Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal
Abstract
:1. Introduction
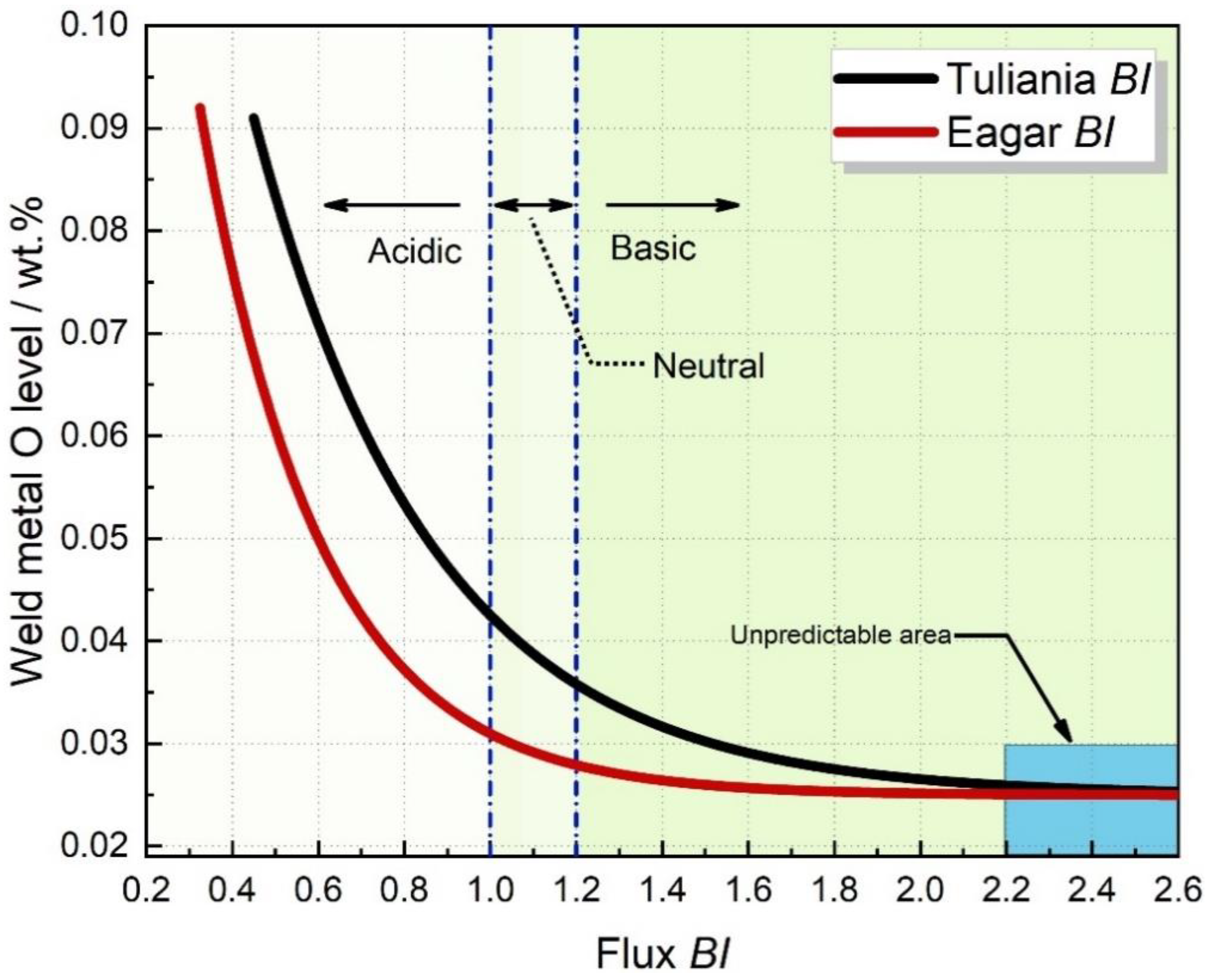
- Some basic oxides, such as FeO and MnO, possess high oxygen potentials, whereas CaO does not, indicating that BI may identify flux oxygen potential inaccurately.
- When BI is higher than 2.2, the predicted oxygen content will hold constant. In other words, the BI models would be invalid in differentiating flux oxygen potential when BI is higher than 2.5 (see the blue shaded area in Figure 1).
- It is still questionable whether CaF2 should be incorporated into the BI formula.
