Microstructure Evolution and Precipitation Behavior in Nb and Nb-Mo Microalloyed Fire-Resistant Steels
Abstract
:1. Introduction
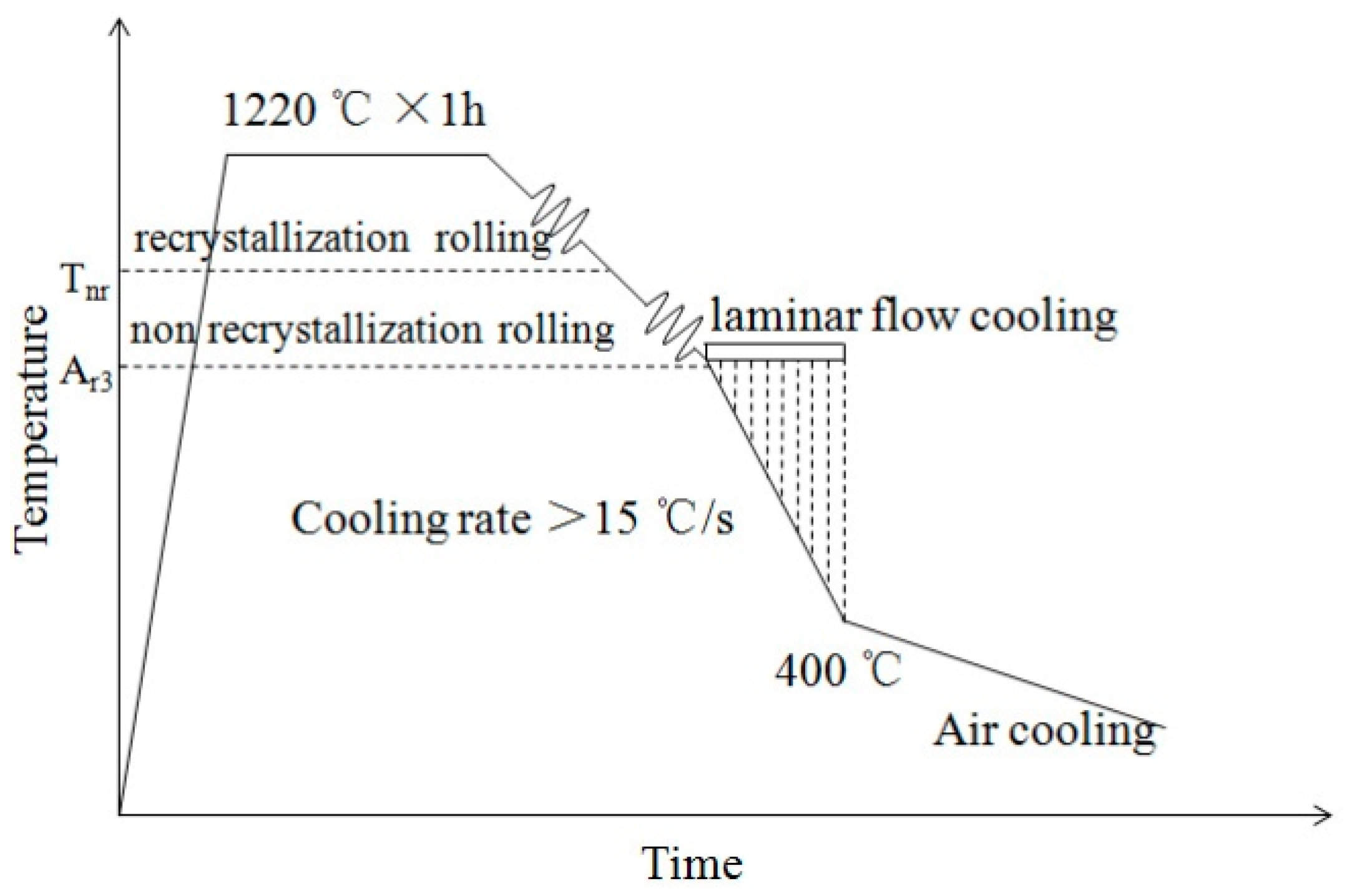
2. Materials and Methods
3. Results and Discussion
3.1. Mechanical Property
3.2. Microstructural Evolution
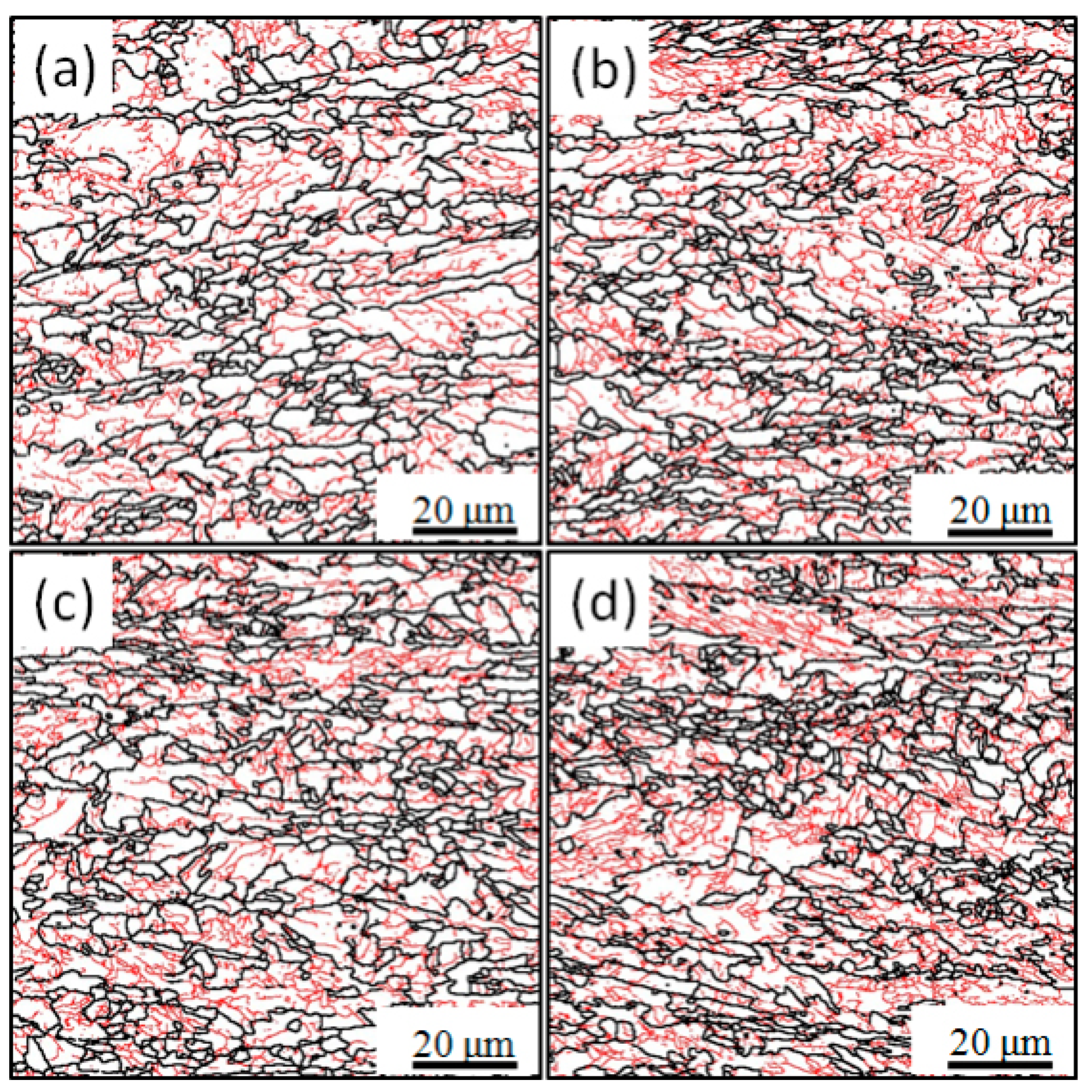
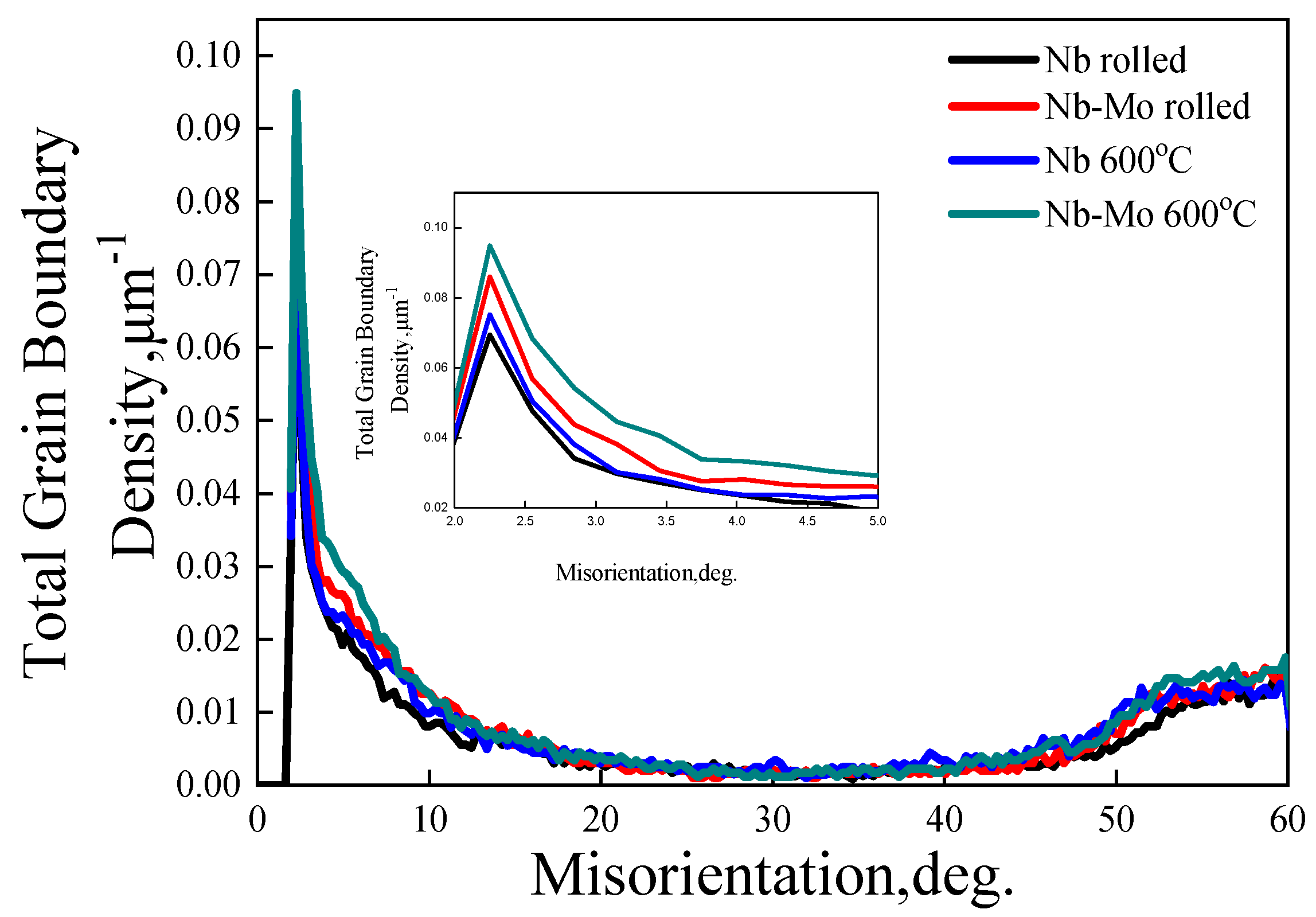
3.3. Grain Boundaries Misorientation
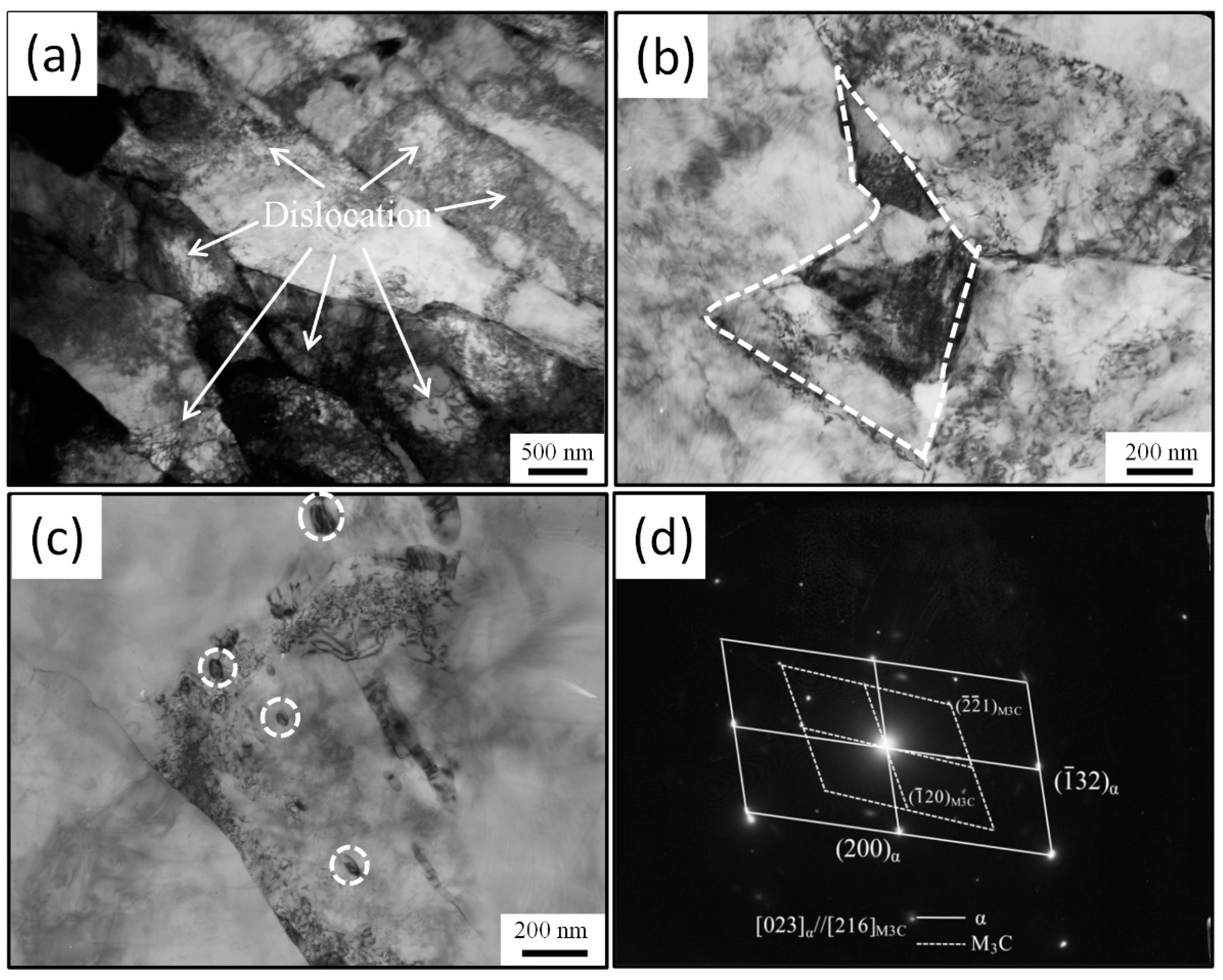
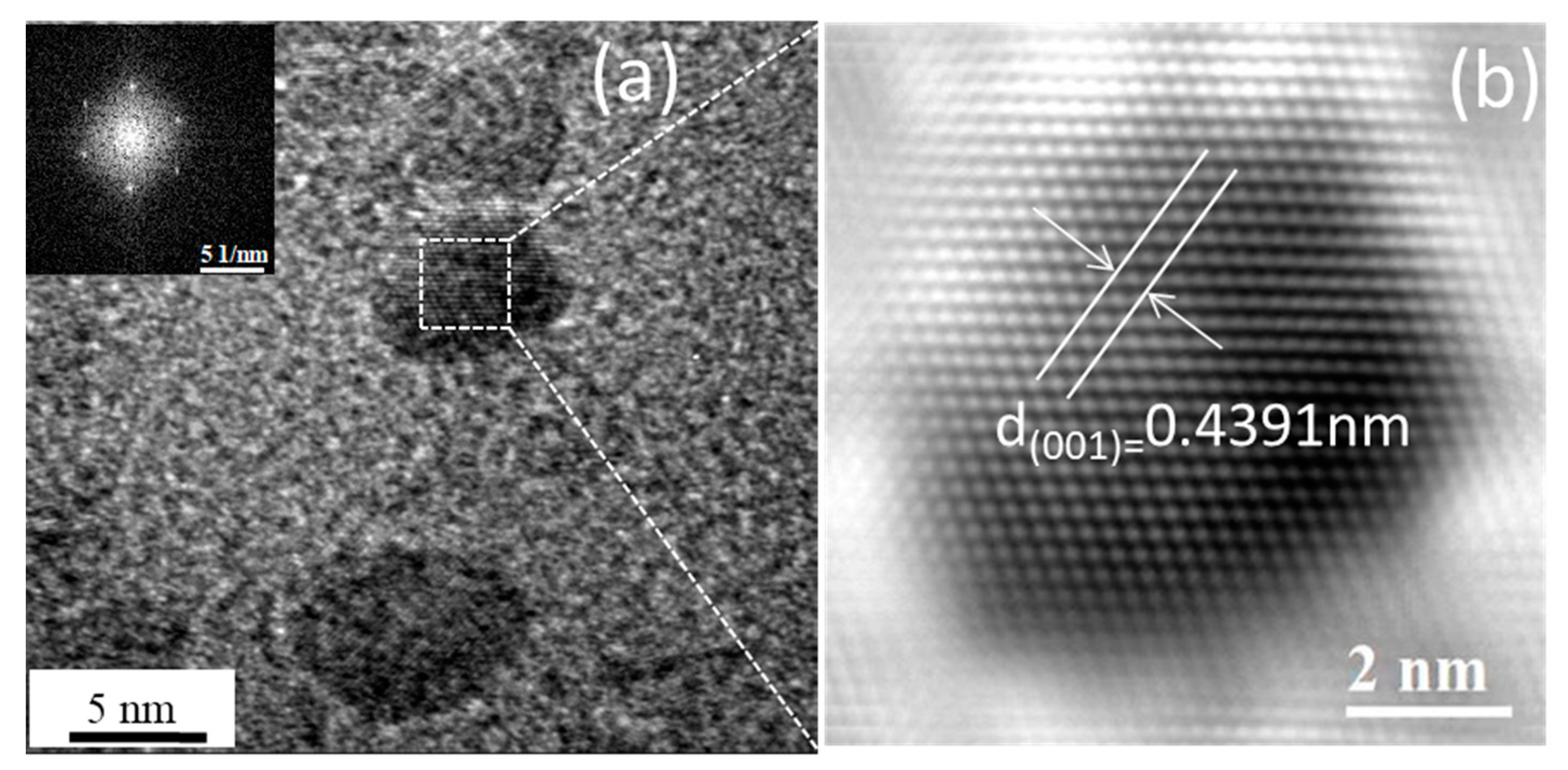
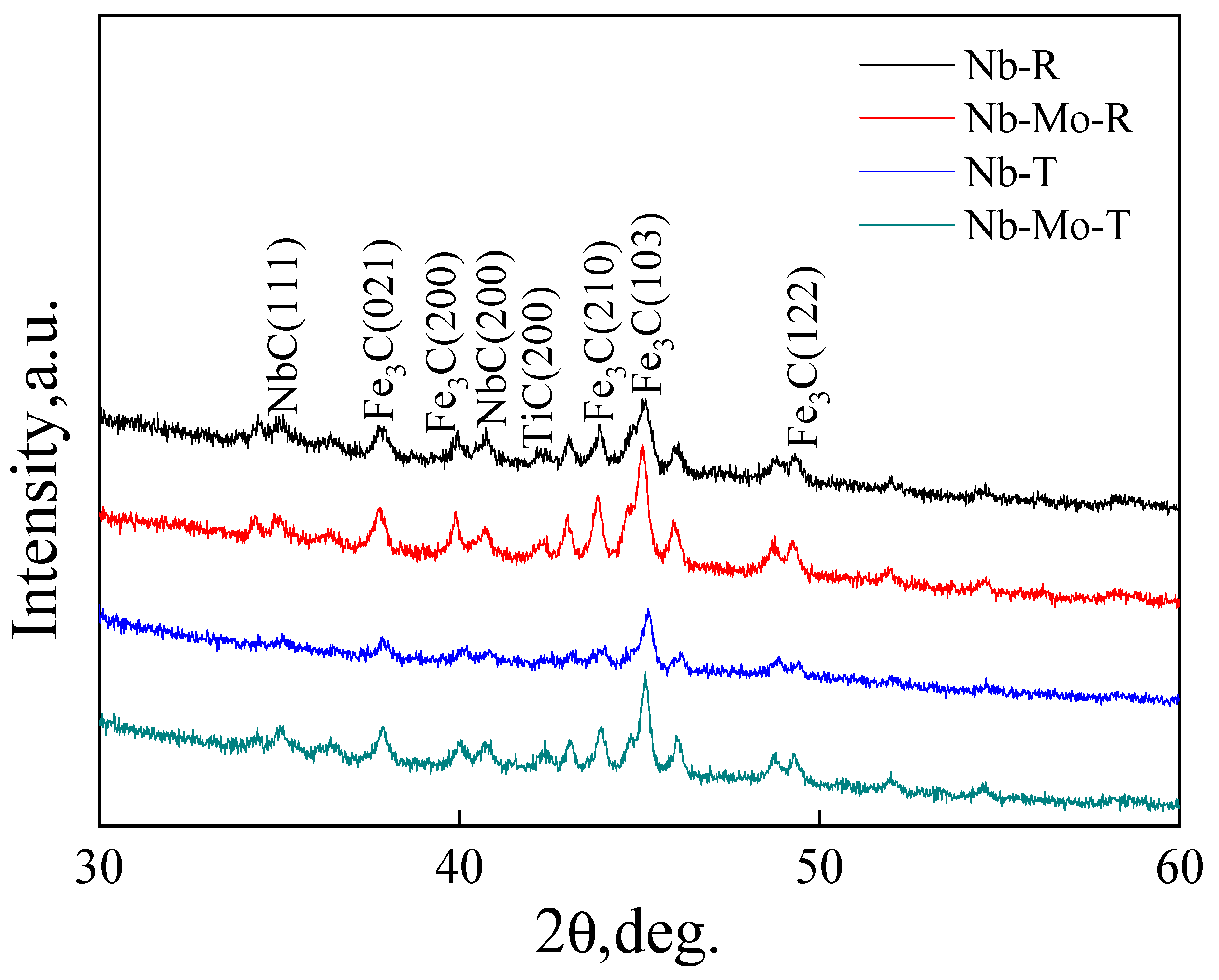
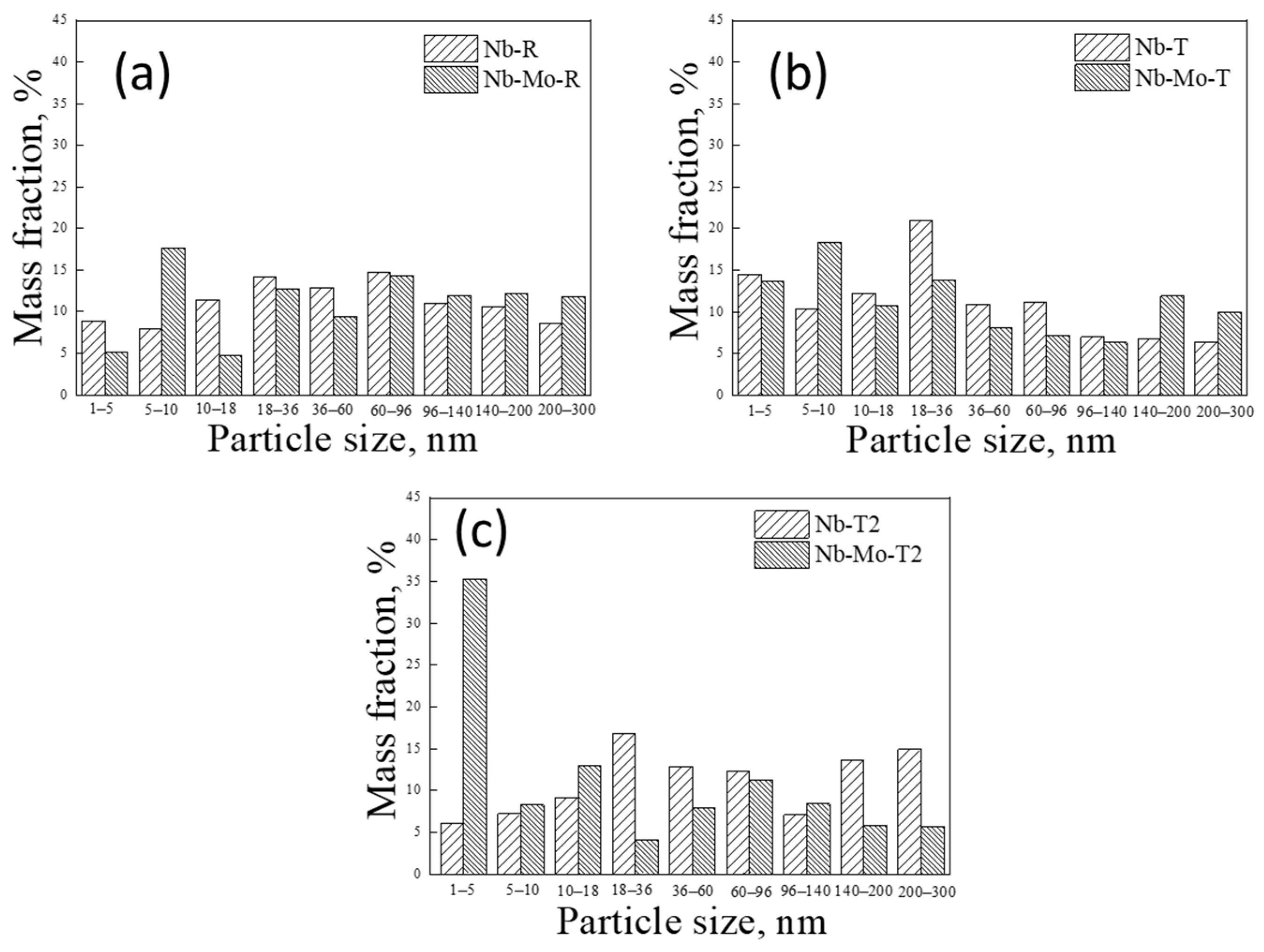
3.4. Precipitation of MC-Type Carbide
4. Conclusions
- Martensite and austenite (M/A) islands in Nb and Nb-Mo microalloyed fire-resistant steel disappear after elevated temperature tension, and cementite and nanometer-sized carbide precipitated.
- The precipitation amount of Nb is nearly equal for the two steels in rolled state; it increased in Nb-Mo steel more obviously than that in Nb steel after tempering treatment at 600 °C, indicating that Mo promotes the precipitation of Nb.
- The controlled element of diffusion during the coursing stage of the (Nb, Mo)C particle can be determined as the Nb atom in Nb-Mo steel, and the amount of dissolved Nb was reduced, which results in decreased coarsening kinetics of (Nb, Mo)C in Nb-Mo steel as compared with