A Comparative Differential Scanning Calorimetry Study of Precipitation Hardenable Copper-Based Alloys with Optimized Strength and High Conductivity
Abstract
1. Introduction
1.1. Copper Alloys Containing Chromium
1.2. Copper Alloys Containing Scandium
1.3. Copper Alloys Containing Hafnium
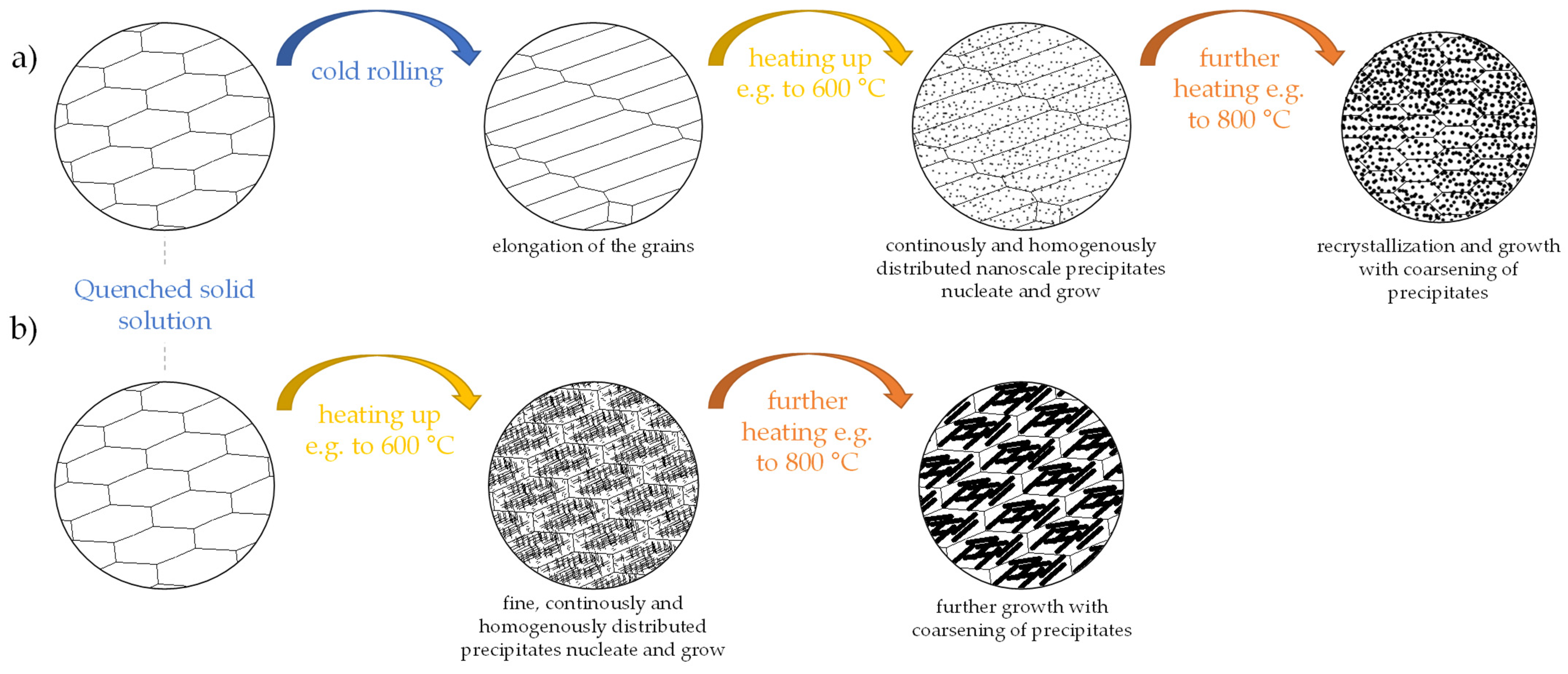
1.4. Analyzing Thermomechanical Processing of Metals Using DSC Measurements
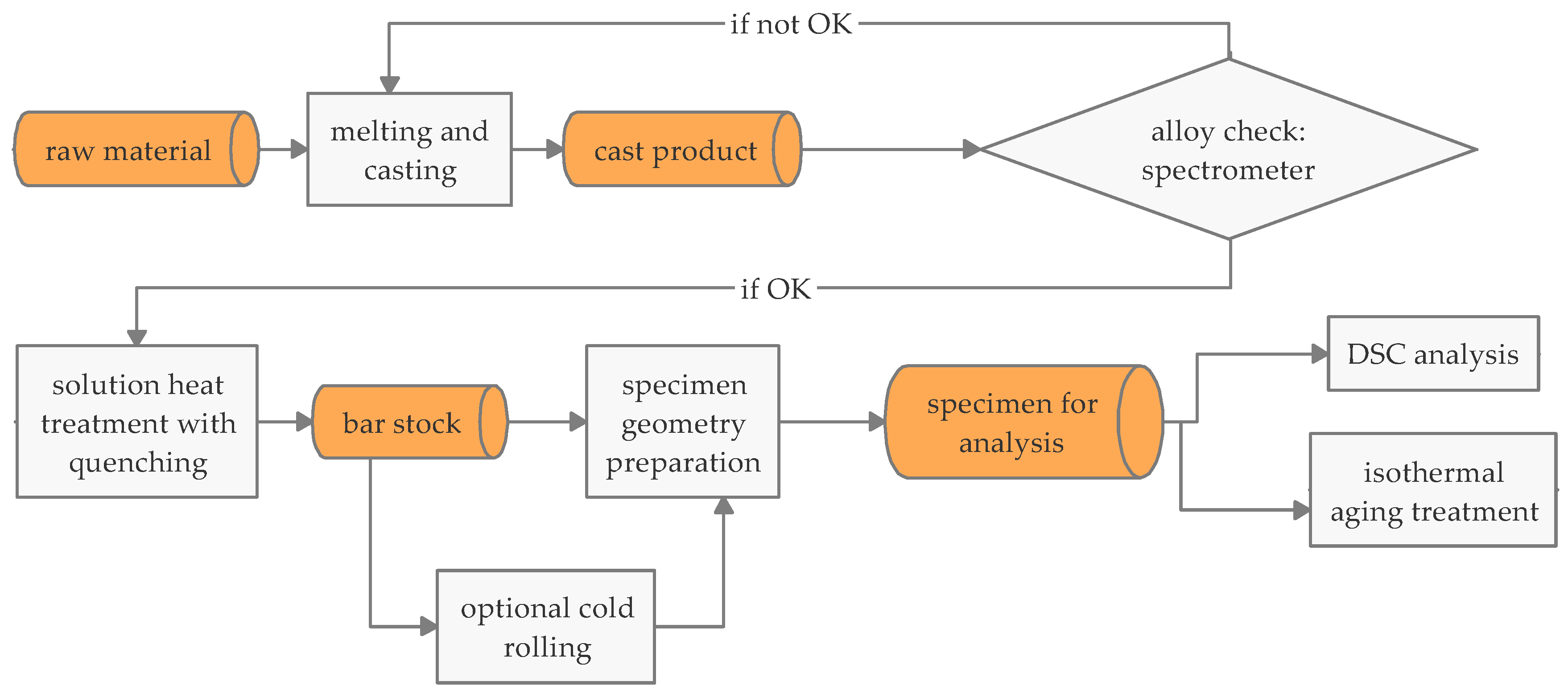
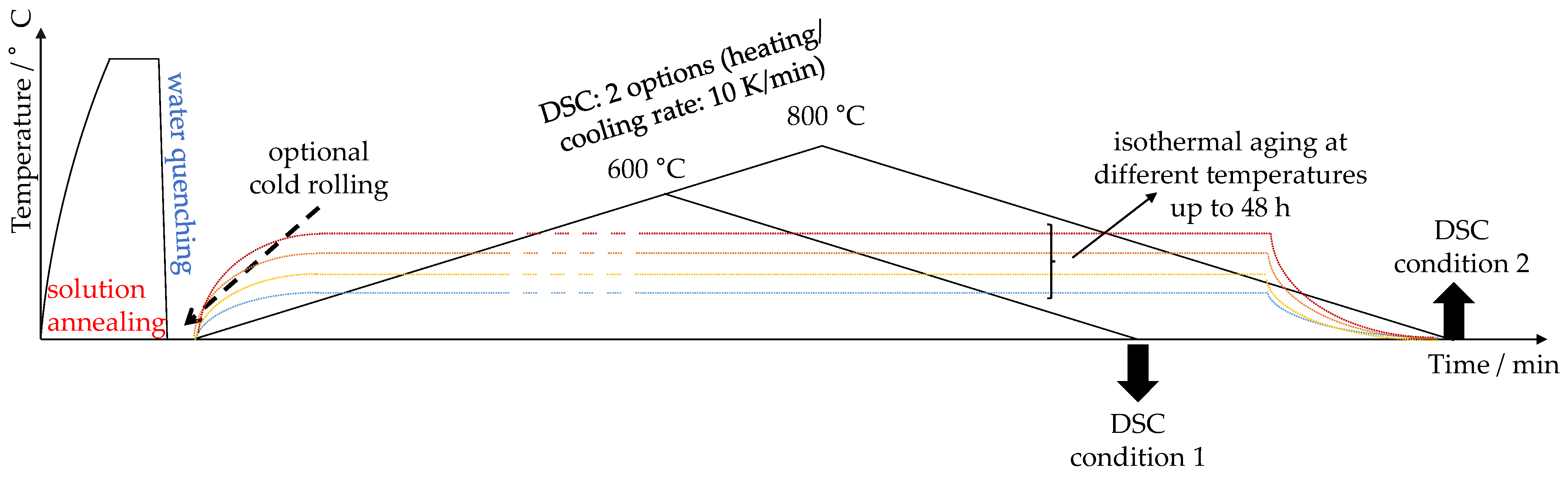
2. Materials and Methods
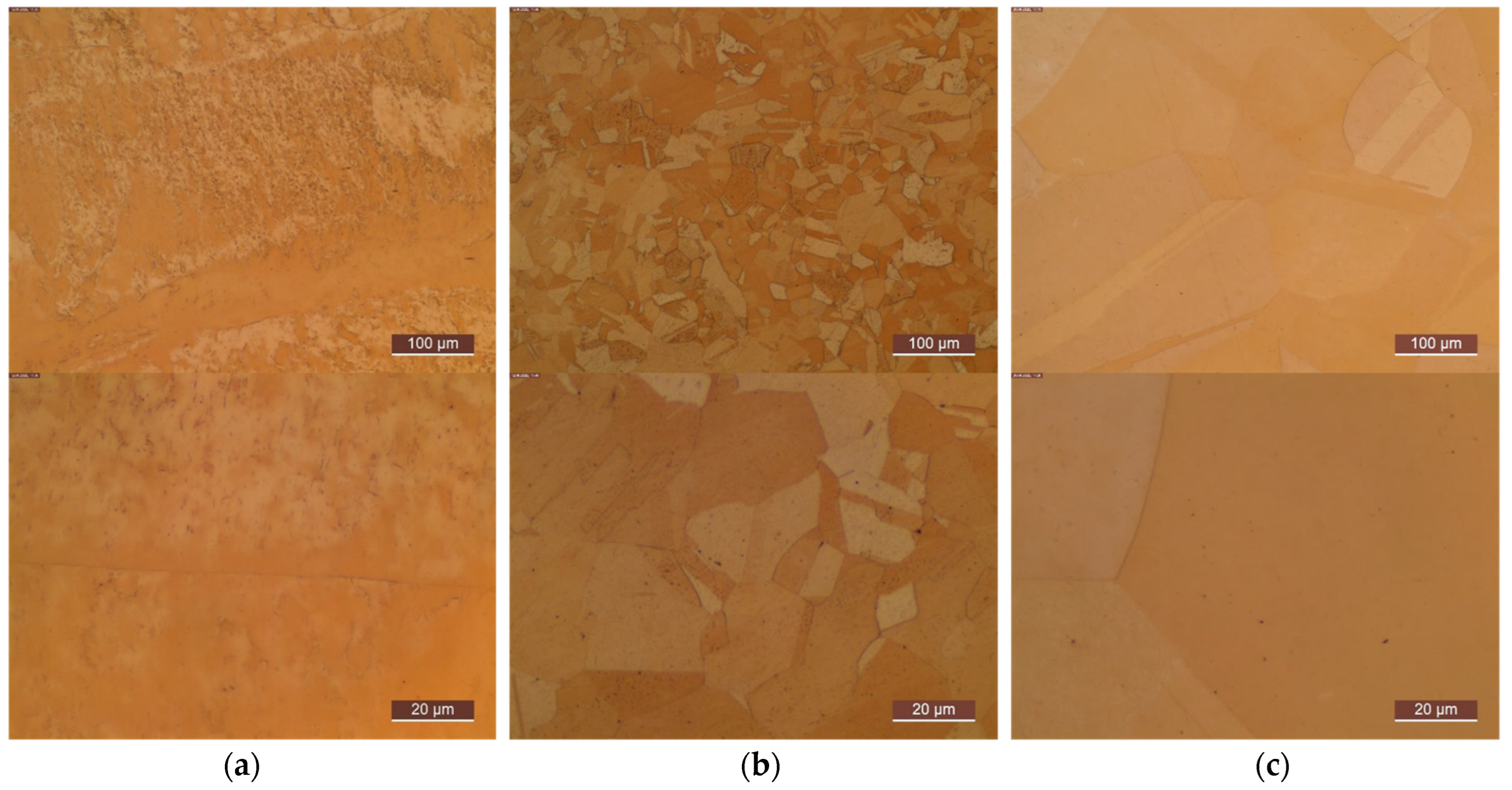
3. Results
