3.2. Thermal Analysis
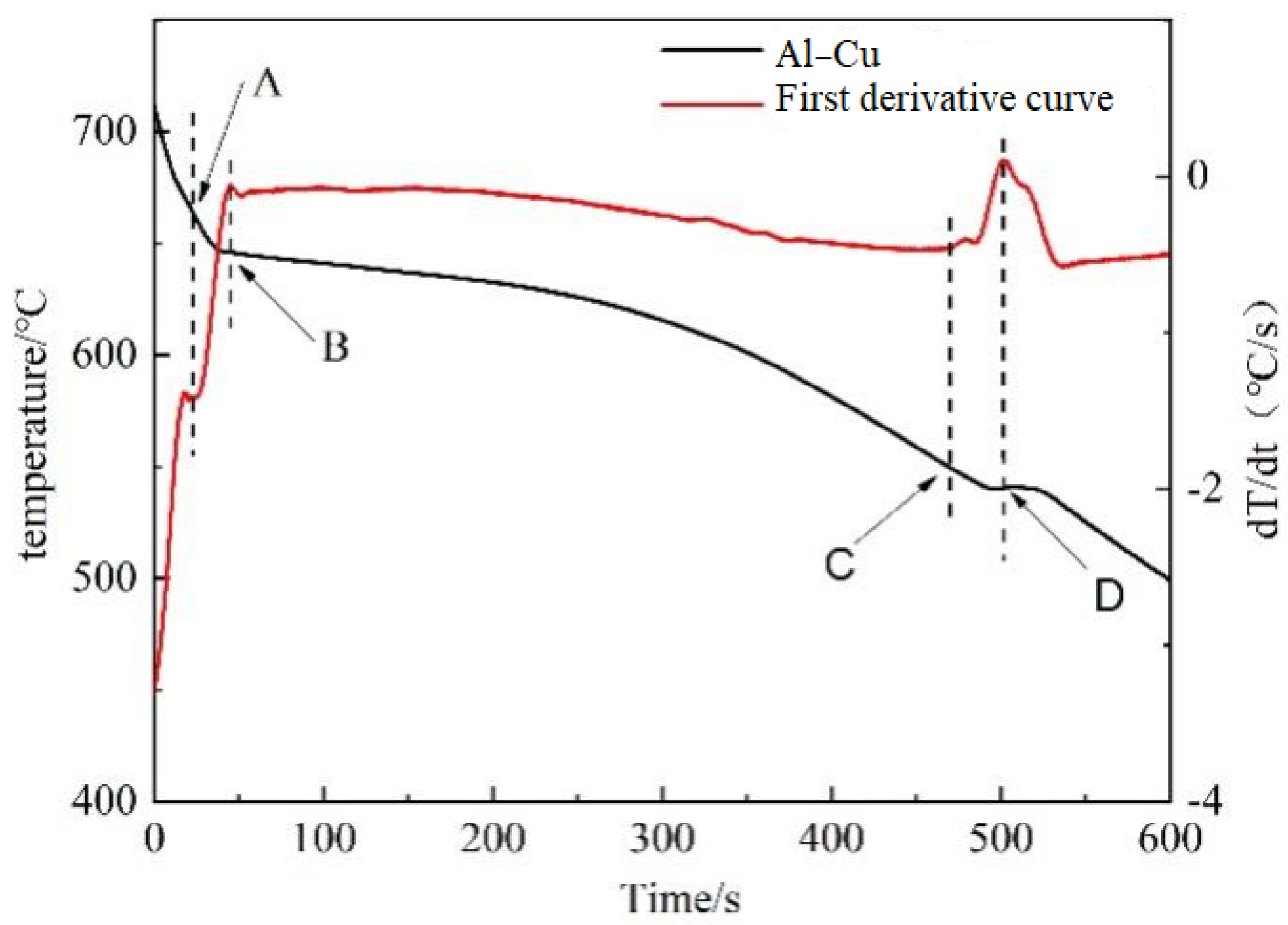
Figure 3 shows the typical solidification curve and first-order differential curve of the ZL205 alloy: A clear turn in the first-order differential curve was observed, and corresponding exothermic peaks were generated. The first exothermic peak (point AB) corresponds to the crystallization process of α-Al. Point A corresponds to the initial nucleation temperature of α-Al, and point B corresponds to the nucleation growth temperature of α-Al. The second exothermic peak (point CD) corresponds to the crystallization process of the CuAl2 phase, and points C and D represent the initial nucleation temperature and growth temperature of the CuAl
2 phase, respectively. On the other hand, the temperature interval between the two points corresponds to the crystallization interval of the corresponding phase.
