Development of Ultrafine–Grained and Nanostructured Bioinert Alloys Based on Titanium, Zirconium and Niobium and Their Microstructure, Mechanical and Biological Properties
Abstract
1. Introduction
2. Materials and Research Methods
2.1. Materials
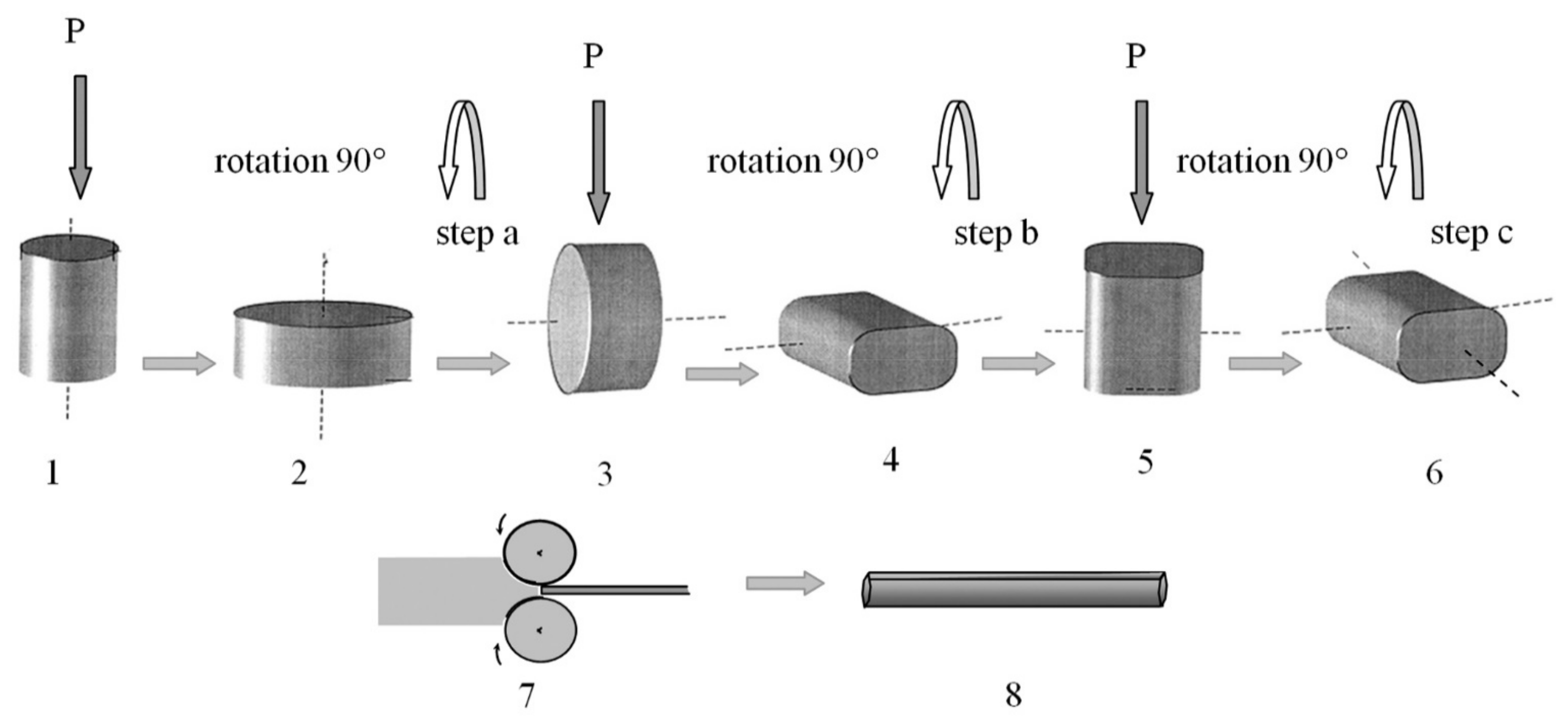
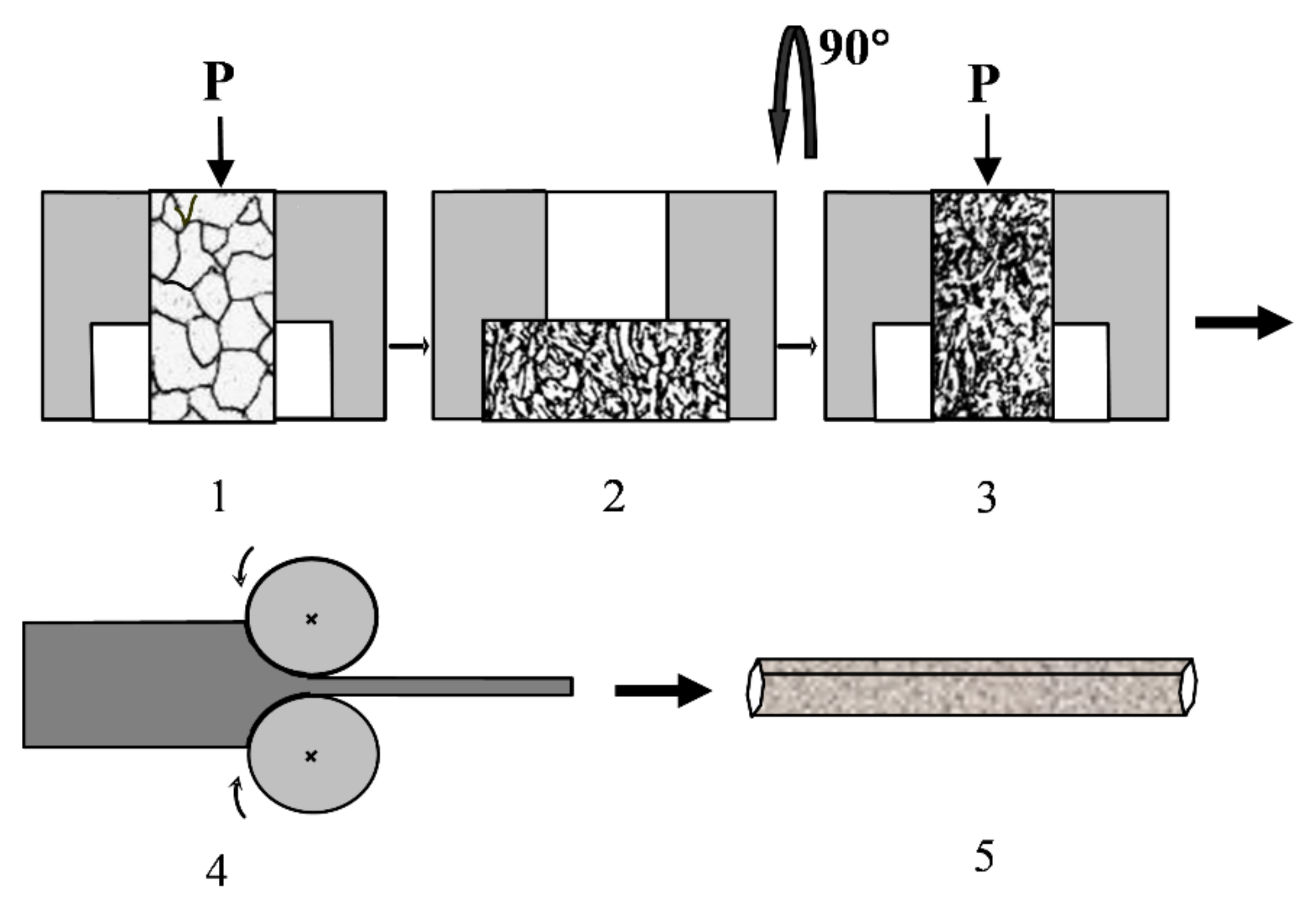
2.2. Technological Aspects of Combined SPD, including MDF or PSC and Subsequent MPGR
2.3. Research Methods
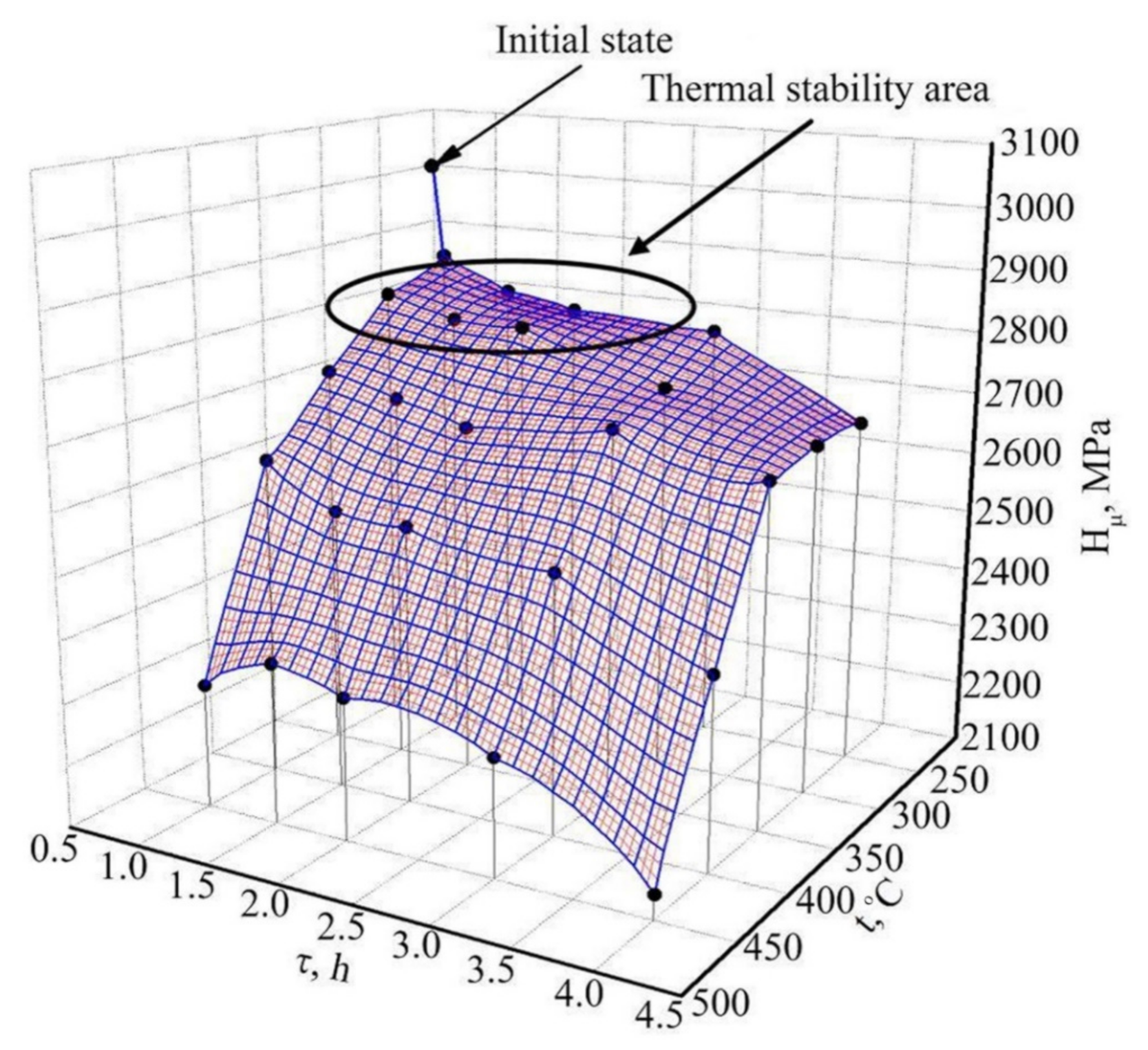
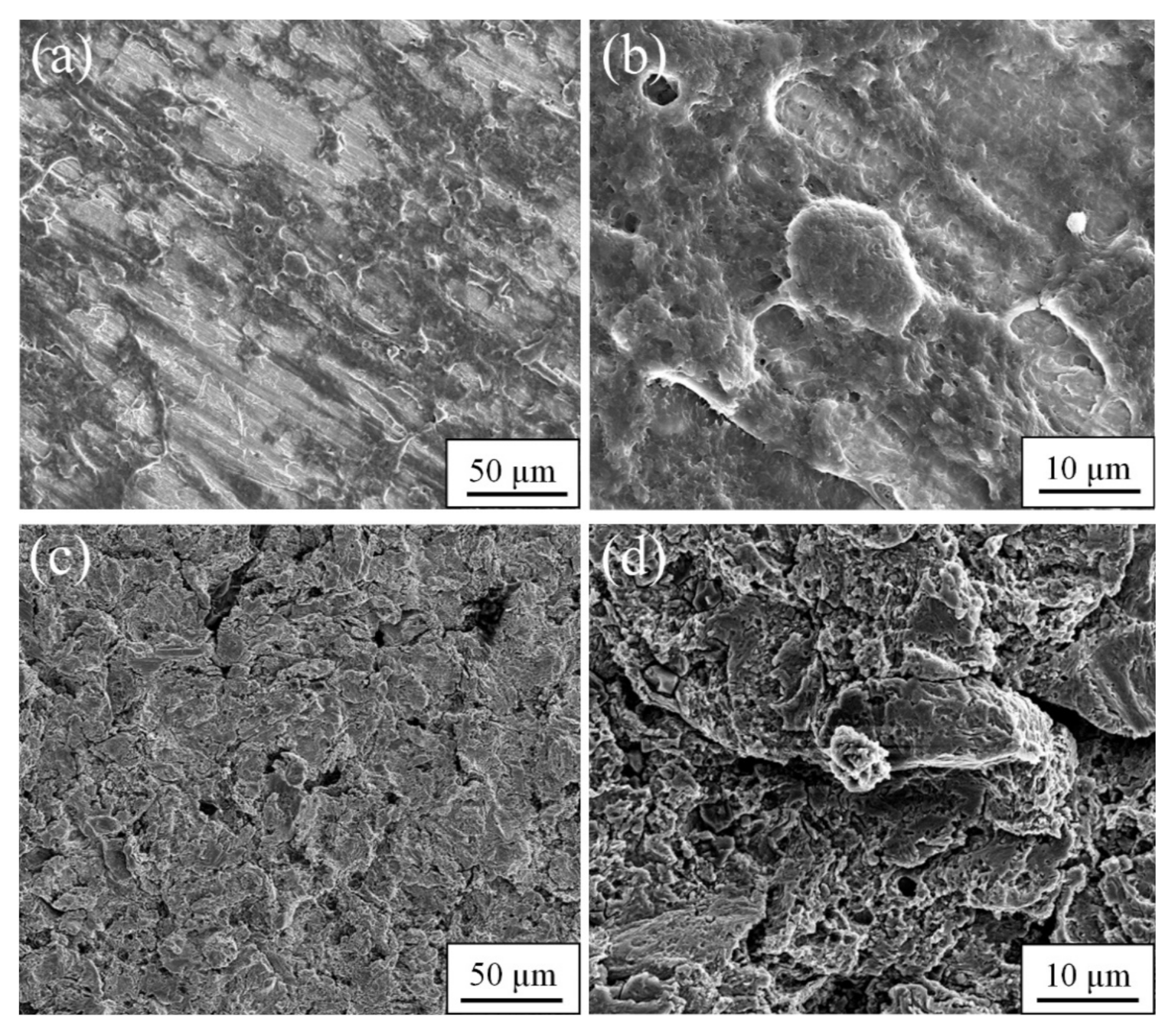
3. Results and Discussion
3.1. Microstructure and Mechanical Properties of Medical Alloys Produced Using SPD Methods
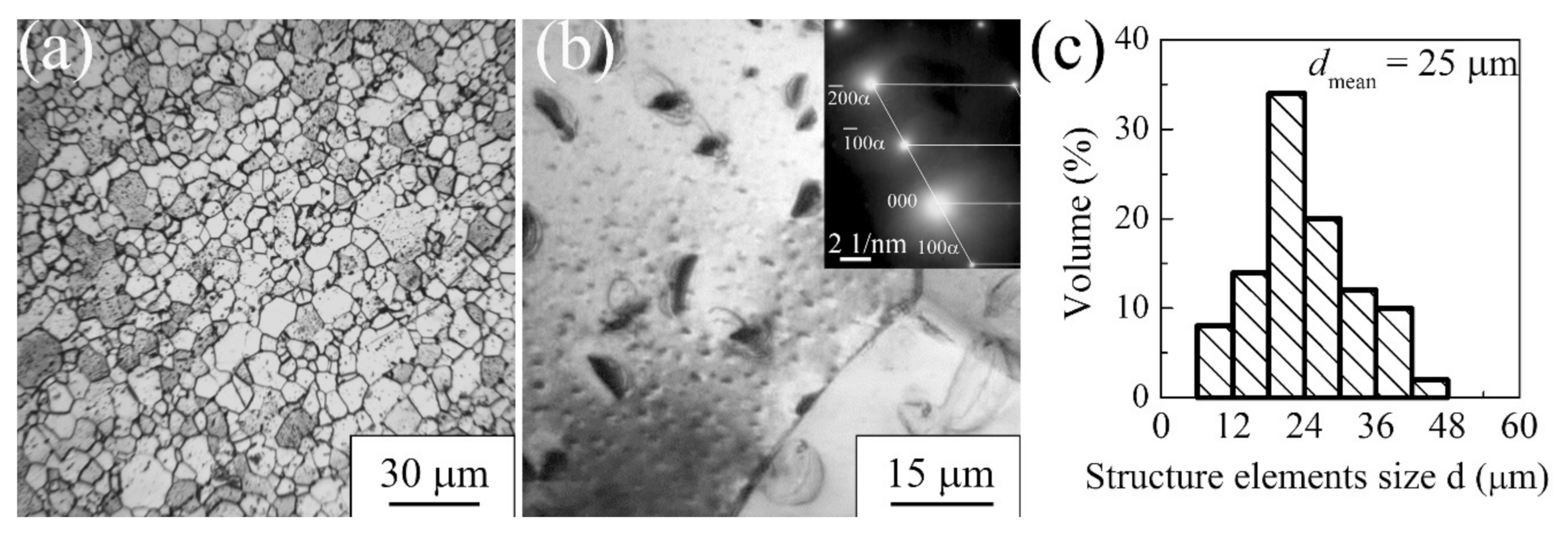
3.1.1. Commercially Pure Titanium
3.1.2. Zr-1Nb Alloy
3.1.3. Ti-45Nb Alloy
3.2. Biological Testing of UFG Titanium, Zr-1Nb, and Ti-45Nb Alloy
MTT Analysis Results
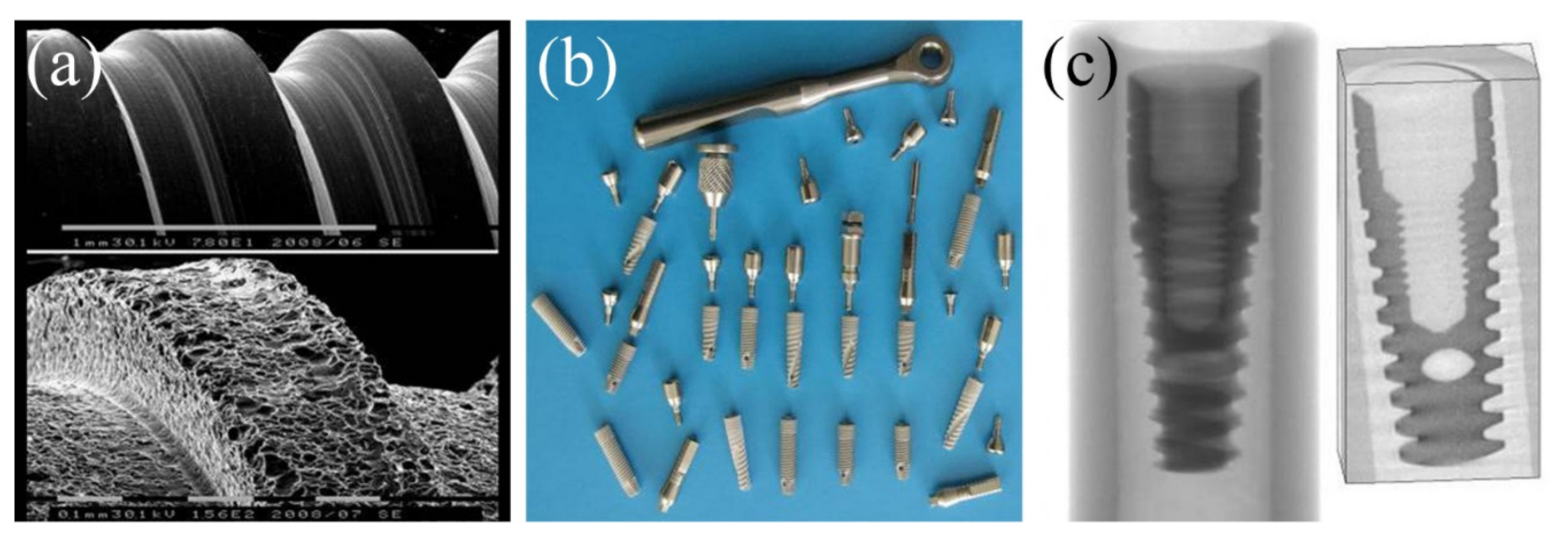
3.3. Development and Medical Applications of Dental Nanostructured Titanium Implants
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathi, A.; Melo, J.S. (Eds.) Advances in Biomaterials for Biomedical Applications, Advanced Structured Materials; Springer: Singapore, 2017; 554p. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Niinomi, M.; Narushima, T.; Nakai, M. (Eds.) Advances in Metallic Biomaterials, Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2015; 351p. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Janbaz, S.; Leeflang, S.M.A.; Lietaert, K.; Weinans, H.H.; Zadpoor, A.A. Rationally designed meta-implants: A combination of auxetic and conventional meta-biomaterials. Mater. Horiz. 2017, 5, 28–35. [Google Scholar] [CrossRef]
- Zhang, B.; Pei, X.; Zhou, C.; Fan, Y.; Jiang, Q.; Ronca, A.; D’Amora, U.; Chen, Y.; Li, H.; Sun, Y.; et al. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load-bearing bone reconstruction. Mater. Des. 2018, 152, 30–39. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants, (II). Etiopathogenesis. Eur. J. Oral Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef]
- Branemark, P.I.; Zarb, G.A.; Albrektsson, T. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence Publishing Co.: Chicago, IL, USA, 1985; p. 350. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef]
