Effect of Al Dross Addition on Temperature Improvements in Molten Steel by Blowing Dry Air
Abstract
1. Introduction
2. Experimental Method
2.1. Dissolution of Al Dross and Cokes in Molten Steel
2.2. Measurement of Molten Steel Temperature by Blowing Dry Air
3. Results and Discussion
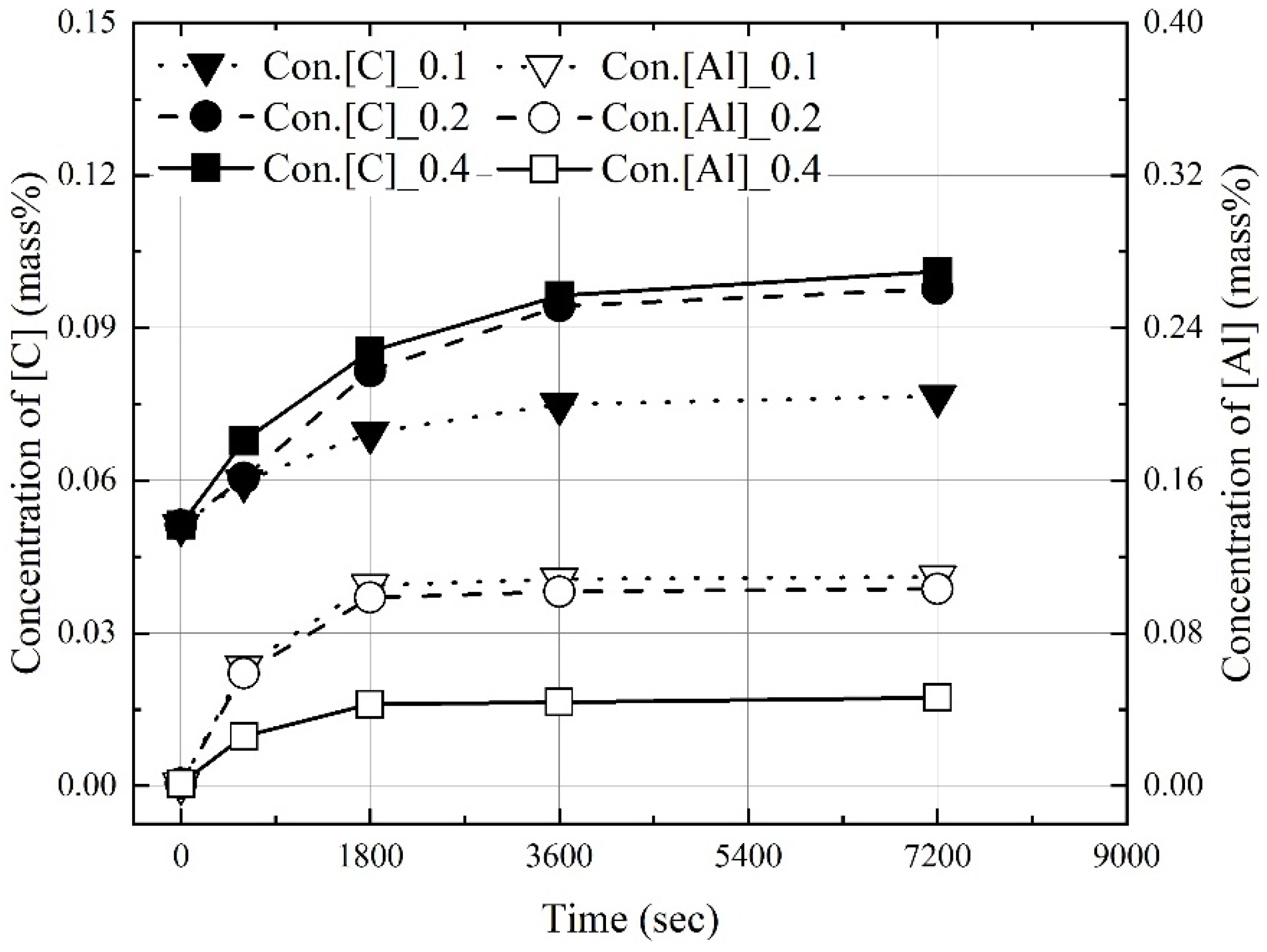
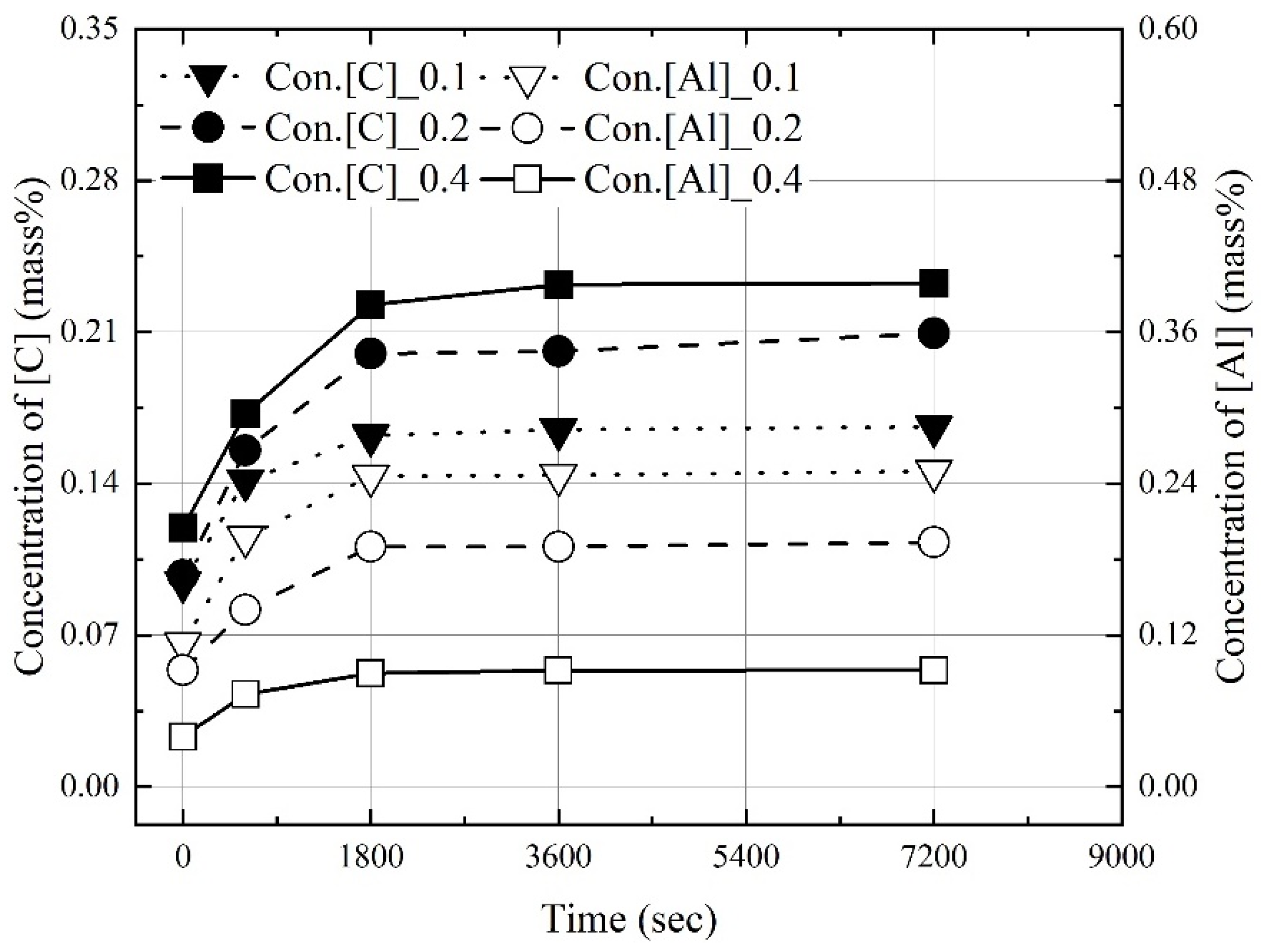
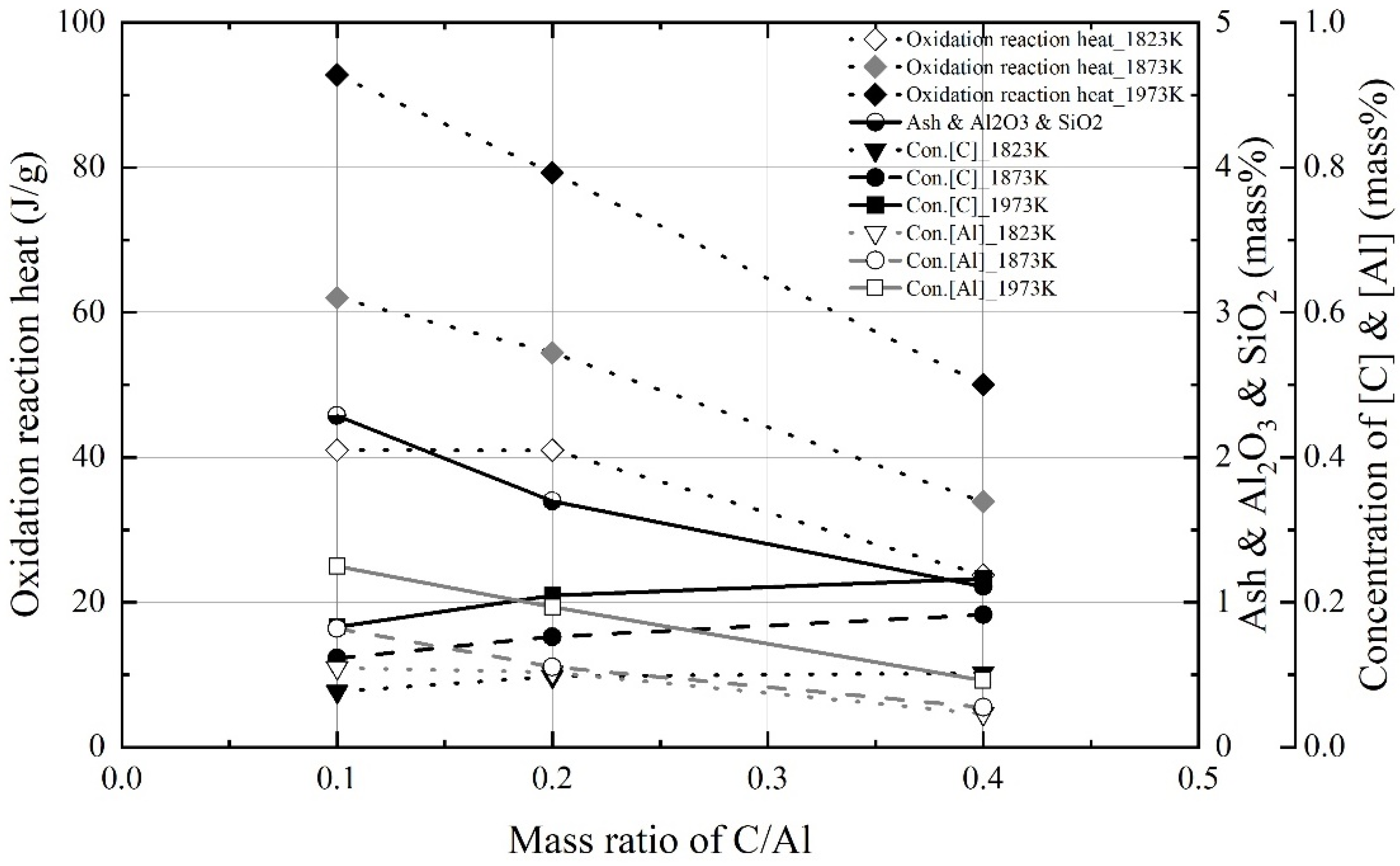
