Abstract
In this work, we report a systematic study on the microstructure evolution of rapid solidified Fe91−xZr5Nb4Bx alloys (x = 10, 15, 20, 25, 30 at%) under melt-spinning conditions. Mechanical and magnetic properties are also evaluated. X-ray diffraction patterns indicate that the microstructure across the compositional series consists of an amorphous matrix with partial crystallization when boron concentration is increased. These features were identified by transmission electron microscopy (TEM). The radial distribution function (RDF) affords to resolve the nearest-neighbor configuration. The tensile and microhardness properties were measured to correlate the microstructural evolution with boron content. On the other hand, the magnetic properties of these alloy series were determined by vibrating sample magnetometry (VSM); the saturation magnetization and Curie temperature showed an increasing tendency when increasing the boron content, reaching values up to 110 Am2kg−1 and 465 K, respectively. In addition to the aforementioned, the coercive field remained constant. All these magnetic properties were correlated with the microstructure features observed by XRD, RDF and TEM.
1. Introduction
Metallic glasses (MGs) have been widely researched since their discovery in the 1960s, when Au-Si alloys were fabricated via rapid solidification [1]. MGs are widely studied due to their outstanding mechanical and magnetic properties. Regarding the former, high hardness and ultimate yield-strength values have been reported [2]. On the order hand, high saturation magnetization (over 1.5 T), low coercive field (below 10 A/m) and great effective permeability (µe) have also been reported in the literature [3,4,5]. Additional aspects such as low manufacturing costs [6], high corrosion resistance [3,5] and great transport properties [4] have also motivated further studies on their development. Nevertheless, the glass-forming ability (GFA) of this alloy family is relatively poor [1,2,3,4,5,6], which may limit further improvements for technological applications. Despite their low GFA, there are several efforts to aim their utilization in electrical and electronic devices related to storage information; for instance, interference devices and integrated service digital networks (ISDN), transformers, computer cores, magnetic heads, magnetic cores, sensors, mutual inductors, transductors, amongst others [7,8,9]. It is well-known and accepted that MGs are classified into two main groups: Fe- and Co-based alloys with a typical soft ferromagnetic behavior. At the same time, Fe-based MGs can be subdivided according to their alloying content which could include metalloids (M = B, Si, and Ge), transition metals (TM = Ti, V, Cr, Co, Ni, Cu, Zr, Nb, Mo) and/or rare-earth (RE) metals [10].
Boron is one of the most utilized metalloid elements in obtaining amorphous alloys due to its high GFA and the fact that it acts as a glass former in Fe-based amorphous alloys [11]. Numerous reports indicate that the optimum range of amorphization with boron in Fe-based alloys is within the range from 12 to 28 at% [12,13]. Nevertheless, it has been reported that in amorphous Fe-Zr-B alloys this constituent could crystallize to a primary phase due its high content [8,14,15,16]. Meanwhile, the use of elements such as Zr and Nb tend to limit the grain growth in this alloy system [17]. It is well-known that the Curie temperature (Tc) strongly depends on the content of metalloid constituents; hence, Fe-B alloys have a closer relationship between boron content and Tc [18], which favors the increase of the latter when incrementing the boron concentration up to a maximum of 35 at% [11]. To understand this behavior, it is necessary to consider the local atomic ordering of the system, i.e., the short-range atomic ordering, which has been studied by means of numerous structural models [19,20,21], as well as with computational developments from pairs-distribution function (PDF) profiles developed on Monte Carlo calculations [22]. An experimental procedure to describe the atomic distribution to the nearest neighbor in amorphous alloys is the radial distribution function (RDF). RDF allows us to describe the average number of atoms between r and (r + dr) from a particular atom. Where the average number of atoms is considered as 4πr2ρ(r)dr and ρ(r) is the number of atoms per unit volume obtained from XRD experiments:
Therefore:
Consequently, K = (4π sin θ)/λ, where θ is the scattering angle and λ the wavelength of the radiation employed; meanwhile, i(K) corresponds to the interference function estimated [23,24,25,26,27].
In this work, we report a systematic study on the microstructural evolution, mechanical and magnetic behavior in high boron content of melt-spun Fe91−xZr5Nb4Bx alloys (x = 10, 15, 20, 25, 30 at%).
