Production of Closed-Cell Foams Out of Aluminum Chip Waste: Mathematical Modeling and Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Experiments
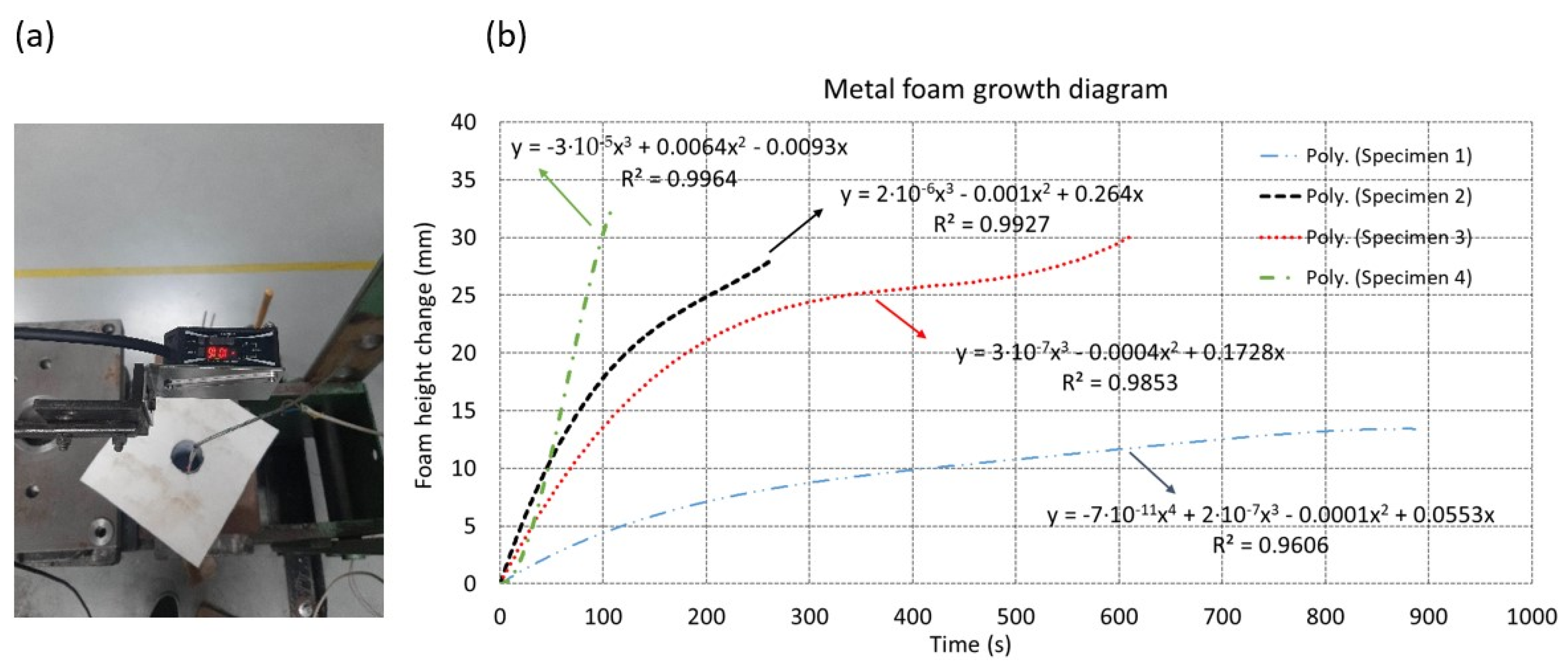
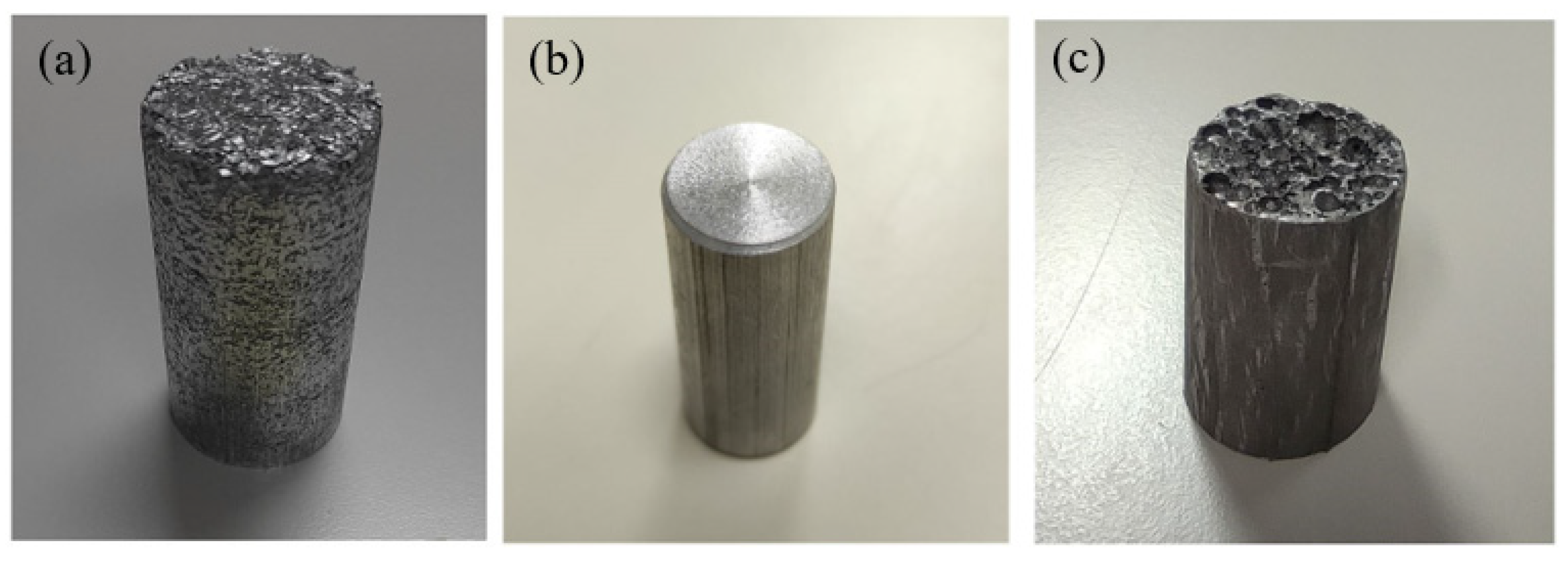
2.2. Experimental Procedure
3. Results and Discussion
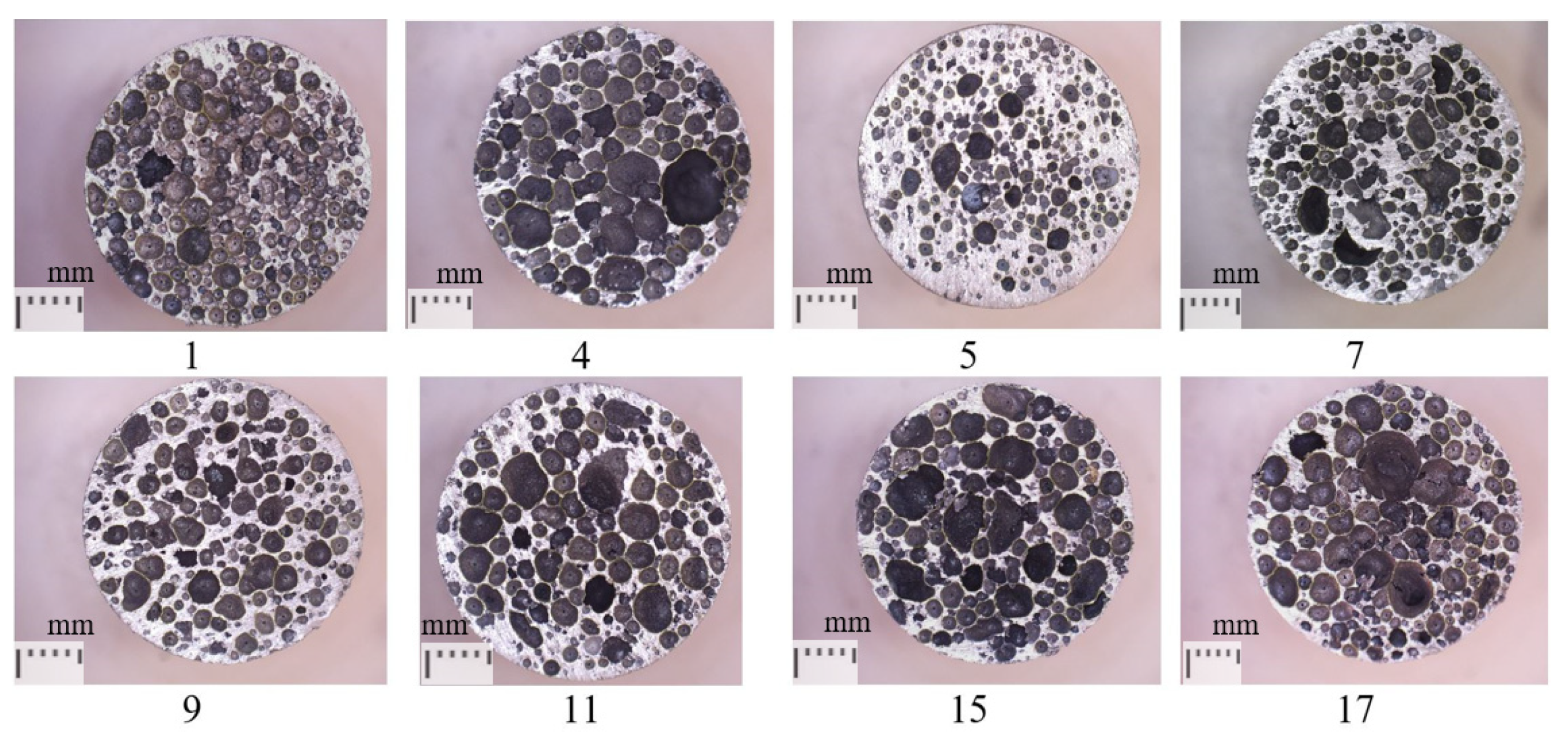
3.1. Pore Size and Inhomogeneity Analysis
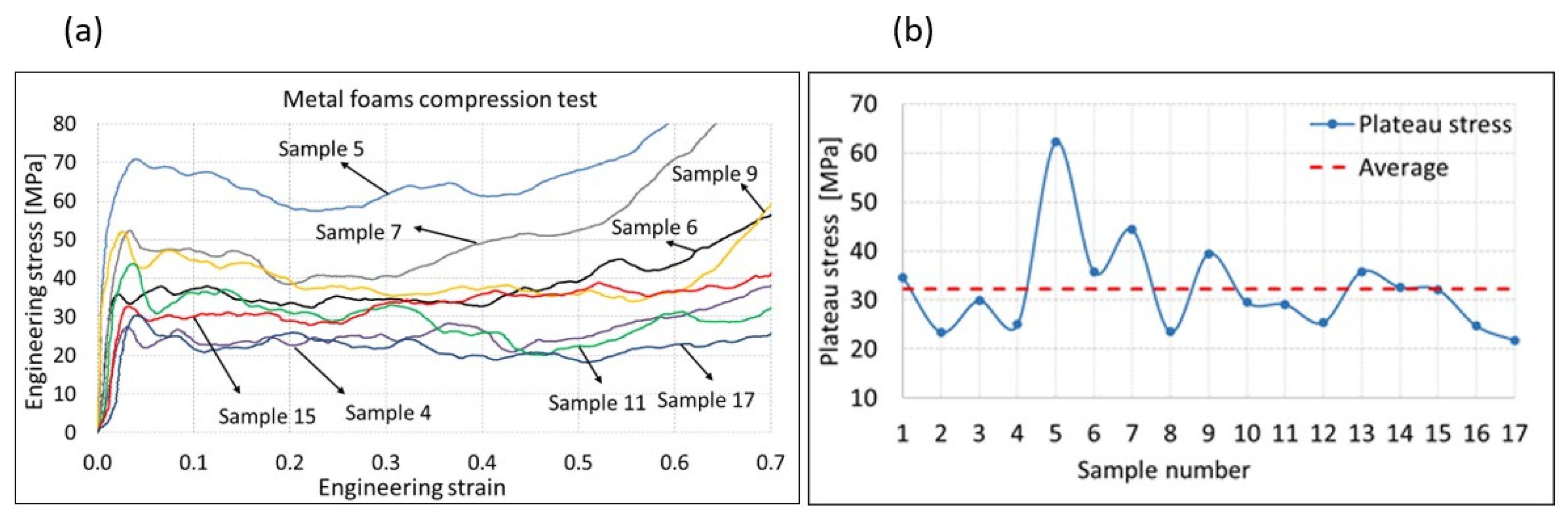
3.2. Compression Test
3.3. Regression Analysis for Density
C + 3.61167·10−4 · B · C − 0.15466 · A2 + 2.10596·10−4· B2
3.4. Regression Analysis for Energy Absorption and Yield Strength
3.5. Regression Analysis for Pore Size
3.6. Regression Analysis for Pore Inhomogeneity
3.7. Optimization of Foam Properties
4. Conclusions
- (1)
