Actual Marine Atmospheric Pre-Corrosion Fatigue Performance of 7075-T73 Aluminum Alloy
Abstract
:1. Introduction
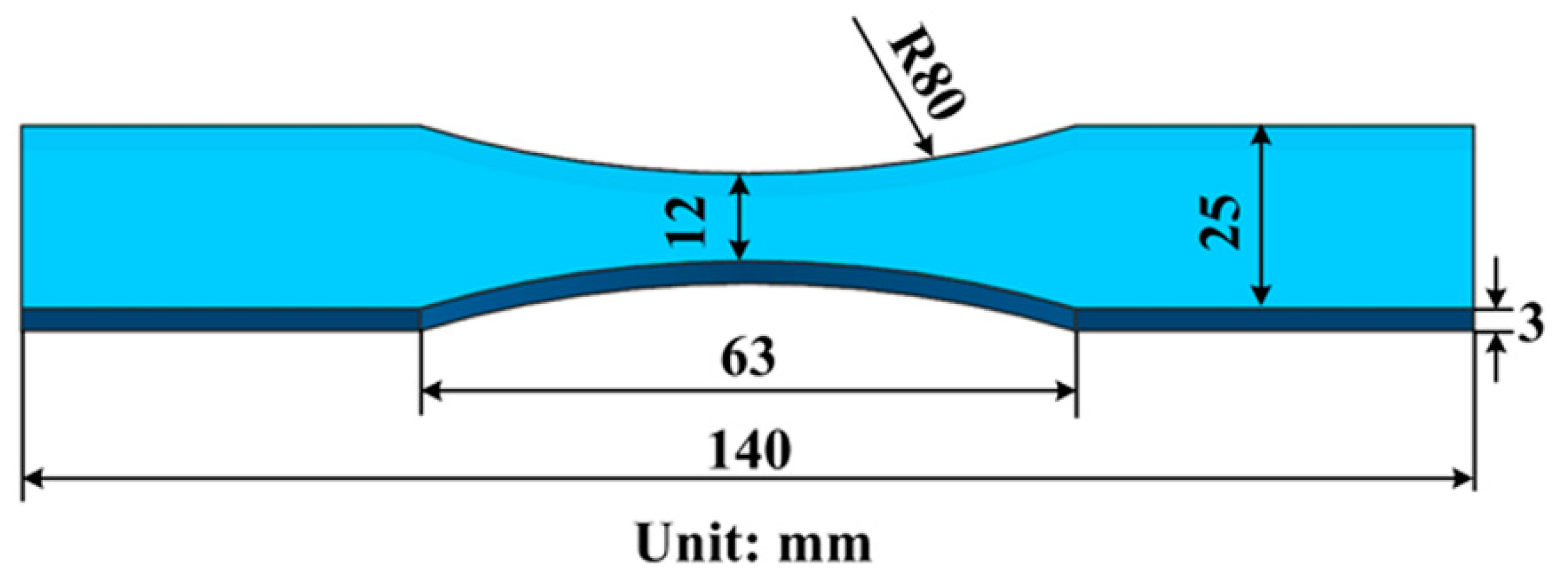
2. Experimental Procedure
3. Results and Discussion
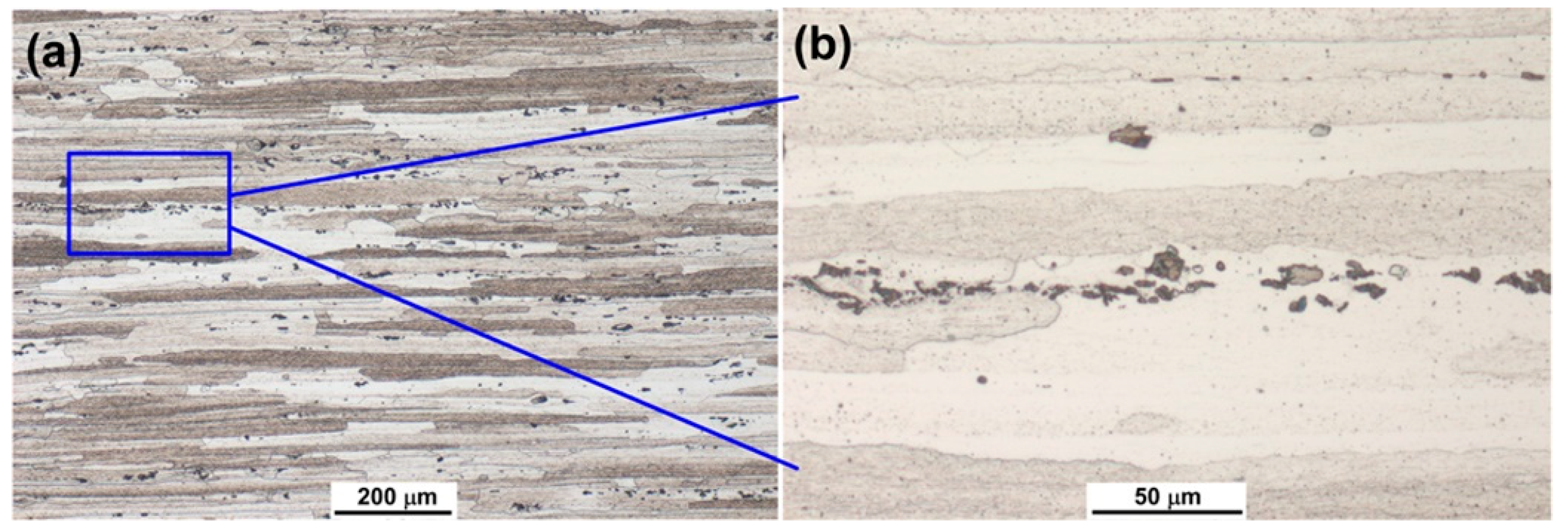
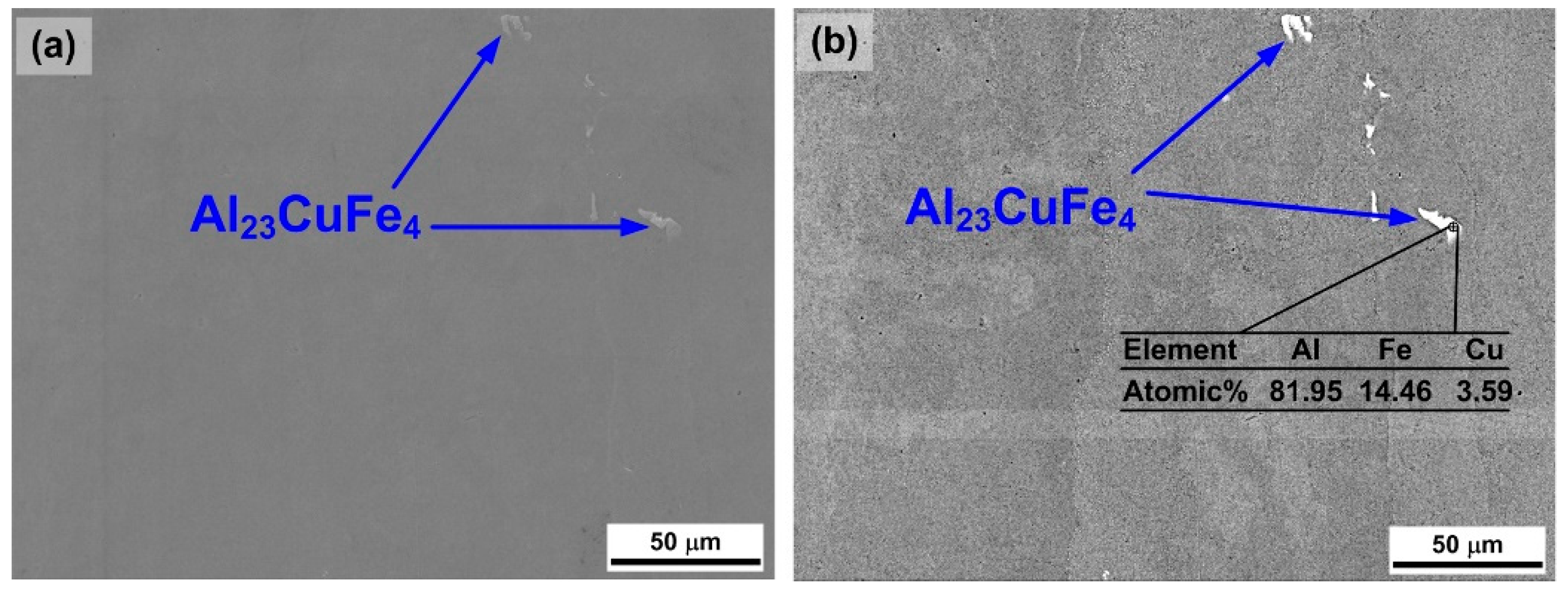
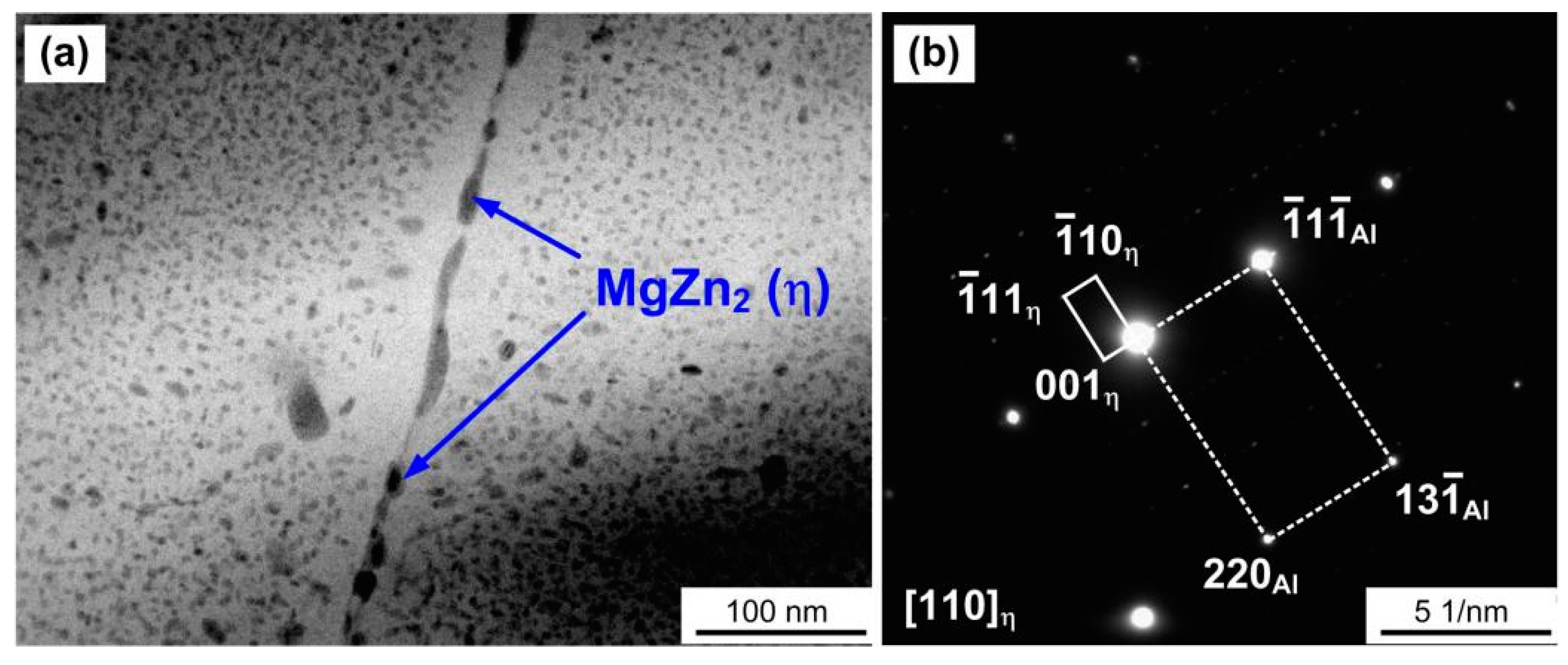
3.1. Microstructures of As-Received 7075 Aluminum Alloy
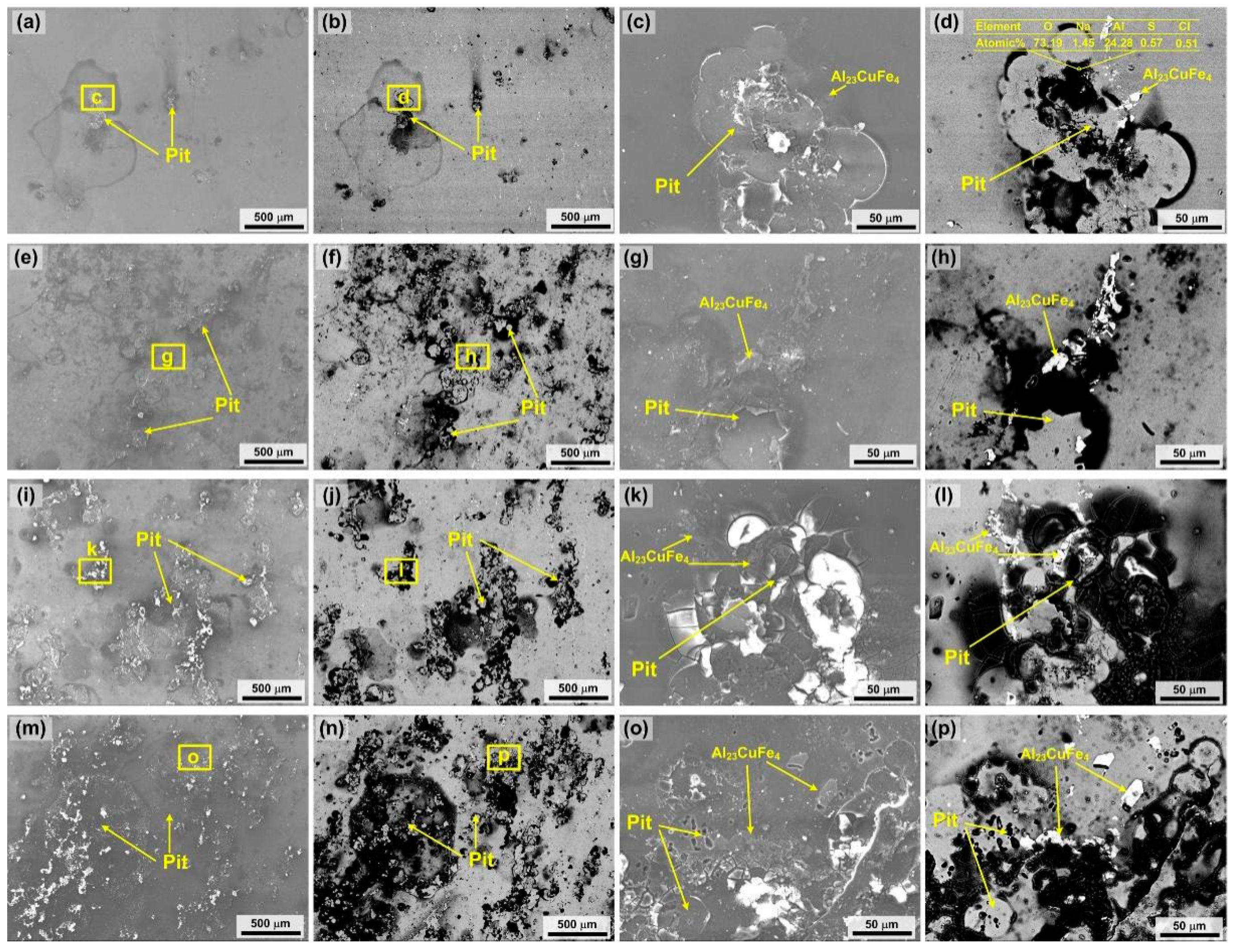
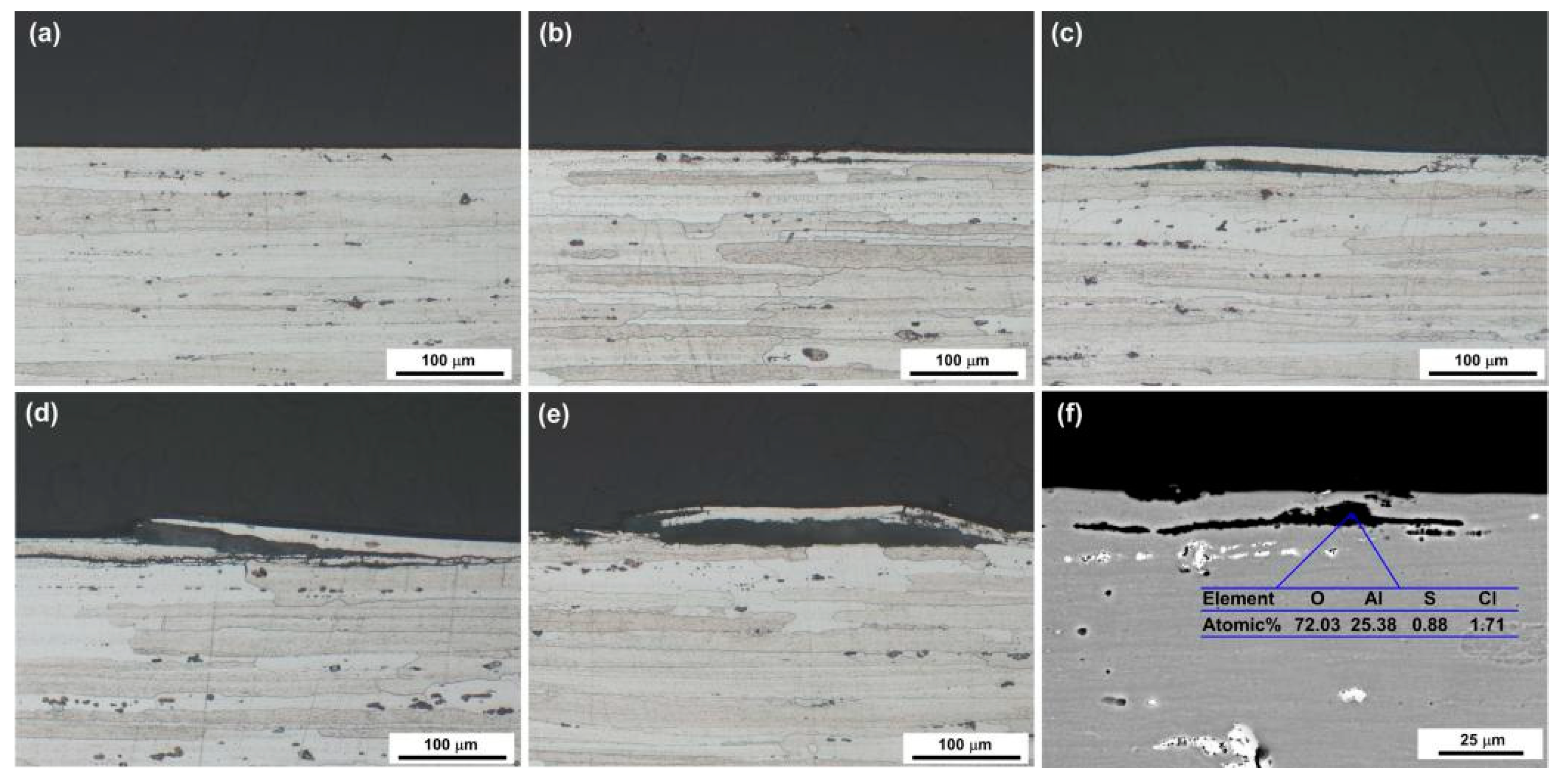
3.2. Corrosion Characteristics
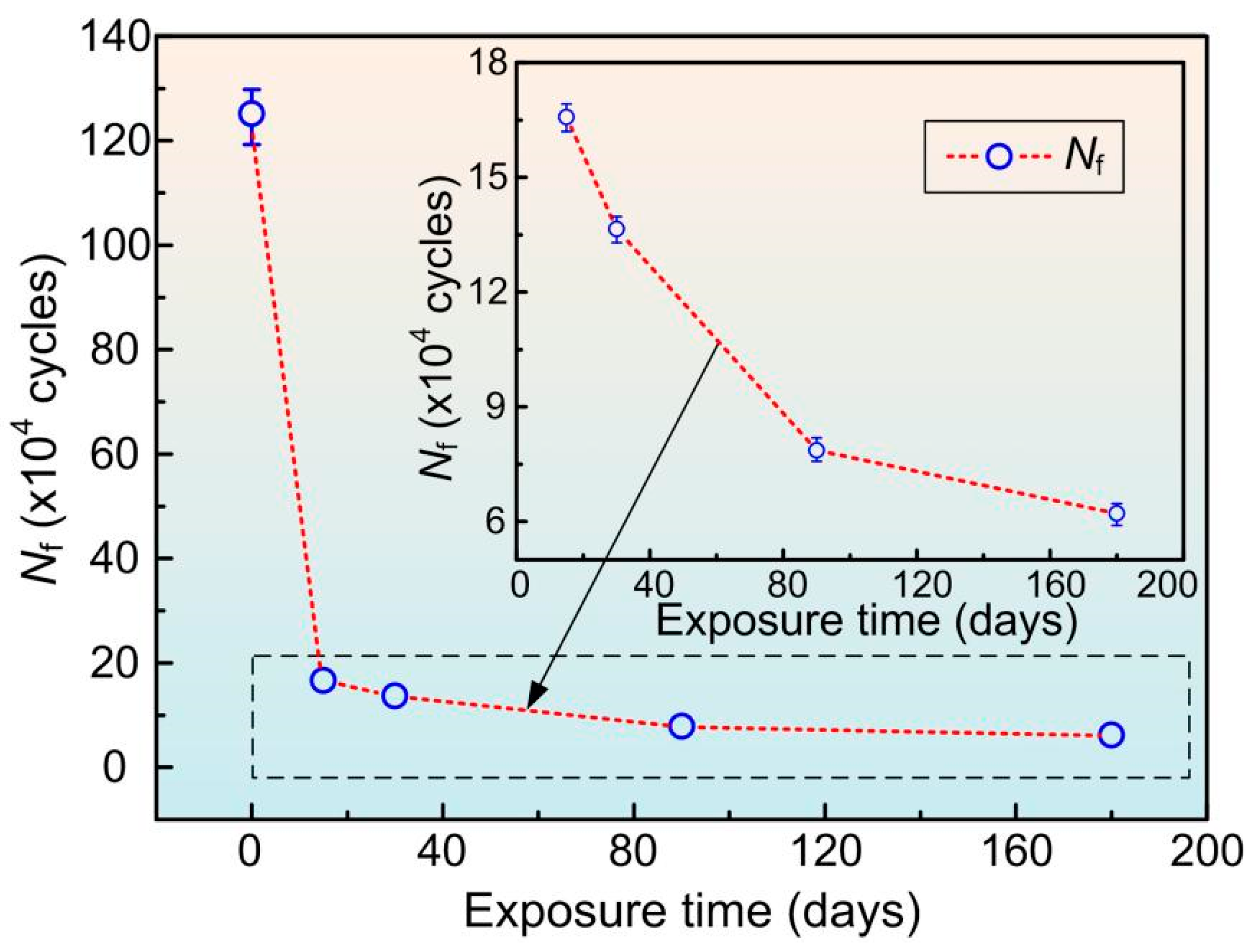
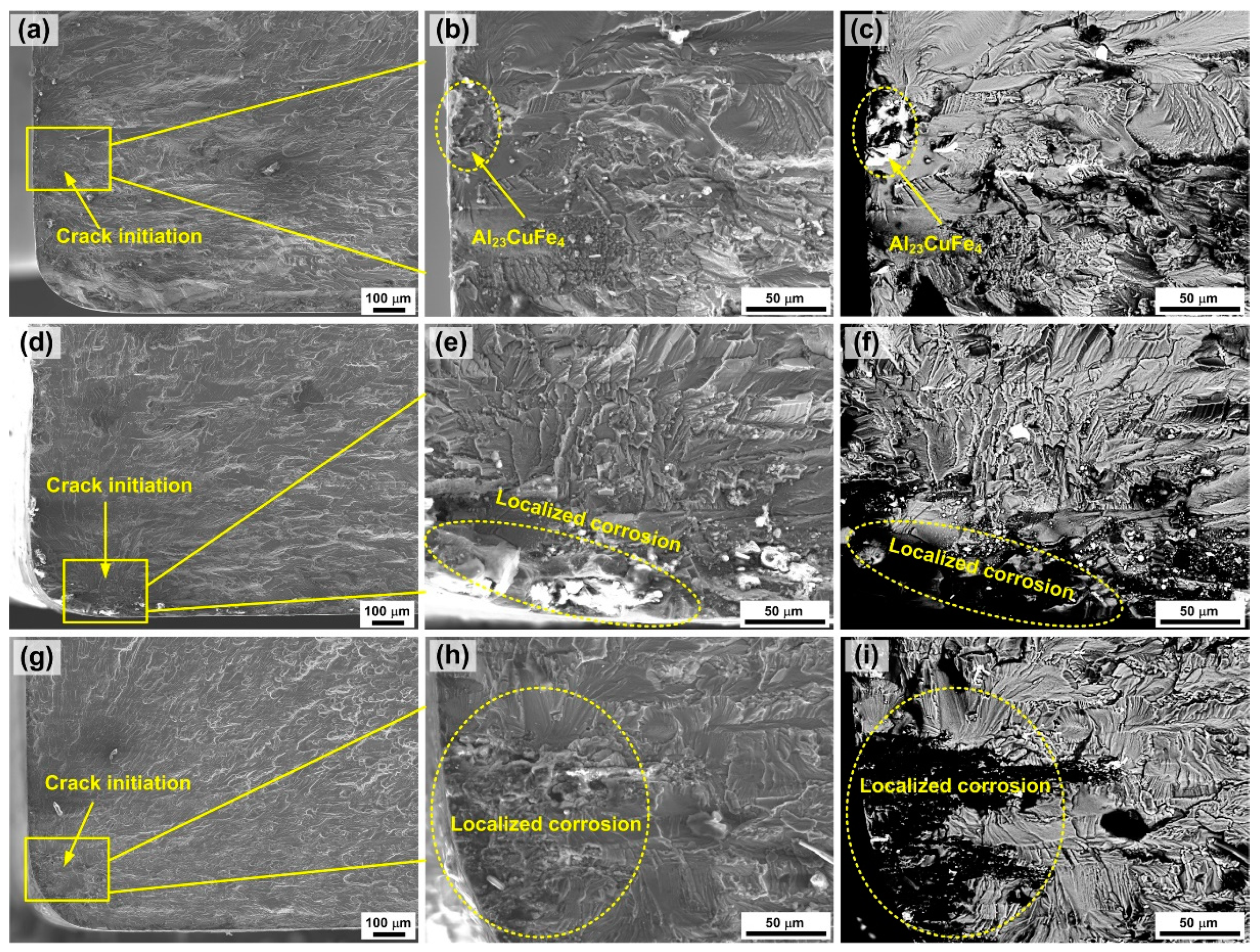
3.3. Fatigue Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, B.; Zhang, S.G. The advancement of 7xxx series aluminum alloys for aircraft structures: A review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Li, Y.; Retraint, D.; Gao, P.; Xue, H.; Gao, T.; Sun, Z. Effect of surface mechanical attrition treatment on torsional fatigue properties of a 7075 aluminum alloy. Metals 2022, 12, 785. [Google Scholar] [CrossRef]