2. Experimental Techniques
Master ingots of each composition Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30 at%) were obtained by means of arc-melting of the pure elemental constituents (>99,9%) in a titanium-gettered argon atmosphere. The ingots were remelted at least five times to ensure chemical homogeneity. Metallic ribbons for all the compositions were prepared from melt-spinning processing in an argon atmosphere using a wheel speed of 35 ms−1. The microstructure of the melt-spun-ribbon alloys was determined by means of X-ray diffraction (XRD) in a Siemens (Munich, Germany) D5000 diffractometer with Co-Kα radiation, λ = 1.7903 Å and step size 0.020° at 35 kV and 20 mA. The radial distribution function (RDF) was calculated from X-ray diffraction pattern (Siemens D500 Mo-Kα radiation, λ = 0.711 Å and step size 0.5°). In addition, the amorphous matrix and partial crystallization of the alloys were verified through transmission electronic microscopy (TEM) in a JEOL ARM 200F (Tokyo, Japan) operating at 200 kV. Selected-area diffraction (SAD) patterns belonging to samples x = 10 and 30 at% B were studied to determine the types of phases present in the alloys. Mechanical behavior was studied by in situ tensile test in a Shimadzu (Kyoto, Japan) equipment with a strain rate of 1 × 10−3 s−1 according to ASTM E8. The microhardness was measured in a Shimadzu HMV-G microdurometer, with a load of 245.2 mN (HV 0.025) for 10 s. Magnetic measurements were carried out on as-quenched alloyed samples by means of a vibrating-sample magnetometer (VSM) in an LDJ Electronics Inc. (Troy, MI, USA) Model., 9600 with a maximum applied field of 1.0 T at room temperature with a sweep rate of 0.0013 T/s. The sensitivity of the equipment is around 10−3 emu. A sphere of pure Ni with magnetic moment and known mass was utilized for calibration. The equipment has a sample holder that is located between the electromagnet and the detector coils. One end of the sample holder is attached to a motor that vibrates the sample at a known frequency. The variation of the magnetic moment of the sample is detected by the equipment due to the voltage induced in the detector coils, which is proportional to the frequency and amplitude of oscillation.
This signal is detected by a lock-in amplifier. The intensity of the external magnetic field is applied by an electromagnet and is measured by a Hall lead connected to the equipment. Additionally, the Curie temperature was determined using magnetic thermogravimetric analysis (MTGA) in a TA Q500 thermobalance with a heating rate of 10 K/min, coupled with an electromagnet.
3. Results and Discussion
3.1. Structural Properties
The X-ray patterns of the melt-spun ribbons Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30 at%) alloys are shown in Figure 1a. It is observed that the specimen with 10 at% B content exhibits a broad peak around 2θ = 51° confirming a fully glassy phase, which is also confirmed by the TEM pattern depicted in Figure 1b. It is therefore demonstrated the glassy nature structure with the attendance of a single halo diffuses. On the other hand, the rest of the Fe91−xZr5Nb4Bx (x = 15–30 at% B) samples show partial crystallization of the amorphous matrix with at a higher B content, exhibiting some obvious peaks that belong to the (001), (100), (101), (002) and (102) planes, which corresponds to the B2Zr phase according to the ICDD file [03-065-3389]. The precipitation of this phase may be due to the fact that B2Zr is a high-temperature intermetallic compound, as reported in the B-Zr equilibrium-phase diagram [28], which can be stabilized by means of rapid solidification process. Likewise, B2Zr is characterized by having a very strong covalent bond, causing the precipitation of this compound to be common in Fe-based alloys [29]. This phase distribution agrees with that reported by Yao et al. in similar alloys [14]. To further identify the crystalline phase of the samples, HR-TEM micrographs were obtained for x = 30 at% B, which are displayed in Figure 1c. These micrographs reveal the random distribution of B2Zr grains, which are embedded in an amorphous matrix. The TEM micrograph (shown as inset) and the corresponding electron diffraction pattern for the sample also confirm that some amount of crystalline phase is present in the specimen.
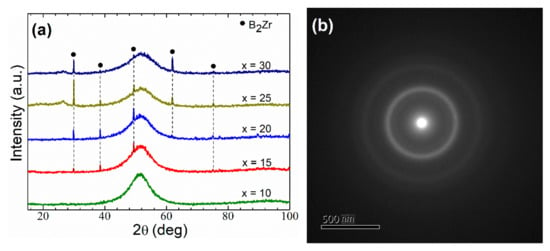
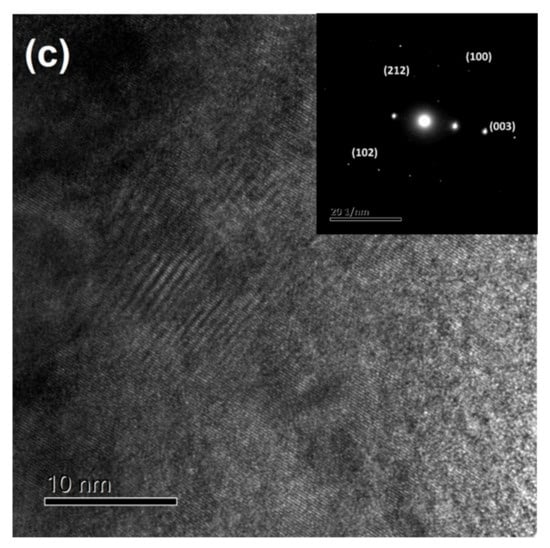
Figure 1.