that of NbC in Nb steel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yong, Q.L. Microalloyed Steels-Physical and Mechanical Metallurgy, Metall; Industry Press: Beijing, China, 1989. [Google Scholar]
- Xiong, Z.P.; Timokhina, I.; Pereloma, E. Clustering, nano-scale precipitation and strengthening of steels. Prog. Mater. Sci. 2021, 118, 100764. [Google Scholar] [CrossRef]
- Yoo, J.; Jo, M.C.; Bian, J.B.; Sohn, S.S.; Lee, S. Effects of Nb or (Nb + Mo) alloying on Charpy impact, bending, and delayed fracture properties in 1.9-GPa-grade press hardening steels. Mater. Charact. 2021, 176, 111133. [Google Scholar] [CrossRef]
- Mao, X.P.; Huo, X.D.; Sun, X.J. Strengthening mechanisms of a new 700MPa hot rolled Ti-microalloyed steel produced by compact strip production. J. Mater. Proc. Tech. 2010, 210, 1660–1666. [Google Scholar] [CrossRef]
- Wu, W.; Cai, M.; Zhang, Z.; Tian, W.; Pan, H. Elevated Temperature Tensile Behavior of a Nb-Mo Microalloyed Medium Mn Alloy under Quasistatic Loads. Metals 2022, 12, 442. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Huo, D.S.; Zhou, Y.Y.; Sui, G.Y.; Jiang, F.C. Effect of Tungsten Addition on Continuous Cooling Transformation and Precipitation Behavior of a High Titanium Microalloyed Steel. Metals 2022, 12, 1649. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Yong, Q.L.; Sun, X.J. An analytical model for the kinetics of strain-induced precipitation in titanium micro-alloyed steels. ISIJ Int. 2012, 52, 1661–1669. [Google Scholar] [CrossRef] [Green Version]
- Claesson, E.; Magnusson, H.; Kohlbrecher, J.; Thuvander, M.; Lindberg, F.; Andersson, M.; Hedström, P. Carbide Precipitation during Processing of Two Low-Alloyed Martensitic Tool Steels with 0.11 and 0.17 V/Mo Ratios Studied by Neutron Scattering, Electron Microscopy and Atom Probe. Metals 2022, 12, 758. [Google Scholar] [CrossRef]
- Park, D.B.; Huh, M.Y.; Shim, J.H.; Suh, J.Y.; Lee, K.H.; Jung, W.S. Strengthening mechanism of hot rolled Ti and Nb microalloyed HSLA steels containing Mo and W with various coiling temperature. Mater. Sci. Eng. A 2013, 560, 528–534. [Google Scholar] [CrossRef]
- Liu, C.Q.; Xiong, F.; Wang, Y.; Cao, Y.X.; Liu, X.B.; Xue, Z.L.; Peng, Q.C.; Peng, L.S. Strengthening mechanism and carbide precipitation behavior of Nb-Mo microalloy medium Mn steel. Metals 2021, 14, 7461. [Google Scholar] [CrossRef]
- Kim, Y.W.; Song, S.W.; Seo, S.J.; Hong, S.J.; Lee, C.S. Development of Ti and Mo micro-alloyed hot-rolled high strength sheet steel by controlling thermomechanical controlled processing schedule. Mater. Sci. Eng. A 2013, 565, 