Abstract
Magnesium (Mg) is a potential candidate for biomedical implants, but its susceptibility to suffer corrosion attack in human body fluid limits its practical use. Thus, alloying Mg with other metal elements is the most effective strategy to improve its mechanical properties and biocompatibility. Herein, we report a Mg-Zn-Co ternary alloy (85-10-5 wt %) synthesized by the mechanical alloying technique. Ball-milling parameters such as ball size and milling time were varied to obtain better alloy properties. After compaction and sintering, the obtained alloy samples were subjected to various characterizations, including grain, scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy, X-ray diffraction (XRD), microhardness and nanoindentation analyses. In vitro biocompatibility analysis of different alloys was also performed with MC3T3-E1 osteoblasts. Grain analysis confirmed the even dispersion of particles, while SEM analysis showed the formation of laminates, spherical and fine particles with an increase in time and varied ball size. XRD results further confirmed the formation of intermetallic compounds. The microhardness of samples was increased with the increase in milling time. The Young’s modulus of ternary alloys obtained from nanoindentation analysis was comparable to the modulus of human bone. Moreover, in vitro analysis with osteoblasts showed that the developed alloys were noncytotoxic and biocompatible.
1. Introduction
Lack of suitable bone implants due to the poor quality of life of patients suffering from bone fractures imposes a significant societal burden [1,2]. Magnesium (Mg) and its alloys have emerged as a potential candidate for biomedical implants because of their unique features of light weight, good elastic modulus, better compressive yield strength and compatibility with those of natural bone [3,4]. The density of Mg (1.738 g/cm3) is slightly lower than that of natural bone (1.8–2.1 g/cm3), but its elastic modulus (45 GPa) is comparable to human bone (7–57 GPa) [5,6,7], which automatically removes the problem of stress shielding and second surgery for Mg implant removal [8]. The fast dissolution rate, resulting in the intrusion of mechanical integrity and premature loss of the implant, is a major drawback associated with Mg and Mg-based alloys [9,10,11,12]. Regarding implant materials, it is therefore critical to effectively control the corrosion degradation rates of alloys. Extensive research is being conducted on different aspects of implant materials such as purification, alloying, improvement of the preparation processes, deformation processing and heat treatment [13].
Alloying is one of the simplest and most effective methods to improve the comprehensive properties of Mg alloys [13,14,15,16]. Zinc (Zn) is a noble harmless metal that exhibits a biocompatible and biodegradable nature in body fluids [9,10]. It is vital for many biological functions and plays a crucial role in the >300 metabolic activities of the human body’s enzymes. It is considered essential for cell division and the synthesis of both proteins and DNA [9]. Zn deficiency in the human body has a detrimental impact on the body’s growth, neuronal development and immunity [12]. Alloying Mg with Zn improves not only the mechanical properties of Mg-based implants but also increases their corrosion resistance [11,12,13]. Moreover, Mg-Zn alloys have the advantages of good biocompatibility and high plasticity. Cobalt (Co) is an essential component of vitamin B12 (hydroxocobalamin) and a fundamental coenzyme of cell mitosis, which plays an important role in the formation of amino acids, neurotransmitters and some proteins to create myelin sheath in nerve cells [13,14]. Co deficiency disturbs vitamin B12 synthesis, which can increase the risk of anemia, thyroid hypofunction and developmental abnormalities in infants [14]. Generally, Mg is alloyed with Co to obtain a lightweight material with inherent load-sensitive abilities [15]. However, in the Mg industry, the presence of elemental Co is generally considered an impurity due to its negative impact on the corrosion behavior of Mg-based alloys [16,17,18,19]. This corrosion-accelerating effect occurs due to the existence of undissolved Co particles in the alloy’s microstructure, which are produced due to the low solubility of Co in Mg, resulting in the formation of active cathodic sites [20]. It has also been reported that a small increase in Co increases the strength values without significantly affecting the corrosion behavior of Mg-based implants [21]. Moreover, it has been stated that the maximum cobalt concentration dissolvable in magnesium is ~5%, making it favorable in the development of an alloy [22].
Mechanical alloying is an interdispersion technique involving repeated cold welding, fracturing and rewelding of powder particles through the high-energy ball-milling process, resulting in the formation of alloy phases. This technique is also capable of synthesizing a variety of equilibrium and nonequilibrium alloy phases, starting with prealloyed powders [23]. Milling time and ball size are important parameters in mechanical alloying. It has been observed that increasing milling time leads to an increase in contamination and the formation of undesirable phases [24]. Thus, milling time is adjusted to ensure equilibrium between the cold-welding and fracturing processes [25]. Mechanical alloying is an effective method to produce metallic alloys with fine microstructure. However, little information is available regarding the production and bulk properties of Mg-based ternary alloys prepared by the mechanical alloying technique.
Herein, we report an Mg-Zn-Co-based ternary alloy (85-10-5 wt %) synthesized by the mechanical alloying technique. Varied ball-milling parameters such as ball size and milling time were employed to develop this alloy. The developed alloy was further characterized with optical microscopy, scanning electron microscopy, EDS, X-ray diffraction (XRD) analysis, microhardness, nanoindentation and biocompatibility analyses.
2. Material and Methods
2.1. Materials
Mg (117 µm), Zn (17.06 µm) and Co (21.7 µm) of 99% purity used for the current study were imported from Premier Industrial Corporation Limited (Mumbai, India). SEM analysis was performed to analyze the morphology of metal powders before further processing, as shown in Figure 1. Initially, the morphology of Mg was in the form of flakes, Zn in the form of fine powder and Co in the form of sponge.