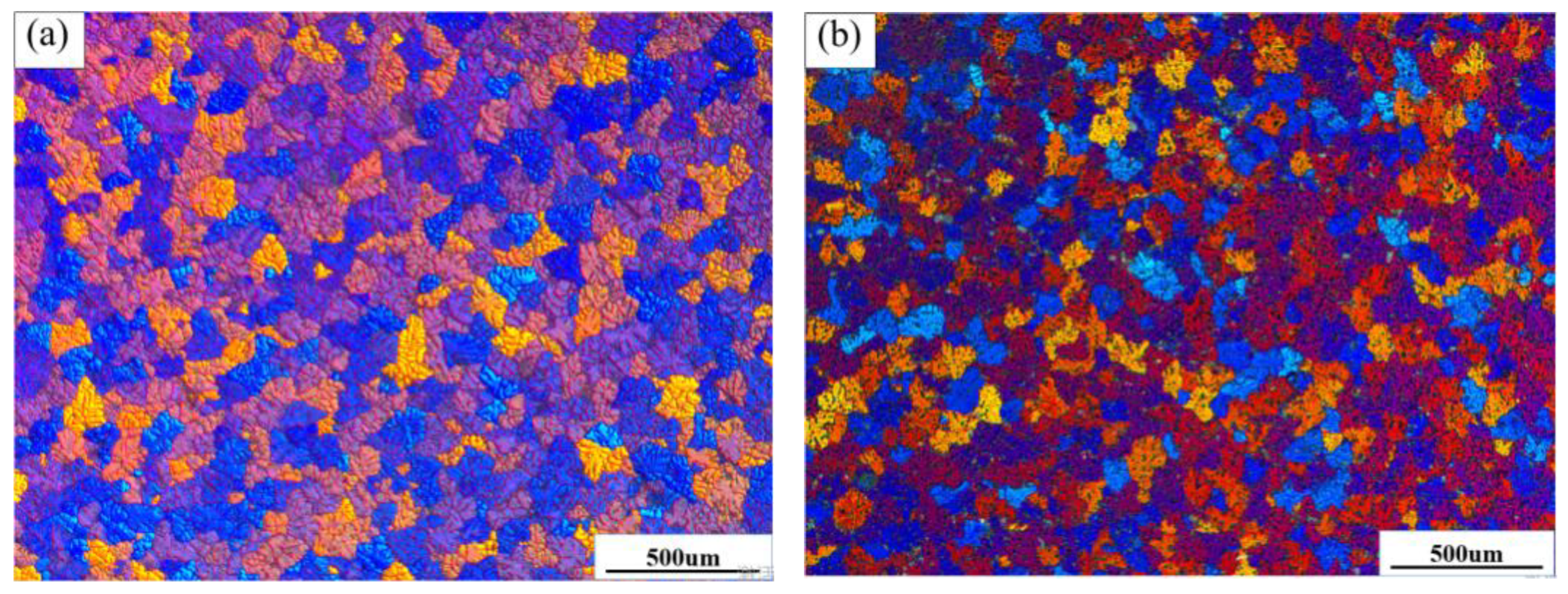
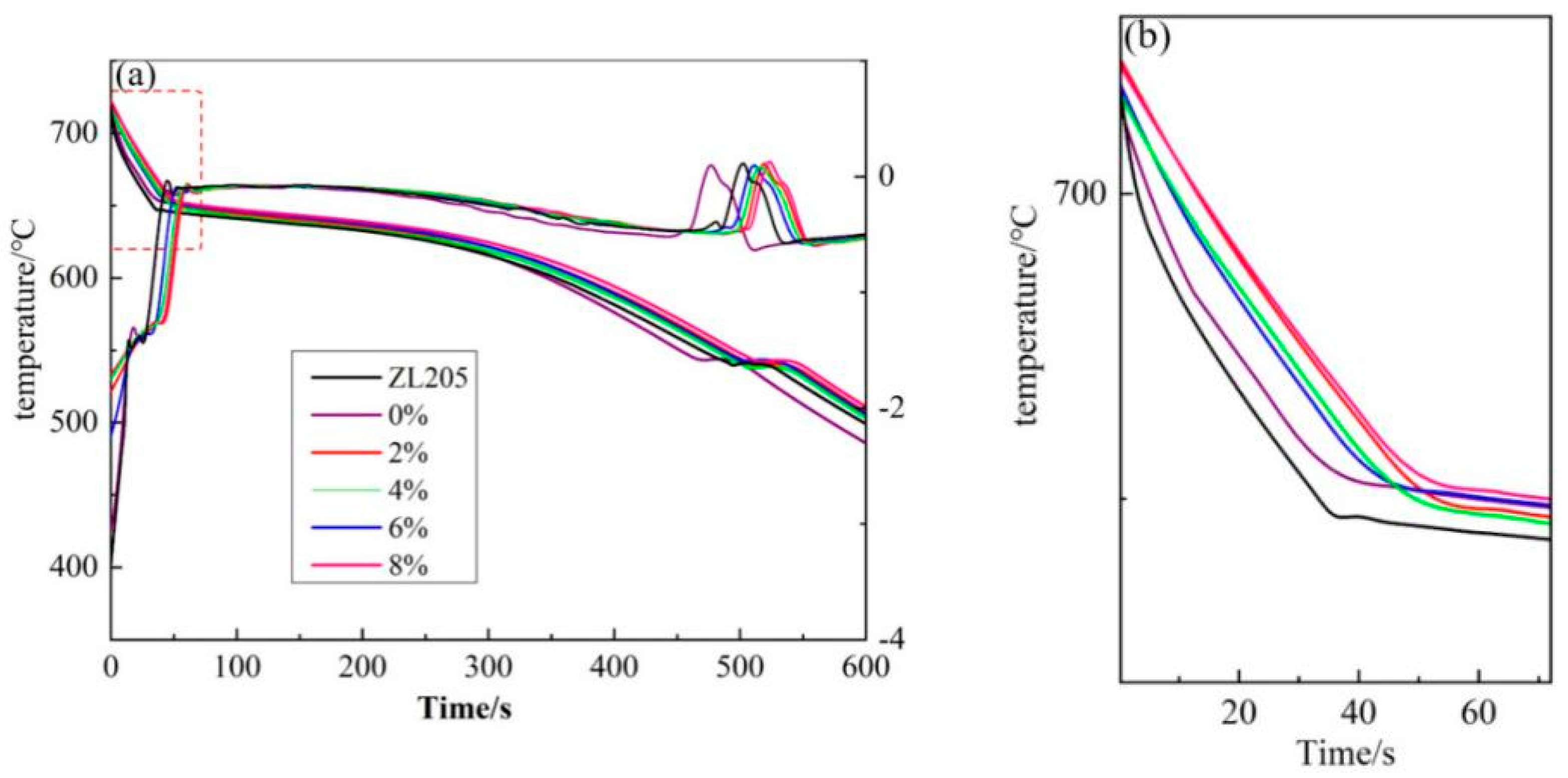
Figure 4 shows the thermal analysis curves of the ZL205 alloy with 0.3 wt% of different Er
2O
3 refiners, and
Figure 4b shows the local enlargement: As the Al
3Ti and TiC phases in the refiners can be used as the core of α-Al heterogeneous nucleation, the increase in the heterogeneous core reduced the supercooling degree required in the nucleation of the ZL205 alloy and improved the nucleation rate of the alloy. The release of a large amount of latent heat of crystallization during nucleation decreases the solidification rate of α-Al during solidification.