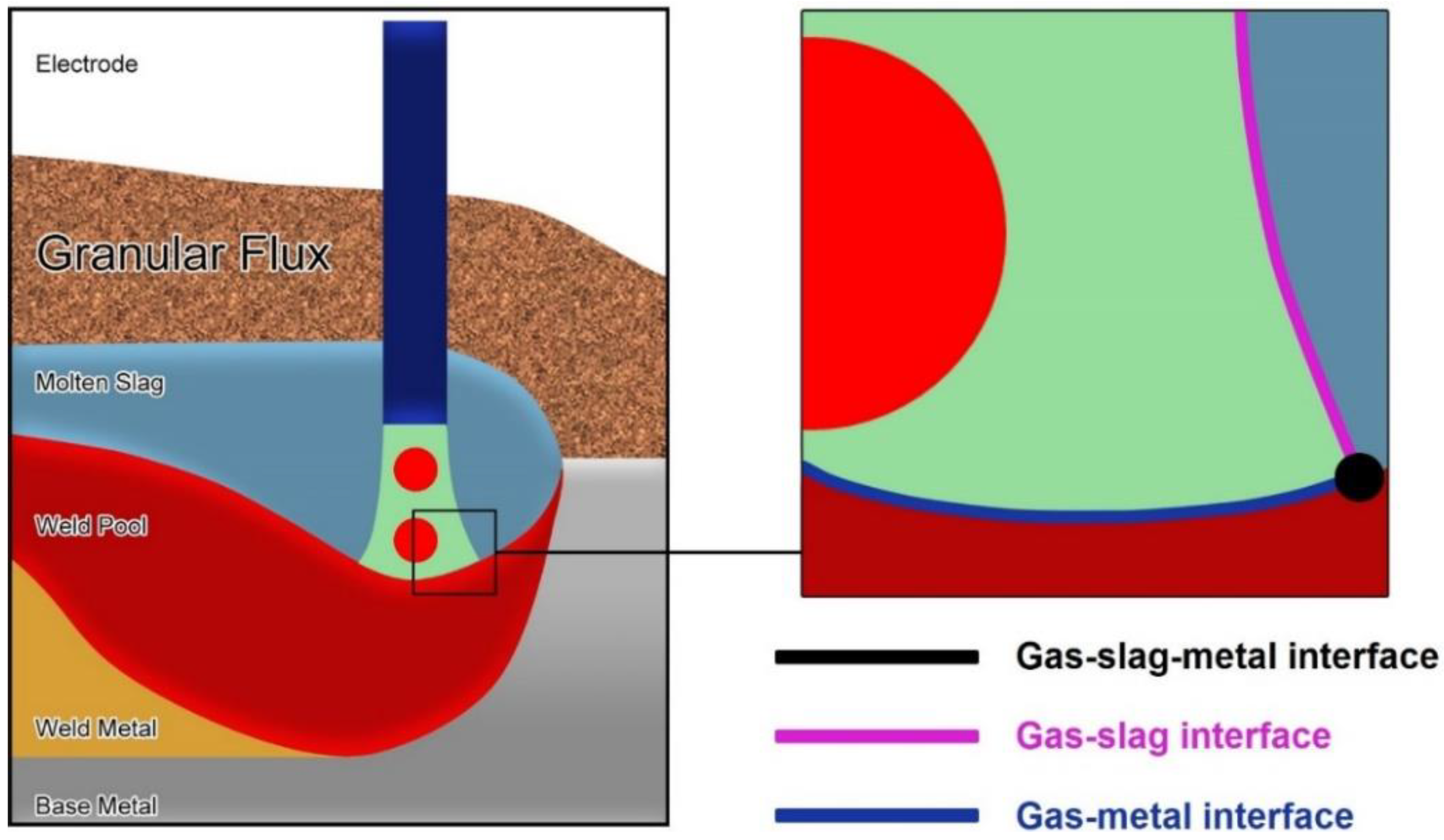
- Since the arc plasma, molten slag, and weld pool are shielded under the flux granular, it is impossible to capture the gases in the arc plasma or to sample the molten slag for analytical purposes.
- The temperature of chemical reactions in SAW is as high as 2000 °C, at which the thermodynamic data remain scarce.
2. Materials and Methods
- FToxid, Fstel, and FactPS databases were selected. Solution phases of ASlag-liq all oxides, S (FToxid-SLAGA), and LIQUID (FStel-Liqu) were selected to model the molten slag and steel phases.
