Impact of Remelting on ZnAl4Cu3 Alloy with Addition of Cd on Selected Technological and Mechanical Properties
Abstract
:1. Introduction
2. Experimental Material and Methodology
2.1. Alloy and Casting Parameters
- -
- Vulcanizer PVM 300 (Figure 1a), in which the vulcanization of the mold takes place at a temperature of 180 °C, under the effect of a pressure 150 bar;
- -
- Melting furnace F50 (Figure 1b) with integrated pyrometer and electronic regulator for setting and maintaining the required melt temperature 420 °C;
- -
- CMRT 300 centrifuge (Figure 1c), which uses centrifugal force to center the silicone mold and fill its cavities with liquid metal.

2.2. Equipment Used and Test Evaluation Methodology
3. Results
3.1. Fluidity Evaluation
3.2. Mechanical Properties
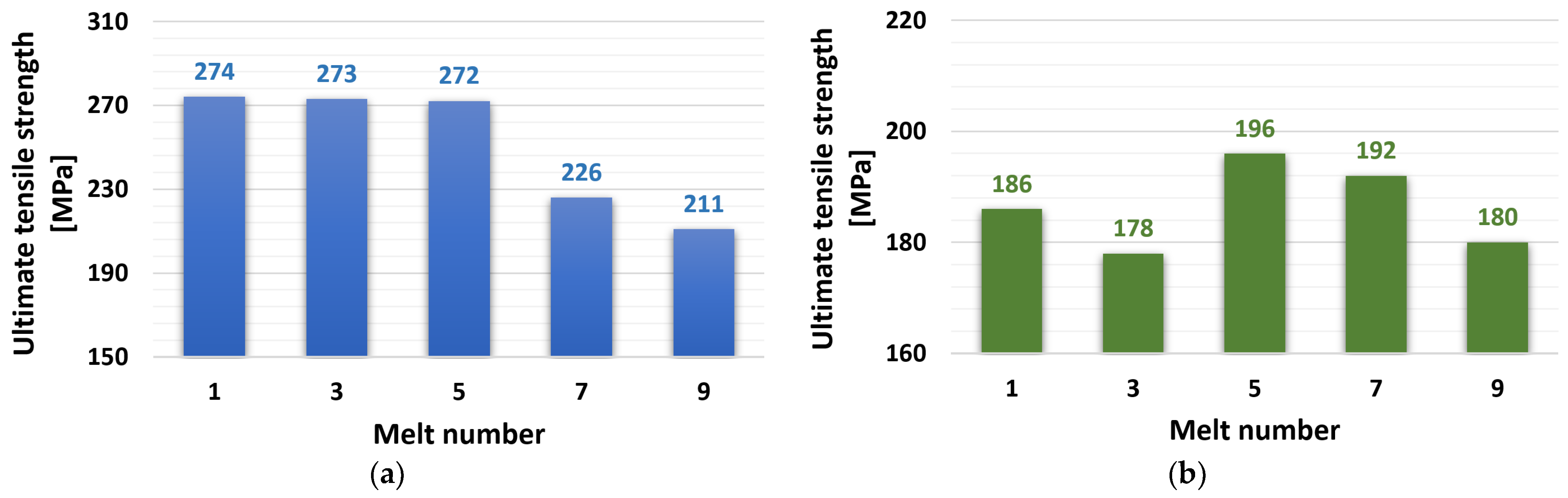
3.2.1. Ultimate Tensile Strength (UTS)
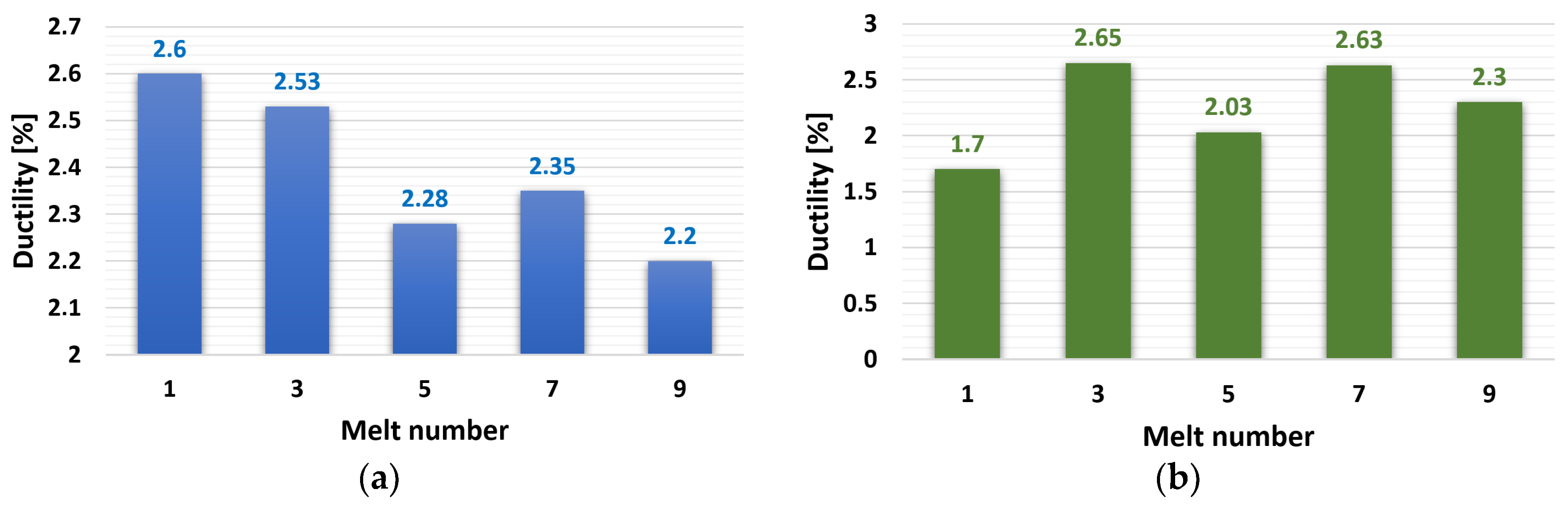
3.2.2. Ductility (A50)
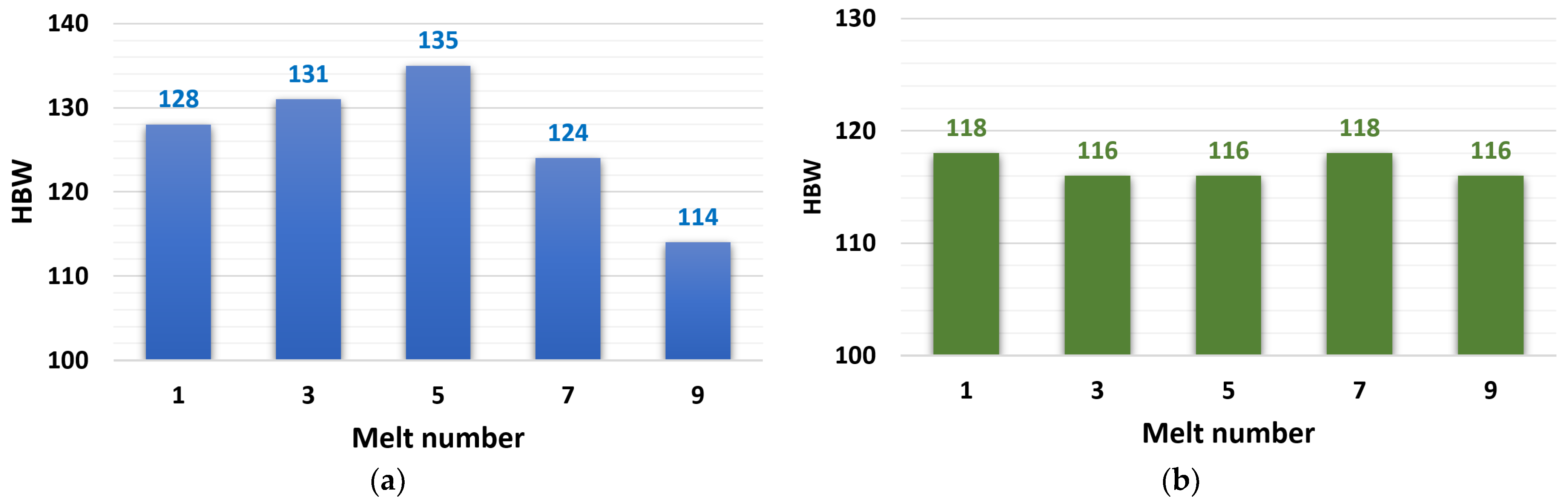