Table 3 shows the solidification characteristic parameters of different specimens corresponding to the first-order differential curves. The initial nucleation temperature (
) of α-Al was 661 °C. By using the Al-TiO
2-C refiner without Er
2O
3, the initial nucleation temperature of α-Al was increased to 666.7 °C, thereby increasing the non-equilibrium liquid-phase line of the alloy. After the addition of the Al-TiO
2-C refiner containing Er
2O
3, the turning point in the first-order differential curve corresponding to the α-Al nucleation disappeared, indicating two rising curves with different slopes, because the rare-earth Al
20Ti
2Er phase generated free rare-earth Er at the reaction temperature [
15], and the effect of rare-earth Er causes the initial nucleation process of α-Al to happen earlier and be longer in duration. However, due to the temperature selection problem, the exact nucleation temperature was not observed in the first-order differential curve.
After the addition of refiners, the eutectic CuAl
2-phase starting nucleation (
) temperature increased overall, and the nucleation temperature reached the maximum value of 552.3 °C after the addition of the Al-TiO
2-C-6%Er
2O
3 refiner. The increase in the nucleation temperature reduced the subcooling required for nucleation. Studies have reported that the eutectic nucleation rate is positively correlated with subcooling [
16] and that the alloy nucleation rate is low in the case of low subcooling. With the increase in the Er
2O
3 content of the refiner, the difference between the growth temperature of the CuAl
2 phase (
) and the starting nucleation temperature Δ