- The equilibrium temperature in SAW of 2000 °C was set.
- Nominal compositions, which refer to the contents considering only the dilution effects of the BM and electrode, were used as the input metal chemistries. Nominal compositions are referenced from the data source given in Table 1.
| Flux | CaF2 | CaO | SiO2 | MnO | FeO | Tuliani BI | Eagar BI | Flux Developers and Data Source |
|---|---|---|---|---|---|---|---|---|
| F-1-1 | 0 | 0 | 40 | 0 | 60 | 0.75 | 0.75 | FeO-SiO2-MnO Indacochea et al. [31] |
| F-1-2 | 0 | 0 | 40 | 10 | 50 | 0.75 | 0.75 | |
| F-1-3 | 0 | 0 | 40 | 20 | 40 | 0.75 | 0.75 | |
| F-1-4 | 0 | 0 | 40 | 30 | 30 | 0.75 | 0.75 | |
| F-1-5 | 0 | 0 | 40 | 40 | 20 | 0.75 | 0.75 | |
| F-1-6 | 0 | 0 | 40 | 50 | 10 | 0.75 | 0.75 | |
| F-2-1 | 0 | 35 | 40 | 25 | 0 | 0.938 | 0.938 | CaO-SiO2-MnO Burck et al. [32] |
| F-2-2 | 0 | 30 | 40 | 30 | 0 | 1.125 | 1.125 | |
| F-2-3 | 0 | 25 | 40 | 35 | 0 | 1.063 | 1.063 | |
| F-2-4 | 0 | 10 | 40 | 50 | 0 | 0.875 | 0.875 | |
| F-2-5 | 0 | 5 | 40 | 55 | 0 | 0.813 | 0.813 | |
| F-3-1 | 35 | 0 | 40 | 25 | 0 | 1.188 | 0.313 | CaF2-SiO2-MnO Burck et al. [32] |
| F-3-2 | 30 | 0 | 40 | 30 | 0 | 1.125 | 0.375 | |
| F-3-3 | 25 | 0 | 40 | 35 | 0 | 1.063 | 0.438 | |
| F-3-4 | 15 | 0 | 40 | 45 | 0 | 0.938 | 0.563 | |
| F-3-5 | 10 | 0 | 40 | 50 | 0 | 0.875 | 0.625 | |
| F-3-6 | 5 | 0 | 40 | 55 | 0 | 0.813 | 0.688 | |
| F-4-1 | 95 | 0 | 5 | 0 | 0 | 19.0 | - | CaF2-SiO2 Dallam et al. [33] |
| F-4-2 | 90 | 0 | 10 | 0 | 0 | 9.0 | - | |
| F-4-3 | 80 | 0 | 20 | 0 | 0 | 4.0 | - | |
