Abstract
The effect of the addition of B2O3 and the CaO/Al2O3 ratios on the CaO–Al2O3–B2O3 melts’ structure was fully analyzed by molecular dynamics (MD) simulations. The results show that, with the increase in CaO/Al2O3 ratios, the charge compensation for [AlO4]5− tetrahedron changed from the oxygen tricluster structure to the charge provided by Ca2+, and the structure of [AlO4]5− tetrahedron was stabilized. The addition of B2O3 reduced the degree of polymerization of the aluminate melt, which, in turn, lowered the melt viscosity; in the systems with CaO/Al2O3 mole ratio less than 1, the added B2O3 mainly existed in the form of [BO3]3−, which reduced the symmetry and the strength of the melt network structures; in the higher CaO/Al2O3 ratio systems, the depolymerization ability of the addition of B2O3 on aluminate network structures was enhanced with the increase in CaO/Al2O3 ratios, and the overall degree of polymerization of the melt decreased. The viscosity measurement shows that B2O3 lowers the viscosity of CaO–Al2O3 melts, which was consistent with the results predicted by MD simulations.
1. Introduction
Recently, advanced high-strength steels with high Al content have attracted much attention, such as transformation induced plasticity (TRIP) steels [1] and twinning induced plasticity (TWIP) steels [2]. However, in the production process, the traditional CaO–SiO2-based slags react strongly with the Al in the molten steel, as shown in Formula (1). Furthermore, the reaction results in significant changes in the physical and chemical properties of molten slags, which impact the lubrication and heat transfer during casting, and severely affects the antegrade of the production process [3,4]. To solve the problem, the CaO–Al2O3-based slags, which have the potential to reduce slag–metal reaction during casting of high-aluminum steels, have been widely developed and applied [5,6]. In the design of CaO–Al2O3-based slags, B2O3 is one of the promising fluxing agents and achieved a good fluxing effect in the application; thus, it is very necessary to fully explore the fluxing mechanism of B2O3 in CaO–Al2O3-based slags.
The fluxing effect and fluxing mechanism of adding B2O3 to CaO–Al2O3 system were considered by several researchers. Wang et al. [7] suggested that, by adding B2O3 to CaO–Al2O3-based slag, it can lower the viscosity of the slag because B2O3 can combine with many kinds of oxides to form low-melting-point eutectics, which decrease the melt point of slag. Akberdin et al. [8] created a mathematical model to study the viscosity of the CaO–Al2O3–B2O3 melt system on the base of experimental data. It was predicted that the viscosity of CaO–Al2O3 melt lowered by adding B2O3. Huang et al. [9] also reported that the viscosity of CaO–Al2O3-based slag lowered with an increase in the content of additive B2O3. They interpreted from the melt structure change that the three-coordinated boron is dominated in these slags, and the networks are loosened with increasing B2O3 content. In fact, B2O3 exists in the molten slag in the form of 2D-plane [BO3]3− groups and 3D-tetrahedron [BO4]5− groups. B2O3 existing in the form of three-coordination, as a simple planar structure, can greatly reduce the symmetry and the strength of the networks, while [BO4]5− tetrahedron can be connected with other tetrahedrons to increase the degree of polymerization (DOP) of the network structure [10]. If B2O3 exists in the form of [BO4]5− tetrahedron, it needs to be charge compensated by the cations in the system, same as the formation of [AlO4]5− tetrahedron. MAS-NMR results [11,12,13] showed that the formation of [AlO4]5− tetrahedron is strongly favored over that of [BO4]5− tetrahedron. In the system with CaO/Al2O3 mole ratio >1, after all the [AlO4]5− tetrahedrons have been fully compensated by the sufficient charges, the excess charges are conducive to the formation of [BO4]5− tetrahedron. In other words, adding B2O3 to CaO–Al2O3-based slag with the CaO/Al2O3 mole ratio >1 increases the DOP of borate network structure because of the formation of more [BO4]5− tetrahedrons. However, we suspect that the addition of B2O3 may not only affect the borate network structure, but also affect the aluminate network that plays a dominant role in the melt. Up until now, whether the addition of B2O3 has a positive or negative impact on the DOP of aluminate network under different CaO/Al2O3 mole ratios remained to be determined. In addition, the aluminate networks in the CaO–Al2O3 slags gradually depolymerize into simpler structural units, with the ratio of CaO/Al2O3 increasing [14,15,16], which has a decisive influence on the properties of aluminate melts. Therefore, the overall change in the DOP of network caused by B2O3 addition and CaO/Al2O3 ratios is worthy of attention. This change in the DOP of network is also closely related to the change in slag viscosity.
