Phase Transformations of 5Cr-0.5Mo-0.1C Steel after Heat Treatment and Isothermal Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Numerical Method
2.2. Experimental Method
3. Results
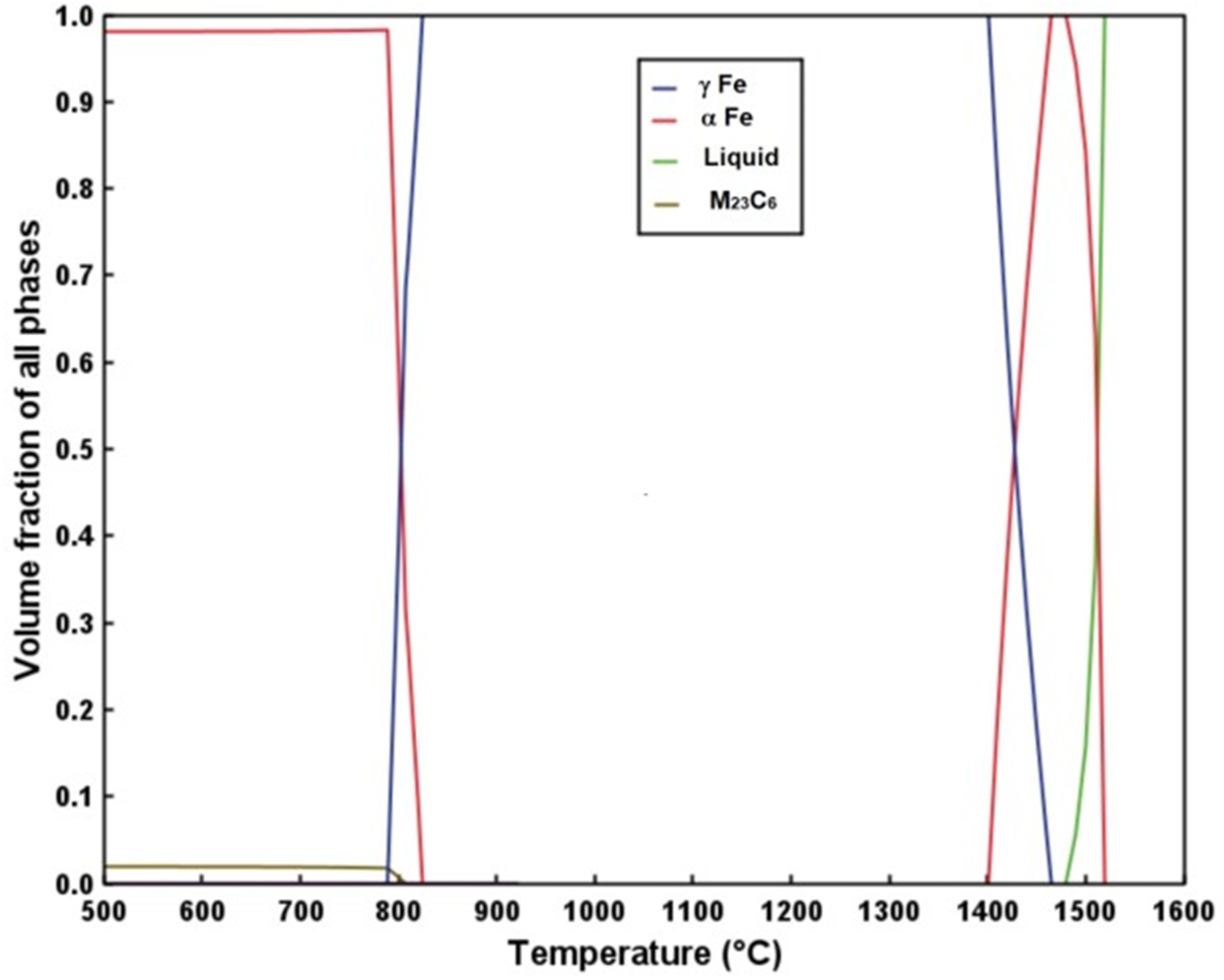
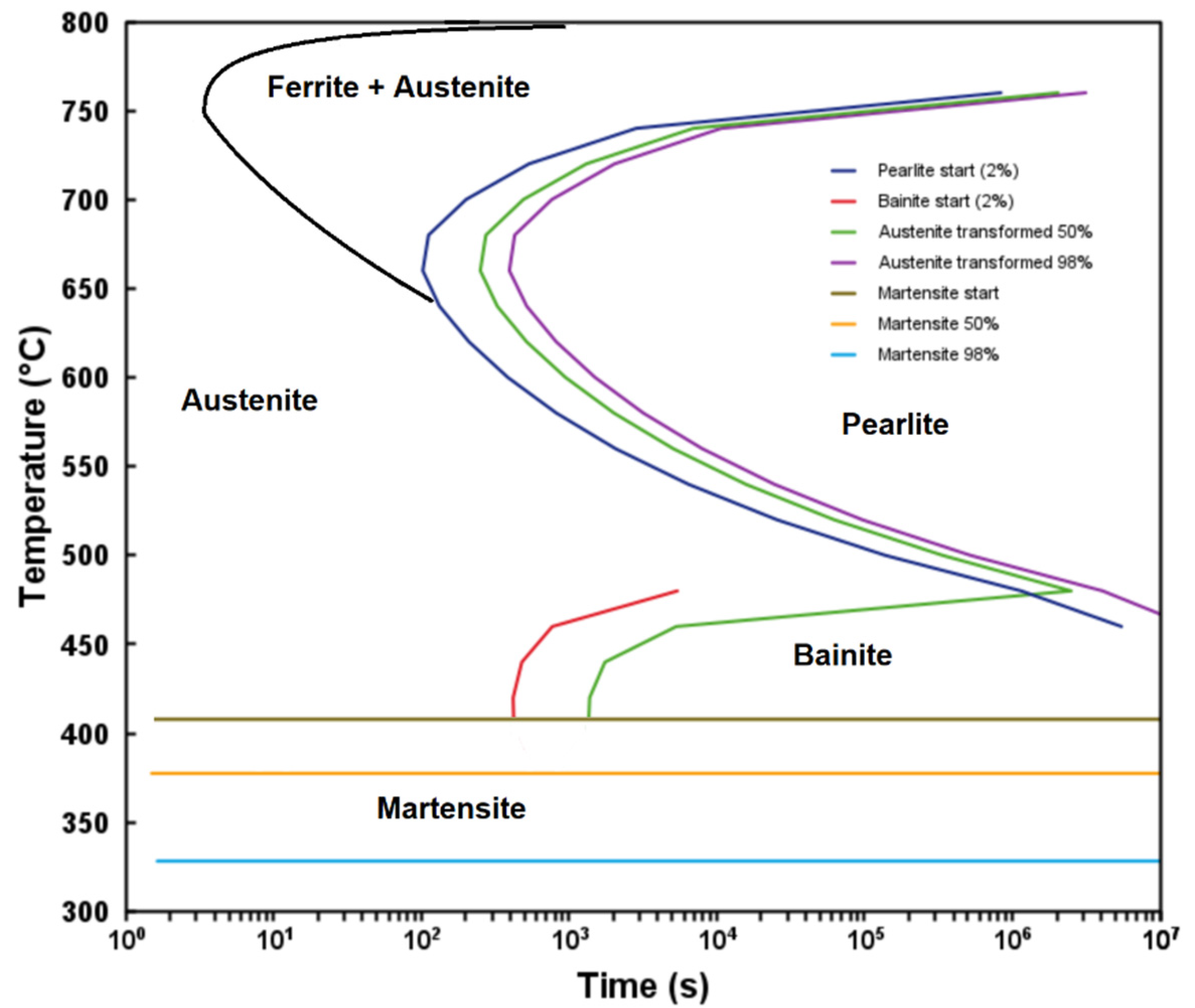
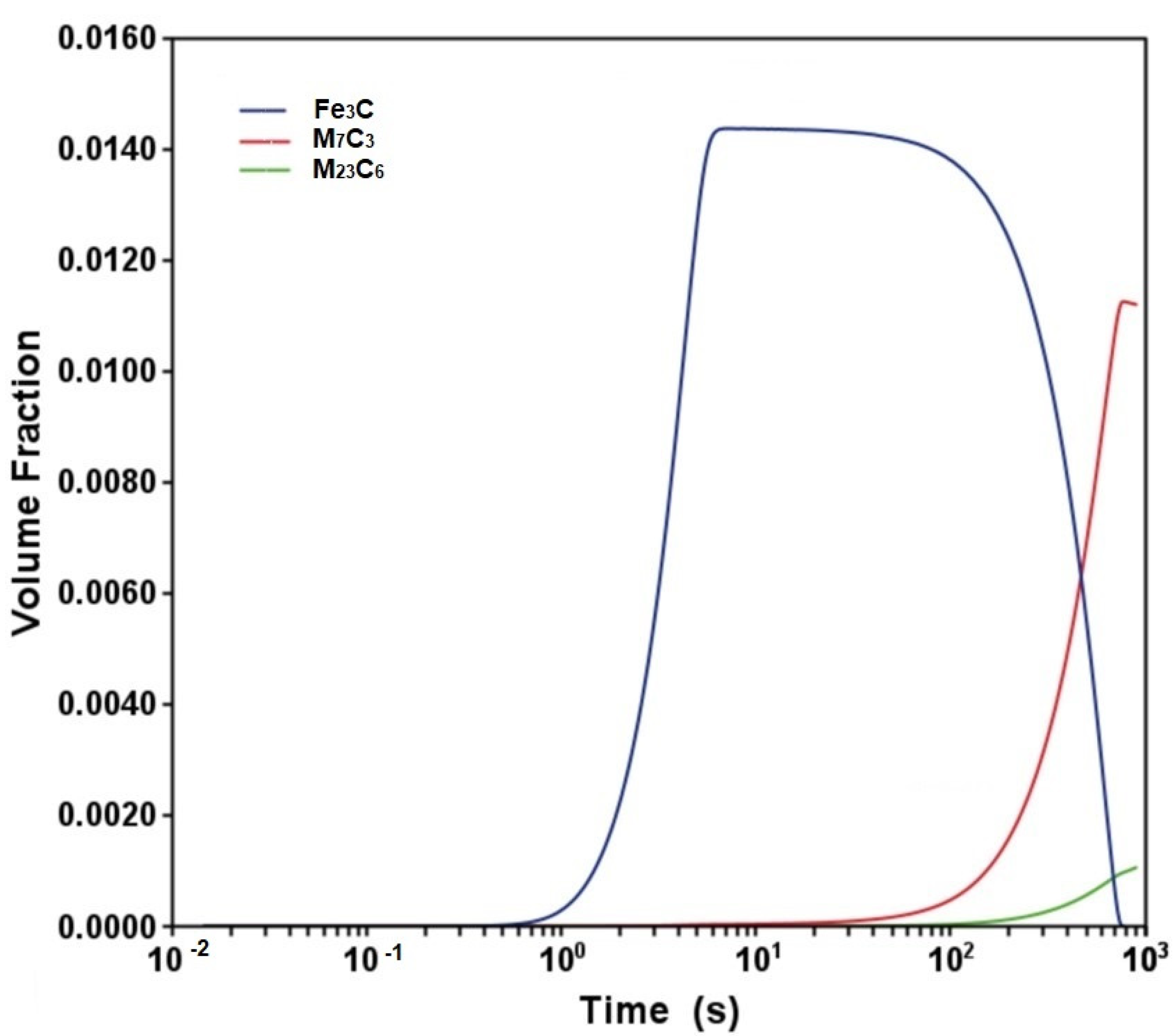
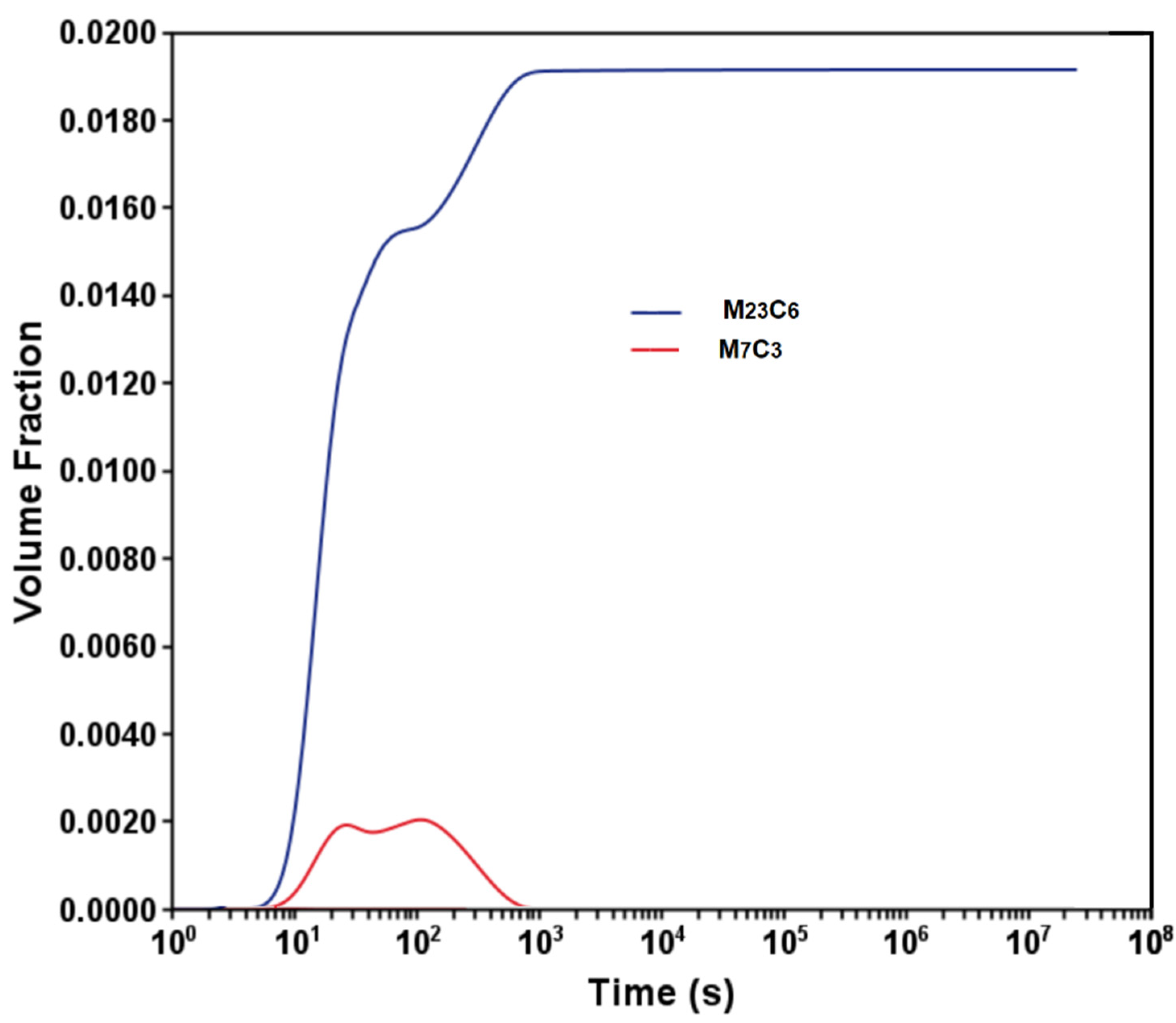
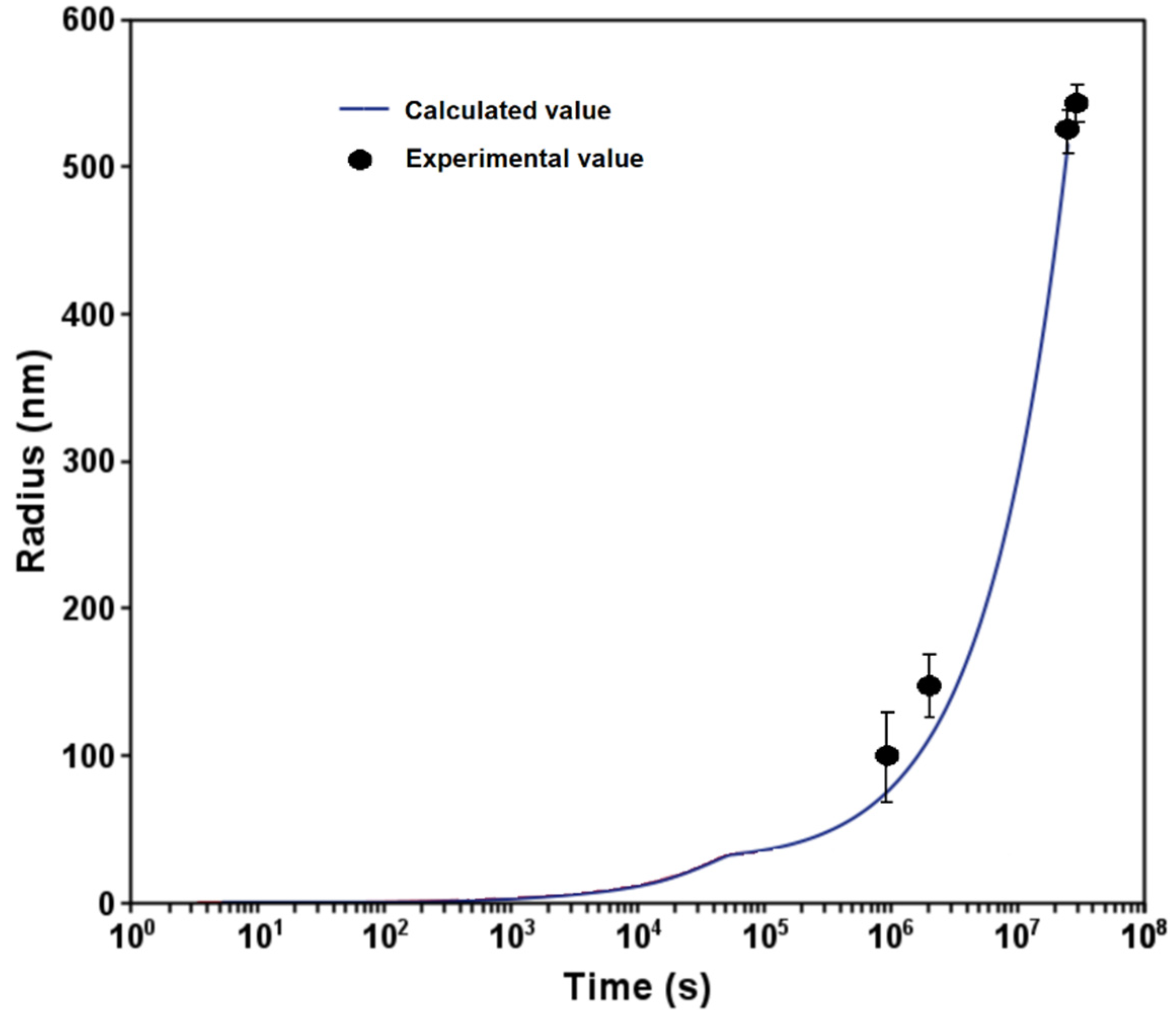
3.1. Phase Stability and TTT Diagram
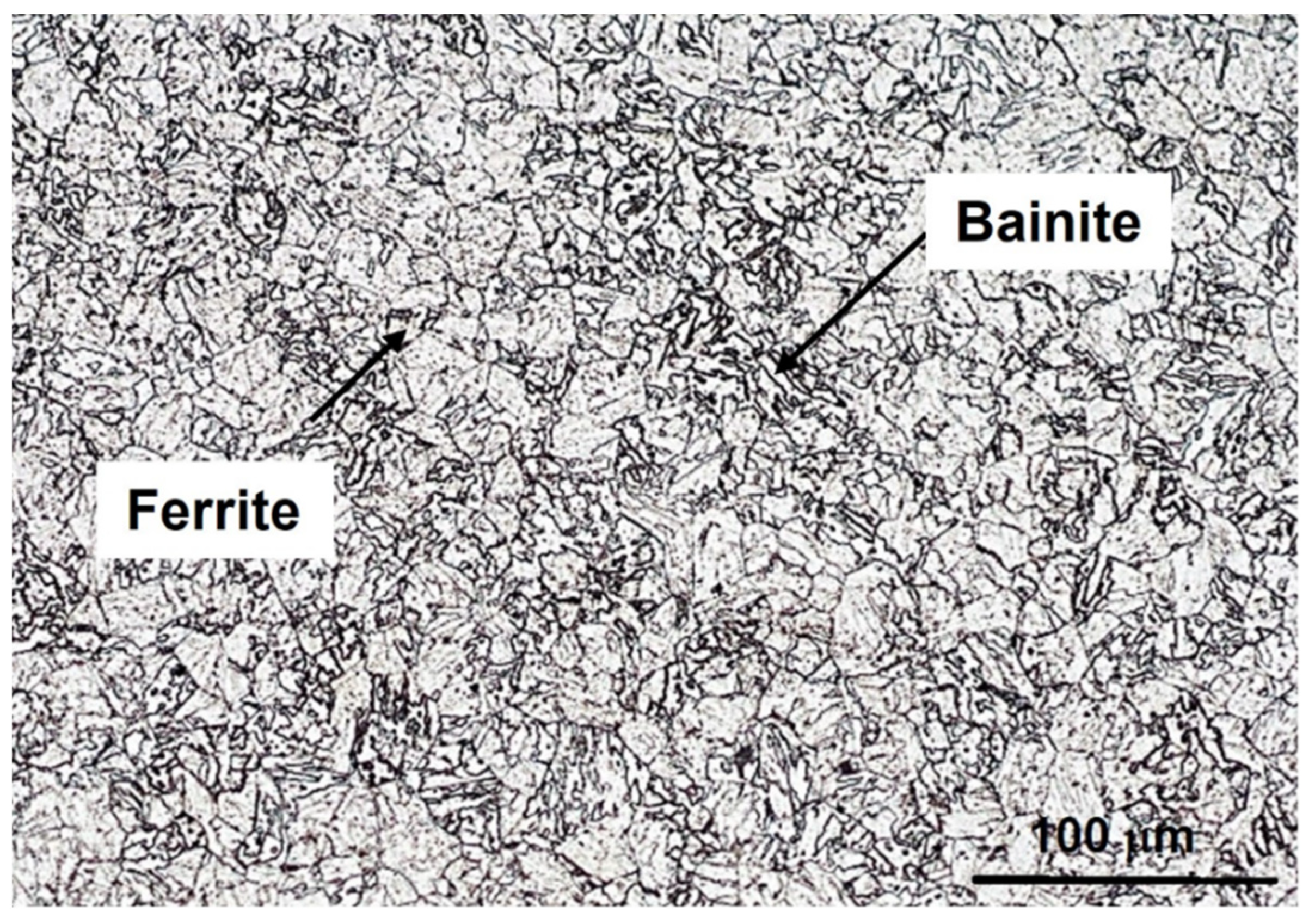
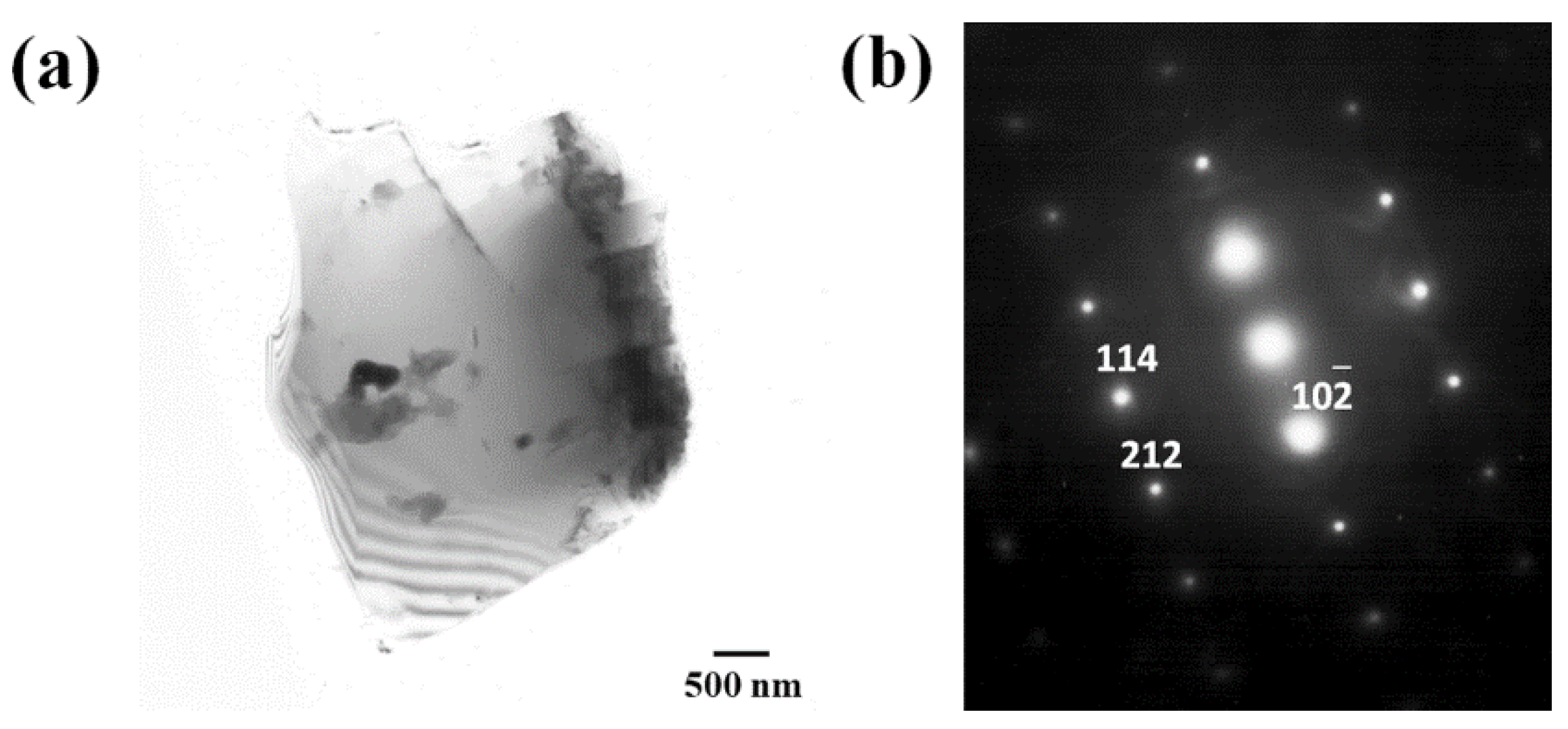
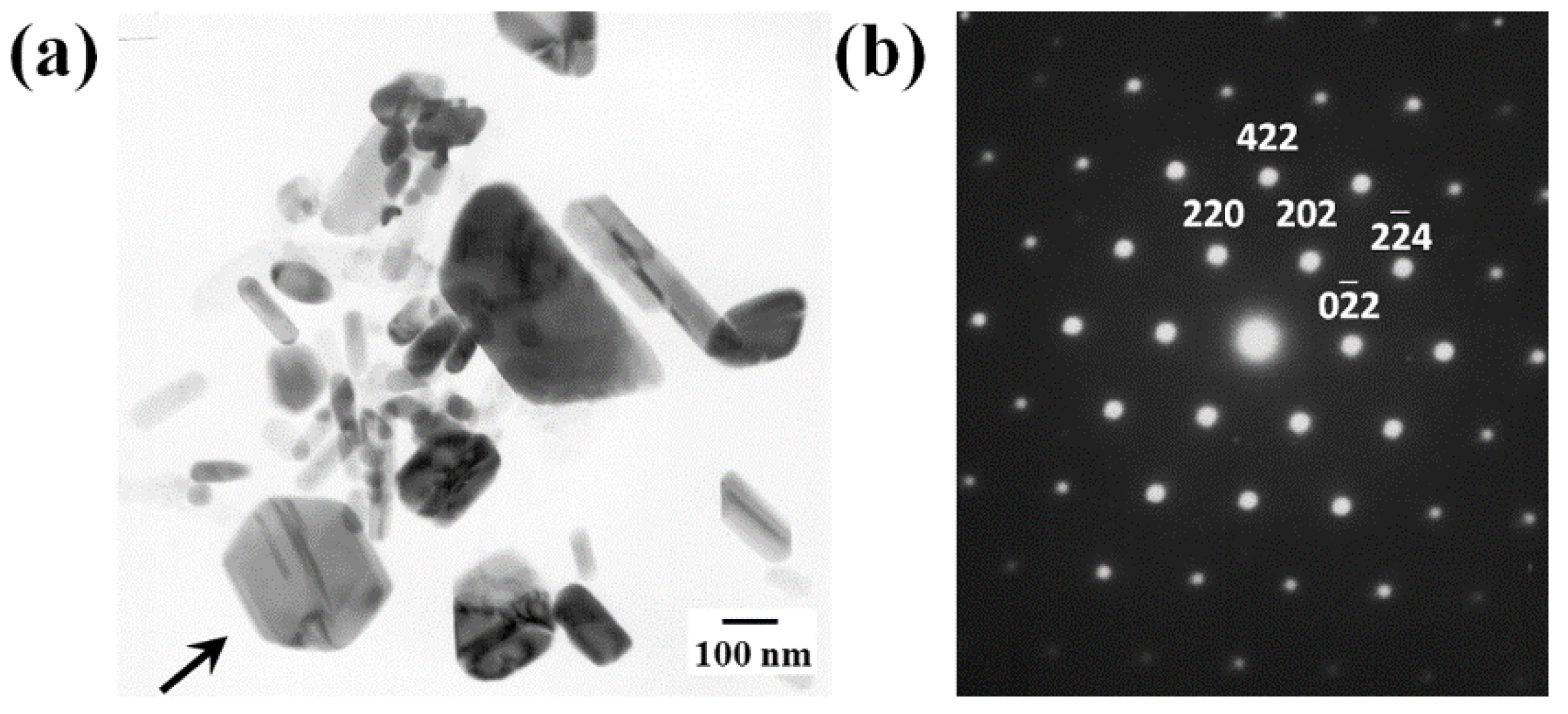
3.2. Microstructure Characterization