(a) XRD pattern and TEM micrograph (b) for the melt-spun amorphous alloys (x = 10 at% B) and (c) region with partial crystallization of the amorphous matrix for x = 30 at% B sample. Inset: TEM micrograph and the corresponding selected-area diffraction pattern indexed.
The formation of short-range ordered structures and consequent microstructural features were observed by means of high-resolution microscopy techniques to determinate their evolution when varying B content, which were also corroborated through the RDF technique, as shown in Figure 2.
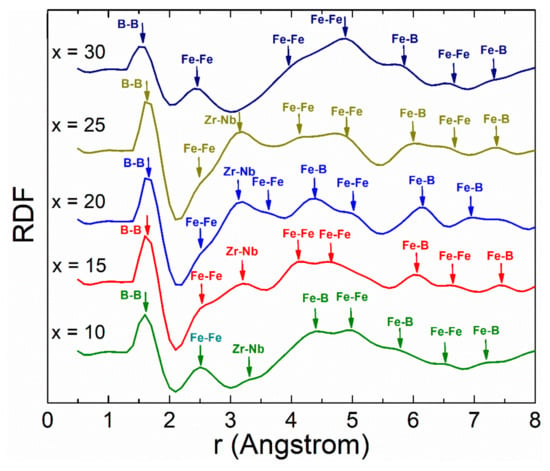
Figure 2.
Radial distribution function (RDF) for Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30 at%) melt-spun ribbons.
From the atom-pair distance information obtained by RDF, the first peaks were attributed to the distances of B-B, Fe-Fe and Zr-Nb for all samples and are detailed below.
The first peak for all samples is located at 1.6 Å, and is associated with a B-B pair, given that the interatomic distance of the boron atom is 1.59 Å. At a distance of 2.5 Å, Fe-Fe pairs were identified as reported by other authors (which are present in all samples) [8,30,31]. However, the second peak exhibits a split for x = 15–25 at% boron. The splitting can be associated with short- and medium-range atomic ordering (MGs), typical behavior rendered by metallic glasses systems [32,33]. Peak 3, located around 3.2 Å, suggests Zr-Nb atomic pairs due to the interatomic distance of Zr (3.23 Å) and Nb (3.3 Å). However, this atomic pair is not present for alloys with x = 30 at% B contents. The aforementioned can be explained as a consequence of the deformation experienced by the Fe-rich regions due to the possible substitution of some atoms that can be replaced by B, Zr or Nb constituents in the cluster. For the fourth peak the splitting is consistent for all samples, reflecting the contribution of Fe-Fe pairs in specimens with x = 15, 25 and 30 at% B. However, for samples with 10 and 20 at% boron content there is no contribution from such atomic pairs. Complementary, peak 5 is only present when the composition includes 10 and 20 at% B and corresponds to a distance of 4.4 Å belonging to Fe-B atom pairs, which have been reported for atomic clusters in metallic glasses [30]. For the remaining alloys (x = 15, 25, and 30 at% B), there is no presence of any peak close to 4.4 Å. Peak 6 has been associated to Fe-Fe pairs according to experimental and theorical results [30,31]. Peak 7 indicates a distance close to 6 Å which can be associated to Fe-B pairs since this matches well with the distance reported by experimental and theoretical studies for such pairs [30]. Peak 8 suggests the formation of Fe-Fe pairs [30,31] with exception of x = 20 at% B. Finally, peak 9 corresponds to Fe-B pairs, which suggests that the increase in the crystalline fraction causes a reduction in the B and Zr content within the cluster, therefore modifying the short-range order. In addition, the assignment of Fe-Fe and Fe-B pairs for the series of alloys is consistent with those proposed for similar Fe-B based alloys [31,34,35,36]. Likewise, the variations of the distances between Fe-Fe and Fe-B pairs indicate slight deformations in the clusters of each alloy, particularly for sample x = 20 at%, where a variation in the interatomic distances is evident in peaks 4 and 9 (see Table 1).