In this study, the basic structural parameters of the CaO–Al2O3–B2O3 melt structure were obtained by molecular dynamics (MD) simulations. The viscosities of CaO–Al2O3–B2O3 melts were obtained by rotating cylinder method. Then, the effects of adding B2O3 and changing the CaO/Al2O3 ratio on the basic structural units and the DOP of the slag were discussed in detail, and the mechanism of the improved slag fluidity was revealed from the perspective of melt structure changes.
2. Materials and Methods
2.1. Simulation Details
In the MD simulations, the approximation of the Buckingham form [17] was used as the pair potential:
where the terms on the right side of the equation represent long-range Coulomb potential term, short-range repulsive potential term, and van der Waals force term, respectively. The potential function is explained in detail in our previous works [18,19,20]. The values of the potential parameter set used in the MD simulations are obtained from [21], as shown in Table 1.

Table 1.
Buckingham potential parameter of particle pairs in this study.
In the setting process of the studied systems, we refer to the theory that [AlO4]5− tetrahedron has a higher charge compensation order than [BO4]5− tetrahedron [11,12,13]. By changing the ratio of CaO/Al2O3, we designed components with insufficient charge compensation for [AlO4]5− tetrahedron by Ca2+ (NO. 1–4), components with sufficient charge compensation for [AlO4]5− tetrahedron by Ca2+ in theory (NO. 5–6), and groups with excessive charge compensation for [AlO4]5− tetrahedron by Ca2+ (NO. 7–10). The compositions of the studied systems are shown in Table 2.

Table 2.
Compositions of the studied CaO–Al2O3 and CaO–Al2O3–B2O3 systems.
The MD simulations were run in the LAMMPS program (version 22 August 2018, Sandia National Labs, Albuquerque, and Temple University, Philadelphia, PA, USA), and the simulation conditions were set up referring to our previous work [18,19,20]. Based on the phase diagram of CaO–Al2O3 binary system [22], the equilibrium temperature of the MD simulation was set to 2150 K, which can ensure that all slag samples can be melted. During the simulation, the initial temperature of the simulation was set to 4273 K, and 22,000 steps were run at this temperature to make the particles in the system mix uniformly and eliminate the influence of the initial state distribution. Then, the temperature of the system was slowly cooled to 2150 K through 100,000 steps. Finally, it continued to run for 80,000 steps at 2150 K to allow the system to reach equilibrium, and samples were drawn during the process. VMD (version 1.9.1, University of Illinois at Urbana-Champaign, Urbana-Champaign, Champaign, IL, USA) and MATLAB (version R2018b, The MathWorks, Inc., Natick, MA, USA) were used to analyze the data of the sampled samples, which can obtain diverse melt structure information.
2.2. Viscosity Measurement
The slags’ composition selected in the viscosity measurement experiment is determined according to the low-temperature composition zone of the CaO–Al2O3 phase diagram [22], as shown in Table 3. The maximum temperature of the experiment is 1823K, which is the high temperature limit of the experimental equipment. In this experiment, the rotating cylinder method was used to measure the viscosity of the molten slag. The experimental equipment was RTW-16 tester (Northeastern University, Shenyang, China), including a test system, a heating system, and a rotating system. The key device of the tester is shown in Figure 1. The slag samples were prepared with pure chemical reagents CaO, Al2O3, and B2O3 (manufacturer: DAMAO chemical reagent factor, Tianjin, China). About 150 g of reagents prepared in proportion was fully mixed and put into a graphite crucible, and the crucible was put into a heating furnace. The specifications of the graphite crucible were the outer diameter of 50 mm, the inner diameter of 40 mm, and the height of 80 mm. A graphite sleeve with the same inner diameter was placed above the graphite crucible to prevent the heating furnace tube and the temperature measuring probe from being oxidized under high temperature conditions. The heating element adopted 6 U-shaped MoSi2 heating elements, and the S-type thermocouple was used to control and measure the furnace temperature. After the temperature calibration, the temperature difference was guaranteed to be within ±0.5 K. The slag sample was heated to 1823 K according to the preset heating program, and the whole process was protected by argon gas, and the slag sample was completely melted by holding the temperature at 1823 K for 30 min. The molybdenum probe and the boom were installed in the center of the furnace tube, and the height of the furnace body was adjusted so that the lowest end of the molybdenum probe was 10 mm from the bottom of the graphite crucible. Then, the measurement system was turned on to measure the viscosity–temperature curve. When the viscosity reached 5 Pa·s, the measurement was stopped and the data were saved. The temperature of the heating furnace was raised to the temperature where the slag reached a low viscosity, and the temperature measuring probe was taken out. The test system was shut down after the furnace temperature had cooled to 573 K, as programed.

Table 3.