- Jung, D.K.; Ha, S.H.; Kim, H.K.; Shin, Y.C. Determination of plastic anisotropy of extruded 7075 aluminum alloy thick plate for simulation of post-extrusion forming. Metals 2021, 11, 641. [Google Scholar] [CrossRef]
- Chen, F.; Qu, H.; Wu, W.; Zheng, J.H.; Qu, S.; Han, Y.; Zheng, K. A physical-based plane stress constitutive model for high strength AA7075 under hot forming conditions. Metals 2021, 11, 314. [Google Scholar] [CrossRef]
- Su, J.X.; Zou, Y.; Chen, K.M.; Wang, Z.Y.; Guan, Q.F. Corrosion mechanism and characteristic of 7075-T6 aluminum alloy panel on airline aircraft. J. Mech. Eng. 2013, 49, 91–96. [Google Scholar] [CrossRef]
- Zhang, R.X.; Zhao, W.D.; Zhang, H.; Yang, W.J.; Wang, G.X.; Dong, Y.L.; Ye, C. Fatigue performance rejuvenation of corroded 7075-T651 aluminum alloy through ultrasonic nanocrystal surface modification. Int. J. Fatigue 2021, 153, 106463. [Google Scholar] [CrossRef]
- Barter, S.A.; Molent, L. Service fatigue cracking in an aircraft bulkhead exposed to a corrosive environment. Eng. Fail. Anal. 2013, 34, 181–188. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, X.; Hu, B.; Chen, L. Equivalent crack size model for pre-corrosion fatigue life prediction of aluminum alloy 7075-T6. Int. J. Fatigue 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Obert, B.; Ngo, K.; Hashemi, J.; Ekwaro-Osire, S.; Sivam, T.P. An investigation of the reduction in tensile strength and fatigue life of pre-corroded 7075-T6 aluminum alloy. J. Mater. Eng. Perform. 2000, 9, 441–448. [Google Scholar] [CrossRef]
- Sankaran, K.K.; Perez, R.; Jata, K.V. Effects of pitting corrosion on the fatigue behavior of aluminum alloy 7075-T6: Modeling and experimental studies. Mater. Sci. Eng. A 2001, 297, 223–229. [Google Scholar] [CrossRef]
