Decorative Multi-Walled Carbon Nanotubes by ZnO: Synthesis, Characterization, and Potent Anti-Toxoplasmosis Activity
Abstract
:1. Introduction
2. Materials and Methods
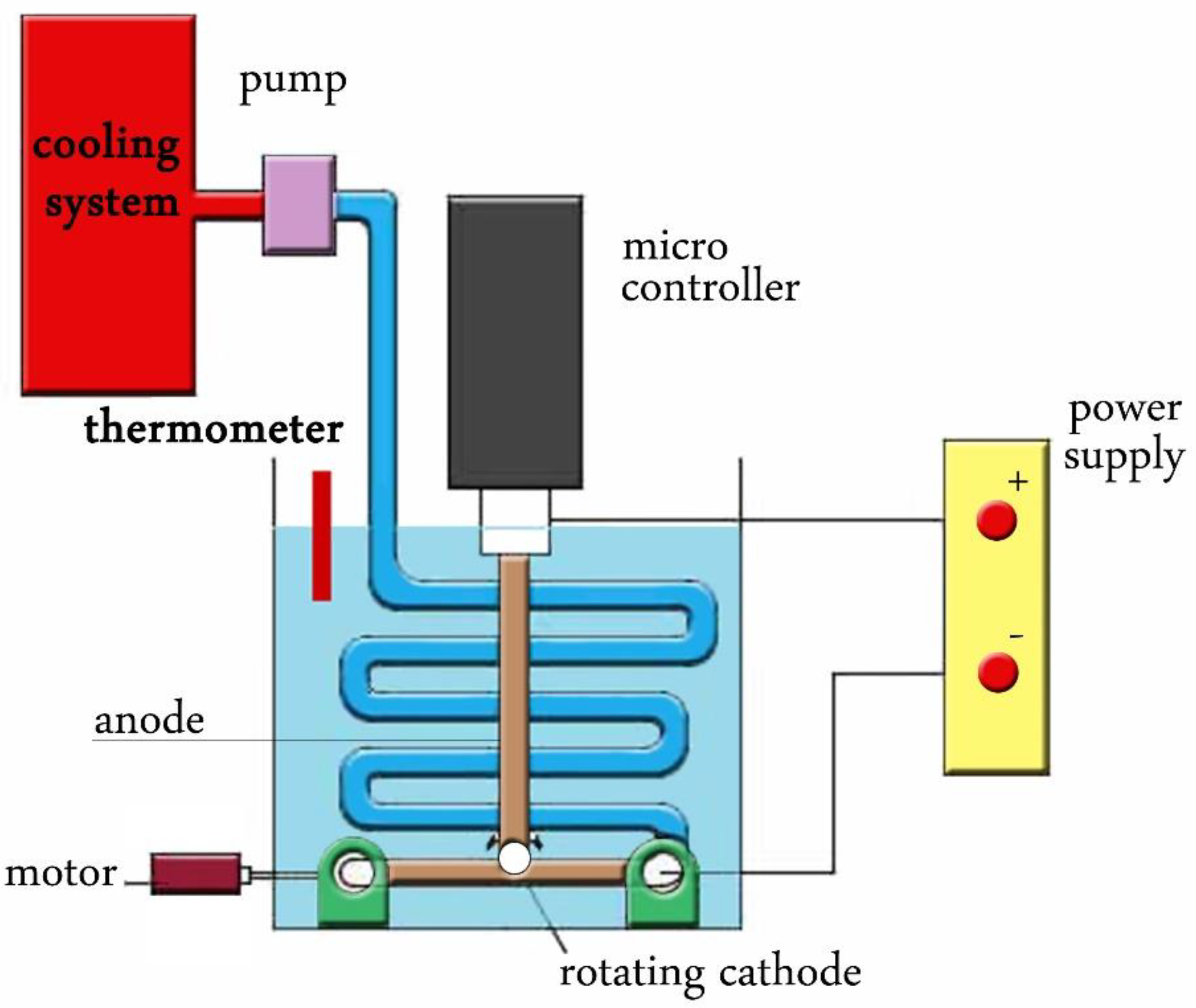
2.1. Nanoparticles Synthesis
2.2. Parasite
2.3. Drugs Preparation
Animal Grouping and Experimental Design
- Group I: Negative control, each mouse received 100 μL normal saline for seven days.
- Group II: Positive (Infected untreated) control, each mouse received 100 μL normal saline (the vehicle of the used drugs) orally by gavage needle starting from the day of infection for seven days.
- Group III: Infected mice received 100 μL of ZnO-NPs at a dose of 10 mg/kg/day orally by gavage needle starting from the day of infection for seven days.
- Group IV: Infected mice received 100 μL of GO-NPs at a dose of 10 mg/kg/day orally by gavage needle starting from the day of infection for seven days.
- Group V: Infected mice received 100 μL of ZnO-MWCNT a dose of 10 mg/kg/day orally by gavage needle starting from the day of infection for seven days.
