The Mechanical Performance of Aluminum Foam Fabricated by Melt Processing with Different Foaming Agents: A Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.2. Structural Characterization
2.3. Mechanical Testing
3. Results and Discussion
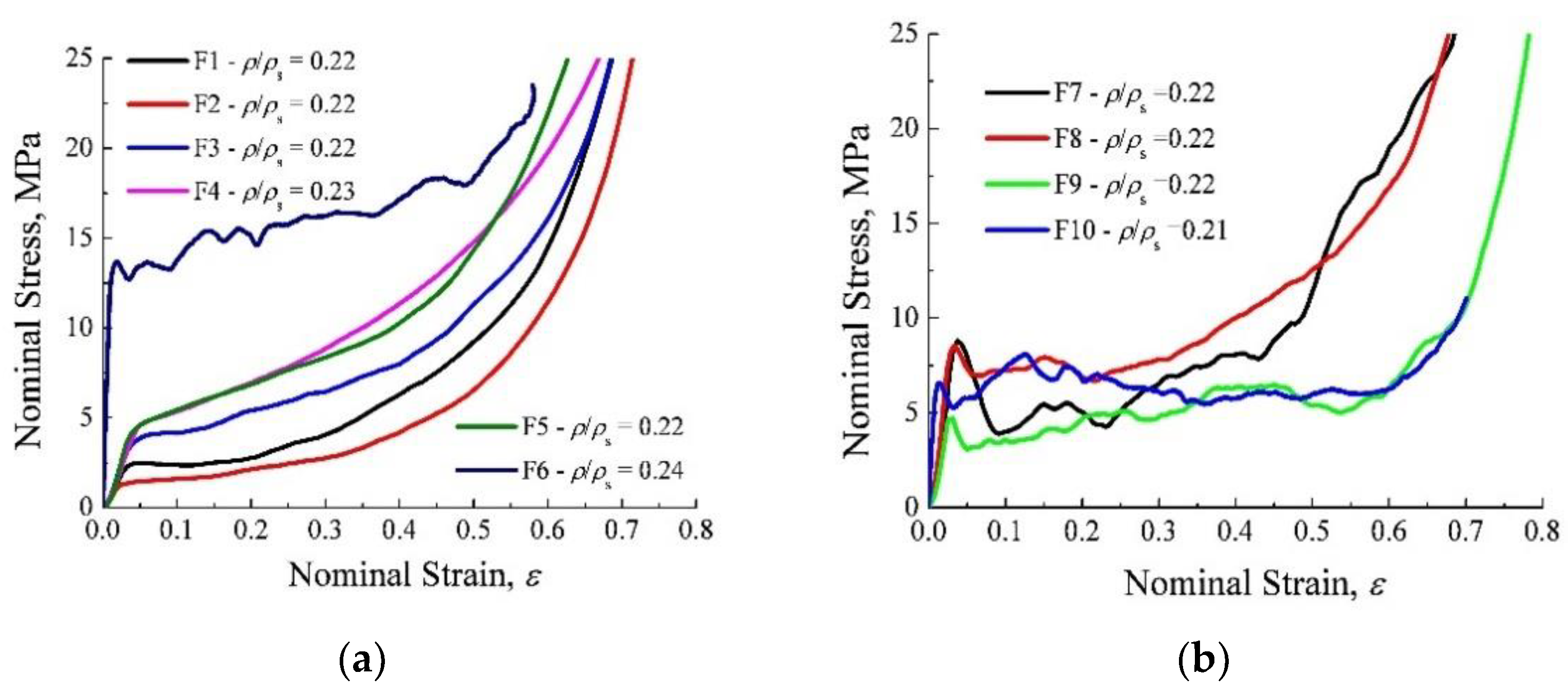
3.1. Compressive Response of Al-Foams
3.2. Compressive Strength of Al-Foams
3.3. Energy Absorption Ability of Al-Foam
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Ashby, M.F.; Evans, A.G.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Metal Foams: A Design Guide; Butterworth Heinemann: Boston, MA, USA, 2000; p. 251. [Google Scholar]
- Byakova, A.V.; Gnyloskurenko, S.V.; Bezimyanniy, Y.B.; Nakamura, T. Closed-cell aluminum foam of improved sound absorption ability: Manufacture and properties. Metals 2014, 4, 445–454. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, H. Electromagnetic interference shielding effectiveness of aluminum foams with different porosity. J. Alloys Compd. 2014, 617, 207–213. [Google Scholar] [CrossRef]
- Zhu, X.; Ai, S.; Lu, X.; Ling, X.; Zhu, L.; Liu, B. Thermal conductivity of closed-cell aluminum foam based on the 3D geometrical reconstruction. Int. J. Heat Mass Transf. 2014, 72, 242–249. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Progr. Mater Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Crupi, V.; Epasto, G.; Guglielmino, E. Impact response of aluminum foam sandwiches for light-weight ship structures. Metals 2011, 1, 98–112. [Google Scholar] [CrossRef]
- Palomba, G.; Epasto, G.; Crupi, V. Lightweight sandwich structures for marine applications: A review. Mech. Adv. Mater. Struct. 2021. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H.-W. Aluminium foam sandwich panels: Manufacture, metallurgy and applications. Adv. Eng. Mater. 2008, 10, 793–802. [Google Scholar] [CrossRef]
- Gama, B.A.; Bogetti, T.A.; Fink, B.K.; Yu, C.-J.; Claar, T.D.; Eifert, H.H.; Gillespie, J.W., Jr. Aluminum foam integral armor: A new dimension of armor design. Compos. Struct. 2001, 52, 381–395. [Google Scholar] [CrossRef]
- Schaeffler, P.; Rajner, W.; Claar, D.; Trendelenburg, T.; Nishimura, H. Production, Properties, and Applications of Alulight® Closed-Cell Aluminum Foams. In Proceedings of the Fifth International Workshop on Advanced Manufacturing Technologies, London, ON, Canada, 16–18 May 2005. [Google Scholar]