Table 1.
Atom-pairs distance and peak positions for Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30 at%) alloys.
The Table 1 and Table 2 compare the positions of the atomic pairs obtained by means of RDF from the literature.

Table 2.
Experimental and calculated profiles for other Fe-based MGs with the available data by other works.
3.2. Mechanical Properties
The tensile behavior of Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30 at%) melt-spun ribbons was tested at room temperature (see Figure 3a). Their mechanical behavior can be correlated to the microstructure evolution. Particularly, the alloy with 10 at% B showed the highest tensile fracture strength, which is greater than 2000 MPa. The high fracture-strength value is usually related with the type and atomic-bonding nature, as well as to the energy enthalpy of mixing [38,39]. For instance, the enthalpies of mixing involved for the Fe-B (ΔH = −26 kJ/mol), Zr-B (ΔH = −71 kJ/mol) and Nb-B (ΔH = −54 kJ/mol) pairs are highly negative in accordance with Takeuchi et al. [40]. The inset micrographs in Figure 3a show the fracture-surface feature after tensile test of the ribbon with 10 at% B.
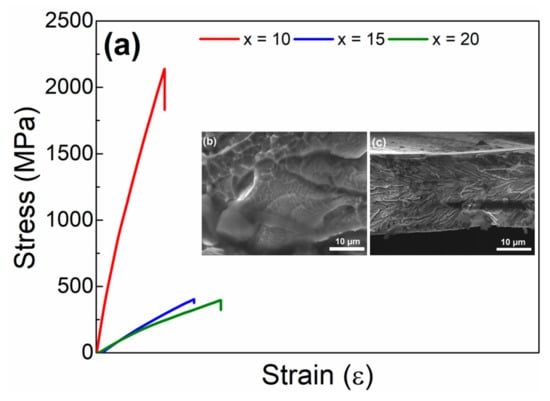
Figure 3.
(a) Tensile stress–strain curve for Fe91−xZr5Nb4Bx (x = 10, 15, 20 at%) melt-spun ribbons. Inset: SEM micrographs of fracture of ribbon tested with 10 at% B where is observed for (b) a well-defined vein pattern and (c) the magnifying micrograph from the previous image with river-like morphology zone.
It can be noticed that the tested sample has a well-defined vein pattern (see Figure 3b) which corresponds to a ductile fracture [41]. Likewise, a river-like morphology can be observed, which is commonly present in materials with good malleability when undergoing tensile stresses, as occurs in bulk metallic glasses [42] (Figure 3c). The presence of these patterns suggests a similar type of fracture that occurs in Fe-based alloys [43]. On the other hand, in alloys with 15 and 20 at% B contents, the yield strength decreased abruptly due to precipitation of the B2Zr intermetallic phase. The last two alloys (x = 25 and x = 30 at% B) were not tested since both rendered a completely brittle behavior.
Figure 4 depicts the Vickers microhardness values for the different B content. From these results, a linear behavior is observed. Such a feature can be related to the presence of an amorphous matrix and partial crystallization previously observed and confirmed by XRD means, i.e., this influence in the hardness behavior can be connected to formation of B2Zr intermetallic compounds which have been reported to alter the mechanical response of metallic materials processed by means of conventional casting or rapid solidification techniques. However, it has been reported that for the quaternary Fe-B-Zr-Nb system, the suitable addition of Nb improves the atomic packing density along with the materials’ hardening [44].
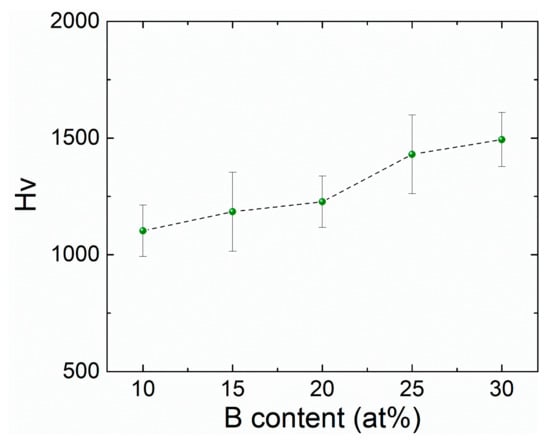
Figure 4.
Variation trend in microhardness for Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30 at%) melt-spun ribbons.
