The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Steel Composition Design
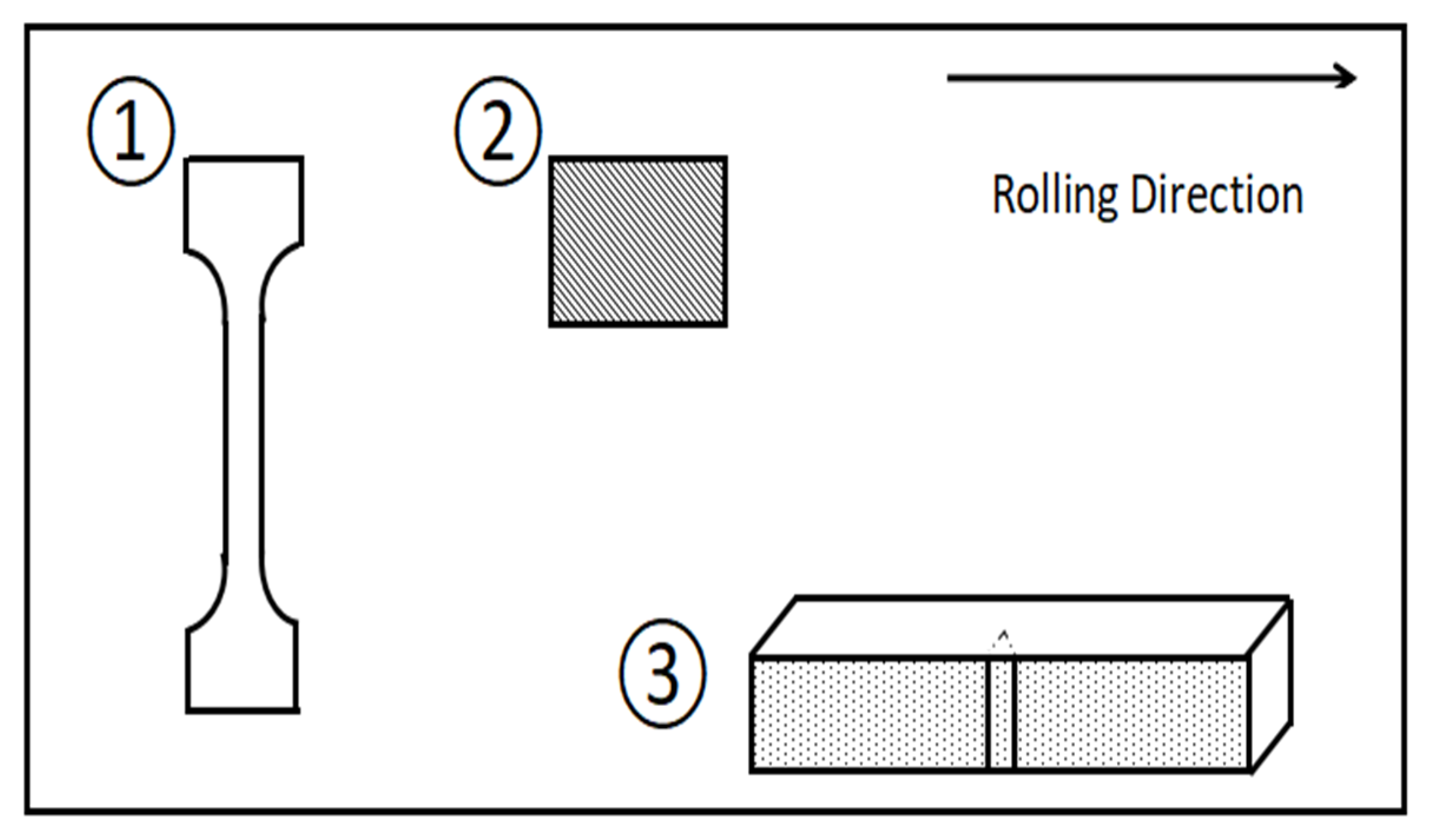
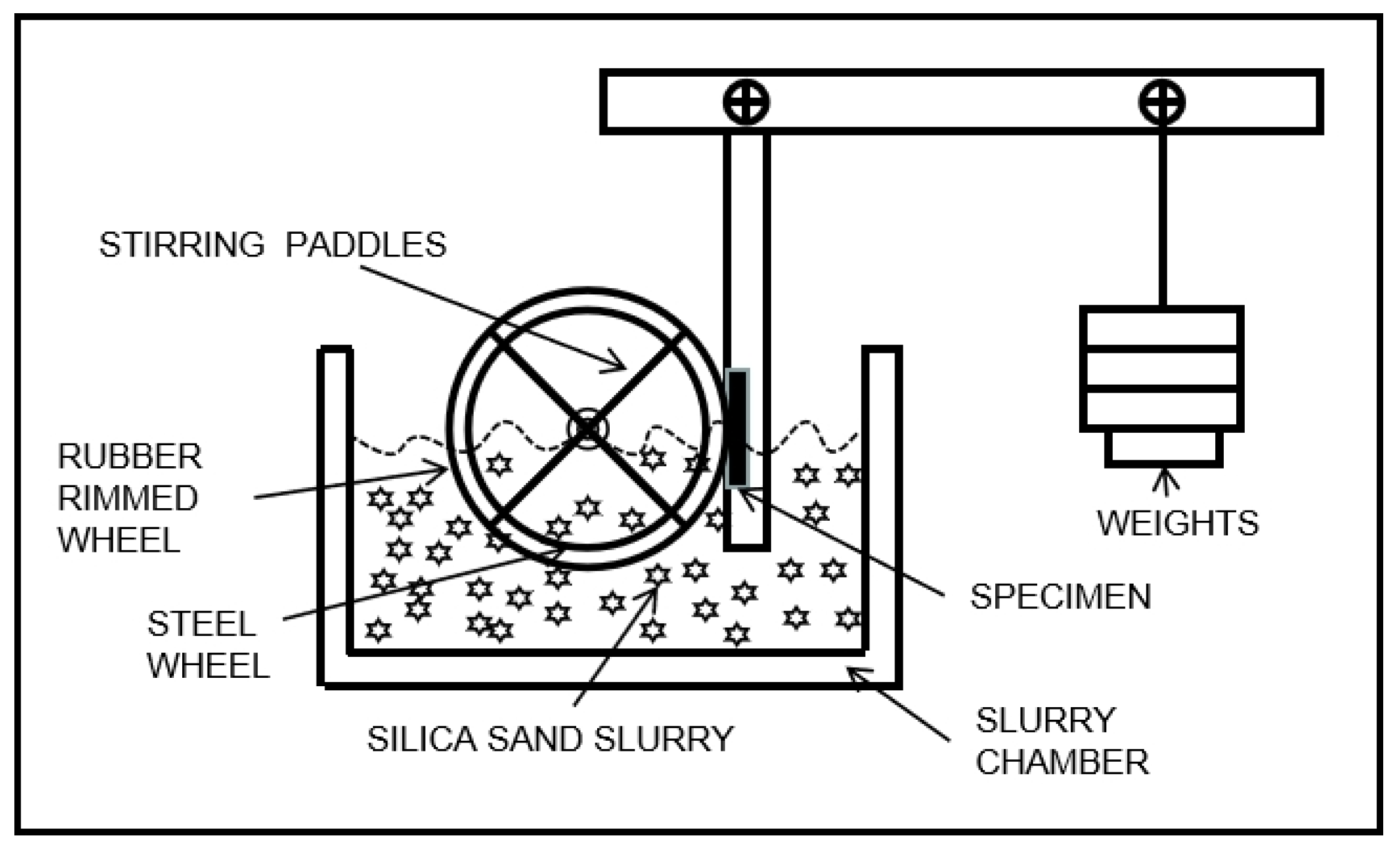
2.2. Test Method
3. Results
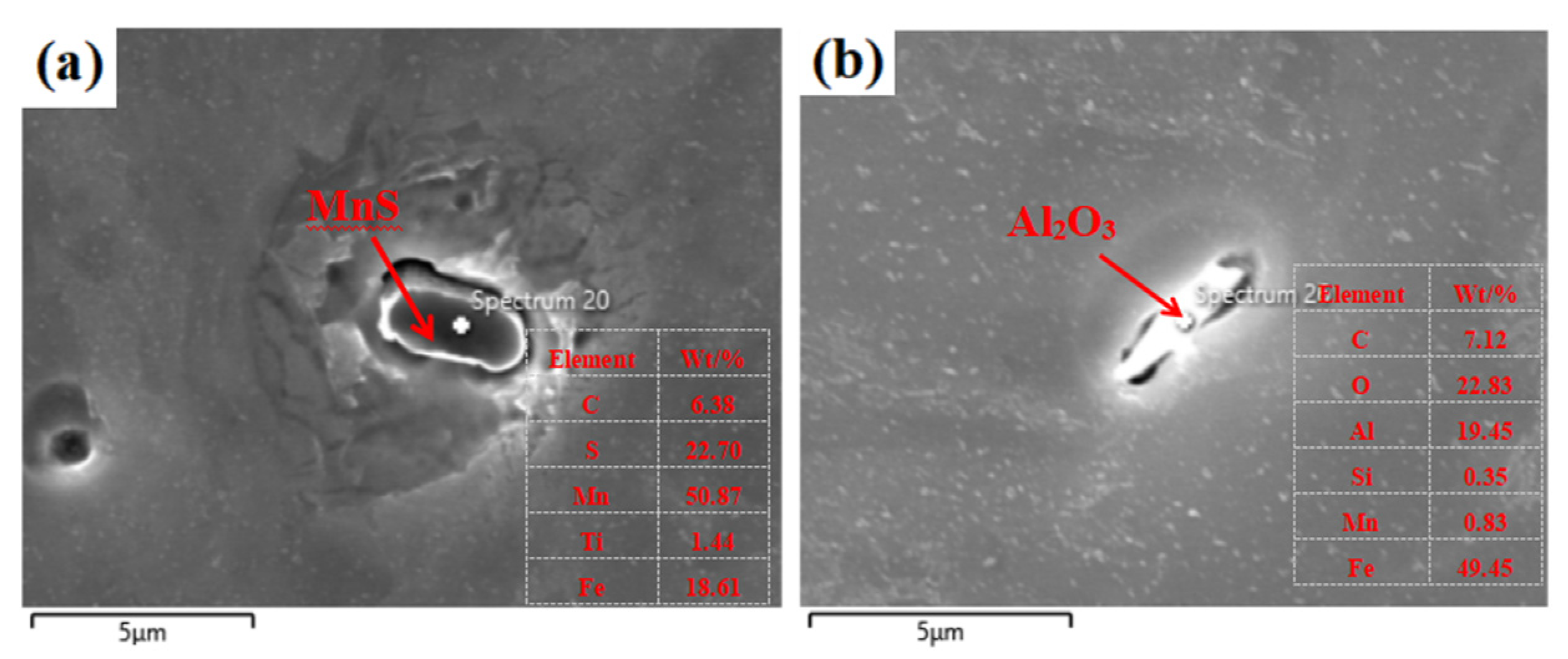