- Banhart, J. Light-metal foams-history of innovation and technological challenges. Adv. Eng. Mater. 2013, 15, 82–111. [Google Scholar] [CrossRef]
- Gibson, L.J. Mechanical behavior of metallic foams. Annu. Rev. Mater. Sci. 2000, 30, 191. [Google Scholar] [CrossRef]
- Nakamura, T.; Gnyloskurenko, S.V.; Sakamoto, K.; Byakova, A.V.; Ishikawa, R. Development of New Foaming Agent for Metal Foam. Mater. Trans. 2002, 43, 1191–1196. [Google Scholar] [CrossRef]
- Byakova, A.V.; Gnyloskurenko, S.V.; Sirko, A.I.; Milman, Y.V.; Nakamura, T. The role of foaming agent in structure and mechanical performance of Al based foams. Mater. Trans. 2006, 47, 2131–2136. [Google Scholar] [CrossRef]
- Byakova, A.V.; Sirko, A.I.; Mykhalenkov, K.V.; Milman, Y.V.; Gnyloskurenko, S.V.; Nakamura, T. Improvements in stabilisation and cellular structure of Al based foams with novel carbonate foaming agent. High Temp. Mater. Processes 2007, 26, 239–246. [Google Scholar] [CrossRef]
- Yu, S.R.; Luo, Y.R.; Liu, J.A. Effects of strain rate and SiC particle on the compressive property of SiCp/AlSi9Mg composite foams. Mater. Sci. Eng. A 2008, 487, 394–399. [Google Scholar] [CrossRef]
- Zhao, W.M.; Zhang, H.; Li, H.P. Study on Fabrication, Defects and Compression Properties of Al Foams at Different Foaming Temperatures. Adv. Mater. Res. 2011, 214, 70–74. [Google Scholar] [CrossRef]
- Byakova, A.V.; Vlasov, A.A.; Gnyloskurenko, S.V.; Kartuzov, I. Method for Making the Blocks of Foamed Aluminum/Aluminum Alloys. UA Patent No. 104367, 27 January 2014. [Google Scholar]
- Kumar, S.; Pandey, O.P. Role of fine size zircon sand ceramic particle on controlling the cell morphology of aluminum composite foams. J. Manuf. Proc. 2015, 3, 172–180. [Google Scholar] [CrossRef]
- Nava, M.G.; Cruz-Ramírez, A.; Rosales, M.Á.; Gutiérrez-Pérez, V.H.; Sánchez-Martínez, A. Fabrication of aluminum alloy foams by using alternative thickening agents via melt route. J. Alloys Compd. 2017, 698, 1009–1017. [Google Scholar] [CrossRef]
- Sutarno, S.; Nugraha, B.; Kusharjanto. Optimization of calcium carbonate content on synthesis of aluminum foam and its compressive strength characteristic. AIP Conf. Proc. 2017, 1805, 060003. [Google Scholar]