3.3. Magnetic Properties
Figure 5a. shows the variation tendency of saturation magnetization (Ms) together with the coercive field (Hc). In Figure 5b, the Curie temperature is evaluated against the B content. As shown in Figure 5a, the Ms exhibits an upward trend with the increasing B concentration reaching values up to 110 Am2kg−1. However, Ms decreases at x = 25 at% B, which is perhaps due to the higher amount of B2Zr formed as indicated by the XRD analysis previously discussed. These results can be associated with the RDF analysis according to estimated distances to the first neighbors of the elemental constituents, which indicate the formation of Fe-pairs. Nevertheless, the decrease in boron content within the cluster due to formation of B2Zr intermetallic phase causes the presence of Fe-rich regions, resulting in a smaller interatomic distance between Fe atoms [45]. The aforementioned does not necessarily indicate an increase in Ms. In accordance with the Bethe–Slater curve, it is suggested that the smaller the interatomic distance between the Fe-Fe pairs, the weaker the response of the exchange interaction [46]. For our alloy with x = 25 at% boron, the decrease in Ms can be a consequence of the rearrangement of Fe pairs affecting the short-range atomic ordering, which in turn reduces the exchange interaction.
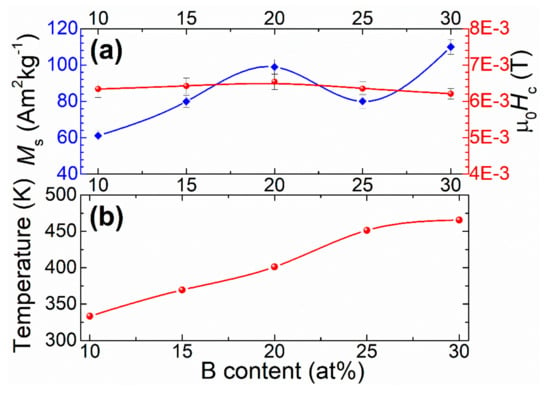
Figure 5.
B-content dependence for Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30 at%) melt-spun ribbons: (a) Ms and Hc; and (b) the change of the Curie temperature. The lines are guide for the eyes.
Figure 5a shows the evolution of Hc as a function of the B content. From this, it can be observed that the coercivity remains practically constant with the variation of boron content.
Figure 5b illustrates the Curie temperature (Tc) variation, which clearly increases systematically with the Boron content. Since Tc strongly depends on the distance between Fe atoms, it is possible to attribute the partial crystallization of the material to increment of B content, which hence favors the rearrangement of the short-range atomic order. The latter determines variation of Tc as it has been reported in Fe-based alloys systems such as Fe-Zr [47], Fe-B [18] and Fe-B-Zr [48], which have a Tc dependence with metalloid content similar to our Fe-Zr-Nb-B alloys. For present alloys, their Tc dependence with boron content can be ascribed to the increase of Fe-Fe exchange interaction caused by a rearrangement of short-range atomic ordering provoked by a decrease in Fe concentration within the amorphous matrix [49]. The variation of the short-range order is consistent with the RDF results, see Section 3.1. The Fe-Fe distance directly influences the exchange integral (Jex) between the Fe atoms and therefore the Tc [50]. Fe-Fe interatomic distances are very sensitive to composition and residual internal stresses [51,52], which also affect Tc.
4. Conclusions
A series of Fe91−xZr5Nb4Bx (x = 10, 15, 20, 25, 30) alloy were characterized to establish the influence of B content on their mechanical and magnetic properties. From XRD analysis, formation of B2Zr intermetallic compounds is confirmed when the B content varies from 15 to 30 at% B. Further analysis utilizing the radial distribution function indicates that for all compositions, the atomic structure undergoes short-range rearrangements, which favor formation of Fe-Fe and Fe-B atomic pairs. The mechanical properties have been correlated to the microstructural features when varying B content. The alloy with 10 at% boron exhibited high tensile-strength facture up to 2100 MPa. Meanwhile, the hardness behavior was lineal, increasing as a function of the B content as a result of the precipitation of the B2Zr intermetallic phase. On the other hand, the magnetic properties showed a soft magnetic response for all alloys. Likewise, the Ms and Tc increased in general when incrementing B content; the Tc behavior is associated with the increment of average iron atomic moments provoked by the decreases in iron concentration. On the other hand, the Ms increased with decreasing iron content, as well as considering the short-range atomic ordering.
