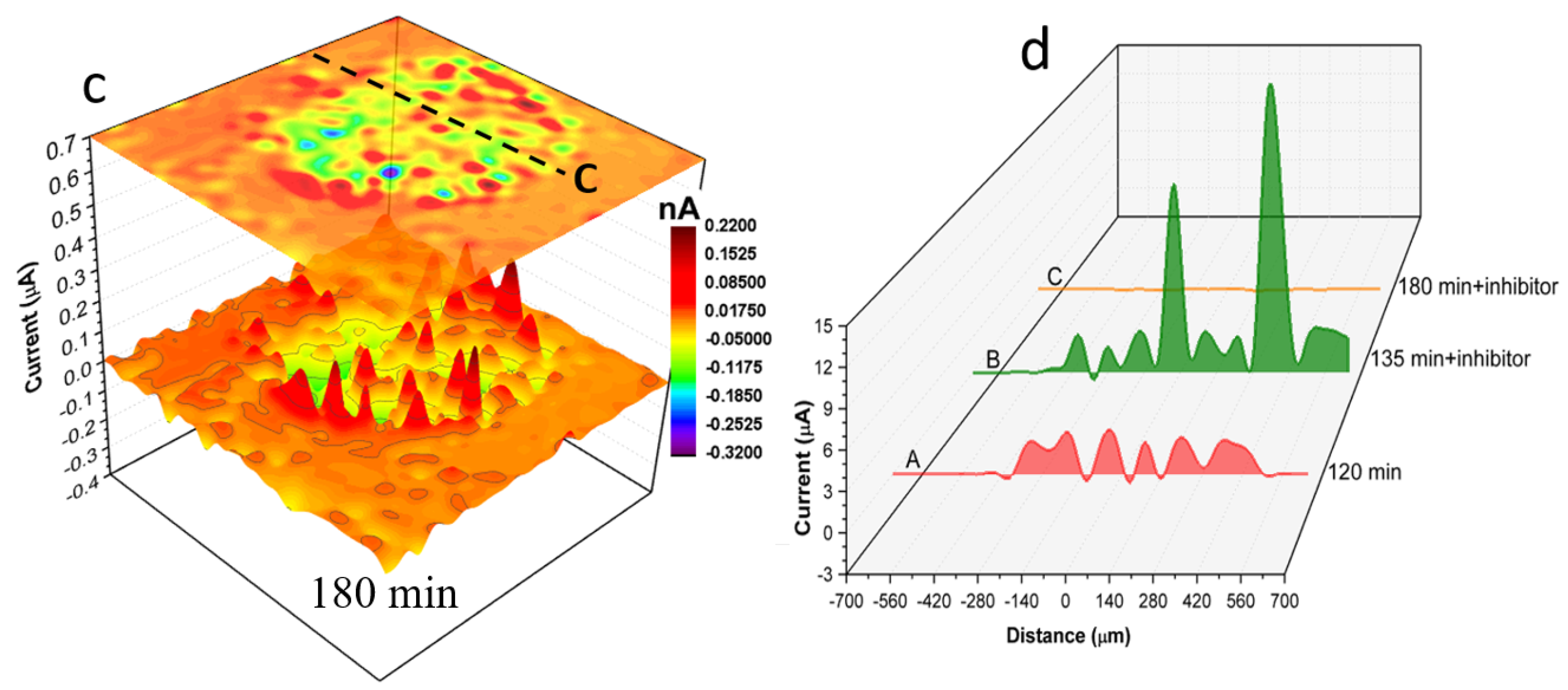
Figure 7 shows SVET ionic current distribution maps of local anodes and cathodes on the surface of the 1 mm AA2024 wire in the electrolyte without (
Figure 7a) and with inhibitor (
Figure 7b,c).
Figure 7a shows the localised ionic current distribution map after 120 min of immersion in solution without inhibitor.
Figure 7b,c show localised ionic current distribution maps at 135 min and 180 min of immersion respectively after the addition of 200 ppm Ce(dpp)
3.
Figure 7d shows time-dependent ionic current line profiles labelled “A” to “C” across black dashed lines shown in
Figure 7a–c. After 120 min of immersion in a solution without inhibitor (
Figure 7a) various active anodes and cathodes are distributed across the wire surface. The most active anodes (generating ionic currents up to 6900 μA) are located in a crescent on the right-hand side of the electrode. Moreover, a considerable localised cathodic activity (as high as −1350 μA) is detected in the vicinity of active anodes and at the wire/epoxy interface. The anodic ionic current increased to about 500 μA with the addition of the inhibitor to the electrolyte and the reaction appears localised on the right-hand side of
Figure 7b. However, after an additional 45 min of exposure, the localised activity was mitigated significantly such that only a few insignificant local cathodes (with a maximum cathodic current of −39 μA) were detectable using SVET. Details of local changes in localised electrochemical activities in the presence and absence of inhibitor molecules can be seen by comparing line profiles shown in
Figure 7d.