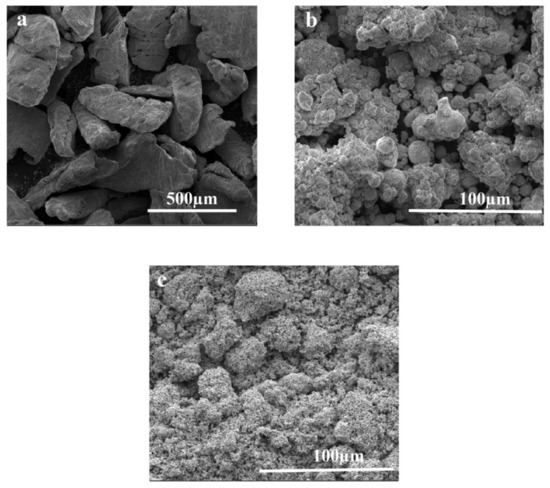
Figure 1.
Morphological analysis of pure constituents of Mg-Zn-Co alloys using SEM: (a) 99.9% pure Mg; (b) 99.9% pure Zn; (c) 99.9% pure Co.
2.2. Alloy Preparation
A planetary ball mill (XQM-2A, Changsha Tianchuang Powder Technology Co., Ltd., Changsha, China) was used for alloy preparation by mechanical alloying. Powder of Mg (117 µm), Zn (17.06 µm) and Co (21.7 µm) was ball milled for 30 h. Stainless steel balls of various sizes were used: Vial 1 was filled with uniform-sized grinding balls of 10 mm diameter, and Vial 2 was filled with variable-sized grinding balls of 2.5, 5 and 10 mm diameter in a proportion of 1:1:2. Metal powders were then weighed in an 85:10:5 ratio (grams of Mg, zinc and Co powder, respectively). A ball-to-powder ratio was maintained at 10:1, and milling was performed at 360 rpm for both uniform- and variable-sized grinding balls. Time intervals were maintained at 7 h, 15 h and 30 h. Stearic acid (3%) and 1–2 drops of alcohol were used as the process control agent (PCA) to reduce cold welding and promote the fracturing of samples [26,27]. Argon gas was used to provide an inert atmosphere during the milling process.
2.2.1. Sintering
Mg-Zn-Co ternary alloy pellets were made using an autopellet machine with 30 MPa force for 15 s. Green samples were sintered in a tube furnace (CY-T1700-50I-T, Zhengzhou CY Scientific Instrument Co., Ltd., Zhengzhou, China) at 550 °C in the presence of argon gas for 90 min. The sintering process provided strength and integrity to the material.
2.2.2. Optical Microscope Analysis
An optical microscope (Leica DMI 5000, Wetzlar, Germany) was used to analyze the particle size distribution, and corresponding software was used for grain analysis. The software is based on the ASTM E112 standard [26]. The acquired microstructure was analyzed using the intercept method, which involves an actual count of the number of grains intercepted by a test line or the number of grain boundary intersections along the test line. The mean linear intercept length ℓ is used to determine the ASTM grain size number G. The precision of the method is a function of the number of intercepts or intersections counted.
2.2.3. Microstructural Analysis
An FEI SEM equipped with the Smart Drift Detector EDS (Inspect S50, Thermo Fisher Scientific, Waltham, MA USA) was used for microstructural analysis. It also revealed information related to the external morphology of the microstructure. The same SEM was used to perform EDS analysis at selected point locations on the sample.
2.2.4. Phase Identification
A diffractometer (D8 DISCOVER, Bruker, Bremen, Germany) with Cu-K∝ radiation was used for the identification of phases of the Mg-Zn-Co alloys. The voltage and current were set to 40 kV and 40 mA, respectively. Diffraction patterns were collected in steps of 0.04°, and 2θ scans were measured and combined to collect diffraction patterns in the range of 16°–76°.
2.2.5. Nanohardness Tests
Nanohardness tests of the sintered samples were carried out by nanoindentation using NHTX S/N: 01-2569. (Needham Heights, MA, USA.) The maximum applied load was 100 mN. The Oliver–Pharr method was used for this indentation test. The samples were held on a metallic steel stub with Crystalbond 509 Amber glue and placed under a microscope for nanohardness measurements. The hardness was measured by making indents at 4 different points, and the elastic modulus was calculated for each indent. The indenter maximum force was 100 mN with a loading and unloading rate of 200 mN/min, and the maximum load was applied for 10 s. The rest time was 10 s. The indenter used for nanoindentation was of the Berkovich type. Berkovich is a three-sided pyramidal nanoindenter. The total included angle of the indenter tip is 142.3°, and the half-angle is 65.35°. The radius of the tip curvature is 150 nm [28].
The Young’s modulus (EIT) was calculated using the following equation offered by Hertz [29].
where Ei and νi are the modulus and Poisson’s ratio of the indenter, respectively. ν and EIT are the parameters of the developed material.
The indentation module MIT defines the relationship between depth and force when a rigid surface is pressed into an elastic half-space. For the Berkovich indenter, MIT is derived using the following equation [30].
where S is the slope of the unloaded curve, A is the contact surface and β is the dimensionless coefficient.
2.2.6. Microindentation
The microhardness of the sintered samples was measured using a Vickers hardness tester (Zwick/Roll Indenter, Model ZHVu, Ulm, Germany) with a load of 150 g/15 s. For this purpose, a diamond indenter was used to measure the hardness at 3 different points on each sample.
2.2.7. Biocompatibility Analysis
Preparation of Extraction Medium
Alloy samples were first sterilized under UV radiation for 2 h. The extraction medium of sample alloys was prepared by submerging in serum-free DMEM for 48 h with a surface area–extraction medium ratio of 1 cm2/mL. The medium was withdrawn and stored at 4 °C.
Cell Culture
Biocompatibility analysis of the Mg-Zn-Co alloys compared to Mg-Zn was performed using mouse MC3T3-E1 cells obtained from American Type Culture Collection (ATCC). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Fisher Scientific, Leicestershire, United Kingdom) supplemented with 10% fetal bovine serum and 5% penicillin/streptomycin. Cells were incubated in a humidified incubator with a 5% CO2 atmosphere at 37 °C.