T increased significantly, and the crystallization interval △T of the ZL205 alloy was only 7.4 °C without the refiner (
Table 3). By the addition of the Al-TiO
2-C refiner, the crystallization temperature of the CuAl
2 phase started to increase up to 543.3 °C, and the crystallization interval increased to 7.7 °C. With the increase in the Er
2O
3 content of the refiner, the crystallization interval further increased and reached the maximum value of 9.3 °C by the addition of 0.3 wt% of Al-TiO
2-C-6%Er
2O
3; this value is greater than that of the alloy without the refiner. Compared with the ZL205 alloy without refiner, the solidification temperature of CuAl
2 phase increased by 1.9 °C; after the continuous increase in the Er
2O
3 content, the interval did not grow continuously, probably because the refinement effect was not further improved by the addition of 8% Er
2O
3, while the content of Al
5Er
3O
12 impurity phase increased [
17]. During the solidification process, the impurity phase segregates at the liquid-phase solidification front, hindering the generation of the CuAl
2 phase and causing a slight decrease in the nucleation temperature at the beginning of the CuAl
2 phase.
3.3. Effect of the Al-TiO2-C-Er2O3 Refiners on the Corrosion Resistance of the ZL205 Alloy
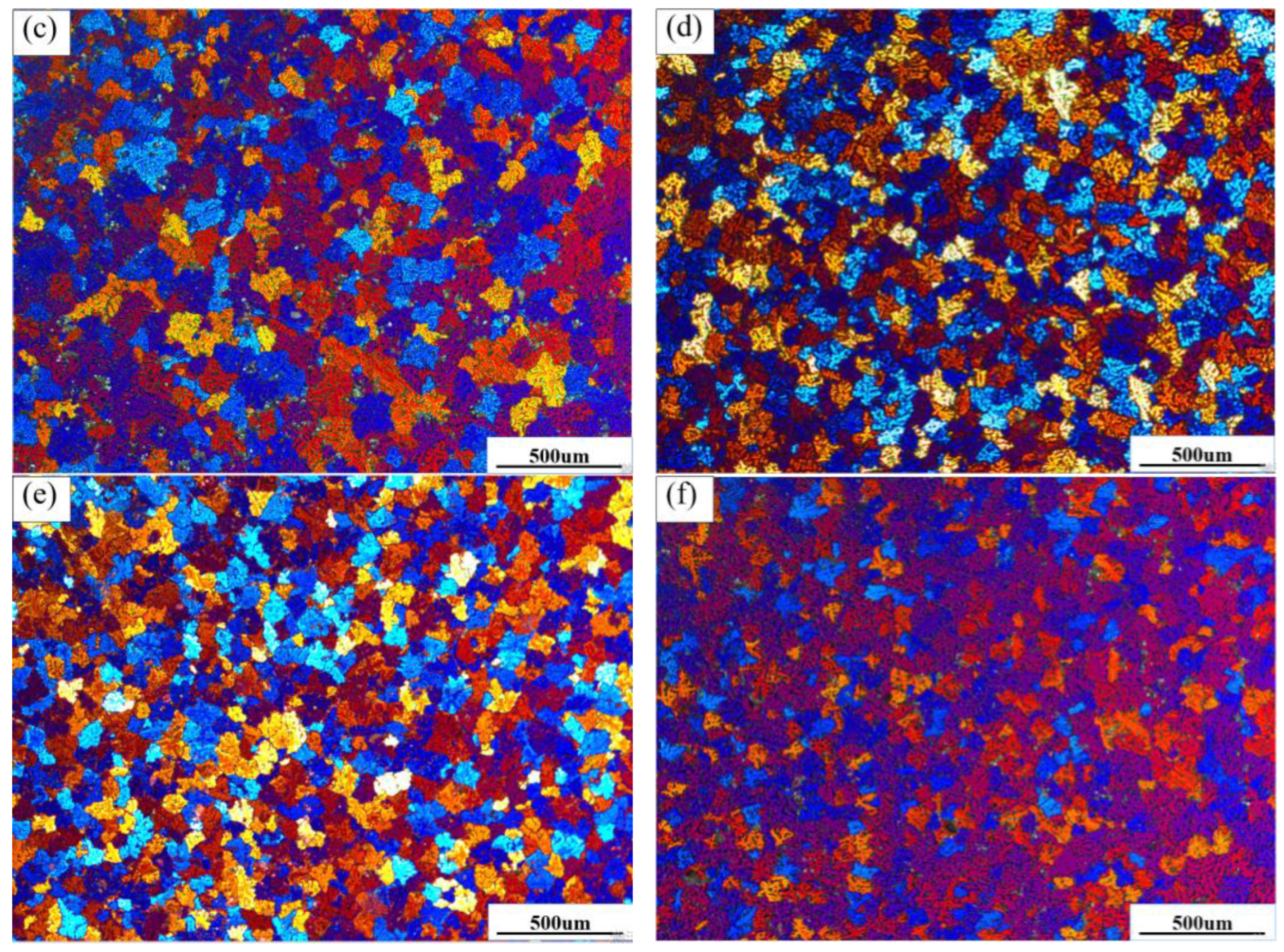
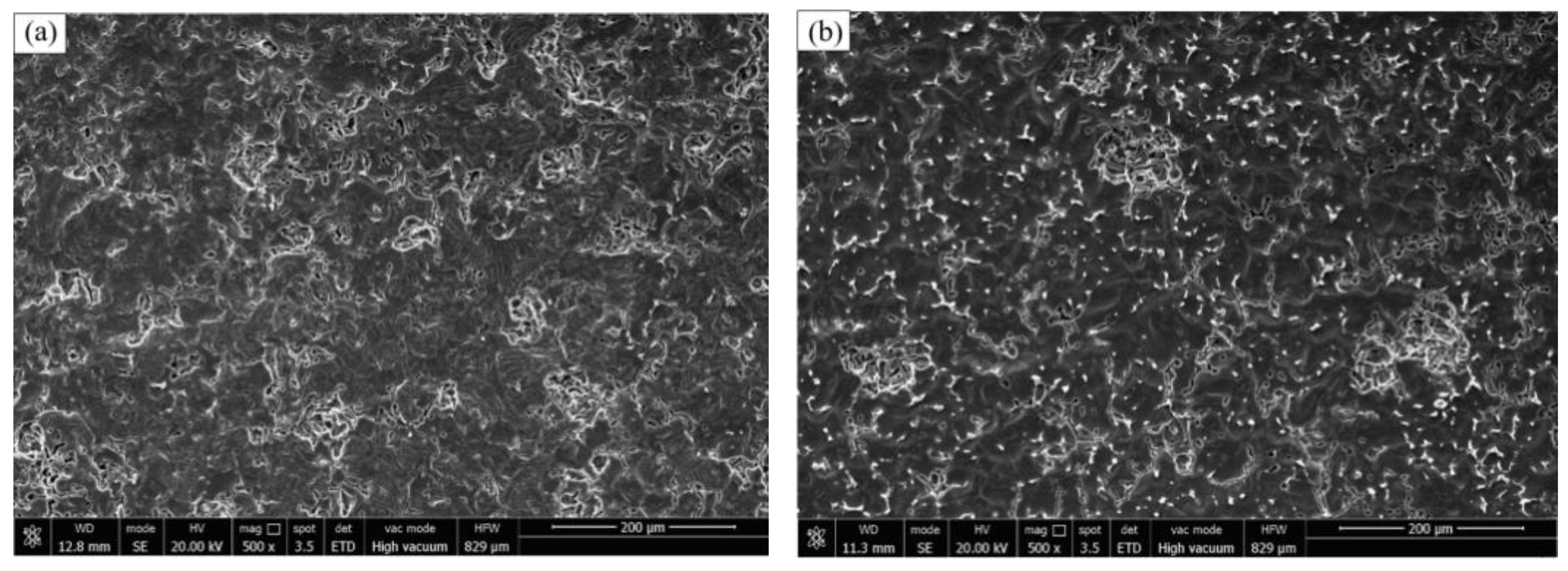
Figure 5a–f show the surface morphology of the ZL205 alloy by the addition of the Al-TiO
2-C-XEr
2O
3 refiner after corrosion in a 3.5% NaCl solution for 30 days to remove the corrosion products. The CuAl