430–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Zhang, J.; Du, Y.; Wang, X.; Wu, H.; Gao, X.H.; Du, L.X. Microstructure, Mechanical Properties, and Fish-Scaling Resistance of a Ti-Nb Microalloyed Hot-Rolled Enamel Steel. Metals 2022, 12, 1970. [Google Scholar] [CrossRef]
- Huang, H.H.; Yang, G.W.; Zhao, G.; Mao, X.P.; Gan, X.L.; Yin, Q.L.; Yi, H. Effect of Nb on the microstructure and properties of Ti-Mo microalloyed high-strength ferritic Steel. Mater. Sci. Eng. A 2018, 736, 148–155. [Google Scholar] [CrossRef]
- Yu, Q.B.; Wang, Z.D.; Liu, X.H.; Wang, G.D. Effect of microcontent Nb in solution on the strength of low carbon steels. Mater. Sci. Eng. A 2004, 379, 384–390. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hui, W.J.; Chen, Z.; Zhang, Y.J.; Zhao, X.L. Effect of vanadium on microstructure and mechanical properties of bainitic forging steel. Mater. Sci. Eng. A 2020, 771, 138653. [Google Scholar] [CrossRef]
- Fang, F.; Hu, X.; Zhou, L.; Jiang, J. Effect of vanadium on microstructure and property of pearlitic steel wire. Mater. Res. Innov. 2015, 19, 394–396. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yang, J.R.; Chen, C.C.; Chen, S.F. Microstructural characterization and strengthening behavior of nanometer sized carbides in Ti-Mo microalloyed steels during continuous cooling process. Mater. Charact. 2016, 114, 18–29. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phases in Steels, Metall; Industry Press: Beijing, China, 2006. [Google Scholar]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of High Strength Hot-rolled Sheet Steel Consisting of Ferrite and Nanometer-sized Carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Shimizu, T.; Funakawa, Y.; Kaneko, S. High Strength Steel Sheets for Automobile Suspension and Chassis Use-High Strength Hot-Rolled Steel Sheets with Excellent Press Formability and Durability for Critical Safety Parts. JFE Tech. Rep. 2004, 4, 25–31. [Google Scholar]
- Hu, B.H.; Cai, Q.W.; Wu, H.B. Influence of Mo on Growth and Coarsening of Nanometer-sized Carbides in Low-alloy Ferritic Steels Containing Ti. J. Iron Steel Res. Int. 2014, 21, 878–885. [Google Scholar] [CrossRef]
- Timokhina, I.; Miller, M.K.; Wang, J.T. On the Ti-Mo-Fe-C atomic clustering during interphase precipitation in the Ti-Mo steel studied by advanced microscopic techniques. Mater. Des. 2016, 111, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Chen, H.; Khorasgani, A.R.; Zhang, B.; Zhang, Y.J.; Wang, Z.Q.; Zhou, X.S.; Wang, W.; Wang, H.R.; Li, T.; et al. Revealing the influence of Mo addition on interphase precipitation in Ti-bearing low carbon steels. Acta Mater. 2022, 223, 117475. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Clark, S.J.; Cai, B.; Venero, D.A.; Yan, K.; Gorley, M.; Surrey, E.; McCartney, D.G.; Sridhar, S.; Lee, P.D. Small-angle neutron scattering reveals the effect of Mo on interphase nano-precipitation in Ti-Mo micro-alloyed steels. Scr. Mater. 2020, 174, 24–28. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Z.D.; Yong, Q.L.; Sun, X.J.; Wang, Z.Q.; Wang, G.D. Precipitation behavior of carbide during heating process in Nb and Nb-Mo microalloyed steels. Acta Metall. Sin. 2015, 51, 315–324. [Google Scholar]
- Jang, J.H.; Lee, C.H.; Heo, Y.U. Stability of (Ti, M)C (M = Nb, V, Mo and W) carbide in steels using first-principles calculations. Acta Mater. 2012, 60, 208–217. [Google Scholar] [CrossRef]
- Lee, W.B.; Hong, S.G.; Park, C.G.; Kim, K.H.; Park, S.H. Influence of Mo on precipitation hardening in hot rolled HSLA steels containing Nb. Scr. Mater. 2000, 43, 319–324. [Google Scholar] [CrossRef]
- Uemori, R.; Chijiiwa, R.; Tamehiro, H.; Morikawa, H. AP-FIM study on the effect of Mo addition on microstructure in Ti-Nb steel. Appl. Surf. Sci. 1994, 76, 255–260. [Google Scholar] [CrossRef]