- DuQuesnay, D.L.; Underhill, P.R.; Britt, H.J. Fatigue crack growth from corrosion damage in 7075-T6511 aluminium alloy under aircraft loading. Int. J. Fatigue 2003, 25, 371–377. [Google Scholar] [CrossRef]
- Jones, K.; Hoeppner, D.W. Pit-to-crack transition in pre-corroded 7075-T6 aluminum alloy under cyclic loading. Corros. Sci. 2005, 47, 2185–2198. [Google Scholar] [CrossRef]
- Genel, K. The effect of pitting on the bending fatigue performance of high-strength aluminum alloy. Scr. Mater. 2007, 57, 297–300. [Google Scholar] [CrossRef]
- Burns, J.T.; Kim, S.; Gangloff, R.P. Effect of corrosion severity on fatigue evolution in Al-Zn-Mg-Cu. Corros. Sci. 2010, 52, 498–508. [Google Scholar] [CrossRef]
- Burns, J.T.; Larsen, J.M.; Gangloff, R.P. Driving forces for localized corrosion-to-fatigue crack transition in Al-Zn-Mg-Cu. Fatigue Fract. Eng. Mater. Struct. 2011, 34, 745–773. [Google Scholar] [CrossRef]
- Zupanc, U.; Grum, J. Effect of pitting corrosion on fatigue performance of shot-peened aluminium alloy 7075-T651. J. Mater. Process. Technol. 2010, 210, 1197–1202. [Google Scholar] [CrossRef]
- Joshi, G.; Mall, S. Crack Initiation and growth from pre-corroded pits in aluminum 7075-T6 under laboratory air and salt water environments. J. Mater. Eng. Perform. 2017, 26, 2293–2304. [Google Scholar] [CrossRef]
- Mcmurtrey, M.D.; Bae, D.; Burns, J.T. Fracture mechanics modelling of constant and variable amplitude fatigue behaviour of field corroded 7075-T6511 aluminium. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 1–18. [Google Scholar] [CrossRef]
- Song, H.P.; Liu, C.C.; Zhang, H.; Du, J.; Yang, X.D.; Leen, S.B. In-situ SEM study of fatigue micro-crack initiation and propagation behavior in pre-corroded AA7075-T7651. Int. J. Fatigue 2020, 137, 105655. [Google Scholar] [CrossRef]