- The production parameters used in the experimental plan were appropriately selected, and all 17 metal foams specimens were successfully produced, where plateau stress, energy absorption, and yield strength were in ranges from 21.8 MPa to 62.3 MPa, 10.9 MJ/m3 to 31.3 MJ/m3, and 14.4 MPa to 64.3 MPa, respectively. The obtained pore perimeter and pore inhomogeneity for all specimens were in the ranges of 3.2 to 7.4 mm and 1.4 to 4.8 mm, respectively.
- (2)
- Energy absorption and yield strength generally increased with the reduction in the height of the metal foams due to the direct link with the metal foam density. For samples foamed with 0.5 and 1 (wt%) TiH2 on 40 mm, 590 °C was more desirable than 610 °C for achieving higher energy absorption. However, for samples foamed at 70 mm when 1 or 1.5 (wt%) of TiH2 was used, a significant increase in energy absorption was observed when 610 °C temperature was used instead of 590 °C.
- (3)
- Three-dimensional optical profilometry was used to additionally evaluate the obtained metal foam quality. According to 3D scanning, the foam cell walls were quite homogeneous without any significant cracks. Pores were homogeneously dispersed across the metal foam cross-section, and the cell wall thickness was uniform for all created pores. Regression analysis was used to statistically analyze and describe the metal foam inhomogeneity.
- (4)
- In accordance with the obtained mathematical models, the foam pore inhomogeneity increased with a higher amount of blowing agent at a 610 °C foaming temperature. This was probably due to the lower semisolid slurry viscosity and higher amount of the blowing agent, which caused some pores to coagulate or to grow rapidly owing to the increased blowing gas pressure. This indicates that pore homogeneity can be reduced if both 1.5 (wt%) of the blowing agent and a 610 °C foaming temperature are selected.
- (5)
- Foaming parameters also influence the pore perimeter. It seems that the foam pore perimeter increases with a lower amount of the blowing agent. The foam pore perimeter was highest with 0.5 (wt%) of the blowing agent and a 610 °C foaming temperature. It seems that at a 610 °C foaming temperature, foam pores increase with the decrease in the amount of the blowing agent for all foaming heights.