2 phase distributed along the grain boundary in the ZL205 alloy was shed during corrosion, and a small amount of pitting also was observed after corrosion. Relatively large corrosion pits were observed on the ZL205 alloy surface without the refiner, and a low number of pits were connected to form larger corrosion pits. A low number of pits were also found to be diffusely distributed around the matrix or grain boundaries. By the addition of the Al-TiO
2-C refiner, the number of pits on the ZL205 surface was significantly reduced, and a low number of granular CuAl
2-phase precipitates were observed at the grain boundaries, thereby significantly improving the corrosion resistance of the ZL205 alloy. By the addition of the Al-TiO
2-C-6%Er
2O
3 refiner to the ZL205 alloy, fewer corrosion pits were observed (
Figure 5e), IGC was significantly reduced, a high number of unexfoliated CuAl
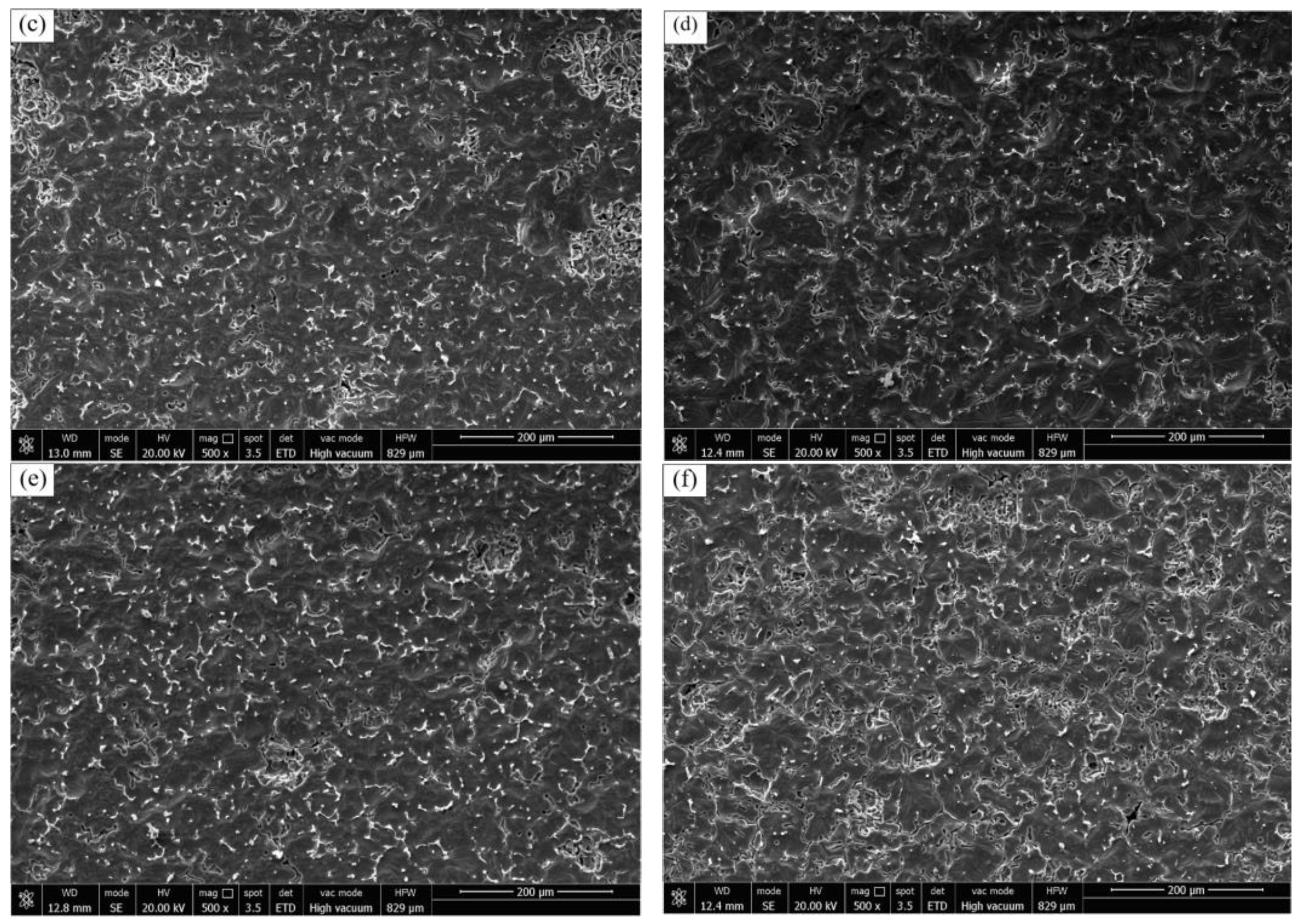
2 phases were observed at the grain boundaries, and the depth of the pits was significantly lower than that of the ZL205 alloy without the refiner. Only marginal localized IGC was caused by the exfoliation of the CuAl
2 phase with the continuous increase in the Er
2O
3 content of the refiner (
Figure 5f), and the number of corrosion pits compared with that of the ZL205 alloy after the addition of the Al-TiO
2-C-6%Er
2O
3 refiner tended to increase, but the corrosion pits did not become larger, but converted to continuous IGC with a diffuse distribution in the matrix. The grain boundaries at the large continuous CuAl
2 phase disappeared, and only a low amount of the granular CuAl
2 phase was present. The corrosion hole was deeper than that of the alloy with a 6% Er
2O
3 refining agent, but, compared with that of the ZL205 alloy corrosion, it was shallow.