4. Discussion
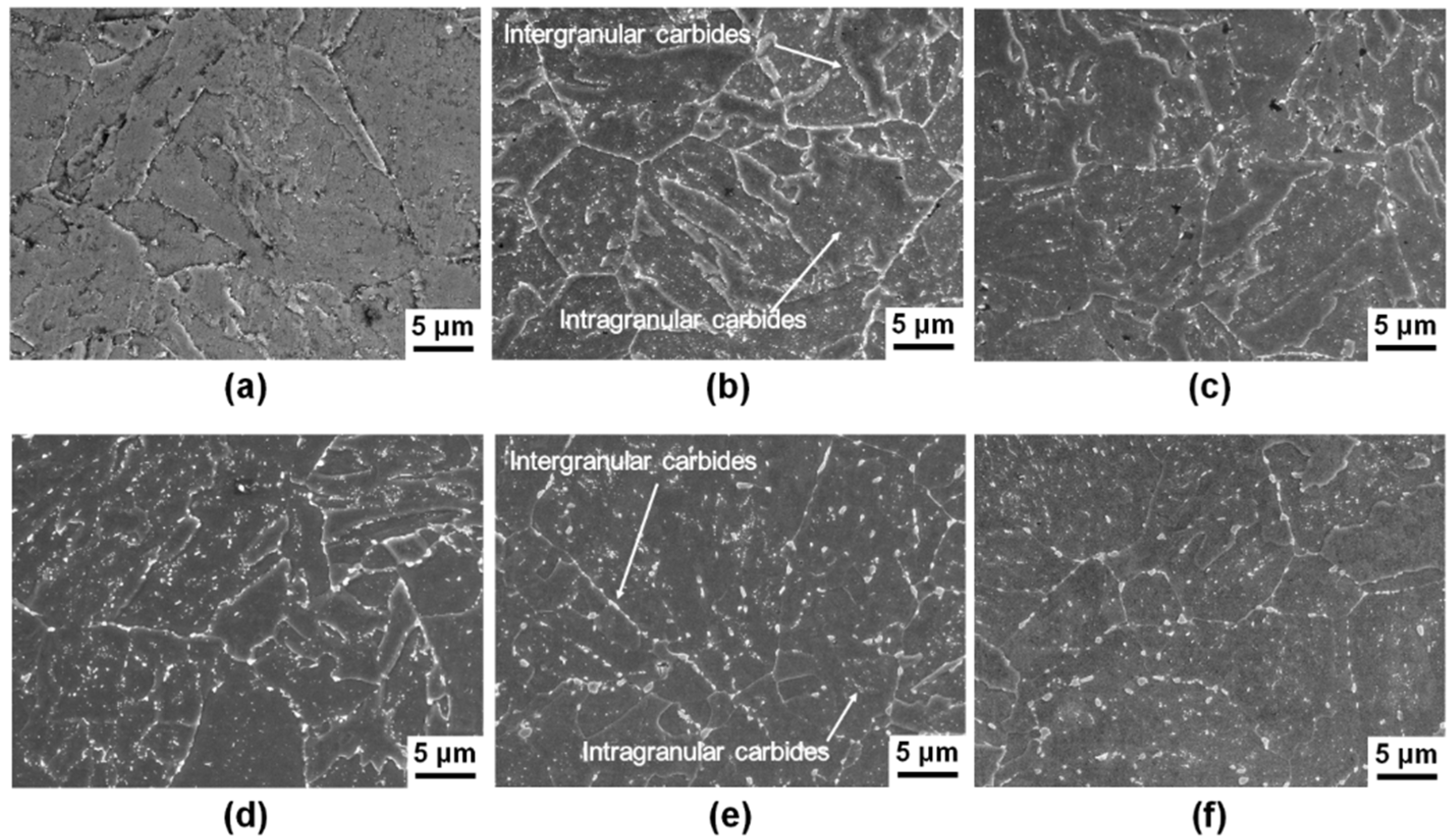
4.1. Characterization of Precipitation Evolution
4.2. Mechanical Characterization
5. Conclusions
- The Calphad-calculated TTT diagram of the steel precisely predicted the microconstituents observed in the as-received steel.
- The cooling stage of the normalizing treatment promoted neither intergranular nor intragranular precipitation of carbides.
- The tempering process at 700 °C originated the precipitation of M7C3 and M23C6 carbides.
- Aging at 600 °C produced the precipitation of M23C6 carbide, which is responsible for the mechanical strength at high temperatures.