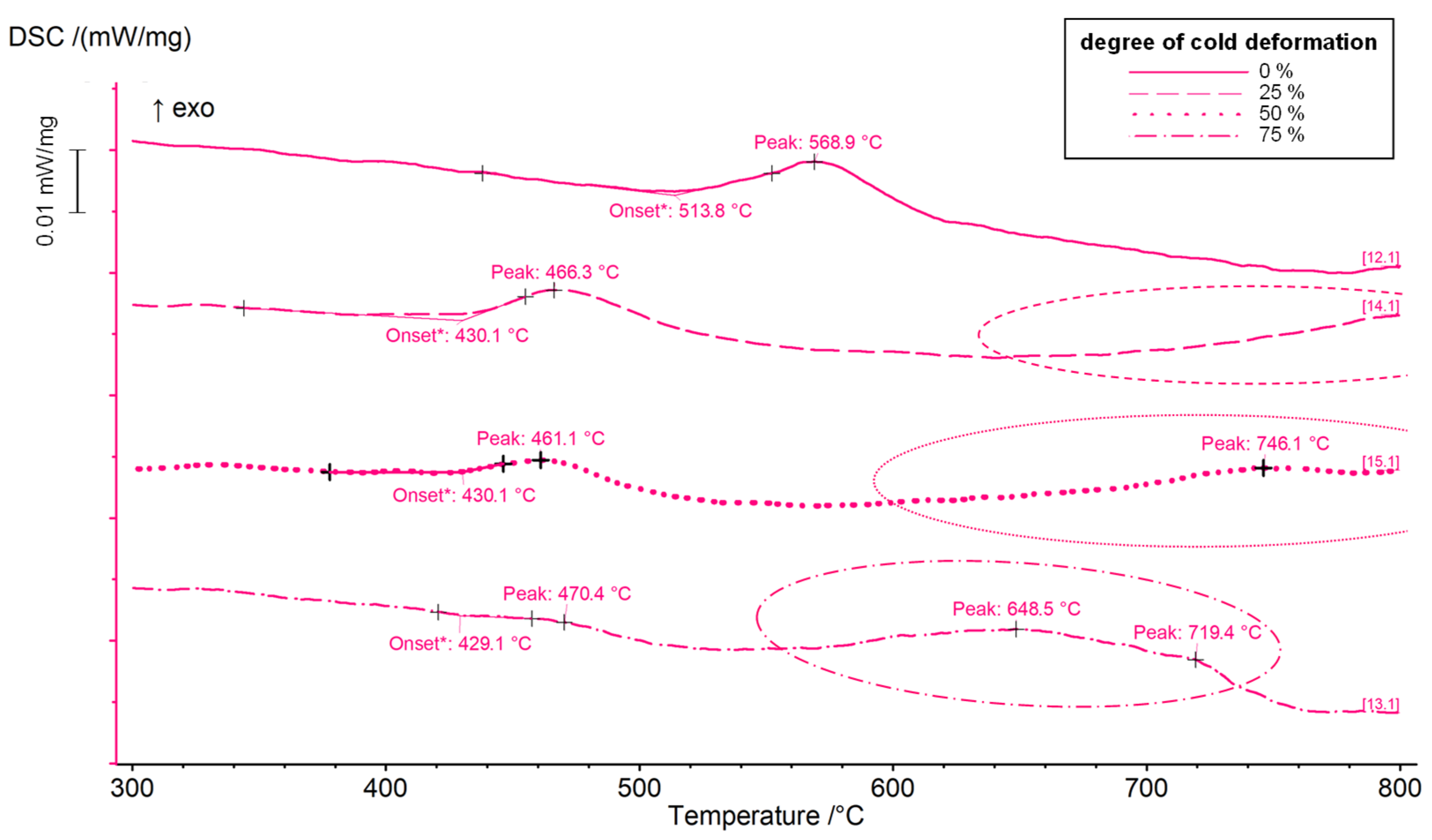
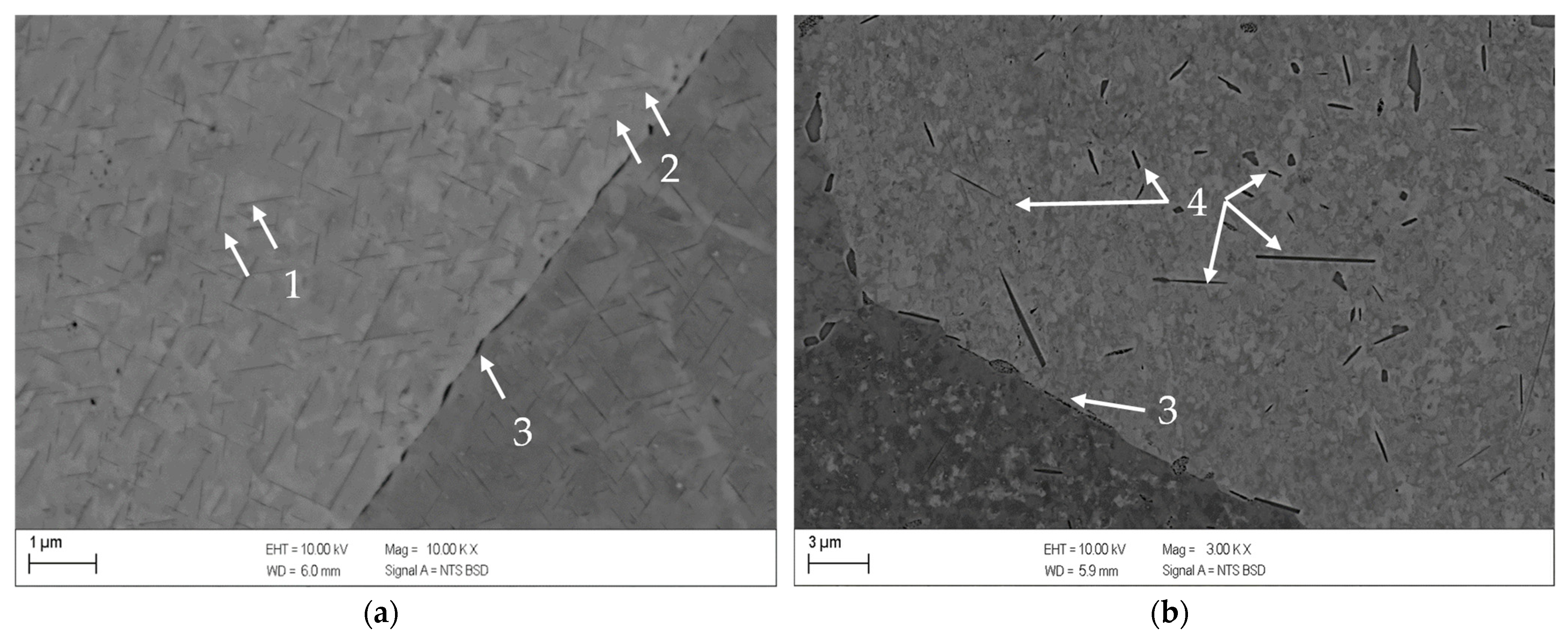
3.1. Results for Copper-Chromium Alloy CuCr0.7
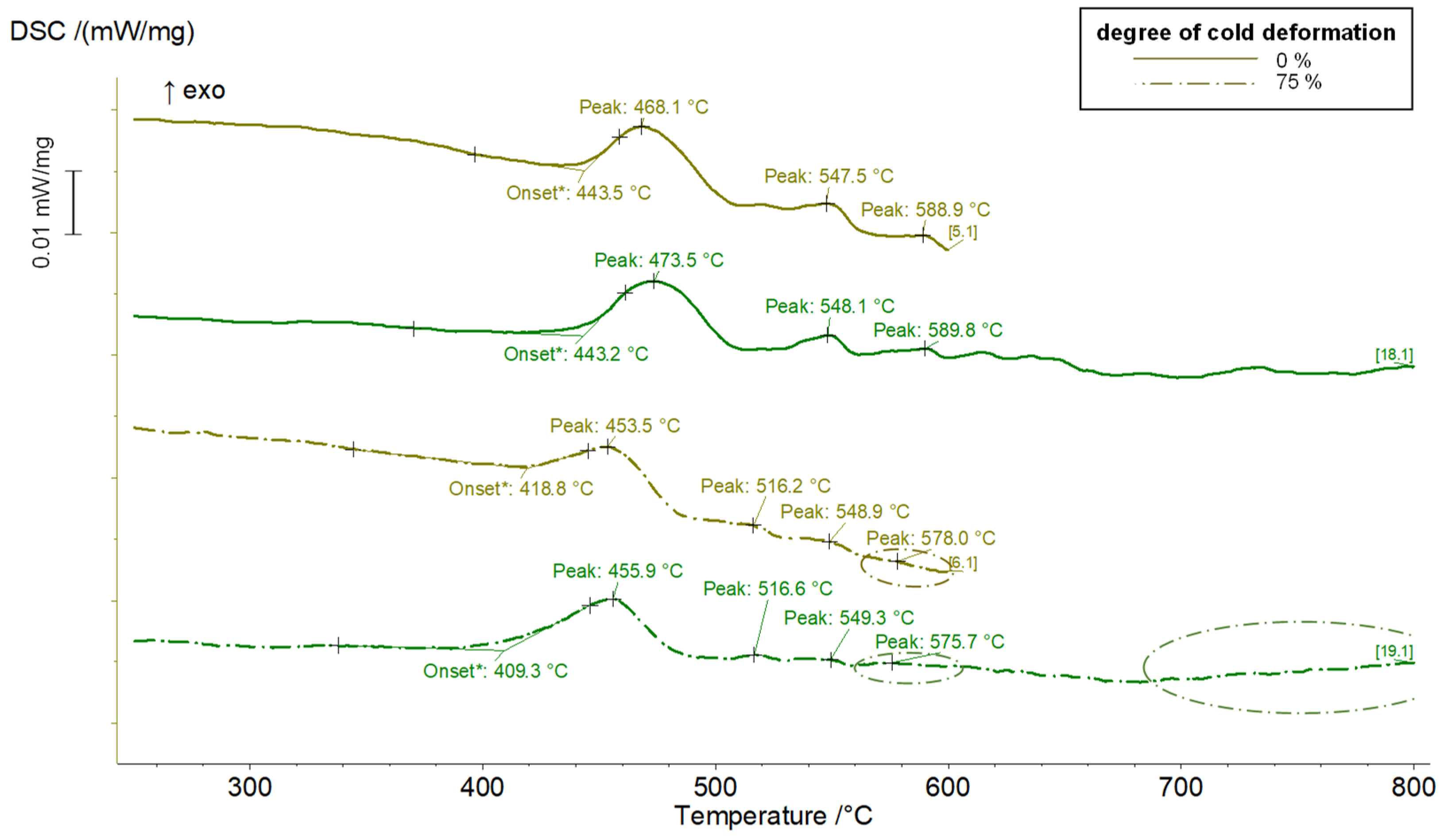
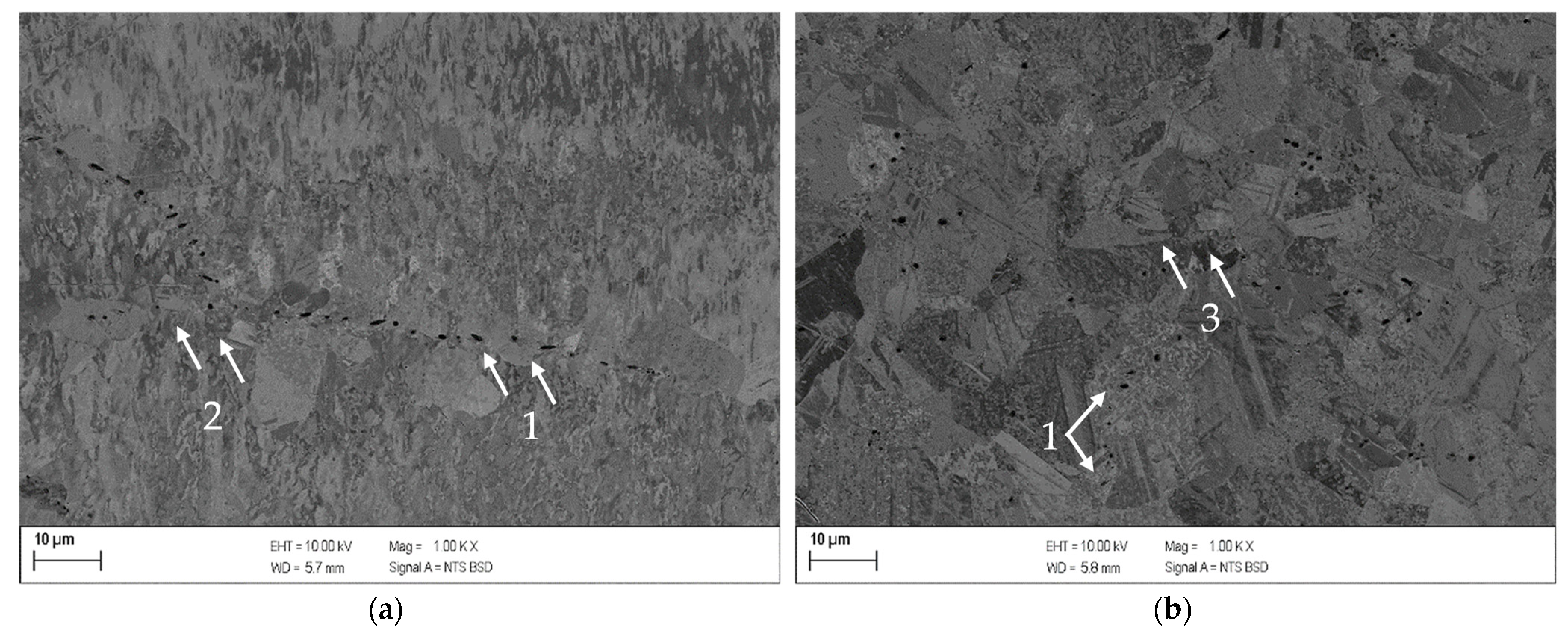
3.2. Results for Copper-Scandium Alloy CuSc0.3
3.3. Results for Copper-Hafnium Alloy CuHf0.7
4. Discussion
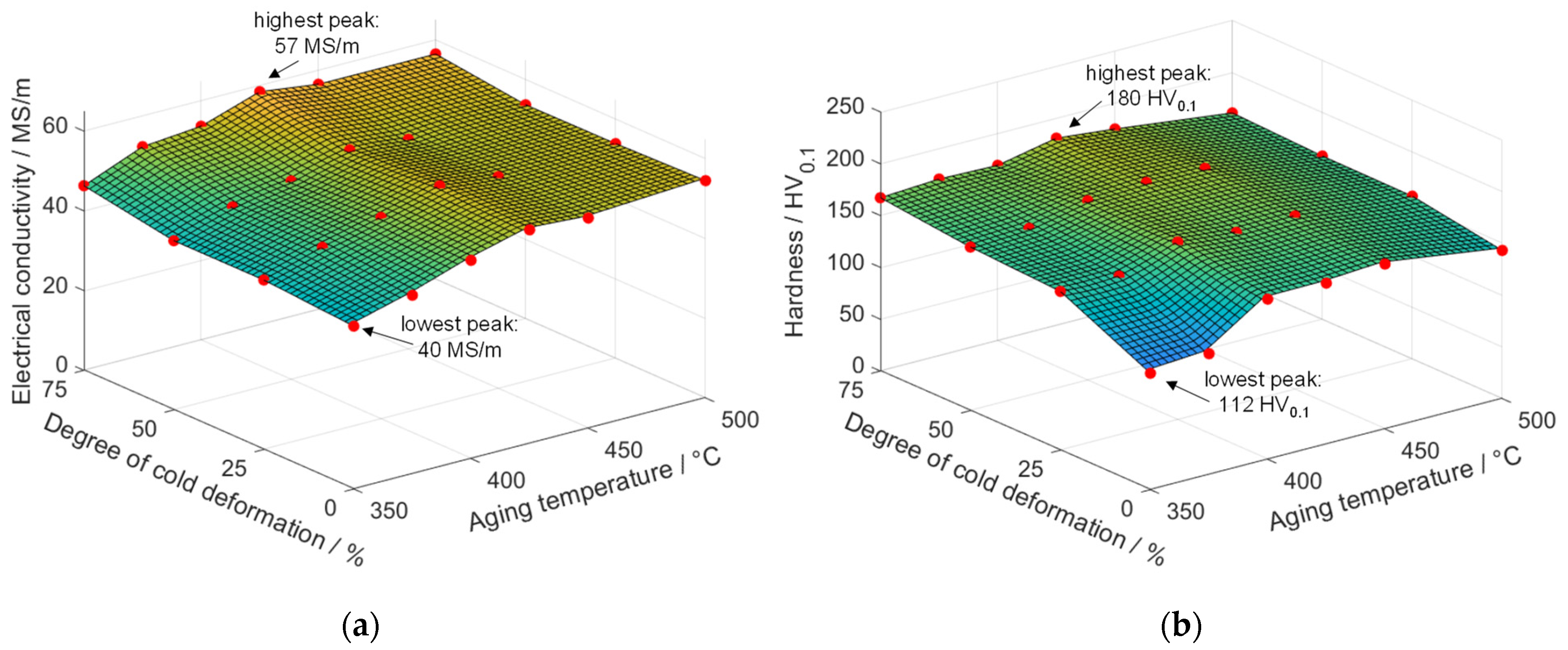
4.1. Influence of Cold Deformation Prior to an Aging Treatment of Low-Alloyed Binary Copper Alloys
4.2. Discussion of the Different Alloying Concepts
5. Conclusions
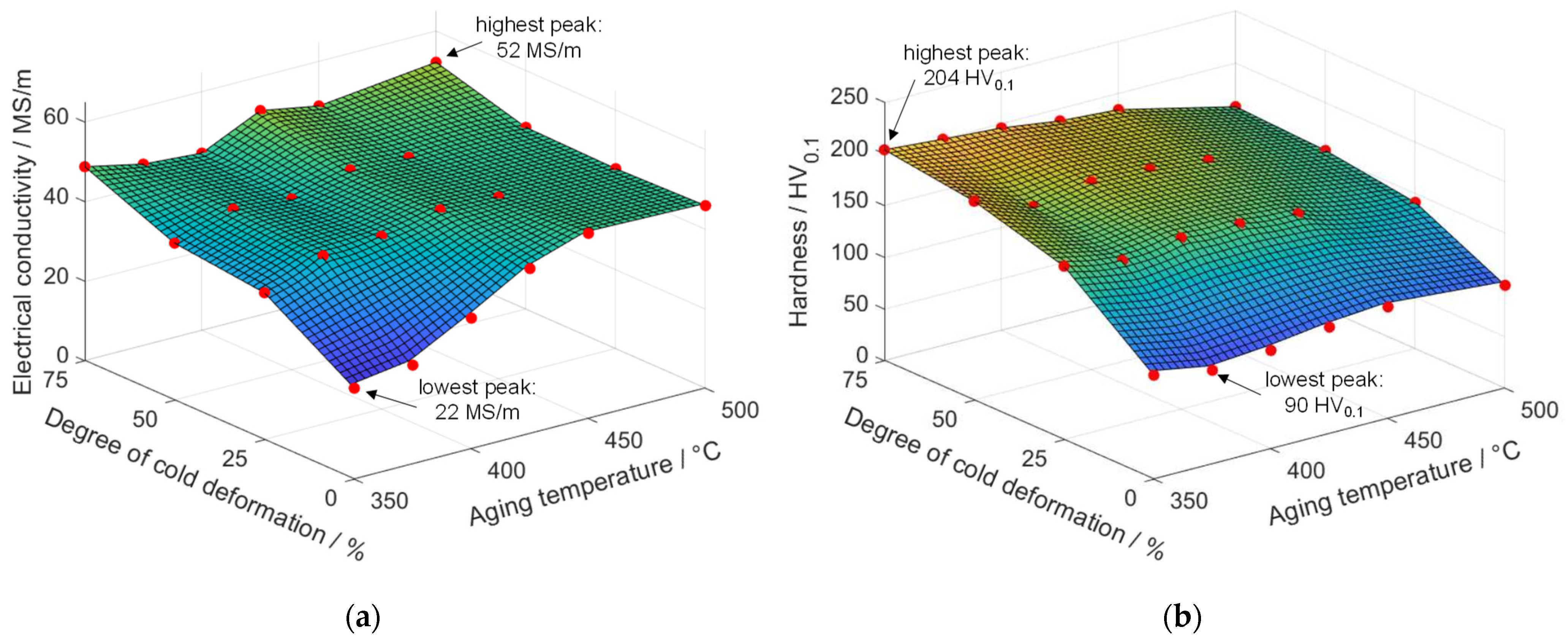
- All alloys, CuCr0.7, CuSc0.3, and CuHf0.7, showed substantial increases in hardness and electrical conductivity due to precipitation effects. A lower aging temperature and a higher degree of cold deformation resulted in an excellent interaction of strengthening effects. Regarding electrical conductivity, which was highly sensitive to the amount of alloying elements in the copper matrix, higher degrees of cold deformation, long aging durations, and enhanced temperatures were favorable due to a maximized segregation of alloying elements from the copper matrix.