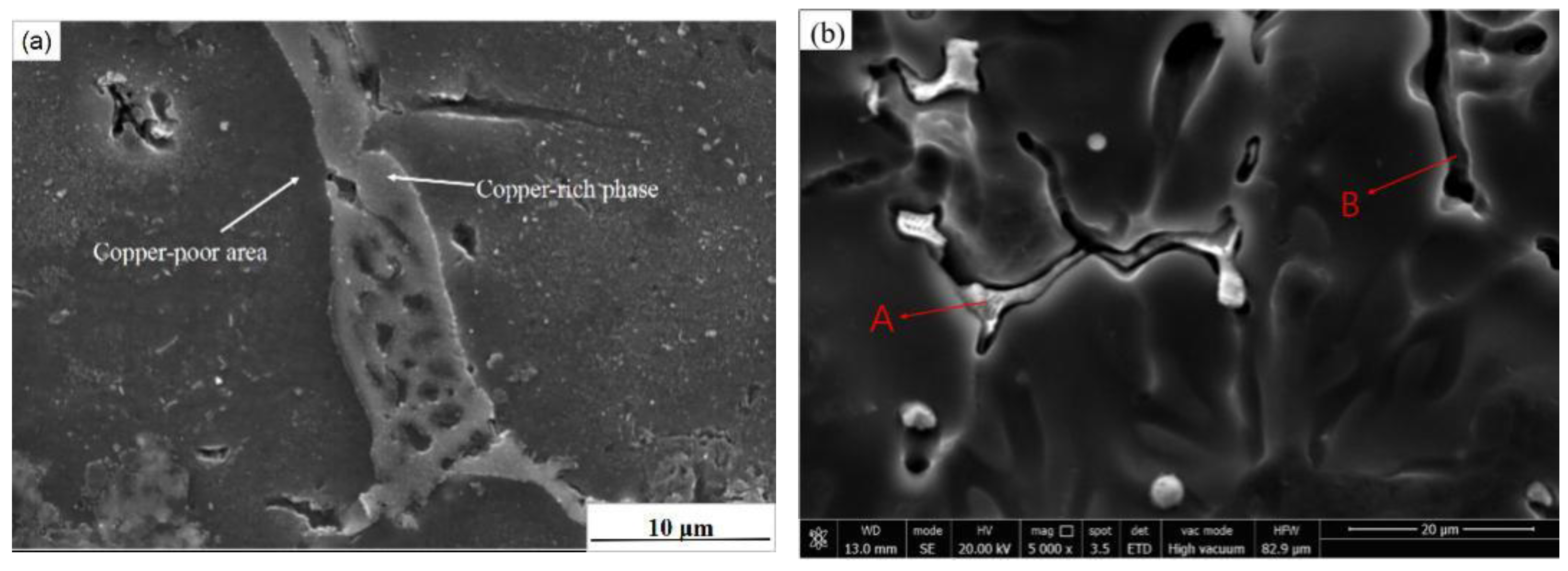
A typical reticulated CuAl
2 phase in the ZL205 alloy was observed as the eutectic reaction is not possible in alloys that are thermodynamically less than 5.8%, but in the ZL205 alloy under practical conditions, the critical Cu solubility and eutectic point also shifted due to the presence of other alloying elements (Mn, Ti, Cd, etc.) and non-stationary cooling (
Figure 6a). As a result, the non-equilibrium eutectic CuAl
2 phases were formed, and these CuAl
2 phases precipitated at grain boundaries, thereby increasing the susceptibility of the alloy to IGC.
IGC occurred mainly due to the low diffusion rate of Cu in aluminum solutions (
Figure 6a), the net CuAl
2 phase precipitated at the grain boundaries corresponded to the Cu-rich zone, and on both sides, a Cu-poor zone was present. When an alloy is in a corrosive environment, a Cu-rich phase (CuAl
2 phase) in a 3.5% NaCl solution potential is generally −0.61 V, while the conventional potential of the 2-series aluminum alloy substrate is generally −0.81 to −0.86 V [
18]; hence, there is a clear potential difference between the copper-rich area and its surrounding copper-poor area. In the local corrosion process, the copper-rich phase as the cathode and the surrounding copper-poor area constituted galvanic corrosion, leading to the anodic dissolution of the surrounding copper-poor area and the formation of IGC.
Figure 6b shows the scanned image of the ZL205 alloy in the NaCl solution after 30 days of corrosion. The CuAl
2 phase in the A region was relatively intact after corrosion, and the surrounding Al matrix was corroded seriously, while for the B region, owing to severe corrosion, when the surrounding Al matrix corroded to a certain depth, the CuAl
2 phase was completely exposed, subsequently falling off after the cleaning of the corrosion product and eventually forming a long deep pit at the grain boundary. A small amount of the CuAl
2 phase was precipitated in the form of plasmas, and a similar corrosion occurred.