Author Contributions
Conceptualization, J.Z.; data curation, J.Z., I.B. and J.A.G.H.; formal analysis, J.Z. and I.B.; investigation, J.Z.; writing—original draft, J.Z.; validation, J.Z. and I.B.; visualization, J.Z. and I.B.; conception of the work, J.Z.; methodology, J.Z. and I.B.; data collection, J.Z. and I.B.; data analysis, J.Z. and I.B.; interpretation, J.Z., I.B. and J.A.G.H.; writing—Review and editing, J.Z. and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
J.Z. is grateful for the scholarship received from Programa de Becas Posdoctorales DGAPA-UNAM for supporting his postdoctoral position at FQ-UNAM. Authors are grateful with Adriana Tejeda, Josue Romero, Omar Novelo, Gabriel Lara, Ruben Mendoza, Eliezer Hernandez, Lourdes Bazán from IIM-UNAM, Mexico and Victor Hugo Lara from UAM, Mexico, for their helpful technical assistance, as well as with Pedro Bosch, for his valuable discussions of results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klement, W.; Willens, R.H.; Duwez, P. Non-Crystalline Structure in Solidified Gold-Silicon Alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.L.; Yavari, A.R.; Greer, A.L. Mechanical Properties of Fe-Based Bulk Glassy Alloys in Fe-B-Si-Nb and Fe-Ga-P-C-B-Si Systems. J. Mater. Res. 2003, 18, 1487–1492. [Google Scholar] [CrossRef]
- Makino, A.; Bitoh, T.; Kojima, A.; Inoue, A.; Masumoto, T. Low Core Losses of Nanocrystalline Fe-Zr-Nb-B Soft Manetic Alloys with High Magnetic Flux Density. Mater. Sci. Eng. A 2001, 304–306, 1083–1086. [Google Scholar] [CrossRef]
- Azuma, D.; Ito, N.; Ohta, M. Recent Progress in Fe-Based Amorphous and Nanocrystalline Soft Magnetic Materials. J. Magn. Magn. Mater. 2020, 501, 166373. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B. Formation and Soft Magnetic Properties of Fe-B-Si-Zr Bulk Glassy Alloys with High Saturation Magnetization above 1.5 T. Mater. Trans. 2002, 43, 2350–2353. [Google Scholar] [CrossRef][Green Version]
- Inoue, A.; Takeuchi, A. Recent Progress in Bulk Glassy Alloys. Mater. Trans. 2002, 43, 1892–1906. [Google Scholar] [CrossRef]
- Liu, E.; Swerts, J.; Couet, S.; Mertens, S.; Tomczak, Y.; Lin, T.; Spampinato, V.; Franquet, A.; Van Elshocht, S.; Kar, G.; et al. [Co/Ni]-CoFeB Hybrid Free Layer Stack Materials for High Density Magnetic Random Access Memory Applications. Appl. Phys. Lett. 2016, 108, 132405. [Google Scholar] [CrossRef]
- Makino, A.; Suzuki, K.; Inoue, A.; Hirotsu, Y.; Masumoto, T. Magnetic Properties and Microstructure of Nanocrystalline Bcc Fe-M-B ( M = Zr, Hf, Nb) Alloys. J. Magn. Magn. Mater. 1994, 133, 329–333. [Google Scholar] [CrossRef]
- Krings, A.; Boglietti, A.; Cavagnino, A.; Member, S.; Sprague, S. Soft Magnetic Material Status and Trends in Electric Machines. IEEE Trans. Ind. Electron. 2017, 64, 2405–2414. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Li, H.X.; Gao, J.E.; Wu, Y.; Lu, Z.P. Effects of Alloying Elements on Glass Formation, Mechanical and Soft-Magnetic Properties of Fe-Based Metallic Glasses. Intermetallics 2011, 19, 1502–1508. [Google Scholar] [CrossRef]
- Valenzuela, R. Magnetic Ceramics, 1st ed.; Cambridge University Press: New York, NY, USA, 1994. [Google Scholar]
- Sun, H.; Wang, Y. Glass Forming Ability, Thermal Stability, and Magnetic Properties of FeCoNiBSi Alloys with Different B Contents. Adv. Mater. Sci. Eng. 2018, 2018, 4841025. [Google Scholar] [CrossRef]