2.4. Parasitological Study
2.4.1. Estimation of the Parasite Count
Parasite Percent Reduction (%R)
2.4.2. Morphological Study of T. gondii Tachyzoites
2.5. Inflammatory Biomarkers
2.6. Histopathological Study
2.7. Statistical Analyses
3. Results and Discussion
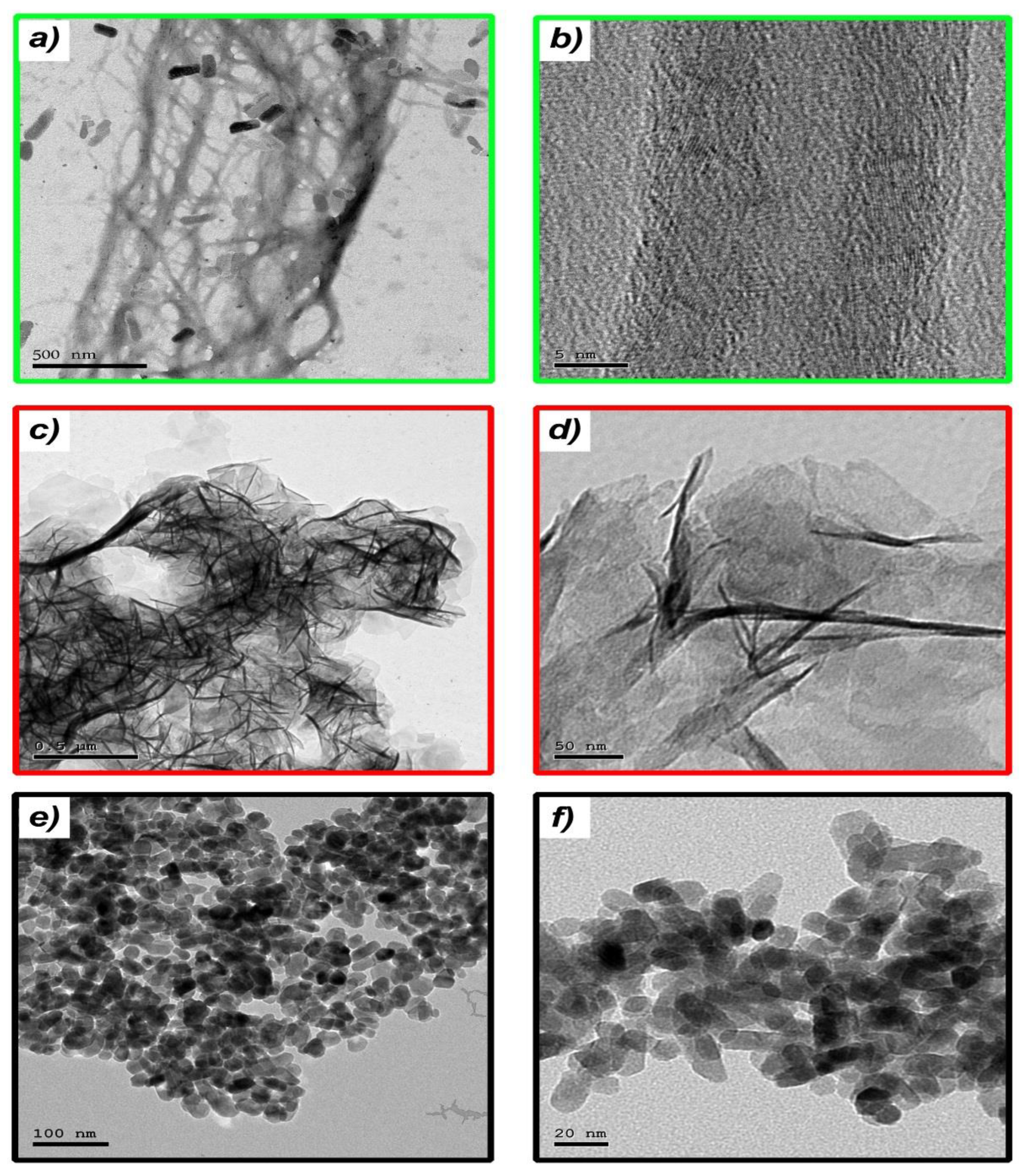
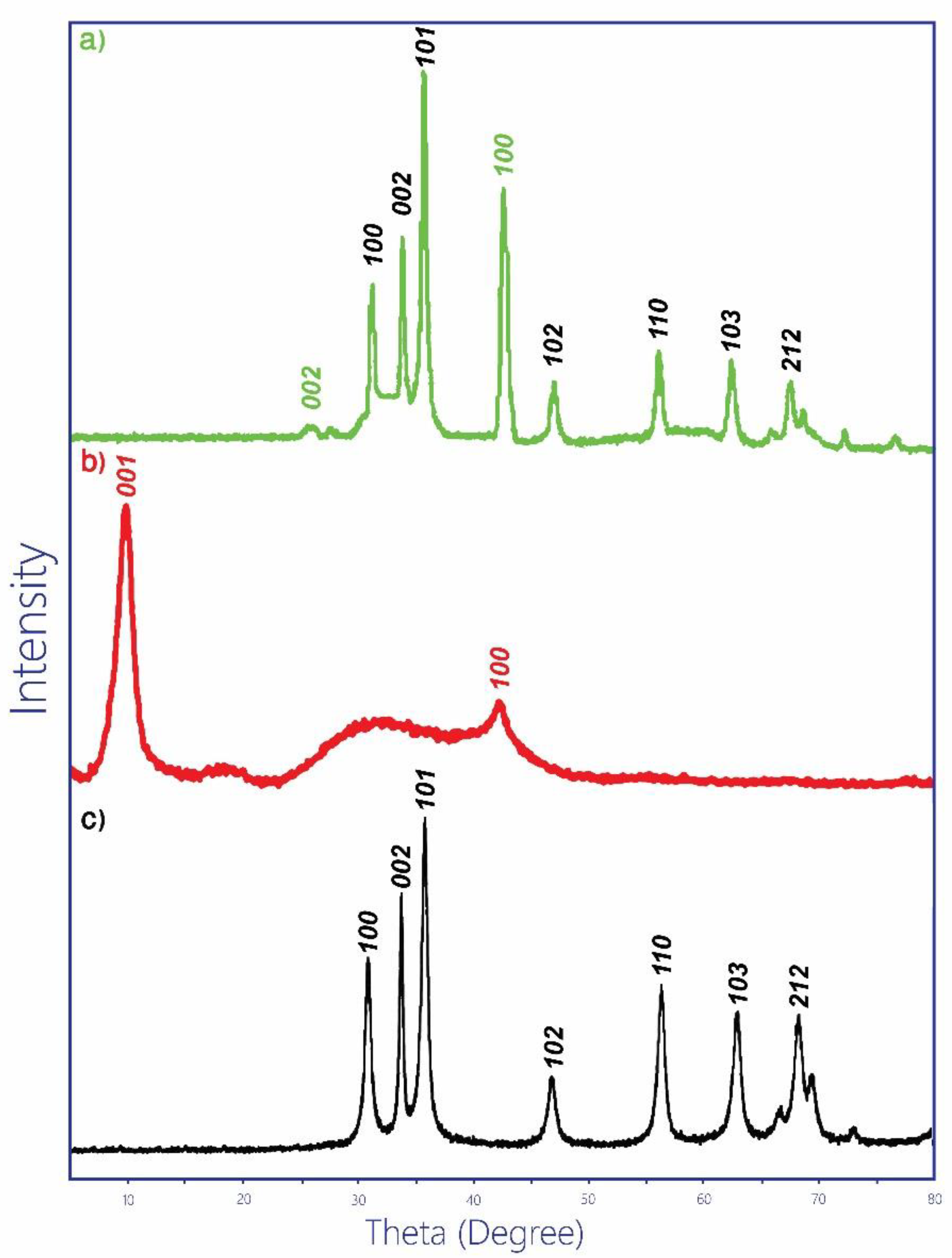
3.1. Nanoparticles Fabrication
3.2. Parasitological Study
3.2.1. Parasite Count and Percent Reduction (%R)
3.2.2. Morphological Study of T. gondii Tachyzoites
3.3. Inflammatory and Anti-Inflammatory Cytokines in Infected and Treated Groups
3.4. Histopathological Studies
3.4.1. Brain
3.4.2. Liver
3.4.3. Spleen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Kamedulski, P.; Kaczmarek-Kedziera, A.; Lukaszewicz, J.P. Influence of intermolecular interactions on the properties of carbon nanotubes. Bull. Mater. Sci. 2018, 41, 76. [Google Scholar] [CrossRef] [Green Version]
- Kamedulski, P.; Gauden, P.A.; Lukaszewicz, J.P.; Ilnicka, A. Effective Synthesis of Carbon Hybrid Materials Containing Oligothiophene Dyes. Materials 2019, 12, 3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Gan, L.; Liu, J.; Yang, X. Carbon dots derived from pea for specifically binding with Cryptococcus neoformans. Anal. Biochem. 2020, 589, 113476. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Y.; Hu, X.H.; Zhang, Y.W.; Wahid, F.; Chu, L.Q.; Jia, S.R.; Zhong, C. Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohydr. Polym. 2020, 229, 115456. