On the Tribocorrosion Behavior of Fe-Mn-Al-C Alloys in Ringer’s Solution
Abstract
:1. Introduction
2. Materials and Methods
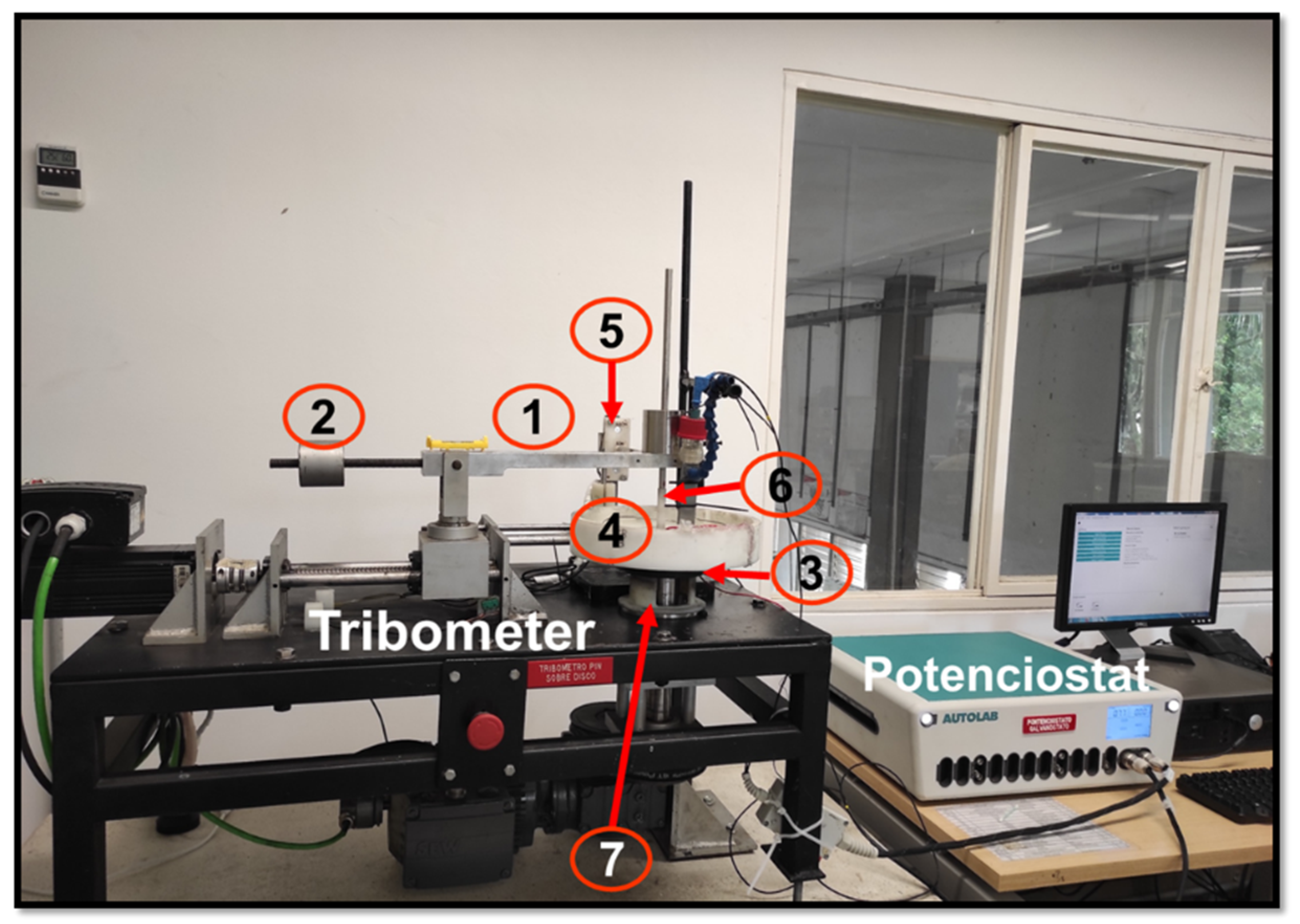
2.1. Tribocorrosion Test
- Level arm with a stem to hold and position the counterbody to the desired radius.
- Counterweight to balance the level arm in a horizontal position without any load.
- Disc, which was attached to a rotating shaft.
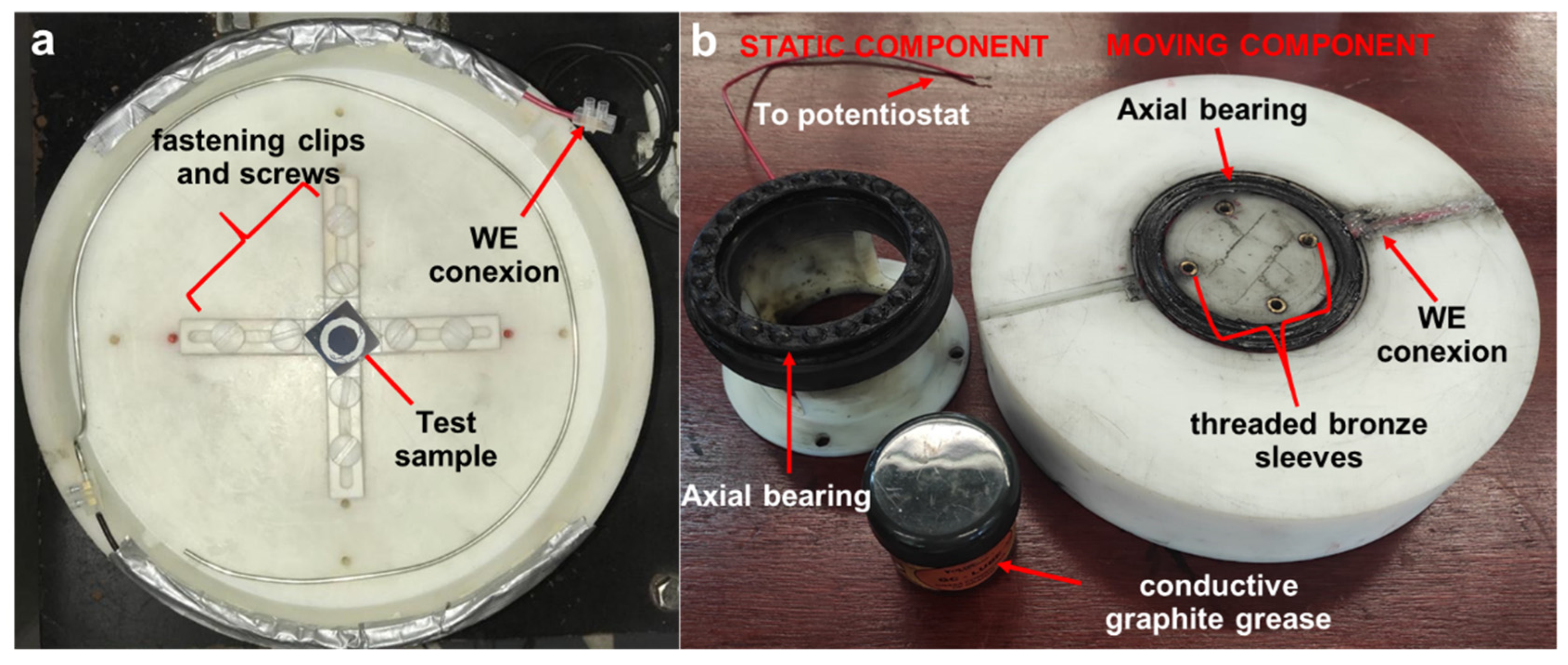
- Corrosion cell was coupled to the rotating disc by means of clamping screws. At the bottom of the cell, the working electrode (test sample) was held by means of fastening clips and screws that were located on four lines at 90° of threaded holes on the cell bottom surface (Figure 2a); the whole part (test sample plus corrosion cell) presented the rotational movement. To enhance the adjustment of the cell to the shaft and avoid subsequent wear of the cell, threaded bronze sleeves were adapted on the lower face of the cell.