| F-4-4 | 70 | 0 | 30 | 0 | 0 | 2.3 | - | |
| F-5-1 | 95 | 0 | 0 | 5 | 0 | - | - | CaF2-MnO Chai et al. [10] |
| F-5-2 | 85 | 0 | 0 | 15 | 0 | - | - | |
| F-5-3 | 78 | 0 | 0 | 22 | 0 | - | - | |
| F-5-4 | 70 | 0 | 0 | 30 | 0 | - | - |
3. Results and Discussion
3.1. Acidic Flux with Low BI (BI = 0.75)
3.2. Flux with Moderate BI (0.3 < BI < 1.2)
3.3. Flux with High BI (BI > 2.3)
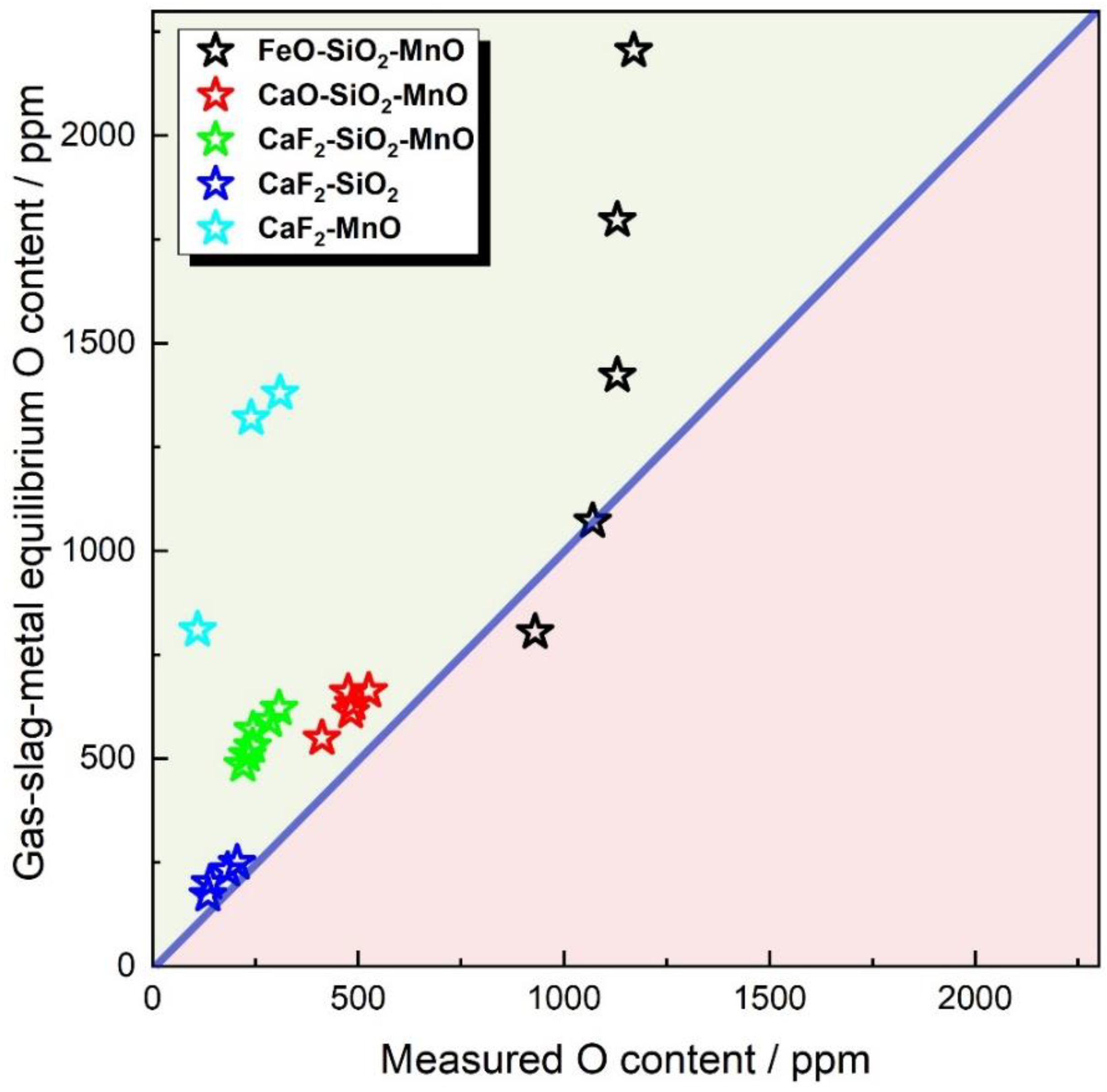
3.4. Bias between Equilibrium and Measured Oxygen Content
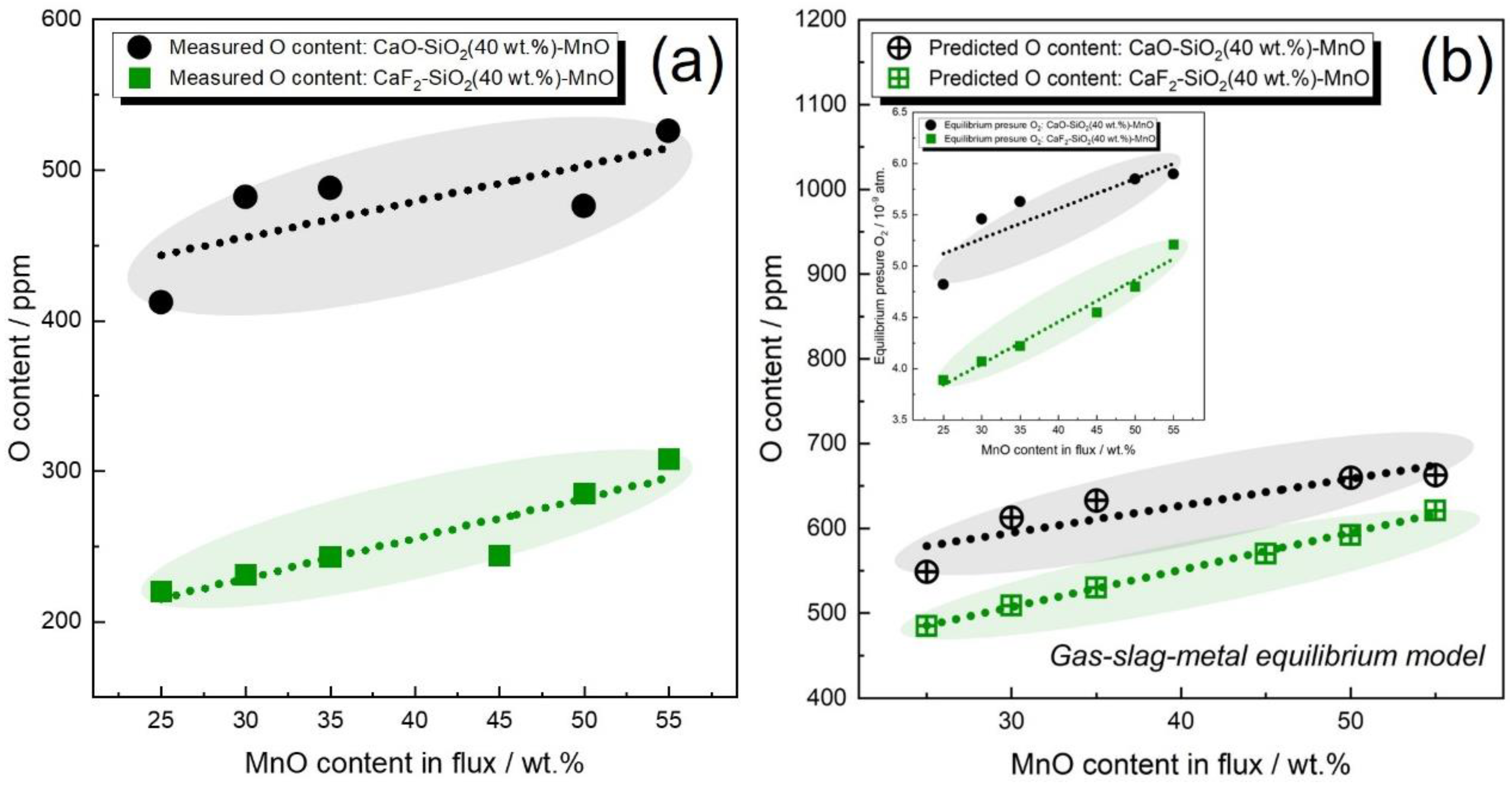
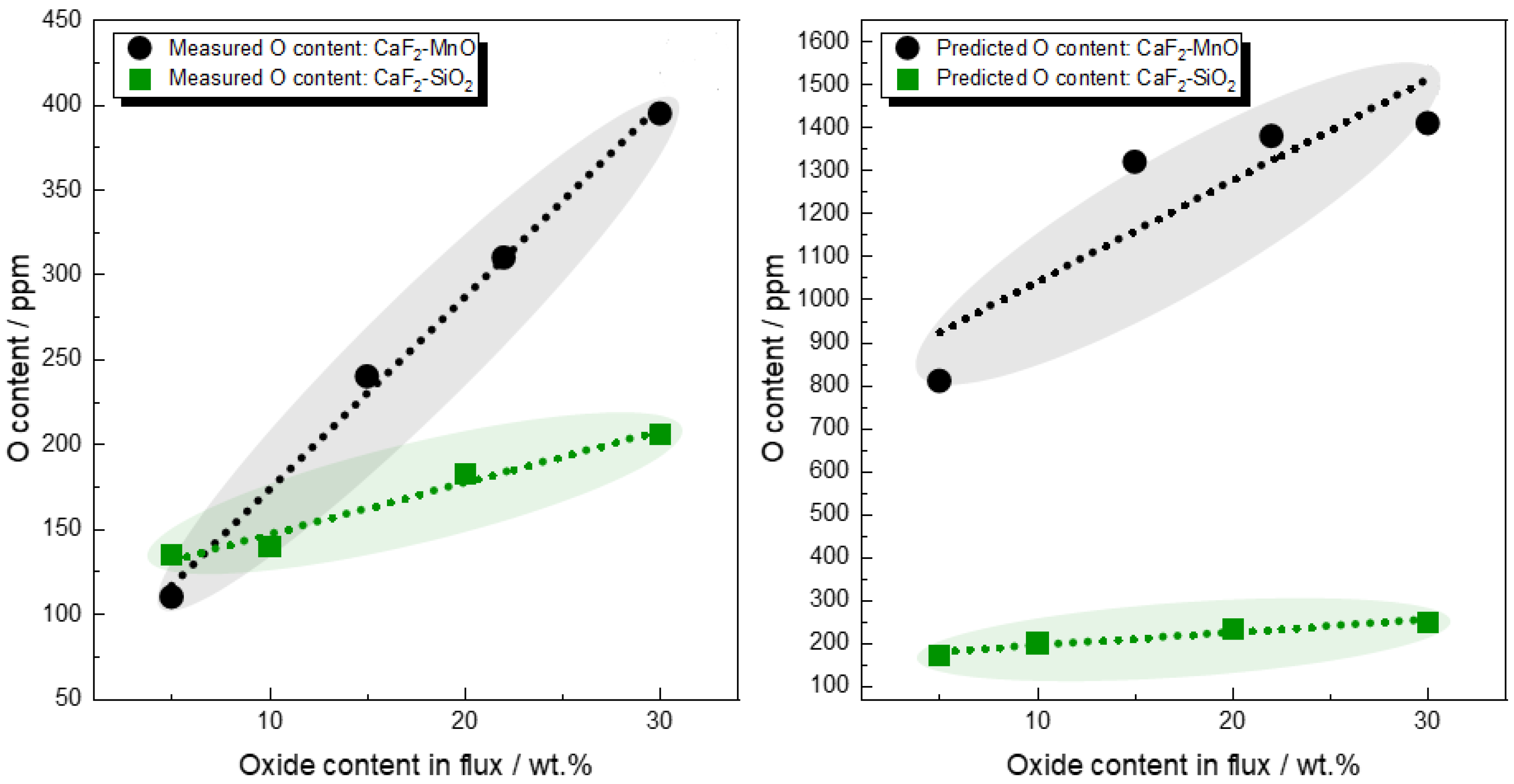
3.5. Role of CaF2 in the Determination of Flux O Potential
4. Conclusions
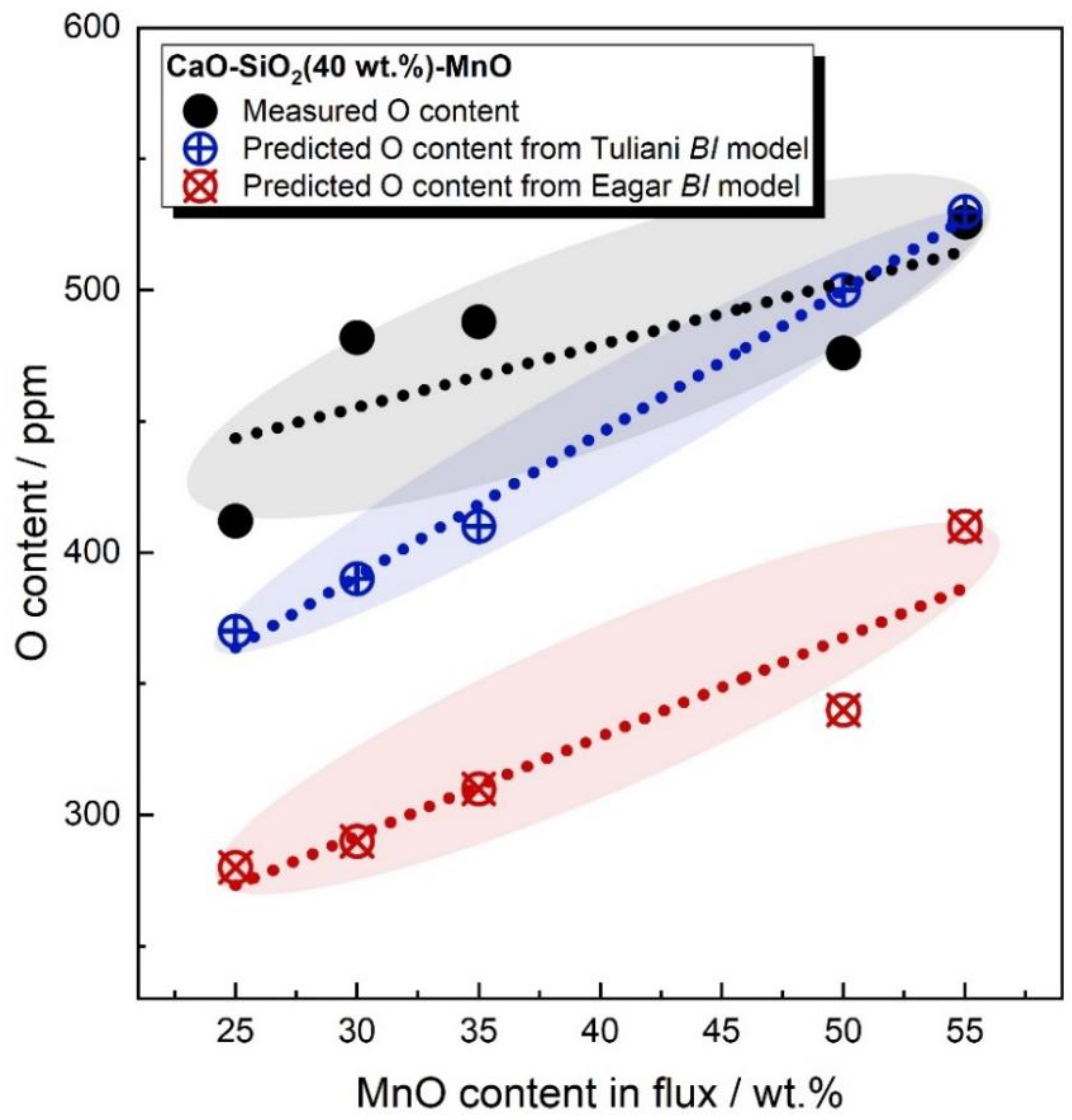
- For CaF2-free fluxes, both Tuliani and Eagar BI models fail to differentiate the oxygen potential of FeO and MnO. For CaF2-enriched fluxes, the Eagar BI model may incorrectly predict the changing trend of WM oxygen content as a function of the flux formula. Additionally, both Tuliani and Eagar BI models fail to differentiate the oxygen potential of SiO2 and MnO in CaF2-based binary fluxes;
- The gas–slag–metal equilibrium model can differentiate the oxygen potential of CaO, FeO, MnO, and SiO2. Additionally, the gas–slag–metal equilibrium model is capable of estimating the changing trend of WM oxygen content, even when BI models are inapplicable.
- In comparison to flux BI, the gas–slag–metal equilibrium oxygen and pO2 is more feasible in differentiating the oxygen potential for fluxes of different formulas.