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef] [Green Version]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Khezerlou, A.; Mirzanajafi-Zanjani, M.; Zolfaghari, H.; Bagheri, V.; Divband, B.; Ehsani, A. Nanoparticles and Zeolites: Antibacterial Effects and their Mechanism against Pathogens. Curr. Pharm. Biotechnol. 2019, 20, 1074–1086. [Google Scholar] [CrossRef]
- De Jong, W.H.; De Rijk, E.; Bonetto, A.; Wohlleben, W.; Stone, V.; Brunelli, A.; Badetti, E.; Marcomini, A.; Gosens, I.; Cassee, F.R. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology 2019, 13, 50–72. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Xu, M.; Cheng, G.; Yang, L.; Guo, Y.; Li, D.; Fang, D.; Zhang, Q.; Liu, H. Facile construction of Ag nanoparticles encapsulated into carbon nanotubes with robust antibacterial activity. Carbon 2018, 130, 775–781. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Synthesis, physical, mechanical and antibacterial properties of nanocomposites based on poly (vinyl alcohol)/graphene oxide–silver nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Ji, Y.; Sun, P.; Li, R.; Xiang, F.; Wang, H.; Yang, Y. Effects of individual and complex ciprofloxacin, fullerene C60, and ZnO nanoparticles on sludge digestion: Methane production, metabolism, and microbial community. Bioresour. Technol. 2018, 267, 46–53. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Elsafi, M.; Sayyed, M.; Abbas, M.; El-Khatib, M. Impact of micro and nano aluminium on the efficiency of photon detectors. Results Phys. 2021, 30, 104908. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Badawi, M.S.; Ghatass, Z.F.; Mohamed, M.M.; Elkhatib, M. Synthesize of Silver Nanoparticles by Arc Discharge Method Using Two Different Rotational Electrode Shapes. J. Clust. Sci. 2018, 29, 1169–1175. [Google Scholar] [CrossRef]