- (6)
- Metal foam optimization, based on derived mathematical models, can be easily performed, but optimization criteria are directly connected with the possible application of the metal foams. In the optimization example provided in this research, the metal foam would have density, energy absorption, yield strength, pore diameter, and pore inhomogeneity values of 0.63 g/cm3, 11.82 MJ/m3, 27.9 MPa, 5.3 mm, and 1.84, respectively. This should be achieved if metal foam with 0.94 (wt%) of the blowing agent is foamed at 590 °C until a 69 mm height is reached.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ashby, M.M.; Evans, A.; Fleck, N.A.; Hutchinson, J.W.; Wadley, H.N.G.; Gibson, L.J. Metal Foams: A Design Guide; Butterworth-Heinemann: Woburn, UK, 2002. [Google Scholar]
- Wang, E.; Sun, G.; Zheng, G.; Li, Q. On multiaxial failure behavior of closed-cell aluminum foams under medium strain rates. Thin Walled Struct. 2021, 160, 107278. [Google Scholar] [CrossRef]
- Ali, H.; Gábora, A.; Naeem, M.M.; Kalácska, G.; Mankovits, T. Effect of the manufacturing parameters on the pore size and porosity of closed-cell hybrid aluminum foams. Int. Rev. Appl. Sci. Eng. 2021, 12, 230. [Google Scholar] [CrossRef]
- Commercial applications of metal foams: Their properties and production. Materials 2016, 9, 85. [CrossRef]
- Wang, E.; Sun, G.; Zheng, G.; Li, Q. Characterization of initial and subsequent yield behaviors of closed-cell aluminum foams under multiaxial loadings. Compos. Part B Eng. 2020, 202, 108247. [Google Scholar] [CrossRef]
- Martín, E.P. Microstructural parameters affecting the compressive response of closed-cell aluminum foams. Mech. Adv. Mater. Struct. 2021, 28, 1–20. [Google Scholar] [CrossRef]
- Grilec, K.; Bunjan, I.; Jakovljević, S. The influence of applied force on aluminium foams energy absorption. Teh. Vjesn. 2021, 28, 1388–1394. [Google Scholar] [CrossRef]
- Banhart, J. Production of Metal Foams. In Beaumont PWR; Zweben, C.H., Ed.; Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2018; pp. 347–363. [Google Scholar]
- Duarte, I.; Vesenjak, M.; Krstulović-Opara, L.; Anžel, I.; Ferreira, J.M. Manufacturing and bending behaviour of in situ foam-filled aluminium alloy tubes. Mater. Des. 2015, 66, 532–544. [Google Scholar] [CrossRef]
- Baumgärtner, F.; Duarte, I.; Banhart, J. Industrialization of Powder Compact Toaming Process. Adv. Eng. Mater. 2000, 2, 168–174. [Google Scholar] [CrossRef]
- Ghaleh, M.M.; Ehsani, N.; Baharvandi, H.R. High-Porosity Closed-Cell Aluminum Foams Produced by Melting Method Without Stabilizer Particles. Int. J. Met. 2021, 15, 899–905. [Google Scholar] [CrossRef]
- Matijašević-Lux, B. Characterisation and Optimisation of Blowing Agent for Making Improved Metal Foams; Technische Universität Berlin: Berlin, Germany, 2006; p. 108. [Google Scholar]
- Madhu, H.H.; Kailas, S.V. Fabrication of localised aluminium foam by a novel polymeric blowing agent. Mater. Charact. 2018, 142, 340–351. [Google Scholar] [CrossRef]