- The measured data confirm that the overall equilibrium could not be attained in SAW, which may provide experimental evidence for assumptions subject to the non-thermodynamic-equilibrium status in SAW processes.
- The sole consideration of CaF2 being a dilutant rather than an active species may underestimate the flux oxygen potential.
Author Contributions
Funding
Conflicts of Interest
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding-A Review. Weld. J. 2019, 98, 283–313. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Coetsee, T.; Mostert, R.J.; Pistorius, P.G.H.; Pistorius, P.C. The Effect of Flux Chemistry on Element Transfer in Submerged Arc Welding: Application of Thermochemical Modelling. J. Mater. Res. Technol. 2021, 11, 2021–2036. [Google Scholar] [CrossRef]
- Coetsee, T. Phase Chemistry of Submerged Arc Welding (SAW) Fluoride Based Slags. J. Mater. Res. Technol. 2020, 9, 9766–9776. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Basu, S.; Wang, C. Impact of Gas Formation on the Transfer of Ti and O from TiO2-bearing Basic-fluoride Fluxes to Submerged Arc Welded Metals: A Thermodynamic Approach. Calphad 2020, 71, 102195. [Google Scholar] [CrossRef]
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Zhang, J.; Leng, J.; Wang, C. Tuning Weld Metal Mechanical Responses via Welding Flux Optimization of TiO2 Content: Application into EH36 Shipbuilding Steel. Metall. Mater. Trans. B 2019, 50, 2083–2087. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Elucidating the Roles of SiO2 and MnO upon Decarburization during Submerged Arc Welding: A Thermodynamic Study into EH36 Shipbuilding Steel. Metall. Mater. Trans. B 2020, 51, 1805–1812. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag Metal Reactions in Binary CaF2-Metal Oxide Welding Fluxes. Weld. J. 1982, 61, 229–232. [Google Scholar]