- Ozaki, T.; Matsumoto, H.; Watanabe, S.; Hanada, S. Beta Ti Alloys with Low Young’s Modulus. Mater. Trans. 2004, 45, 2776–2779. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Guo, S.; Meng, Q.; Zhao, X.; Wei, Q.; Xu, H. Design and fabrication of a metastable β-type titanium alloy with ultralow elastic modulus and high strength. Sci. Rep. 2015, 5, 14688. [Google Scholar] [CrossRef]
- Amarnath, G.; Muddugangadhar, B.; Tripathi, S.; Dikshit, S.; Divya, M.S. Biomaterials for Dental Implants: An Overview. Int. J. Oral Implant. Clin. Res. 2011, 2, 13–24. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Padovan, S.; Chiesa, M.; Poggio, C.; Scribante, A. Influence of Different Surface Pretreatments on Shear Bond Strength of an Adhesive Resin Cement to Various Zirconia Ceramics. Materials 2020, 13, 652. [Google Scholar] [CrossRef]
- Zardiackas, L.; Freese, H.; Kraay, M. (Eds.) Titanium, Niobium, Zirconium, and Tantalum for Medical and Surgical Applications; ASTM: West Conshohocken, PA, USA, 2006; p. 265. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications, Engi-neering Materials; Springer: Berlin/Heidelberg, Germany, 2001; pp. 145–162. [Google Scholar]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Xu, L.-J.; Xiao, S.-L.; Tian, J.; Chen, Y.-Y.; Huang, Y.-D. Microstructure and dry wear properties of Ti-Nb alloys for dental prostheses. Trans. Nonferrous Met. Soc. China 2009, 19, s639–s644. [Google Scholar] [CrossRef]
- Hermann, R.; Calin, M.; Büchner, B.; Eckert, J. Elastic constants of single crystalline β-Ti70Nb30. Scr. Mater. 2012, 66, 198–201. [Google Scholar] [CrossRef]
- Gepreel, M.A.-H.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Reck, A.; Pilz, S.; Thormann, U.; Alt, V.; Gebert, A.; Călin, M.; Heiß, C.; Zimmermann, M. Effects of thermomechanical history and environment on the fatigue behavior of (β)-Ti-Nb implant alloys. MATEC Web Conf. 2018, 165, 06001. [Google Scholar] [CrossRef][Green Version]
- Zhou, F.; Wang, B.; Qiu, K.; Lin, W.; Li, L.; Wang, Y.; Nie, F.; Zheng, Y. Microstructure, corrosion behavior and cytotoxicity of Zr–Nb alloys for biomedical application. Mater. Sci. Eng. C 2012, 32, 851–857. [Google Scholar] [CrossRef]
- ASTM F2384-10; Standard Specification for Wrought Zirconium 2.5 Niobium Alloy for Surgical Implant Applications (UNS R60901). ANSI: New York, NY, USA, 2016.