- Timerkaev, B.A.; Shakirov, B.R.; Timerkaeva, D.B. Creation of Silicon Nanostructures in Electric Arc Discharge. High Energy Chem. 2019, 53, 162–166. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y.; Zhu, J. Temperature and catalyst effects on the production of amorphous carbon nanotubes by a modified arc discharge. Carbon 2005, 43, 2907–2912. [Google Scholar] [CrossRef]
- Su, Y.; Yang, Z.; Wei, H.; Kong, E.S.-W.; Zhang, Y. Synthesis of single-walled carbon nanotubes with selective diameter distributions using DC arc discharge under CO mixed atmosphere. Appl. Surf. Sci. 2011, 257, 3123–3127. [Google Scholar] [CrossRef]
- Biró, L.; Horváth, Z.; Szalmás, L.; Kertész, K.; Wéber, F.; Juhász, G.; Radnóczi, G.; Gyulai, J. Continuous carbon nanotube production in underwater AC electric arc. Chem. Phys. Lett. 2003, 372, 399–402. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Lin, Y.-S.; Ku, H.-C.; Lee, H.-L. Study on the Characteristics of Zinc Oxide Nanocolloid Prepared Using Electrical Spark Discharge Method. J. Clust. Sci. 2021, 33, 145–150. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hotea, I.; Olariu, T.R.; Jones, J.L.; Darabus, G. Epidemiological review of toxoplasmosis in humans and animals in Romania. Parasitology 2014, 141, 311–325. [Google Scholar] [CrossRef]