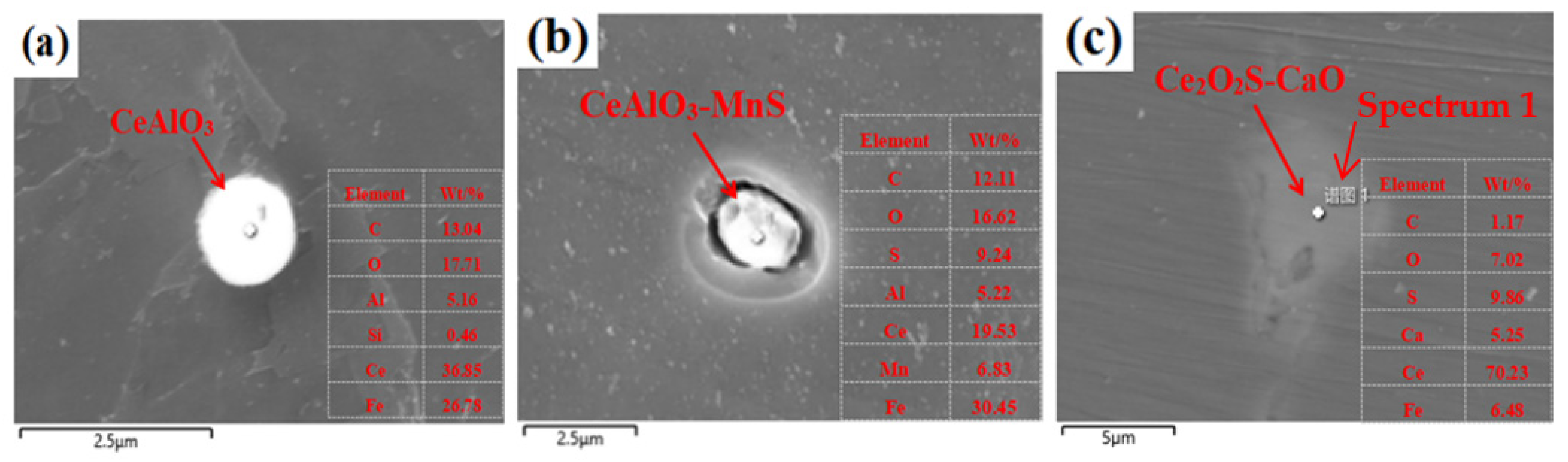
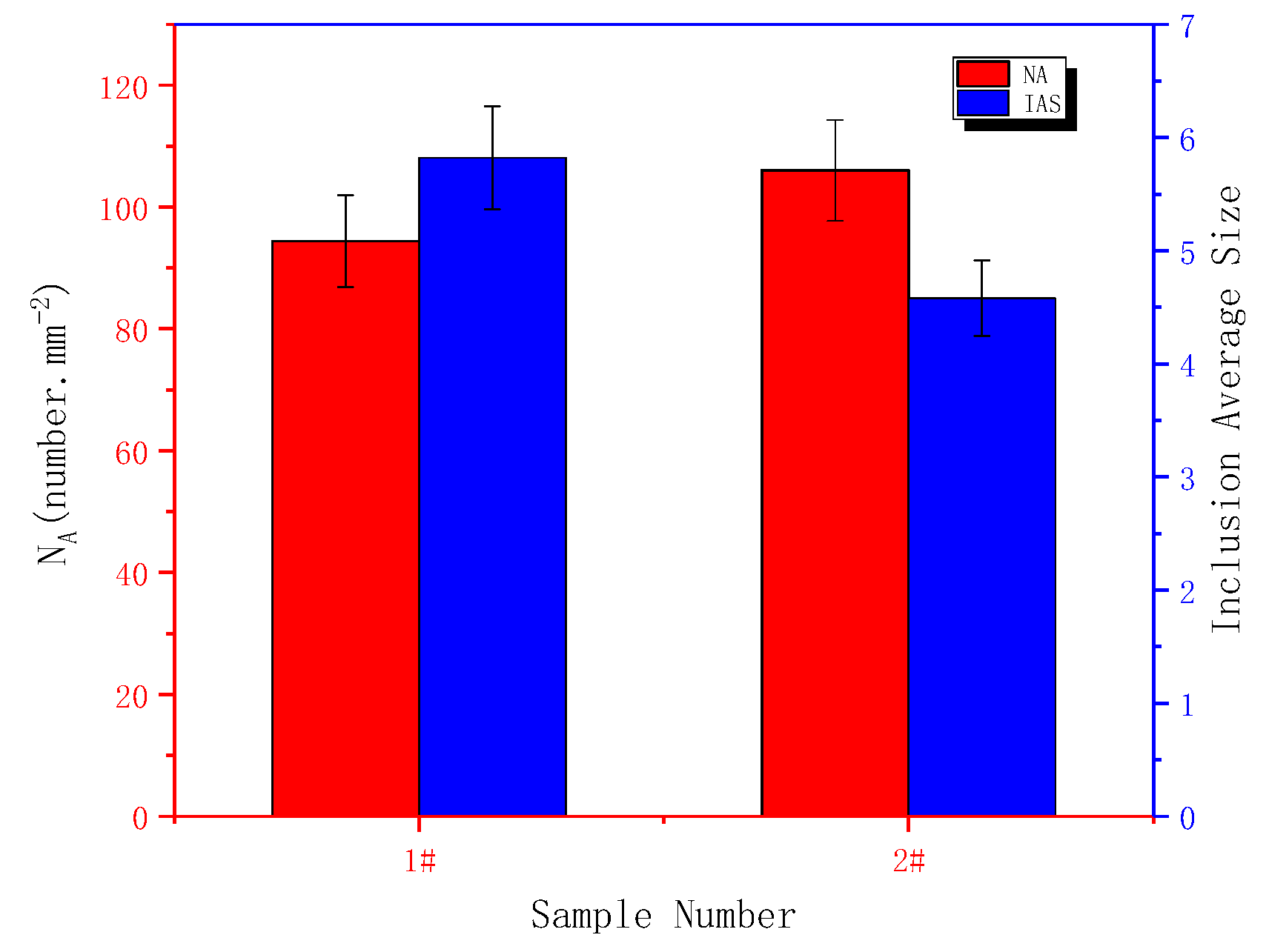
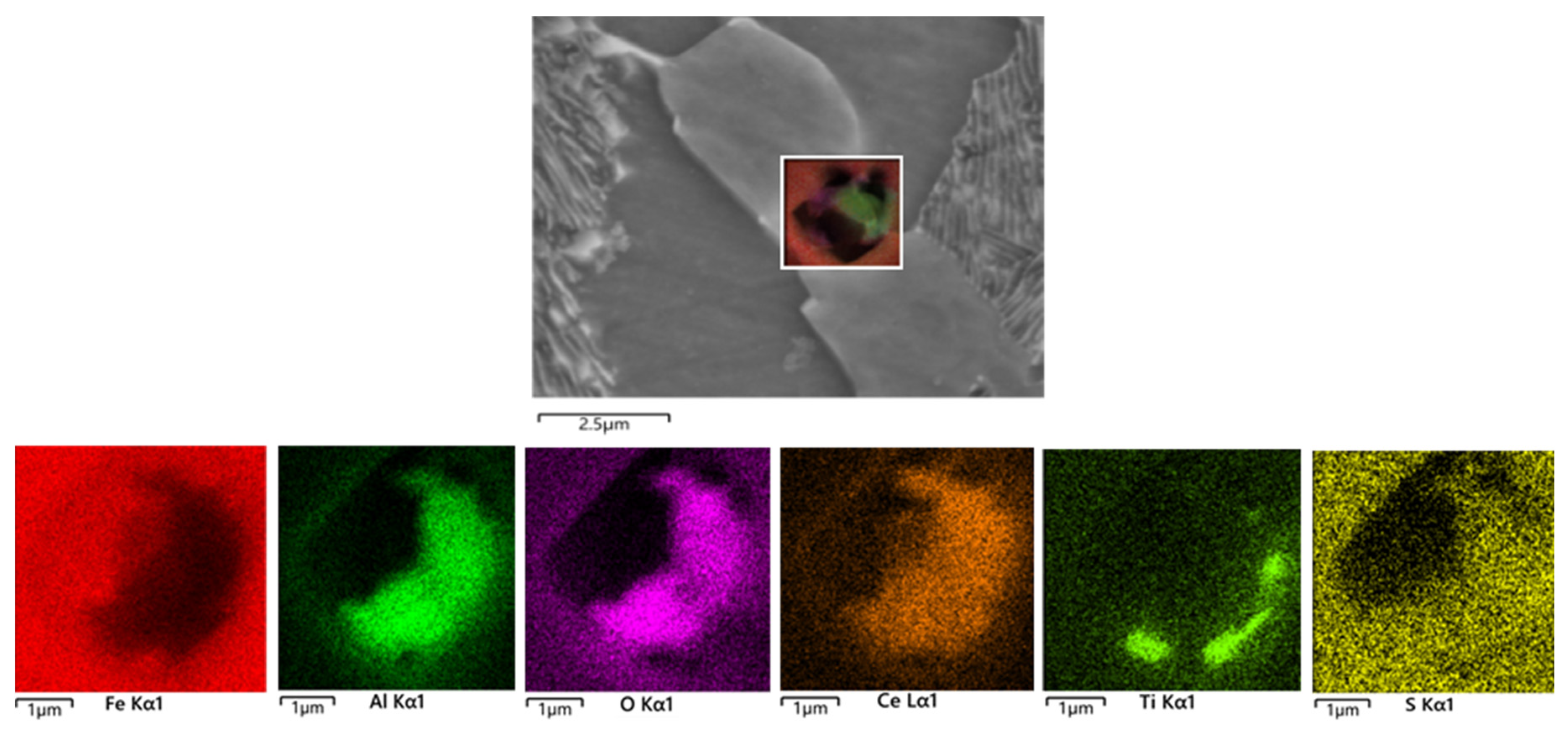
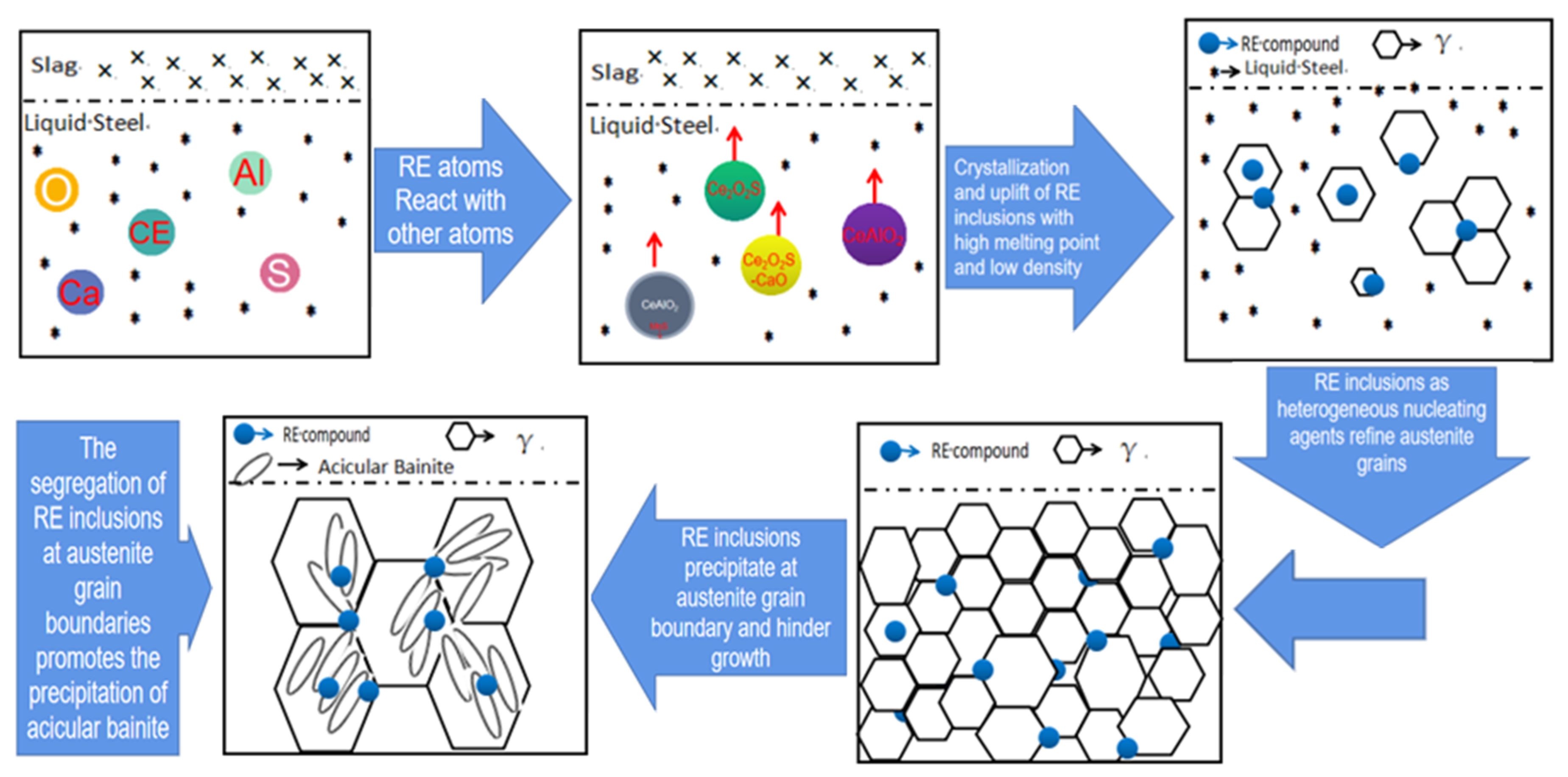
3.1. Effect of Cerium on the Morphology of Inclusion