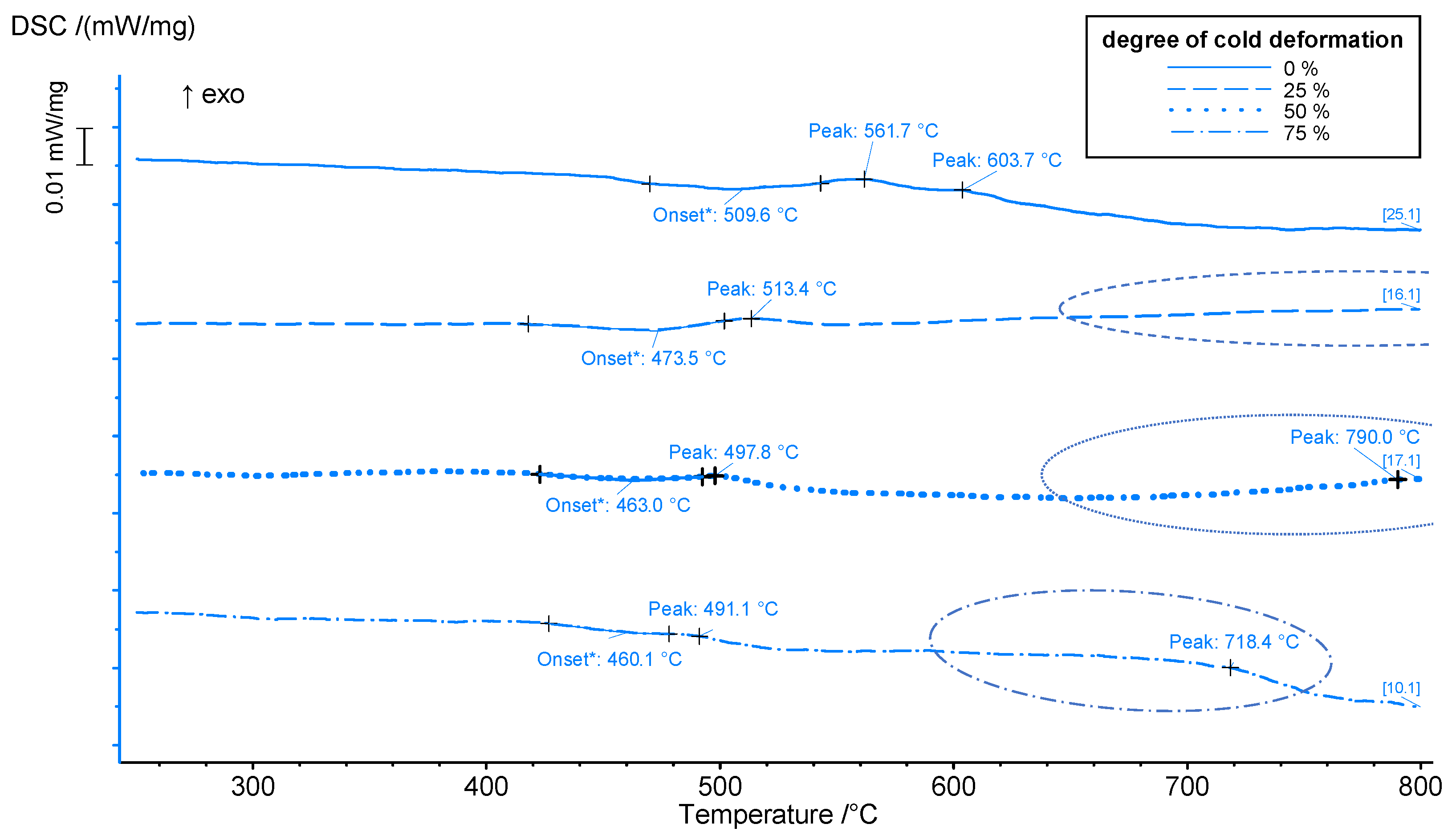
- The time and temperature-dependent solid-state reactions resulted in different onset, peak temperatures of the continuously heated DSC measurements, and optimal aging temperatures in isothermal aging experiments. The enhanced heating rate during the DSC method shifted the effects to higher temperatures.
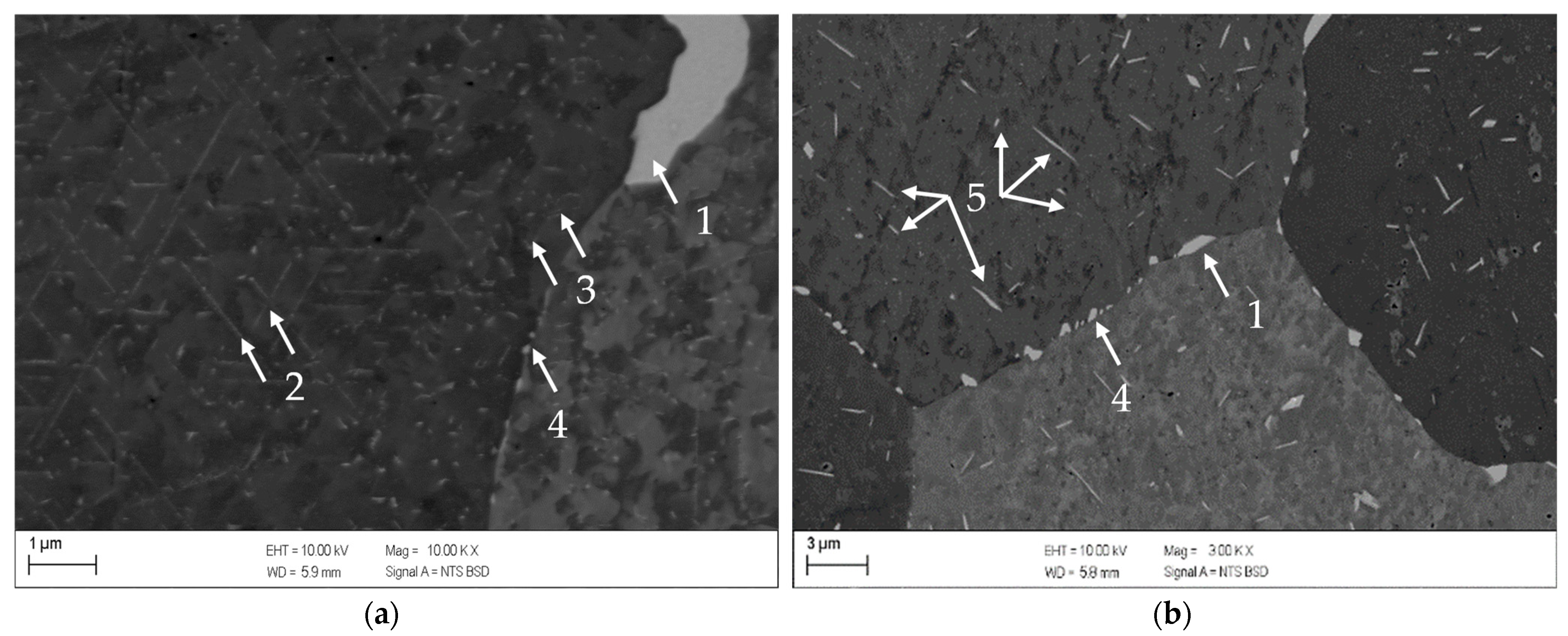
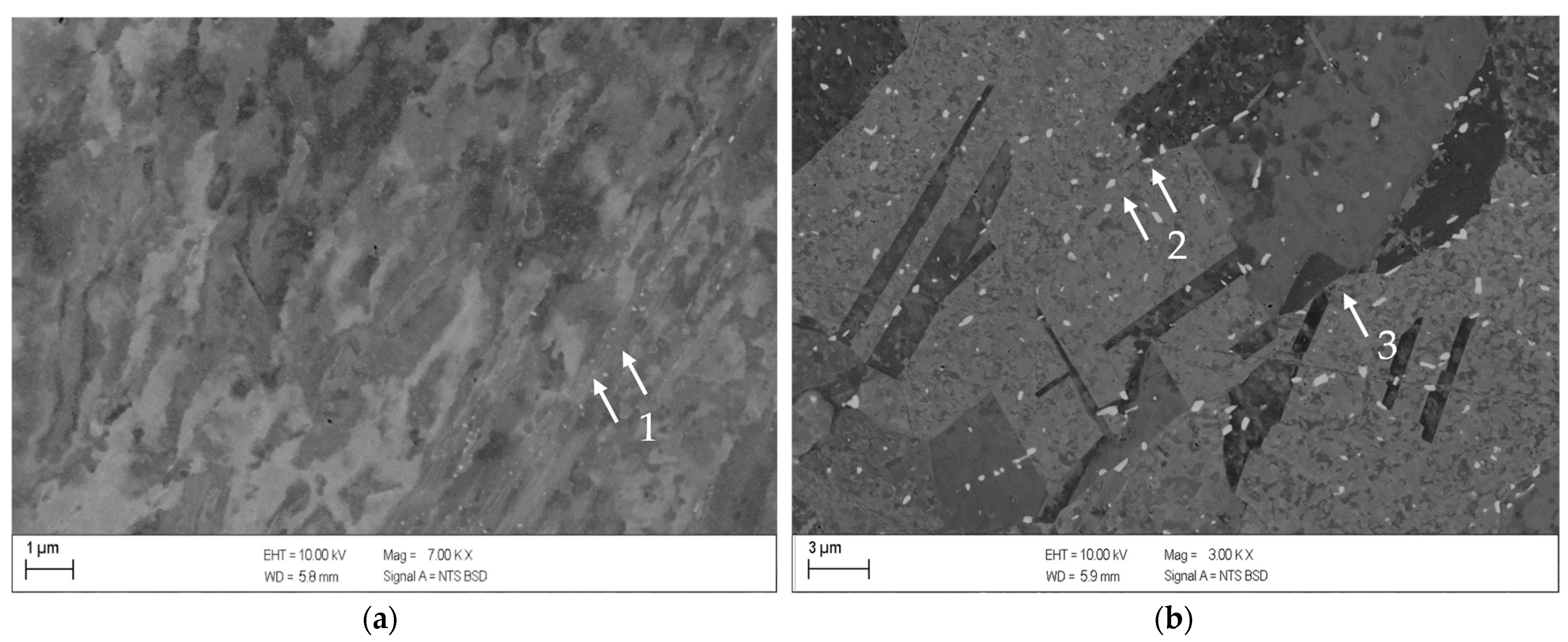
- The alloys CuSc0.3 and CuHf0.7 showed comparable precipitation behavior, including the onset and peak temperatures which reacted susceptible to cold deformation prior to the aging process. A cold rolled supersaturated solid solution promoted the precipitation of fine homogenously distributed precipitates. The difference between as-cast temperature treated specimens and all other cold rolled options was the most substantial. The degree of cross-section reduction due to cold rolling did not have a huge impact but continuously followed the trend to promote the precipitation reaction.
- CuCr0.7 appeared to be mainly influenced by the aging temperature. An initial cold rolling shifted the solid-state reactions to lower temperatures, but in a considerably lower degree compared to CuSc0.3 and CuHf0.7.