MTT Assay
To analyze the cytotoxicity potential of the samples, MTT cell viability assay was performed as reported previously [31]. Mouse MC3T3-E1 cells were seeded in a 96-well plate with a density of 1 × 106 cells/mL. Cells were allowed to attach for 24 h. The medium was replaced with the extraction medium of 7hr-U and 30hr-U samples of Mg-Zn-Co alloys for a period of 72 h, separately. Cells cultured with normal DMEM were kept as control. Six wells were assigned to each group. MTT cell viability assay was performed to evaluate the cytotoxicity effect of both alloys after 72 h incubation. Morphology and attachment of cells were observed under a light microscope, and images were captured using an Olympus fluorescence microscope. A 10 μL volume of MTT was subjected to each well and incubated for 4 h. After 4 h, MTT crystals were dissolved in 100 μL of dimethyl sulfoxide per well and transferred to an ELISA reader plate. The absorbance of each well was measured at a 490 nm wavelength using the ELISA plate reader. Percent cell viability was calculated using the following formula.
Cell Viability = ODSample/ODControl × 100
Statistical analysis was performed using GraphPad Prism 5. To analyze the group data, one-way ANOVA with Tukey’s post-hoc test was used, and p < 0.05, p < 0.01 and p < 0.001 were considered statistically significant. The results are shown as mean ± SEM.
3. Results and Discussion
3.1. Effect of Milling Time on Particle Dispersion of Unsintered Mg-Zn-Co Alloy
Mg-Zn-Co alloy samples prepared using uniform-sized steel balls (10 mm diameter) are designated as Xhr-U, while samples prepared by variable-sized (2.5, 5, 10 mm diameter) steel balls are designated as Xhr-V. In both Xhr-U and Xhr-V samples, X corresponds to milling time (X = 7 h or 15 h or 30 h). Dispersion of particles of Xhr-U and Xhr-V samples shows that the maximum percentage count of particles is obtained in the 30hr-U and 30hr-V samples, whereas the zig-zag behavior observed for 15 h of milling is due to the mixture of various sizes of particles. At 15 h of milling, the aggregate had fine and coarse particles compared to the particle size obtained after 7 h of milling. This effect can be attributed to the mechanism of mechanical alloying (Figure 2).
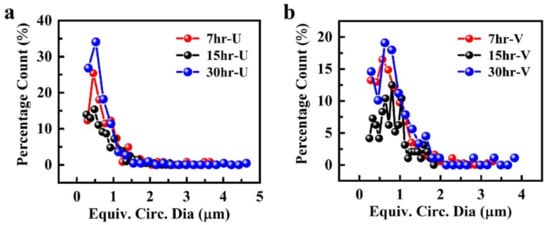
Figure 2.
Graphical representation of particle size distribution in Mg-Zn-Co alloy samples, prepared via: (a) uniform-sized ball milling (Xhr-U); (b) variable-sized ball milling (Xhr-V).
For both uniform- and variable-sized grinding balls (Figure 2), 7 h of milling time was not enough for the size reduction of particles; instead, more particle distribution was gained after 15 h of milling time. Proper dispersion of particles was not achieved at 7 h due to the shorter milling time. When milling time was increased to 15 h, particles were more dispersed, with a combination of fine and coarse sizes resulting in a zig-zag pattern observed in the graph. When milling time was further increased to 30 h, both types of samples showed their best behavior. Dispersion of different sizes of particles is further shown in Figure 3.
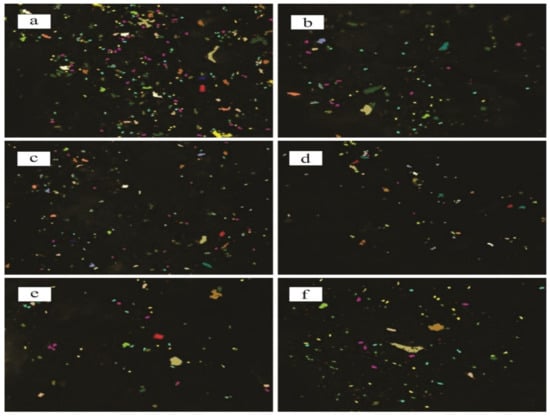
Figure 3.
Microstructure analysis of different samples of Mg-Zn-Co alloys using ASTM-E112; (a) 7hr-U; (b) 7hr-V; (c) 15hr-U; (d) 15hr-V; (e) 30hr-U; (f) 30hr-V. Particles shown with different colors represent percentage count with respect to equivalent circumference diameter.
Particles with a diameter <1 µm were counted and presented as a percentage count of Xhr-U and Xhr-V samples in Table 1. In both cases, 30 h of milling gave the highest percentage of fine particles.

Table 1.
Optical microscopy data of particle size.
The mechanism of mechanical milling involves welding, fracturing and rewelding of particles [32]. During the milling process, the interaction between the grinding medium and powder particles generates heat, which causes the welding of particles. This phenomenon is called cold welding [33]. When milling time is increased from 7 to 30 h, the fineness of particles increases as they become trapped within the grinding balls, which ultimately causes the fracturing of agglomerated particles into fine particles. Particle-to-particle interaction causes the formation of intermetallic compounds, which will be studied in detail in subsequent sections.