- Song, H.P.; Liu, C.C.; Zhang, H.; Yang, X.D.; Chen, Y.J.; Leen, S.B. Experimental investigation on damage evolution in pre-corroded aluminum alloy 7075-T7651 under fatigue loading. Mater. Sci. Eng. A 2021, 799, 140206. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, T.; He, Y.T.; Du, X.; Ma, B.L.; Zhang, T.Y. Long-term atmospheric pre-corrosion fatigue properties of epoxy primer-coated 7075-T6 aluminum alloy structures. Int. J. Fatigue 2019, 129, 105225. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Zhang, Y.; Knight, S. Heat treatment of 7xxx series aluminium alloys—Some recent developments. Trans. Nonferr. Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Pao, P.S.; Feng, C.R.; Gil, S.J. Corrosion fatigue crack initiation in aluminum alloys 7075 and 7050. Corrosion 2000, 56, 1022–1031. [Google Scholar] [CrossRef]
- Pao, P.S.; Gill, S.J.; Feng, C.R. On fatigue crack initiation from corrosion pits in 7075-T7351 aluminum alloy. Scr. Mater. 2000, 43, 391–396. [Google Scholar] [CrossRef]
- Birbilis, N.; Cavanaugh, M.K.; Buchheit, R.G. Electrochemical behavior and localized corrosion associated with Al7Cu2Fe particles in aluminum alloy 7075-T651. Corros. Sci. 2006, 48, 4202–4215. [Google Scholar] [CrossRef]
- Wang, Z. Handbook of Aluminum Alloy and Its Working; Central South University Press: Changsha, China, 2000. [Google Scholar]
- Mcnaughtan, D.; Worsfold, M.; Robinson, M.J. Corrosion product force measurements in the study of exfoliation and stress corrosion cracking in high strength aluminium alloys. Corros. Sci. 2003, 45, 2377–2389. [Google Scholar] [CrossRef]
- Robinson, M.J.; Jackson, N.C. The influence of grain structure and intergranular corrosion rate on exfoliation and stress corrosion cracking of high strength Al-Cu-Mg alloys. Corros. Sci. 1999, 41, 1013–1028. [Google Scholar] [CrossRef]
| Element | Zn | Mg | Cu | Fe | Ti | Si | Cr | Mn | Al |
|---|---|---|---|---|---|---|---|---|---|