3.2.3. HBW Hardness Evaluation
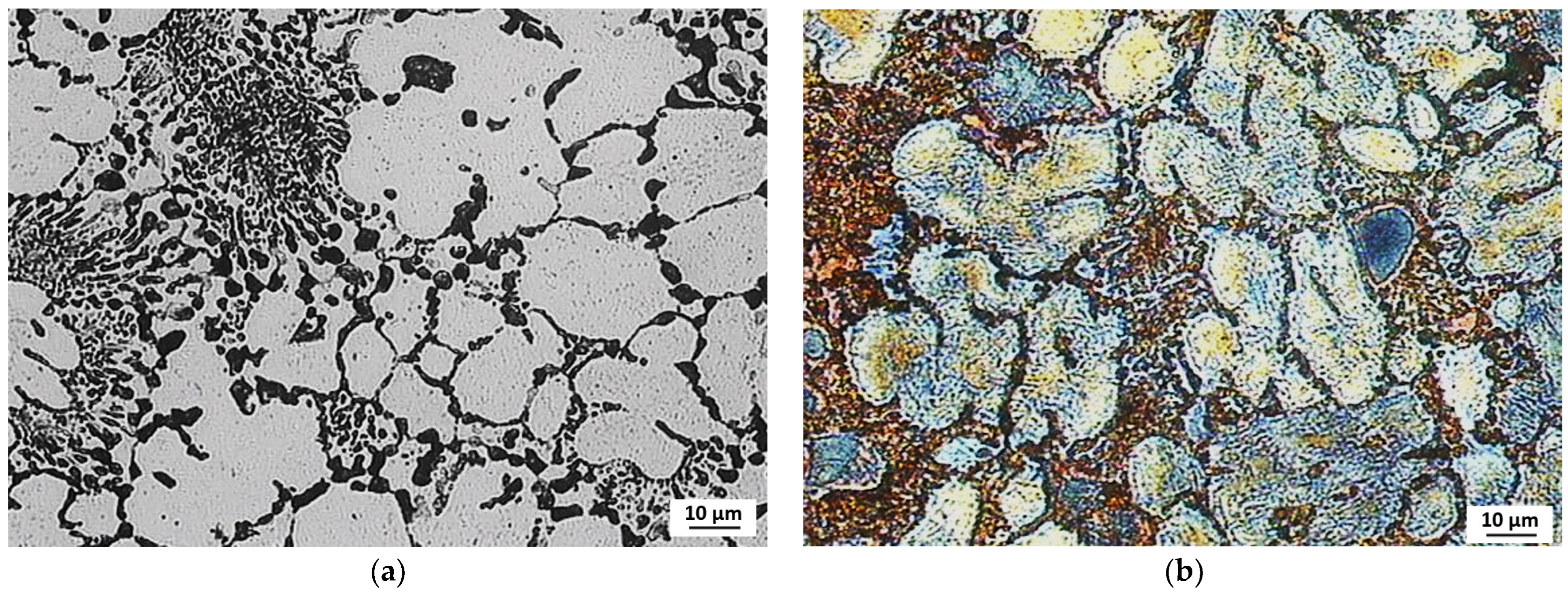
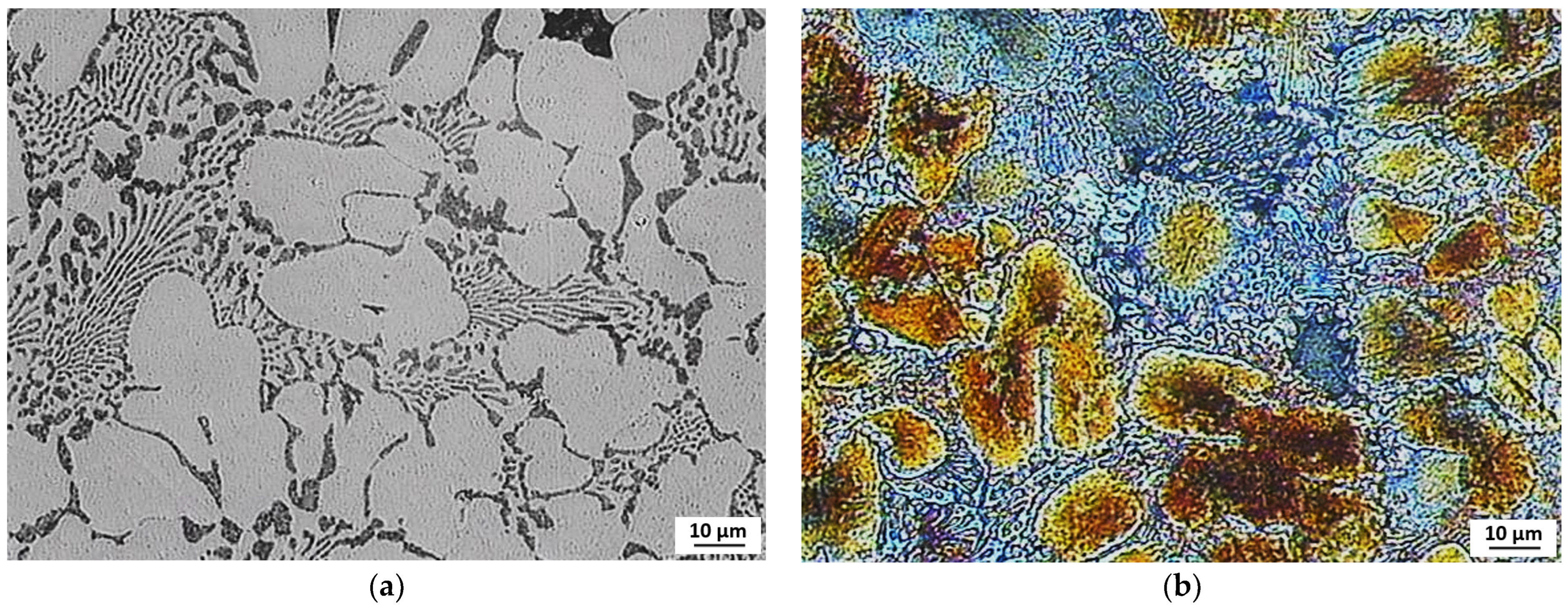
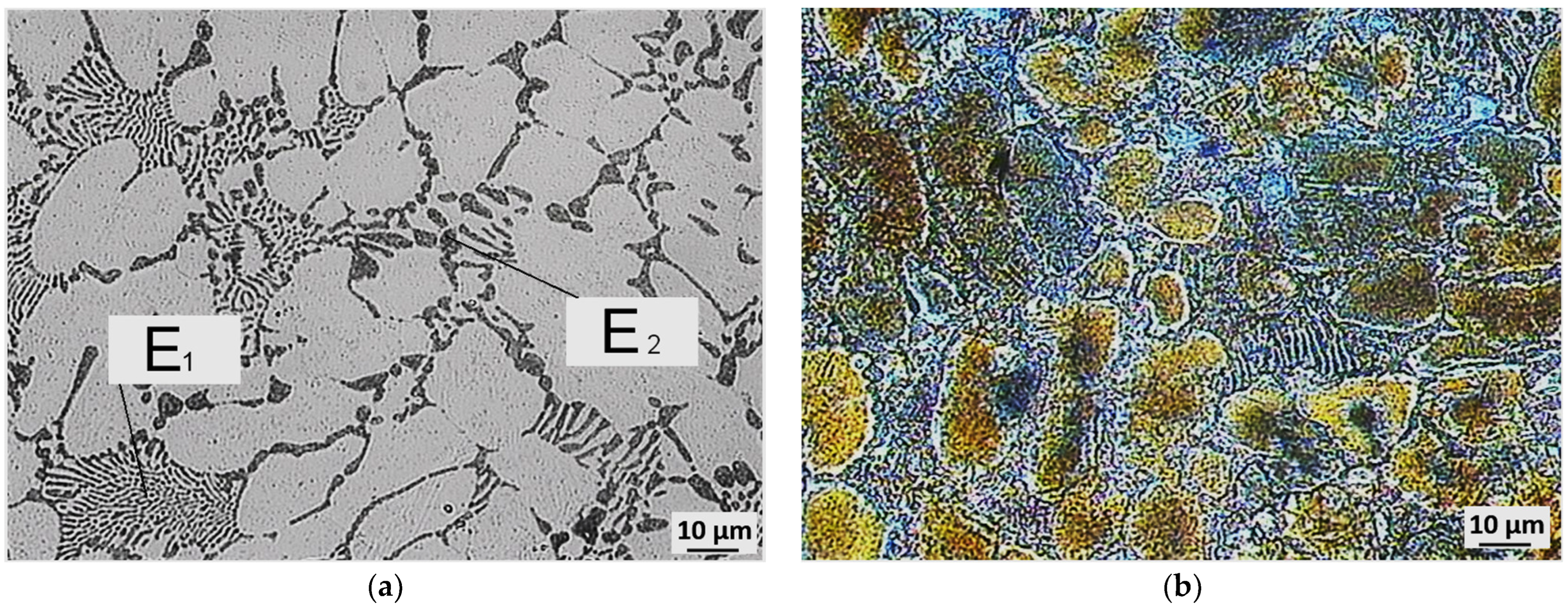
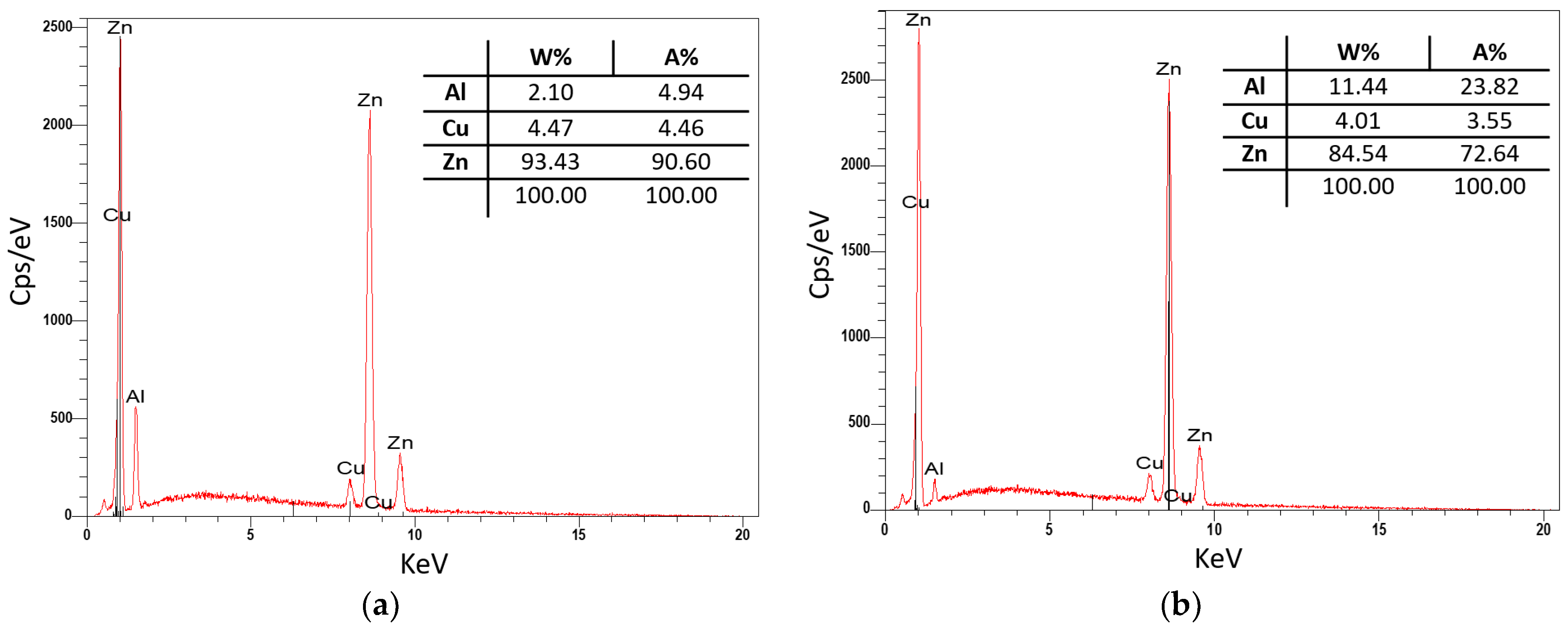
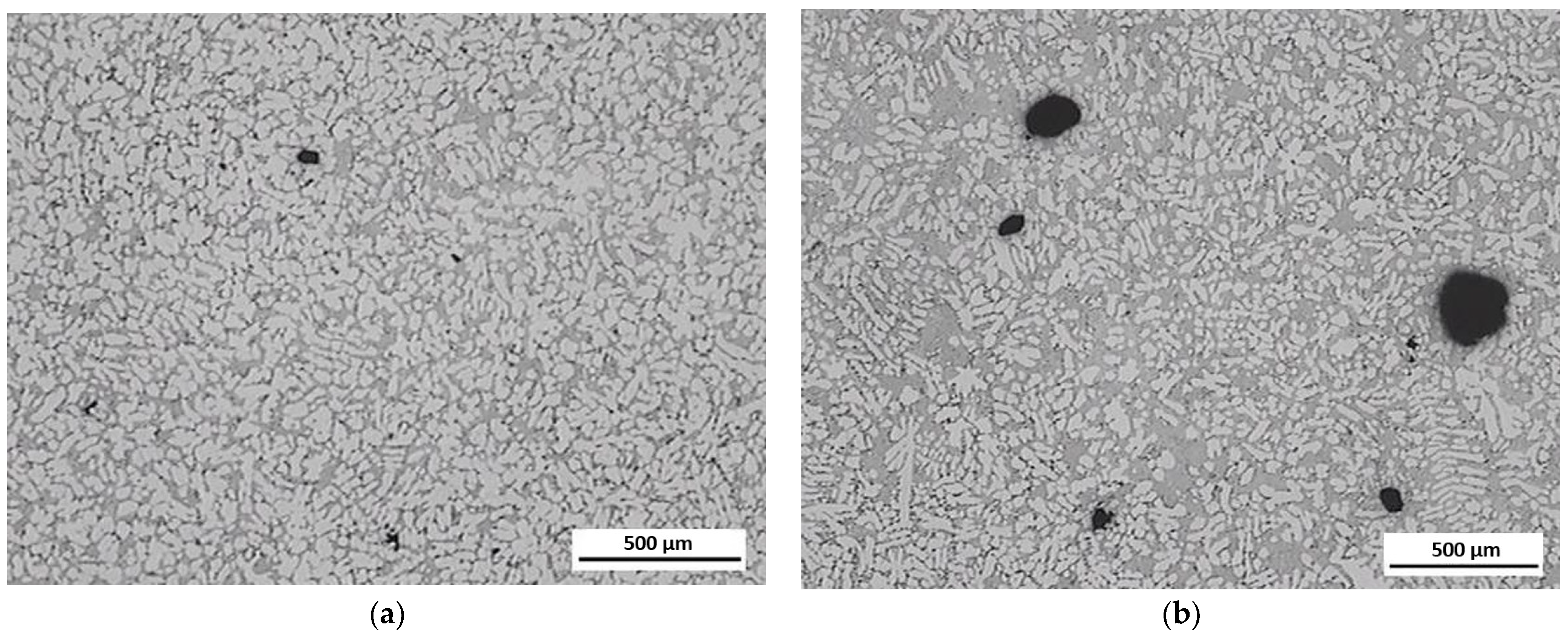
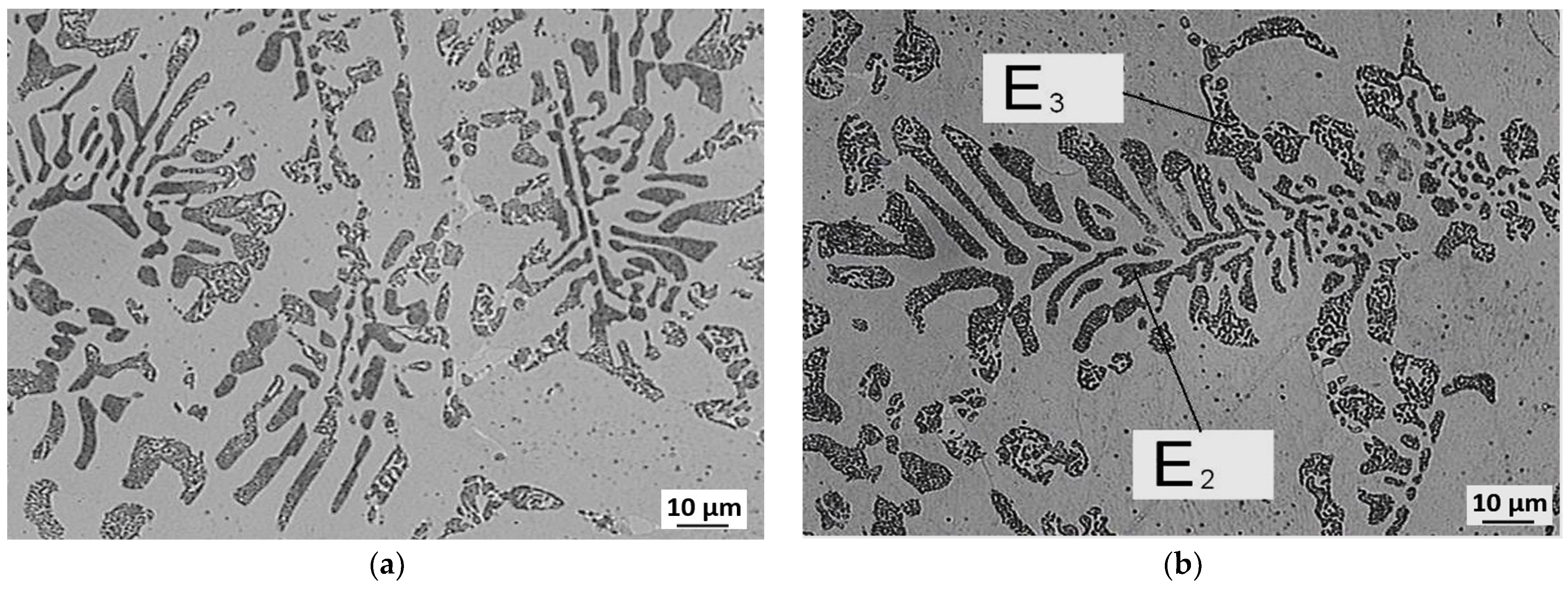
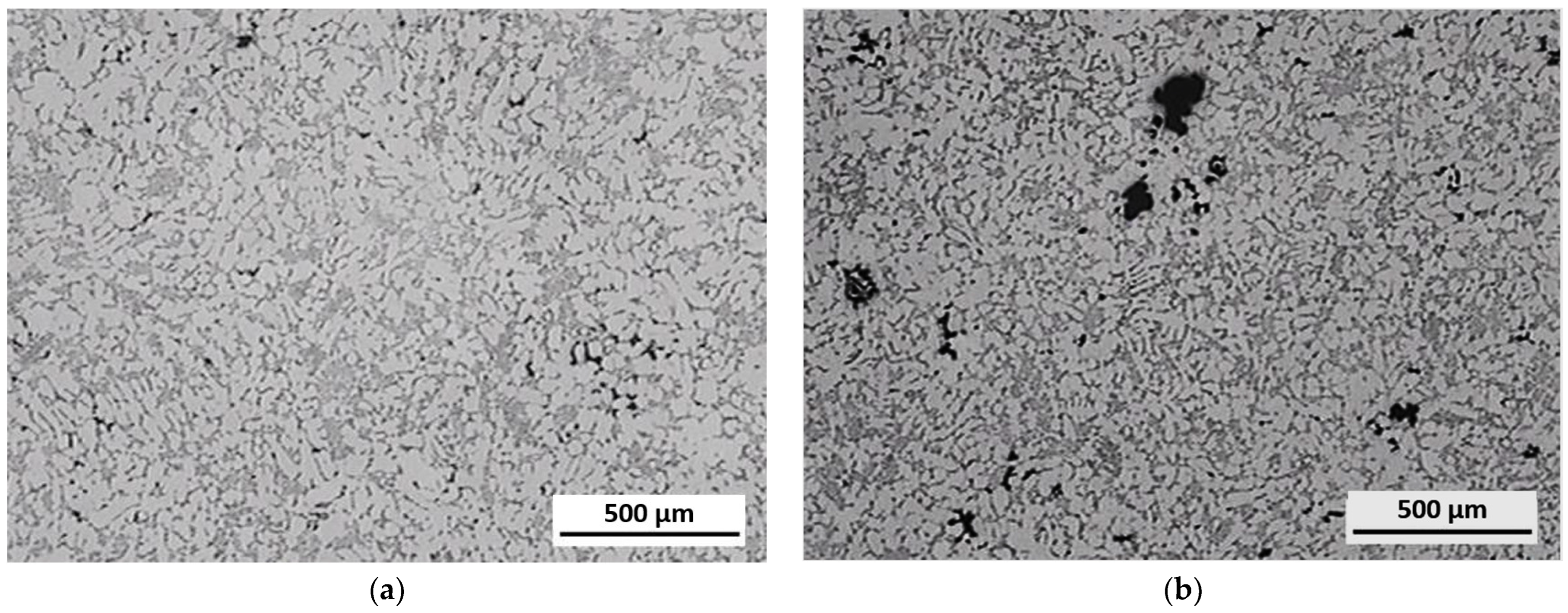
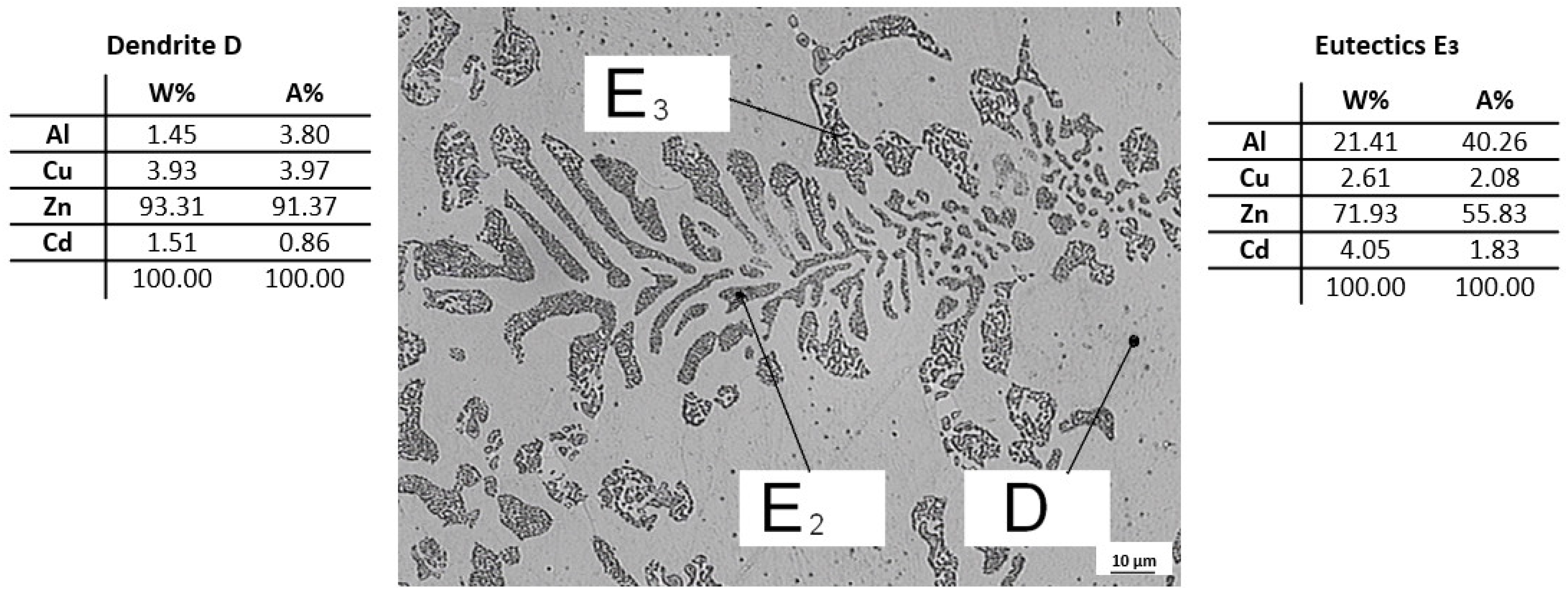
3.3. Evaluation of Microstructure
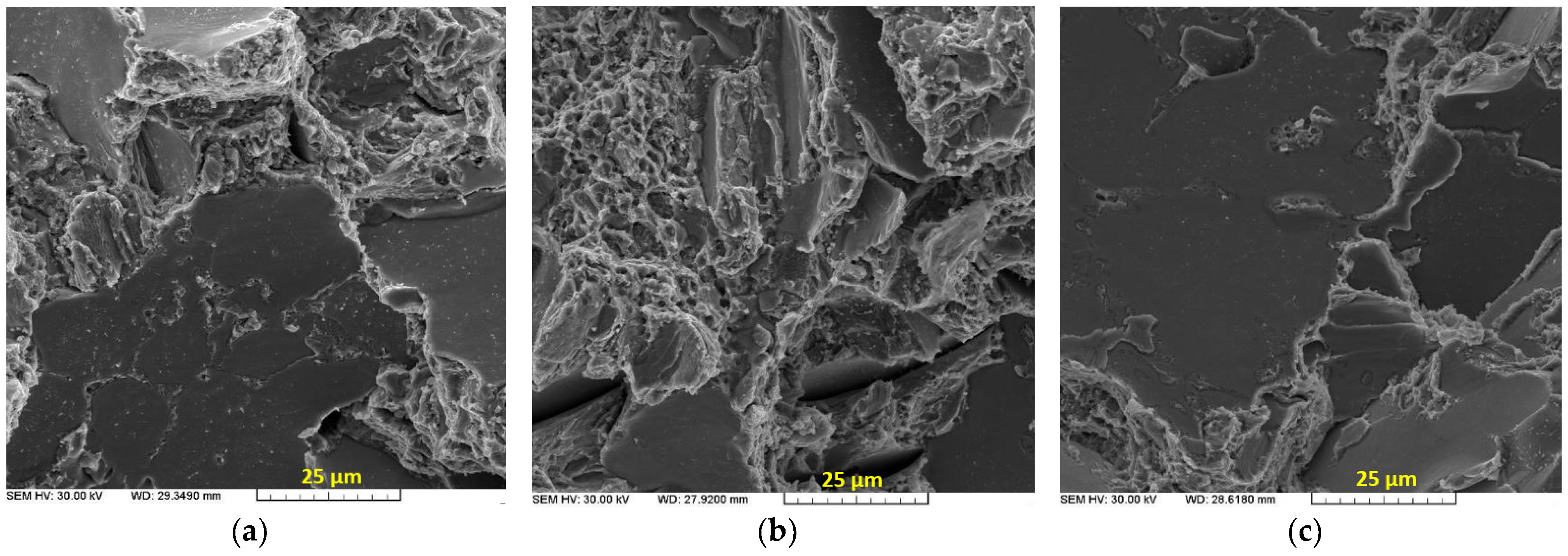
3.4. Fractographic Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shurkin, P.; Belov, N.; Akopyan, T.; Karpova, Z. Recycling-oriented design of the Al-Zn-Mg-Ca alloys. Mater. Proc. 2021, 3, 7. [Google Scholar] [CrossRef]
- Tan, R.; Khoo, H. Zinc Casting and Recycling. Int. J. Life Cycle Assess 2005, 10, 211–218. [Google Scholar] [CrossRef]
- Westing, E.; Savran, V.; Hofman, J. Recycling of Metals from Coatings; Materials Innovation Institute M2i: Delft, The Netherlands, 2013. [Google Scholar]