3.2. Effect of Ball Size and Milling Time on Morphological Alterations of Unsintered Mg-Zn-Co Alloy
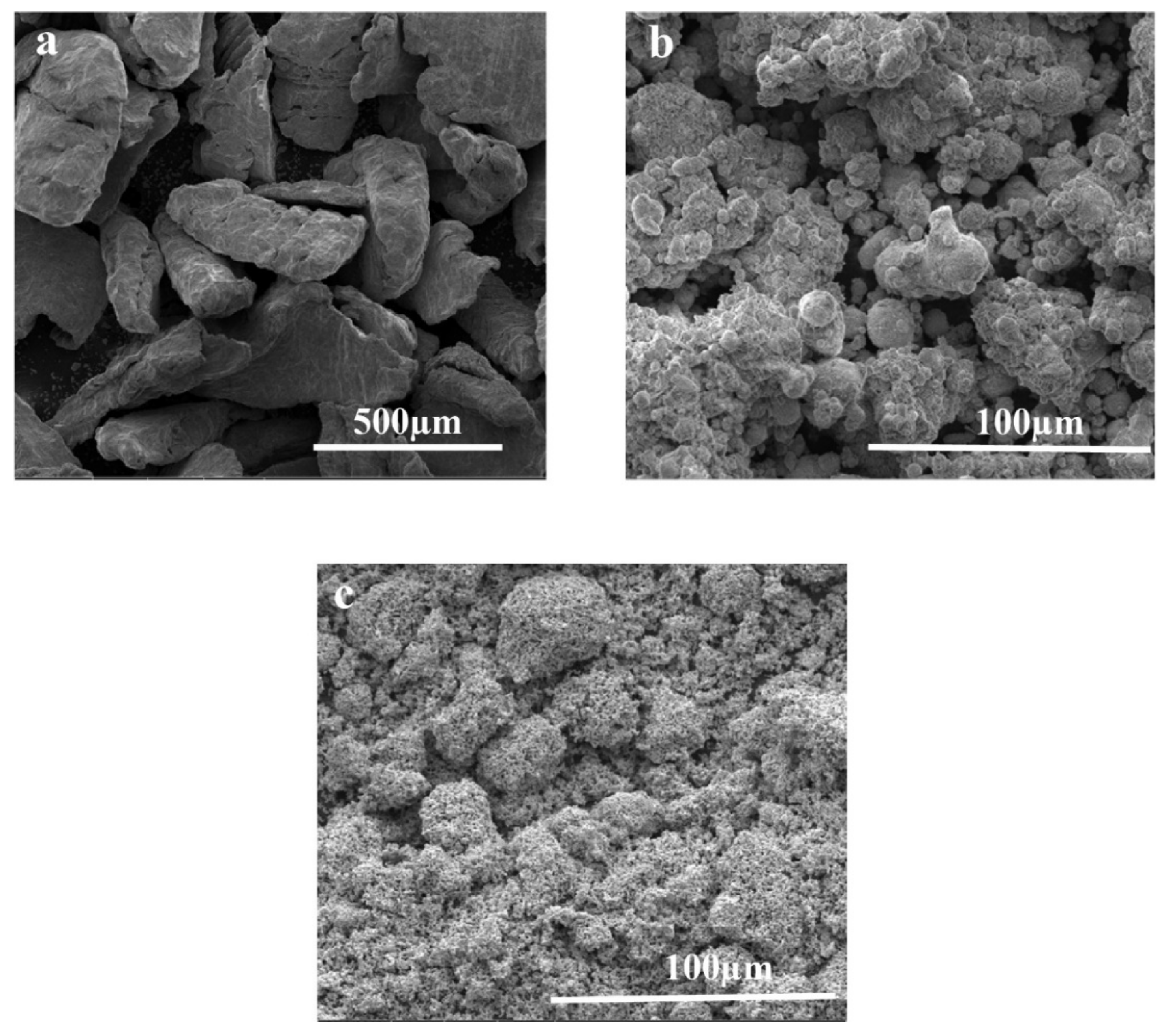
SEM microscopy was performed to analyze the morphology of the developed Mg-Zn-Co ternary alloy. SEM images of the Mg-Zn-Co alloy depicted significant morphological alterations compared to raw materials. The time-dependent increase in milling time significantly affected the morphology of both types of Xhr-U and Xhr-V samples, as shown in Figure 4. Mg-Zn-Co alloy powder combines to form a laminate structure after milling for 7 h (Figure 4a,b). The intermediate stage of milling (15 h) consists of laminates, as well agglomeration of particles (Figure 4c,d). When milling time continues to increase and reaches 30 h, it is found that the laminates convert into small clusters due to repeated fracture and cold welding (Figure 4e,f). However, the changes in Xhr-U at specific milling time were similar to the Xhr-V sample.
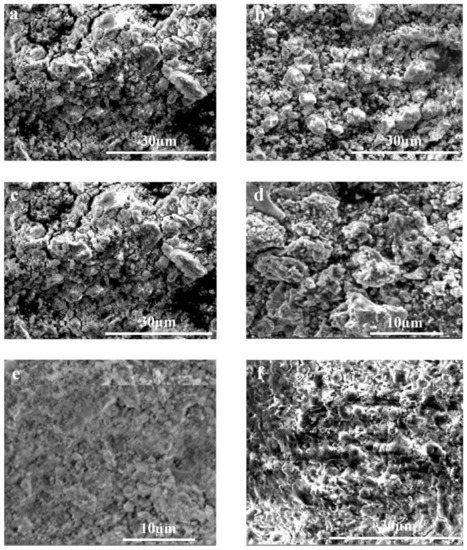
Figure 4.
Morphological analysis of different samples of Mg-Zn-Co alloys using SEM: (a) 7hr-U; (b) 7hr-V; (c) 15hr-U; (d) 15hr-V; (e) 30hr-U; (f) 30hr-V.
In the initial stages of mechanical alloying, random-sized particles are formed due to the fracturing and rewelding of particles. Powder particles are welded with each other due to heat produced by the collision of particles and form agglomerates. As milling time increases, the fracturing of agglomerates also occurs, which causes a further reduction in particle size. This phenomenon is also reported by Supriyanto et al. [34].