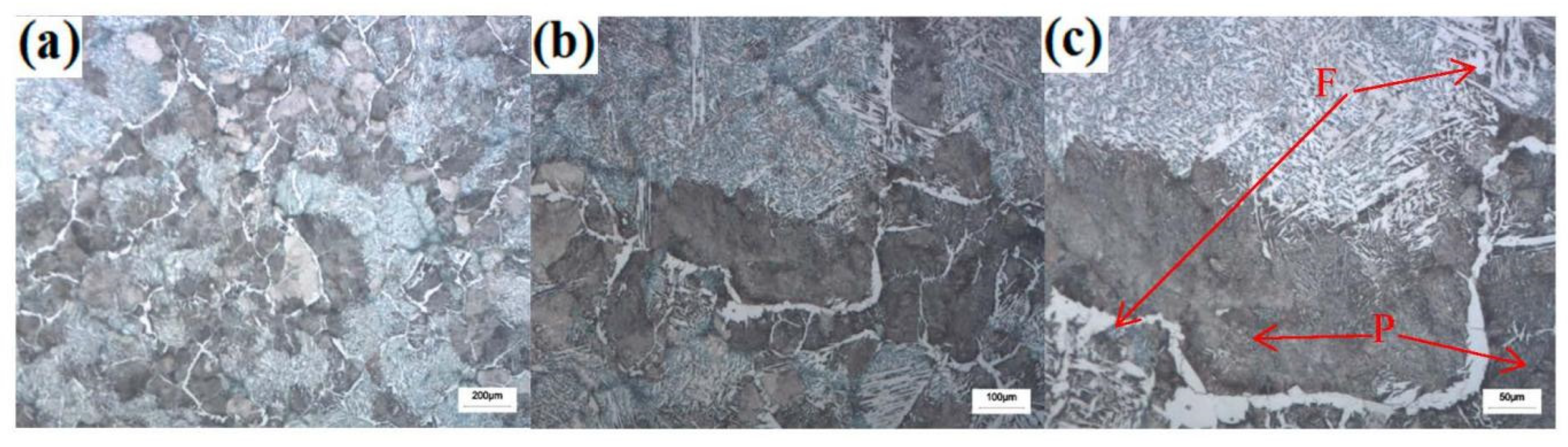
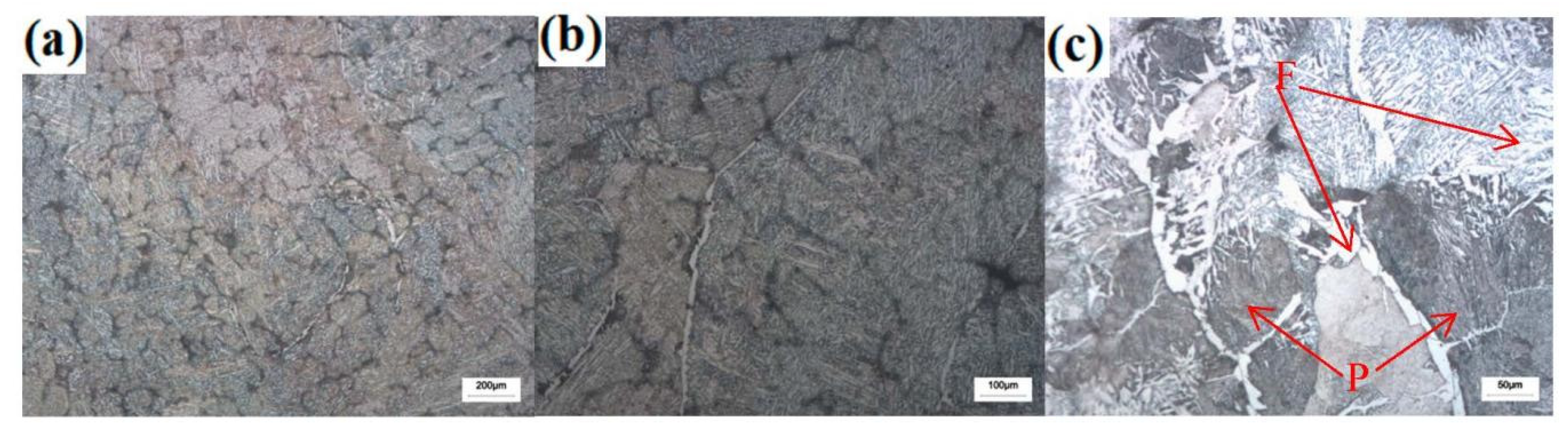
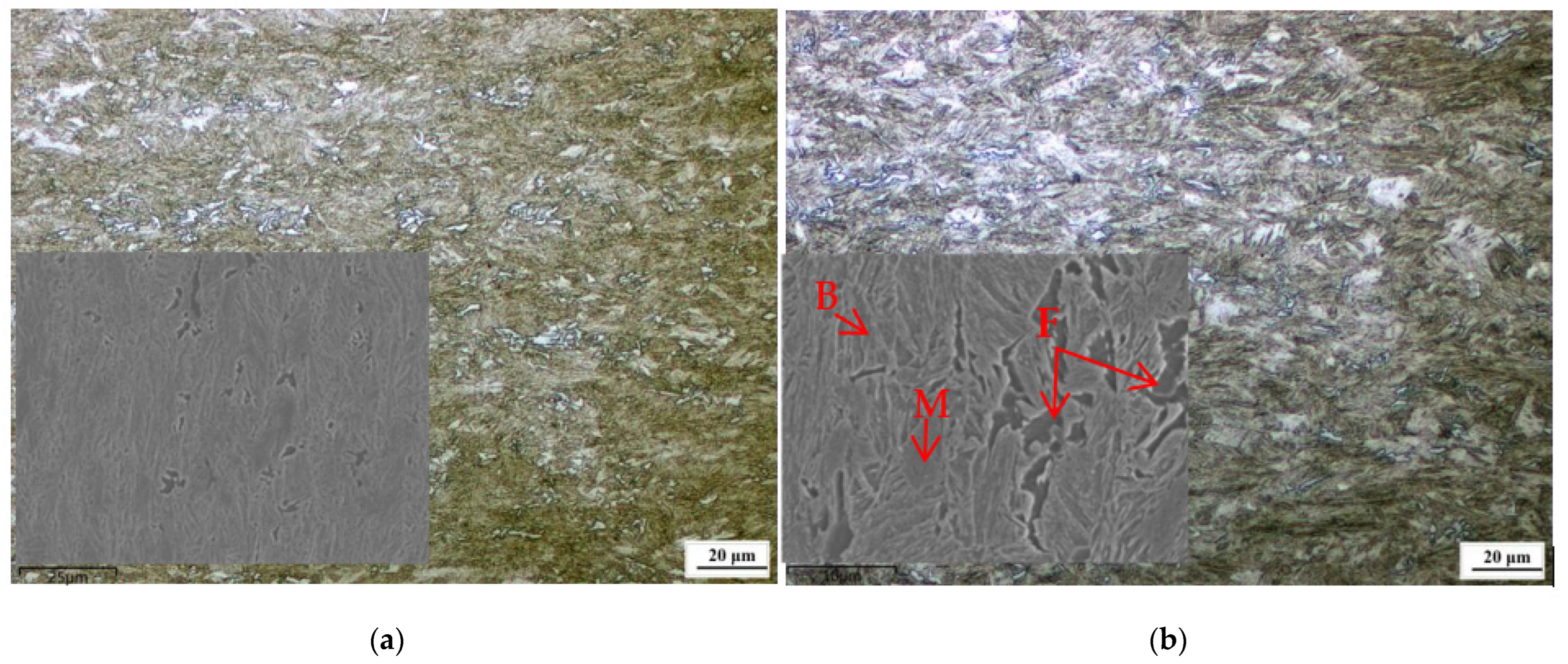
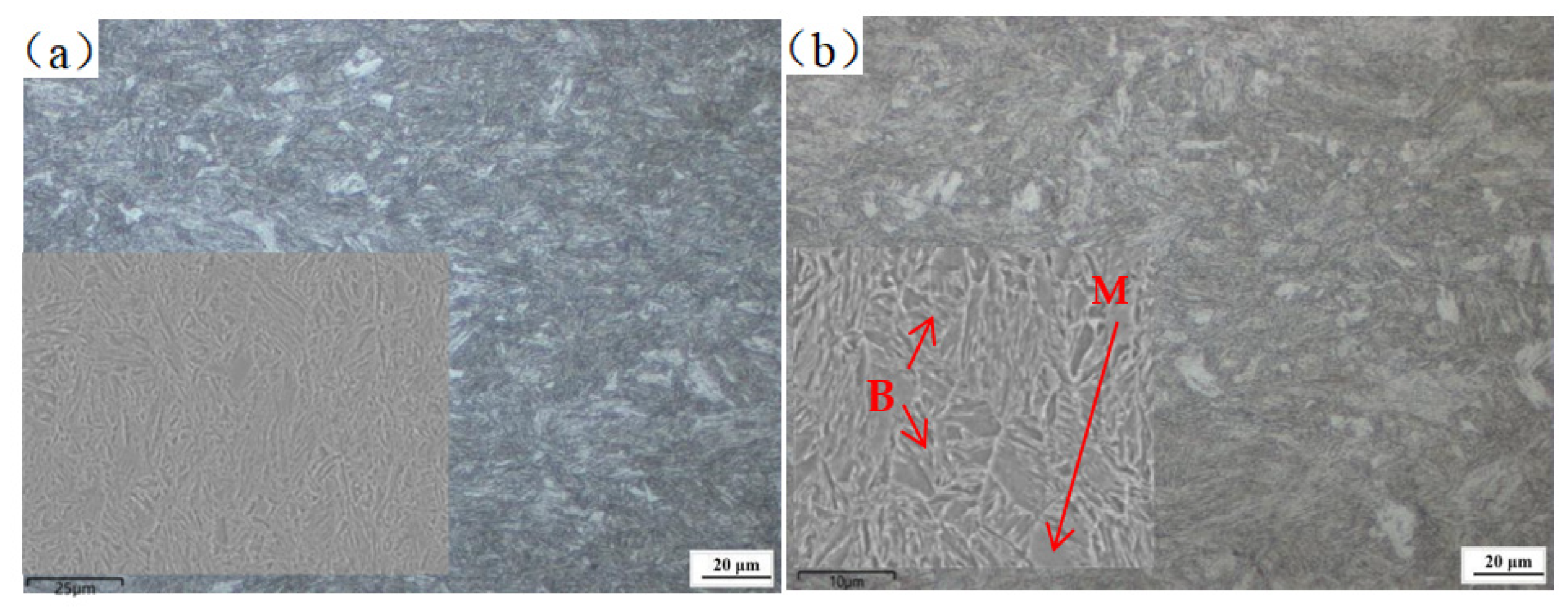
3.2. Effect of Cerium on Microstructure Transformation