- Lynch, R.F. Zinc: Alloying, Thermomechanical Processing, Properties, and Applications. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 9869–9883. [Google Scholar]
- Dudek, P.; Piwowońska, J. Influence of Titanium on the Microstructure and Mechanical Properties of Foundry Zinc Alloy. J. Mater. Eng. Perform. 2022. [Google Scholar] [CrossRef]
- Kaya, M.; Hussaini, S.; Kursunoğlu, S. Critical review on secondary zinc resources and their recycling technologies. Hydrometallurgy 2020, 195, 105362. [Google Scholar] [CrossRef]
- Lu, X.; Miki, T.; Sasaki, Y.; Nagasaka, T. Thermodynamic criteria of alloying elements elimination during recycling end-of-life zinc-based products by remelting. Resources. Conserv. Recycl. 2022, 176, 105913. [Google Scholar] [CrossRef]
- Rollez, D.; Pola, A.; Prenger, F. Zinc alloy family for foundry purposes. World Metall. 2015, 68, 354–358. [Google Scholar]
- SM International Committee. Properties and Selection: NonferrousAlloys and Special Purpose Materials, ASM Metals Handbook, 10th ed.; ASM International: Almere, The Netherlands, 1990; Volume 2, p. 1300. [Google Scholar]
- Li, B.J.; Chao, C.G. Aging Kinetics of Heat-Treated Zn–4Al–3CuAlloy. Scr. Mater. 1999, 41, 143–147. [Google Scholar] [CrossRef]
- da Silva, F.C.; Kazmierczak, K.; da Costa, C.E.; Milan, J.C.G.; Torralba, J.M. Zamak 2 Alloy Produced by Mechanical Alloying and Consolidated by Sintering and Hot Pressing. J. Manuf. Sci. Eng. 2017, 139, 091011. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxfordshire, UK, 2015; pp. 243–247. [Google Scholar]
- Al-Maharbi, M.; Karaman, I.; Purcek, G. Flow response of a severe plastically deformed two-phase zinc–aluminum alloy. Mater. Sci. Eng. A 2010, 527, 518–525. [Google Scholar] [CrossRef]
- Pürçek, G. Improvement of mechanical properties for Zn–Al alloys using equal-channel angular pressing. J. Mater. Process. Technol. 2005, 169, 242–248. [Google Scholar] [CrossRef]