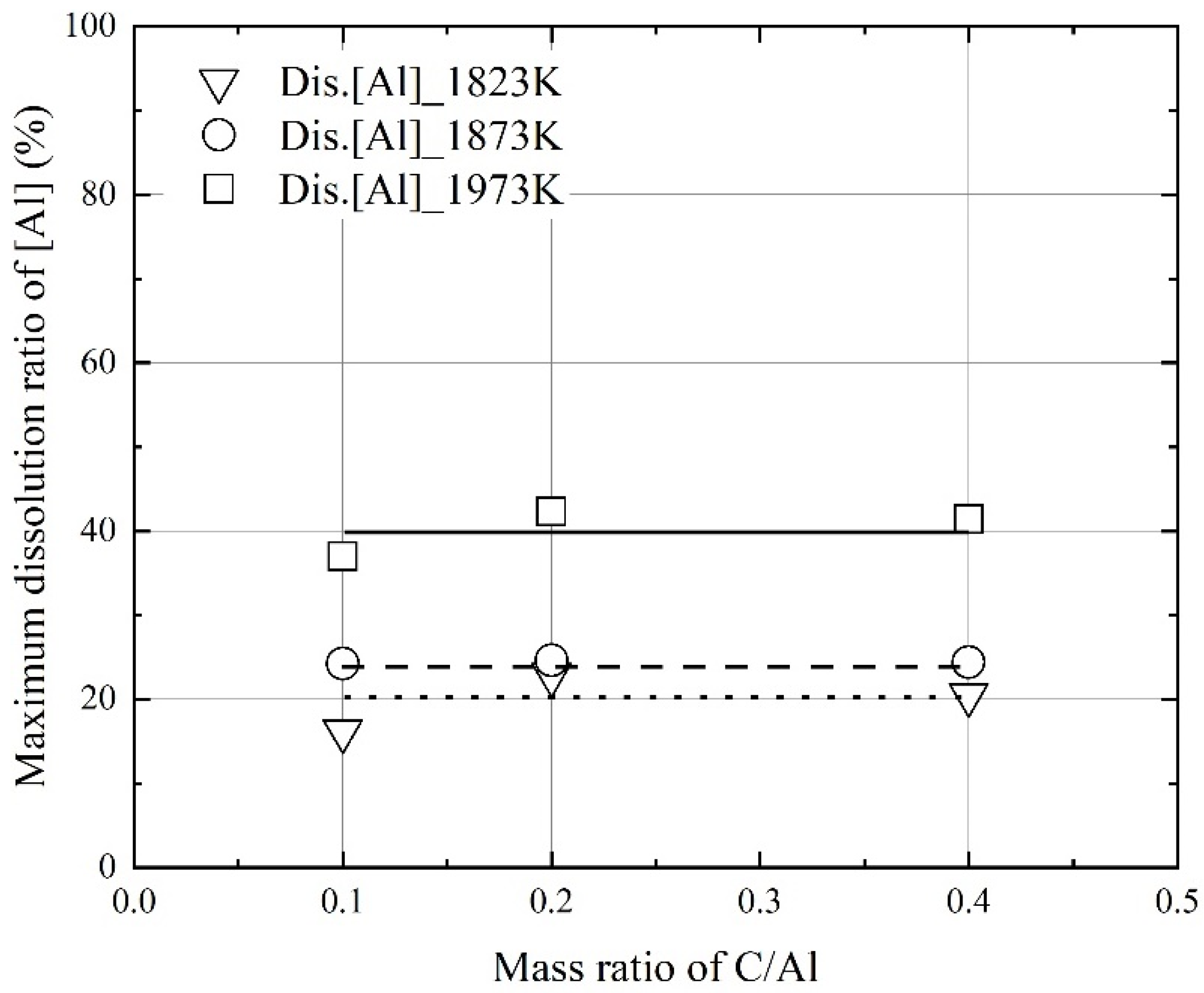
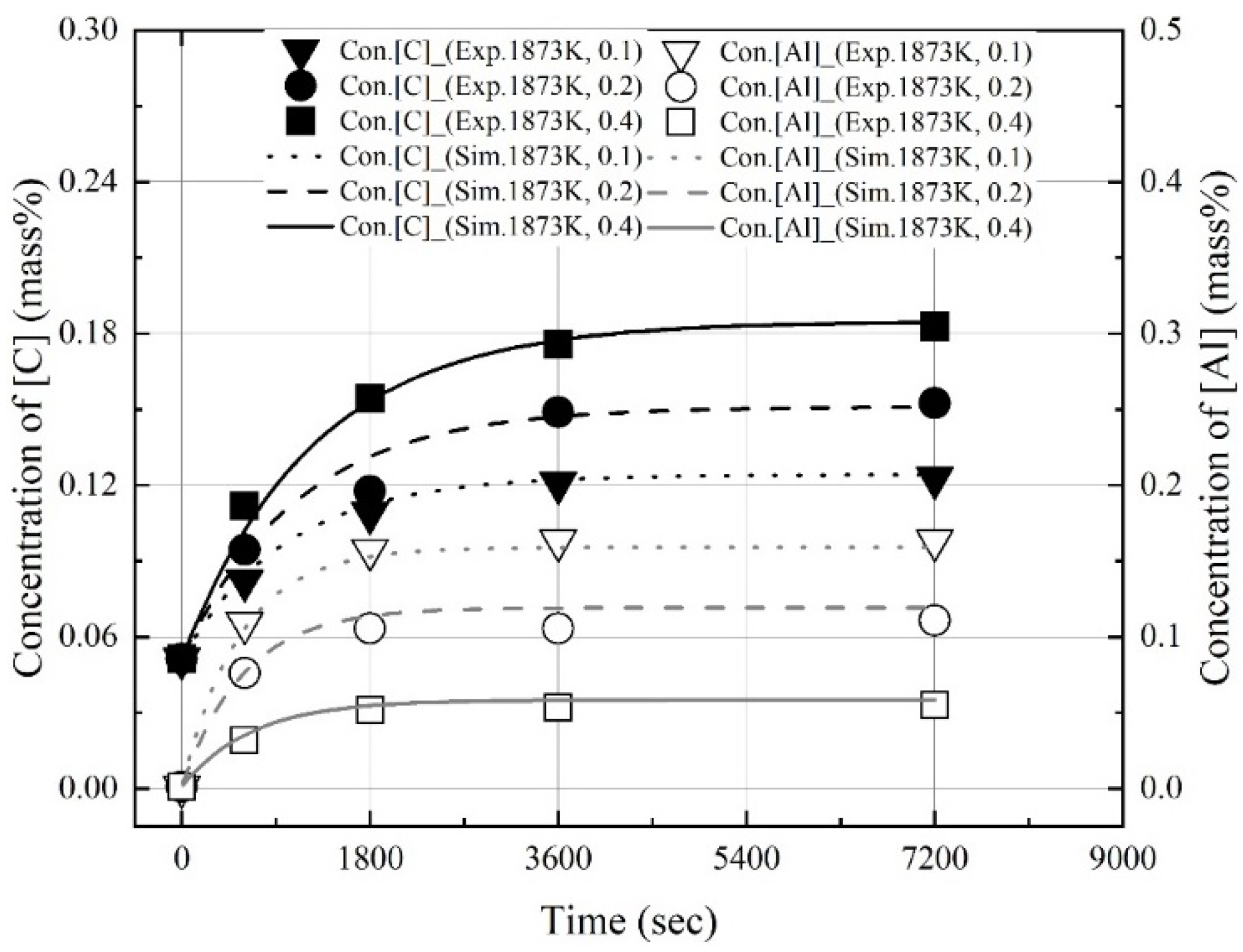
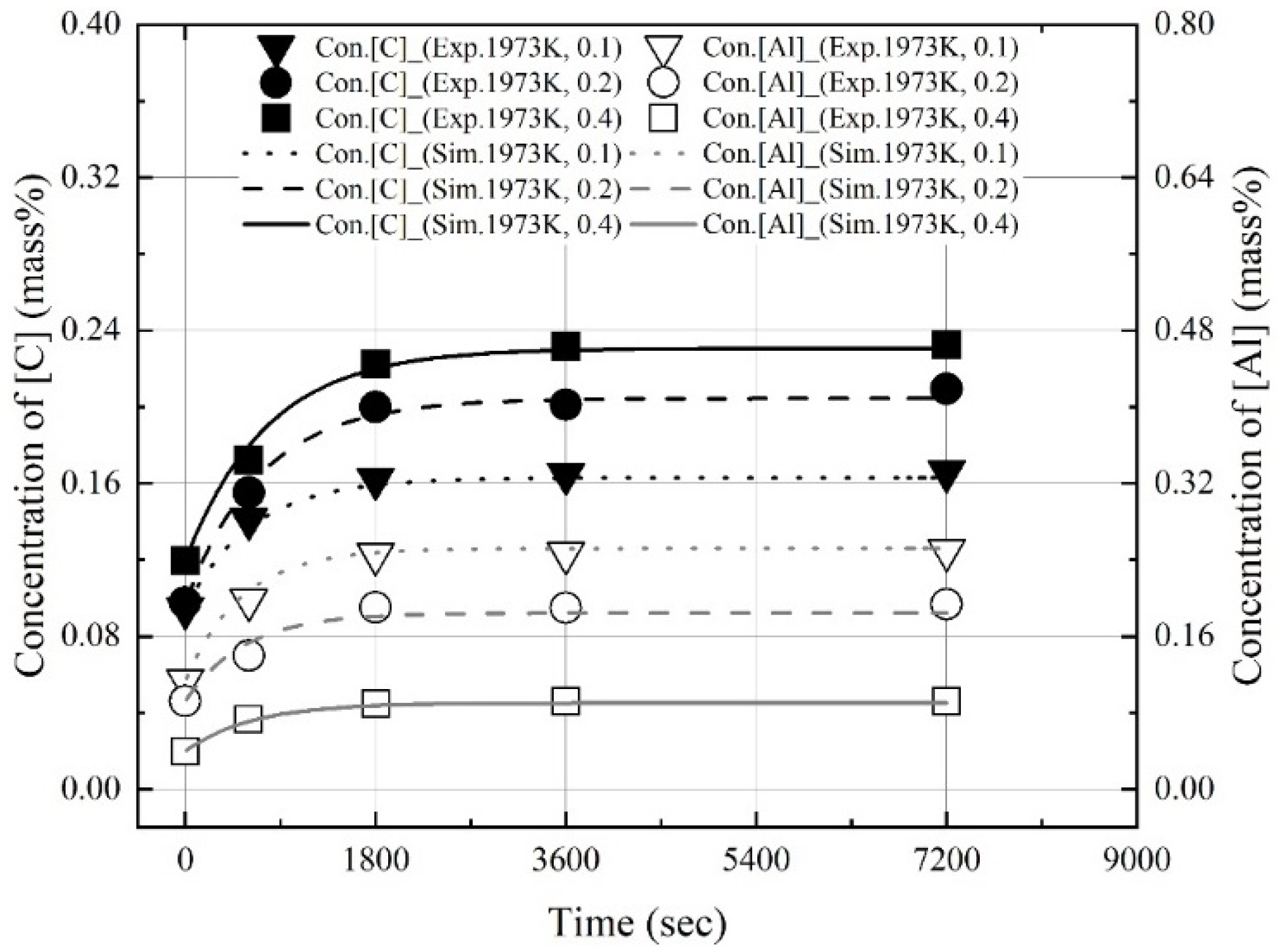
3.1. Dissolution of Al Dross and Coke in Molten Steel
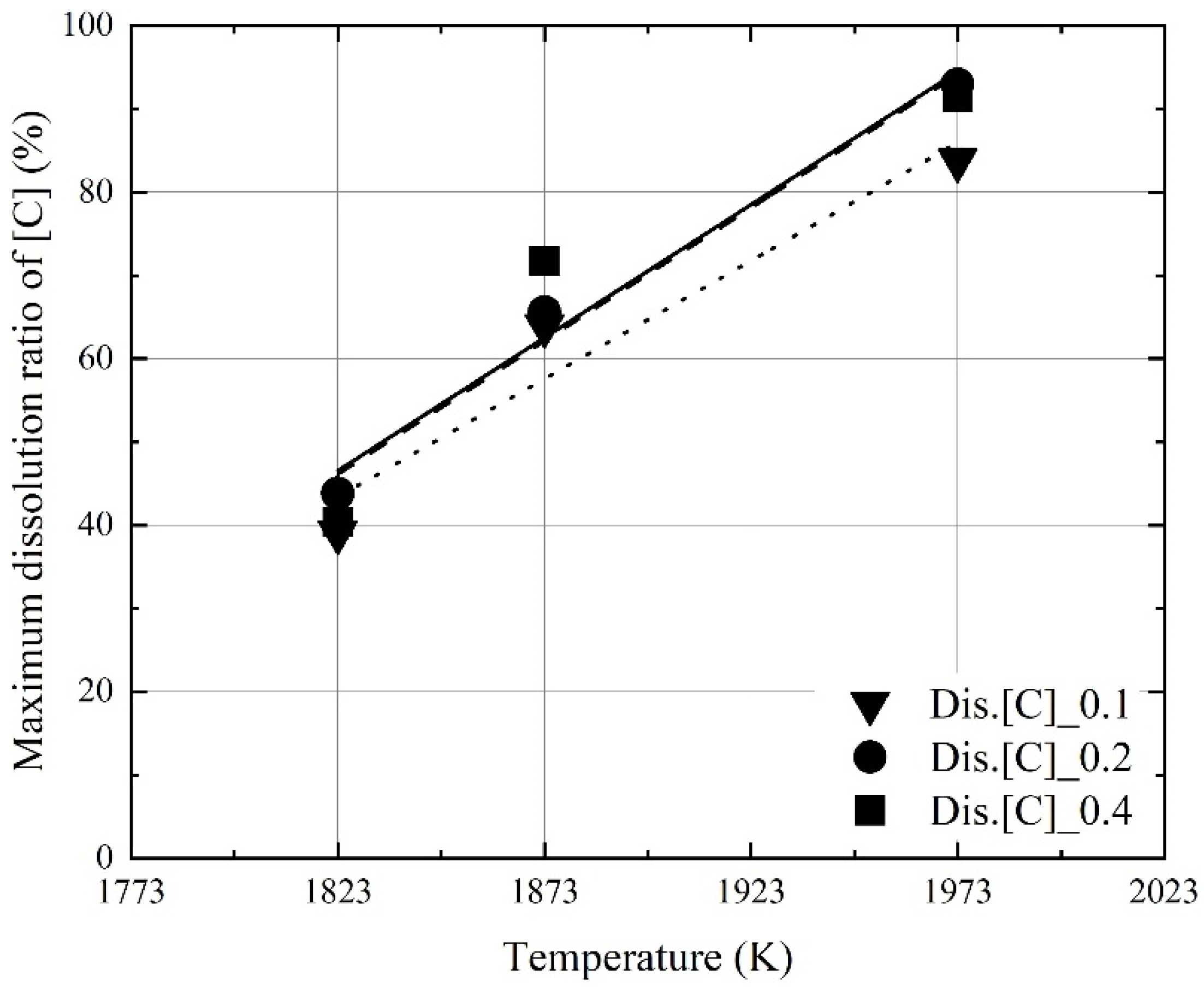
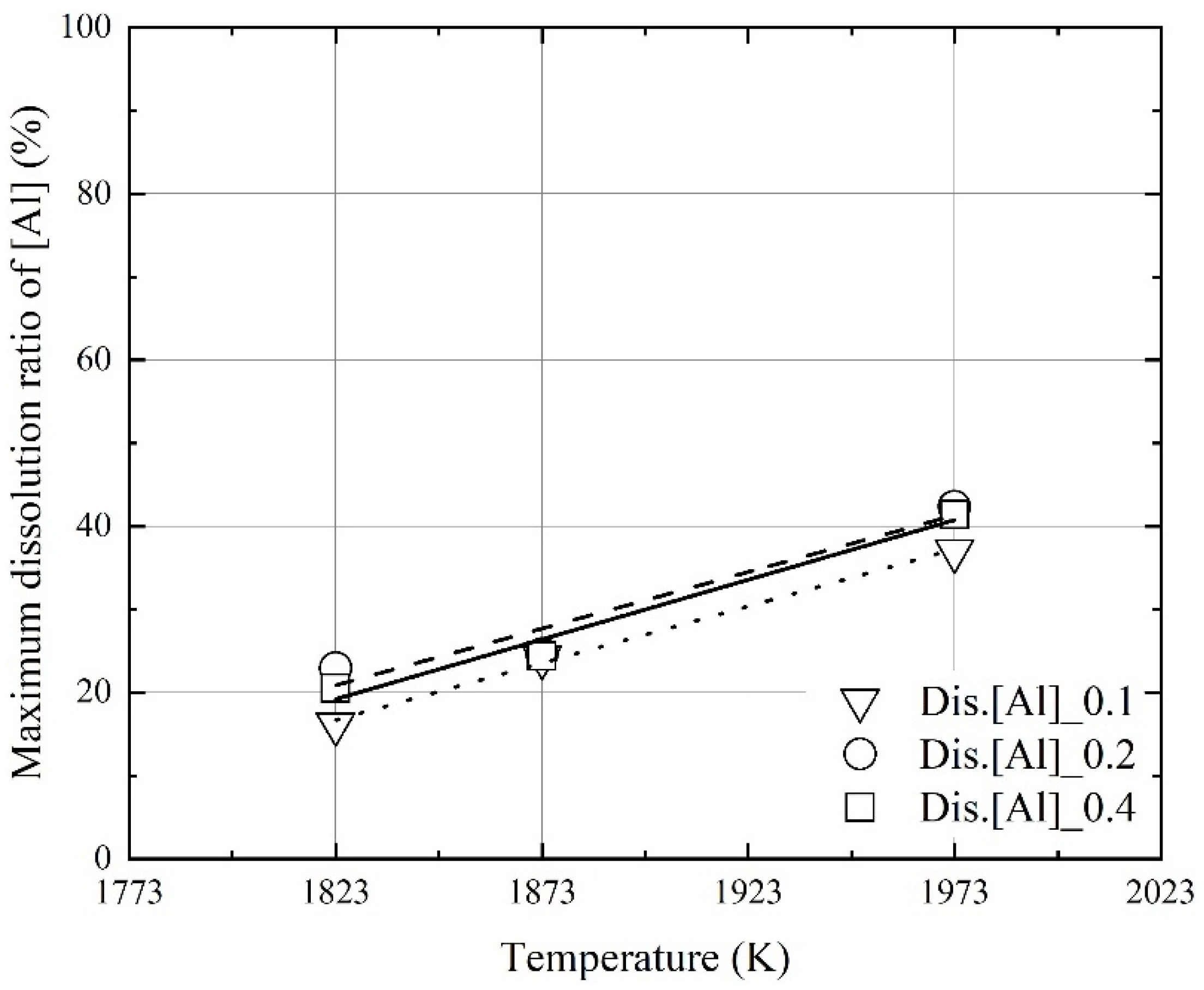
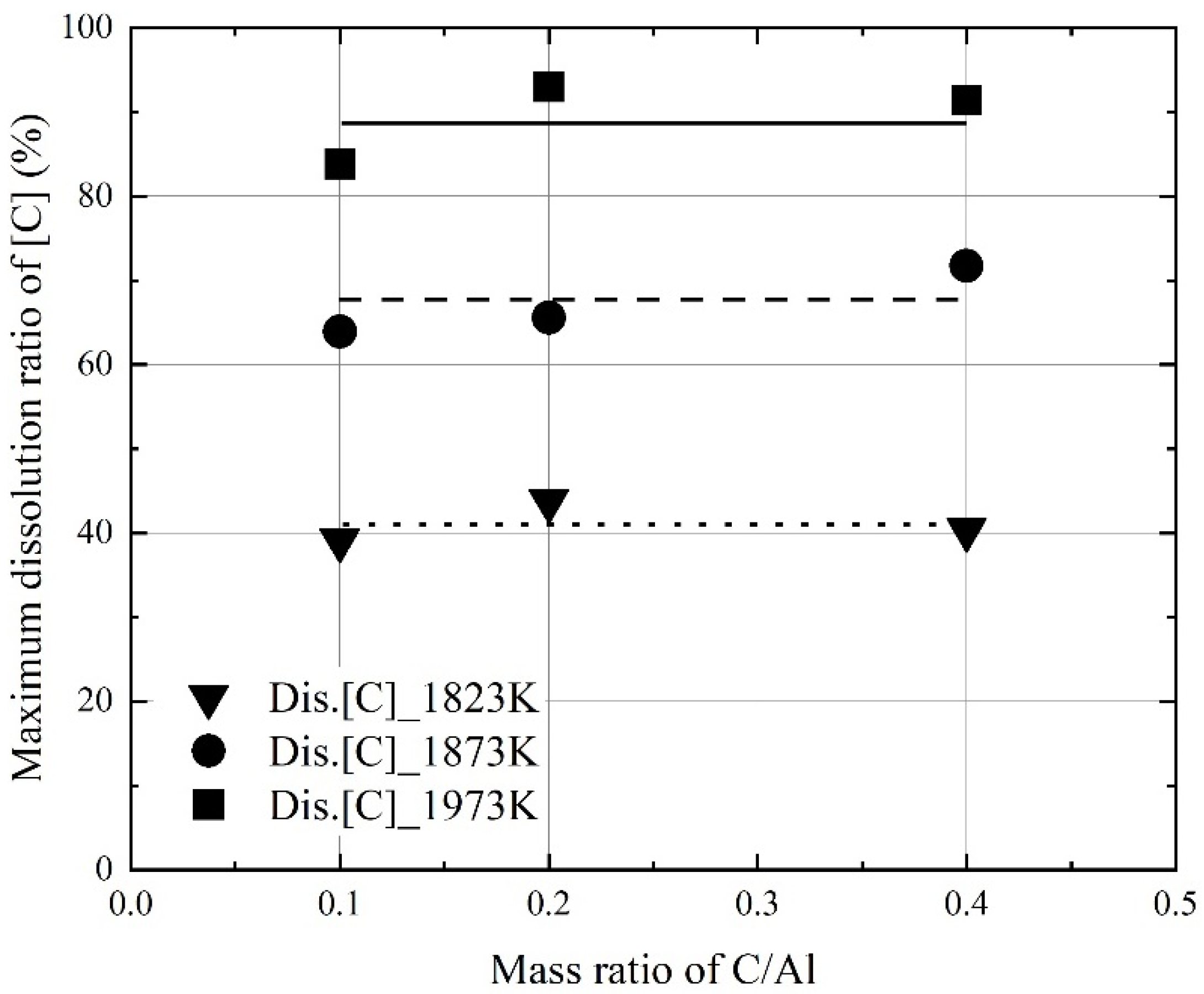
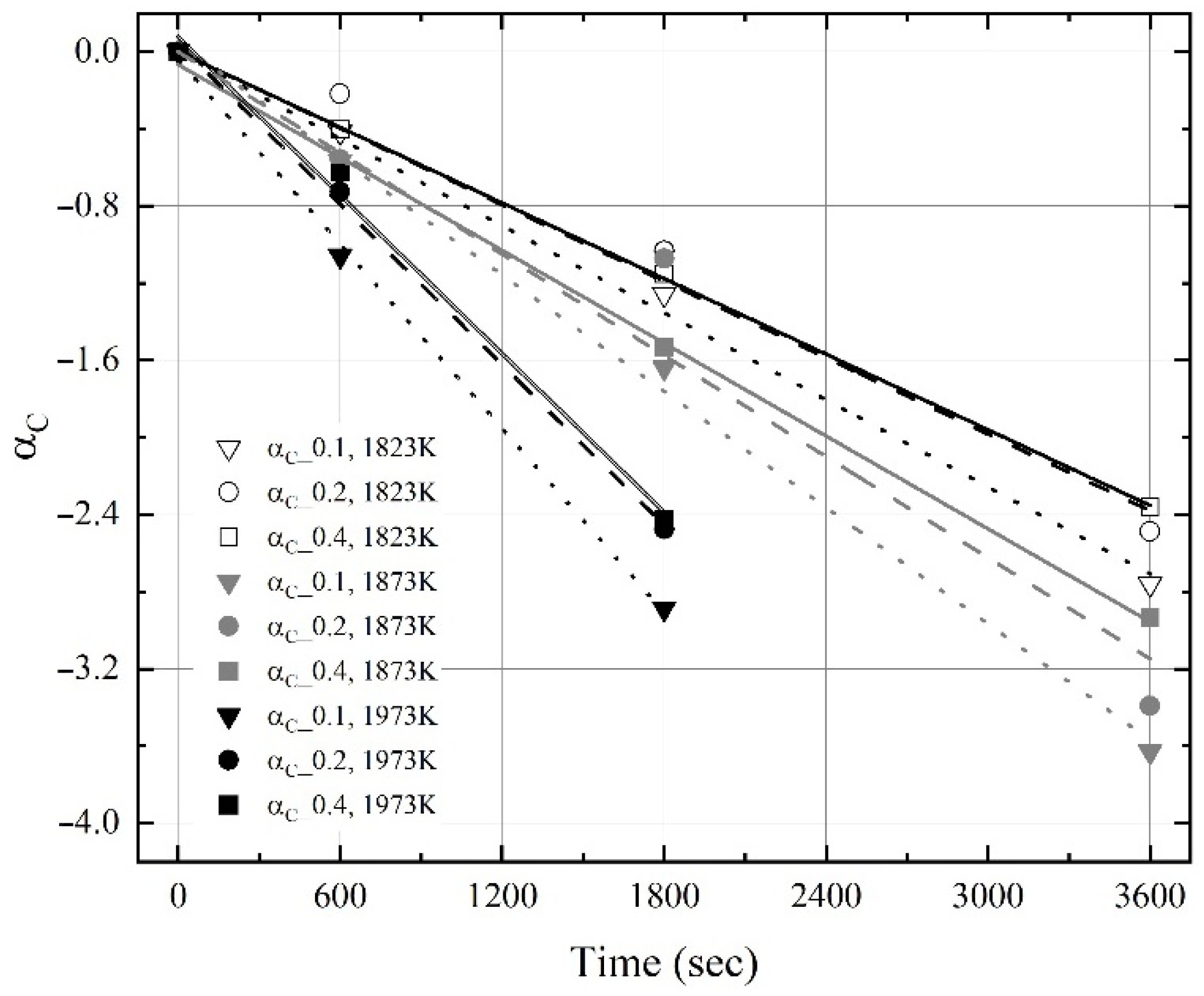
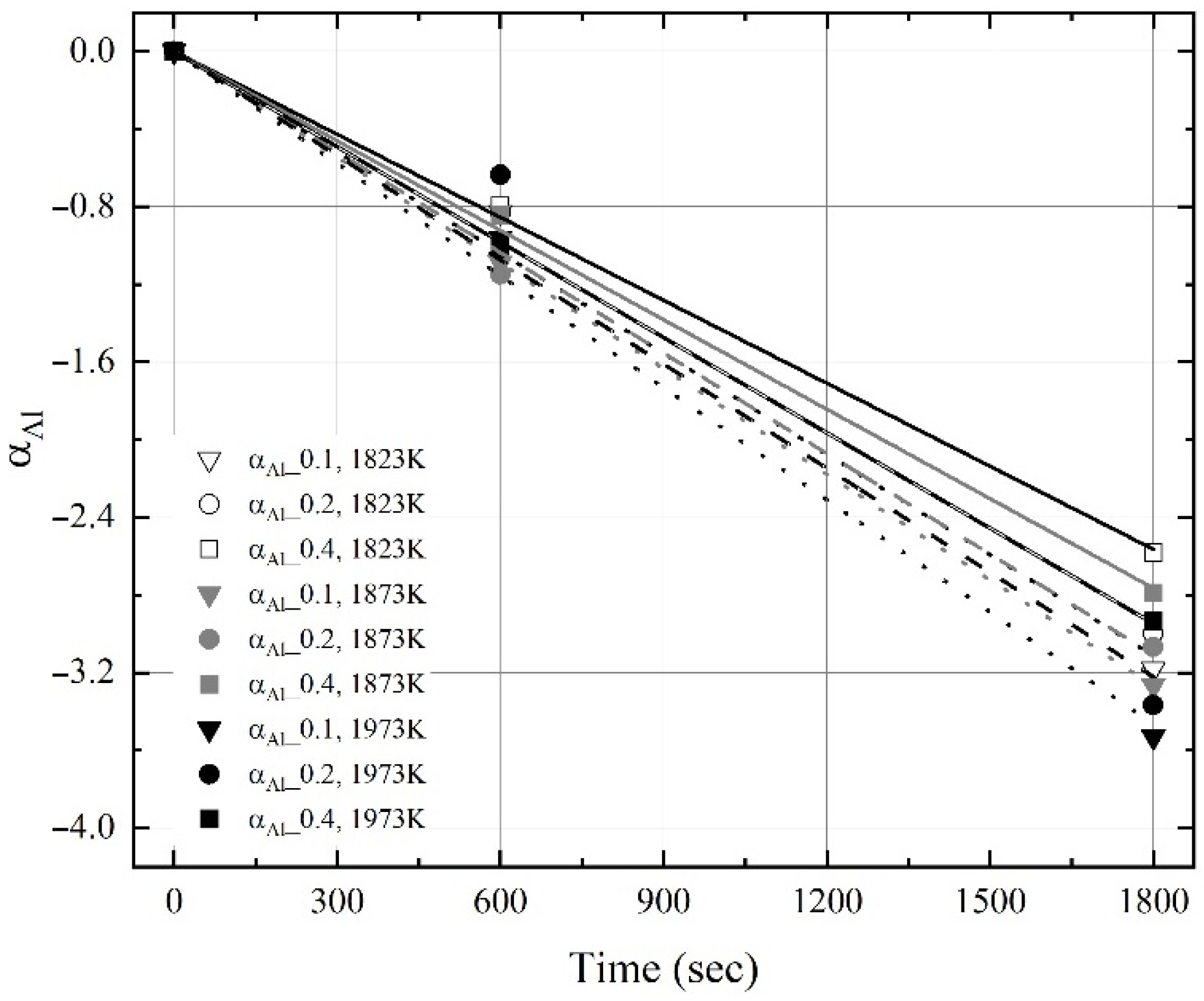
3.2. Kinetics for Dissolution of Al and C from Al Dross and Coke Mixture in Molten Steel