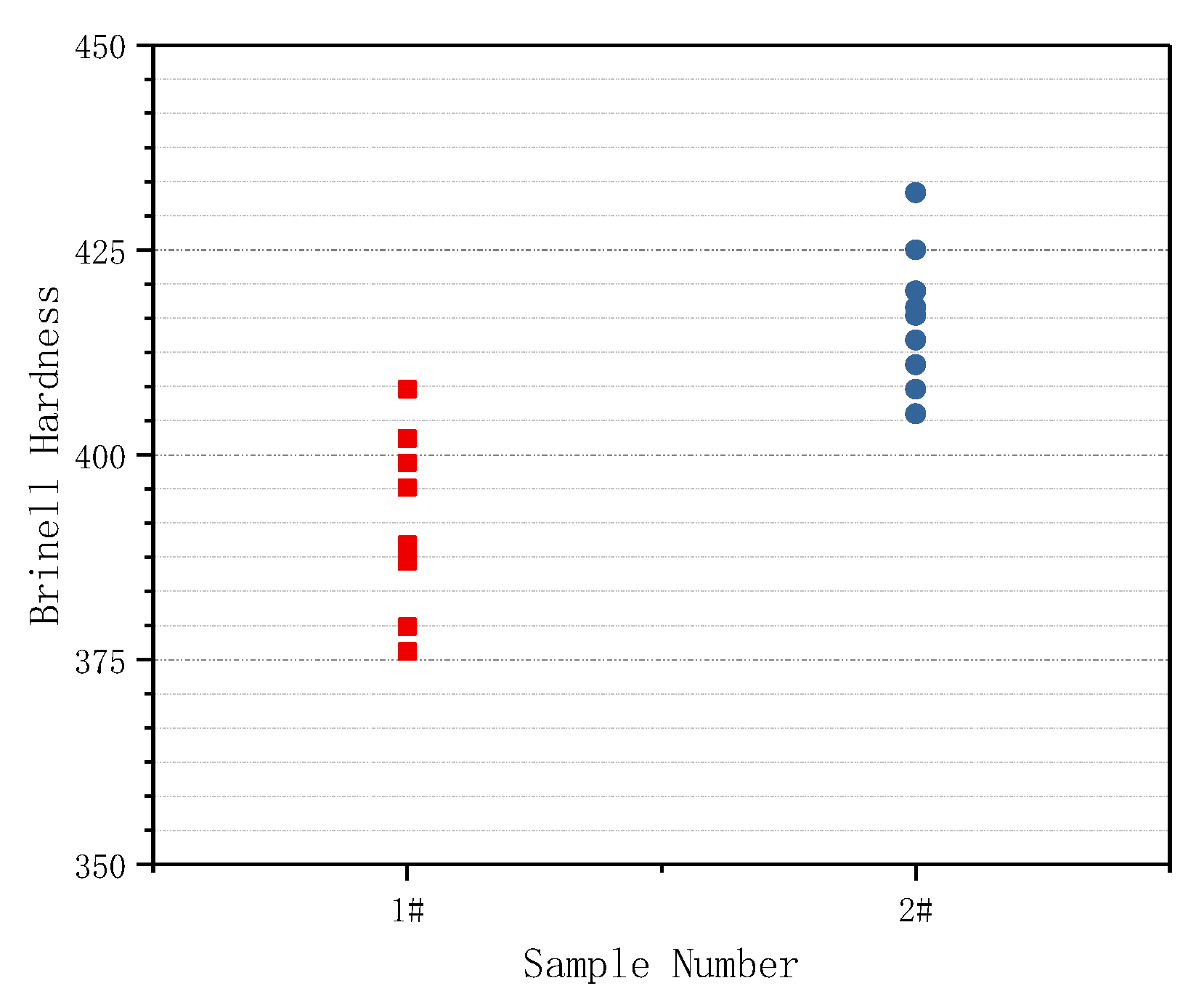
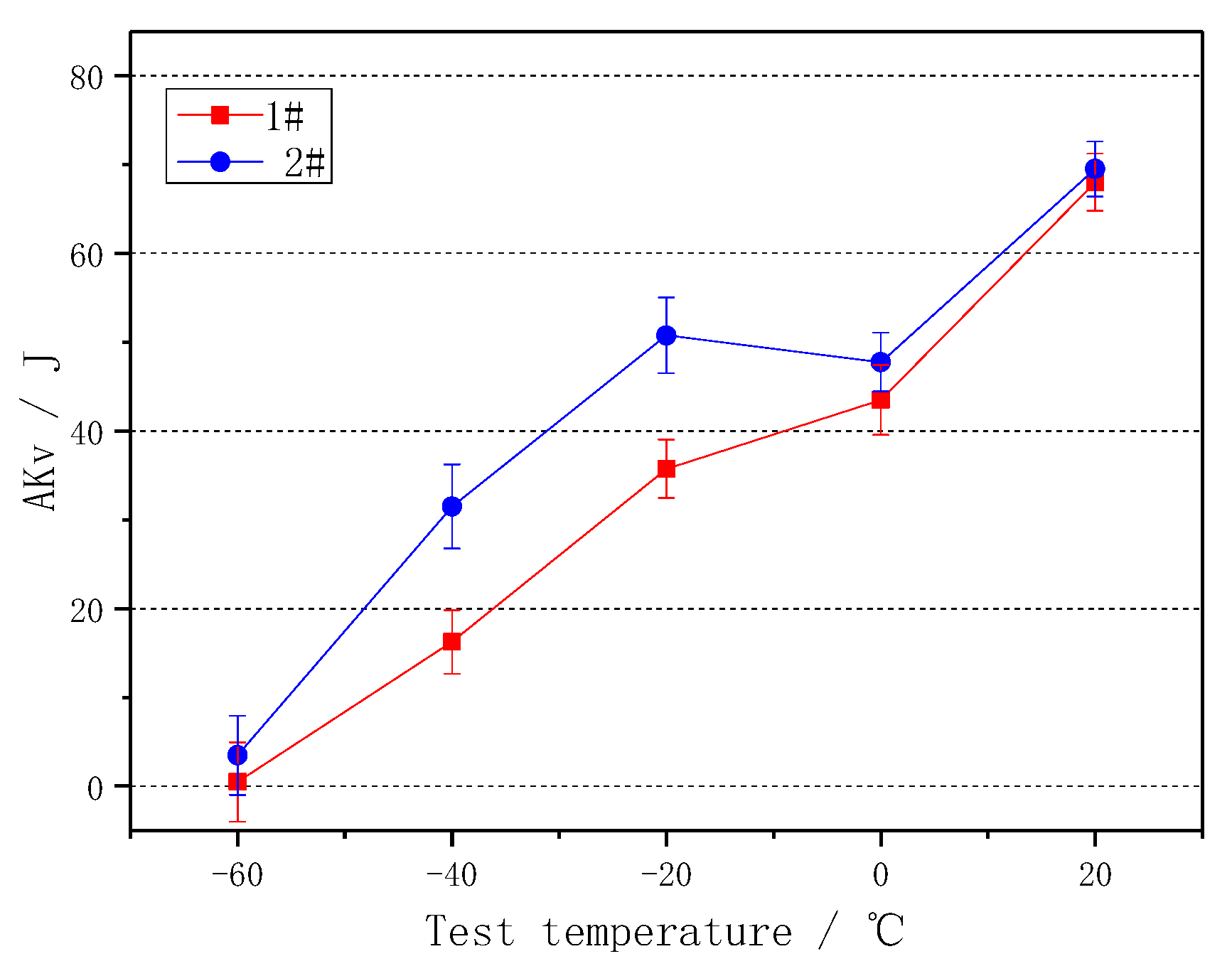
3.3. Effect of Cerium on the Mechanical Properties
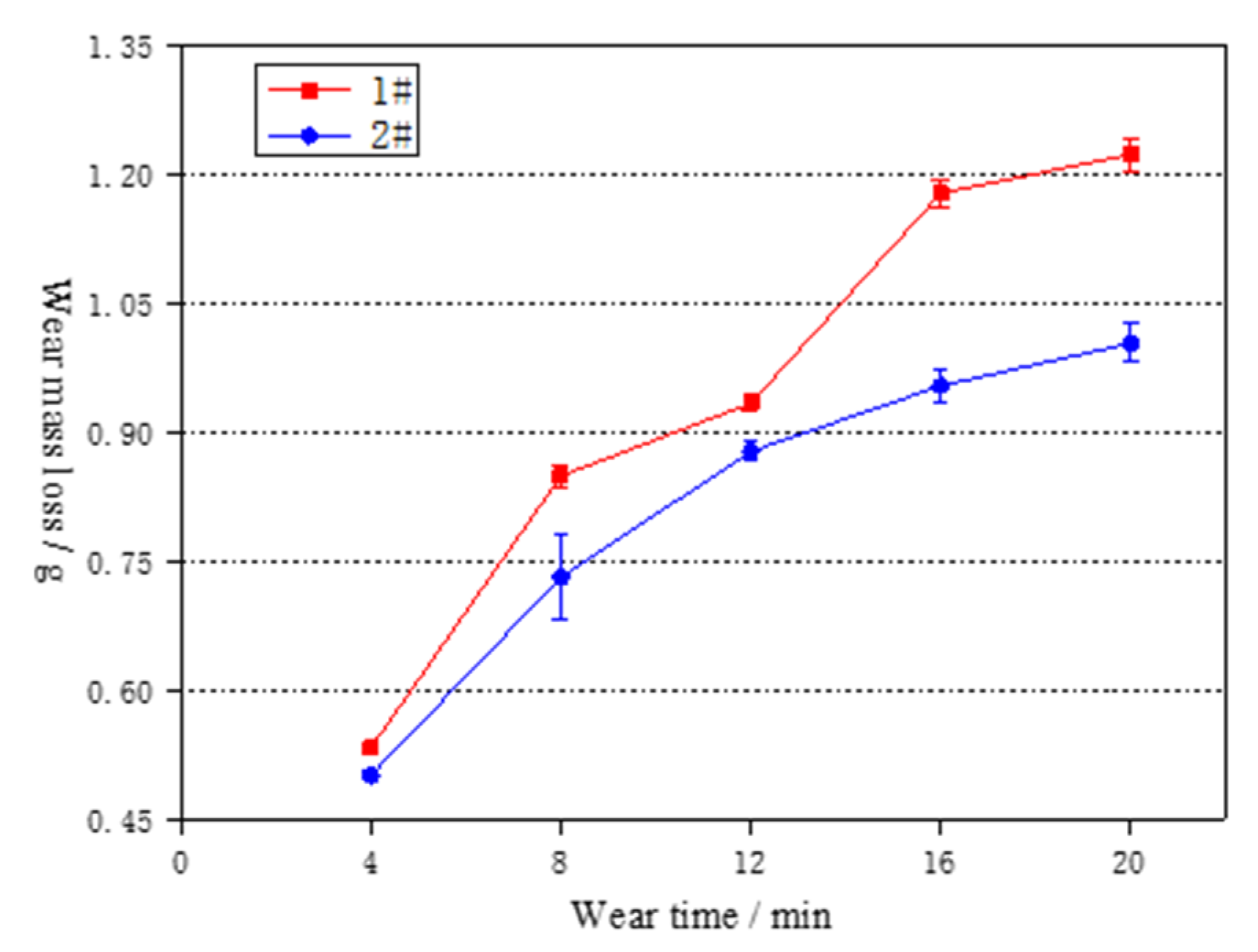