- Load cell holder to hold the load cells and to measure the tangential force generated by the tribological pair.
- Counterbody holder to hold the ZrO3 ball.
- The last part of the device corresponds to the electrical adaptation in which another device was built to keep the electrical contact between the potentiostat and the working electrode during the tests (Figure 2b). The main element to transmit the electric current was an axial bearing. A wire was welded to the bearing in the moving component to make contact to the working electrode and another one to the static component to link to the potentiostat. To improve the electrical conductivity between both parts, conductive graphite grease was applied periodically.
- Corrosion test to measure the corrosion rate, C0: the samples were immersed into the solution during 20 min until a stable open circuit potential (OCP) was obtained. After this time, a potential sweep of 200 mV was applied above and below the OCP value (OCP ± 200 mV) at step size of 0.15 mV and at a scan rate of 0.16 mV/s according to the ASTM G5 standard [31]. From this measurement, corrosion current density (Icorr) and corrosion potential (Ecorr) were obtained by means of the Tafel extrapolation method. The values were computed by means of linear fitting. The rules that were applied to the extrapolation methods according to some authors [32,33]: at least one of the branches of the polarization curve should exhibit Tafel behavior over at least one decade of current density and the extrapolation should start at least 50 to 100 mV away from Ecorr.
- Pure sliding wear test under cathodic protection to obtain the wear rate, W0: a load of 10 N was applied to the sphere–sample contact, which corresponds to mean pressures of 1304, 1287 and 1271 MPa estimated using the Hertz contact theory and corresponding to an initial contact radius about 0.0605, 0.0608 mm and 0.00613 mm for the 0Al, 3Al and 8Al, respectively. However, these contact pressures are enough to produce plastic deformation of the material. Tangential speed was 2 mm/s and the positioning radius of the counterbody was fixed in about 8 mm.
- 3.
- Wear-corrosion test to find the total wear rate, T: the wear test described in item (2) was repeated without cathodic protection, i.e., with the test sample exposed to the electrolyte without applying any potential and the evolution of the open circuit potential was studied without the polarization of the sample. The OCP recording was started and measured during 20 min before starting the wear test. At the end of the wear test, the behavior of the OCP was measured during the same time interval as the beginning.
- 4.
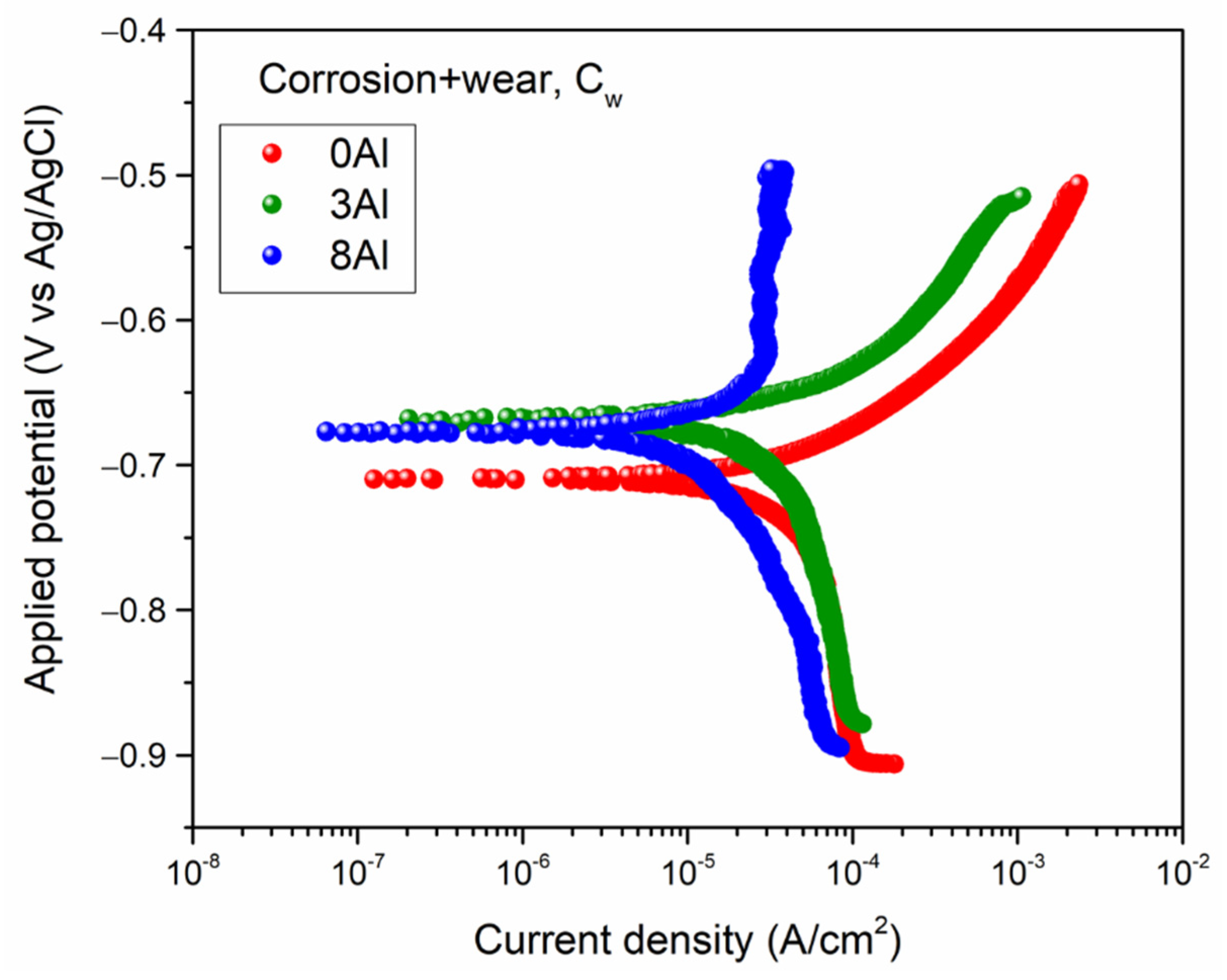
- Wear-corrosion test to find the corrosion rate under the action of wear, Cw: the tests described in items (1) and (2) were repeated without cathodic protection. The wear test was started and the OCP was allowed to stabilize for 20 min (the time should be long enough to allow the potential to stabilize, but it should not be long either to avoid excessive wear of the sample). After 20 min, the linear polarization test was started and at the end, the sample was removed to avoid further loss of dimensions and for further analysis. From this measurement, corrosion current density (Icorr) and corrosion potential (Ecorr) were obtained by means of Tafel extrapolation method.