Compositions of the viscosity measurement samples (wt pct) with different CaO/Al2O3 mole ratios.
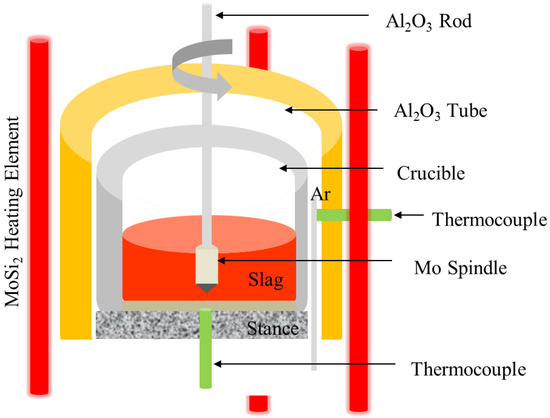
Figure 1.
Schematic illustration of the apparatus for viscosity measurement.
3. Results and Discussion
3.1. Radial Distribution Function (RDF) and Co-Ordination Number (CN) Distribution
The RDFs and CNs of different particle pairs in CaO–Al2O3–B2O3 melt are shown in Figure 2. In the RDFs curve, the abscissa value corresponding to the first peak in the curve represents the average bond length of the particle pair. It can be seen from Figure 2a that the average bond length of Al-O, Ca-O, B-O, and O-O is 1.76, 2.33, 1.36, and 2.76 Å, respectively. L. Cormier et al. [23] proposed the Al-O and Ca-O bond length in calcium aluminosilicate glasses is 1.76 and 2.35 Å by neutron diffraction. The B-O bond length in soda-boric oxide glass obtained by X-ray diffraction patterns is 1.37 Å [24]. The bond length values in this study are in good agreement with the experimental data, indicating that the MD simulations are reliable and accurate. Further analysis showed that the bond length of each particle pair in this study was not affected by the change in the CaO/Al2O3 ratio and the addition of B2O3, as shown in Table 4.
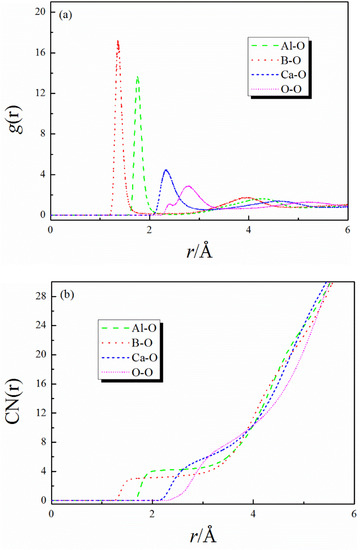
Figure 2.
(a) RDFs and (b) CNs of different particle pairs in CaO–Al2O3–B2O3 melt.

Table 4.
Bond length of different particle pairs with different CaO/Al2O3 mole ratio systems in this study.
In the CNs curve, the ordinate value corresponding to the flat platform in the curve represents the co-ordination number of the particle pair. The co-ordination numbers of different particle pairs have changed significantly with the change in CaO/Al2O3 ratios and the addition of B2O3, as shown in Table 5. The co-ordination numbers of Al-O gradually decrease with the increase in CaO/Al2O3 ratios and the addition of B2O3, and is closer to 4, which indicates the proportion of the [AlO4]5− structure in the system is increasing. The higher CaO/Al2O3 ratios in the system provide sufficient charge compensation, and the Ca2+ preferentially charge compensates the [AlO4]5− prior to charge compensating [BO4]5− [11,12,13]. Moreover, the addition of B2O3 introduces more nonbridging oxygen (NBO) or bridging oxygen (BO) that may participate in the aluminate network into the system. The above factors all contribute to the formation of [AlO4]5− structure. The co-ordination numbers of Ca-O decrease with the increase in CaO/Al2O3 ratios, while they increase with the addition of B2O3. This opposite trend is related to the different roles played by Ca2+ in the melt structure: modifying the network structure as a network modifier or providing charge compensation for [AlO4]5− and [BO4]5− structure. When Ca2+ plays a role in modifying the network, a Ca-NBO structure is formed in the system. Hannon and Parker [25] reported that the co-ordination number of Ca-NBO is 4. When Ca2+ plays the role of charge compensation, one Ca2+ simultaneously charges two tetrahedrons, and the co-ordination number of Ca-BO is 8. When the CaO/Al2O3 ratios increases, CaO introduces more free oxygen (FO) to depolymerize the network structure, and more Ca2+ ions are needed to modify the network structure. At this time, the systems form more Ca-NBO structures with lower co-ordination. The co-ordination numbers of Ca-O decrease. By the addition of B2O3 to the system, the more tetrahedrons ([AlO4]5− and [BO4]5−) that need charge compensation are formed, resulting in an increase in the amount of Ca-BO with a higher co-ordination structure. The co-ordination numbers of Ca-O increase. The co-ordination numbers of B-O increase from 3.17 to 3.21 with the increase in CaO/Al2O3 ratios in the range where the CaO/Al2O3 ratio is less than 1; this slight increase indicates that the degree of conversion of [BO3]3− to [BO4]5− is very low, even if more cations have been added to these systems, and the priority of the structure of [BO3]3− in the system is still stable. The co-ordination numbers of B-O increase from 3.21 to 3.32 with the further increase in CaO/Al2O3 ratios. This is because excessive Ca2+ ions can charge compensate for the formation of [BO4]5− structure in this range of CaO/Al2O3 ratios, and the degree of conversion of [BO3]3− to [BO4]5− is enhanced.