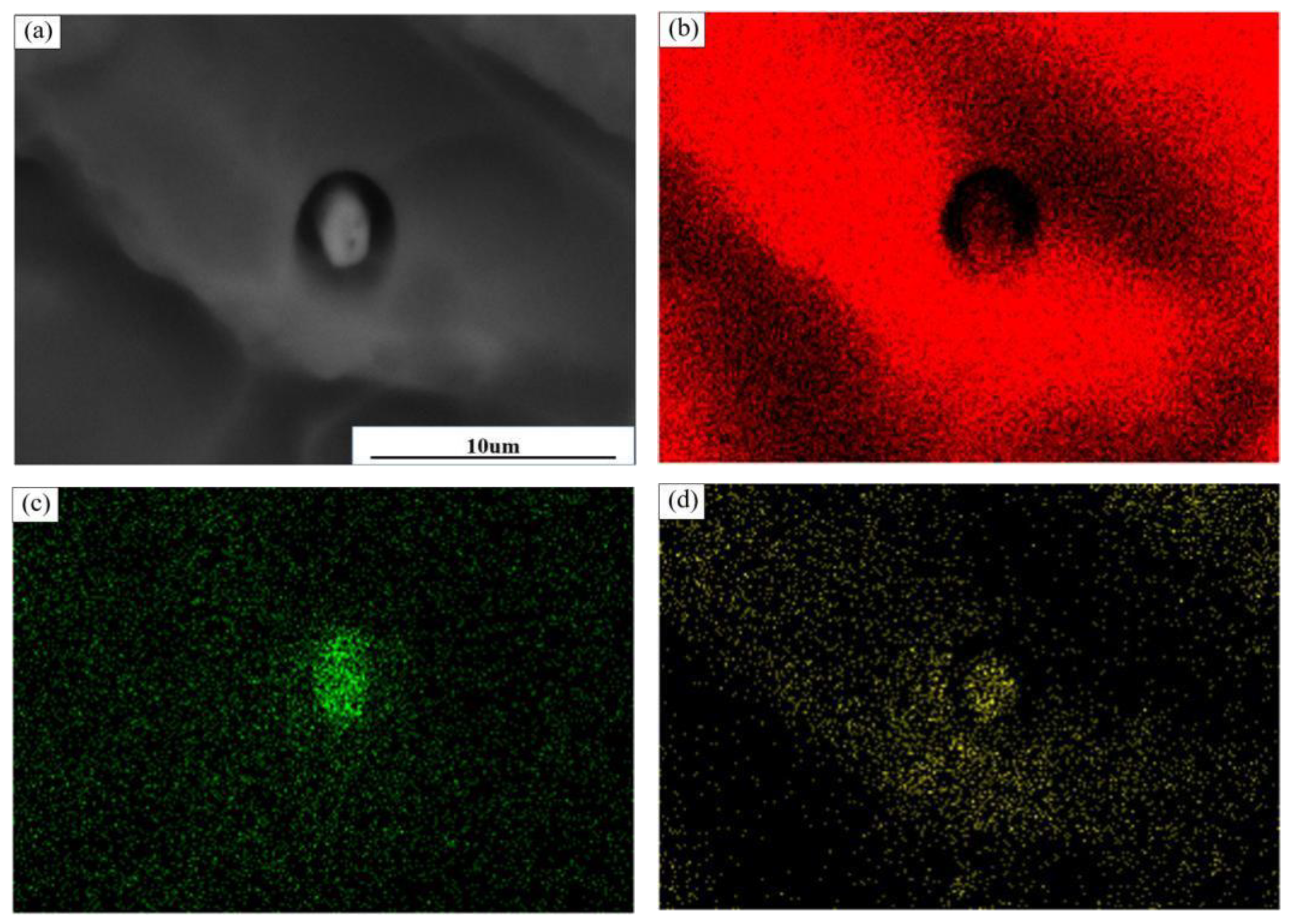
Figure 7 shows the change in the morphology from the intermetallic compound to plasmas after corrosion, and
Figure 7b–d shows the distribution of each element. It can be seen that there are obvious deep pits around the particles; the deep pits at the Al element are significantly reduced, and its corrosion starts from around the particles. There is an obvious potential difference between the Cu-poor area around the particles and the particles containing Cu elements, so that the Cu-poor area around the particles has corrosion priority; with the increase in corrosion time, corrosion gradually extends downward, and finally around the particles, the formation of craters occurs, as shown in the figure.
In addition to being sensitive to IGC, the ZL205 alloy is also sensitive to pitting corrosion, which forms an un-dense oxide film layer on its surface. Meanwhile, the biased CuAl
2, second-phase particles, and some impurities affect the integrity of the oxide film, but the large amount of enriched Cl
− destroys the passivation film of the material and eventually leads to pitting corrosion. The corrosion products mainly consist of Al(OH)
3. The reduction of O
2 and H
2O mainly occurs at the cathode, while the dissolution of Al mainly occurs at the anode. The redox of oxygen occurs mainly as follows:
Owing to the destruction of the passivation film, the Al matrix around the particles was highly soluble in the corrosive environment, generating Al
3+ and eventually reacting with the OH
− generated at the cathode to form an Al(OH)
3 precipitate. The reaction was as follows:
A large amount of OH
− and Al
3+ generated Al(OH)
3 precipitates, which were attached on the outside of the etch pit, and these precipitates halted the exchange of material inside and outside the pit. A high amount of Cl
− was present inside the pit, while Al
3+ hydrolysis generated a large number of H
+ to reduce the internal pH, resulting in the dissolution of the internal matrix Al; it eventually expanded the pit continuously, because pitting occurred mostly at the grain boundary precipitation. A part of the pitting pits connected together after a certain period of development or with IGC. These pitting pits converged together, the corrosion products were cleaned, and the grain boundary near the corrosion of the serious CuAl
2 phase and intergranular corrosion and the joint action of pitting corrosion were produced, as
Figure 5a, in the larger corrosion pits.
3.4. Electrochemical Analysis of the ZL205 Alloy by the Al-TiO2-C-XEr2O3 Refining Agent
Figure 8 shows the dynamic polarization curve of the ZL205 alloy in a 3.5% NaCl solution after the addition of the Al-TiO
2-C-XEr
2O
3 refiner: The shape of the polarization curve was the same, and the polarization curve shifted to the left, indicating that the overall corrosion rate after the addition of the refiner tends to reduce the dynamic polarization curve parameters for the Tafel extrapolation simulation of the self-corrosion current.
Table 4 summarizes the results of the self-corrosion current density (i
corr) and self-corrosion potential (E
corr). Compared with that of the original ZL205 alloy, the self-corrosion potential of the ZL205 alloy increased after the addition of the refiner because the refiner improved the porosity, segregation, and other defects of the ZL205 alloy and reduced its corrosion sensitivity. With the increase in the Er
2O
3 content of the Al-TiO
2-C-XEr
2O
3 refiner, the self-corrosion current density first reduced and then increased, reaching a minimum of 6.26 × 10
−5 A/cm
2 by the addition of the Al-TiO
2-C-6%Er
2O
3 refiner; this value is about 77.8% less than that of the ZL205 alloy without the refiner (2.83 × 10
−5 A/cm
2). This result indicated that the corrosion rate of the material in the 3.5% NaCl solution is slower and that the corrosion resistance is better because, when the Er
2O
3 content of the refining agent is 6%, the refining agent exerts the best effect on alloy refinement, and rare-earth Er plays a purifying role on the material; simultaneously, the CuAl
2 phase precipitated at the grain boundaries was evenly distributed, the number of active atoms then increased, the ability of the material surface passivation film generation increased, and the final material corrosion resistance was enhanced. Moreover, at an extremely high Er
2O
3 content of the refiner, the corrosion resistance of the alloy was reduced because of the excessive Al
5O
12Er
3 particles in the refiner, which increased the corrosion sites in the alloy.