- Buczynski, B.; Kory, M.; Steiner, R.; Kittinger, T.; Ramsier, R. Bacterial adhesion to zirconium surfaces. Colloids Surfaces B Biointerfaces 2003, 30, 167–175. [Google Scholar] [CrossRef]
- Narayan, R.J.; Hobbs, L.W.; Jin, C.; Rabiei, A. The use of functionally gradient materials in medicine. JOM 2006, 58, 52–56. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Zhilyaev, A.P.; Langdon, T.G. Bulk Nanostructured Materials: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; 456p. [Google Scholar] [CrossRef]
- Elias, C.N.; Meyers, M.A.; Valiev, R.Z.; Monteiro, S.N. Ultrafine grained titanium for biomedical applications: An overview of performance. J. Mater. Res. Technol. 2013, 2, 340–350. [Google Scholar] [CrossRef]
- Purcek, G.; Yapici, G.; Karaman, I.; Maier, H. Effect of commercial purity levels on the mechanical properties of ultrafine-grained titanium. Mater. Sci. Eng. A 2010, 528, 2303–2308. [Google Scholar] [CrossRef]
- Sergueeva, A.; Stolyarov, V.; Valiev, R.; Mukherjee, A. Advanced mechanical properties of pure titanium with ultrafine grained structure. Scr. Mater. 2001, 45, 747–752. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, W.; Yang, X.; Langdon, T.G. Microstructure and properties of pure titanium processed by equal-channel angular pressing at room temperature. Scr. Mater. 2008, 59, 542–545. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, J.; Gubicza, J.; Ungár, T.; Wang, Y.M.; Ma, E.; Valiev, R.Z. Nanostructures in Ti processed by severe plastic deformation. J. Mater. Res. 2003, 18, 1908–1917. [Google Scholar] [CrossRef]
- Meredith, C.S.; Khan, A.S. Texture evolution and anisotropy in the thermo-mechanical response of UFG Ti processed via equal channel angular pressing. Int. J. Plast. 2012, 30–31, 202–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Figueiredo, R.B.; Alhajeri, S.N.; Wang, J.T.; Gao, N.; Langdon, T.G. Structure and mechanical properties of commercial purity titanium processed by ECAP at room temperature. Mater. Sci. Eng. A 2011, 528, 7708–7714. [Google Scholar] [CrossRef]
- Rosochowski, A. (Ed.) Severe Plastic Deformation Technology; Whittles Publishing: Scotland, UK, 2017; 264p. [Google Scholar]
- Gzyl, M.; Rosochowski, A.; Pesci, R.; Olejnik, L.; Yakushina, E.; Wood, P. Mechanical Properties and Microstructure of AZ31B Magnesium Alloy Processed by I-ECAP. Metall. Mater. Trans. A 2013, 45, 1609–1620. [Google Scholar] [CrossRef]
- Qarni, M.J.; Sivaswamy, G.; Rosochowski, A.; Boczkal, S. Effect of incremental equal channel angular pressing (I-ECAP) on the microstructural characteristics and mechanical behavior of commercially pure titanium. Mater. Des. 2017, 122, 385–402. [Google Scholar] [CrossRef]
- Zhilyaev, A.P.; Langdon, T.G. Using high-pressure torsion for metal processing: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Dimić, I.; Cvijović-Alagić, I.; Völker, B.; Hohenwarter, A.; Pippan, R.; Veljović, D.; Rakin, M.; Bugarski, B. Microstructure and metallic ion release of pure titanium and Ti–13Nb–13Zr alloy processed by high pressure torsion. Mater. Des. 2016, 91, 340–347. [Google Scholar] [CrossRef]
- Mohammed, M.T. Mechanical and wear properties of HPT-biomedical titanium: A review. Biomater. Biomech. Bioeng. 2015, 2, 185–196. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Salishchev, G.; Galeyev, R.; Valiakhmetov, O.; Mironov, S.; Semiatin, S. Production of submicrocrystalline structure in large-scale Ti–6Al–4V billet by warm severe deformation processing. Scr. Mater. 2004, 51, 1147–1151. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Salishchev, G.; Galeyev, R.; Maekawa, K. Mechanical Properties of Ti–6Al–4V Titanium Alloy with Submicrocrystalline Structure Produced by Severe Plastic Deformation. Mater. Trans. 2005, 46, 2020–2025. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Kudryavtsev, E.; Kostjuchenko, S.; Malysheva, S.; Salishchev, G. Strength and ductility-related properties of ultrafine grained two-phase titanium alloy produced by warm multiaxial forging. Mater. Sci. Eng. A 2012, 536, 190–196. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Salishchev, G.; Łojkowski, W. Strengthening of a Ti–6Al–4V titanium alloy by means of hydrostatic extrusion and other methods. Mater. Sci. Eng. A 2009, 515, 43–48. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Lojkowski, W.; Mazur, A.; Salishchev, G. Structure and properties of hydrostatically extruded commercially pure titanium. Mater. Sci. Eng. A 2010, 527, 5596–5603. [Google Scholar] [CrossRef]
- Topolski, K.; Garbacz, H.; Wieciński, P.; Pachla, W.; Kurzydłowski, K. Mechanical properties of titanium processed by hydrostatic extrusion. Arch. Met. Mater. 2012, 57, 863–867. [Google Scholar] [CrossRef]
- Dinda, G.; Rösner, H.; Wilde, G. Synthesis of bulk nanostructured Ni, Ti and Zr by repeated cold-rolling. Scr. Mater. 2005, 52, 577–582. [Google Scholar] [CrossRef]
- Nocivin, A.; Raducanu, D.; Cinca, I.; Trisca-Rusu, C.; Butu, M.; Thibon, I.; Cojocaru, V.D. X-ray Diffraction Study and Texture Evolution for a Ti-Nb-Ta Biomedical Alloy Processed by Accumulative Roll Bonding. J. Mater. Eng. Perform. 2015, 24, 1587–1601. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Abbasi, S.; Morakabati, M.; Karjalainen, L.; Porter, D. Effect of cold rolling and subsequent annealing on grain refinement of a beta titanium alloy showing stress-induced martensitic transformation. Mater. Sci. Eng. A 2018, 731, 465–478. [Google Scholar] [CrossRef]
- Li, S.J.; Cui, T.C.; Li, Y.L.; Hao, Y.L.; Yang, R. Ultrafine-grained β-type titanium alloy with nonlinear elasticity and high ductility. Appl. Phys. Lett. 2008, 92, 043128. [Google Scholar] [CrossRef]
- Xu, Y.; Yi, D.; Liu, H.; Wu, X.; Wang, B.; Yang, F. Effects of cold deformation on microstructure, texture evolution and mechanical properties of Ti–Nb–Ta–Zr–Fe alloy for biomedical applications. Mater. Sci. Eng. A 2012, 547, 64–71. [Google Scholar] [CrossRef]
- Cojocaru, V.; Raducanu, D.; Gloriant, T.; Gordin, D.; Cinca, I. Effects of cold-rolling deformation on texture evolution and mechanical properties of Ti–29Nb–9Ta–10Zr alloy. Mater. Sci. Eng. A 2013, 586, 1–10. [Google Scholar] [CrossRef]
- Gabrion, X.; Thibaud, S.; Zang, Y.; Charbonnier, P.; Laheurte, P.; Gaillard, Y. Effect of a hyper deformation drawing process on mechanical behaviour of thin wires of Ti-26Nb (at.%) alloys. Mater. Des. 2017, 120, 273–279. [Google Scholar] [CrossRef]
- Sordi, V.L.; Ferrante, M.; Kawasaki, M.; Langdon, T.G. Microstructure and tensile strength of grade 2 titanium processed by equal-channel angular pressing and by rolling. J. Mater. Sci. 2012, 47, 7870–7876. [Google Scholar] [CrossRef]
- Fan, Z.; Jiang, H.; Sun, X.; Song, J.; Zhang, X.; Xie, C. Microstructures and mechanical deformation behaviors of ultrafine-grained commercial pure (grade 3) Ti processed by two-step severe plastic deformation. Mater. Sci. Eng. A 2009, 527, 45–51. [Google Scholar] [CrossRef]
- Stolyarov, V.V.; Zhu, Y.T.; Lowe, T.C.; Valiev, R.Z. Microstructure and properties of pure Ti processed by ECAP and cold extrusion. Mater. Sci. Eng. A 2001, 303, 82–89. [Google Scholar] [CrossRef]
- Valiev, R.; Semenova, I.P.; Jakushina, E.; Latysh, V.; Rack, H.J.; Lowe, T.C.; Petruželka, J.; Dluhoš, L.; Hrušák, D.; Sochová, J. Nanostructured SPD Processed Titanium for Medical Implants. Mater. Sci. Forum 2008, 584-586, 49–54. [Google Scholar] [CrossRef]
- Semenova, I.P.; Korshunov, A.I.; Salimgareeva, G.K.; Latysh, V.V.; Yakushina, E.B.; Valiev, R.Z. Mechanical behavior of ultrafine-grained titanium rods obtained using severe plastic deformation. Phys. Met. Met. 2008, 106, 211–218. [Google Scholar] [CrossRef]
- Li, T.; Kent, D.; Sha, G.; Dargusch, M.S.; Cairney, J.M. Precipitation of the α-phase in an ultrafine grained beta-titanium alloy processed by severe plastic deformation. Mater. Sci. Eng. A 2014, 605, 144–150. [Google Scholar] [CrossRef]
- Zherebtsov, S.V.; Dyakonov, G.S.; Salishchev, G.A.; Salem, A.; Semiatin, S.L. The Influence of Grain Size on Twinning and Microstructure Refinement During Cold Rolling of Commercial-Purity Titanium. Met. Mater. Trans. A 2016, 47, 5101–5113. [Google Scholar] [CrossRef]
- Edalati, K.; Matsubara, E.; Horita, Z. Processing Pure Ti by High-Pressure Torsion in Wide Ranges of Pressures and Strain. Met. Mater. Trans. A 2009, 40, 2079–2086. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sulkowski, B.; Waitz, T.; Ozaltin, K.; Chrominski, W.; Pukenas, A.; Horky, J.; Lewandowska, M.; Skrotzki, W.; Zehetbauer, M. Mechanical properties, structural and texture evolution of biocompatible Ti–45Nb alloy processed by severe plastic deformation. J. Mech. Behav. Biomed. Mater. 2016, 62, 93–105. [Google Scholar] [CrossRef]
- Elmay, W.; Prima, F.; Gloriant, T.; Bolle, B.; Zhong, Y.; Patoor, E.; Laheurte, P. Effects of thermomechanical process on the microstructure and mechanical properties of a fully martensitic titanium-based biomedical alloy. J. Mech. Behav. Biomed. Mater. 2013, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Eroshenko, A.; Sharkeev, Y.; Khimich, M.; Glukhov, I.; Uvarkin, P.; Tolmachev, A.; Mairambekova, A. Microstructure of ultrafine-grained Ti-40 wt.% Nb alloy after annealing. Lett. Mater. 2020, 10, 54–59. (In Russian) [Google Scholar] [CrossRef]
- Ozaltin, K.; Chrominski, W.; Kulczyk, M.; Panigrahi, A.; Horky, J.; Zehetbauer, M.; Lewandowska, M. Enhancement of mechanical properties of biocompatible Ti–45Nb alloy by hydrostatic extrusion. J. Mater. Sci. 2014, 49, 6930–6936. [Google Scholar] [CrossRef]
- Völker, B.; Jäger, N.; Calin, M.; Zehetbauer, M.; Eckert, J.; Hohenwarter, A. Influence of testing orientation on mechanical properties of Ti45Nb deformed by high pressure torsion. Mater. Des. 2017, 114, 40–46. [Google Scholar] [CrossRef]
- Panigrahi, A.; Bönisch, M.; Waitz, T.; Schafler, E.; Calin, M.; Eckert, J.; Skrotzki, W.; Zehetbauer, M. Phase transformations and mechanical properties of biocompatible Ti–16.1Nb processed by severe plastic deformation. J. Alloys Compd. 2015, 628, 434–441. [Google Scholar] [CrossRef]
- Ma, J.; Karaman, I.; Kockar, B.; Maier, H.; Chumlyakov, Y. Severe plastic deformation of Ti74Nb26 shape memory alloys. Mater. Sci. Eng. A 2011, 528, 7628–7635. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z.; Yagi, S.; Matsubara, E. Allotropic phase transformation of pure zirconium by high-pressure torsion. Mater. Sci. Eng. A 2009, 523, 277–281. [Google Scholar] [CrossRef]