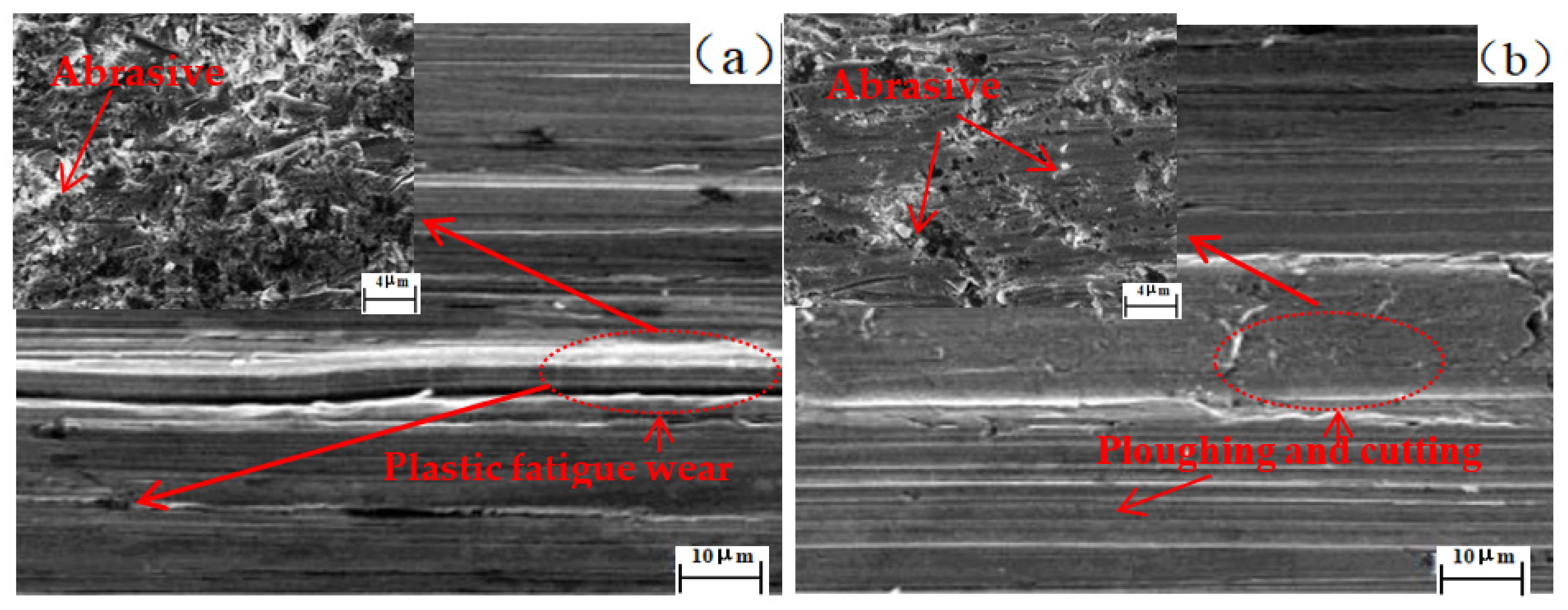
3.4. Effect of Cerium on the Wear Resistance Property
4. Discussion
5. Conclusions
- Adding Ce with a mass fraction of 0.0030% to the low alloy wear-resistant tested steel formed spheroidal CeAlO3 and CeAlO3-MnS with a diameter ≤2.5 μm and elliptic Ce2S2O-CaO with length ≤5 μm, refined the inclusion size, globalized the inclusion morphology and enhanced the binding force of the inclusion to the matrix.