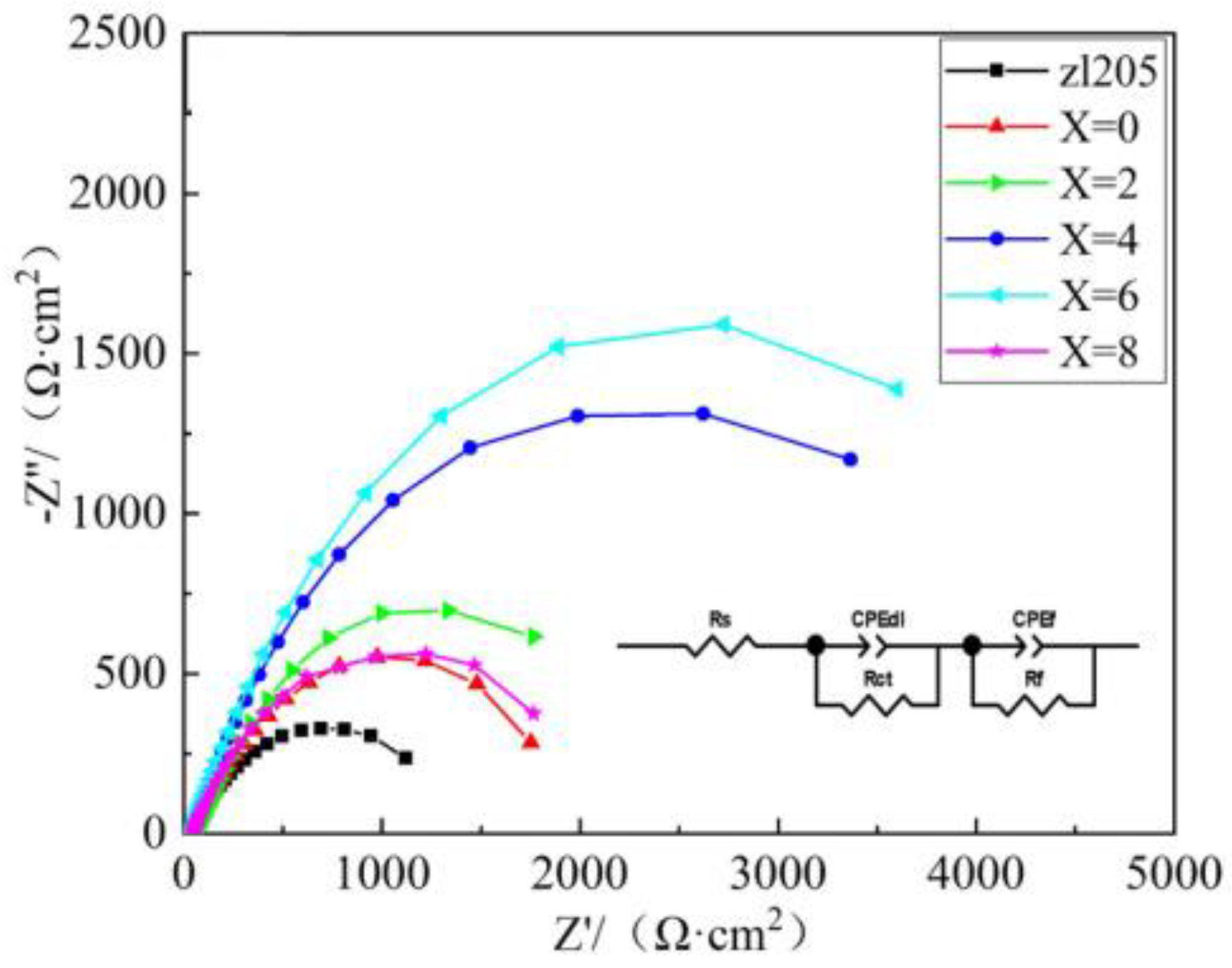
Figure 9 shows the Nyquist plot of the ZL205 alloy in a 3.5% NaCl solution: the Nyquist plots of all specimens revealed a high-frequency region of a capacitive resistance arc, indicating that in the test process, the specimen and corrosion solution produced a charge transfer, and the generated passivation zone was not clear, which is consistent with the polarization curve measurement results.
Figure 10 shows the fitting results of the EIS data for fitting: Without the addition of the refining agent, the ZL205 alloy exhibited the lowest polarization resistance, and with the increase in the Er
2O
3 content of the refining agent, the polarization resistance first increased and then decreased. In particular, at an Er
2O
3 content of 6% in the refining agent, the impedance was 1808.62 Ω·cm
2, and that of the original ZL205 alloy was 480.42 Ω·cm
2, corresponding to a difference of 376.7%. With the increase in the Er
2O
3 content to 8% in the Al-TiO
2-C-Er
2O
3 refiner, the impedance decreased significantly. A high impedance reflected that the solution current density is low; therefore, for the ZL205 alloy, with an Er
2O
3 content of 6% in the refiner, the alloy corrosion process exhibited the best inhibition effect, which was consistent with the polarization curve and corrosion morphology observation.