- Pérez-Prado, M.; Gimazov, A.; Ruano, O.; Kassner, M.; Zhilyaev, A. Bulk nanocrystalline ω-Zr by high-pressure torsion. Scr. Mater. 2008, 58, 219–222. [Google Scholar] [CrossRef]
- Zhilyaev, A.; Sabirov, I.; González-Doncel, G.; Molina-Aldareguía, J.; Srinivasarao, B.; Pérez-Prado, M. Effect of Nb additions on the microstructure, thermal stability and mechanical behavior of high pressure Zr phases under ambient conditions. Mater. Sci. Eng. A 2011, 528, 3496–3505. [Google Scholar] [CrossRef]
- Rogachev, S.O.; Rozhnov, A.B.; Nikulin, S.A.; Rybal’Chenko, O.V.; Gorshenkov, M.V.; Chzhen, V.G.; Dobatkin, S.V. Effect of torsion conditions under high pressure on the structure and strengthening of the Zr–1% Nb alloy. Phys. Met. Met. 2016, 117, 371–377. [Google Scholar] [CrossRef]
- Companhoni, M.V.P.; Matheus, J.R.G.; Marcondes, T.L.; Pinto, A.L. Analysis of microstructure and microhardness of Zr-2.5Nb processed by High-Pressure Torsion (HPT). J. Mater. Sci. 2012, 47, 7835–7840. [Google Scholar] [CrossRef]
- Grabovetskaya, G.P.; Stepanova, E.N.; Mishin, I.P.; Vinokurov, V.A. Effect of deformation and heat treatment on the structure, the mechanical properties, and the fracture characteristics of an ultrafine-grained Zr–1Nb alloy. Russ. Met. 2017, 2017, 271–278. [Google Scholar] [CrossRef]
- Kukareko, V.A.; Kopylov, V.I.; Kononov, A.G.; Rogachev, S.O.; Nikulin, S.A.; Dobatkin, S.V. Structural transformations during heating of a Zr-2.5% Nb alloy subjected to equal-channel angular pressing. Russ. Met. 2010, 2010, 642–647. [Google Scholar] [CrossRef]
- Straumal, B.; Gornakova, A.; Mazilkin, A.; Fabrichnaya, O.; Kriegel, M.; Baretzky, B.; Jiang, J.-Z.; Dobatkin, S. Phase transformations in the severely plastically deformed Zr–Nb alloys. Mater. Lett. 2012, 81, 225–228. [Google Scholar] [CrossRef]
- Terent’ev, V.F.; Dobatkin, S.V.; Nikulin, S.A.; Kopylov, V.I.; Prosvirnin, D.V.; Rogachev, S.O.; Bannykh, I. Fatigue strength of submicrocrystalline Ti and Zr–2.5% Nb alloy after equal channel angular pressing. Kov. Mater. 2011, 49, 65–73. [Google Scholar] [CrossRef]
- Nikulin, S.; Rozhnov, A.; Rogachev, S.; Khatkevich, V.; Turchenko, V.; Khotulev, E. Investigation of structure, phase composition, and mechanical properties of Zr-2.5% Nb alloy after ECAP. Mater. Lett. 2016, 169, 223–226. [Google Scholar] [CrossRef]
- Sharkeev, Y.; Eroshenko, A.; Kulyashova, K.; Fortuna, S.; Suvorov, K.; Epple, M.; Prymak, O.; Sokolova, V.; Chernousova, S. Microstructure, mechanical and biological properties of zirconium alloyed with niobium after severe plastic deformation. Mater. Und Werkst. 2013, 44, 198–204. [Google Scholar] [CrossRef]
- Kovalevskaya, Z.G.; Khimich, M.A.; Belyakov, A.V.; Shulepov, I.A. Evaluation of Physical and Mechanical Properties of Structural Components of Ti-Nb Alloy. Adv. Mater. Res. 2014, 1040, 39–42. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Legostaeva, E.V.; Vavilov, V.P.; Skripnyak, V.A.; Belyavskaya, O.A.; Eroshenko, A.Y.; Glukhov, I.A.; Chulkov, A.O.; Kozulin, A.A. Regular Features of Stage Formation in the Stress-strain Curves and Microstructure in the Zone of Fracture of Coarse-Grained and Ultrafine-Grained Titanium and Zirconium Alloys. Sov. Phys. J. 2019, 62, 1349–1356. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Legostaeva, E.V.; Eroshenko, Y.A.; Khlusov, I.A.; Kashin, O.A. The Structure and Physical and Mechanical Properties of a Novel Biocomposite Material, Nanostructured Titanium–Calcium-Phosphate Coating. Compos. Interfaces 2009, 16, 535–546. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Vavilov, V.P.; Belyavskaya, O.A.; Skripnyak, V.A.; Nesteruk, D.A.; Kozulin, A.A.; Kim, V.M. Analyzing Deformation and Damage of VT1-0 Titanium in Different Structural States by Using Infrared Thermography. J. Nondestruct. Eval. 2016, 35, 42. [Google Scholar] [CrossRef]
- Humphreys, J.; Rohrer, G.S.; Rollet, A. Recrystallization and Related Annealing Phenomena, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; 734p. [Google Scholar]
- Sharkeev, Y.P.; Eroshenko, A.Y.; Danilov, V.I.; Tolmachev, A.I.; Uvarkin, P.V.; Abzaev, Y.A. Microstructure and Mechanical Properties of Nanostructured and Ultrafine-Grained Titanium and the Zirconium Formed by the Method of Severe Plastic Deformation. Sov. Phys. J. 2014, 56, 1156–1162. [Google Scholar] [CrossRef]
- Sharkeev, Y.; Vavilov, V.; Skrypnyak, V.; Legostaeva, E.; Eroshenko, A.Y.; Belyavskaya, O.; Ustinov, A.; Klopotov, A.; Chulkov, A.; Kozulin, A.; et al. Research on the processes of deformation and failure in coarse- and ultrafine-grain states of Zr1–Nb alloys by digital image correlation and infrared thermography. Mater. Sci. Eng. A 2020, 784, 139203. [Google Scholar] [CrossRef]
- Bannikov, M.V.; Naimark, O.B.; Oborin, V.A. Experimental investigation of crack initiation and propagation in high- and gigacycle fatigue in titanium alloys by study of morphology of fracture. Frat. Integrita Struttrale 2016, 35, 50–56. [Google Scholar] [CrossRef]
- Naimark, O.; Palin-Luc, T. Gigacycle fatigue: Non-local and scaling aspects of damage localization, crack initiation and propagation. Procedia Struct. Integr. 2016, 2, 1143–1148. [Google Scholar] [CrossRef][Green Version]
- Betekhtin, V.I.; Kadomtsev, A.; Narykova, M.; Bannikov, M.; Abaimov, S.; Akhatov, I.; Palin-Luc, T.; Naimark, O.B. Experimental and theoretical study of multiscale damage-failure transition in very high cycle fatigue. Phys. Mesomech. 2017, 20, 78–89. [Google Scholar] [CrossRef]
- Glezer, A.M.; Kozlov, E.V.; Koneva, N.A.; Popova, N.A.; Kurzina, I.A. Plastic Deformation of Nanostructured Materials; CRC Press: Boca Raton, FL, USA, 2017; 334p. [Google Scholar] [CrossRef]
- Legostaeva, E.V.; Egorkin, V.S.; Sinebryukhov, S.L.; Eroshenko, A.Y.; Lyamina, G.V.; Komarova, E.; Gnedenkov, S.V.; Sharkeev, Y.P. Nanostructured titanium: Structure, mechanical and electrochemical properties. Inorg. Mater. Appl. Res. 2014, 5, 44–53. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- ASTM E1382-97; Standard Test Methods for Determining Average Grain Size Using Semiautomatic and Automatic Image Analysis. ANSI: New York, NY, USA, 2010. [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Dirras, G.; Tingaud, D.; Ueda, D.; Hocini, A.; Ameyama, K. Dynamic Hall-Petch versus grain-size gradient effects on the mechanical behavior under simple shear loading of β-titanium Ti-25Nb-25Zr alloys. Mater. Lett. 2017, 206, 214–216. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Mei, W.; Sun, J. Role of grain size in tensile behavior in twinning-induced plasticity β Ti-20V-2Nb-2Zr alloy. Mater. Charact. 2016, 120, 263–267. [Google Scholar] [CrossRef]
- Li, Y.; Bushby, A.J.; Dunstan, D.J. The Hall–Petch effect as a manifestation of the general size effect. Proc. R. Soc. A 2016, 472, 20150890. [Google Scholar] [CrossRef]
- Murakam, Y.; Nomoto, T.; Ueda, T. Factors influencing the mechanism of superlong fatigue failure in steels. Fatigue Fract. Eng. Mater. Struct. 1999, 22, 581–590. [Google Scholar] [CrossRef]
- Mughrabi, H. Specific features and mechanisms of fatigue in the ultrahigh-cycle regime. Int. J. Fatigue 2006, 28, 1501–1508. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Kukareko, V.A.; Zinchenko, V.F.; Eroshenko, A.Y.; Kuchina, A.S. Increasing the cyclic life of submicrocrystalline and large-grain titanium in high-intensity ionic implantation. Steel Transl. 2010, 40, 729–732. [Google Scholar] [CrossRef]
- Naimark, O.B.; Sharkeev, Y.P.; Mairambekova, A.M.; Bannikov, M.V.; Eroshenko, A.Y.; Vedernikova, A.I. Failure mechanisms of titanium VT1−0 and zirconium alloy E110 in ultrafine-grained, fine-grained and coarse-grained states under cyclic loading in gigacycle regime. Lett. Mater. 2018, 8, 317–322. [Google Scholar] [CrossRef]
- Eroshenko, A.Y.; Mairambekova, A.M.; Sharkeev, Y.P.; Kovalevskaya, Z.G.; Khimich, M.A.; Uvarkin, P.V. Structure, phase composition and mechanical properties in bioinert zirconium-based alloy after severe plastic deformation. Lett. Mater. 2017, 7, 469–472. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Eroshenko, A.Y.; Danilov, V.I.; Glukhov, I.A.; Tolmachev, A.I. Production of ultrafine-grain bioinert alloys. Steel Transl. 2015, 45, 116–119. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Skripnyak, V.A.; Vavilov, V.P.; Legostaeva, E.V.; Kozulin, A.A.; Chulkov, A.O.; Eroshenko, A.Y.; Belyavskaya, O.A.; Glukhov, I.A. Special Aspects of Microstructure, Deformation and Fracture of Bioinert Zirconium and Titanium-Niobium Alloys in Different Structural States. Sov. Phys. J. 2018, 61, 1718–1725. [Google Scholar] [CrossRef]
- Kovalevskaya, Z.G.; Ivanov, Y.F.; Perevalova, O.B.; Klimenov, V.A.; Uvarkin, P.V. Study of microstructure of surface layers of low-carbon steel after turning and ultrasonic finishing. Phys. Met. Met. 2013, 114, 41–53. [Google Scholar] [CrossRef]
- Kondo, R.; Nomura, N.; Suyalatu; Tsutsumi, Y.; Doi, H.; Hanawa, T. Microstructure and mechanical properties of as-cast Zr–Nb alloys. Acta Biomater. 2011, 7, 4278–4284. [Google Scholar] [CrossRef]
- Bönisch, M.; Panigrahi, A.; Calin, M.; Waitz, T.; Zehetbauer, M.; Skrotzki, W.; Eckert, J. Thermal stability and latent heat of Nb–rich martensitic Ti-Nb alloys. J. Alloys Compd. 2017, 697, 300–309. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Kovalevskaya, Z.G.; Khimich, M.A.; Bataev, V.A.; Zhu, Q.F.; Belyakov, A.V.; Gluhov, I.A. Phase Transformations of the Ti-40% Nb Alloy Under External Influence. Key Eng. Mater. 2016, 683, 174–180. [Google Scholar] [CrossRef]
- Colings, E.W. (Ed.) Physical Metallurgy of Titanium Alloys; American Society for Metals, Metals Park: Geauga County, OH, USA, 1984; 261p. [Google Scholar]
- Eroshenko, A.Y.; Sharkeev, Y.P.; Glukhov, I.A.; Uvarkin, P.V.; Mairambekova, A.M.; Tolmachev, A.I. The Influence of Dimesions and Phase State of Structural Elements on Mechanical Properties of Binary Alloys of the Ti–Nb and Zr–Nb Systems. Sov. Phys. J. 2019, 61, 1899–1907. [Google Scholar] [CrossRef]
- Sharkeev, Y.; Vavilov, V.; Skripnyak, V.A.; Belyavskaya, O.; Legostaeva, E.; Kozulin, A.; Chulkov, A.; Sorokoletov, A.; Eroshenko, A.; Kuimova, M. Analyzing the Deformation and Fracture of Bioinert Titanium, Zirconium and Niobium Alloys in Different Structural States by the Use of Infrared Thermography. Metals 2018, 8, 703. [Google Scholar] [CrossRef]
- Company Certificate ATI 45Nb™ Alloy, Allegheny Technologies Incorporated (ATI), Batch-No.TSC-522069. Available online: https://www.atimetals.com (accessed on 6 May 2022).