| Weight fraction (%) | 5.57 | 2.58 | 1.51 | 0.14 | 0.024 | <0.05 | 0.20 | 0.045 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Xiang, L.; Tao, J.; Chen, Q.; Liu, J.; Zhong, Y. Actual Marine Atmospheric Pre-Corrosion Fatigue Performance of 7075-T73 Aluminum Alloy. Metals 2022, 12, 874. https://doi.org/10.3390/met12050874
Shi L, Xiang L, Tao J, Chen Q, Liu J, Zhong Y. Actual Marine Atmospheric Pre-Corrosion Fatigue Performance of 7075-T73 Aluminum Alloy. Metals. 2022; 12(5):874. https://doi.org/10.3390/met12050874
Chicago/Turabian StyleShi, Laixin, Lin Xiang, Jianquan Tao, Qiang Chen, Jun Liu, and Yong Zhong. 2022. "Actual Marine Atmospheric Pre-Corrosion Fatigue Performance of 7075-T73 Aluminum Alloy" Metals 12, no. 5: 874. https://doi.org/10.3390/met12050874
APA StyleShi, L., Xiang, L., Tao, J., Chen, Q., Liu, J., & Zhong, Y. (2022). Actual Marine Atmospheric Pre-Corrosion Fatigue Performance of 7075-T73 Aluminum Alloy. Metals, 12(5), 874. https://doi.org/10.3390/met12050874