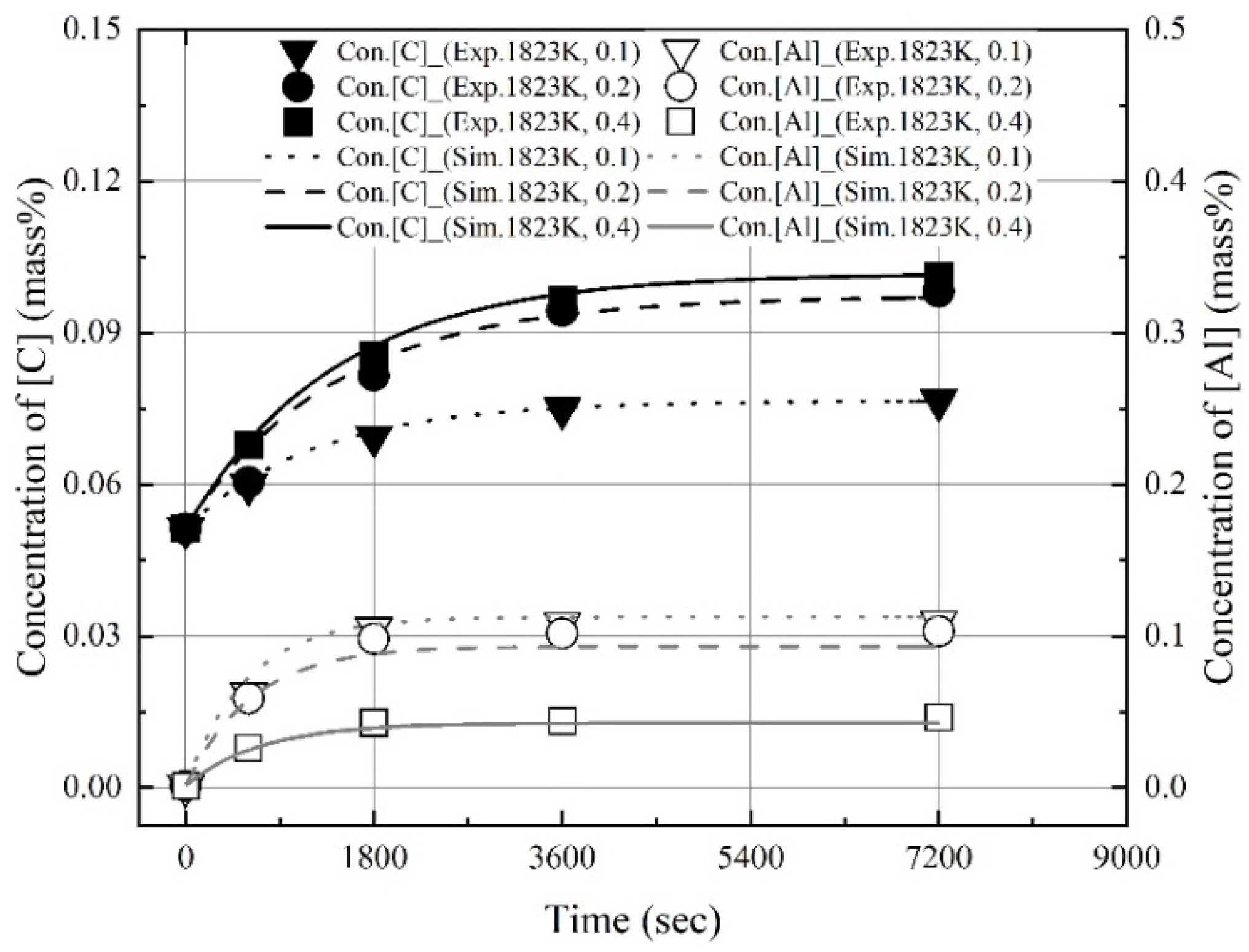
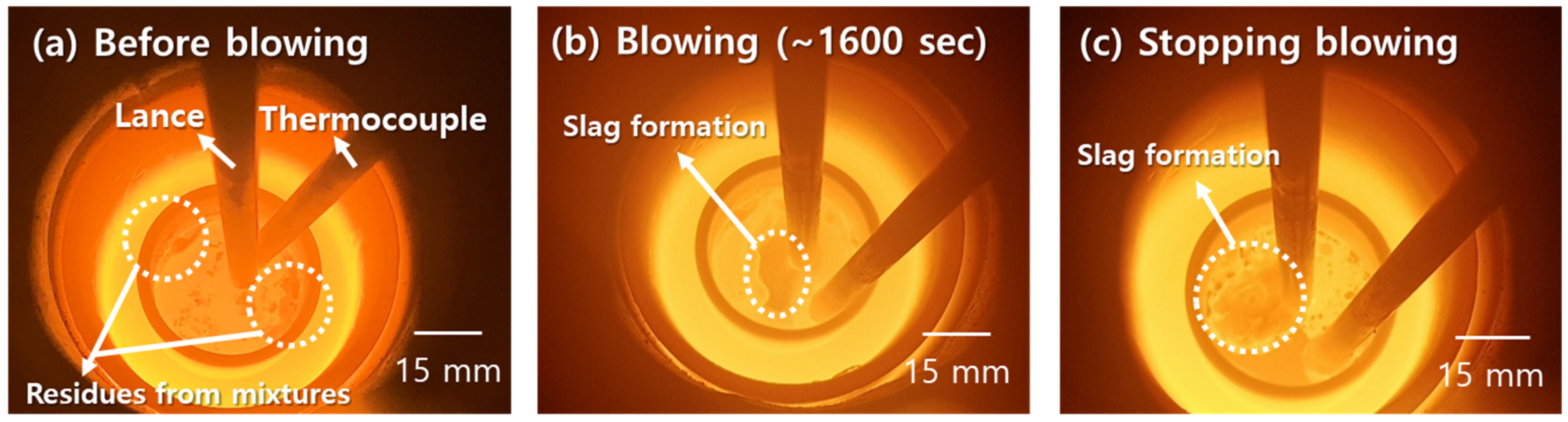
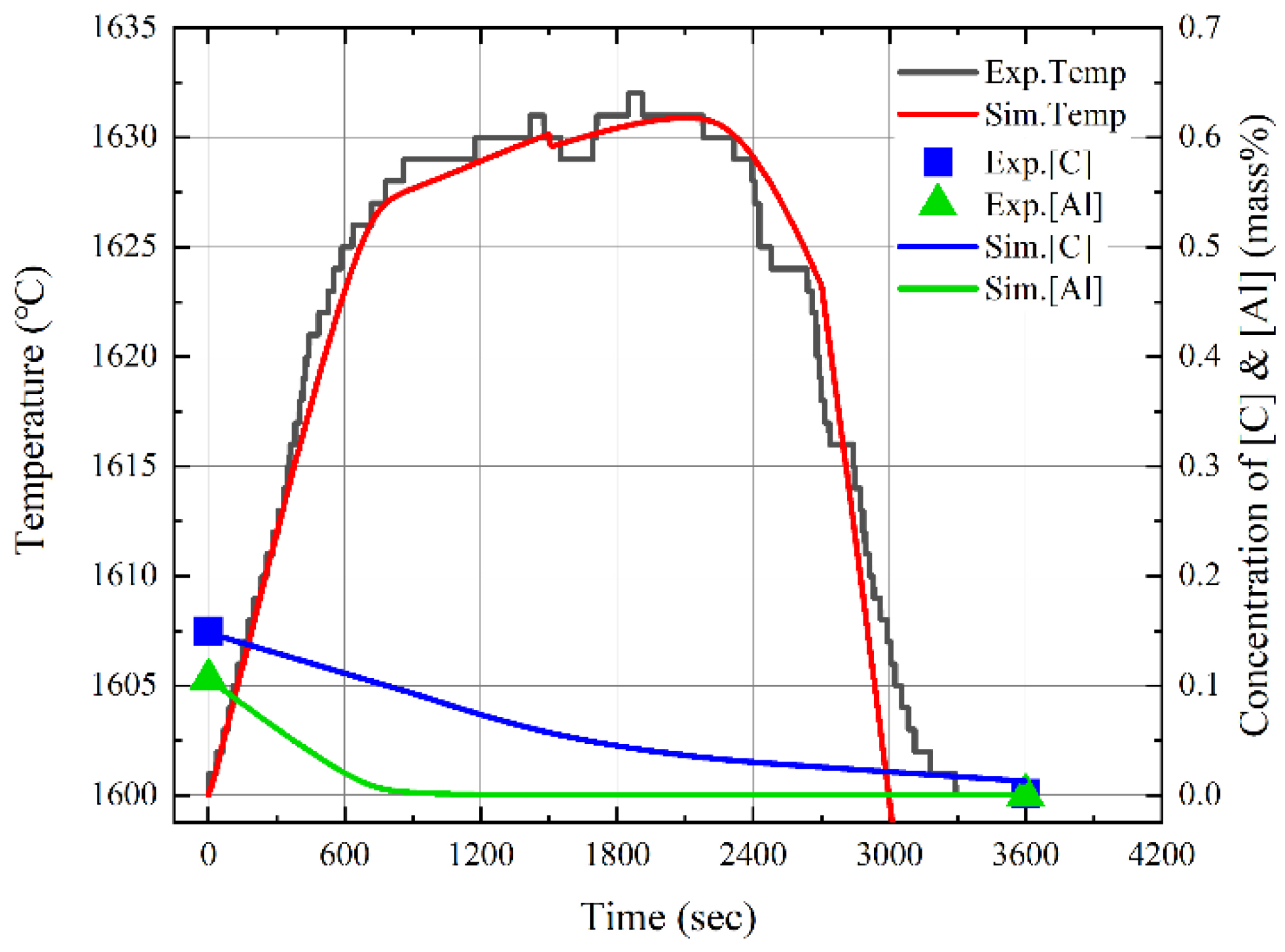
3.3. Changes in Molten Steel Temperature by Blowing Dry Air after Dissolution of Al Dross and Coke Mixture
4. Conclusions
- (1)
- The dissolution experiments of the coke and Al dross mixtures were conducted at 1823–1973 K. The dissolution concentrations of carbon and aluminum in molten steel increased with the reaction time and molten steel temperature. The dissolution concentration of aluminum in molten steel was constant after 1800 s. At 1823 K and 1873 K, the dissolution concentration of carbon in molten steel was constant after 3600 s. At 1973 K, and the dissolution concentration of carbon in molten steel remained constant after 1800 s. The theoretical oxidation heat was calculated using the maximum dissolution concentrations of carbon and aluminum in molten steel.
- (2)
- At a constant mixing ratio of coke and Al dross, the maximum dissolution ratio of carbon and aluminum in molten steel increased with the molten steel temperature. At a constant molten steel temperature, the effect of the mixing ratio on the maximum dissolution ratio of carbon and aluminum was not significant. The relationship between the maximum dissolution ratio of carbon and aluminum in molten steel and the molten steel temperature was obtained.
- (3)
- The dissolution rate constants of carbon and aluminum increased with the molten steel temperature. The dissolution concentrations of the coke and Al dross were calculated using the dissolution rate constants of carbon and aluminum, and the calculated values were in good agreement with the experimental data.