Table 5.
Co-ordination numbers of different particle pairs with different CaO/Al2O3 mole ratio systems in this study.
Moreover, to investigate the effect of the B2O3 addition on the DOP of the entire network under different CaO/Al2O3 ratios, the changes in the co-ordination numbers of Al-Al and O-O are focused on. As the most important network former particles in the melt structure, the larger the co-ordination numbers of Al-Al and O-O are, the more complex the network structure of the melts is. Within the range of CaO/Al2O3 ratios set in this study, the co-ordination numbers of Al-Al and O-O decrease with the increase in CaO/Al2O3 ratios and the addition of B2O3. It shows that the aluminate network structure that plays a dominant role in the entire network structure is simplified with the increase in the CaO/Al2O3 ratios and the addition of B2O3, which becomes loose in spatial distribution. It is worth noting that, as the CaO/Al2O3 ratio increases, the effect of adding B2O3 to the CaO–Al2O3 melts to reduce the co-ordination number of O-O is weakened. This is because more [BO4]5− structures are formed in the system, which will increase the co-ordination number of O-O in borate network. Combining the changes in aluminate and borate network, the decrease trend of the overall co-ordination number of O-O is weakened. At high CaO/Al2O3 ratios, the co-ordination number of O-O before and after adding B2O3 is the same. Even so, the O-O structure of the CaO–Al2O3 melts and the CaO–Al2O3–B2O3 melts is different. In the CaO–Al2O3–B2O3 melts, there is a large amount of 2D-plane [BO3]3− structure in the overall network structure because of the addition of B2O3, and the DOP of the network structure is weaker than the CaO–Al2O3 melts. Therefore, the addition of B2O3 to the CaO–Al2O3 melts can reduce the DOP of the overall network structure in this study, which probably means that the viscosity lowers.
3.2. Distribution of Oxygen Types
It can be seen from Figure 3 and Figure 4 that there are free oxygen, non-bridging oxygen, bridging oxygen, and oxygen triclusters (TO) in the CaO–Al2O3 and CaO–Al2O3–B2O3 system. TO is the characteristic structure in Al2O3-contained systems. In the TO structure, it can be assumed that the third [AlO4]5− tetrahedron connecting the shared oxygen does not require charge compensation. In this way, when there are insufficient cations to charge compensation for the [AlO4]5− structure, three [AlO4]5− tetrahedrons share one apex oxygen that can balance the negative overcharge of the corresponding [AlO4]5− tetrahedrons [26,27]. With the increase in CaO/Al2O3 ratios, the content of cations increases and gradually replaces the TO structure as the main form of charge compensation for the [AlO4]5− structure. In theory, all the [AlO4]5− tetrahedrons can be compensated by the charge provided by Ca2+ at CaO/Al2O3 = 1. At this time, the system does not need deformed TO structure to balance the excess charge. Therefore, the change trend of TO appears to be a turning point at CaO/Al2O3 = 1 (see Figure 3b and Figure 4b).
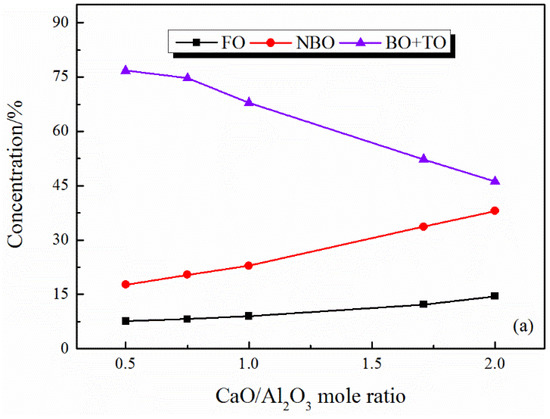
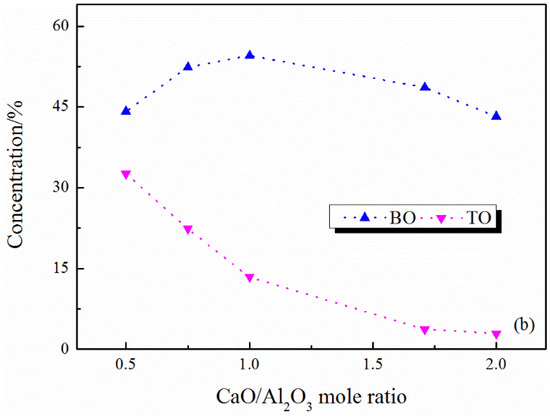
Figure 3.