3.3. Effect of Ball Size and Milling Time on Chemical Composition of Sintered Mg-Zn-Co Alloy
The developed Mg-Zn-Co ternary alloy was pressed to form pellets, sintered to enhance their mechanical properties and structural integrity. The chemical composition of elements in the different Xhr-U and Xhr-V samples is shown in Table 2. Mechanical synthesis indicated the presence of unreacted components due to the affinity of alloying elements with each other. EDS data also showed the formation of some intermetallic compounds such as Mg62 Zn20 Co17. Chemical composition analysis therefore concludes that the solid-state diffusion did not reach its peak during the mechanical alloying process, causing the instability of the alloy [35].

Table 2.
EDS results of Mg-Zn-Co alloy samples.
3.4. Effect of Milling Time on Formation of Intermetallic Compounds of Mg-Zn-Co Alloy
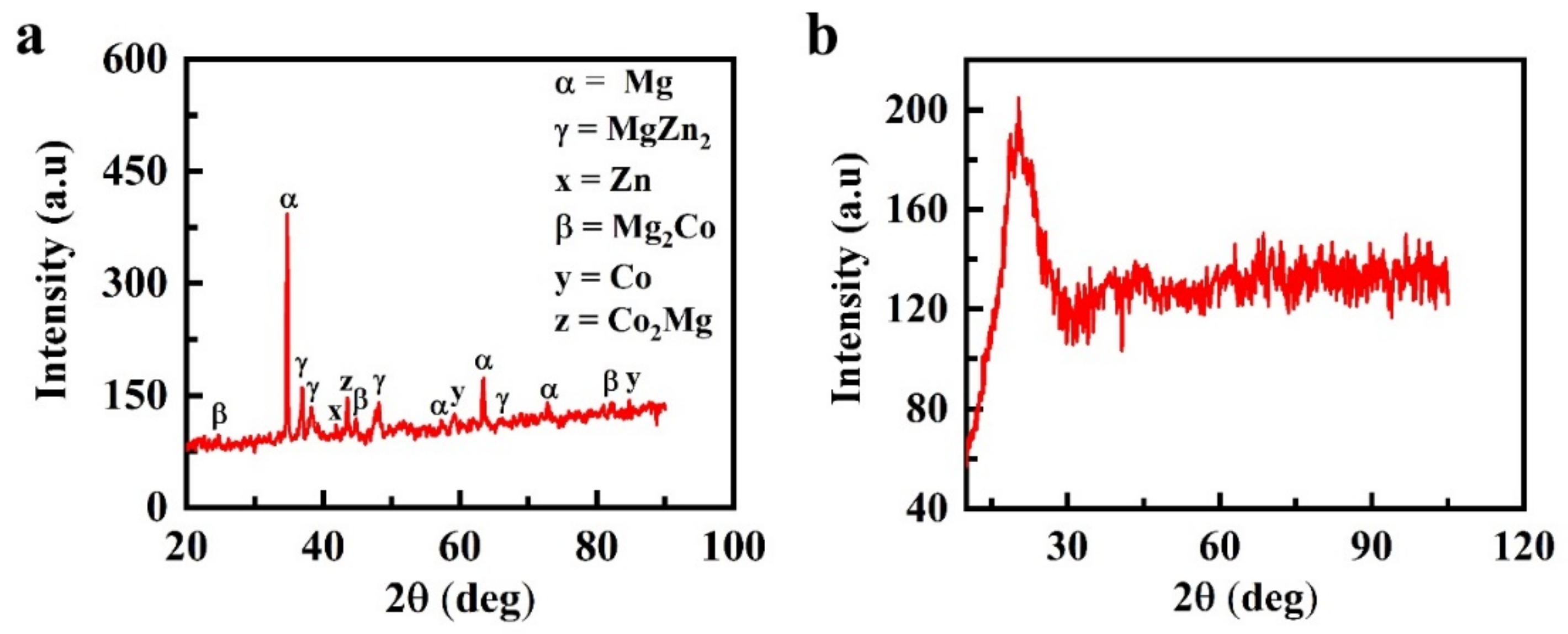
Analysis of the formation of intermetallic compounds performed with XRD showed the formation of a crystalline structure in the 7hr-U sample compared to the amorphous structure of the 30hr-U sample (Figure 5). Figure 5a depicts the identification of Mg peaks at 2θ = 35°, 36°, 57°, 63° and 72° along with peaks of MgZn2 at 2θ = 36.5°, 38.5°, 66° and a Zn peak at 2θ = 42° [36]. In addition, a Co2Mg peak was observed at 44°, and Mg2Co indicated peaks at 25°, 45°, 80° and 81°, whereas peaks of Co were formed at 2θ of 60° and 85° [34,37].
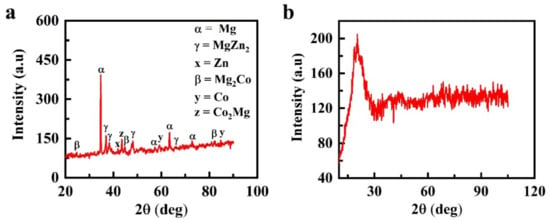
Figure 5.
Formation of intermetallic compound analysis of Mg-Zn-Co alloys using XRD: (a) 7hr-U; (b) 30hr-U.
As expected, the peaks with the highest intensities belong to the primary Mg-crystals occupying the largest peak of the sample surface area. Zn exhibits limited solubility in Mg. According to the literature, Zn only shows 6.2 wt % solubility in the Mg phase even at eutectic temperature (340 °C) [37]. Thus, with the further addition of Zn in the Mg matrix, the solubility limit of Zn is exceeded, and it restricts α-Mg, which results in the formation of a secondary phase of intermetallic γ-MgZn2.