- The reduction in steel hardness can be attributed to the bainite transformation and diffusion-controlled coarsening of M23C6 carbide.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gneezy, V.C.; Ugwuegbu, C.C.; Mark, U. Physical metallurgy of modern creep-resistant steel for steam power plants: Microstructure and phase transformations. J. Metall. 2016, 3, 5468292. [Google Scholar] [CrossRef]
- Bhadeshia, H.-K.D.H.; Strange, A.; Gooch, D.J. Ferritic power plant steels: Remanent life assessment and approach to equilibrium. Inter. Mater. Rev. 1998, 43, 45–69. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Heat-Resistant Materials; ASM International: Materials Park, OH, USA, 1997; pp. 50–75. [Google Scholar]
- Bhadeshia, H.K.D.H. Design of ferritic creep-resistant steels. ISIJ Int. 2001, 41, 626–640. [Google Scholar] [CrossRef]
- Viswanathan, R. Effect of stress and temperature on the creep and rupture behavior of a 1.25 Pct.Chromium-0.5 Pct. molybdenum steel. Met. Trans. 1977, 8, 877–884. [Google Scholar] [CrossRef]
- Saucedo-Muñoz, M. Precipitation kinetics of carbides during cyclical and isothermal aging of 2.25Cr–1Mo steel and its effect on mechanical properties. J. Irons Steel Res. Int. 2021, 28, 1282–1290. [Google Scholar] [CrossRef]
- Das, S.; Vora, J.; Patel, V.; Andersson, J.; Pimenov, D.Y.; Giasin, K. Elucidating the effect of step cooling heat treatment on the properties of 2.25 Cr–1.0 Mo steel welded with a combination of GMAW techniques incorporating metal-cored wires. Materials 2021, 14, 14206033. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, F. History of power plants and progress in heat resistant steels. ISIJ Int. 2001, 41, 612–625. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, K.; Sterner, G.; Mason, P. Modeling Precipitation Kinetics During Heat Treatment with Calphad-Based Tools. J. Mater. Eng. Perform. 2014, 23, 4193–4196. [Google Scholar] [CrossRef]
- Costa e Silva, A. Challenges and opportunities in thermodynamic and kinetic modeling microalloyed HSLA steels using computational thermodynamics. Calphad 2020, 68, 101720. [Google Scholar] [CrossRef]
- Saucedo-Muñoz, M.; Ortiz-Mariscal, A.; Lopez-Hirata, V.; Villegas-Cardenas, J.; Soriano-Vargas, O.; Avila-Davila, E.O. Precipitation analysis of as-cast HK40 steel after isothermal aging. Int. J. Min. Metall. Mater. 2017, 24, 1125–1133. [Google Scholar] [CrossRef]
- Lopez-Hirata, V.M.; Hernandez-Santiago, F.; Saucedo-Muñoz, M.L.; Dorantes-Rosales, H.J.; Paniagua-Mercado, A.M. Analysis of β´(Cu4Ti) Precipitation during isothermal aging of a Cu-4wt.%Ti alloy. Mater. Res. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Thermo-Calc. Thermo-Calc Prisma Software TCFE 11 and MOBFE6 Steels/ Fe Alloys Databases. Available online: https://thermocalc.com/content/uploads/Documentation/Databases/Mobility/mobfe7-technical-info.pdf (accessed on 21 June 2022).