Distribution of oxygen types in CaO–Al2O3 melts with different CaO/Al2O3 mole ratios: (a) the initial distribution; (b) the refined distribution of networked structural oxygen.
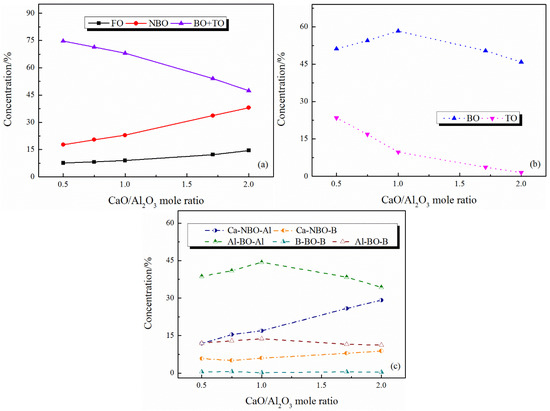
Figure 4.
Distribution of oxygen types in CaO–Al2O3–B2O3 melts with different CaO/Al2O3 mole ratios: (a) the initial distribution; (b) the refined distribution of networked structural oxygen; (c) the refined distribution of nonbridging oxygen and bridging oxygen.
The content of FO and NBO both increase with the increase in CaO/Al2O3 ratio, while the networked structural oxygen (BO and TO) decreases (see Figure 3a and Figure 4a). These results suggest that the network structure of the system is simplified with the CaO/Al2O3 ratio increases. Subdividing the networked structural oxygen into BO and TO, it can be found that the proportion of BO increases with the increase in CaO/Al2O3 ratio when the CaO/Al2O3 ratio is less than 1, as Figure 3b and Figure 4b show. As shown in Figure 5, with the addition of CaO within this range, there is a large amount of TO transform into BO, and this transition is stronger than the transition of BO to non-networked structural oxygen (NBO and FO), thus showing an increase in BO content. In addition, Figure 4c shows the detailed analysis of the distribution of BO and NBO types in the CaO–Al2O3–B2O3 system; it can be seen that the B-BO-B structure content is extremely small, and the added B2O3 mainly exists in the B-BO-Al structure, that is, the B-O structure participates in the Al-O network structure, and borate and aluminate do not exist separately.
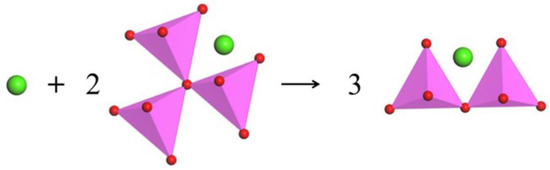
Figure 5.
Schematic diagram of the transition from oxygen triclusters to bridging oxygen with the addition of CaO; green ball: Ca2+; pink tetrahedron: [AlO4]5− tetrahedron.
3.3. Distribution of Qn (Al) Species
Al3+ mainly exists in the form of tetrahedrons in this study. The [AlO4]5− tetrahedron structural units can be represented by Qn (Al), where the superscript n represents the number of networked structural oxygen. As shown in Figure 6 and Figure 7, with the increase in CaO/Al2O3 ratios, the amount of Q4 decreases, while the amounts of Q2 and Q3 increase accordingly, which is consistent with the report by Shu et al. [15]. It suggested that the Qn (Al) units in both the CaO–Al2O3 system and the CaO–Al2O3–B2O3 system are simplified with the increase in the CaO/Al2O3 ratios, the aluminate network structure is depolymerized.
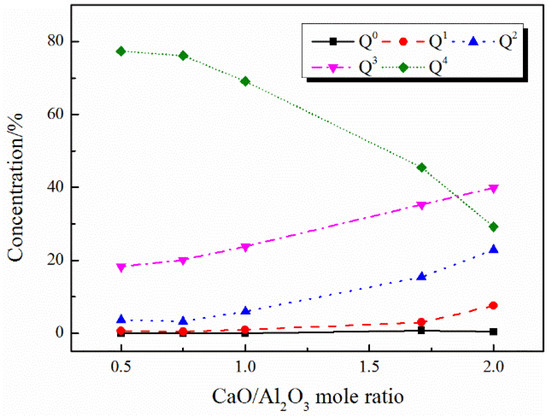
Figure 6.