- The alternative alloying concepts containing scandium and hafnium show excellent benefits due to recrystallization appearing at conspicuously higher temperatures, compared to the benchmark alloy CuCr0.7.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
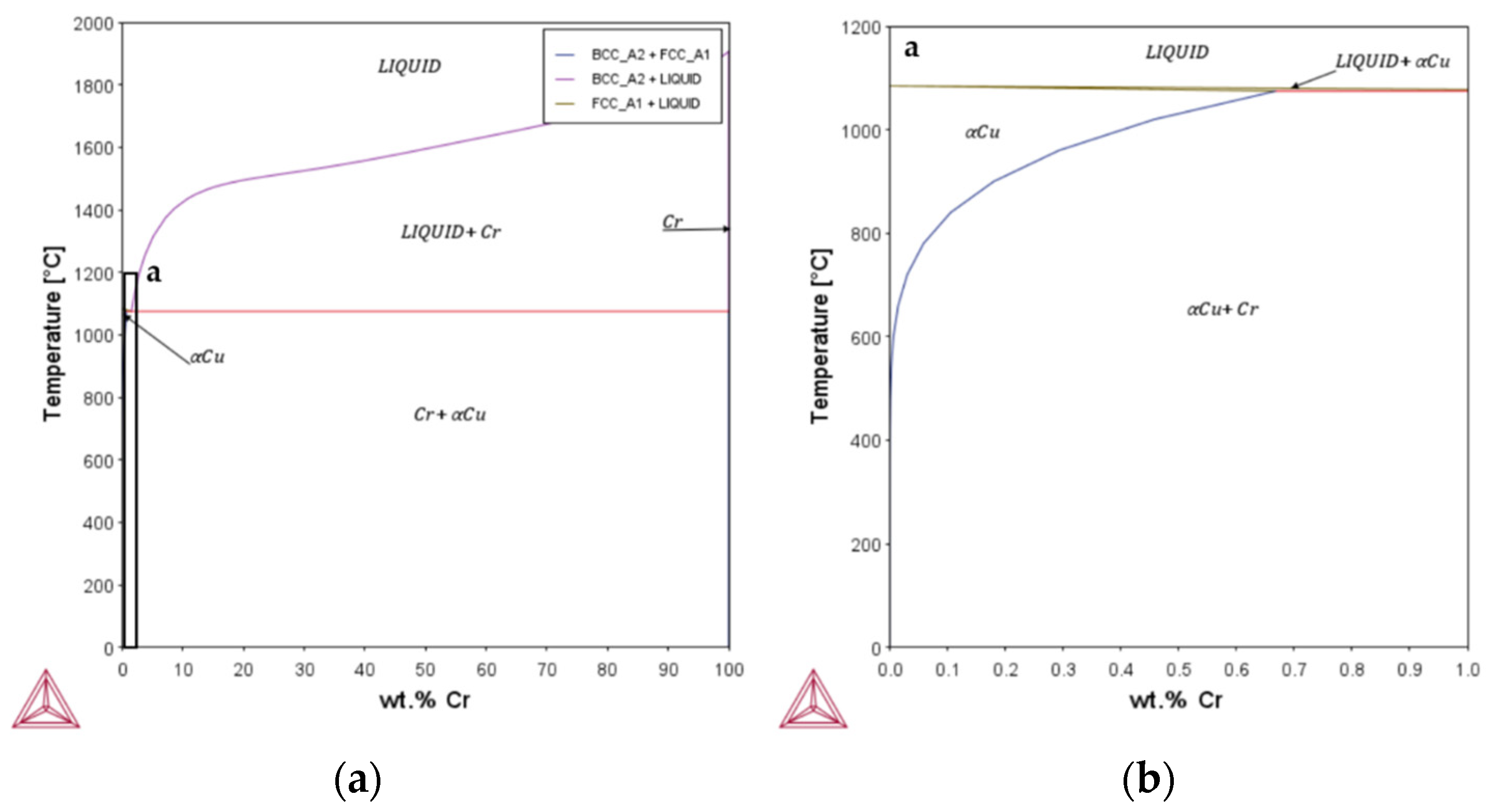

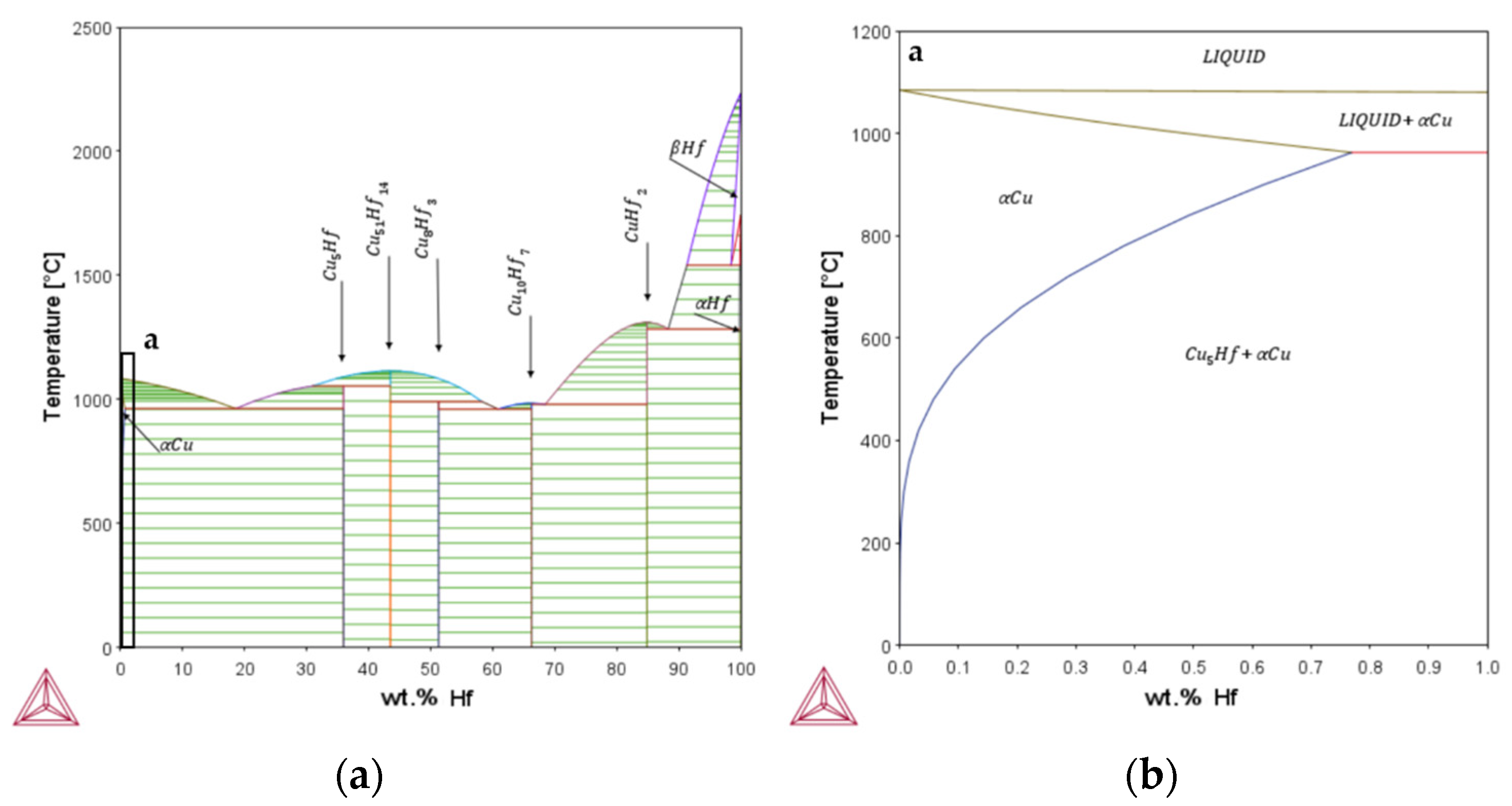
Appendix B
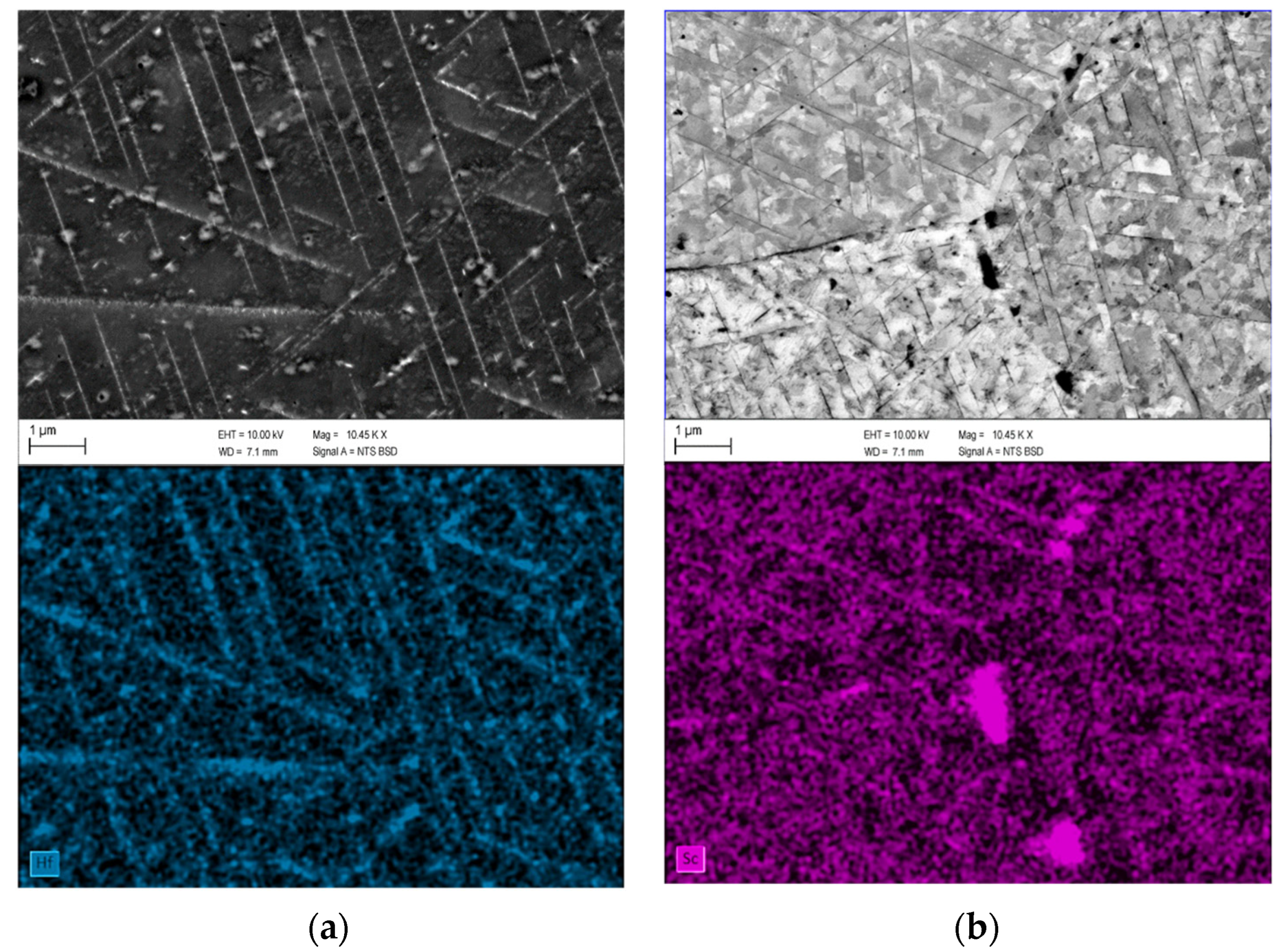
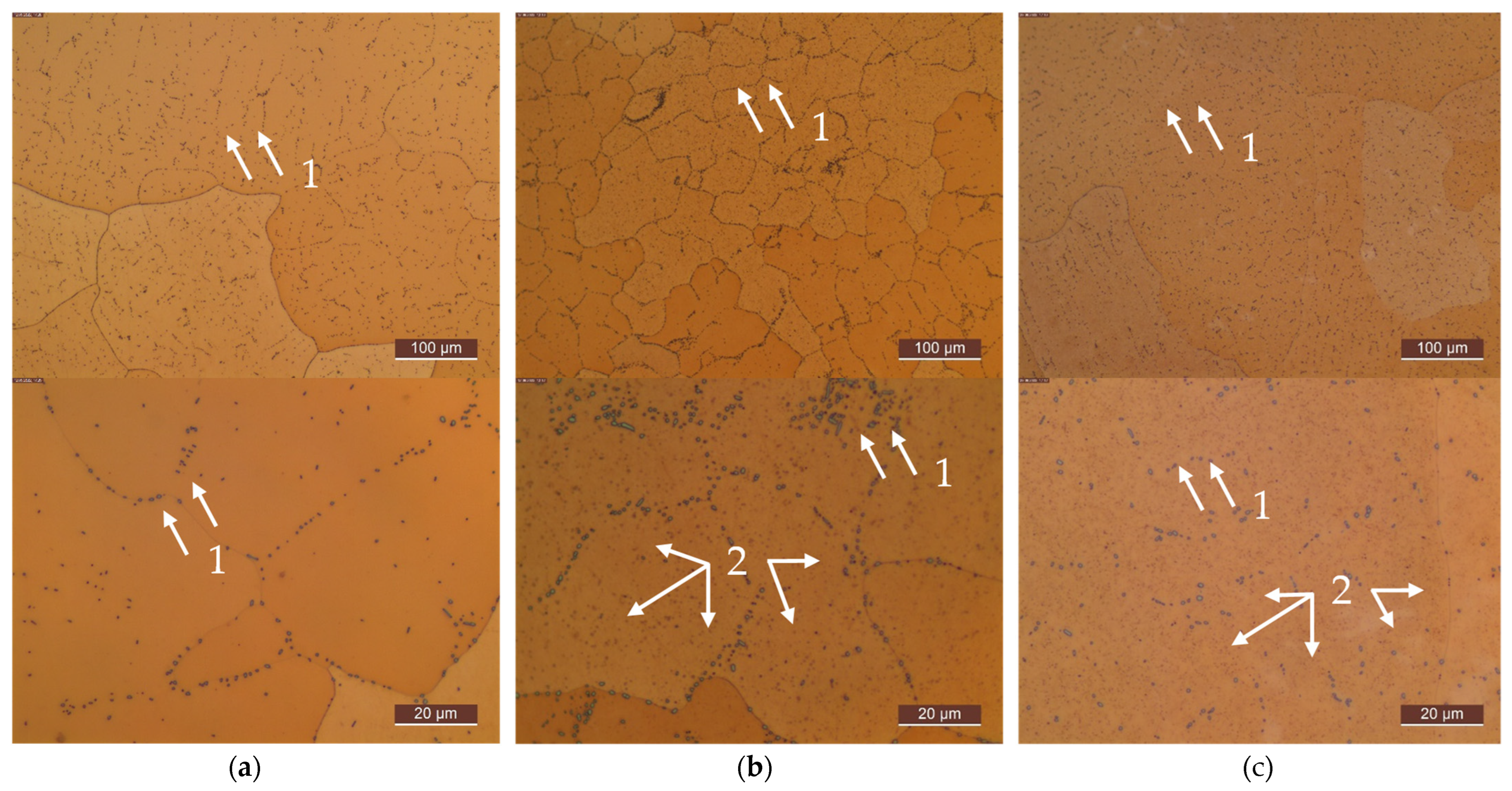

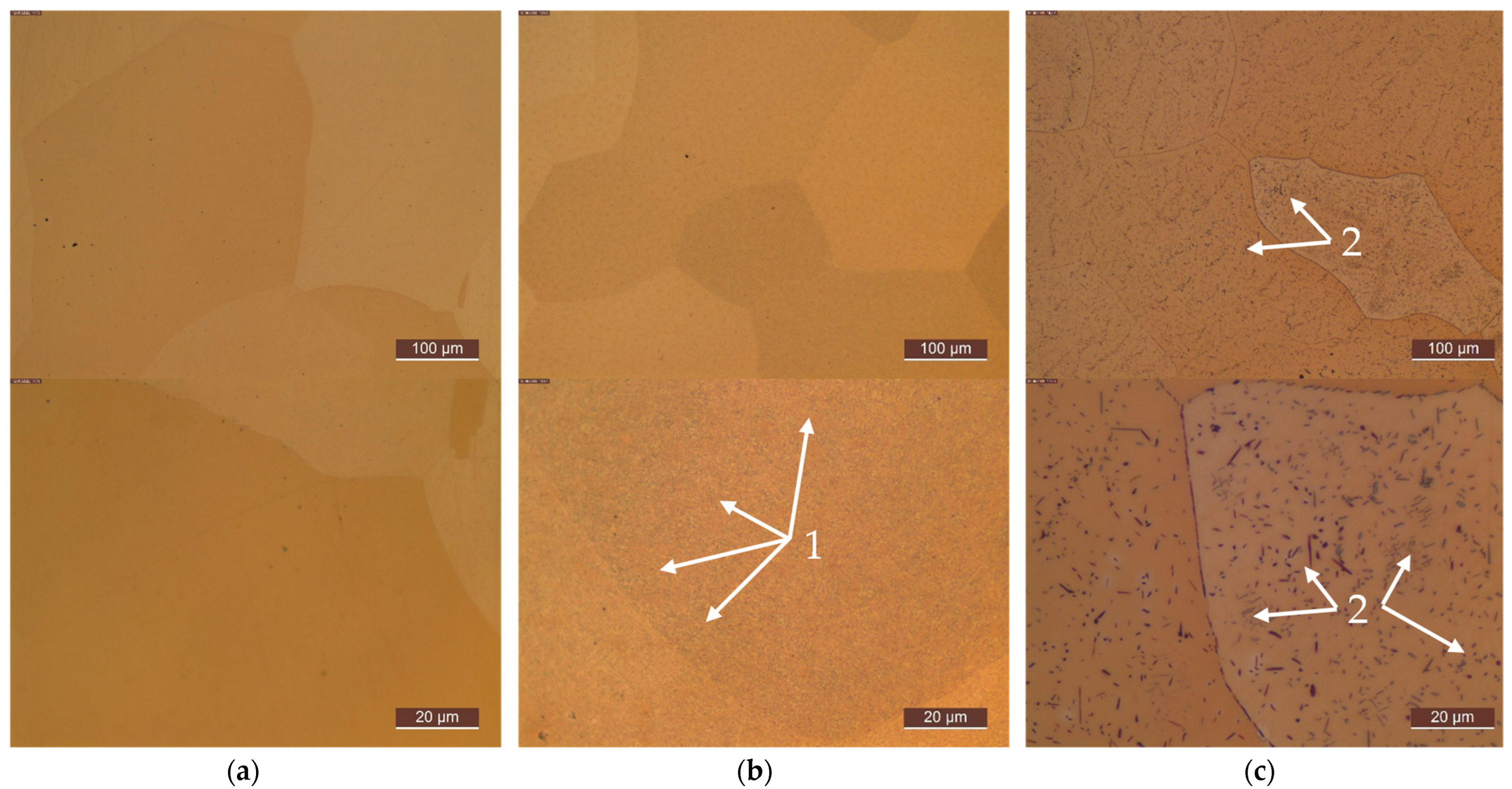
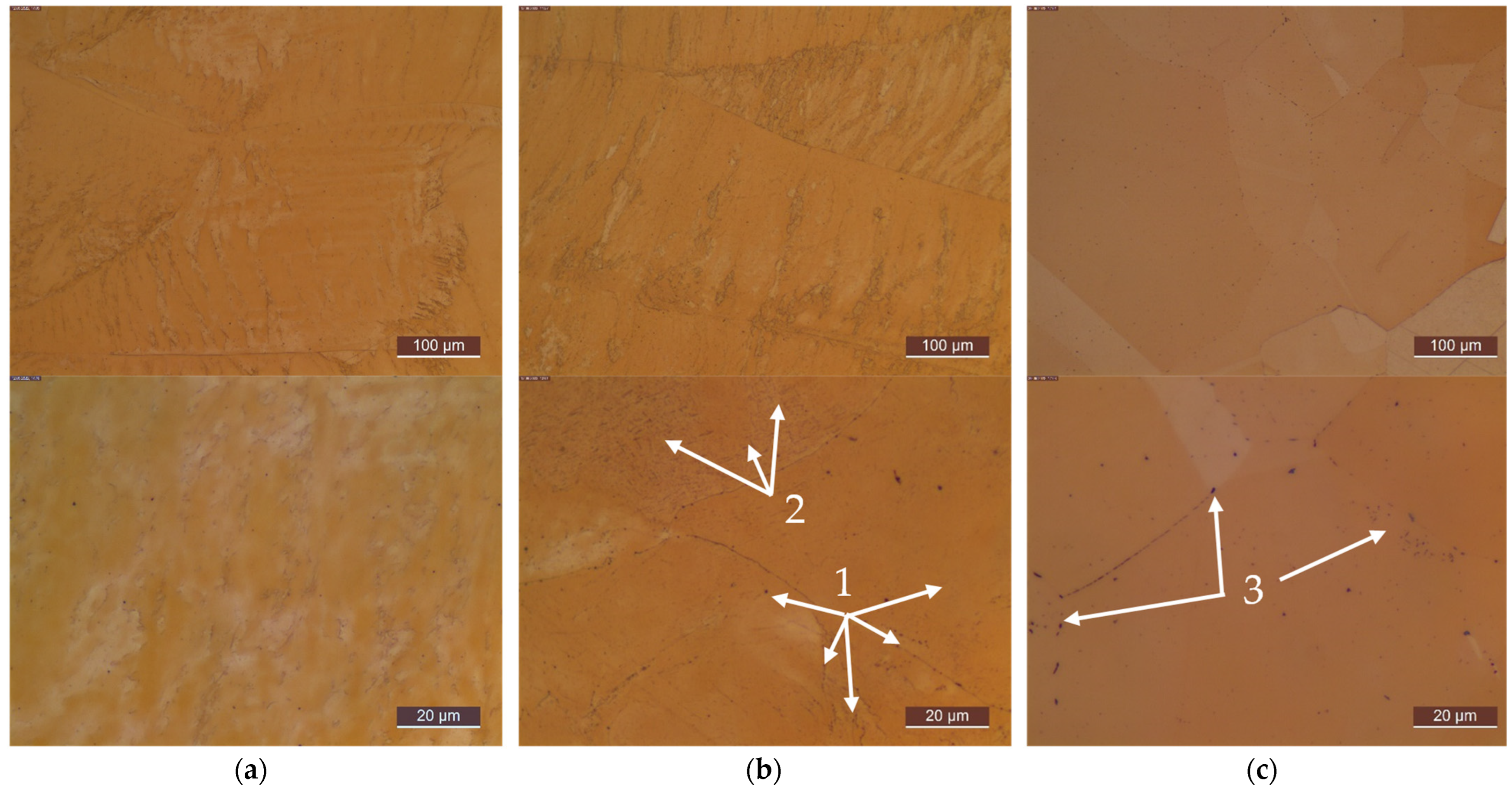

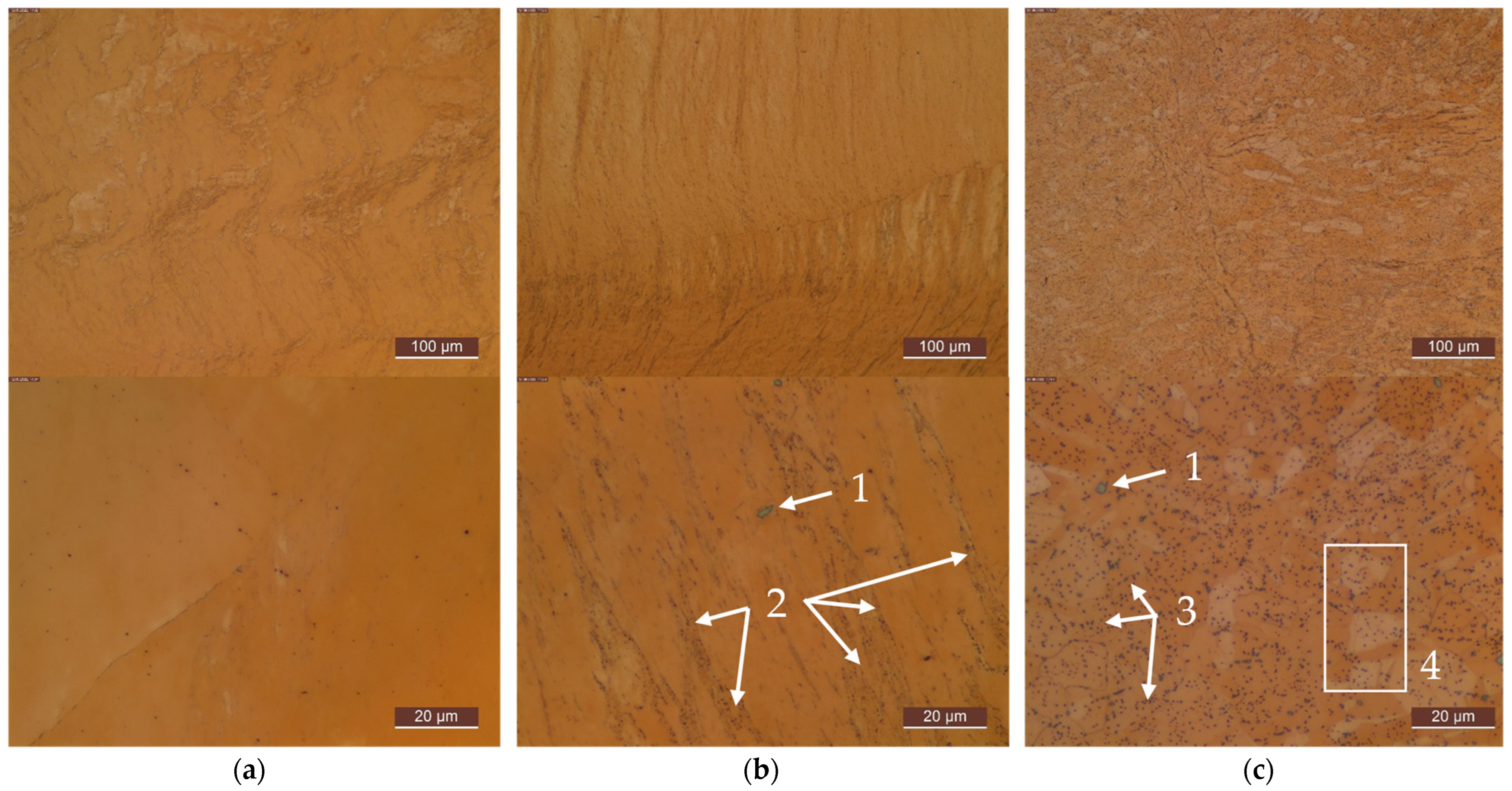
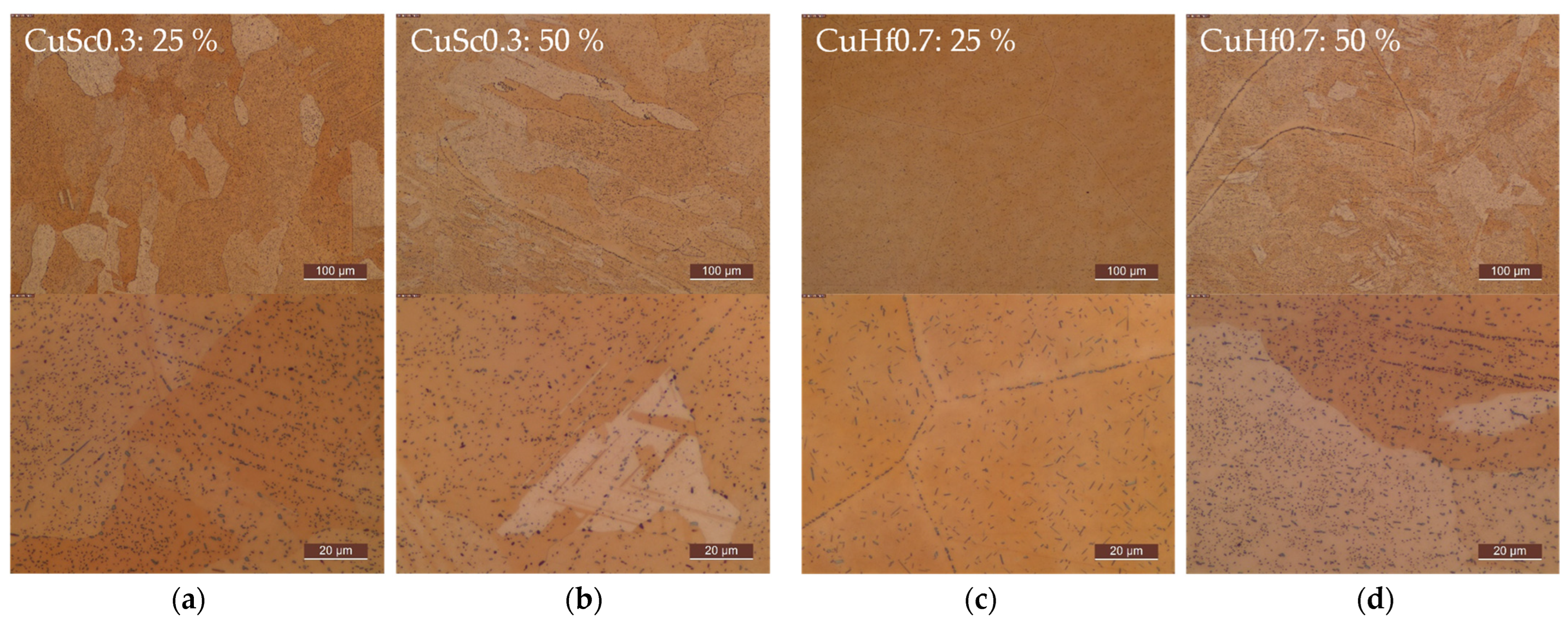
Appendix C


References
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. (Eds.) Phase Transformations in Metals and Alloys, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781003011804. [Google Scholar]
- Dies, K. Kupfer und Kupferlegierungen in der Technik; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-48932-7. [Google Scholar]
- Davis, J.R. Metals Handbook: Non-Ferrous Alloys and Special-Purpose Materials, 10th ed.; ASM International: Novelty, OH, USA, 1990; ISBN 0871703785. [Google Scholar]
- Gottstein, G. Materialwissenschaft und Werkstofftechnik: Physikalische Grundlagen, 4th ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-36602-4. [Google Scholar]
- Freudenberger, J.; Heilmaier, M. Materialkunde der Nichteisenmetalle und -Legierungen, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; ISBN 9783527822546. [Google Scholar]
- Chakrabarti, D.J.; Laughlin, D.E. The Cr-Cu (Chromium-Copper) system. Bull. Alloy Phase Diagr. 1984, 5, 59–68. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Wu, P.; Mi, X. Study on improvement of conductivity of Cu-Cr-Zr alloys. Rare Met. 2007, 26, 124–130. [Google Scholar] [CrossRef]
- Peng, L.; Xie, H.; Huang, G.; Xu, G.; Yin, X.; Feng, X.; Mi, X.; Yang, Z. The phase transformation and strengthening of a Cu-0.71 wt% Cr alloy. J. Alloys Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Chbihi, A.; Sauvage, X.; Blavette, D. Atomic scale investigation of Cr precipitation in copper. Acta Mater. 2012, 60, 4575–4585. [Google Scholar] [CrossRef]
- Liu, J.; Hou, M.; Yang, H.; Xie, H.; Yang, C.; Zhang, J.; Feng, Q.; Wang, L.; Meng, L.; Wang, H. In-situ TEM study of the dynamic interactions between dislocations and precipitates in a Cu-Cr-Zr alloy. J. Alloys Compd. 2018, 765, 560–568. [Google Scholar] [CrossRef]
- Wan, X.; Xie, W.; Chen, H.; Tian, F.; Wang, H.; Yang, B. First-principles study of phase transformations in Cu–Cr alloys. J. Alloys Compd. 2021, 862, 158531. [Google Scholar] [CrossRef]
- Bodyakova, A.; Mishnev, R.; Belyakov, A.; Kaibyshev, R. Effect of chromium content on precipitation in Cu–Cr–Zr alloys. J. Mater. Sci. 2022, 57, 13043–13059. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, L.; Huang, G.; Xie, H.; Mi, X.; Liu, X. Effects of Mg addition on the microstructure and softening resistance of Cu–Cr alloys. Mater. Sci. Eng. A 2020, 776, 139009. [Google Scholar] [CrossRef]
- Holzwarth, U.; Stamm, H. The precipitation behaviour of ITER-grade Cu–Cr–Zr alloy after simulating the thermal cycle of hot isostatic pressing. J. Nucl. Mater. 2000, 279, 31–45. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Ge, Y.; Wang, J.; Cui, J.-Z. Effect of processing and heat treatment on behavior of Cu-Cr-Zr alloys to railway contact wire. Met. Mater. Trans. A 2006, 37, 3233–3238. [Google Scholar] [CrossRef]
- Xie, H.; Mi, X.; Huang, G.; Gao, B.; Yin, X.; Li, Y. Effect of thermomechanical treatment on microstructure and properties of Cu-Cr-Zr-Ag alloy. Rare Met. 2011, 30, 650–656. [Google Scholar] [CrossRef]
- Predel, B. Cu-Sc (Copper-Scandium): Group IV Physical Chemistry; Springer GmbH: Heidelberg, Germany, 1994; Volume 5D. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Laughlin, D.E.; Chakrabarti, D.J. The Cu-Sc (Copper-Scandium) System. Bull. Alloy Phase Diagr. 1988, 9, 378–382. [Google Scholar] [CrossRef]
- Goncharuk, L.V.; Sidorko, V.R. Thermodynamic properties of scandium-copper compounds. Sov. Powder Met. Ceram. 2006, 45, 72–75. [Google Scholar] [CrossRef]
- Shubin, A.B.; Shunyaev, K.Y. Copper-scandium system: Thermodynamic properties of intermetallics and liquid alloys. Russ. Metal. 2010, 2010, 672–677. [Google Scholar] [CrossRef]
- Franczak, K.; Kwaśniewski, P.; Kiesiewicz, G.; Zasadzińska, M.; Jurkiewicz, B.; Strzępek, P.; Rdzawski, Z. Research of mechanical and electrical properties of Cu–Sc and Cu–Zr alloys. Archiv. Civ. Mech. Eng. 2020, 20, 28. [Google Scholar] [CrossRef]