- Yan, J.; Agren, J.; Jeppsson, J. Pearlite in multicomponent steels: Phenomenological steady-state modeling. Met. Mater. Trans. 2020, 51, 1978–2001. [Google Scholar] [CrossRef]
- Stormvinter, A.; Borgenstam, A.; Agren, J. Thermodynamically based prediction of the martensite start temperature for commercial steels. Met. Mater. Trans. 2012, 43, 3870–3879. [Google Scholar] [CrossRef]
- Leach, L.; Kolmskog, P.; Hönglund, L.; Hillert, M.; Borgenstam, A. Critical driving forces for formation of bainite. Met. Mater. Trans. 2018, 49, 4509–4520. [Google Scholar] [CrossRef]
- Thermo-Calc. Thermo-Calc Software TCFE Steels 11/ Fe Alloys Database. Available online: https://thermocalc.com/content/uploads/Documentation/Databases/Thermodynamic/tcfe12-technical-info.pdf (accessed on 21 June 2022).
- ASTM Standard E 18-15; Standard Test Methods for Rockwell Hardness of Metallic Materials. ASTM: West Conshohocken, PA, USA, 2016.
- ASTM E 112-19; Standard Test Methods for Determining Average Grain Size. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM E 562-19; Standard Test Method for Determining Volume Fraction by Systematic Manual Point Count. ASTM: West Conshohocken, PA, USA, 2019.
- Rouault, M.A.; Herpin, P.; Fruchart, M.R. Etude cristallographique des carbures Cr7 C3 et Mn7 C3. Ann. Chim. 1970, 5, 461–470. [Google Scholar]
- Bowman, A.L.; Arnold, G.P.; Storms, E.K.; Nereson, N.G. The crystal structure of Cr23 C6. Acta Cryst. B 1972, 28, 3102–3103. [Google Scholar] [CrossRef]
- Kostorz, K. Phase Transformations in Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 309–407. [Google Scholar]



| C | Cr | Mo | Mn | Si | Cu | Ni | Ti |
|---|---|---|---|---|---|---|---|
| 0.10 | 4.50 | 0.46 | 0.36 | 0.35 | 0.05 | 0.12 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saucedo-Muñoz, M.L.; Lopez-Hirata, V.M.; Dorantes-Rosales, H.J.; Villegas-Cardenas, J.D.; Rivas-Lopez, D.I.; Beltran-Zuñiga, M.; Ferreira-Palma, C.; Moreno-Palmerin, J. Phase Transformations of 5Cr-0.5Mo-0.1C Steel after Heat Treatment and Isothermal Exposure. Metals 2022, 12, 1378. https://doi.org/10.3390/met12081378
Saucedo-Muñoz ML, Lopez-Hirata VM, Dorantes-Rosales HJ, Villegas-Cardenas JD, Rivas-Lopez DI, Beltran-Zuñiga M, Ferreira-Palma C, Moreno-Palmerin J. Phase Transformations of 5Cr-0.5Mo-0.1C Steel after Heat Treatment and Isothermal Exposure. Metals. 2022; 12(8):1378. https://doi.org/10.3390/met12081378
Chicago/Turabian StyleSaucedo-Muñoz, Maribel L., Victor M. Lopez-Hirata, Hector J. Dorantes-Rosales, Jose D. Villegas-Cardenas, Diego I. Rivas-Lopez, Manuel Beltran-Zuñiga, Carlos Ferreira-Palma, and Joel Moreno-Palmerin. 2022. "Phase Transformations of 5Cr-0.5Mo-0.1C Steel after Heat Treatment and Isothermal Exposure" Metals 12, no. 8: 1378. https://doi.org/10.3390/met12081378
APA StyleSaucedo-Muñoz, M. L., Lopez-Hirata, V. M., Dorantes-Rosales, H. J., Villegas-Cardenas, J. D., Rivas-Lopez, D. I., Beltran-Zuñiga, M., Ferreira-Palma, C., & Moreno-Palmerin, J. (2022). Phase Transformations of 5Cr-0.5Mo-0.1C Steel after Heat Treatment and Isothermal Exposure. Metals, 12(8), 1378. https://doi.org/10.3390/met12081378