- The addition of Ce in steel significantly refined the size of the as-cast structure, prevented the transformation of proeutectoid ferrite of overcooled austenite and contributed to the formation of bainite ferrite.
- The addition of Ce can improve the yield strength, yield ratio and surface hardness of the tested steel, especially the impact toughness at −40 °C~−20 °C.
- With the prolongation of the test time, the inclined wear amount of the tested steel first increases and then decreases; after adding Ce, the inflection point of the wear loss rate decreases in advance and the wear amount reduces under the same wear time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- An, X.Y. Development and Industrialization of High Strength Low-Alloy Wear Resistant Steel; Metallurgical Industry Press: Beijing, China, 2014. [Google Scholar]
- Lindroos, M.; Valtonen, K.; Kemppainen, A.; Laukkanen, A.; Holmberg, K.; Kuokkala, V.-T. Wear behavior and work hardening of high strength steels in high stress abrasion. Wear 2015, 32, 322–323. [Google Scholar] [CrossRef]
- Dong, X.C.; Zhang, H. Microstructure and Mechanical properties of welded joint of NM450 wear-resistant steel plate. Mater. Mech. Eng. 2018, 42, 45–47. [Google Scholar]
- Wang, L.M. Application of Rare Earth Elements in Low Alloy and Alloy Steel; Metallurgical Industrial Press: Beijing, China, 2016; pp. 15–30. [Google Scholar]
- Li, G.; Lu, M.G.; Lan, P.; Tang, H.Y.; Zhi, J.G.; Zhang, J.Q. Research progress of rare earth Ce on improvement of microstructure and homogeneity of as-cast steel. J. Iron Steel Res. 2018, 30, 80–85. [Google Scholar]
- Su, C.; Feng, G.H. Effect of rare earth on low temperature impact toughness of NM400 wear-resistant coil plate. J. Iron Steel Res. 2012, 33, 1292–1294. [Google Scholar]
- Parthiban, R.; Chowdhury, S.G.; Harikumar, K.C.; Sankaran, S. Evolution of microstructure and its influence on tensile properties in thermo-mechanically controlled processed (TMCP) quench and partition (Q&P) steel. Mater. Sci. Eng. A 2017, 705, 376–384. [Google Scholar]
- Li, X.L.; Wang, Z.D. Effect of one step Q&P Process on Microstructure and Mechanical Properties of a Dual Martensite Steel. Acta Metall. Sin. 2015, 51, 537–544. [Google Scholar]
- Li, H.; Mi, Z.L. Inflence of partition time on mechanical properties and microstructure of Q&P steel. Mater. Rep. 2017, 31, 84–86. [Google Scholar]
- Li, J.; Jia, J. Effect of one-step partitioning process on microstructure and properties of low alloy wear-resistant stee. Mater. Rep. 2019, 33, 3114–3116. [Google Scholar]
- Su, C.; Feng, G.H. Research on development of low alloy NM400 wear-resistant coil plate based on 2250 mm hot continuous rolling mill. Iron Steel 2021, 56, 127–130. [Google Scholar]
- Wen, B.; Song, B. Effect of austenitizing temperature on microstructure in 16Mn steel treated by cerium. Int. J. Miner. Metall. Mater. 2011, 18, 653–656. [Google Scholar] [CrossRef]
- Hao, F.F.; Liao, B. Effects of rare earth oxide on hardfacing metal microstructure of medium carbon steel and its refinement mechanism. J. Rare Earths 2011, 29, 610–615. [Google Scholar] [CrossRef]
- Liang, Y.L.; Tan, Q.B. Effect of rare earth on CCT curves and microstructure of Mn-RE bainite steel. Heat Treat. Met. 1995, 30, 52. [Google Scholar]
- Saastamoinen, A.; Kaijalainen, A.; Porter, D.; Suikkanen, P. The effect of thermomechanical treatment and tempering on the subsurface microstructure and bendability of direct-quenched low-carbon strip steel. Mater. Charact. 2017, 10, 20. [Google Scholar] [CrossRef]
- Zhang, C.L.; Fu, H.G.; Ma, S.Q. Effect of Mn content on microstructure and properties of wear resistant bainitic steell. Mater. Res. Express 2019, 6, 18–20. [Google Scholar]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Feng, X.; Liu, S.; Niu, H.; Tang, Z. Equilibria between cerium or neodynium and oxygen in molten iron. Matallurgical Trans. 1990, 21, 295. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, L.J.; Guo, J.B.; Hu, X.J.; Chou, K.C. Thermodynamic analysis of cerium inclusion formed in spring steel used in fastener of high-speed railway. Chin. J. Nonferrous Met. 2013, 23, 721–724. [Google Scholar]
- Wang, L.M.; Du, T.; Lu, X.L.; Zheng, B.; Gai, Y. Thermodynamics and application of rare earth elements in steel. J. Chin. Rare Earth Soc. 2003, 21, 251–254. [Google Scholar]
- Chen, J.X. Hand Book of Common Datas and Graph in Steel Making; Metallurgical Industry Press: Beijing, China, 2010; pp. 757–761. [Google Scholar]