- Almutairi, T.M.; Rezki, N.; Aouad, M.R.; Hagar, M.; Bakr, B.A.; Hamed, M.T.; Moneer, E.A. Exploring the Antiparasitic Activity of Tris-1, 3, 4-Thiadiazoles against Toxoplasma gondii-Infected Mice. Molecules 2022, 27, 2246. [Google Scholar] [CrossRef]
- Wohlfert, E.A.; Blader, I.J.; Wilson, E.H. Brains and Brawn: Toxoplasma Infections of the Central Nervous System and Skeletal Muscle. Trends Parasitol. 2017, 33, 519–531. [Google Scholar] [CrossRef]
- Mordue, D.G.; Monroy, F.; La Regina, M.; Dinarello, C.A.; Sibley, L.D. Acute Toxoplasmosis Leads to Lethal Overproduction of Th1 Cytokines. J. Immunol. 2001, 167, 4574–4584. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.S.; Shin, J.-H.; Yang, J.-P.; Jung, B.-K.; Lee, S.H.; Shin, E.-H. Characteristics of Infection Immunity Regulated by Toxoplasma gondii to Maintain Chronic Infection in the Brain. Front. Immunol. 2018, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Thiptara, A.; Kongkaew, W.; Bilmad, U.; Bhumibhamon, T.; Anan, S. Toxoplasmosis in piglets. Ann. Acad. Sci. 2006, 1081, 336–338. [Google Scholar] [CrossRef]
- Penido, M.L.D.O.; Nelson, D.L.; Vieira, L.; Coelho, P.M.Z. Schistosomicidal activity of alkylaminooctanethiosulfuric acids. Memórias Inst. Oswaldo Cruz 1994, 89, 595–602. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, A.; Bondouk, I.; Omar, K.; Hamdy, A. Impact of changing electrodes dimensions and different ACs on the characteristics of nano composites NZnO/MWCNTs prepared by the arc discharge method. Surf. Interfaces 2022, 29, 101736. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Elsafi, M.; Ghoneim, N.I.; Toderaş, M.; Sayyed, M.I.; Mohafez, H.; Islam, M.A.; Khandaker, M.U.; El-Khatib, M. Water Treatment from MB Using Zn-Ag MWCNT Synthesized by Double Arc Discharge. Materials 2021, 14, 7205. [Google Scholar] [CrossRef] [PubMed]