- Eroshenko, A.Y.; Sharkeev, Y.P.; Khimich, M.A.; Uvarkin, P.V.; Tolmachev, A.I.; Glukhov, I.A.; Legostaeva, E.V. Effect of Prolonged Thermal Exposure on Microstructure and Mechanical Properties of Zr–1 wt.% Nb and Ti–45 wt.% Nb Ultrafine-Grained Bioinert Alloys. Sov. Phys. J. 2021, 63, 1846–1853. [Google Scholar] [CrossRef]
- Lai, M.; Li, T.; Raabe, D. ω phase acts as a switch between dislocation channeling and joint twinning- and transformation-induced plasticity in a metastable β titanium alloy. Acta Mater. 2018, 151, 67–77. [Google Scholar] [CrossRef]
- Marketing in Dentistry. Chicago Center for Modern Dentistry. 2005. Available online: https://www.chicago-center.com (accessed on 5 May 2022).
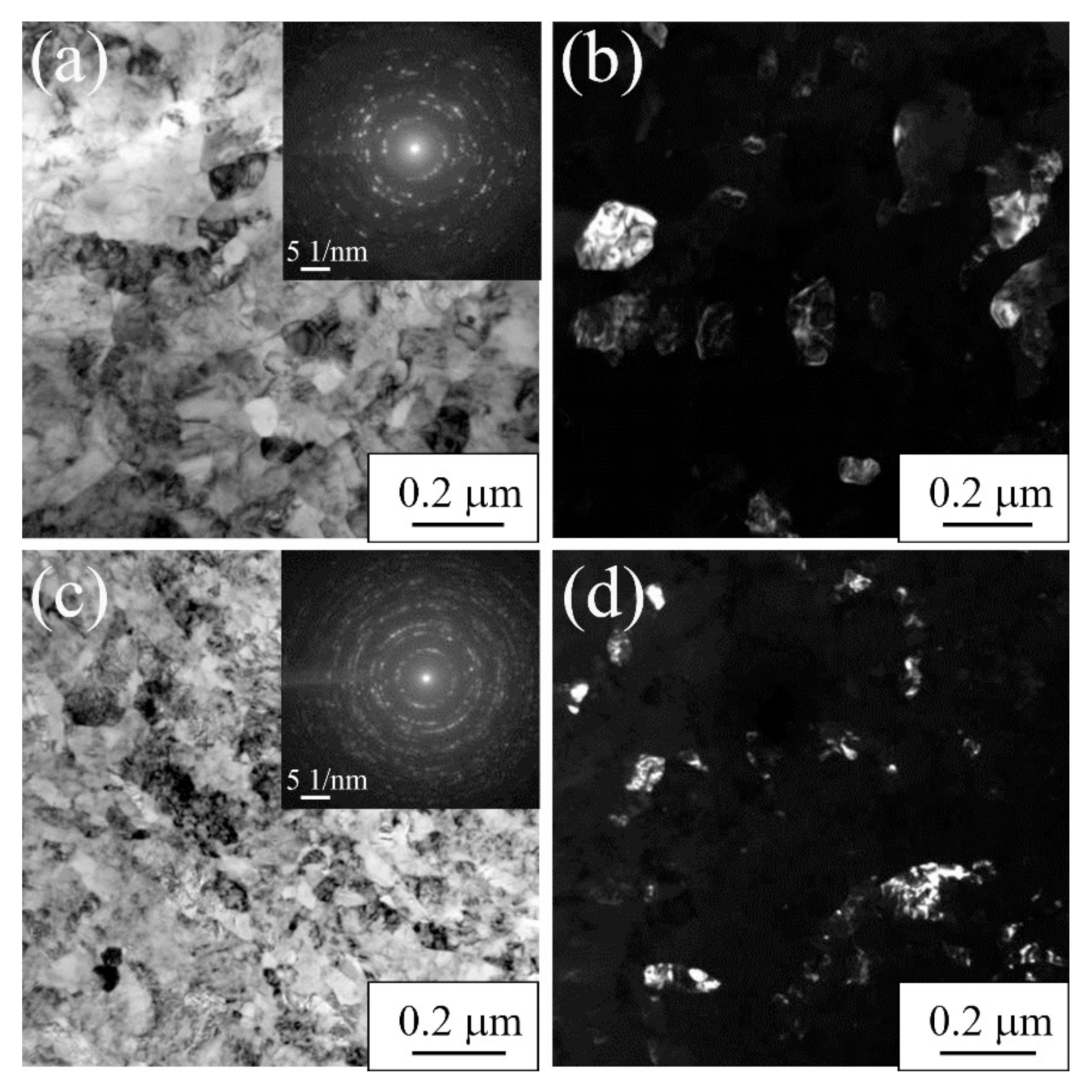
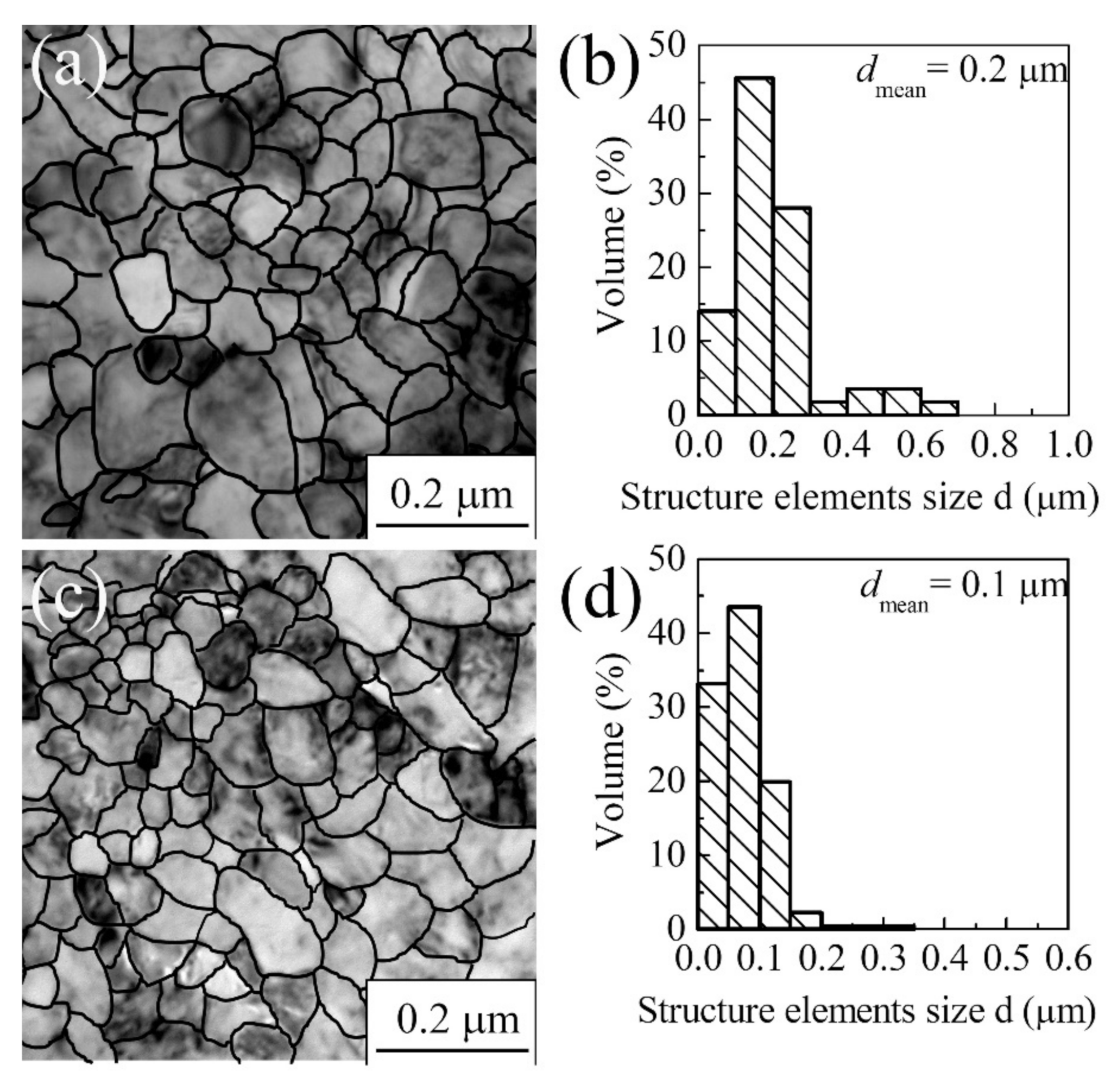
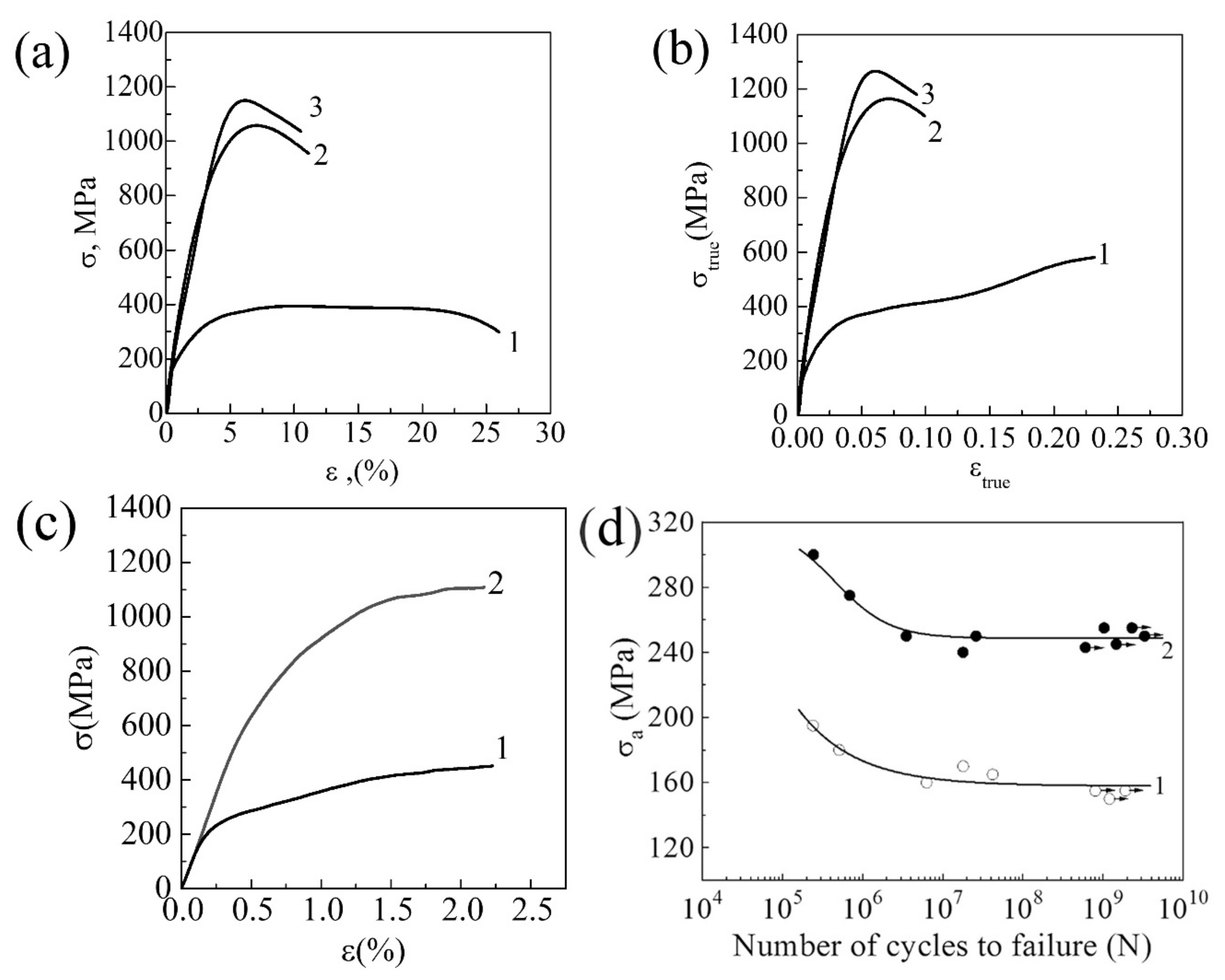
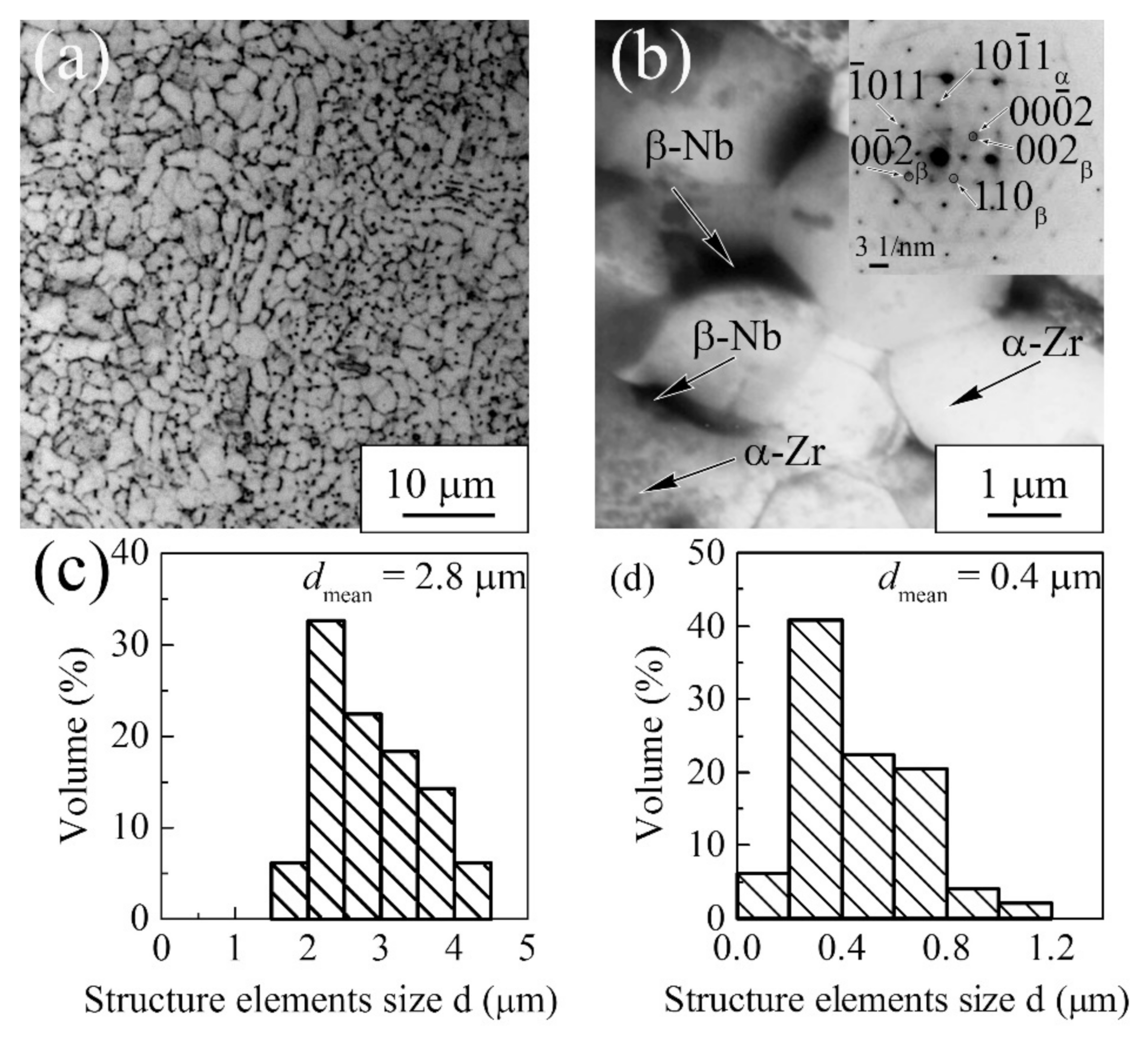

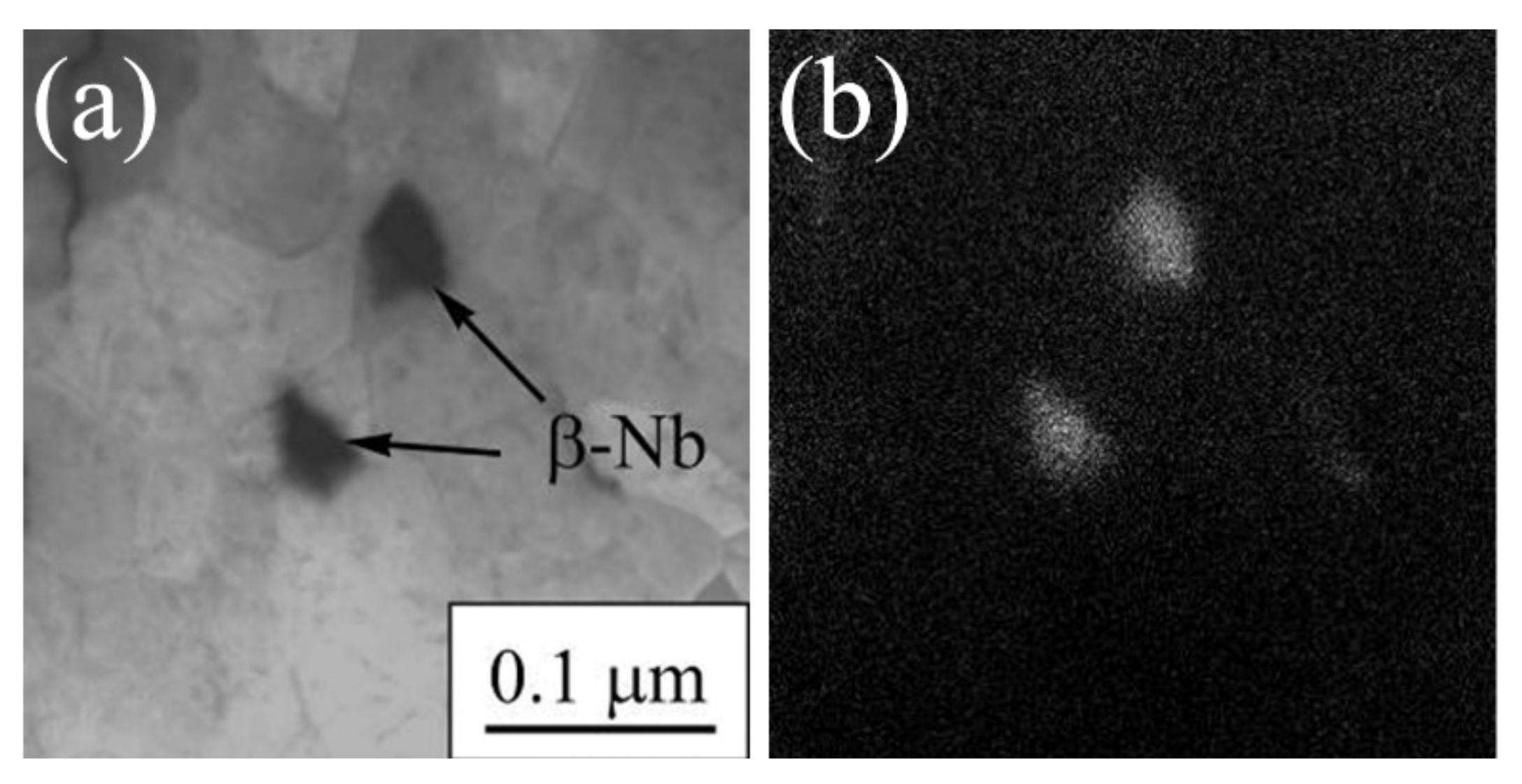
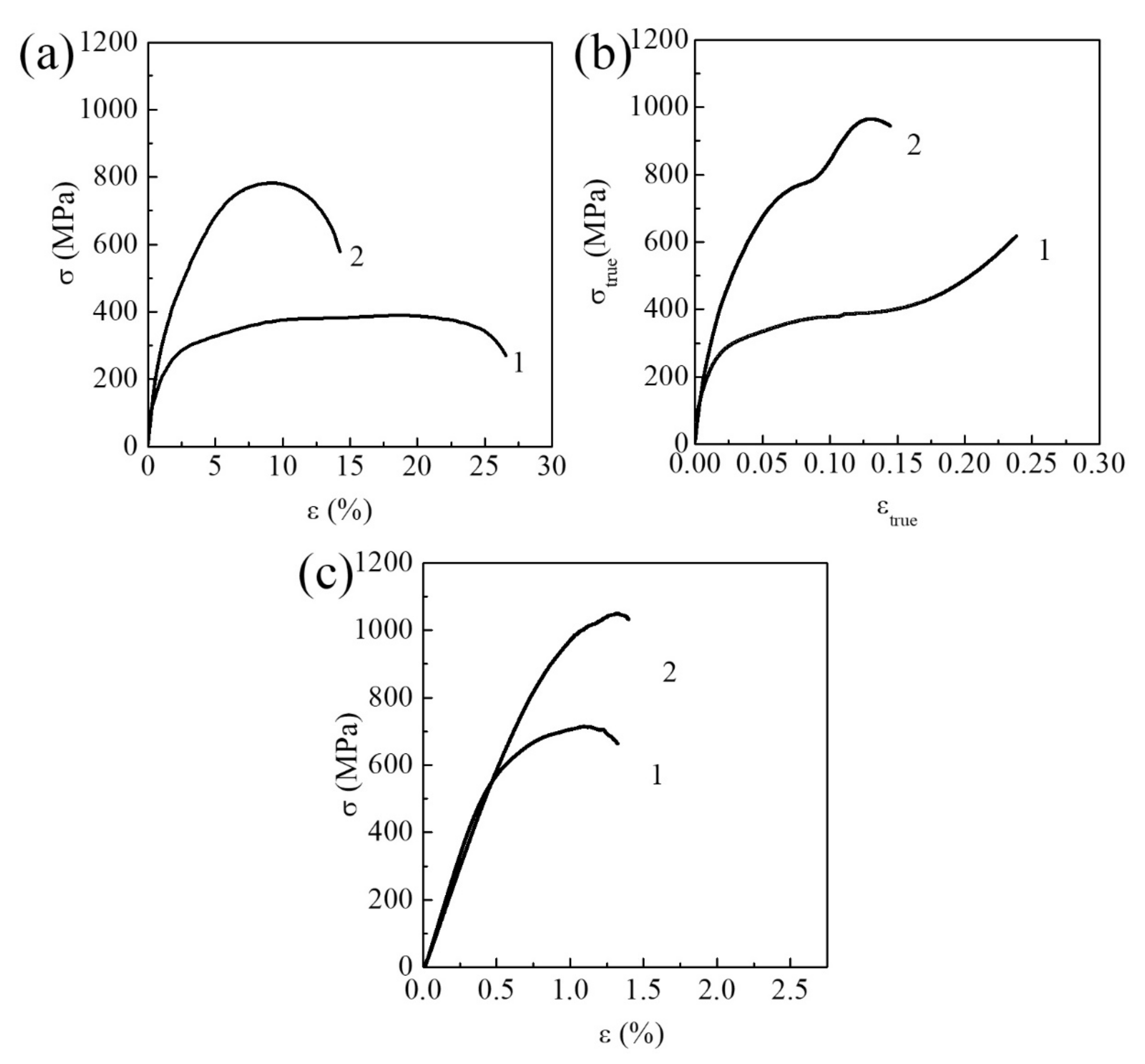