2.2. Statistical Analysis
3. Results and Discussion
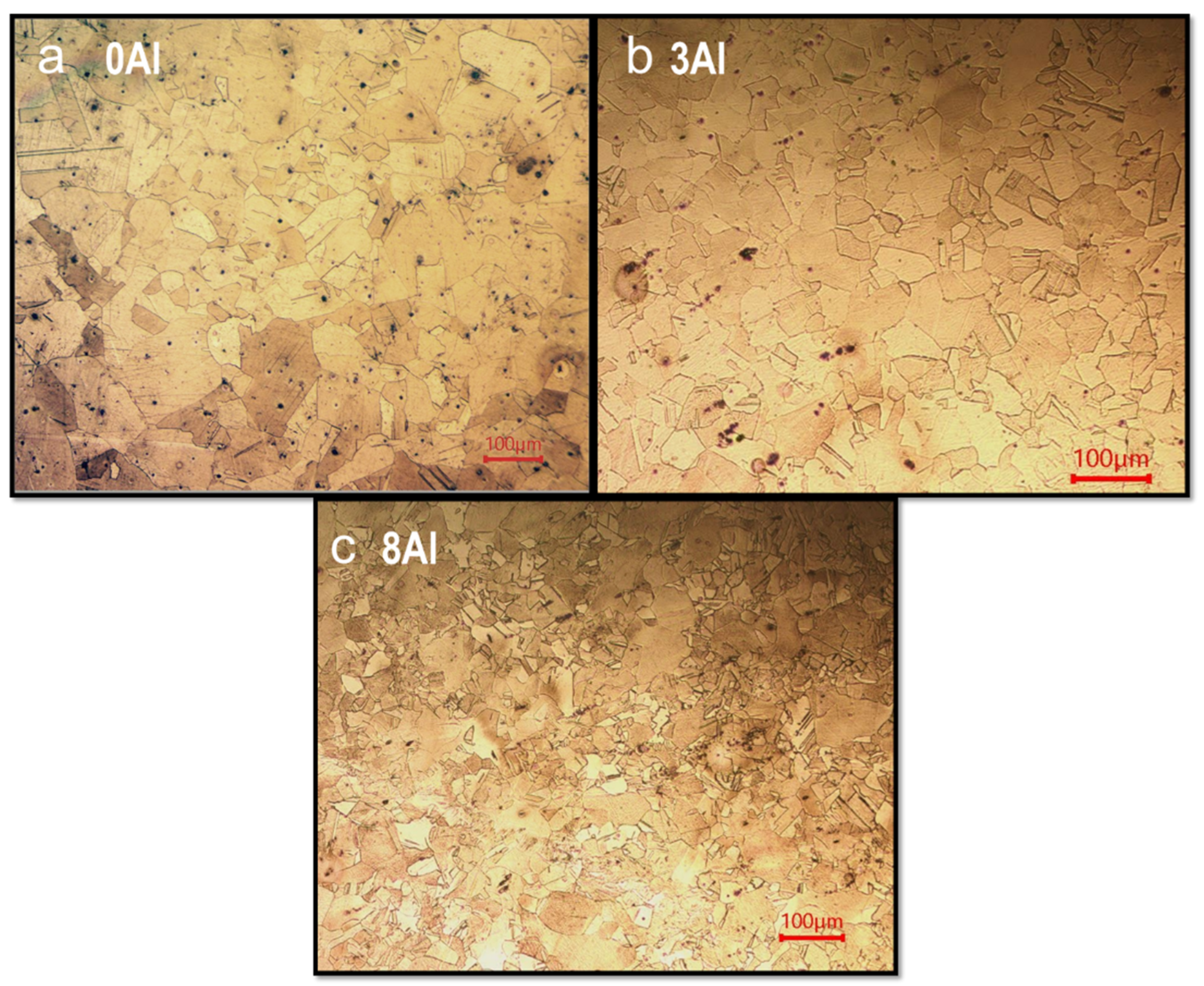
3.1. Microstructural Characterization
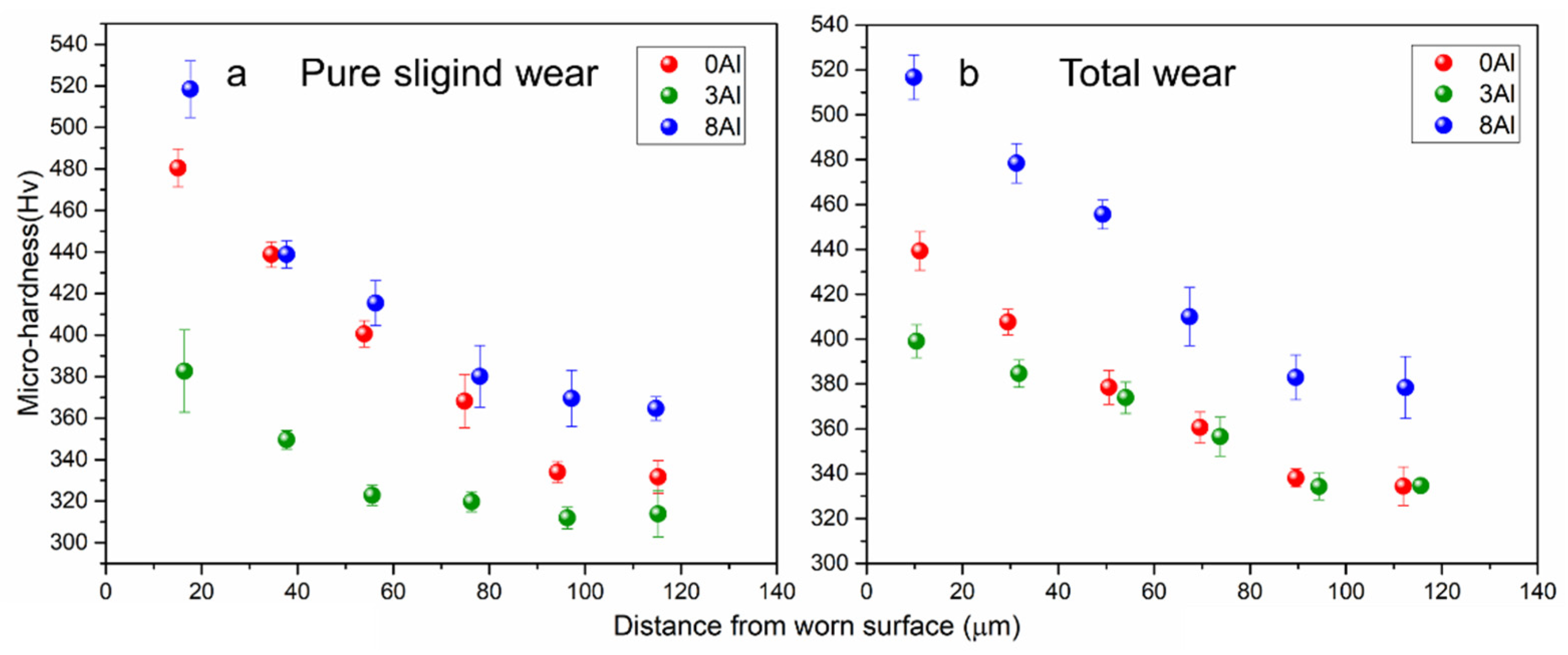
3.2. Hardness and Micro-Hardness
3.3. Corrosion Measurements, C0
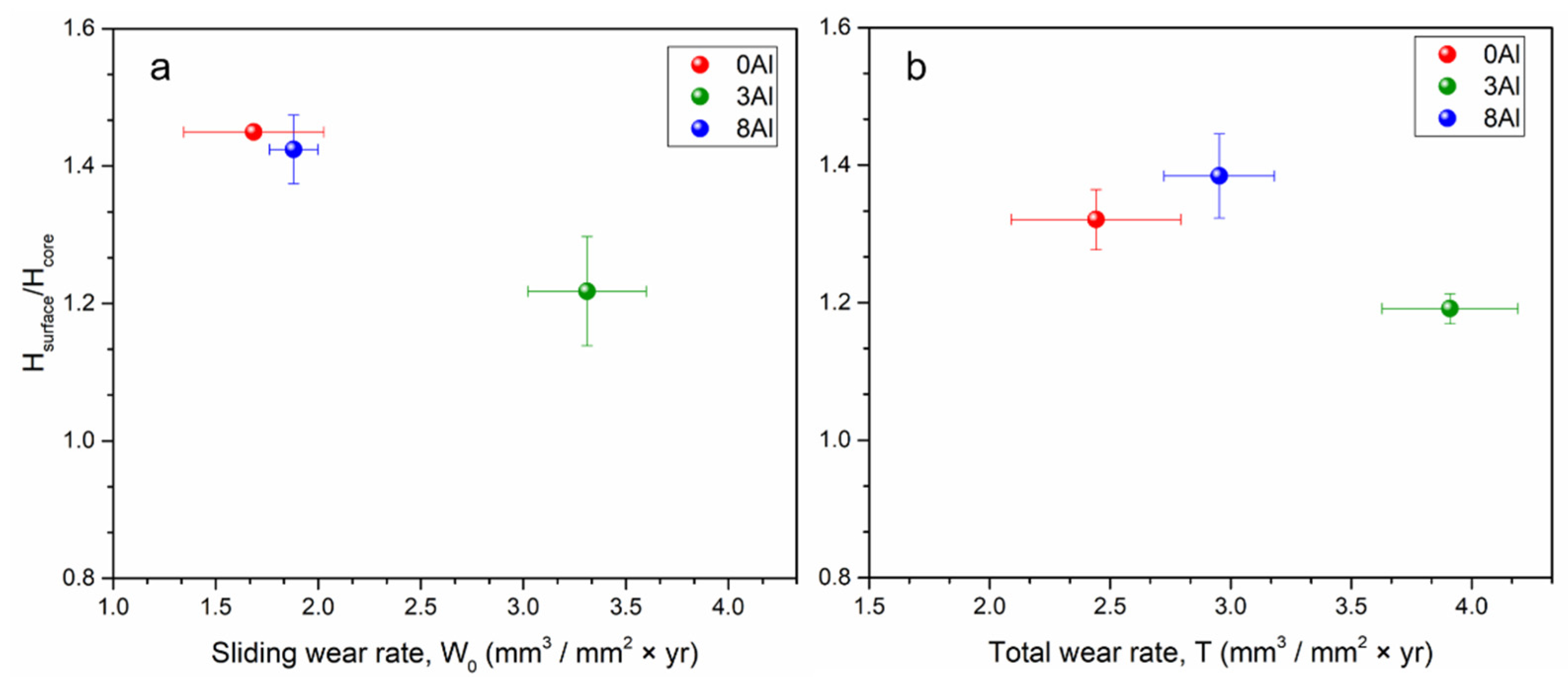
3.4. Pure Sliding Wear Measurements, W0
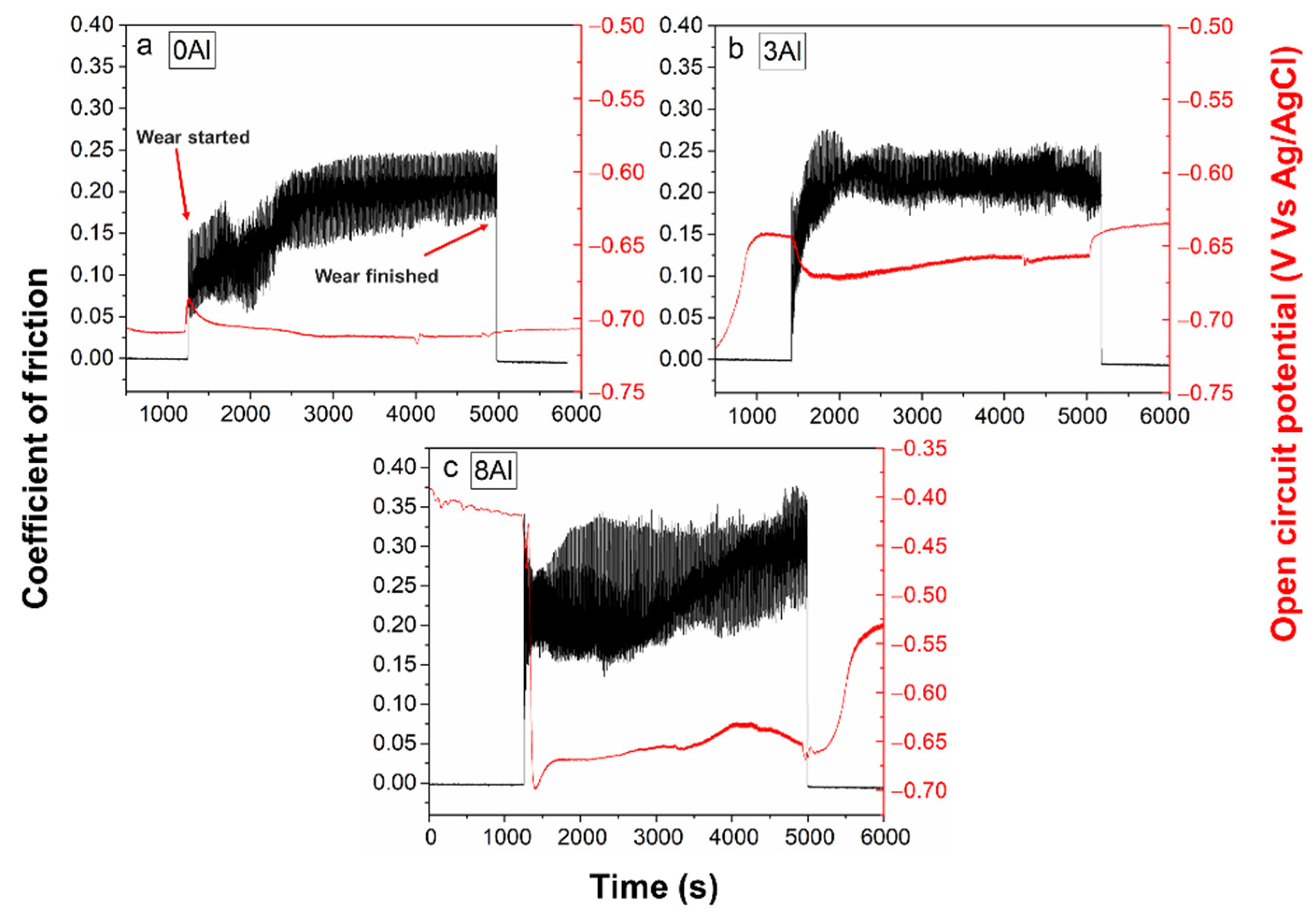
3.5. Total Wear Measurements, T
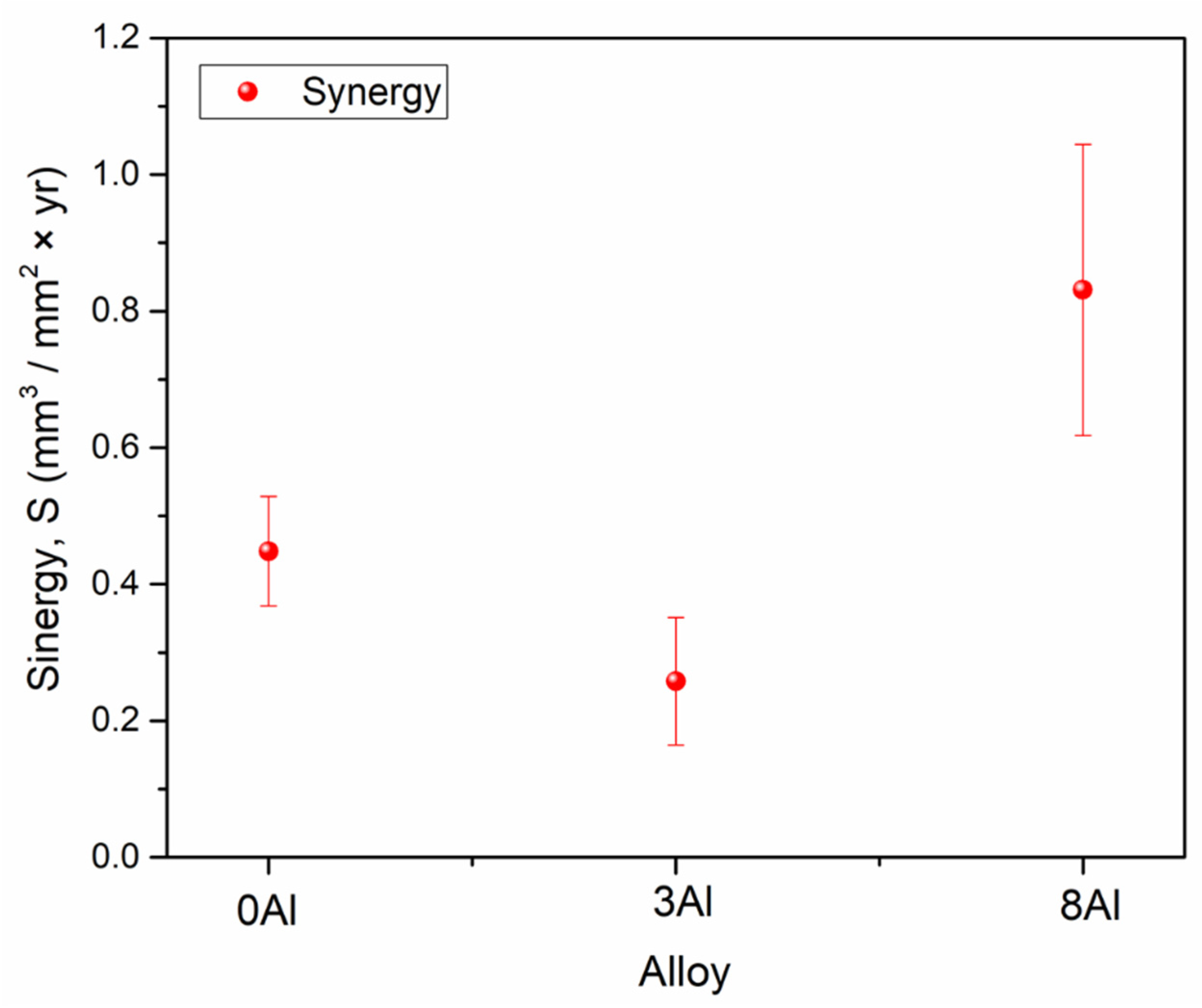
3.6. Corrosion + Wear Measurements, Cw
4. Conclusions
- The 0Al alloy showed the highest tendency to corrosion and the 8Al alloy showed the lowest tendency to corrosion and corrosion rate, which is due to the fact that Al reduces the ability to form other type of oxides and impurities and promotes the formation of a stable oxide on the surface.
- Total wear rates increased compared to the pure sliding wear test, indicating that corrosion reduces the wear resistance of the materials in the evaluated system. In addition, for all materials, the largest contribution to the total wear rate was due to pure sliding wear.
- The change in the wear rate due to corrosion provided the main contribution to the synergism and was greater for the 8Al alloy.
- An increase in micro-hardness after wear (total and pure) with respect to the initial micro-hardness was observed, which indicates the formation of a hardened layer produced by plastic deformation. The layer was not supported by the base material, mainly in the 3Al alloy, producing a greater removal of the material due to the consecutive friction with the counterbody.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbasi, M.; Kheirandish, S.; Kharrazi, Y.; Hejazi, J. The Fracture and Plastic Deformation of Aluminum Alloyed Hadfield Steels. Mater. Sci. Eng. A 2009, 513–514, 72–76. [Google Scholar] [CrossRef]
- Bronz, A.V.; Kaputkin, D.E.; Kaputkina, L.M.; Kindop, V.E.; Svyazhin, A.G. Mechanical and Physical Properties of Cast Fe-Mn-Al-C-N Alloys. Steel Transl. 2015, 44, 804–807. [Google Scholar] [CrossRef]