- Enloe, C.M.; Findley, K.O.; Parish, C.M.; Miller, M.K.; DeCooman, B.C.; Speer, J.G. Compositional evolution of microalloy carbonitrides in a Mo-bearing microalloyed steel. Scr. Mater. 2013, 68, 55–58. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, H.; Guo, C.H.; Liu, W.B.; Yang, Z.G.; Sun, X.J.; Zhang, Z.Y.; Jiang, F.C. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Chijiiwa, R.; Yoshida, Y.; Uemori, R.; Tamehiro, H.; Funato, K.; Horii, Y. Development and practical application of fire-resistant steel for buildings. Nippon Steel Tech. Rep. 1993, 58, 47–55. [Google Scholar]
- Zhang, Z.Y.; Sun, X.J.; Wang, Z.Q.; Li, Z.D.; Yong, Q.L.; Wang, G.D. Carbide precipitation in austenite of Nb-Mo-bearing low-carbon steel during stress relaxation. Mater. Lett. 2015, 159, 249–252. [Google Scholar] [CrossRef]
- Cao, Y.B.; Xiao, F.R.; Qiao, G.Y.; Huang, C.J.; Zhang, X.B.; Wu, Z.X.; Liao, B. Strain-induced precipitation and softening behaviors of high Nb microalloyed steels. Sci. Eng. A 2012, 552, 502–513. [Google Scholar] [CrossRef]


| Steel | C | Mn | P | S | Si | Mo | Ti | Nb | N | Al | B | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nb | 0.036 | 1.35 | 0.0034 | 0.0057 | 0.024 | - | 0.010 | 0.10 | ~0.004 | 0.012 | 0.0012 | Bal. |
| Nb-Mo | 0.042 | 1.38 | 0.0040 | 0.0060 | 0.016 | 0.19 | 0.015 | 0.10 | ~0.004 | 0.014 | 0.0010 | Bal. |
| Steels | Rm/MPa | Rp0.2/MPa | A/% | Rm (600 °C)/MPa | Rp0.2 (600 °C)/MPa | Rp0.2 (600 °C)/Rp0.2(RT) |
|---|---|---|---|---|---|---|