- Rajak, D.D.; Kumaraswamidhas, L.L.; Das, S. On the influence of porosity and pore size on AlSi17 alloy foam using artificial neural network. Ciencia. Tecnol. Mater. 2017, 29, 14–21. [Google Scholar] [CrossRef]
- Banhart, J.; Baumeister, J. Weber M Powder Metallurgical Technology for the Production of Metallic Foams Experimental procedure Preparation of metal foams. Proc. Eur. Conf. Adv. PM Mater. 1995, 201–208. [Google Scholar]
- Shiomi, M.; Imagama, S.; Osakada, K.; Matsumoto, R. Fabrication of aluminium foams from powder by hot extrusion and foaming. J. Mater. Process. Technol. 2010, 210, 1203–1208. [Google Scholar] [CrossRef]
- Papantoniou, I.I.; Pantelis, D.D.; Manolakos, D.E. Powder metallurgy route aluminium foams: A study of the effect of powder morphology, compaction pressure and foaming temperature on the porous structure. Procedia. Struct. Integr. 2018, 10, 243–248. [Google Scholar] [CrossRef]
- Stacey, M. Aluminium Recyclability and Recycling; Cwningen Press: Llundain, UK, 2015. [Google Scholar]
- Kumar, N.; Bharti, A. Review on Powder Metallurgy: A Novel Technique for Recycling and Foaming of Aluminium-Based Materials. Powder Met. Met. Ceram. 2021, 60, 52–59. [Google Scholar] [CrossRef]
- Duflou, J.J.; Tekkaya, A.A.; Haase, M.; Welo, T.; Vanmeensel, K.; Kellens, K.; Dewulf, W.; Paraskevas, D. Environmental assessment of solid state recycling routes for aluminium alloys: Can solid state processes significantly reduce the environmental impact of aluminium recycling? CIRP Ann. Manuf. Technol. 2015, 64, 37–40. [Google Scholar] [CrossRef]
- Gronostajski, J.; Marciniak, H.; Matuszak, A. New methods of aluminium and aluminium-alloy chips recycling. J. Mater. Process. Technol. 2000, 106, 34–39. [Google Scholar] [CrossRef]
- Hangai, Y.; Kobayashi, R.; Suzuki, R.; Matsubara, M.; Yoshikawa, N. Aluminum foam-filled steel tube fabricated from aluminum burrs of die-castings by friction stir back extrusion. Metals 2019, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, S.; Kobashi, M.; Kanetake, N. Producing technology of aluminum foam from machined chip waste. Mater. Trans. 2006, 47, 2125–2130. [Google Scholar] [CrossRef] [Green Version]
- Kanetake, N.; Kobashi, M.; Tsuda, S. Foaming behavior of aluminum precursor produced from machined chip waste. Adv. Eng. Mater. 2008, 10, 840–844. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.D.; Hou, S.; Yang, D.; Nieh, T.; Lu, Z. Cellular structure and energy absorption of Al–Cu alloy foams fabricated via a two-step foaming method. Mater. Des. 2020, 196, 1–8. [Google Scholar] [CrossRef]
- Raj, R.R.; Daniel, B.S.S. Prediction of compressive properties of closed-cell aluminum foam using artificial neural network. Comput. Mater. Sci. 2008, 43, 767–773. [Google Scholar] [CrossRef]
- Lela, B.; Krolo, J.; Grgić, K.; Sonja, J.; Dumanić, I. Production of Closed Cell Foams from the Aluminium Chip Waste; Croatian society for mechanical technologies: Split, Croatia, 2021; pp. 95–100. [Google Scholar]