- Ramos, J.; Piamba, J.F.; Sánchez, H.; Pérez Alcazar, G.A. Mossbauer and XRD Characterization of the Phase Transformations in a Fe-Mn-Al-C-Mo-Si-Cu as Cast Alloy during Tribology Test. Hyperfine Interact. 2015, 232, 119–126. [Google Scholar] [CrossRef]
- Kim, J.-K.; De Cooman, B.C. Stacking Fault Energy and Deformation Mechanisms. Mater. Sci. Eng. A 2016, 676, 216–231. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Valdés, J.; Rodriguez, L.A.; Reyes, D.; Snoeck, E.; Rodríguez, S.A.; Coronado, J.J. Elucidating the Role of Κ-Carbides in Fe–Mn–Al–C Alloys on Abrasion Wear. Tribol. Int. 2019, 135, 421–431. [Google Scholar] [CrossRef]
- Kim, J.K.; Estrin, Y.; De Cooman, B.C. Constitutive Modeling of the Stacking Fault Energy-Dependent Deformation Behavior of Fe-Mn-C-(Al) TWIP Steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 5919–5924. [Google Scholar]
- Kim, J.; Estrin, Y.; De Cooman, B.C. Application of a Dislocation Density-Based Constitutive Model to Al-Alloyed TWIP Steel. Metall. Mater. Trans. A 2013, 44A, 4168–4182. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Aguilar, Y.; Valdés, J.; Rodríguez, S.A.; Coronado, J.J. Effect of Normal Load on Abrasive Wear Resistance and Wear Micromechanisms in FeMnAlC Alloy and Other Austenitic Steels. Wear 2016, 348–349, 61–68. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Estrin, Y.; Kim, S.K. Twinning-Induced Plasticity (TWIP) Steels. Acta Mater. 2018, 142, 283–362. [Google Scholar] [CrossRef]
- Bouaziz, O.; Allain, S.; Scott, C.P.; Cugy, P.; Barbier, D. High Manganese Austenitic Twinning Induced Plasticity Steels: A Review of the Microstructure Properties Relationships. Curr. Opin. Solid State Mater. Sci. 2011, 15, 141–168. [Google Scholar] [CrossRef]
- Zambrano, O.A. Stacking Fault Energy Maps of Fe-Mn-Al-C Steels: Effect of Temperature, Grain Size and Variations in Compositions. J. Eng. Mater. Technol. 2016, 138, 041010. [Google Scholar] [CrossRef]
- Dai, Q.X.; Wang, A.D.; Cheng, X.N. Stacking Fault Energy of Cryogenic Austenitic Steel. J. Iron Steel Res. 2002, 14, 34–37. [Google Scholar]