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding. ASM Int. ASM Handb. 1993, 6, 55–63. [Google Scholar] [CrossRef]
- Lao, Y.; Gao, Y.; Deng, F.; Wang, Q.; Li, G. Effects of Basicity and CaF2 on the Viscosity of CaF2-CaO-SiO2 Slag for Electroslag Remelting Process. Metall. Res. Technol. 2019, 116, 638. [Google Scholar] [CrossRef]
- Jindal, S.; Chhibber, R.; Mehta, N. Effect of Flux Constituents and Basicity Index on Mechanical Properties and Microstructural Evolution of Submerged Arc Welded high Strength Low Alloy Steel. Mater. Sci. Forum 2013, 738–739, 242–246. [Google Scholar] [CrossRef]
- Liby, A.; Dixon, R.; Olson, D. Welding: Theory and Practice; Elsevier Science Publishers: Amsterdam, The Netherlands, 1990; pp. 151–169. [Google Scholar]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Palm, J. How Fluxes Determine the Metallurgical Properties of Submerged Arc Welds. Weld. J. 1972, 51, 358. [Google Scholar]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions During Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Liu, H.; Wang, Z.; Wang, C. Addressing Weld Metal Compositional Variations in EH36 Shipbuilding Steel Processed by CaF2-SiO2-CaO-TiO2 Fluxes. Metall. Mater. Trans. B 2022, 53, 1329–1334. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Thermodynamic Evaluation of Element Transfer Behaviors for Fused CaO-SiO2-MnO Fluxes Subjected to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2021, 52, 1937–1944. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Jung, I.-H. Overview of the applications of thermodynamic databases to steelmaking processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Park, J.H. Solidification structure of CaO-SiO2-MgO-Al2O3(-CaF2) systems and computational phase equilibria: Crystallization of MgAl2O4 spinel. Calphad 2007, 31, 428–437. [Google Scholar] [CrossRef]
- Park, J.H. Formation of CaZrO3 at the interface between CaO-SiO2-MgO-CaF2(-ZrO2) slags and magnesia refractories: Computational and experimental study. Calphad 2007, 31, 149–154. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.; Petersen, S. FactSage Thermochemical Software and Databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Cong, W.; Zhang, J. Fine-tuning Weld Metal Compositions via Flux Optimization in Submerged Arc Welding: An Overview. Acta Metall. Sin. 2022, 57, 1126–1140. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Prediction of Weld-metal Composition during Flux-shielded Welding. J. Mater. Energy Syst. 1983, 5, 160–164. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Ssubmerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Assessment of Weld Metal Compositional Prediction Models Geared Towards Submerged Arc Welding: Case Studies Involving CaF2-SiO2-MnO and CaO-SiO2-MnO Fluxes. Mater. Trans. B 2021, 52, 2404–2415. [Google Scholar] [CrossRef]