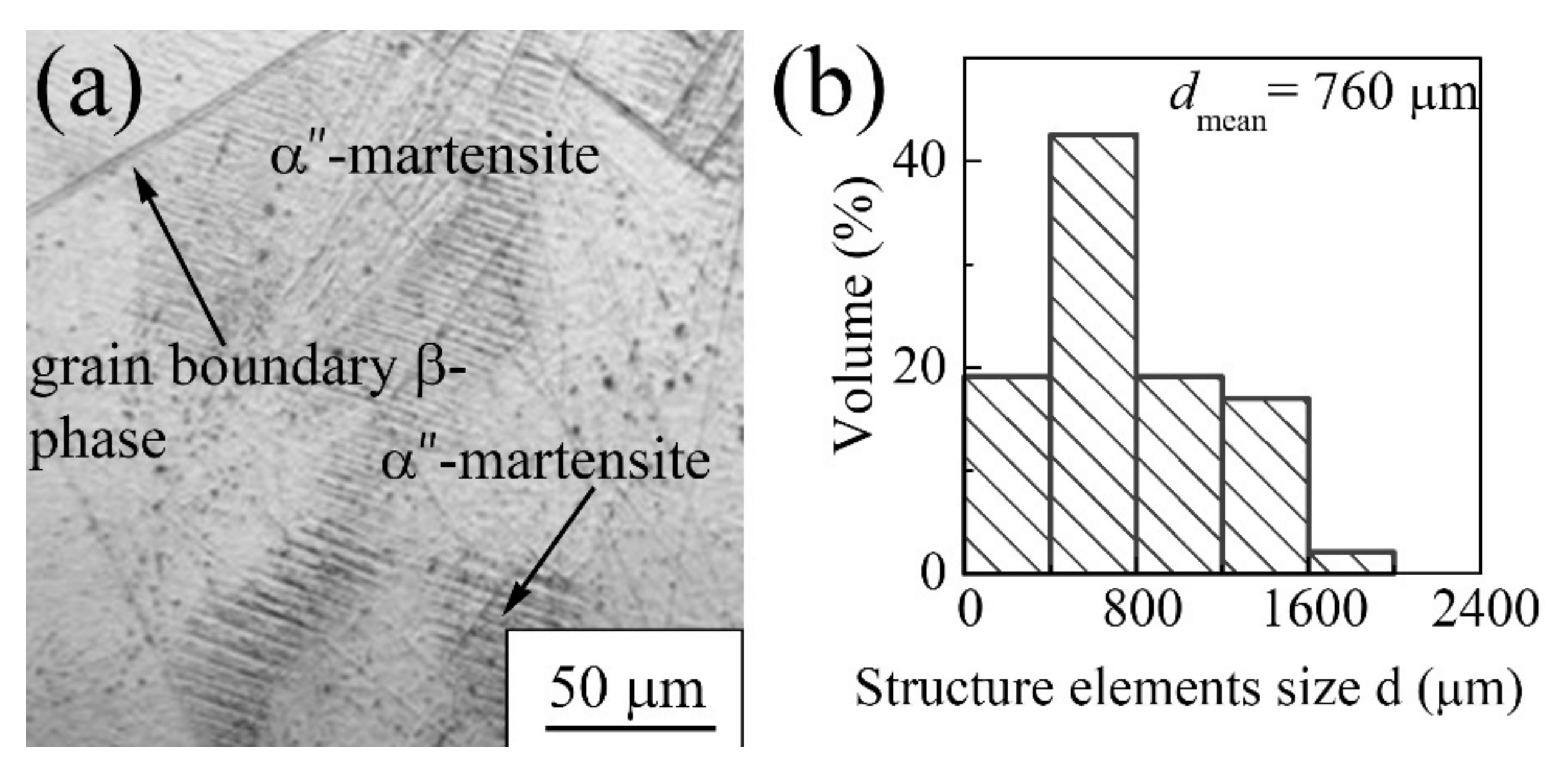
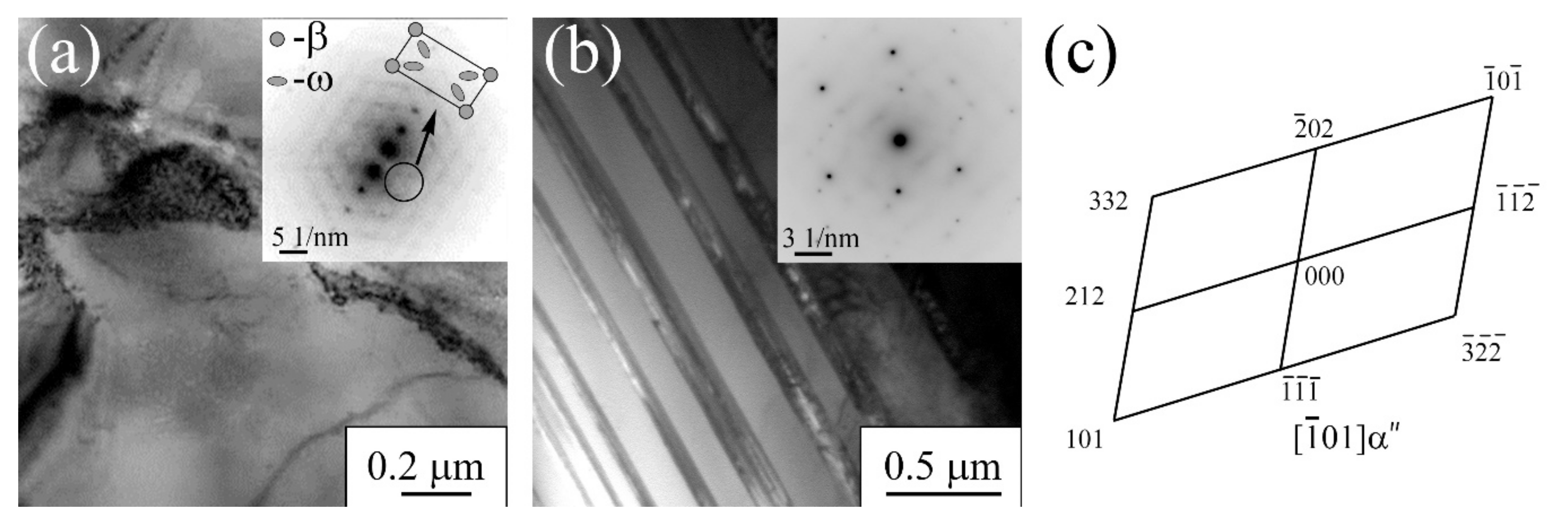

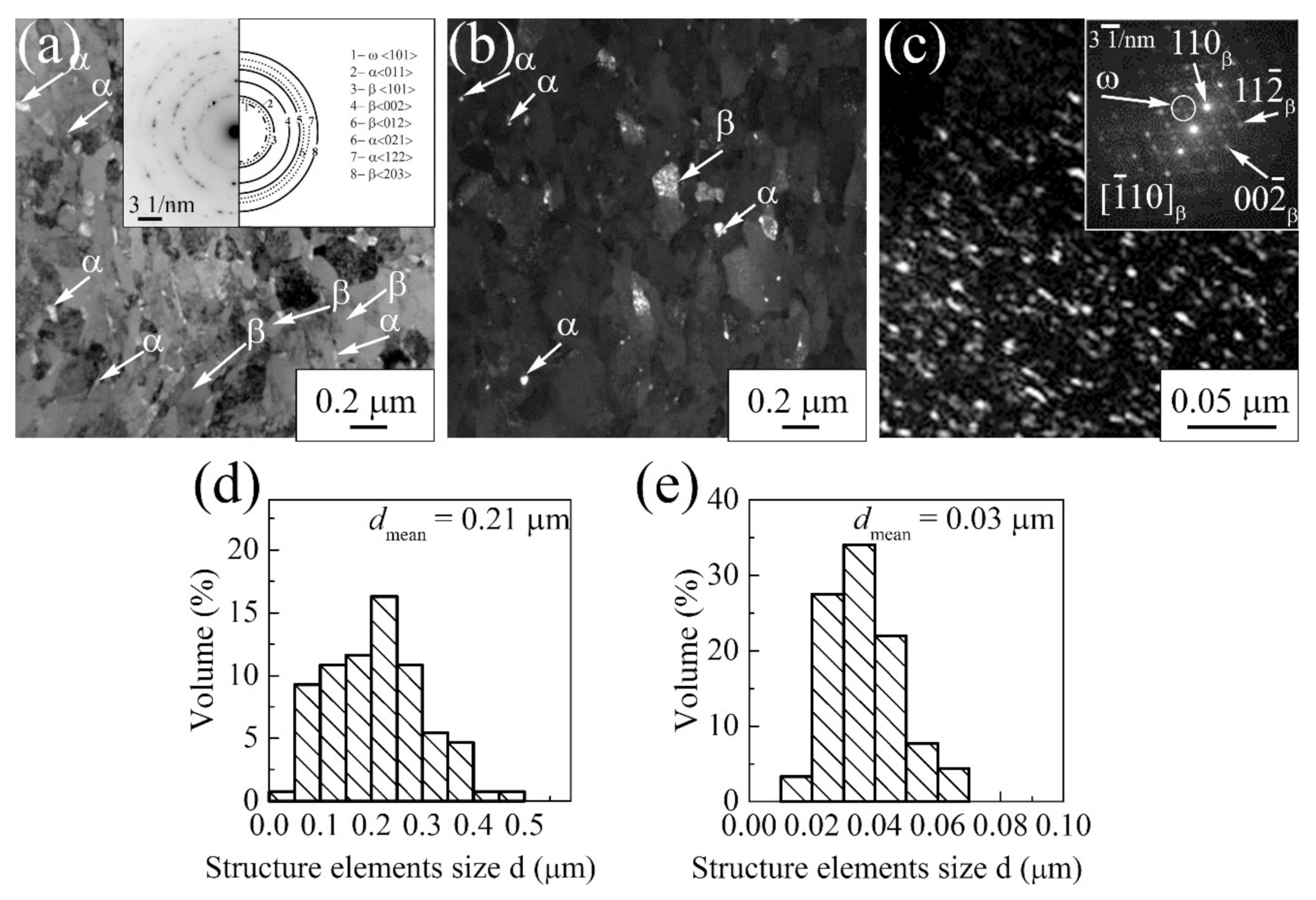
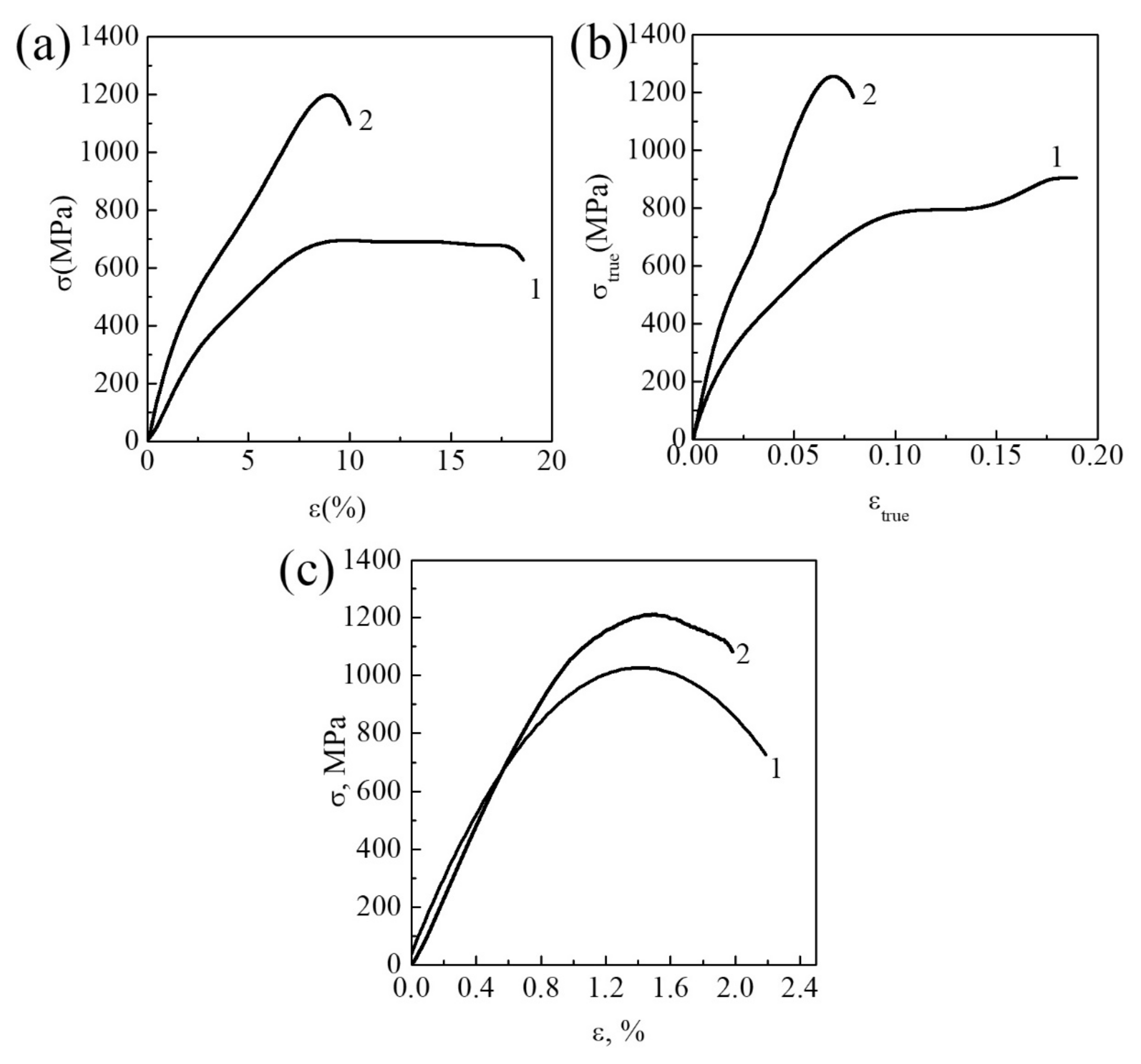
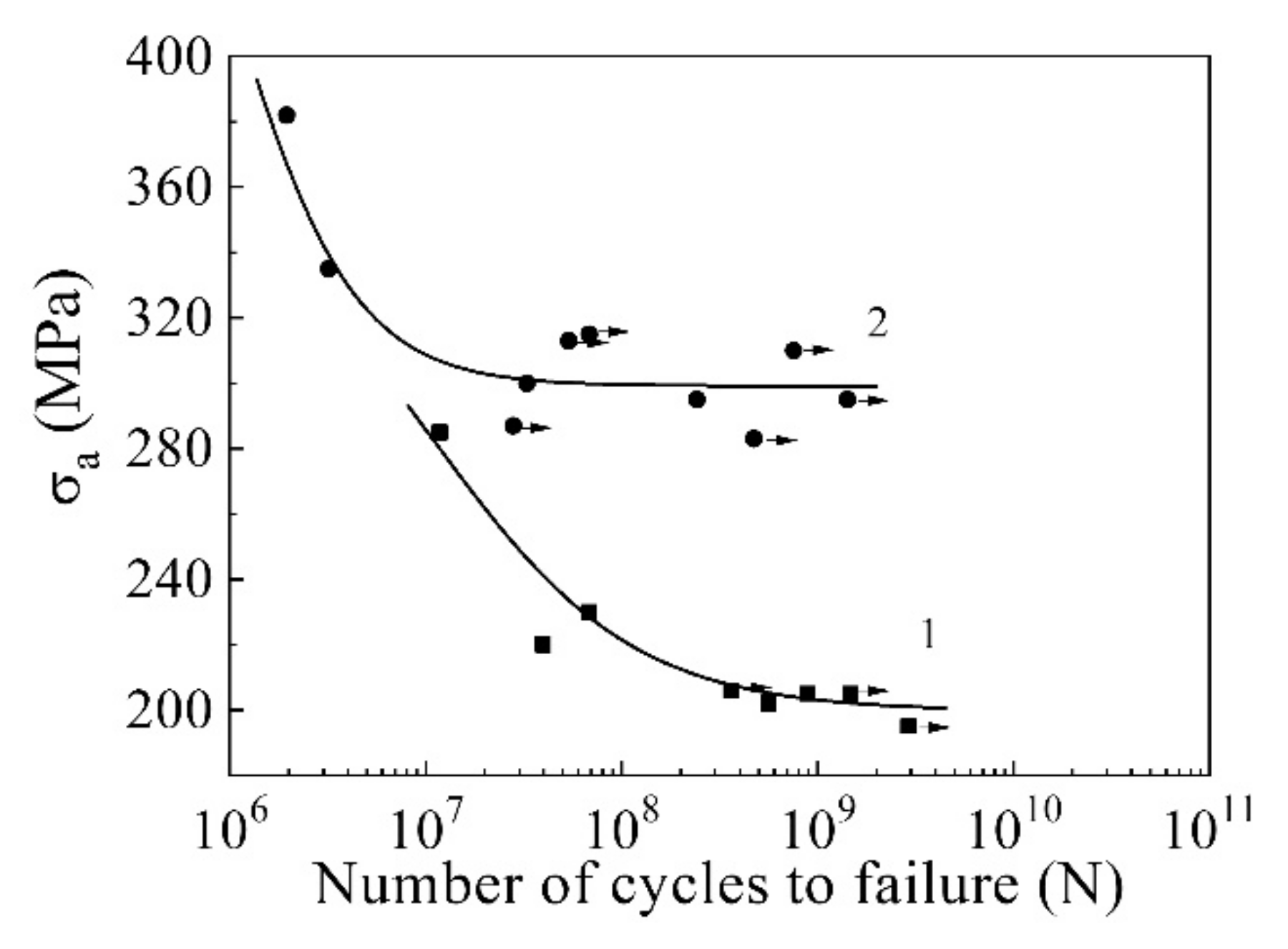

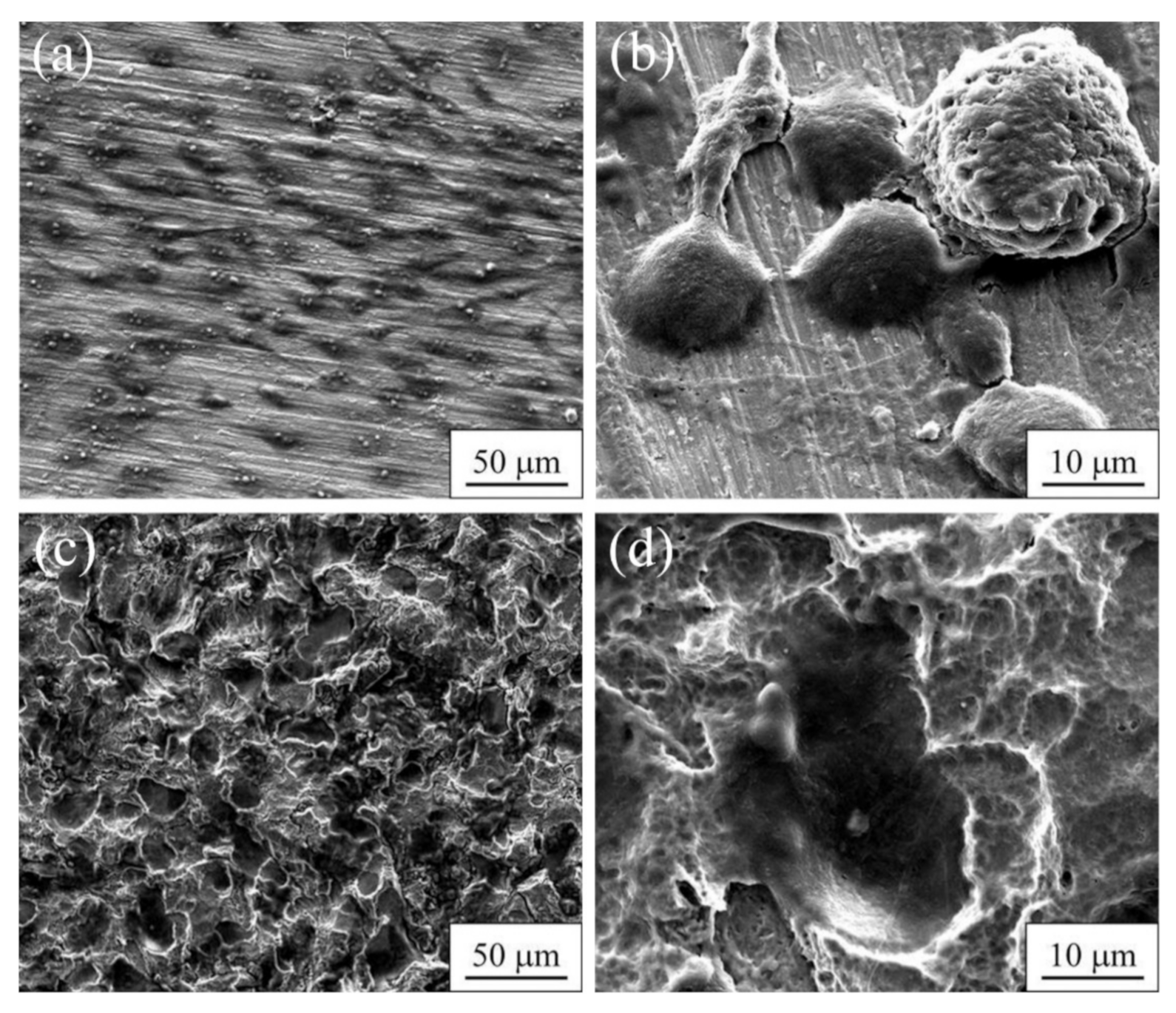

| Materials | σ0.2/YS, MPa | σUTS, MPa | ε, % | σf, MPa | Hμ, MPa | E, GPa |
|---|---|---|---|---|---|---|
| Pure Ti CG state | 270 | 400 | 23 | 280 | 1800 | 122 |
| UFG Ti (MDF and MPGR) | 870 | 1070 | 7 | 300 | 2700–2800 | 117 |
| NS Ti (PSC and MPGR) | 1100 | 1160 | 6 | 580 | 3000–3200 | 100 |
| Grade 4 | ≥480 | ≥550 | 15 | 470 | 2200 | 114 |
| Ti–6Al–4V | 1010 | 1100 | ≥8 | 570 | 3500 | 88–116 |
| Ti–6Al–7Nb | 820 | 940 | 7 | – | 2000 | 110–120 |
| Materials | σ0.2/YS, MPa | σUTS, MPa | ε, % | σf, MPa | Hμ, MPa | E, GPa |
|---|---|---|---|---|---|---|
| Zr-1Nb SG state | 230 | 390 | 26 | 100 | 1540 | 68 |
| UFG Zr-1Nb | 380 | 790 | 12 | 170 | 2800 | 66 |
| Zr (in billets) | 305 | 390 | 15 | – | – | 95 |