- Kim, K.Y.; Noh, T.H.; Kang, I.K.; Kang, T. Microstructural Change upon Annealing FeZrB Alloys with Different Boron Contents. Mater. Sci. Eng. A 1994, 179–180, 552–556. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, Y.; Si, L.; Tan, H.; Li, Y. Boron Content Dependence of Crystallization, Glass Forming Ability and Magnetic Properties in Amorphous Fe-Zr-B-Nb Alloys. J. Alloys Compd. 2004, 370, 1–7. [Google Scholar] [CrossRef]
- Yao, B.; Si, L.; Tan, H.; Zhang, Y.; Li, Y. Effects of High Boron Content on Crystallization, Forming Ability and Magnetic Properties of Amorphous Fe91-XZr5B XNb4 Alloy. J. Non. Cryst. Solids 2003, 332, 43–52. [Google Scholar] [CrossRef]
- Suzuki, K.; Kataoka, N.; Inoue, A.; Masumoto, T.; Makino, A. High Saturation Magnetization and Soft Magnetic Properties of Bcc Fe-Zr-B and Fe-Zr-B-M (M = Transition Metal) Alloys with Nanoscale Grain Size. Mater. Trans. JIM 1991, 32, 93–102. [Google Scholar] [CrossRef]
- Herzer, G. Chapter 3 Nanocrystalline Soft Magnetic Alloys. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 1997; Volume 10, pp. 415–462. [Google Scholar] [CrossRef]
- Hasegawa, R.; Ray, R. Iron-boron Metallic Glasses. J. Appl. Phys. 2008, 49, 4174. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Miracle, D.B.; Egami, T.; Flores, K.M.; Kelton, K.F. Structural Aspects of Metallic Glasses. MRS Bull. 2007, 32, 629–634. [Google Scholar] [CrossRef]
- Miracle, D.B. Efficient Local Packing in Metallic Glasses. J. Non-Cryst. Solids 2004, 342, 89–96. [Google Scholar] [CrossRef]
- Lee, C.M.; Park, K.W.; Lee, B.J.; Shibutani, Y.; Lee, J.C. Structural Disordering of Amorphous Alloys: A Molecular Dynamics Analysis. Scr. Mater. 2009, 61, 911–914. [Google Scholar] [CrossRef]
- Warburg, E. Magnetische Untersuchungen. Ann. Phys. 1881, 249, 141–164. [Google Scholar] [CrossRef]
- Klug, H.; Alexander, L. X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley-Interscience Publications: Hoboken, NJ, USA, 1974. [Google Scholar]
- Wollmann, L.; Chadov, S.; Kübler, J.; Felser, C. Magnetism in Cubic Manganese-Rich Heusler Compounds. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 90, 214420. [Google Scholar] [CrossRef]
- Luborsky, F. Ferromagnetic Materials. In Handbook of Magnetic Materials, 1st ed.; North-Holland: Amsterdam, The Netherlands, 1980; Volume 1. [Google Scholar]
- Valenzuela, R. 3. Soft Ferromagnetic Amorphous and Nanocrystalline Alloys. In Advances in Non-Crystalline Solids: Metallic Glass Formation, Magnetic Properties and Amorphous Carbon Films, 1st ed.; Paladai, E.S.G., Ed.; Transworld Rersearch Network: Trivandrum, India, 2010; pp. 59–83. [Google Scholar]
- Okamoto, H.; Emeritus, P.; Schlesinger, M.E.; Mueller, E.M. ASM Handbook, Volume 3: Alloy Phase Diagrams; ASM: Almere, The Netherlands, 2016; ISBN 9781627080705. [Google Scholar]
- Mihalkovič, M.; Widom, M. Cohesive Energies of Fe-Based Glass-Forming Alloys. arXiv 2004, arXiv:cond-mat/0405298. [Google Scholar]
- Aykol, M.; Mekhrabov, A.O.; Akdeniz, M.V. Nano-Scale Phase Separation in Amorphous Fe-B Alloys: Atomic and Cluster Ordering. Acta Mater. 2009, 57, 171–181. [Google Scholar] [CrossRef]
- Ohkubo, T.; Kai, H.; Makino, A.; Hirotsu, Y. Structural Change of Amorphous Fe90Zr7B3 Alloy in the Primary Crystallization Process Studied by Modern Electron Microscope Techniques. Mater. Sci. Eng. A 2001, 312, 274–283. [Google Scholar] [CrossRef]
- Finney, J.L. Modelling the Structures of Amorphous Metals and Alloys. Nature 1977, 266, 309–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Mattern, N.; Eckert, J. Atomic Structure and Transport Properties of Cu50Zr 45Al5 Metallic Liquids and Glasses: Molecular Dynamics Simulations. J. Appl. Phys. 2011, 110, 093506. [Google Scholar] [CrossRef]