Mg and Co have a hexagonal structure at room temperature, due to which the reflexes of both metals partially overlap at 2θ = 81°. The XRD database used in this work clearly indicates the existence of an intermetallic compound of the types Co2Mg and Mg2Co in the specimen [38]. The diffraction peaks of Mg widen with the increase in milling time from 7 to 30 h, and their intensity decreases, which occurs due to the refinement and microstrain of grains [23,39].
It can be concluded that the initial local deformation of the sample occurs under the action of the ball-milling medium, which generates high dislocation densities. Due to the combination of these dislocations, small-angle grain boundaries will be formed. When these small-angle grain boundaries are transformed to large-angle grain boundaries, further grain refinement occurs. Continuous collision and squeezing of milling balls and powder particles enhances the microstrain and causes serious plastic deformation.
In contrast, Figure 5b shows that in the 30hr-U sample with 30 h of milling time, the diffraction peaks disappeared. In the diffraction position of Mg, only a single broad and diffused halo pattern was detected, indicating that the powder particles existed in the amorphous form. The above-mentioned intermetallic compounds were also formed for the variable-sized ball diameter because the same process parameters were followed. Amorphization by mechanical alloying occurs when the free energy of the hypothetical amorphous phase GA is lower than that of the crystalline phase GC, i.e., the GA < GC crystalline phase has lower energy than the amorphous phase, but its free energy can be increased by a variety of crystal defects such as dislocations, grain boundaries and stacking faults. If an intermetallic compound has formed, then additional energy can be introduced by disordering the crystal lattice. By this approach, it is possible to obtain a situation when
where GD is the free energy increase due to the introduction of crystal defects [40].
GA < GC + GD
3.5. Effect of Ball Size and Milling Time on Microhardness of Sintered Mg-Zn-Co Alloy
Figure 6 shows the microhardness of Xhr-U and Xhr-V. The average hardness values obtained from the multiple indents represent the obtained results by applying six indents at random places. In the case of both Xhr-U and Xhr-V samples, an increase in average hardness values was observed when milling time increased from 7 to 15 h. In the initial stage of milling, the Vickers microhardness increases with the increase in milling time due to due to work hardening of alloy powder [37]. According to the mechanism of grain refinement, the internal strain change occurs in alloy powder prior to the formation of crystallite size. Thus, the initial stage of mechanical alloying plays an important role in improving microhardness.
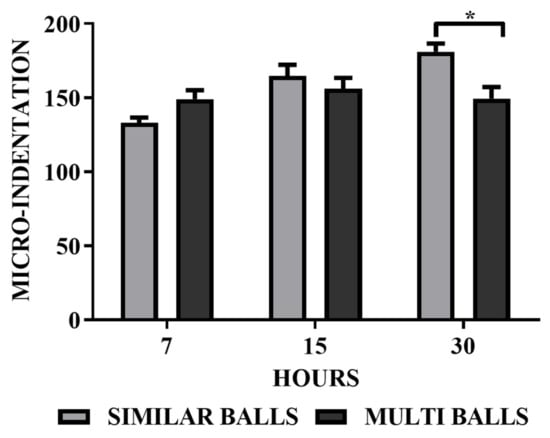
Figure 6.
Microhardness analysis of Mg-Zn-Co alloy using micro-Vickers for both Xhr-U and Xhr-V. (* p < 0.05).
The phenomenon of particle dispersion also supports this, because coarse and fine particles were dispersed evenly in the case of 15 h of milling time both for uniform and variable ball size diameters. This effect is more vivid in the case of variable-diameter balls, and a random trend can be observed in Figure 2. In the case of similar diameter balls, the microhardness of the sample increased significantly when milling time increased from 15 to 30 h, which may be due to the amorphous phase formation [37,41]. The increase in microhardness may be due to the solid solution strengthening with the decrease in particle size, intense intermixing of constituents and the increase in milling time, a characteristic of mechanical alloying [25,42]. An increase in the hardness value results in an increase in the wear resistance of the ternary alloy [42].
The decrease in the hardness value in the case of samples processed by variable-diameter grinding balls may be due to the inherent characteristics of the milling effect of the variable-diameter balls. Up to a certain milling time and particle size to ball ratio, milling is effective and results in a continuous size reduction and an increase in hardness. After a certain time and below a particular particle size, the particles become recessed from impact due to the variable gap size between milling as the mass of the small-sized ball begins resisting further milling and supporting the agglomeration of particles, as shown in Figure 4 for the 30hr-V sample. The resulting sample would be less dense and show lower hardness values, as observed in Figure 6 [43,44].
3.6. Effect of Milling Time on Young’s Modulus of Mg-Zn-Co Alloy
The specimens 7hr-U and 30hr-U were nanoindented at four different points to indirectly calculate the Young’s modulus value at each specific point. The average Young’s modulus obtained was ≈27.12 GPa and 20.66 GPa, respectively. Moreover, in contrast to the Young’s moduli of pure Mg (41–45 GPa) and Mg-Zn (42.3 GPa) alloys, the elastic modulus of developed Mg-Zn-Co alloys was comparable to the modulus of human bone (7–30 GPa) [5,6]. These results are in accordance with the literature, stating that a decrease in the size and irregular shape of particles decreases the elastic modulus of the developed alloys. Mechanical alloying of Mg-Zn-Co milled powders with reduced sizes and irregular shapes may result in the formation of pores. It has been stated that irregular porosity will reduce the Young’s modulus of the alloy [44,45,46]. The low Young’s modulus of the developed implant material makes it a potential candidate for biomedical applications [41,47].