- Indacochea, J.; Blander, M.; Shah, S. Submerged Arc Welding: Evidence for Electrochemical Effects on the Weld Pool. Weld. J. 1989, 68, 11–14. [Google Scholar]
- Burck, P.; Indacochea, J.; Olson, D. Effects of Welding Flux Additions on 4340 Steel Weld Metal Composition. Weld. J. 1990, 3, 115–122. [Google Scholar]
- Dallam, C.; Liu, S.; Olson, D. Flux Composition Dependence of Microstructure and Toughness of Submerged Arc HSLA Weldments. Weld. J. 1985, 64, 140–151. [Google Scholar]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2-MnO Fluxes under High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 885–890. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2 Fluxes Subject to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 16–21. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-TiO2 Fluxes in EH36 Shipbuilding Steel during High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 1953–1957. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530. https://doi.org/10.3390/met12091530
Shao G, Liu Z, Fan J, Guo Y, Xu Q, Zhang J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals. 2022; 12(9):1530. https://doi.org/10.3390/met12091530
Chicago/Turabian StyleShao, Guoyou, Zhongqiu Liu, Jun Fan, Yan Guo, Qiong Xu, and Jin Zhang. 2022. "Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal" Metals 12, no. 9: 1530. https://doi.org/10.3390/met12091530
APA StyleShao, G., Liu, Z., Fan, J., Guo, Y., Xu, Q., & Zhang, J. (2022). Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals, 12(9), 1530. https://doi.org/10.3390/met12091530