- Titelman, G.; Gelman, V.; Bron, S.; Khalfin, R.; Cohen, Y.; Bianco-Peled, H. Characteristics and microstructure of aqueous colloidal dispersions of graphite oxide. Carbon 2005, 43, 641–649. [Google Scholar] [CrossRef]
- Bigdeli, F.; Morsali, A.; Retailleau, P. Syntheses and characterization of different zinc(II) oxide nano-structures from direct thermal decomposition of 1D coordination polymers. Polyhedron 2010, 29, 801–806. [Google Scholar] [CrossRef]
- Khalil, A.M.; El-Khatib, A.M.; El-Khatib, M. Synthesis of hexagonal nanozinc by arc discharge for antibacterial water treatment. Surf. Innov. 2019, 8, 165–171. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Yousef, N.; Ghatass, Z.; Badawi, M.S.; Mohamed, M.; Elkhatib, M. Synthesized Silver Carbon Nanotubes and Zinc Oxide Nanoparticles and their Ability to Remove Methylene Blue Dye. J. Nano Res. 2019, 56, 1–16. [Google Scholar] [CrossRef]
- Saadatmand, M.; Al-Awsi, G.R.L.; Alanazi, A.D.; Sepahvand, A.; Shakibaie, M.; Shojaee, S.; Mohammadi, R.; Mahmoudvand, H. Green synthesis of zinc nanoparticles using Lavandula angustifolia Vera. Extract by microwave method and its prophylactic effects on Toxoplasma gondii infection. Saudi J. Biol. Sci. 2021, 28, 6454–6460. [Google Scholar] [CrossRef]