Distribution of Qn (Al) species in CaO–Al2O3 melts with different CaO/Al2O3 mole ratios.
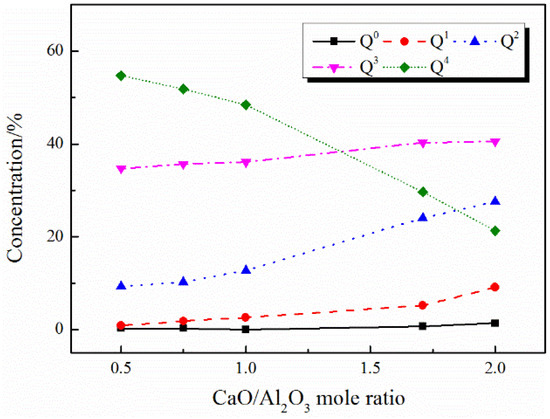
Figure 7.
Distribution of Qn (Al) species in CaO–Al2O3–B2O3 melts with different CaO/Al2O3 mole ratios.
Table 6 shows the effect of the addition of B2O3 on the aluminate network under different CaO/Al2O3 ratios. At each CaO/Al2O3 ratio, the addition of B2O3 decreases the content of Q4 and increases the content of other lower DOP structural units, which indicates that the addition of B2O3 to the CaO–Al2O3 melts cause the depolymerization of the aluminate network. Furthermore, we studied to what extent Q4 can be depolymerized by the addition of B2O3 under each CaO/Al2O3 ratio, as shown in Figure 8. With the increase in CaO/Al2O3 ratios, the proportions of lower DOP structural units are gradually increasing in the structural units transformed from Q4, which indicate that B2O3 has a stronger depolymerization effect on Qn (Al) in the high CaO/Al2O3 ratio system. In the analysis of the co-ordination number of the network formers, we have pointed out that the overall DOP of the network structure decreases with the addition of B2O3. The depolymerization of the Qn (Al) units, which play an absolutely dominant role in the entire network structure, also indicated that the DOP of the entire network structure decreases and the viscosity of the slags lowers.

Table 6.
The effect of the addition of B2O3 on the aluminate network under different CaO/Al2O3 mole ratios.
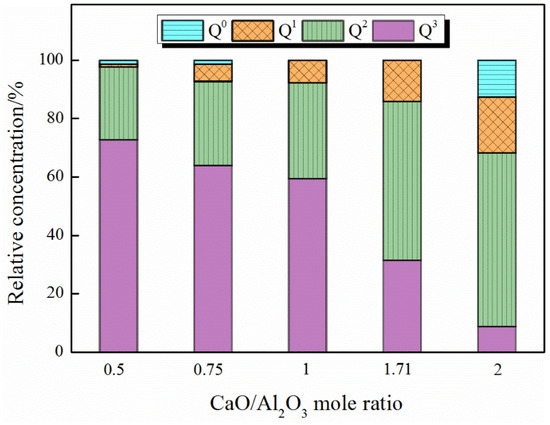
Figure 8.
The effect of B2O3 addition on the degree of transformation of Q4 under different CaO/Al2O3 mole ratios.
3.4. Effect of B2O3 on Viscosity of CaO–Al2O3 Slag
Figure 9 shows the effect of B2O3 on the viscosity of CaO–Al2O3 slags from 1773 K to 1823 K. By comparing the measured viscosity value of the CaO–Al2O3–B2O3 system with the CaO–Al2O3 system, it can be found that the addition of B2O3 can effectively lower the viscosity of CaO–Al2O3 slag within the CaO/Al2O3 ratio range of the viscosity measurement. Although the temperature set in the experiment is different from the MD simulation, the magnitude relationship between the viscosity of the slag in the comparison group does not change above the turning point temperature, so that the magnitude relationship of viscosity at experimental temperature has reference significance for the magnitude relationship of viscosity at MD temperature. The effects of B2O3 addition on viscosity in measurement are consistent with our MD simulations.
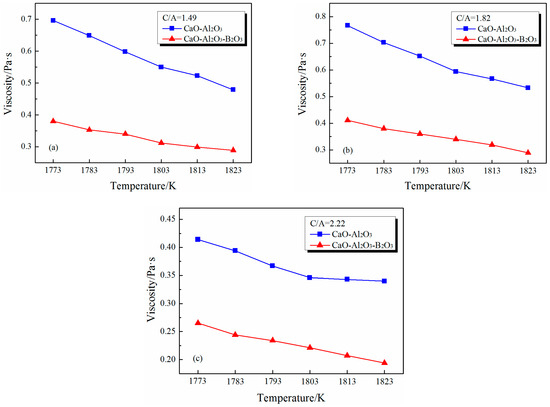
Figure 9.