Figure 8a shows the corrosion morphology of the region that showed the highest anodic activity before and after the inhibitor addition; the area is highlighted by a white dashed rectangle in
Figure 7b. As can be seen, the anodic area consists of regions with cracked layers of corrosion product mixed with inhibitor deposits, corrosion caps, and domes.
Figure 8b shows the morphology of the corrosion dome and surrounding cracked surface film highlighted in
Figure 8a. To investigate the morphology and composition of a few microns below the domes, layers were removed by a fine wet-grinding stage using a 4000 SiC paper (
Figure 8c). EDS was performed on the corrosion dome (
Figure 8d) and the sub-dome corroded/inhibited microstructure (
Figure 8e). To diminish the possible chance of contamination of the subsurface corroded microstructure (e.g., pit mouth) by polishing debris, the surface was gently rinsed with ethanol during the grinding stage. EDS result (
Figure 8d) shows that corrosion domes contain small levels of Mn, Cu, Cl, P, and Fe together with higher levels of Al, Ce, and O. The corroded microstructure beneath the domes (
Figure 8c) mainly contained de-alloyed S-phase particles together with oxygen-rich Cu and Ce clusters with no evidence of P from dpp anions. The result here shows that considerable levels of Ce and O (from oxide/hydroxide compounds) can be found at the mouth of the pit/IGC network suggesting a pH above neutral at this particular site. Previous research indicated that Ce cations interact with areas of cathodic activity where higher concentrations of hydroxyl ions (higher pH values) are present to form Ce oxyhydroxides [
37,
38]. Generally, by comparing the corroded area (covered by a film of cracked corrosion products and domes) and the sub-film corroded microstructure, the following findings can be summarised:
(i) Swelling and cracking of corrosion/inhibitor products occurs mainly on top of active cathodes (e.g., de-alloyed S-phase remnants [
36]) and/or anodes. These dehydrate and crack during drying of the wire surface after removal of the specimen from the inhibited solution.
(ii) Domes of corrosion/inhibition products that have formed over clusters of particles including de-alloyed S-phase and AlCuFeMn(Si) IM particles that are commonly known as the cause of stable pitting. If this site is a stable pit then it is possible that the dome of corrosion product forms over small H
2 bubbles attached to the pit mouth leading to its shut-down of H
2 gas evolution. Details of In-situ observation of domes and active/passive H
2 emitting sites on various size AA2024-T3 wires were detected in a previous work [
27].
Figure 8.
Corrosion morphology of the wire after a total of 180 min of SVET immersion in both uninhibited (for 120 min) and inhibited (200 ppm Ce(dpp)3) 0.05 M NaCl open-to-air solution. (a) SE-SEM image of a fraction of the wire surface highlighted by a white dashed rectangle in 10b. (b) SE-SEM image of corrosion domes highlighted by a red dashed circle in (a). (c) SE-SEM image of sub-domes corroded/inhibited microstructure revealed after fine grinding of the surface shown in (b), (d) and (e) EDS spectra of spots highlighted by blue and red stars in (b) and (c), respectively. The scale bar length in (b) and (c) is 10 μm.
While SVET detects potential fields related to various micron scale electrochemical processes such as pitting, SECM, under oxygen reduction competition mode at OCP, can detect electrochemical reactions relating to the oxygen reduction on and surrounding actively corroding microstructure. In saying that, maximum competition (minimum sensed current by the UME) will occur when the UME tip sits above a highly cathodic S-phase remnant for instance. It is well-known that during the OCP corrosion of AA2024-T3 in near-neutral aqueous solutions, the predominant anodic reaction is aluminium dissolution while the cathodic process is the oxygen reduction reaction which preferentially takes place on cathodic IMPs (e.g., Al-Cu-Mn-Fe-Si type particles) and is strongest over fully de-alloyed S-phase particle remnants [
1].