- Purcek, G.; Altan, B.S.; Miskioglu, I.; Ooi, P.H. Processing of eutectic Zn–5% Al alloy by equal-channel angular pressing. J. Mater. Process. Technol. 2004, 148, 279–287. [Google Scholar] [CrossRef]
- da Costa, E.M.; da Costa, C.E.; Vecchia, F.D.; Rick, C.; Scherer, M.; dos Santos, C.A.; Dedavid, B.A. Study of the Influence of Copper andMagnesium Additions on the Microstructure Formation of Zn–Al HypoeutecticAlloys. J. Alloys Compd. 2009, 488, 89–99. [Google Scholar] [CrossRef]
- Wu, Z.; Sandlöbes, S.; Wu, L.; Hu, W.; Gottstein, G.; Korte-Kerzel, S. Mechanical behaviour of Zn–Al–Cu–Mg alloys: Deformation mechanisms of as-cast microstructures. Mater. Sci. Eng. A 2016, 651, 675–687. [Google Scholar] [CrossRef]
- Lakomá, R.; Čamek, L.; Lichý, P.; Kroupová, I.; Radovský, F.; Obzina, T. Some Possibilities of Using Statistical Methods While Solving Poor Quality Production. Arch. Foundry Eng. 2021, 21, 18–22. [Google Scholar]