- (4)
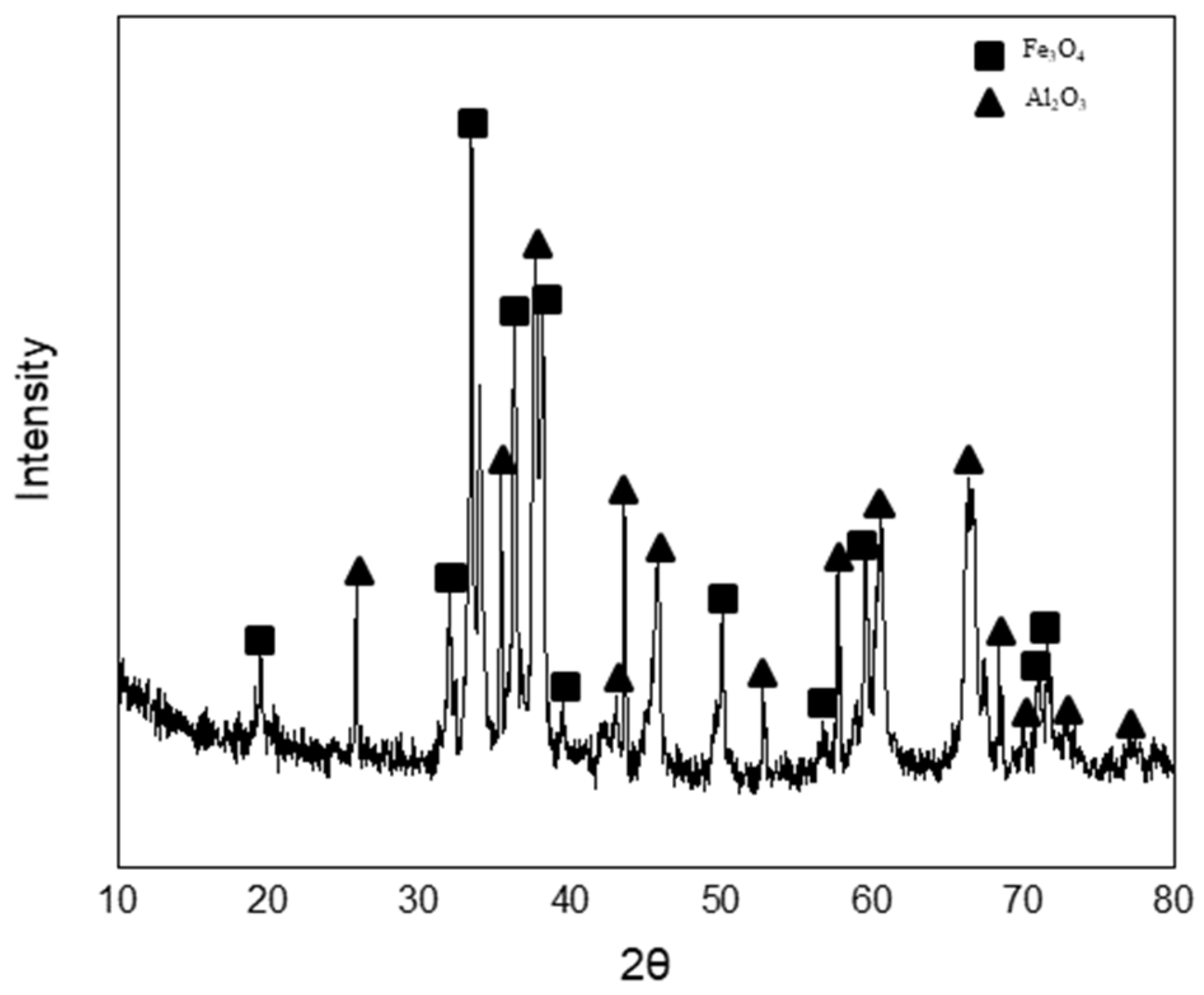
- After the dissolution of the coke and Al dross mixtures, the molten steel temperature was measured and simulated by blowing dry air into the melt at a starting temperature of 1873 K. The molten steel temperature increased significantly immediately after the blowing of dry air started, owing to the oxidation of aluminum. Subsequently, the molten steel temperature increased gradually, due to the combustion of carbon. A slag phase was formed at approximately 1600 s after the start of the blowing of dry air, and it became thicker with the continued blowing of dry air. The slag phase was composed of Fe3O4 and Al2O3, as determined using XRD. The simulation results for the changes in the temperature and composition of molten steel showed good agreement with the measured results.
Funding
Conflicts of Interest
References
- Hansen, J.; Sato, M.; Hearty, P.; Ruedy, R.; Kelley, M.; Masson-Delmotte, V.; Russell, G.; Tselioudis, G.; Cao, J.; Rignot, E.; et al. Ice melt, sea level rise and superstorms: Evidence from paleoclimate data, climate modeling, and modern observations that 2 °C global warming could be dangerous. Atmos. Chem. Phys. 2016, 16, 3761–3812. [Google Scholar] [CrossRef]
- Figueres, C.; Quere, C.L.; Mahindra, A.; Bäte, O.; Whiteman, G.; Peters, G.; Guan, D. Emissions are still rising: Ramp up the cuts. Nature 2018, 564, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.Y.; Choi, G.Y.; Lee, E.M.; Lee, S.K. A Decomposition Analysis of Domestic Carbon Dioxide Emissions Related to Industry Structure and Energy Mix in Korea. J. Environ. Policy Adm. 2020, 28, 153–182. [Google Scholar]
- Xu, C.; Cang, D. A Brief Overview of Low CO2 Emission Technologies for Iron and Steel Making. J. Iron Steel Res. Int. 2010, 17, 1–7. [Google Scholar] [CrossRef]
- Oh, D.W.; Park, H.-k.; Park, T.-j.; Im, S.K. New Energy Technology of Electric Arc Furnace in Steel Making Industry. In Proceedings of the KIEE Conference 2003, Kwangwon-do, Korea, 21–23 July 2003; pp. 71–73. [Google Scholar]
- Matoba, S.; Banya, S. Equilibrium of carbon and oxygen in molten iron saturated with carbon. Testu-to-Hagane 1957, 43, 790–796. [Google Scholar] [CrossRef]
- Kim, D.-H.; Paek, M.-K.; Kim, T.-J.; Won, S.-Y.; Pak, J.-J. Carbon Solubility in Liquid Iron Containing V, Mo and Ni. Mater. Trans. 2014, 55, 610–615. [Google Scholar] [CrossRef]
- Kitchener, J.A.; Bockris, J.O.M.; Spratt, D.A. The influence of sulphur on the solubility and activity coefficient of carbon. Trans. Faraday Soc. 1952, 48, 608–617. [Google Scholar] [CrossRef]
- Wu, C.; Sahajwalla, V. Dissolution Rates of Coals and Graphite in Fe-C-S Melts in Direct Ironmaking: Influence of Melt Carbon and Sulfur on Carbon Dissolution. Metall. Mater. Trans. B 2000, 31B, 243–251. [Google Scholar] [CrossRef]
- Cham, S.T.; Sakurovs, R.; Sun, H.; Sahajwalla, V. Influence of Temperature on Carbon Dissolution of Cokes in Molten Iron. ISIJ Int. 2006, 46, 652–659. [Google Scholar] [CrossRef][Green Version]
- Jang, D.; Kim, T.; Shin, M.; Lee, J. Kinetics of Carbon Dissolution of Coke in Molten Iron. Metall. Mater. Trans. B 2012, 43B, 1308–1314. [Google Scholar] [CrossRef]