Figure 9 shows SECM current distribution maps revealing relative locations of localised positive and negative current excursions due to localised reduction of dissolved oxygen without (
Figure 9a) and with inhibitor (
Figure 9b,c) in 0.05 M NaCl solution. Positive current excursions represent higher than the background level of oxygen reduction. This is attributed to the convective flow of fresh solution into the region above anodes due to hydrogen evolution thus providing a greater oxygen supply than available by oxygen diffusion and supply from regions with higher concentration of unconsumed oxygen. No attempt is made here to cross-correlate the distribution of these peaks on a one-to-one basis to anodic sites on the surface, but their presence does reflect anodic activity in the vicinity of the electrode during scanning. This also indicates that Equation (1) is not valid at these sites since it is based only on the diffusion of oxygen into the sensing region under the electrode and not on the convective flow. With this perspective in mind, it can be seen in
Figure 9a, that major anodic activity appears across the wire surface although more active anodes are mainly located at the peripheral region of the wire. Following 120 min of exposure to 0.05 M NaCl solution, the maximum anodic and cathodic currents were measured to be about 4 and −1 μA, respectively. Given the length of immersion time and since the observed IGC and H
2 evolution were localised, the corrosion would be in the propagation stage thus growing and penetrating into susceptible microstructures both laterally and vertically.
Following inhibitor addition (after 135 min of immersion;
Figure 9b), the maximum cathodic and so-called convection-related anodic currents were increased by about 4 and 6 times, respectively. Here, the SECM results again revealed the inhibitor activation (mostly through changes in localised cathodic activities) behaviour in support of WBE and SVET findings. The initial convection-related anodic activity decreased after inhibitor addition and was confined to the annular region of the wire consistent with this part of the microstructure being more prone to pitting initiation and propagation [
27]. As exposure time increased to 180 min (
Figure 9c), the overall electrochemical activity of the surface was reduced showing only a few slightly active regions and cathodes, mostly near the edge of the wire indicating considerably declined cathodic and hence anodic activities across wire microstructure.
Figure 9d shows time-dependent current line profiles labelled “A” to “C” across the black dashed lines shown in
Figure 9a–c.
Figure 10 shows the surface morphology of the AA2024 wire after completion of SECM measurements discussed in
Figure 9. Most of the heavily attacked sites showing pitting and IGC and relatively large plumes of corrosion product in dark grey are situated in the annular region of the wire (
Figure 10a). Compared to the corrosion morphology of the SVET specimen (
Figure 7), most of the pit mouths were open and no pronounced corrosion rings were observed. This difference in corrosion morphologies is likely to be related to SECM and SVET probe-surface distances (100 µm in SVET as compared to 15 µm in SECM), and the introduction of a localised highly alkaline environment at the UME tip in the proximity of the surface. Compared to SVET, smaller tip-to-surface distances in SECM may lead to physical removal and displacement of corrosion and inhibition products during surface scans.
Figure 10b,c show SE-/BSE-SEM images of the region highlighted with a white dashed rectangle in
Figure 10a. This region was chosen as a typical example of a heavily corroded site in the annular region. Pits, corrosion plumes, and caps (e.g., highlighted by a red triangle) were all observed within the dashed rectangle. EDS was performed on some of these features (
Figure 10d,e) to determine the extent of inhibitor interaction with corrosion sites. Plumes of corrosion products have been observed before [
27] and attributed to subsurface S-phase dealloying. A plume surrounded by pits (
Figure 10d) also on a corrosion cap (
Figure 10e) very close to the heavily attacked area. The EDS analysis of the corrosion plume showed trace levels of Mg and Cu (likely from subsurface S-phase IMPs) and higher levels of Al, Cl, and O elements (
Figure 10d) showing that inhibitor molecules have not suppressed the plume formation mechanism which is not clearly known. Whereas the EDS analysis of the corrosion cap (
Figure 10e) reveals the presence of Al, Ce, P, Cl, O, Mg, and Cu elements. In this case, inhibitor deposits were found on top of the corrosion product cap covering trenched and de-alloyed S-phase particles [
36] where there is greater cathodic activity due to the presence of Cu (irrespective of its formation source). This also indicates that S-phase remnants covered by gel products were still active after 120 min of corrosion and could still take part in cathodic reactions possibly at slower rates.
3.3. Discussion on Multiscale Inhibition by Integrating WBE, SVET and SECM Data
The overall qualitative behaviour of the individual electrodes measured using SVET and SECM are similar to that observed by the WBE. The addition of 200 ppm Ce(dbp)3 appears to suppress corrosion after about 60 to 70 min in both the SVET and WBE experiments, respectively. This indicates that the inhibition mechanism shows similar time dependence on a scale range going from perhaps 10 μm in the SVET to over 1 cm for the WBE experiment. This appears to be due to the contraction of large-scale anodes and cathodes observed using the WBE to a local scale of tens of microns. The reason for this is that when the inhibitor reacts and suppresses anodic and cathodic activity, the resultant current densities required by each are small which significantly reduces the physical scale from which anodes and cathodes balance. Clearly, this scale is much smaller than individual electrodes as seen in the SVET measurements, so inter-electrode activity ceases in the WBE.