- Turchanin, M.A. Phase equilibria and thermodynamics of binary copper systems with 3d-metals. I. The copper-scandium system. Powder Metall Met Ceram 2006, 45, 143–152. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lv, L. Quantum chemical calculations of thermodynamic and mechanical properties of the intermetallic phases in copper–scandium alloy. J. Theor. Comput. Chem. 2017, 16, 1750056. [Google Scholar] [CrossRef]
- Hao, Z.; Xie, G.; Liu, X.; Tan, Q.; Wang, R. The precipitation behaviours and strengthening mechanism of a Cu-0.4 wt% Sc alloy. J. Mater. Sci. Technol. 2022, 98, 1–13. [Google Scholar] [CrossRef]
- Arzhavitin, V.M.; Korotkova, I.M.; Sytin, V.I. Grain-boundary internal friction of yttrium- or scandium-microalloyed copper. Russ. Metal. 2016, 2016, 229–234. [Google Scholar] [CrossRef]
- Dölling, J.; Henle, R.; Prahl, U.; Zilly, A.; Nandi, G. Copper-Based Alloys with Optimized Hardness and High Conductivity: Research on Precipitation Hardening of Low-Alloyed Binary CuSc Alloys. Metals 2022, 12, 902. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Qin, N.; Zhang, Y.; Li, S.; Xiao, Z.; Lei, Q.; Li, Z. Effects of minor rare earths on the microstructure and properties of Cu-Cr-Zr alloy. J. Alloys Compd. 2020, 847, 155762. [Google Scholar] [CrossRef]
- Okamoto, H. Cu-Hf (Copper-Hafnium). J. Phase Equilib. Diffus. 2007, 28, 583–584. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Zhu, M.; Wang, H.; Wei, H. Physical Properties and Precipitate Microstructures of Cu-Hf Alloys at Different Processing Stages. Scanning 2018, 2018, 3653987. [Google Scholar] [CrossRef]
- Li, R.; Zhang, S.; Zou, C.; Kang, H.; Wang, T. The roles of Hf element in optimizing strength, ductility and electrical conductivity of copper alloys. Mater. Sci. Eng. A 2019, 758, 130–138. [Google Scholar] [CrossRef]
- Saarivirta, M.J. High conductivity copper alloys—Part 1. Met. Ind. 1963, 103, 685–688. [Google Scholar]
- Watanabe, R. Study on Copper-Rich Copper-Hafnium Alloys. J. Jpn. Inst. Met. 1966, 30, 754–759. [Google Scholar] [CrossRef]
- Saarivirta, M.J. High conductivity copper alloys—Part 2. Met. Ind. 1963, 103, 716–718. [Google Scholar]
- Saarivirta, M.J. High conductivity copper alloys—Part 3. Met. Ind. 1963, 103, 758–760. [Google Scholar]
- Watanabe, S.; Kleppa, O.J. Thermochemistry of alloys of transition metals: Part IV. Alloys of copper with scandium, yttrium, lanthanum, and lutetium. MTB 1984, 15, 357–368. [Google Scholar] [CrossRef]
- Ghosh, G. First-principles calculations of structural energetics of Cu–TM (TM=Ti, Zr, Hf) intermetallics. Acta Mater. 2007, 55, 3347–3374. [Google Scholar] [CrossRef]
- Shangina, D.V.; Ivanov, N.I.; Bochvar, N.R.; Dobatkin, S.V. Resistance of the Contact Welding Electrodes Made of a Cu–0.7% Cr–0.9% Hf Alloy with an Ultrafine-Grained Structure. Russ. Metal. 2018, 2018, 815–819. [Google Scholar] [CrossRef]
- Shangina, D.V.; Bochvar, N.R.; Dobatkin, S.V. The effect of alloying with hafnium on the thermal stability of chromium bronze after severe plastic deformation. J. Mater. Sci. 2012, 47, 7764–7769. [Google Scholar] [CrossRef]
- Stolbovsky, A.; Popov, V.; Falahutdinov, R. The Structure of Annealed Hafnium Bronze Subjected to Severe Plastic Deformation by High Pressure Torsion. IOP Conf. Ser. Mater. Sci. Eng. 2020, 969, 12087. [Google Scholar] [CrossRef]
- Stolbovsky, A.; Popov, V.; Popova, E.; Falahutdinov, R.; Murzinova, S.; Shorokhov, E. Thermal stability of hafnium bronze subjected to dynamic channel angular pressing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 966, 12031. [Google Scholar] [CrossRef]
- Yi, G.; Zhang, X.; Qin, J.; Ning, J.; Zhang, S.; Ma, M.; Liu, R. Mechanical, electronic and thermal properties of Cu5Zr and Cu5Hf by first-principles calculations. J. Alloys Compd. 2015, 640, 455–461. [Google Scholar] [CrossRef]
- Shangina, D.; Maksimenkova, Y.; Bochvar, N.; Serebryany, V.; Raab, G.; Vinogradov, A.; Skrotzki, W.; Dobatkin, S. Influence of alloying with hafnium on the microstructure, texture, and properties of Cu–Cr alloy after equal channel angular pressing. J. Mater. Sci. 2016, 51, 5493–5501. [Google Scholar] [CrossRef]
- Bochvar, N.R.; Rybalchenko, O.V.; Shangina, D.V.; Dobatkin, S.V. Effect of equal-channel angular pressing on the precipitation kinetics in Cu-Cr-Hf alloys. Mater. Sci. Eng. A 2019, 757, 84–87. [Google Scholar] [CrossRef]
- Shangina, D.; Maksimenkova, Y.; Bochvar, N.; Serebryany, V.; Raab, G.; Vinogradov, A.; Skrotzki, W.; Dobatkin, S. Structure and Properties of Cu Alloys Alloying with Cr and Hf after Equal Channel Angular Pressing. AMR 2014, 922, 651–656. [Google Scholar] [CrossRef]