3.7. Effect of Milling Time on Biocompatibility of Mg-Zn-Co Alloy
Morphometric and cell attachment analysis showed that cells treated with the extract medium of both 7hr-U and 30hr-U were relatively normal and comparable except for some variations in the shape of cells (Figure 7). The average viability of more than 75% of cells cultured in the extract medium of both 7hr-U and 30hr-U alloys further verified their noncytotoxic and biocompatible nature. It has been previously reported that alloying Mg-Zn with other biocompatible metals has an excellent effect in reducing its cytotoxicity. Co is a trace element in the human body. It is an important component of vitamin B12 and is involved in the synthesis of amino acids, protein or neurotransmitters. The results of biocompatibility studies further confirm that the addition of Co to Mg-Zn enhances the corrosion resistance within the living system, leading to reduced cytotoxicity [46,47,48,49].
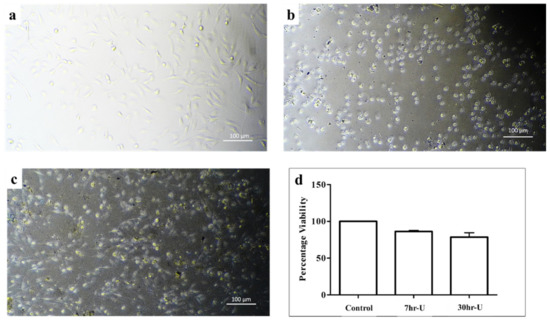
Figure 7.
In vitro biocompatibility analysis of different mechanically alloyed Mg-Zn-Co alloys. MC3T3-E1 cells were used to analyze biocompatibility test of different combinations. Cells cultured in extract media were analyzed for morphological analysis: (a) control; (b) 7hr-U; (c) 30hr-U. (d) Graphical representation of MTT cell cytotoxicity analysis of cells cultured in different extraction media of alloys compared to control media.
4. Conclusions
This work demonstrated the effect of mechanical alloying parameters such as ball size and milling time on the development of Mg-Zn-Co alloys. Based on the results, it is therefore concluded that these parameters have a significant effect on the microstructure, mechanical properties and biocompatibility of Mg-Zn-Co ternary alloys. After milling, dispersion of particles not only reduced the elastic modulus of the developed Mg-Zn-Co alloys comparable to the modulus of the natural bone but also improved its biocompatibility, therefore making it a favorable and promising material for biomedical applications.
Author Contributions
Conceptualization, M.K., R.A., A.T.; methodology, S.M., A.T.; software, S.M., A.T.; validation, S.M., A.T.; formal analysis, M.K., R.A.; investigation, S.M., A.T.; resources, M.K., R.A., A.T.; data curation, S.M.; writing—original draft preparation, S.M., A.T.; writing—review and editing, M.K., R.A., A.T.; supervision, R.A., A.T.; project administration, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding and carried out on the funds provided by University of the Punjab, Lahore, Pakistan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In Vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R.; Fraser, H.L. A novel combinatorial approach for understanding microstructural evolution and its relationship to mechanical properties in metallic biomaterials. Acta Biomater. 2007, 3, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Salleh, E.M.; Zuhailawati, H.; Ramakrishnan, S. Synthesis of biodegradable Mg-Zn alloy by mechanical alloying: Statistical prediction of elastic modulus and mass loss using fractional factorial design. Trans. Nonferrous Met. Soc. China 2018, 28, 687–699. [Google Scholar] [CrossRef]
- Homayun, B.; Afshar, A. Microstructure, mechanical properties, corrosion behavior and cytotoxicity of Mg–Zn–Al–Ca alloys as biodegradable materials. J. Alloys Compd. 2014, 607, 1–10. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, M.; Sajjadi, S.A.; Azadbeh, M. An investigation on the variations occurring during Ni3Al powder formation by mechanical alloying technique. J. Alloys Compd. 2010, 497, 171–175. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kerstetter, J.E. Nutrition in bone health revisited: A story beyond calcium. J. Am. Coll. Nutr. 2000, 19, 715–737. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable orthopedic magnesium-calcium (MgCa) alloys, processing, and corrosion performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical characteristics, in vitro degradation, cytotoxicity, and antibacterial evaluation of Zn-4.0 Ag alloy as a biodegradable material. Int. J. Mol. Sci. 2018, 19, 755. [Google Scholar] [CrossRef] [Green Version]
- Ortega, R.; Bresson, C.; Fraysse, A.; Sandre, C.; Devès, G.; Gombert, C.; Tabarant, M.; Bleuet, P.; Seznec, H.; Simionovici, A.; et al. Cobalt distribution in keratinocyte cells indicates nuclear and perinuclear accumulation and interaction with magnesium and zinc homeostasis. Toxicol. Lett. 2009, 188, 26–32. [Google Scholar] [CrossRef]
- Battaglia, V.; Compagnone, A.; Bandino, A.; Bragadin, M.; Rossi, C.A.; Zanetti, F.; Colombatto, S.; Grillo, M.A.; Toninello, A. Cobalt induces oxidative stress in isolated liver mitochondria responsible for permeability transition and intrinsic apoptosis in hepatocyte primary cultures. Int. J. Biochem. Cell Biol. 2009, 41, 586–594. [Google Scholar] [CrossRef]
- Klose, C.; Mroz, G.; Angrisani, G.L.; Kerber, K.; Reimche, W.; Bach, F.W. Casting process and comparison of the properties of adapted load-sensitive magnesium alloys. Prod. Eng. 2013, 7, 35–41. [Google Scholar] [CrossRef]
- Kammer, C.; Aluminium-Zentrale, D. Magnesium Taschenbuch, 1st ed.; Kammer, C., Ed.; Aluminium-Verlag: Düsseldorf, Germany, 2000; Chapter 8; pp. 155–188. [Google Scholar]