- Dumanić, I.; Krolo, J.; Lela, B.; Jozić, S. Thermomechanical treatment of A380 aluminum alloy for globular thixo feedstock. Materwiss Werksttech 2020, 51, 1047–1057. [Google Scholar] [CrossRef]






| Specimen | Blowing Agent TiH2 | Temperature | Density | Relative Density |
|---|---|---|---|---|
| (wt%) | (°C) | (g/cm3) | ||
| 1 | 0.25 | 590 | 0.909 | 0.34 |
| 2 | 0.25 | 610 | 0.804 | 0.3 |
| 3 | 0.5 | 590 | 0.688 | 0.26 |
| 4 | 0.5 | 610 | 0.764 | 0.28 |
| Level of Exp. Run | Blowing Agent TiH2 | Foam Height | Temperature | Density | Energy Absorption | Plateau Stress | Rp0.2 | Pore Perimeter | Pore Inhomogeneity |
|---|---|---|---|---|---|---|---|---|---|
| (wt%) | (mm) | (°C) | (g/cm3) | (MJ/m3) | (MPa) | (MPa) | (mm) | (S.D.) | |
| 1 | 1.00 | 55.00 | 600.00 | 0.813 | 16.758 | 34.6 | 32.0 | 4.8 | 1.61 |
| 2 | 0.50 | 40.00 | 600.00 | 1.021 | 21.7823 | 23.5 | 58.0 | 4.3 | 2.05 |
| 3 | 1.00 | 55.00 | 600.00 | 0.764 | 14.6647 | 30.0 | 32.7 | 5.2 | 2.14 |
| 4 | 0.50 | 70.00 | 600.00 | 0.651 | 11.8967 | 25.0 | 25.2 | 6.2 | 2.61 |
| 5 | 1.00 | 40.00 | 590.00 | 1.148 | 31.3413 | 62.3 | 64.3 | 3.2 | 1.41 |
| 6 | 1.00 | 55.00 | 600.00 | 0.821 | 17.3468 | 35.7 | 35.7 | 5.4 | 2.91 |
| 7 | 1.00 | 40.00 | 610.00 | 0.993 | 22.3228 | 44.4 | 50.9 | 4.3 | 2.54 |
| 8 | 1.00 | 70.00 | 590.00 | 0.640 | 11.2354 | 23.5 | 25.4 | 5.5 | 1.90 |
| 9 | 1.50 | 40.00 | 600.00 | 0.930 | 19.8079 | 39.5 | 38.0 | 4.8 | 1.72 |
| 10 | 0.50 | 55.00 | 590.00 | 0.786 | 15.4102 | 29.5 | 38.7 | 5.4 | 3.33 |
| 11 | 1.50 | 55.00 | 590.00 | 0.753 | 14.8613 | 29.1 | 41.4 | 5.2 | 2.19 |
| 12 | 1.00 | 70.00 | 610.00 | 0.703 | 12.5716 | 25.4 | 25.3 | 4.8 | 2.20 |
| 13 | 1.50 | 55.00 | 610.00 | 0.749 | 17.5522 | 35.8 | 33.0 | 4.8 | 4.81 |
| 14 | 1.00 | 55.00 | 600.00 | 0.811 | 15.6664 | 32.6 | 23.7 | 5.1 | 2.59 |
| 15 | 1.00 | 55.00 | 600.00 | 0.777 | 15.6003 | 32.1 | 30.1 | 6.1 | 2.87 |
| 16 | 0.50 | 55.00 | 610.00 | 0.853 | 11.269 | 24.8 | 14.4 | 7.4 | 2.11 |
| 17 | 1.50 | 70.00 | 600.00 | 0.610 | 10.8603 | 21,8 | 25.9 | 5.2 | 2.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krolo, J.; Lela, B.; Grgić, K.; Jozić, S. Production of Closed-Cell Foams Out of Aluminum Chip Waste: Mathematical Modeling and Optimization. Metals 2022, 12, 933. https://doi.org/10.3390/met12060933
Krolo J, Lela B, Grgić K, Jozić S. Production of Closed-Cell Foams Out of Aluminum Chip Waste: Mathematical Modeling and Optimization. Metals. 2022; 12(6):933. https://doi.org/10.3390/met12060933
Chicago/Turabian StyleKrolo, Jure, Branimir Lela, Karla Grgić, and Sonja Jozić. 2022. "Production of Closed-Cell Foams Out of Aluminum Chip Waste: Mathematical Modeling and Optimization" Metals 12, no. 6: 933. https://doi.org/10.3390/met12060933
APA StyleKrolo, J., Lela, B., Grgić, K., & Jozić, S. (2022). Production of Closed-Cell Foams Out of Aluminum Chip Waste: Mathematical Modeling and Optimization. Metals, 12(6), 933. https://doi.org/10.3390/met12060933