- LeHuy, H.; L’Espérance, G. Ageing characteristics of dendritic and non-dendritic (stir-cast) Zn-Al alloy (ZA-27). J. Mater. Sci. 1991, 26, 559–568. [Google Scholar] [CrossRef]
- Pola, A.; Tocci, M.; Goodwin, F.E. Review of Microstructures and Properties of Zinc Alloys. Metals 2020, 10, 253. [Google Scholar] [CrossRef] [Green Version]
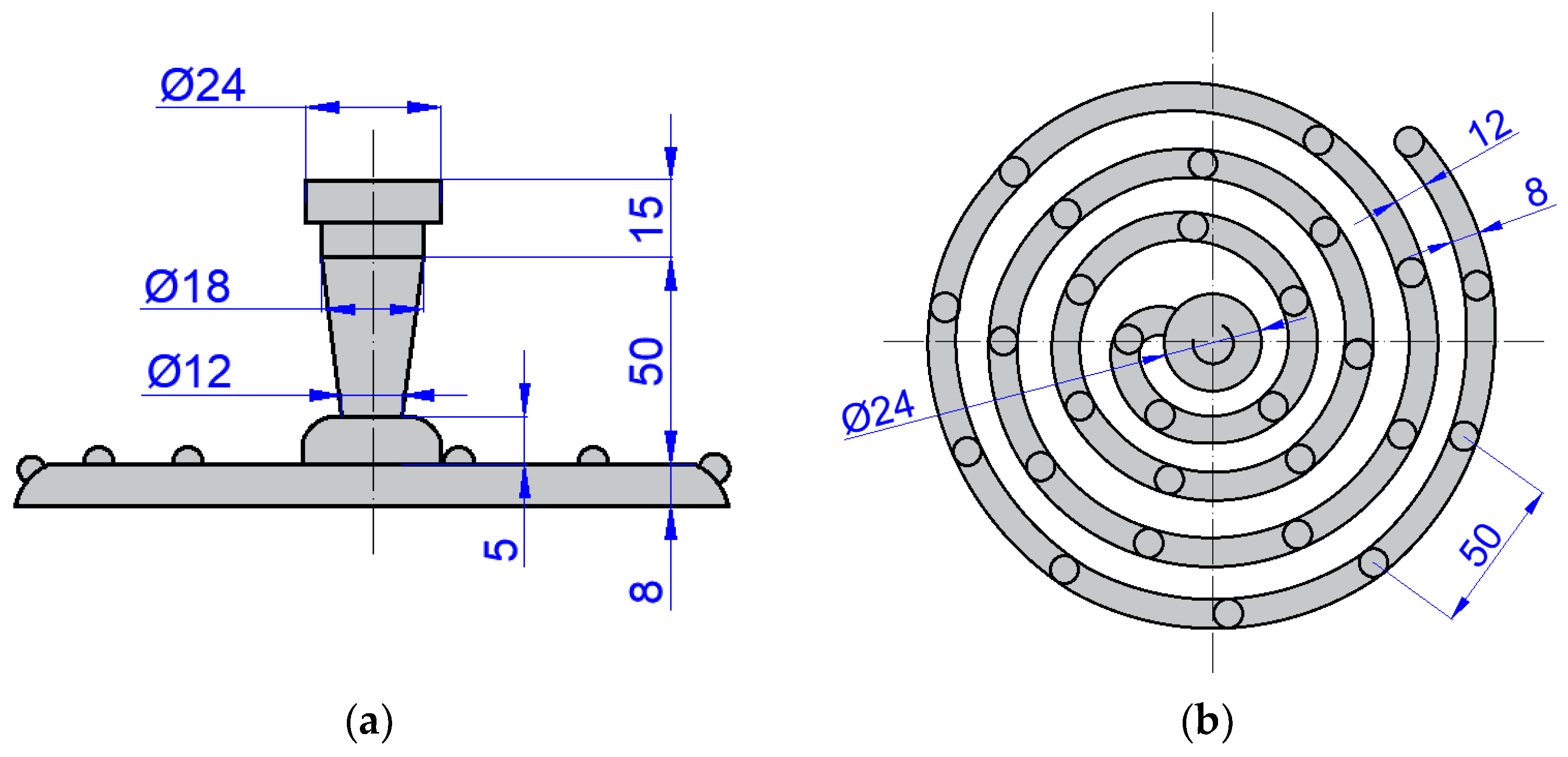


| Element Content (wt.%) | |||
|---|---|---|---|
| Al | Cu | Mg | Zn |
| 3.93 | 3.06 | 0.047 | rest |
| Permissible Elements Content (wt.%) | |||
| Pb | Cd | Sn | Fe |
| 0.0037 | 0.002 | 0.0078 | 0.010 |
| Rm (MPa) | A50 (%) | Hardness HBS | |||
|---|---|---|---|---|---|
| HPDC | Sand Mold | HPDC | Sand Mold | HPDC | Sand Mold |
| 355 | 215 | 5 | 2 | 102 | 90 |
| Temperature of the cast metal | Tk = 420 ± 5 °C |
| Initial temperature of the mold | Tf = 60 ± 3 °C |
| Rounds per minute of the mold | n = 700 rpm |
| Solidification time | t = 30 s |