- Waudby, P.E. Rare earth additions to steel. Int. Met. Rev. 1978, 23, 74. [Google Scholar] [CrossRef]
- Ohashi, T.; Hiromota, T.; Fu, J.H.; Nuri, Y.; Asano, K. Effect of oxides on nucleation behaviour in supercooled iron. Tetsu-Hagane 2010, 62, 614. [Google Scholar] [CrossRef]
- Huang, C.; Song, B.; Mao, J.H.; Zhao, P. The mathematic research of wetting angled during heterogenous nucleation. Sci. China Ser. E Eng. Mater. Sci. 2004, 34, 737. [Google Scholar]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogenous nucleation behavior of liquid iron. Metall. Mater. Trans. 1970, 1, 1987. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Lü, W. Influence of rare earth elements on martensitic transformation in low carbon steel. Iron Steel 1995, 30, 52. [Google Scholar]
- Jiang, Y.Y.; Wang, Z.D.; Deng, X.T. Effect of trace rare earth Ce on martensitic transformation behavior of ultra-high strength low alloy steel. Iron Steel 2020, 55, 86–88. [Google Scholar]
- Zhang, J.Z.; Li, D.Z.; Gao, S.Q.; Zhang, L.F. Study of the properties and thermo-dynamic of CeC2 in steel. J. Gui-Zhou Inst. Technol. 1995, 2, 72. [Google Scholar]
- Zhang, C.L.; Fu, H.G. The effect of silicon on microstructure and wear resistance in bainitic steel. Trans. Indian Inst. Met. 2019, 72, 1231–1244. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Shi, Q.; Lin, Q. Effects of cerium on microalloying in low sulfur Nb-Ti-bearing Steel. J. Chin. Rare Earth Soc. 2004, 22, 666–668. [Google Scholar]
- Costin, W.L.; Lavigne, O.; Kotousov, A. A study on the relationship between microstructure and mechanical properties of acicular ferrite and upper bainite. Mater. Sci. Eng. A 2016, 663, 193–203. [Google Scholar] [CrossRef]
- Kang, S.; Speer, J.G.; Regier, R.W.; Nako, H.; Kennett, S.C.; Findley, K.O. The analysis of bainitic ferrite microstructure in microalloyed plate steels through quantitative characterization of intervariant boundaries. Mater. Sci. Eng. A 2016, 669, 459–468. [Google Scholar] [CrossRef]

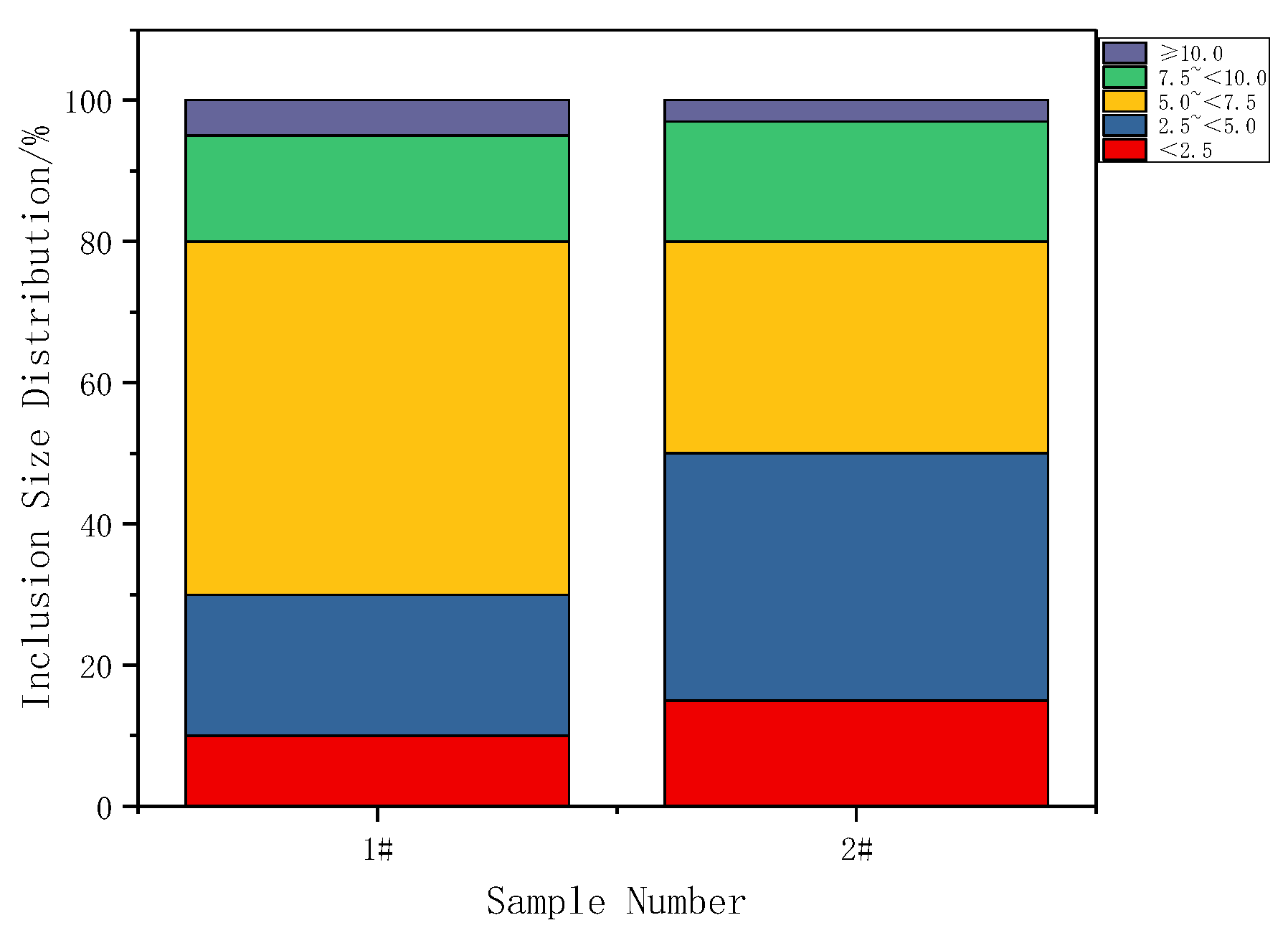

| Sample | C | Si | Mn | Al | P | S | Nb + Ti | Cr | O | Ce (Addition) | Ce (Content) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardox400 | ≤0.32 | ≤0.70 | ≤1.60 | ≥0.015 | ≤0.025 | ≤0.010 | / | ≤2.50 | / | / | / |
| 1# | 0.21 | 0.62 | 1.54 | 0.055 | 0.013 | 0.003 | 0.045 | 0.42 | 0.0030 | / | / |
| 2# | 0.22 | 0.60 | 1.57 | 0.060 | 0.015 | 0.003 | 0.042 | 0.42 | 0.0025 | 0.0100 | 0.0030 |
| Sample | Yield Strength/MPa | Tensile Strength/MPa | Elongation A50/% | Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | |
| 1# | 850 | 1020 | 935 | 1340 | 1400 | 1370 | 9 | 13 | 11 | 0.65 | 0.71 | 0.68 |
| 2# | 980 | 1100 | 1040 | 1380 | 1450 | 1415 | 11 | 14 | 12.5 | 0.70 | 0.76 | 0.73 |
| C | Si | Mn | Al | P | S | Cr | O | Ce | |
|---|---|---|---|---|---|---|---|---|---|
| Ce | −0.077 | - | 0.13 | −2.25 | 1.746 | −39.8 | - | −5.03 | −0.003 |
| O | −0.45 | −0.131 | −0.012 | −3.9 | −0.07 | −0.133 | −0.04 | −0.2 | −0.57 |
| S | 0.11 | 0.063 | −0.026 | 0.035 | 0.029 | −0.028 | −0.011 | −0.27 | −0.231 |
| Al | 0.091 | 0.0051 | 0.012 | 0.045 | 0.05 | 0.03 | 0.025 | −6.6 | −0.043 |
| Reaction Equation | ΔGθ/(J⋅mol−1) | ΔG/(KJ⋅mol−1) |
|---|---|---|
| −930.9 | ||
| −532.6 | ||
| −572.5 | ||
| −202.7 | ||
| −189 | ||
| −67.3 | ||
| 9.7 |
| Heterogeneous Nucleation Particle | Melting Points/°C | Degree of Nucleation Overcooling/°C | Equilibrium Constant Angle/° | Mismatch Degree/% | |
|---|---|---|---|---|---|
| δ | |||||
| Al2O3 | 2054 | 13.9 | 20.5 | 8.0 | 15.5 |
| CeAlO3 | 2050 | * | * | 3.1 | 4.7 |
| Ce2O2S | 1949 | 4.5 | * | 1.2 | 8.5 |
| Ce2O3 | 1692 | 3.0 | 9.4 | 5.0 | 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Feng, G.; Zhi, J.; Zhao, B.; Wu, W. The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel. Metals 2022, 12, 1358. https://doi.org/10.3390/met12081358
Su C, Feng G, Zhi J, Zhao B, Wu W. The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel. Metals. 2022; 12(8):1358. https://doi.org/10.3390/met12081358
Chicago/Turabian StyleSu, Cheng, Guanghong Feng, Jianguo Zhi, Bo Zhao, and Wei Wu. 2022. "The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel" Metals 12, no. 8: 1358. https://doi.org/10.3390/met12081358
APA StyleSu, C., Feng, G., Zhi, J., Zhao, B., & Wu, W. (2022). The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel. Metals, 12(8), 1358. https://doi.org/10.3390/met12081358