- Heidari Ghaleh, M.; Ehsani, N.; Baharvandi, H.R. Compressive Properties of A356 Closed-Cell Aluminum Foamed with a CaCO3 Foaming Agent Without Stabilizer Particles. Met. Mater. Int. 2020, 27, 3856–3861. [Google Scholar] [CrossRef]
- Byakova, A.; Kartuzov, I.; Gnyloskurenko, S.; Nakamura, T. The Role of Foaming Agent and Processing Route in Mechanical Performance of Fabricated Aluminum Foams. Procedia Mater. Sci. 2014, 4, 104–109. [Google Scholar] [CrossRef][Green Version]
- Gnyloskurenko, S.; Nakamura, T.; Byakova, A.; Podrezov, Y.; Ishikawa, R.; Maeda, M. Development of Lightweght Al alloy and Technique. Can. Metall. Q. 2005, 44, 7–12. [Google Scholar] [CrossRef]
- Milman, Y.V.; Byakova, A.V.; Sirko, A.I.; Gnyloskurenko, S.V.; Nakamura, T. Improvement of structure and deformation behavior of high-strength Al-Zn-Mg foams. Mater. Sci. Forum 2006, 519–521, 573–578. [Google Scholar] [CrossRef]
- Gnyloskurenko, S.V.; Byakova, A.V.; Sirko, A.I.; Dudnyk, A.O.; Milman, Y.V.; Nakamura, T. Advanced Structure and Deformation Pattern of Al-based Alloys with Calcium Carbonate Agent. In Porous Metals and Metallic Foams; Lefebvre, L.P., Banhart, J., Dunand, D.C., Eds.; DEStech Publications, Inc.: Lancaster, PA, USA, 2008; pp. 399–402. [Google Scholar]
- Byakova, A.V.; Gnyloskurenko, S.V.; Vlasov, A.N.; Semenov, N.M.; Yevych, Y.M.; Zatsarna, O.V.; Danilyuk, V.M. Effect of Cell Wall Ductility and Toughness on Compressive Response and Strain Rate Sensitivity of Aluminium Foam. Adv. Mater. Sci. Eng. 2019, 2019, 3474656. [Google Scholar] [CrossRef]
- Kriszt, B.; Foroughi, K.; Faure, H.P. Degischer, Behaviour of aluminium foam under uniaxial compression. Mater. Sci. Technol. 2000, 16, 792–796. [Google Scholar] [CrossRef]
- Markaki, A.E.; Clyne, T.W. The effect of cell wall microstructure on the deformation and fracture of aluminum-based foams. Acta Mater. 2001, 49, 1677–1686. [Google Scholar] [CrossRef]



| Foam Code | Parent Alloy and Processing Additives, (wt.%) | Microstructure of the Cell Wall Material | Contaminating Side Products | Solid Yield Strength, σys (MPa) |
|---|---|---|---|---|
| F1 | Al + 1TiH2 + 1Ca | α-Al + E (Al + Al3Ti + Al4Ca) | Particles: TiH2/ TiAl3/TiAl2 | 42.8 ± 4.89 |