- Swedin, L.; Arrighi, R.; Andersson-Willman, B.; Murray, A.; Chen, Y.; Karlsson, M.C.I.; Georén, S.K.; Tkach, A.V.; Shvedova, A.A.; Fadeel, B.; et al. Pulmonary exposure to single-walled carbon nanotubes does not affect the early immune response against Toxoplasma gondii. Part. Fibre Toxicol. 2012, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [Green Version]
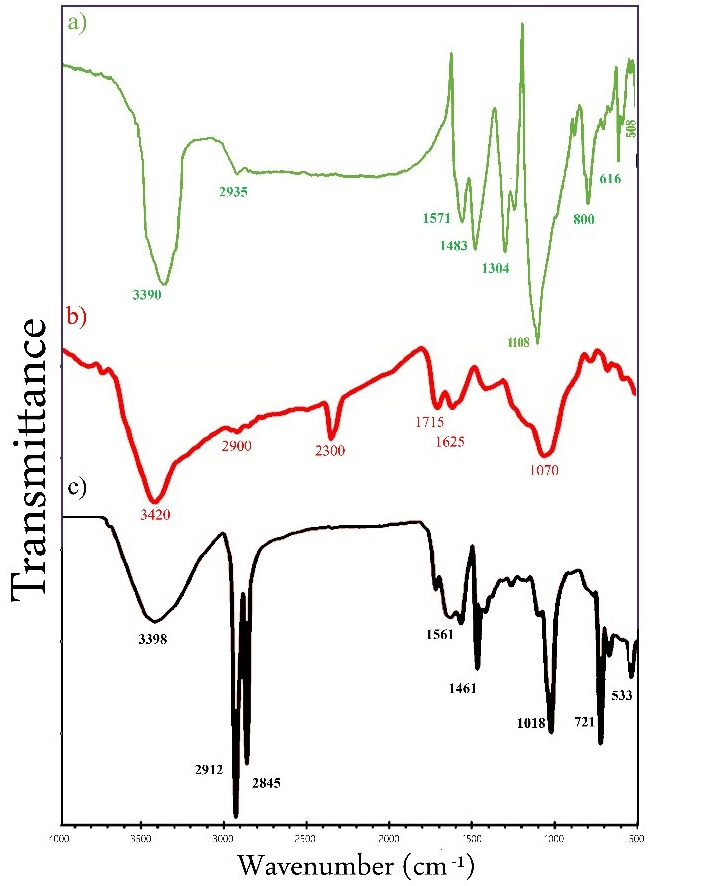
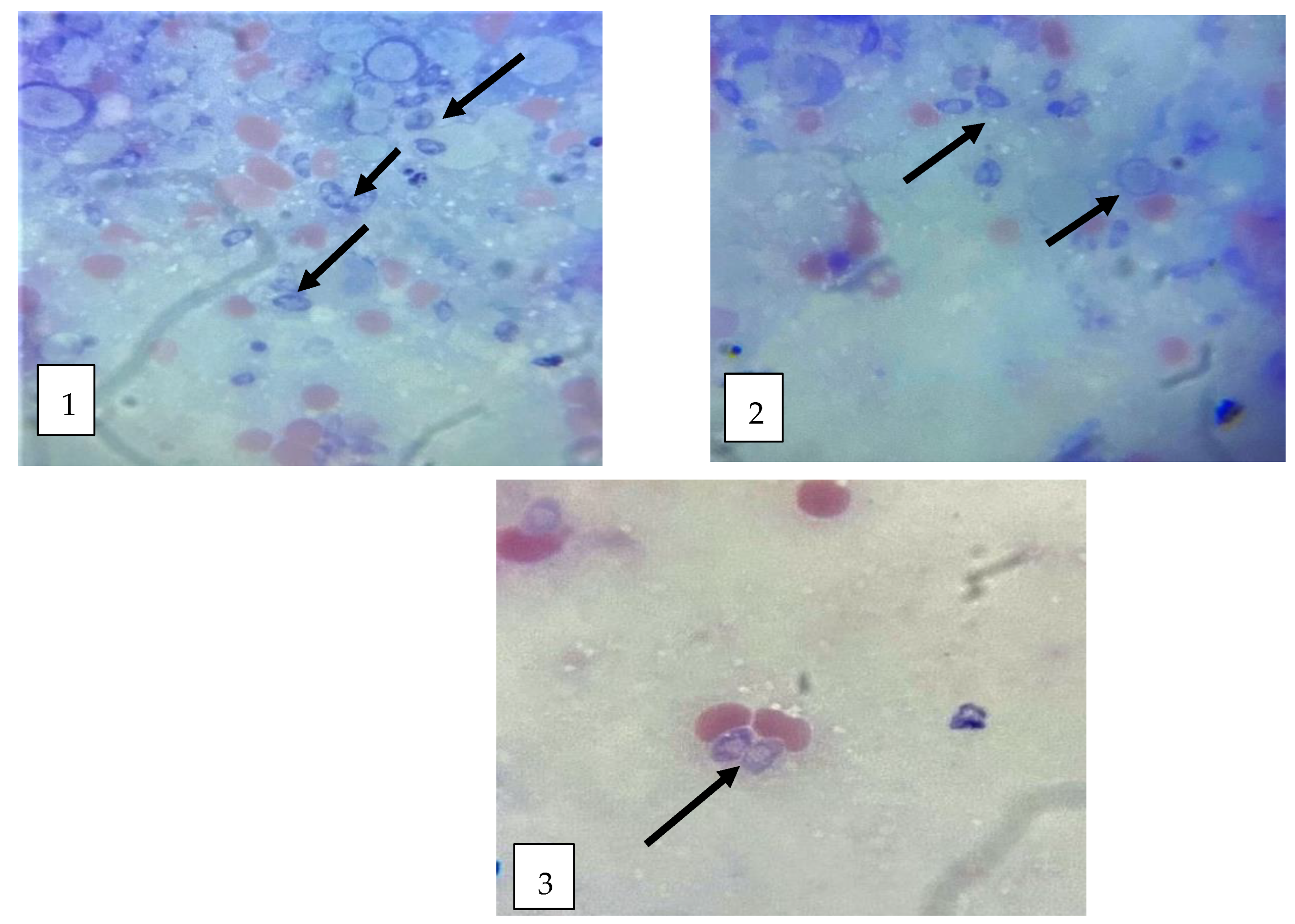

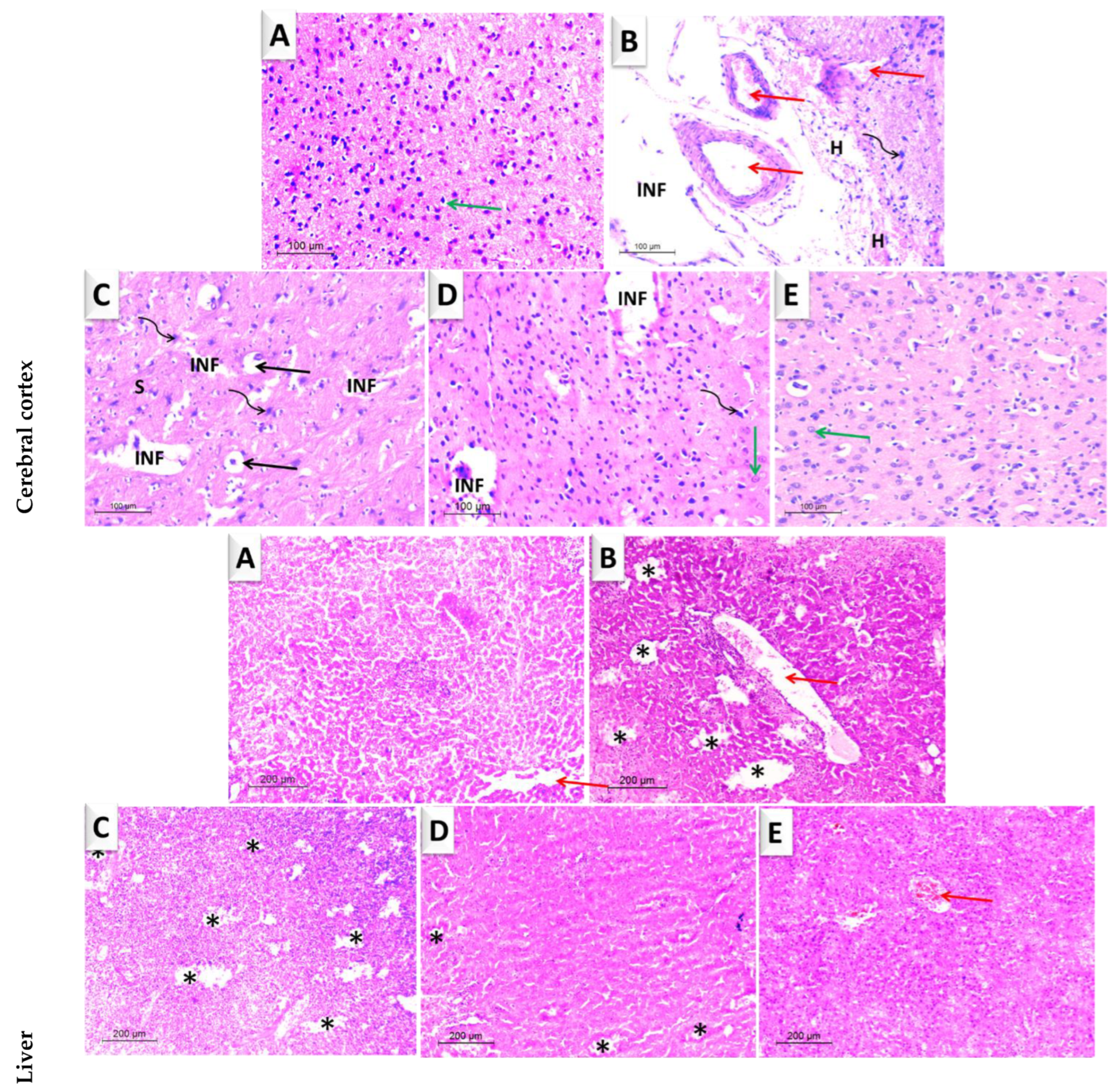
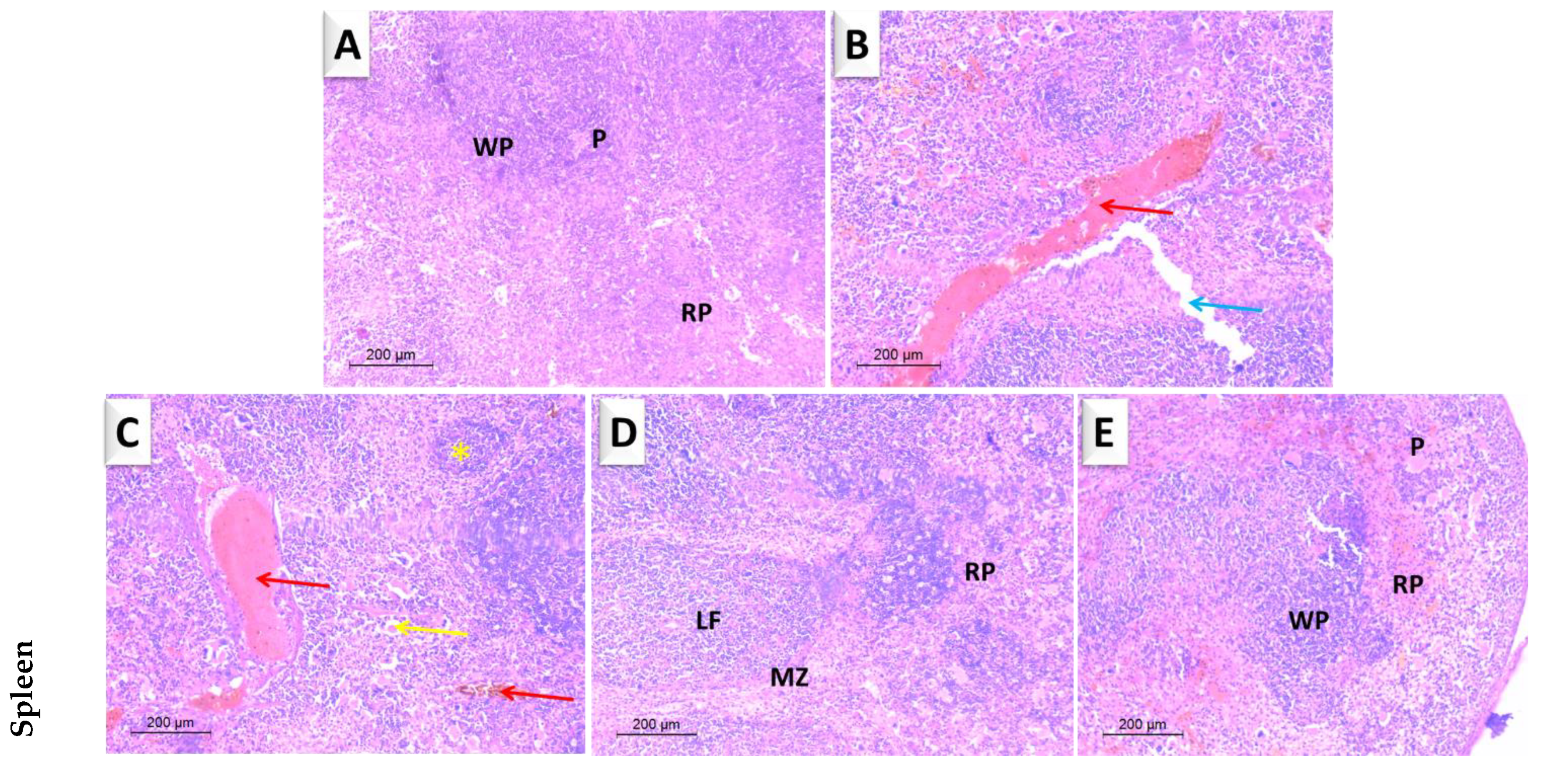
| ZnO-MWCNT | GO-NPs | ZnO-NPs | |||
|---|---|---|---|---|---|
| Frequency (cm−1) | Band Refer to | Frequency (cm−1) | Band Refer to | Frequency (cm−1) | Band Refer to |
| 3390 | -OH | 3420 | -OH | 3398 | -OH group |
| 2935 | -C=H | 2912 | -C=H | ||
| 1571 | -C=C | 2900 | -C=H | 2845 | -OH group |
| 1483 | -C-H | 2300 | 1561 | -C=C | |
| 1304 | -OH | 1715 | -C=O | 1461 | -C=O |
| 1108 | -C=O | 1018 | -C=O | ||
| 800 | -C-H | 1625 | -C=C | 721 | Zn-O |
| 616 | Zn-O | 533 | Zn-O | ||
| 508 | Zn-O | 1070 | -C-C | ||
| Groups | Liver (n = 10) | Spleen (n = 10) | Brain (n = 10) | |
|---|---|---|---|---|
| Group II a | Mean ± SD | 16.7 ± 0.67 b,c,d | 9.7 ± 0.67 b,c,d | 2.8 ± 0.60 b,c,d |
| Group III | Mean ± SD | 9.9 ± 0.9 a,c,d | 6.1 ± 0.99 a,c | 1.3 ± 0.46 a,c |
| R1% | 41 | 37 | 54 | |
| Group IV | Mean ± SD | 12.2 ± 1.14 a,b,d | 7.6 ± 0.97 a,b,d | 2 ± 0.45 a,b |
| R2% | 27 | 22 | 29 | |
| Group V | Mean ± SD | 8 ± 1.05 a,b,c | 5.3 ± 0.82 a,c | 1.1± 0.54 a,c |
| R3% | 52 | 45 | 61 | |
| F | 146.1 | 48.1 | 20.2 | |
| p | <0.001 | <0.001 | <0.001 |
| Mice Groups | TNF-ɑ (ng/mL) | IL-10 (ng/mL) | IL-6 (pg/mL) | IL-1β (ng/mL) |
|---|---|---|---|---|