| Alloy | Al (wt.%) | Cu (wt.%) | Mg (wt.%) | Pb (wt.%) | Cd (wt.%) | Sn (wt.%) | Fe (wt.%) | Zn (wt.%) |
|---|---|---|---|---|---|---|---|---|
| P3 | 3.92 | 2.92 | 0.040 | 0.0036 | 0.002 | 0.0078 | 0.011 | rest |
| P5 | 3.97 | 2.92 | 0.042 | 0.0042 | 0.002 | 0.0079 | 0.012 | rest |
| P7 | 3.91 | 2.98 | 0.039 | 0.0040 | 0.002 | 0.0091 | 0.011 | rest |
| P9 | 4.04 | 2.85 | 0.045 | 0.0047 | 0.003 | 0.0077 | 0.0072 | rest |
| Alloy | Al (wt.%) | Cu (wt.%) | Mg (wt.%) | Pb (wt.%) | Cd (wt.%) | Sn (wt.%) | Fe (wt.%) | Zn (wt.%) |
|---|---|---|---|---|---|---|---|---|
| C1 | 3.92 | 2.42 | 0.036 | 0.0047 | 3.39 | 0.0096 | 0.0150 | rest |
| C3 | 3.84 | 2.41 | 0.035 | 0.0048 | 3.38 | 0.0110 | 0.0078 | rest |
| C5 | 3.82 | 2.37 | 0.035 | 0.0051 | 3.50 | 0.0110 | 0.0065 | rest |
| C7 | 3.55 | 2.54 | 0.031 | 0.0046 | 2.99 | 0.0120 | 0.0140 | rest |
| C9 | 3.76 | 2.35 | 0.035 | 0.0050 | 3.38 | 0.0110 | 0.0078 | rest |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolibruchová, D.; Bruna, M.; Matejka, M. Impact of Remelting on ZnAl4Cu3 Alloy with Addition of Cd on Selected Technological and Mechanical Properties. Metals 2022, 12, 1180. https://doi.org/10.3390/met12071180
Bolibruchová D, Bruna M, Matejka M. Impact of Remelting on ZnAl4Cu3 Alloy with Addition of Cd on Selected Technological and Mechanical Properties. Metals. 2022; 12(7):1180. https://doi.org/10.3390/met12071180
Chicago/Turabian StyleBolibruchová, Dana, Marek Bruna, and Marek Matejka. 2022. "Impact of Remelting on ZnAl4Cu3 Alloy with Addition of Cd on Selected Technological and Mechanical Properties" Metals 12, no. 7: 1180. https://doi.org/10.3390/met12071180
APA StyleBolibruchová, D., Bruna, M., & Matejka, M. (2022). Impact of Remelting on ZnAl4Cu3 Alloy with Addition of Cd on Selected Technological and Mechanical Properties. Metals, 12(7), 1180. https://doi.org/10.3390/met12071180