- Dumay, A.; Chateau, J.P.; Allain, S.; Migot, S.; Bouaziz, O. Influence of Addition Elements on the Stacking-Fault Energy and Mechanical Properties of an Austenitic Fe-Mn-C Steel. Mater. Sci. Eng. A 2008, 483–484, 184–187. [Google Scholar] [CrossRef]
- Park, K.T.; Jin, K.G.; Han, S.H.; Hwang, S.W.; Choi, K.; Lee, C.S. Stacking Fault Energy and Plastic Deformation of Fully Austenitic High Manganese Steels: Effect of Al Addition. Mater. Sci. Eng. A 2010, 527, 3651–3661. [Google Scholar] [CrossRef]
- Abbasi, M.; Kheirandish, S.; Kharrazi, Y.; Hejazi, J. On the Comparison of the Abrasive Wear Behavior of Aluminum Alloyed and Standard Hadfield Steels. Wear 2010, 268, 202–207. [Google Scholar] [CrossRef]
- Arana, J.L. Tribocorrosion of Passive Materials: A Review on Test Procedures and Standards. Int. J. Corros. 2018, 2018, 7345346. [Google Scholar]
- Mischler, S. Triboelectrochemical Techniques and Interpretation Methods in Tribocorrosion: A Comparative Evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical Materials and Techniques to Improve the Tribological, Mechanical and Biomedical Properties of Orthopedic Implants—A Review Article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Mathew, M.T.; Wimmer, M.A.; Pourzal, R.; Fischer, A.; Srinivasa Pai, P. Significance of Tribocorrosion in Biomedical Applications: Overview and Current Status. Adv. Tribol. 2009, 2009, 250986. [Google Scholar] [CrossRef]
- Azzi, M.; Szpunar, J.A. Tribo-Electrochemical Technique for Studying Tribocorrosion Behavior of Biomaterials. Biomol. Eng. 2007, 24, 443–446. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R.P. Metals for Biomedical Applications. In Biomedical Engineering—From Theory to Applications; Fazel-Rezai, R., Ed.; InTech: London, UK, 2011; pp. 411–430. [Google Scholar]
- Zhang, Y.S.; Zhu, X.M. Electrochemical Polarization and Passive Film Analysis of Austenitic Fe-Mn-Al Steels in Aqueous Solutions. Corros. Sci. 1999, 41, 1817–1833. [Google Scholar] [CrossRef]