- Buchmann, W. Festigkeitseigenschaften. In Magnesium und Seine Legierungen, 2nd ed.; Beck, A., Altwicker, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Chapter 5; pp. 101–271. [Google Scholar]
- Schultze, W. Chemisches Verhalten, Korrosion und Oberflächenschutz. In Magnesium und SeineLegierungen, 2nd ed.; Beck, A., Altwicker, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Chapter 6; pp. 272–312. [Google Scholar]
- Shaw, B.A. Corrosion Resistance of Magnesium Alloys. In ASM Handbook Volume 13A: Corrosion: Fundamentals, Testing, and Protection, 1st ed.; Cramer, S.D., Covino, B.S., Eds.; ASM International: Novelty, OH, USA, 2003; pp. 692–696. [Google Scholar]
- Klose, C.; Demminger, C.; Mroz, G.; Reimche, W.; Bach, F.W.; Maier, H.J.; Kerber, K. Influence of cobalt on the properties of load-sensitive magnesium alloys. Sensors 2013, 13, 106–118. [Google Scholar] [CrossRef]
- Hawke, D.L. Corrosion Behavior. In ASM Specialty Handbook: Magnesium and Magnesium Alloys, 1st ed.; Avedesian, M., Baker, H., Eds.; ASM International: Novelty, OH, USA, 1999; pp. 194–210. [Google Scholar]
- Wetherill, J.P. Magnesium Alloy. U.S. Patent 1,880,614, 4 October 1932. [Google Scholar]
- Benjamin, J.S.; Volin, T.E. The Mechanism of Mechanical Alloying. Metall. Trans. 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E. Nou® R, Contreras MA, Moore JJ. J. Mater Res. 1999, 14, 377–378. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A novel technique to synthesize advanced materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, C.C.; Cavin, O.B.; McKamey, C.G.; Scarbrough, J.O. Preparation of “amorphous” Ni60Nb40 by mechanical alloying. Appl. Phys. Lett. 1983, 43, 1017–1019. [Google Scholar] [CrossRef]
- Francavilla, A.; Zienkiewicz, O.C. A note on numerical computation of elastic contact problems. Int. J. Numer. Methods Eng. 1975, 9, 913–924. [Google Scholar] [CrossRef]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Chaudry, U.M.; Farooq, A.; Malik, A.; Nabeel, M.; Sufyan, M.; Tayyeb, A.; Asif, S.; Inam, A.; Elbalaawy, A.; Hafez, E.; et al. Biodegradable properties of AZ31-0.5 Ca magnesium alloy. Mater. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Raducanu, D.; Cojocaru, V.D.; Nocivin, A.; Hendea, R.; Ivanescu, S.; Stanciu, D.; Trisca-Rusu, C.; Drob, S.I.; Cojocaru, E.M. Mechanical Alloying Process Applied for Obtaining a New Biodegradable Mg-xZn-Zr-Ca Alloy. Metals 2022, 12, 132. [Google Scholar] [CrossRef]
- ASTM E112-13; Standard Test Methods for Determining Average Grain Size. ASTM International: West Conshohocken, PA, USA, 2013.
- Supriyanto, A.A.; Daud, A.R. Effect of milling time on microstructure of mechanically alloyed al-Ti powders in AIP conference proceedings. Am. Inst. Phys. 2010, 1202, 117–121. [Google Scholar]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304, 151–158. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Salleh, E.M.; Ramakrishnan, S.; Hussain, Z. Synthesis of biodegradable Mg-Zn alloy by mechanical alloying: Effect of milling time. Procedia Chem. 2016, 19, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.; Bhor, R.; Pai, K.; Sen, D.; Mazumder, S.; Ghosh, K.; Kolekar, Y.; Ramana, C. Cobalt nanoparticles for biomedical applications: Facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl. Surf. Sci. 2017, 414, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Sidambe, A.T.; Figueroa, I.A.; Hamilton HG, C.; Todd, I. Metal injection moulding of CP-Ti components for biomedical applications. J. Mater. Processing Technol. 2012, 212, 1591–1597. [Google Scholar] [CrossRef]
- Sharma, S.; Vaidyanathan, R.; Suryanarayana, C. Criterion for predicting the glass-forming ability of alloys. Appl. Phys. Lett. 2007, 90, 111915. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X. Microstructural evolution, thermal stability and microhardness of the Nb–Ti–Si-Based alloy during mechanical alloying. Metals 2018, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Rios, J.; Restrepo, A.; Zuleta, A.; Bolívar, F.; Castaño, J.; Correa, E.; Echeverria, F. Effect of ball size on the microstructure and morphology of mg powders processed by high-energy ball milling. Metals 2021, 11, 1621. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, K.L.; Xu, Z.M.; Lee, H.B. Corrosion and Biocompatibility Behavior of the Micro-Arc Oxidized AZ31B Alloy in Simulated Body Fluid. Int. J. Electrochem. Sci. 2020, 15, 6405–6424. [Google Scholar] [CrossRef]
- Kim, H.N.; Kim, J.W.; Kim, M.S.; Lee, B.H.; Kim, J.C. Effects of Ball Size on the Grinding Behavior of Talc Using a High-Energy Ball Mill. Minerals 2019, 9, 668. [Google Scholar] [CrossRef] [Green Version]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd alloy after mechanical alloying. Materials 2021, 14, 226. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Klassen, T.; Ivanov, E. Synthesis of nanocomposites and amorphous alloys by mechanical alloying. J. Mater. Sci. 2011, 46, 6301–6315. [Google Scholar] [CrossRef]
- Lee, S.H.; Todai, M.; Tane, M.; Hagihara, K.; Nakajima, H.; Nakano, T. Biocompatible low Young’s modulus achieved by strong crystallographic elastic anisotropy in Ti–15Mo–5Zr–3Al alloy single crystal. J. Mech. Behav. Biomed. Mater. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Liu, Q.; Zhao, W.; Yang, Q.; Wang, J.; Jiang, D. In Vitro Studies on Mg-Zn-Sn-based Alloys Developed as a New Kind of Biodegradable Metal. Materials 2021, 14, 1606. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).