- Yamauchi, T.; Hamada, Y.; Hermann, H.; Mattern, N. Analytic Approach to the Structure of Amorphous Iron-Boron Alloys. J. Phys. F Met. Phys. 1986, 16, 131. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Y.; Qiang, J.; Zhang, G.; Dong, C.; Häussler, P. Composition Formulas of Fe–B Binary Amorphous Alloys. J. Non. Cryst. Solids 2016, 432, 453–458. [Google Scholar] [CrossRef]
- Matsubara, E.; Sato, S.; Imafuku, M.; Nakamura, T.; Koshiba, H.; Inoue, A.; Waseda, Y. Structural Study of Amorphous Fe70M10B20 (M=Zr, Nb and Cr) Alloys by X-ray Diffraction. Mater. Sci. Eng. A 2001, 312, 136–144. [Google Scholar] [CrossRef]
- Matsubara, E.; Sato, S.; Imafuku, M.; Nakamura, T.; Koshiba, H.; Inoue, A.; Waseda, Y. Retraction: Anomalous X-ray Scattering Study of Amorphous; (M = Zr, Nb, and Cr) Alloys. Mater. Trans. JIM 2000, 41, 1379–1384. [Google Scholar] [CrossRef]
- Si, J.J.; Wang, T.; Wu, Y.D.; Cai, Y.H.; Chen, X.H.; Wang, W.Y.; Liu, Z.K.; Hui, X.D. Cr-Based Bulk Metallic Glasses with Ultrahigh Hardness. Appl. Phys. Lett. 2015, 106, 251905. [Google Scholar] [CrossRef]
- Xu, T.; Jian, Z.; Chang, F.; Zhuo, L.; Shi, M.; Zhu, M.; Xu, J.; Liu, Y.; Zhang, T. Synthesis of Fe75Cr5(PBC)20 Bulk Metallic Glasses with a Combination of Desired Merits Using Industrial Ferro-Alloys without High-Purity Materials. J. Alloys Compd. 2017, 699, 92–97. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Joshi, S.S.; Samimi, P.; Ghamarian, I.; Katakam, S.; Collins, P.C.; Dahotre, N.B. Tensile Behavior of Laser Treated Fe-Si-B Metallic Glass. J. Appl. Phys. 2015, 118, 164904. [Google Scholar] [CrossRef]
- Liu, F.J.; Yao, K.F.; Ding, H.Y. Fe-Based Glassy Alloys with High Iron Content and High Saturation Magnetization. Intermetallics 2011, 19, 1674–1677. [Google Scholar] [CrossRef]
- Sharma, S.; Suryanarayana, C. Effect of Nb on the Glass-Forming Ability of Mechanically Alloyed Fe-Ni-Zr-B Alloys. Scr. Mater. 2008, 58, 508–511. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Zhang, T.; Inoue, A. Effect of Nb Addition on Glass-Forming Ability, Strength, and Hardness of Fe-B-Zr Amorphous Alloys. Mater. Res. Bull. 1999, 34, 915–920. [Google Scholar] [CrossRef]
- Chen, H.S. Glassy Metals. Rep. Prog. Phys. 1980, 43, 353. [Google Scholar] [CrossRef]
- Cullity, D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ohnuma, S.; Nose, M.; Masumoto, T. Magnetic Properties of (Fe, Co and Ni)-Zr Amorphous Alloys. IEEE Trans. Magn. 1980, 16, 1129–1131. [Google Scholar] [CrossRef]
- Brzózka, K.; Ślawska-Waniewska, A.; Jezuita, K. Mössbauer Studies of FeZrB(Cu) Amorphous Alloys. J. Magn. Magn. Mater. 1996, 160, 255–256. [Google Scholar] [CrossRef]
- Chen, H.S. Correlation between the Thermal Stability and Activation Energy of Crystallization in Metallic Glasses. Appl. Phys. Lett. 1976, 29, 12. [Google Scholar] [CrossRef]
- Davies, H.A.A.; Luborsky, F.E.; Liebermann, H.H.; Finney, J.L.L.; Wagner, C.N.J.; Suzuki, K.; Egami, T.; Messmer, R.P.; Hague, C.F.; Oelhafen, P.; et al. Amorphous Metallic Alloys; Butterworths: London, UK; Boston, MA, USA, 1983; Volume 33, ISBN 9780408110303. [Google Scholar]
- Barandiarán, J.; Gorria, P.; Orúe, I.; Fdez-Gubieda, M.; Plazaola, F.; Hernando, A. Tensile Stress Dependence of the Curie Temperature and Hyperfine Field in Fe-Zr-B-(Cu) Amorphous Alloys. Phys. Rev. B 1996, 54, 3026. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Gubieda, M.L.; García-Arribas, A.; Barandiarán, J.M.; López Antón, R.; Orue, I.; Gorria, P.; Pizzini, S.; Fontaine, A. Local Structure and Ferromagnetic Character of Fe-B and Fe-P Amorphous Alloys. Phys. Rev. B 2000, 62, 5746. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).