- Hamada, A.S.; Karjalainen, L.P.; El-Zeky, M.A. Effect of Anodic Passivation on the Corrosion Behaviour of Fe-Mn-Al Steels in 3.5%NaCl; Elsevier B.V.: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Chen, P.C.; Chao, C.G.; Liu, T.F. A Novel High-Strength, High-Ductility and High-Corrosion-Resistance FeAlMnC Low-Density Alloy. Scr. Mater. 2013, 68, 380–383. [Google Scholar]
- Hsueh, S.J.; Huang, J.Y.; Chao, C.G.; Juang, J.Y.; Liu, T.F. Mechanical Behavior and Electrochemical Stability of Gas-Nitrided FeMnAlC Alloy in Simulated Body Fluid. Mater. Lett. 2018, 216, 150–153. [Google Scholar]
- Villamizar, J.; Blanco, K.; Rivera, M.; Vargas, A.; Aperado, W.; Vera, E.; Pineda, Y. Evaluacion del Desempeño Triboquimico de Aleaciones Fermanal en Solucion de Hank’s. Rev. Colomb. Mater. 2013, 5, 331–341. [Google Scholar]
- Tsay, G.; Lin, C.; Chao, C.; Liu, T. A New Austenitic FeMnAlCrC Alloy with High-Strength, High-Ductility, and Moderate Corrosion Resistance. Mater. Trans. 2010, 51, 2318–2321. [Google Scholar]
- Montero-Ocampo, C.; Lopez, H.; Salinas Rodriguez, A. Effect of Compressive Straining on Corrosion Resistance of a Shape Memory Ni-Ti Alloy in Ringer’s Solution. J. Biomed. Mater. Res. 1996, 32, 583–591. [Google Scholar] [CrossRef]
- Kumar, S.; Narayanan, T.S.N.S. Electrochemical Characterization of B-Ti Alloy in Ringer’s Solution for Implant Application. J. Alloys Compd. 2009, 479, 699–703. [Google Scholar] [CrossRef]
- G119-93; Standard Guide for Determining Synergism between Wear and Corrosion. ASTM International: West Conshohocken, PA, USA, 1994; pp. 1–7.
- G5; Making Potentiodynamic Anodic Polarization. ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–8.
- Berradja, A. Electrochemical Techniques for Corrosion and Tribocorrosion Monitoring: Methods for the Assesment of Corrosion Rates. In Corrosion Inhibitors; InTech: London, UK, 2019. [Google Scholar] [CrossRef]
- Badea, G.E.; Caraban, A.; Sebesan, M.; Dzitac, S.; Cret, P.; Setel, A. Polarisation Measurements Used for Corrosion Rates Determination. J. Sustenable Energy 2010, 1, 1–4. [Google Scholar]
- E112-12; Standard Test Methods for Determining Average Grain Size. ASTM: West Conshohocken, PA, USA, 2010; pp. 1–27.