- Kulik, G.J.; Daley, J.C. Aluminum Dross Processing in the 90’s. In Proceedings of the Second International Symposium-Recycling of Metals and Engineered Materials, Williamsburg, VA, USA, 28–31 October 1990; pp. 427–438. [Google Scholar]
- Cassells, J.M.; Rusin, P.A.; Young, T.L.; Greene, M.G. Removal and reuse of aluminum dross solid waste. In Proceedings of the Technical Sessions Presented by the TMS Light Metals Committee at the 122nd TMS Annual Meeting, Denver, CO, USA, 21–25 February 1993; pp. 1075–1081. [Google Scholar]
- Park, H.; Lee, H.; Lee, J. Preparation of Castable Refractories by Recycling of Aluminum Dross. J. Korean Inst. Resour. Recycl. 2003, 12, 46–53. [Google Scholar]
- Park, H.; Lee, H.; Kim, J.; Yoon, E. Pretreatment for Recycling of Domestic Aluminum Dross. J. Korean Inst. Resour. Recycl. 1996, 5, 14–20. [Google Scholar]
- Kim, Y.-H.; Yoo, J.-M. A study on Reduction of Iron oxide from slag in the EAF Process. J. Korean Inst. Resour. Recycl. 2016, 25, 54–59. [Google Scholar] [CrossRef][Green Version]
- Snyder, P.E.; Seltz, H. The Heat of Formation of Aluminum oxide. J. Am. Chem. Soc. 1945, 67, 683–685. [Google Scholar] [CrossRef]
- Chase, M.W., Jr. NIST-JANAF Themochemical Tables, 4th ed.; American Institute of Physics: Washington, DC, USA, 1998; p. 641. [Google Scholar]
- Kitamura, S.; Kitamura, T.; Shibata, K.; Mizukami, Y.; Mukawa, S.; Nakagawa, J. Effect of Stirring Energy, Temperature and Flux Composition on Hot Metal Dephosphorization Kinetics. ISIJ Int. 1991, 31, 1322–1328. [Google Scholar] [CrossRef]
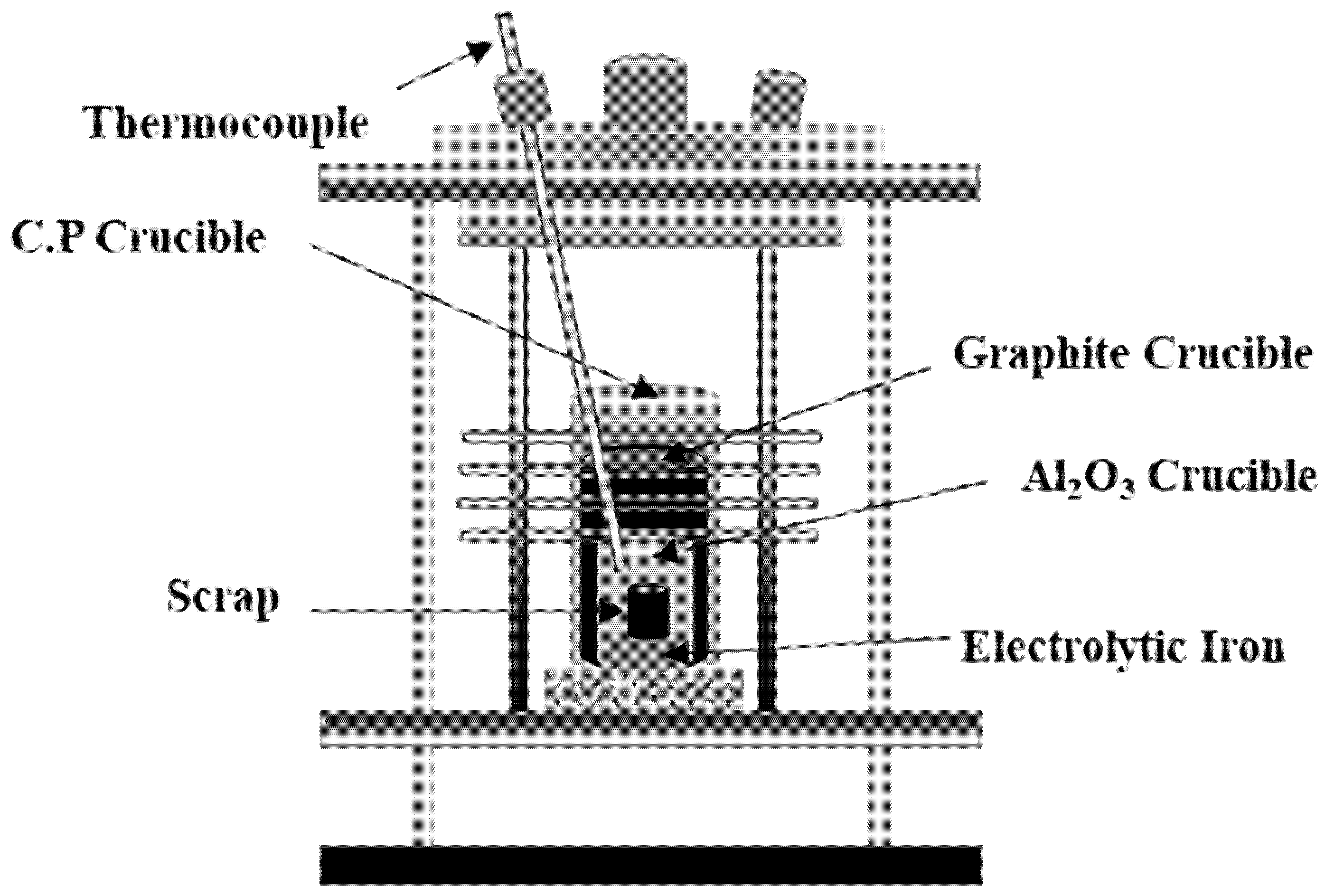
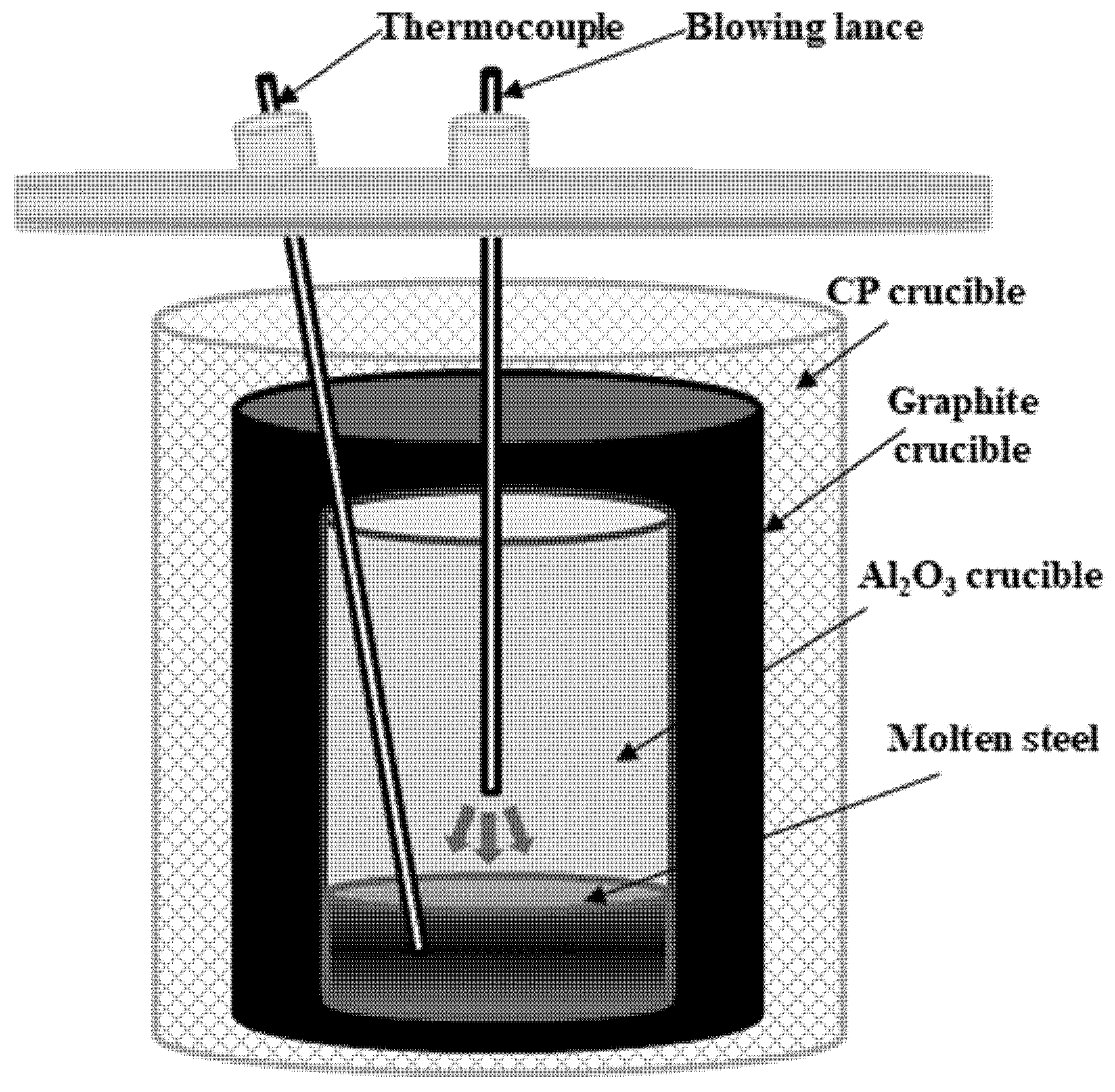


| Fixed Carbon | Volatile | Moisture | Ash |
|---|---|---|---|
| 86.06 | 2.1 | 0.93 | 10.91 |
| Al | Fe | Sn | Zn | SiO2 | Al2O3 |
|---|---|---|---|---|---|
| 27.02 | 0.16 | 0.01 | 0.03 | 7.00 | 65.78 |
| Mixture | Coke: Al Dross | ||
|---|---|---|---|
| Ratio (%) | 80:20 | 60:40 | 40:60 |
| C/Al | 0.4 | 0.2 | 0.1 |
| Temperature (K) | 1873 |
| Atmosphere (mL/min) | Ar (500) |
| Blowing gas (mL/min) | Dry Air (100) |
| Dissolution time (s) | 3600 |
| Blowing time (s) | 3600 |
| Starting Temperature (K) | 1873 |
| Gas flow rate (Nm3/min) | 0.0001 (dried air, M = 29 g/mol) |
| Weight of metal (g) | 90 |
| Inner diameter of nozzle (m) | 0.008 (single nozzle) |
| Height of the nozzle tip (m) | 0.01 |
| Inner diameter of crucible (m) | 0.035 |
| Blowing time (s) | 2400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J. Effect of Al Dross Addition on Temperature Improvements in Molten Steel by Blowing Dry Air. Metals 2022, 12, 1170. https://doi.org/10.3390/met12071170
Kim S-J. Effect of Al Dross Addition on Temperature Improvements in Molten Steel by Blowing Dry Air. Metals. 2022; 12(7):1170. https://doi.org/10.3390/met12071170
Chicago/Turabian StyleKim, Sun-Joong. 2022. "Effect of Al Dross Addition on Temperature Improvements in Molten Steel by Blowing Dry Air" Metals 12, no. 7: 1170. https://doi.org/10.3390/met12071170
APA StyleKim, S.-J. (2022). Effect of Al Dross Addition on Temperature Improvements in Molten Steel by Blowing Dry Air. Metals, 12(7), 1170. https://doi.org/10.3390/met12071170