| Materials | σ0,2/YS, MPa | σUTS, MPa | ε, % | σf, MPa | Hμ, MPa | E, GPa |
|---|---|---|---|---|---|---|
| Ti-45Nb (CG state) | 345 | 710 | 14.5 | 280 | 2300 | 50 |
| UFG Ti-45Nb | 415 | 1180 | 6 | 380 | 3100 | 58 |
| Ti-45Nb industrial | 450 | 655 | 10 | 300 | – | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharkeev, Y.; Eroshenko, A.; Legostaeva, E.; Kovalevskaya, Z.; Belyavskaya, O.; Khimich, M.; Epple, M.; Prymak, O.; Sokolova, V.; Zhu, Q.; et al. Development of Ultrafine–Grained and Nanostructured Bioinert Alloys Based on Titanium, Zirconium and Niobium and Their Microstructure, Mechanical and Biological Properties. Metals 2022, 12, 1136. https://doi.org/10.3390/met12071136
Sharkeev Y, Eroshenko A, Legostaeva E, Kovalevskaya Z, Belyavskaya O, Khimich M, Epple M, Prymak O, Sokolova V, Zhu Q, et al. Development of Ultrafine–Grained and Nanostructured Bioinert Alloys Based on Titanium, Zirconium and Niobium and Their Microstructure, Mechanical and Biological Properties. Metals. 2022; 12(7):1136. https://doi.org/10.3390/met12071136
Chicago/Turabian StyleSharkeev, Yurii, Anna Eroshenko, Elena Legostaeva, Zhanna Kovalevskaya, Olga Belyavskaya, Margarita Khimich, Matthias Epple, Oleg Prymak, Viktoriya Sokolova, Qifang Zhu, and et al. 2022. "Development of Ultrafine–Grained and Nanostructured Bioinert Alloys Based on Titanium, Zirconium and Niobium and Their Microstructure, Mechanical and Biological Properties" Metals 12, no. 7: 1136. https://doi.org/10.3390/met12071136
APA StyleSharkeev, Y., Eroshenko, A., Legostaeva, E., Kovalevskaya, Z., Belyavskaya, O., Khimich, M., Epple, M., Prymak, O., Sokolova, V., Zhu, Q., Sun, Z., & Zhang, H. (2022). Development of Ultrafine–Grained and Nanostructured Bioinert Alloys Based on Titanium, Zirconium and Niobium and Their Microstructure, Mechanical and Biological Properties. Metals, 12(7), 1136. https://doi.org/10.3390/met12071136