- Frommeyer, G.; Drewes, E.J.; Engl, B. Physical and Mechanical Properties of Iron-Aluminium-(Mn, Si) Lightweight Steels. Rev. Métall. 2000, 97, 1245–1253. [Google Scholar] [CrossRef]
- Sutou, Y.; Kamiya, N.; Umino, R.; Ohnuma, L.; Ishida, K. High-Strength Fe-20Mn-Al-C-Based Alloys with Low Density. ISIJ Int. 2010, 50, 893–899. [Google Scholar] [CrossRef]
- Sato, K.; Tagawa, K.; Inoue, Y. Modulated Structure and Magnetic Properties of Age-Hardenable Fe-Mn-Al-C Alloys. Metall. Trans. A 1990, 21, 5–11. [Google Scholar]
- Choo, W.K.; Kim, J.H.; Yoon, J.C. Microstructural Change in Austenitic Fe-30.0wt%Mn-7.8wt%Al-1.3wt%C Initiated by Spinodal Decomposition and Its Influence on Mechanical Properties. Acta Metall. 1997, 45, 4877–4885. [Google Scholar]
- Choi, W.S.; De Cooman, B.C.; Sandlöbes, S.; Raabe, D. Size and Orientation Effects in Partial Dislocation-Mediated Deformation of Twinning-Induced Plasticity Steel Micro-Pillars. Acta Mater. 2015, 98, 391–404. [Google Scholar] [CrossRef]
- Castañeda, J.A.; Zambrano, O.A.; Alcázar, G.A.; Rodríguez, S.A.; Coronado, J.J. Stacking Fault Energy Determination in Fe-Mn-Al-c Austenitic Steels by X-ray Diffraction. Metals 2021, 11, 1701. [Google Scholar]
- Lehnhoff, G.R.; Findley, K.O.; De Cooman, B.C. The Influence of Silicon and Aluminum Alloying on the Lattice Parameter and Stacking Fault Energy of Austenitic Steel. Scr. Mater. 2014, 92, 19–22. [Google Scholar]
- Yoo, J.D.; Hwang, S.W.; Park, K.T. Origin of Extended Tensile Ductility of a Fe-28Mn-10Al-1C Steel. Metall. Mater. Trans. A 2009, 40, 1520–1523. [Google Scholar] [CrossRef]
- Xing, J.; Wei, Y.; Hou, L. An Overview of the Effects of Alloying Elements on the Properties of Lightweight Fe-(15–35) Mn-(5–12) Al-(0.3–1.2) C Steel. Jom 2018, 70, 929–937. [Google Scholar]
- Dieudonné, T.; Marchetti, L.; Wery, M.; Miserque, F.; Tabarant, M.; Chêne, J.; Allely, C.; Cugy, P.; Scott, C.P. Role of Copper and Aluminum on the Corrosion Behavior of Austenitic Fe-Mn-C TWIP Steels in Aqueous Solutions and the Related Hydrogen Absorption. Corros. Sci. 2014, 83, 234–244. [Google Scholar]
- Sarlak, M.; Shahrabi, T.; Zamanzade, M. Investigation of Calcareous Deposits Formation on Copper and 316L Stainless Steel under Cathodic Polarization in Artificial Seawater. Prot. Met. Phys. Chem. Surfaces 2009, 45, 216–222. [Google Scholar]
- Hartt, W.H.; Kim, K. Characteristics of Cathodic Protection and Calcareous Deposits for Type 316L Stainless Steel in Simulated Deep Sea Conditions. In Proceedings of the NACE-Corrosion Conference, San Diego, CA, USA, 11–16 March 2006. [Google Scholar]
- Hughes, D.A. Microstructural Evolution in a Non-Cell Forming Metal: AlMg. Acta Metall. Mater. 1993, 41, 1421–1430. [Google Scholar]
- Grajcar, A.; Plachci, A.; Topolska, S.; Kciuk, M. Effect of Thermomechanical Treatment on the Corrosion Behaviour of Si- and Al-Containing High-Mn Austenitic Steel with Nb and Ti Micro-Additions. Mater. Technol. 2015, 49, 889–894. [Google Scholar] [CrossRef]
- Di Schino, A.; Barteri, M.; Kenny, J.M. Grain Size Dependence of Mechanical, Corrosion and Tribological Properties of High Nitrogen Stainless Steels. J. Mater. Sci. 2003, 38, 3257–3262. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Kwon, O.; Chin, K.G. State-of-the-Knowledge on TWIP Steel. Mater. Sci. Technol. 2012, 28, 513–527. [Google Scholar]
- Mao, B.; Chu, S.; Wang, S. Effect of Grain Size on the Friction-Induced Martensitic Transformation and Tribological Properties of 304 Austenite Stainless Steel. Metals 2020, 10, 1246. [Google Scholar]
- Zhang, X.; Sawaguchi, T.; Ogawa, K.; Yin, F.; Zhao, X. Orientation Dependence of Variant Selection and Intersection Reactions of ε Martensite in a High-Manganese Austenitic Steel. Philos. Mag. Lett. 2011, 91, 563–571. [Google Scholar]
- Farias, M.C.M.; Souza, R.M.; Sinatora, A.; Tanaka, D.K. The Influence of Applied Load, Sliding Velocity and Martensitic Transformation on the Unlubricated Sliding Wear of Austenitic Stainless Steels. Wear 2007, 263, 773–781. [Google Scholar] [CrossRef]






| Alloy | Composition (wt.%) | |||