- Shangina, D.V.; Terent’ev, V.F.; Prosvirnin, D.V.; Antonova, O.V.; Bochvar, N.R.; Gorshenkov, M.V.; Raab, G.I.; Dobatkin, S.V. Mechanical Properties, Fatigue Life, and Electrical Conductivity of Cu-Cr-Hf Alloy after Equal Channel Angular Pressing. Adv. Eng. Mater. 2018, 20, 1700536. [Google Scholar] [CrossRef]
- Dobatkin, S.V.; Shangina, D.V.; Bochvar, N.R.; Terent’ev V., F.; Prosvirnin, D.V.; Putinceva, M.N.; Purcek, G.; Yanar, H.; Alsaran, A.; Raab, G.I. Enhanced mechanical and service properties of ultrafinegrained copper-based alloys with Cu, Zr and Hf additives. Mater. Sci. Non-Equilib. Phase Transform. 2017, 3, 3–5. [Google Scholar]
- Reinbach, R. Hafnium als Legierungselement in Kupfer, Eisen und Nickel. Z. Met. 1960, 51, 292–293. [Google Scholar] [CrossRef]
- Birol, Y. DSC analysis of the precipitation reaction in AA6005 alloy. J. Therm. Anal. Calorim. 2008, 93, 977–981. [Google Scholar] [CrossRef]
- Lang, P.; Povoden-Karadeniz, E.; Falahati, A.; Kozeschnik, E. Simulation of the effect of composition on the precipitation in 6xxx Al alloys during continuous-heating DSC. J. Alloys Compd. 2014, 612, 443–449. [Google Scholar] [CrossRef]
- Bourezg, Y.I.; Abib, K.; Azzeddine, H.; Bradai, D. Kinetics of Cr clustering in a Cu-Cr-Zr alloy processed by equal-channel angular pressing: A DSC study. Thermochim. Acta 2020, 686, 178550. [Google Scholar] [CrossRef]
- Kočiško, R.; Kvačkaj, T.; Kováčová, A.; Šimčák, D.; Bidulský, R.; Lupták, M.; Vlado, M.; Pokorný, I. The mechanical properties of OFHC copper and CuCrZr alloys after asymmetric rolling at ambient and cryogenic temperatures. Open Eng. 2018, 8, 426–431. [Google Scholar] [CrossRef]
- Abib, K.; Larbi, F.H.; Rabahi, L.; Alili, B.; Bradai, D. DSC analysis of commercial Cu–Cr–Zr alloy processed by equal channel angular pressing. Trans. Nonferrous Met. Soc. China 2015, 25, 838–843. [Google Scholar] [CrossRef]
- Li, X.; Starink, M.J. Analysis of Precipitation and Dissolution in Overaged 7xxx Aluminium Alloys Using DSC. Mater. Sci. Forum. 2000, 2000, 1071–1076. [Google Scholar] [CrossRef]
- Lomakin, I.; Castillo-Rodríguez, M.; Sauvage, X. Microstructure, mechanical properties and aging behaviour of nanocrystalline copper–beryllium alloy. Mater. Sci. Eng. A 2019, 744, 206–214. [Google Scholar] [CrossRef]
- Pfeffer, K.; Eisenbart, M.; Klotz, U.E.; Preußner, J.; Weber, M.; Helm, D. Charakterisierung des Ausscheidungszustandes von Cu-Ni-Si-Legierungen nach unterschiedlichen Wärmebehandlungen. Metall 2013, 67, 500–504. [Google Scholar]
- Azzeddine, H.; Bourezg, Y.I.; Khereddine, A.Y.; Baudin, T.; Helbert, A.-L.; Brisset, F.; Kawasaki, M.; Bradai, D.; Langdon, T.G. An investigation of the stored energy and thermal stability in a Cu–Ni–Si alloy processed by high-pressure torsion. Philos. Mag. 2020, 100, 688–712. [Google Scholar] [CrossRef]
- Hayoune, A.; Titouche, N. A DSC Investigation of the Effects of Cold Deformation and Low Temperatures Aging on the Microstructural Stability of a Peak Aged (PA) Al 6061 Alloy. AMM 2013, 432, 32–38. [Google Scholar] [CrossRef]
- Bever, M.B.; Holt, D.L.; Titchener, A.L. The stored energy of cold work. Prog. Mater. Sci. 1973, 17, 5–177. [Google Scholar] [CrossRef]
- Kodetová, V.; Vlach, M.; Čížek, J.; Cieslar, M.; Bajtošová, L.; Kudrnová, H.; Leibner, M.; Šíma, V. Early Stages of Precipitation in Mould-Cast, Cold-Rolled and Heat-Treated Aluminium Alloy AA7075 with Sc,Zr-Addition. Acta Phys. Pol. A 2020, 137, 250–254. [Google Scholar] [CrossRef]
- Quainoo, G.K.; Yannacopoulos, S. The effect of cold work on the precipitation kinetics of AA6111 aluminum. J. Mater. Sci. 2004, 39, 6495–6502. [Google Scholar] [CrossRef]
- Long, D.C.; Ohmori, Y.; Nakai, K. Effects of Cold Rolling on the Aging Kinetics in an Al–Mg–Si Based Commercial Alloy. Mater. Trans. JIM 2000, 41, 690–695. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, L.; Jia, Y.; Liu, L.; Zhang, X. Precipitation kinetics of 2519A aluminum alloy based on aging curves and DSC analysis. Trans. Nonferrous Met. Soc. China 2014, 24, 3076–3083. [Google Scholar] [CrossRef]
- Naimi, A.; Yousfi, H.; Trari, M. Influence of cold rolling degree and ageing treatments on the precipitation hardening of 2024 and 7075 alloys. Mech. Time Depend. Mater. 2013, 17, 285–296. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayaganthan, R.; Pancholi, V.; Gupta, M. A DSC study on the precipitation kinetics of cryorolled Al 6063 alloy. Mater. Chem. Phys. 2010, 122, 188–193. [Google Scholar] [CrossRef]
- Blessto, B.; Sivaprasad, K.; Muthupandi, V.; Arumugam, M. DSC analysis on AA2219 plates processed by cryorolling and coldrolling. Mater. Res. Express 2019, 6, 1065c9. [Google Scholar] [CrossRef]
- Dölling, J.; Zilly, A. Niedriglegierte festigkeitsoptimierte Kupferbasislegierungen mit hohen Leitfähigkeitseigenschaften: Untersuchung des Potentials binärer CuSc-Legierungen. Metall 2021, 75, 328–331. [Google Scholar]