| Nb | 618 | 511 | 23 | 368 | 315 | 0.62 |
| Nb-Mo | 642 | 550 | 19 | 450 | 395 | 0.72 |
| Steels | Number of Elements in MC wt % | Precipitate Ratio of Nb and Mo, % | ||
|---|---|---|---|---|
| Nb | Mo | Nb | Mo | |
| Nb-R | 0.025 | - | 25 | - |
| Nb-Mo-R | 0.026 | 0.003 | 26 | 1.6 |
| Nb-T | 0.054 | - | 54 | - |
| Nb-Mo-T | 0.064 | 0.024 | 64 | 13 |
| Nb-T2 | 0.076 | - | 76 | - |
| Nb-Mo-T2 | 0.078 | 0.028 | 78 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, Z.; Li, Z.; Sun, X. Microstructure Evolution and Precipitation Behavior in Nb and Nb-Mo Microalloyed Fire-Resistant Steels. Metals 2023, 13, 112. https://doi.org/10.3390/met13010112
Zhang Z, Wang Z, Li Z, Sun X. Microstructure Evolution and Precipitation Behavior in Nb and Nb-Mo Microalloyed Fire-Resistant Steels. Metals. 2023; 13(1):112. https://doi.org/10.3390/met13010112
Chicago/Turabian StyleZhang, Zhengyan, Zhenqiang Wang, Zhaodong Li, and Xinjun Sun. 2023. "Microstructure Evolution and Precipitation Behavior in Nb and Nb-Mo Microalloyed Fire-Resistant Steels" Metals 13, no. 1: 112. https://doi.org/10.3390/met13010112
APA StyleZhang, Z., Wang, Z., Li, Z., & Sun, X. (2023). Microstructure Evolution and Precipitation Behavior in Nb and Nb-Mo Microalloyed Fire-Resistant Steels. Metals, 13(1), 112. https://doi.org/10.3390/met13010112