| F2 | Al + 2CaCO3 + 1Ca | α-Al + E (Al + Al4Ca) | Fine particles CaO | 43.5 ± 7.12 |
| F3 | Al-7Si + 1.5TiH2 + 1Ca | α-Al (Ti) + E (Al-Si) | Particles: TiH2/ TiAl3, Al3Ti (Si), Al2CaSi2 | 220 ± 20.54 |
| F4 | Al-7Si + 2CaCO3 | α-Al + E (Al-Si) | Fine particles CaO | 140 ± 15.67 |
| F5 | Al-1Mg-0.6Si + 1.5TiH2 + 1Ca | α-Al (Ti) + E (Al + Mg2Si + S(Al2CuMg) + CuAl2) + E (α-Al + Al4Ca+ Al2CaSi2 + Al4CaCu + Al3Ti) | Particles: TiH2/ TiAl3, Al3Ti | 124 ± 14.82 |
| F6 | Al-1Mg-0.6Si + 2CaCO3 | α-Al + E {α-Al + CuAl2}, α-Al + E {α-Al + S(Al2CuMg)}, α-Al + E {α-Al + Mg2Si} | Fine particles CaO | 105 ± 7.25 |
| F7 | Al-5.5Zn-3.0Mg (Sc,Zr)+ 1.5TiH2 + 1Ca | α-Al(Ti) + T(AlCuMgZnCaTi) | Particles: TiH2/ TiAl3, Al3(ScZr) | 200 ± 28.24 |
| F8 | Al-5.5Zn-3.0Mg (Sc,Zr) + 2CaCO3 | α-Al + T(AlCuMgZn) | Particles Al3(ScZr) Fine particles CaO | 213 ± 15.14 |
| F9 | Al-6Zn-2.3Mg + 1.5TiH2 + 1Ca | α-Al(Ti) + T(AlCuMgZnCaTi)/ M(AlCuMgZnCaTi)/S (CuMgAl2CaTi) | Particles: TiH2/ TiAl3 | 220 ± 15.00 |
| F10 | Al-6Zn-2.3Mg + 2CaCO3 | α-Al + T(AlCuMgZn)/ M(AlCuMgZn)/S(CuMgAl2) | Fine particles CaO | 252 ± 10.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byakova, A.; Gnyloskurenko, S.; Vlasov, A.; Yevych, Y.; Semenov, N. The Mechanical Performance of Aluminum Foam Fabricated by Melt Processing with Different Foaming Agents: A Comparative Analysis. Metals 2022, 12, 1384. https://doi.org/10.3390/met12081384
Byakova A, Gnyloskurenko S, Vlasov A, Yevych Y, Semenov N. The Mechanical Performance of Aluminum Foam Fabricated by Melt Processing with Different Foaming Agents: A Comparative Analysis. Metals. 2022; 12(8):1384. https://doi.org/10.3390/met12081384
Chicago/Turabian StyleByakova, Alexandra, Svyatoslav Gnyloskurenko, Andrey Vlasov, Yan Yevych, and Nikolay Semenov. 2022. "The Mechanical Performance of Aluminum Foam Fabricated by Melt Processing with Different Foaming Agents: A Comparative Analysis" Metals 12, no. 8: 1384. https://doi.org/10.3390/met12081384
APA StyleByakova, A., Gnyloskurenko, S., Vlasov, A., Yevych, Y., & Semenov, N. (2022). The Mechanical Performance of Aluminum Foam Fabricated by Melt Processing with Different Foaming Agents: A Comparative Analysis. Metals, 12(8), 1384. https://doi.org/10.3390/met12081384