Effect of B2O3 addition on the viscosity of CaO–Al2O3 systems: (a) CaO/Al2O3 mole ratio = 1.49; (b) CaO/Al2O3 mole ratio = 1.82; (c) CaO/Al2O3 mole ratio = 2.22.
4. Conclusions
In the present study, the effects of adding B2O3 and changing the CaO/Al2O3 ratio on the basic structural units and the DOP of the slag were discussed in detail, and the mechanism of the improved slag fluidity was revealed from the perspective of melt structure changes. The obtained results are summarized as follows:
(1) With the increase in the CaO/Al2O3 ratios, the proportion of nonstructural oxygen (free oxygen and nonbridging oxygen) increases; correspondingly, the proportion of networked structural oxygen (bridging oxygen and oxygen triclusters) decreases, the Qn (Al) units become depolymerized, and the viscosity of the slag lowers.
(2) The main form of charge compensation for [AlO4]5− tetrahedron changes from the oxygen triclusters’ structure to the charge provided by Ca2+ with the increase in the CaO/Al2O3 ratios, and the deformed space structure is reduced, which can help stabilize the structure of [AlO4]5− tetrahedrons
(3) In the system where the CaO/Al2O3 ratio is less than 1, the added B2O3 mainly exists in the form of [BO3]3−, which reduces the symmetry and the strength of the melt network’s structure and lowers the viscosity of the slag.
(4) In the system where the CaO/Al2O3 ratio is more than 1, the depolymerization ability of the addition of B2O3 on Qn (Al) is enhanced with the increase in CaO/Al2O3 ratios, the depolymerization of the aluminate network structure is dominant in the overall change in the network structure, and the viscosity of the slag lowers.
Author Contributions
Data curation, X.Z. and C.L.; Funding acquisition, C.L. and M.J.; Investigation, X.Z. and M.J.; Methodology, X.Z. and C.L.; Project administration, C.L. and M.J.; Software, X.Z.; Writing—original draft, X.Z. and C.L.; Writing—review and editing, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (grant numbers 51874082 and U1908224).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zaefferer, S.; Ohlert, J.; Bleck, W. A study of microstructure, transformation mechanisms and correlation between microstructure and mechanical properties of a low alloyed TRIP steel. Acta Mater. 2004, 52, 2765–2778. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Kwon, O.; Chin, K. State-of-the-knowledge on TWIP steel. Mater. Sci. Technol. 2012, 28, 513–527. [Google Scholar] [CrossRef]
- Moon, K.H.; Park, M.S.; Yoo, S.; Park, J.K.; Cho, J.W.; Shin, G. Molten mold flux technology for continuous casting of the ULC and TWIP steel. In Proceedings of the 8th Pacific Rim International Conference on Advanced Materials and Processing, Waikoloa, HI, USA, 4–9 August 2013; Marquis, F., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Grässel, O.; Krüger, L.; Frommeyer, G.; Meyer, L.W. High strength Fe-Mn-(Al, Si) TRIP/TWIP steels development-properties-application. Int. J. Plast. 2000, 16, 1391–1409. [Google Scholar] [CrossRef]
- Lu, B.; Chen, K.; Wang, W.; Jiang, B. Effects of Li2O and Na2O on the crystallization behavior of lime-alumina-based mold flux for casting high-Al steels. Metall. Mater. Trans. B 2014, 45, 1496–1509. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Shao, H. Review of non-reactive CaO–Al2O3-based mold fluxes for casting high-aluminum steel. J. Iron Steel Res. Int. 2019, 26, 336–344. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Zhu, H.; Yan, Y.; Zhao, Y. Effect of B2O3 and CaF2 on viscosity of ladle refining slag. Adv. Mater. Res. 2011, 295–297, 2647–2650. [Google Scholar] [CrossRef]
- Akberdin, A.; Konurov, U.; Kim, A.; Isagulov, A.; Saitov, R.; Sultangaziyev, R. Viscosity and electric conductivity of melt system of CaO-Al2O3-B2O3. Metalurgija 2016, 55, 313–316. [Google Scholar]