- Bauch, J.; Rosenkranz, R. Physikalische Werkstoffdiagnostik: Ein Kompendium Wichtiger Analytikmethoden für Ingenieure und Physiker; Springer Vieweg: Berlin, Germany, 2017; ISBN 978-3-662-53951-4. [Google Scholar]
- Vončina, M.; Medved, J.; Kores, S.; Xie, P.; Schumacher, P.; Li, J. Precipitation microstructure in Al-Si-Mg-Mn alloy with Zr additions. Mater. Charact. 2019, 155, 109820. [Google Scholar] [CrossRef]
- Heugue, P.; Larouche, D.; Breton, F.; Massinon, D.; Martinez, R.; Chen, X.-G. Precipitation Kinetics and Evaluation of the Interfacial Mobility of Precipitates in an AlSi7Cu3.5Mg0.15 Cast Alloy with Zr and V Additions. Metals 2019, 9, 777. [Google Scholar] [CrossRef]
- Funamizu, Y.; Watanabe, K. Interdiffusion in the Al–Cu System. Trans. JIM 1971, 12, 147–152. [Google Scholar] [CrossRef]
- Barrena, M.I.; Gómez de Salazar, J.M.; Pascual, L.; Soria, A. Determination of the kinetic parameters in magnesium alloy using TEM and DSC techniques. J. Therm. Anal. Calorim. 2013, 113, 713–720. [Google Scholar] [CrossRef]
- Petzow, G. Metallographisches, Keramographisches, Plastographisches Ätzen, 7th ed.; Gebrüder Borntraeger: Stuttgart, Germany, 2015; ISBN 9783443230197. [Google Scholar]
- Russell, K.C. Nucleation in solids: The induction and steady state effects. Adv. Colloid Interface Sci. 1980, 13, 205–318. [Google Scholar] [CrossRef]
- Perez, M.; Dumont, M.; Acevedo-Reyes, D. Implementation of classical nucleation and growth theories for precipitation. Acta Mater. 2008, 56, 2119–2132. [Google Scholar] [CrossRef]
- Dalan, F.C.; de Lima Andreani, G.F.; Travessa, D.N.; Faizova, S.; Fiazov, I.A.; Cardoso, K.R. Effect of ECAP Processing on Hardness, Electrical Conductivity, and Precipitation Kinetics of the Cu-0.81Cr-0.07Zr Alloy. J. Electron. Mater. 2021, 50, 6171–6182. [Google Scholar] [CrossRef]
- Milkereit, B.; Starink, M.J.; Rometsch, P.A.; Schick, C.; Kessler, O. Review of the Quench Sensitivity of Aluminium Alloys: Analysis of the Kinetics and Nature of Quench-Induced Precipitation. Materials 2019, 12, 4083. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.D. Modelling the evolution of particle size distribution during nucleation, growth and coarsening. J. Mater. Sci. Technol. 2004, 20, 441–448. [Google Scholar] [CrossRef]
- Paul, A.; Divinski, S.V. (Eds.) Handbook of Solid State Diffusion: Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128042878. [Google Scholar]
- Watanabe, H.; Miyamoto, K.; Kunimine, T.; Monzen, R.; Muramatsu, N.; Nomura, K.; Ueno, S. Effect of Thermo-Mechanical Treatment on Electrical Conductivity and Strength of Cu–0.29 mass%Zr Alloy Wires. Mater. Trans. 2021, 62, 1710–1715. [Google Scholar] [CrossRef]
- Peng, L.; Xie, H.; Huang, G.; Li, Y.; Yin, X.; Feng, X.; Mi, X.; Yang, Z. The phase transformation and its effects on properties of a Cu−0.12 wt% Zr alloy. Mater. Sci. Eng. A 2015, 633, 28–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, R.; Peng, C.; Feng, Y.; Wang, X.; Wu, X.; Cai, Z. Effect of elevated-temperature annealing on microstructureand properties of Cu−0.15Zr alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3772–3784. [Google Scholar] [CrossRef]
- Nakashima, K.; Miyamoto, K.; Kunimine, T.; Monzen, R.; Muramatsu, N. Precipitation behavior of Cu–Zr compounds in a Cu-0.13 wt%Zr alloy. J. Alloys Compd. 2020, 816, 152650. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dölling, J.; Kracun, S.F.; Prahl, U.; Fehlbier, M.; Zilly, A. A Comparative Differential Scanning Calorimetry Study of Precipitation Hardenable Copper-Based Alloys with Optimized Strength and High Conductivity. Metals 2023, 13, 150. https://doi.org/10.3390/met13010150
Dölling J, Kracun SF, Prahl U, Fehlbier M, Zilly A. A Comparative Differential Scanning Calorimetry Study of Precipitation Hardenable Copper-Based Alloys with Optimized Strength and High Conductivity. Metals. 2023; 13(1):150. https://doi.org/10.3390/met13010150
Chicago/Turabian StyleDölling, Julia, Stefanie Felicia Kracun, Ulrich Prahl, Martin Fehlbier, and Andreas Zilly. 2023. "A Comparative Differential Scanning Calorimetry Study of Precipitation Hardenable Copper-Based Alloys with Optimized Strength and High Conductivity" Metals 13, no. 1: 150. https://doi.org/10.3390/met13010150
APA StyleDölling, J., Kracun, S. F., Prahl, U., Fehlbier, M., & Zilly, A. (2023). A Comparative Differential Scanning Calorimetry Study of Precipitation Hardenable Copper-Based Alloys with Optimized Strength and High Conductivity. Metals, 13(1), 150. https://doi.org/10.3390/met13010150