The results presented here show that at 200 ppm concentration, Ce(dpp)
3 does not fully shut down all localised activities on a corroding AA2024-T3 wire with fully developed pits and IGC sites.
Table 2 gives a summary of the maximum ratio of anodic to cathodic current probed by WBE, SVET, and SECM after inhibitor addition (concentrations at which these events happened are also included) and their associated corrosion/inhibition morphologies. It should be noted that because of the convective nature of the SECM response over anodic regions, no figures for this technique are presented in
Table 2.
Previous inhibitor studies show a common inhibition mechanism for Ce(dbp)
3, Ce(dpp)
3, and Pr(dpp)
3 inhibitors. Markley and co-workers [
24] have proposed that RE organophosphates only partially dissociate in solution and most likely exist as complexes or polynuclear species instead of discrete ionic species. They have suggested that the partial dissociation of the dpp ligand from Ce(dpp)
3 (Ce(dpp)
3 → Ce (dpp)
(3−n)n+ +
n(dpp
−) (
n ≤ 3)) provides for a time-dependent and solution pH dependant response to surface conditions. On the other hand, Soestbergen et al. [
39] reported that Ce(dbp)
3 undergoes complete dissociation over a pH range of 1 to 9. In the case of the lower pH (1 to 2) the dbp anion undergoes protonation increasing the availability of free Ce
3+ cations and at higher pH (>9) precipitation of Ce(OH)
3 occurs. It is also conceivable that protonation of dbp anions may increase the presence of hydrogen ions (leading to an H
+ gradient and transport to regions with higher dbp anion concentration) in the vicinity of anodic sites thus facilitating further dissolution thus acting as an activating agent. Over cathodic IMPs where alkaline pH values are sustained, a reaction of Ce ions with excess hydroxyl ions can occur which facilitates film formation in the alkaline environment above active cathodes. Diffusion of OH ions beyond the locality of the cathodes may result in Ce hydroxide formation more generally on the surface providing more widespread protection. This is the classic island growth model described by Arnott et al. [
40]. As a consequence, precipitation of Ce oxide/hydroxide products happens once the solubility product is reached although mixed precipitates such as Ce(dpp)
(3−n)(OH)
n may also form. Depending on the initial surface condition, the film formation might not fully cover the whole surface and thus, non-covered sites may continue to take part in localised corrosion reactions.
A critical phenomenon observed in this work is corrosion activation in the presence of lower concentrations of the inhibitor (e.g., 50 and 100 ppm). This has also been reported for other inhibitors such as RE salts, CeCl
3 (as AA2024 corrosion inhibitor) [
22,
35], and chromate (applied to Al alloys) [
41]. In the case of oxidizing inhibitors such as chromate at very low concentrations, the activation is thought to be related to the Cr
6+ to Cr
3+ reduction reaction resulting in Al oxidation. As discussed above activation at low concentrations of inhibitor may occur through a range of possibilities:
Increase in hydrogen ion activity due to consumption of hydrogen ions in the protonation of dpp anion in the acidic anolyte solution.
The reaction of dpp with aluminium ions in the electrolyte at the anodes.
Reaction of Ce with hydroxyl ions in solution (i.e., generation of Ce(OH)n3−n) near cathodic sites thus increasing the activity for oxygen reduction over cathodes.
Formation of other cerium species such as peroxo complexes during oxygen reduction over cathodes. These may facilitate superoxide or peroxide generation and increase the rate of these first steps in the reduction of oxygen in a fashion similar to conversion coatings.
The other aspect of note with these results is that the incubation period observed with the RE-organo compounds is not evident here. Specifically, cathodic activity has often been observed some 60 min to 24 h after exposure of aluminium to an electrolyte containing RE-organo compounds whereas anodic activity is evident after a short time (usually 30 min). Results presented here show that inhibition of anodic and cathodic activity seems to occur in parallel. This conclusion suggests that the delay in the onset of cathodic activity is probably related to the time taken to develop the “right” conditions on the surface for inhibition. This further suggests that cathodic suppression is probably via a secondary reaction such as the oxygen reduction reaction and pH increase at cathodic sites which may take some time to establish high enough pH levels for the precipitation of cerium compounds. The onset of cathodic reactions may be further impeded by surface microstructural changes such as the de-alloying of S-phase particles and other Cu-enrichment processes over IMPs. Thus, the interval during which corrosion is happening before the addition of inhibitor or release of inhibitive components becomes a controlling factor. In the previous inhibitor studies, inhibitors were incorporated into the corrosive medium initially, but the current study confirms that the delay in cathodic inhibition is related to corrosion processes and not inhibitor activity.