|---|---|---|---|---|
| Mn | Al | C | Fe | |
| 0Al | 20.5 | 0.0 | 0.87 | Balance |
| 3Al | 22.2 | 3.5 | 0.84 | Balance |
| 8Al | 22.1 | 8.3 | 0.89 | Balance |
| Composition | Weight (g) |
|---|---|
| NaCl | 9.00 |
| KCl | 0.43 |
| CaCl2 dihydrate | 0.24 |
| NaHCO3 | 0.20 |
| Alloy | Spectrum | O | S | Mn | Al | Fe | Mo | N |
|---|---|---|---|---|---|---|---|---|
| 0Al | 1 | 11.30 | - | 18.18 | - | 68.61 | 1.91 | |
| 2 | 10.57 | 0.69 | 19.73 | - | 69.01 | - | ||
| 12.01 | 0.83 | 19.18 | 67.99 | - | ||||
| 8.89 | - | 18.73 | 72.37 | - | ||||
| 3 | 8.20 | - | 18.79 | 73.01 | - | |||
| 3Al | 1 | 11.95 | - | 13.58 | 27.91 | 39.96 | - | 6.60 |
| 2 | - | - | 22.77 | 2.56 | 74.67 | - | - | |
| 3 | 11.38 | - | 19.19 | 4.27 | 65.16 | - | - | |
| 4 | - | - | 24.87 | 2.94 | 72.19 | - | - | |
| 8Al | 1 | 10.21 | - | 12.56 | 28.80 | 38.41 | - | 10.02 |
| 2 | 5.80 | - | 20.73 | 10.25 | 63.22 | - | - | |
| 3 | - | - | 22.13 | 14.10 | 63.77 | - | - | |
| 4 | - | - | 23.73 | 7.64 | 68.63 | - | - | |
| 5 | - | - | 22.30 | 8.37 | 69.33 | - | - |
| Alloy | Hardness (HV196.1 N/15 s) | Micro-Hardness (HV0.98 N/15 s) | Group 1 |
|---|---|---|---|
| 0Al | 201 ± 7 | 325 ± 13 | A |
| 3Al | 207 ± 2 | 333 ± 12 | A |
| 8Al | 280 ± 9 | 378 ± 11 | B |
| Alloy | Corrosion Potential Ecorr (V vs. Ag/AgCl) | Group 1 | Corrosion Rate (mm3/mm2 yr) | Group 1 |
|---|---|---|---|---|
| 0Al | −0.70 ± 0.02 | A | 0.34 ± 0.07 | A |
| 3Al | −0.59 ± 0.04 | B | 0.32 ± 0.09 | A, B |
| 8Al | −0.38 ± 0.07 | C | 0.22 ± 0.04 | B |
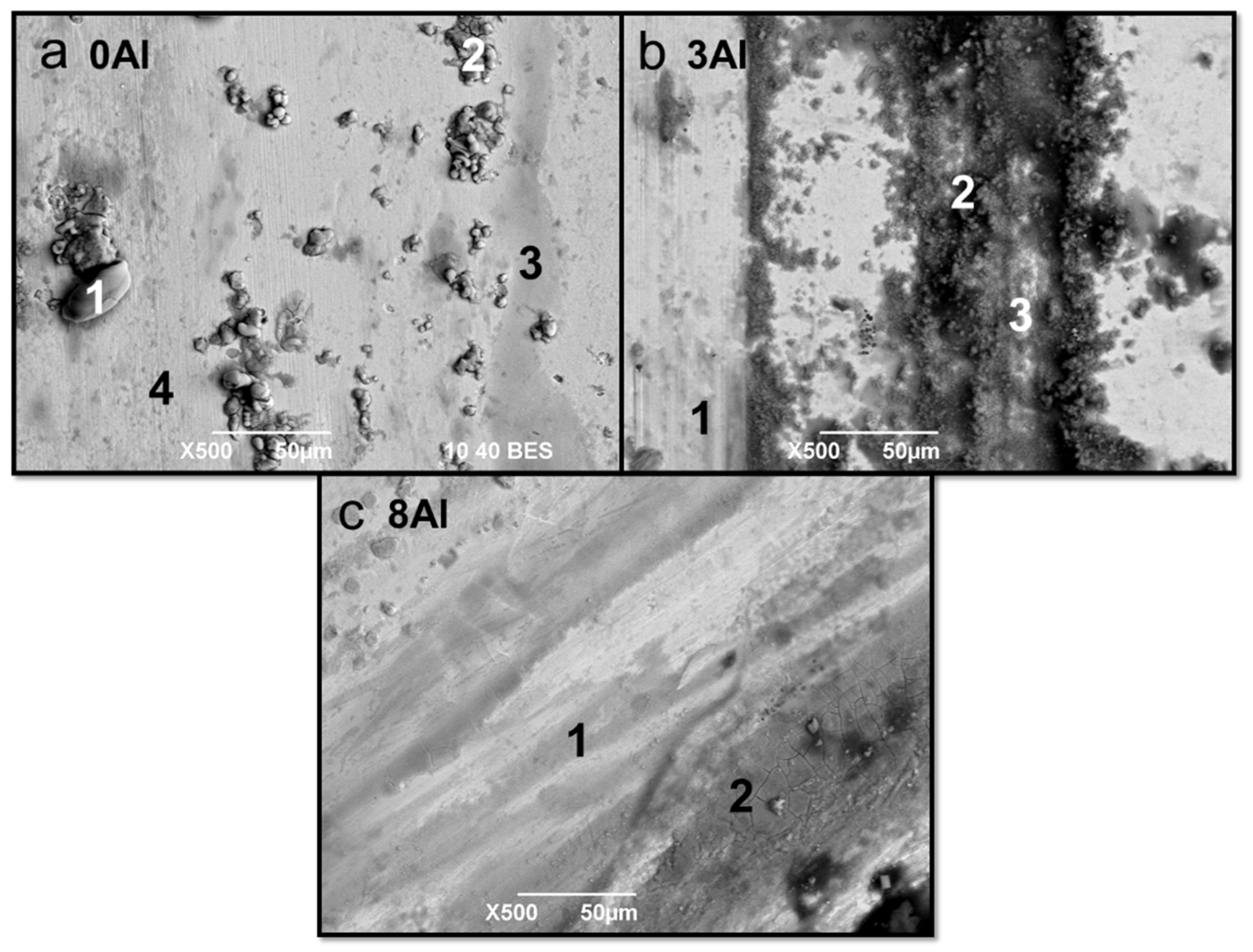
| Alloy | Spectrum | C | O | Al | Cl | Ca | Mn | Fe |
|---|---|---|---|---|---|---|---|---|
| 0Al | 1 | 7.88 | 6.94 | - | - | - | 20.97 | 64.30 |
| 2 | 18.07 | 10.37 | - | - | - | 20.28 | 63.26 | |
| 3 | 23.23 | 36.39 | - | - | 19.80 | 5.62 | 14.96 | |
| 3Al | 1 | - | 36.35 | - | 14.03 | 1.63 | 9.56 | 38.43 |
| 2 | - | 51.17 | - | 3.63 | 31.53 | 2.75 | 10.92 | |
| 3 | 5.57 | - | 2.35 | - | - | 21.22 | 70.87 | |
| 4 | 16.97 | 22.03 | 1.17 | 0.39 | 8.11 | 12.71 | 38.62 | |
| 8Al | 1 | - | 8.34 | 5.60 | - | - | 19.12 | 66.94 |
| 2 | 12.89 | 54.11 | - | - | 33.00 | - | - | |
| 3 | 11.98 | 58.56 | - | - | 29.56 | - | - | |
| 4 | 14.83 | 36.01 | - | - | 20.66 | 7.36 | 20.14 |
| Alloy | Spectrum | C | O | Al | Cl | Ca | Na | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0Al | 1 | - | 29.67 | - | 10.98 | - | - | 14.32 | 45.03 |
| 2 | 5.69 | 24.64 | - | - | - | - | 14.54 | 55.13 | |
| 3 | 7.70 | 14.28 | - | - | - | 2.05 | 15.65 | 60.32 | |
| 4 | 3.95 | 7.84 | - | - | - | 1.05 | 17.80 | 69.36 | |
| 3Al | 1 | 6.62 | 15.11 | 2.28 | - | - | 2.73 | 15.77 | 57.47 |
| 2 | 48.59 | 17.58 | 0.52 | 0.71 | 7.99 | 1.74 | 5.49 | 17.36 | |
| 3 | 14.63 | 11.62 | 1.62 | - | 3.88 | 1.20 | 17.25 | 49.80 | |
| 8Al | 1 | 29.60 | 19.91 | 2.86 | 2.81 | - | - | 8.83 | 35.15 |
| 2 | 15.32 | 24.73 | 4.24 | 1.44 | - | 4.56 | 9.53 | 40.18 |
| Alloy | Corrosion Potential Ecorr (V vs. Ag/AgCl) | Group 1 | Corrosion Rate (mm3/mm2 yr) | Group 1 |
|---|---|---|---|---|
| 0Al | −0.71 ± 0.01 | A | 0.40 ± 0.04 | A |
| 3Al | −0.66 ± 0.07 | B | 0.40 ± 0.10 | A, B |
| 8Al | −0.67 ± 0.01 | C | 0.24 ± 0.10 | B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barona-Osorio, G.M.; Teran, L.A.; Rodríguez, S.A.; Coronado, J.J. On the Tribocorrosion Behavior of Fe-Mn-Al-C Alloys in Ringer’s Solution. Metals 2022, 12, 1339. https://doi.org/10.3390/met12081339
Barona-Osorio GM, Teran LA, Rodríguez SA, Coronado JJ. On the Tribocorrosion Behavior of Fe-Mn-Al-C Alloys in Ringer’s Solution. Metals. 2022; 12(8):1339. https://doi.org/10.3390/met12081339
Chicago/Turabian StyleBarona-Osorio, Gisselle M., Leonel A. Teran, Sara A. Rodríguez, and John J. Coronado. 2022. "On the Tribocorrosion Behavior of Fe-Mn-Al-C Alloys in Ringer’s Solution" Metals 12, no. 8: 1339. https://doi.org/10.3390/met12081339
APA StyleBarona-Osorio, G. M., Teran, L. A., Rodríguez, S. A., & Coronado, J. J. (2022). On the Tribocorrosion Behavior of Fe-Mn-Al-C Alloys in Ringer’s Solution. Metals, 12(8), 1339. https://doi.org/10.3390/met12081339