| Group I (Normal) | 1.91 ± 0.41 | 1.32 ± 0.19 | 395.43 ± 0.82 | 1.45 ± 0.52 |
| Group II (Infected untreated) | 3.23 ± 0.58 | 2.36 ± 0.25 | 637.90 ± 0.29 | 11.24 ± 0.33 |
| Group III (ZnO) | 2.03 ± 1.07 | 1.65 ± 0.31 | 539.83 ± 1.03 | 9.43 ± 0.97 |
| Group IV (GO) | 2.60 ± 0.27 | 2.11 ± 0.22 | 548.64 ± 0.30 | 7.47 ± 0.29 |
| Group V (ZnO-MWCNT) | 2.00 ± 0.38 | 1.49 ± 0.93 | 450.80 ±0.64 | 6.12 ± 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Humaidi, J.Y.; Hagar, M.; Bakr, B.A.; Elwakil, B.H.; Moneer, E.A.; El-Khatib, M. Decorative Multi-Walled Carbon Nanotubes by ZnO: Synthesis, Characterization, and Potent Anti-Toxoplasmosis Activity. Metals 2022, 12, 1246. https://doi.org/10.3390/met12081246
Al-Humaidi JY, Hagar M, Bakr BA, Elwakil BH, Moneer EA, El-Khatib M. Decorative Multi-Walled Carbon Nanotubes by ZnO: Synthesis, Characterization, and Potent Anti-Toxoplasmosis Activity. Metals. 2022; 12(8):1246. https://doi.org/10.3390/met12081246
Chicago/Turabian StyleAl-Humaidi, Jehan Y., Mohamed Hagar, Basant A. Bakr, Bassma H. Elwakil, Esraa Abdelhamid Moneer, and Mostafa El-Khatib. 2022. "Decorative Multi-Walled Carbon Nanotubes by ZnO: Synthesis, Characterization, and Potent Anti-Toxoplasmosis Activity" Metals 12, no. 8: 1246. https://doi.org/10.3390/met12081246
APA StyleAl-Humaidi, J. Y., Hagar, M., Bakr, B. A., Elwakil, B. H., Moneer, E. A., & El-Khatib, M. (2022). Decorative Multi-Walled Carbon Nanotubes by ZnO: Synthesis, Characterization, and Potent Anti-Toxoplasmosis Activity. Metals, 12(8), 1246. https://doi.org/10.3390/met12081246