- Huang, X.; Liao, J.; Zheng, K.; Hu, H.; Wang, F.; Zhang, Z. Effect of B2O3 addition on viscosity of mould slag containing low silica content. Ironmak. Steelmak. 2014, 41, 67–74. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z. Structural roles of boron and silicon in the CaO-SiO2-B2O3 glasses using FTIR, Raman, and NMR spectroscopy. Metall. Mater. Trans. B 2015, 46, 1549–1554. [Google Scholar] [CrossRef]
- Zheng, Q.; Youngman, R.E.; Hogue, C.L.; Mauro, J.C.; Potuzak, M.; Smedskjaer, M.M.; Yue, Y. Structure of boroaluminosilicate glasses: Impact of [Al2O3]/[SiO2] ratio on the structural role of sodium. Phys. Rev. B 2012, 86, 054203. [Google Scholar] [CrossRef]
- Kwinda, T.I.; Koppka, S.; Sander, S.A.H.; Kohns, R.; Enke, D. Effect of Al2O3 on phase separation and microstructure of R2O-B2O3-Al2O3-SiO2 glass system (R = Li, Na). J. Non-Cryst. Solids 2020, 531, 119849. [Google Scholar] [CrossRef]
- Yamashita, H.; Yoshino, H.; Nagata, K.; Inoue, H.; Nakajin, T.; Maekawa, T. Nuclear magnetic resonance studies of alkaline earth phosphosilicate and aluminoborosilicate glasses. J. Non-Cryst. Solids 2000, 270, 48–59. [Google Scholar] [CrossRef]
- Kim, G.H.; Sohn, I. Role of B2O3 on the viscosity and structure in the CaO-Al2O3-Na2O-based system. Metall. Mater. Trans. B 2014, 45, 86–95. [Google Scholar] [CrossRef]
- Shu, Q.; Li, P.; Zhang, X.; Chou, K. Thermodynamics and structure of CaO-Al2O3-3 mass pct B2O3 slag at 1773 K (1500 °C). Metall. Mater. Trans. B 2016, 47, 3527–3532. [Google Scholar] [CrossRef]
- Li, J.; Chou, K.; Shu, Q. Structure and viscosity of CaO-Al2O3-B2O3 based mould fluxes with varying CaO/Al2O3 mass ratios. ISIJ Int. 2020, 60, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Mead, R.N.; Mountjoy, G. A molecular dynamics study of the atomic structure of (CaO)x(SiO2)1−x glasses. J. Phys. Chem. B 2006, 110, 14273–14278. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Jiang, M. Effect of fluorine on melt structure for CaO-SiO2-CaF2 and CaO-Al2O3-CaF2 by molecular dynamics simulations. ISIJ Int. 2020, 60, 2176–2182. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Jiang, M. Effect of Na ions on melt structure and viscosity of CaO-SiO2-Na2O by molecular dynamics simulations. ISIJ Int. 2021, 61, 1389–1395. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Jiang, M. Molecular dynamics simulations of melt structure properties of CaO-Al2O3-Na2O slag. Metall. Mater. Trans. B 2021, 52, 2604–2611. [Google Scholar] [CrossRef]
- Bi, Z.; Li, K.; Jiang, C.; Zhang, J.; Ma, S.; Sun, M.; Wang, Z.; Li, H. Performance and transition mechanism from acidity to basicity of amphoteric oxides (Al2O3 and B2O3) in SiO2-CaO-Al2O3-B2O3 system: A molecular dynamics study. Ceram. Int. 2021, 47, 12252–12260. [Google Scholar] [CrossRef]
- Allibert, M.; Gaye, H.; Geiseler, J.; Janke, D.; Keene, B.J.; Kirner, D.; Kowalski, M.; Lehmann, J.; Mills, K.C.; Neuschütz, D.; et al. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995; p. 39. [Google Scholar]
- Cormier, L.; Neuville, D.R.; Calas, G. Structure and properties of low-silica calcium aluminosilicate glasses. J. Non-Cryst. Solids 2000, 274, 110–114. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. X-ray diffraction study of soda-boric oxide glass. J. Am. Ceram. Soc. 1938, 21, 287–293. [Google Scholar] [CrossRef]
- Hannon, A.C.; Parker, J.M. The structure of aluminate glasses by neutron diffraction. J. Non-Cryst. Solids 2000, 274, 102–109. [Google Scholar] [CrossRef]
- Tandia, A.; Timofeev, N.T.; Mauro, J.C.; Vargheese, K.D. Defect-mediated self-diffusion in calcium aluminosilicate glasses: A molecular modeling study. J. Non-Cryst. Solids 2011, 357, 1780–1786. [Google Scholar] [CrossRef]
- Stebbins, J.F.; Oglesby, J.V.; Kroeker, S. Oxygen triclusters in crystalline CaAl4O7 (grossite) and in calcium aluminosilicate glasses: 17O NMR. Am. Miner. 2001, 86, 1307–1311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).