The Formation Mechanisms and Evolution of Multi-Phase Inclusions in Ti-Ca Deoxidized Offshore Structural Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
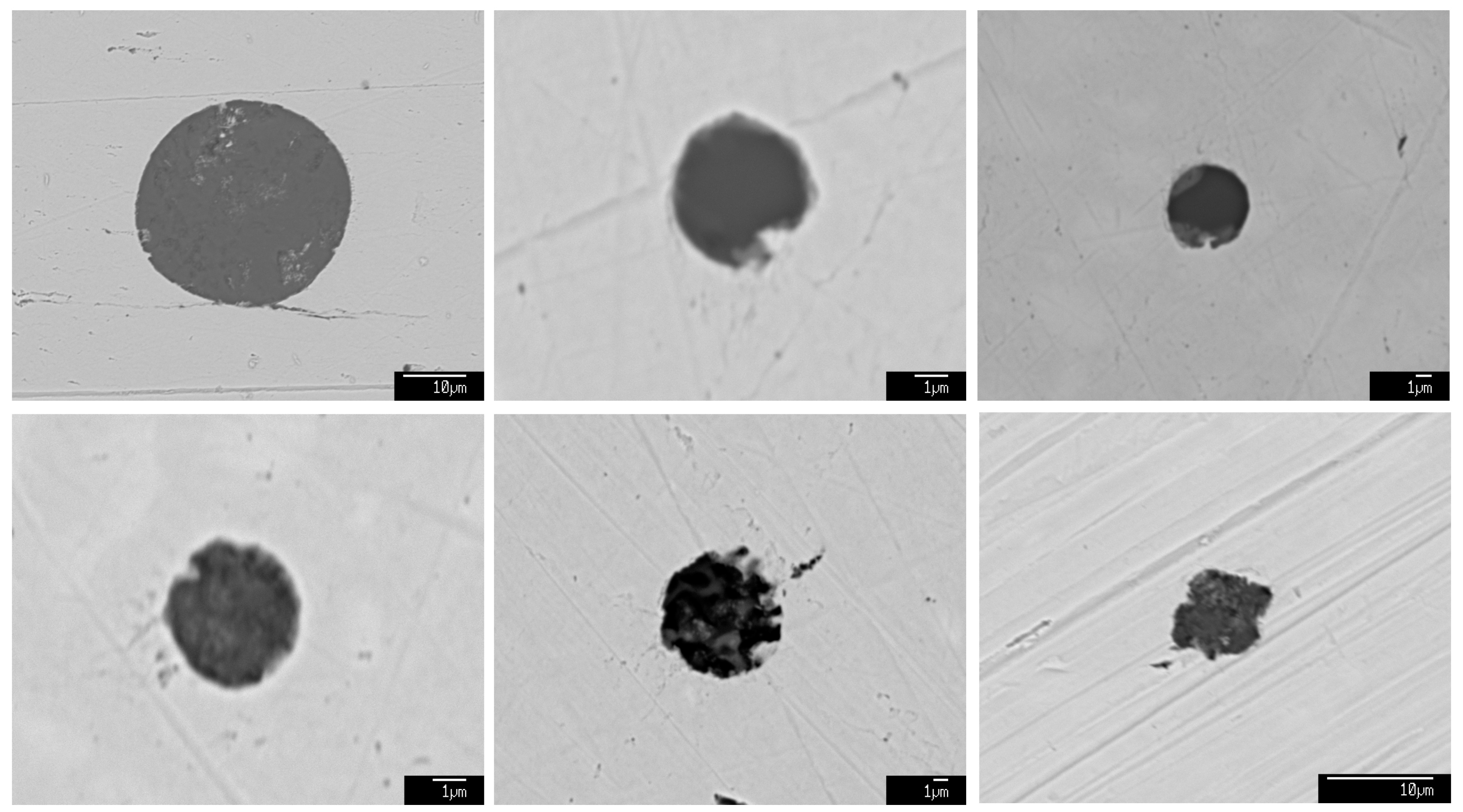
3.1. Characteristics of the Inclusions at Different Stages
3.2. Number Density and Size Distribution of Inclusions
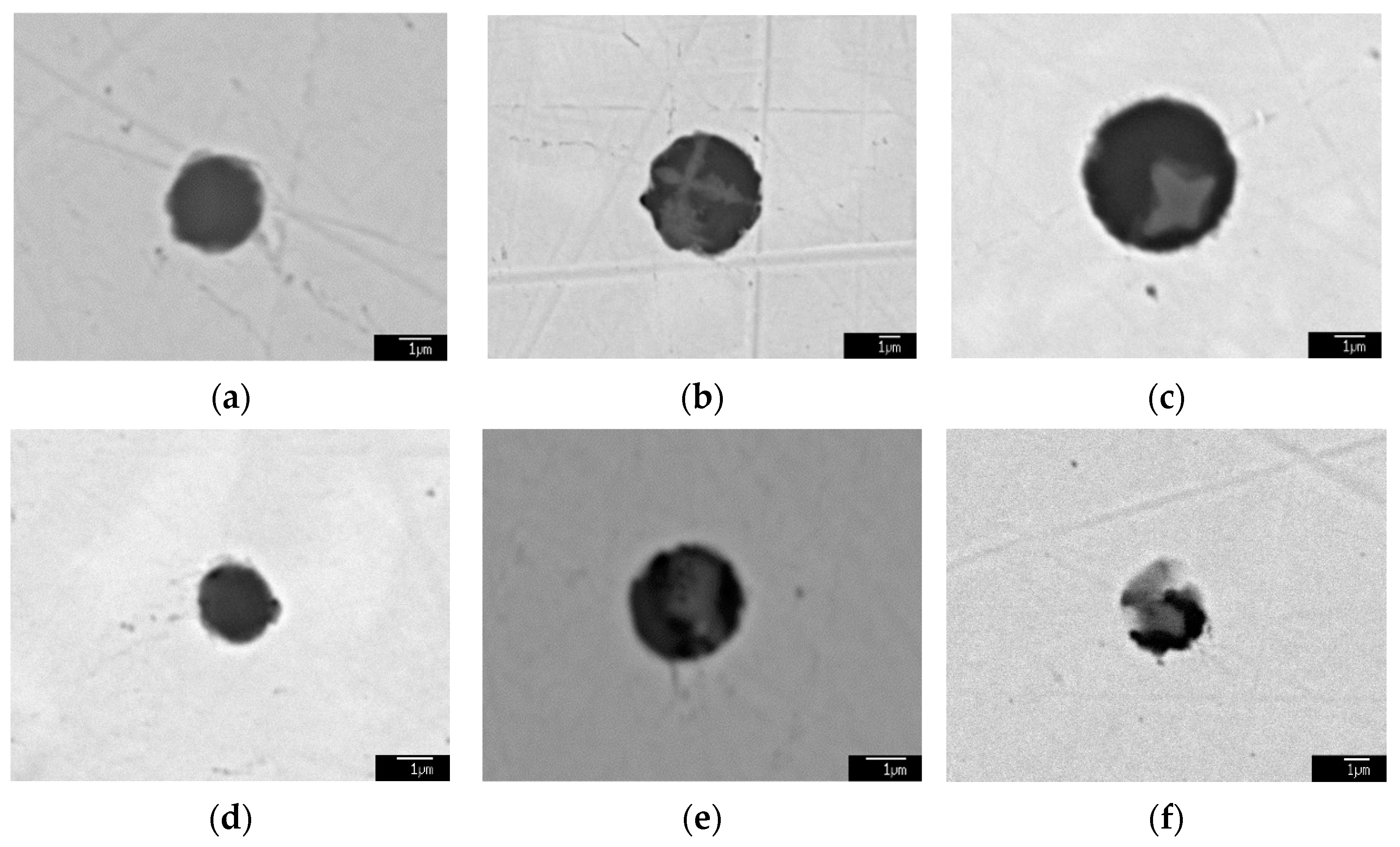
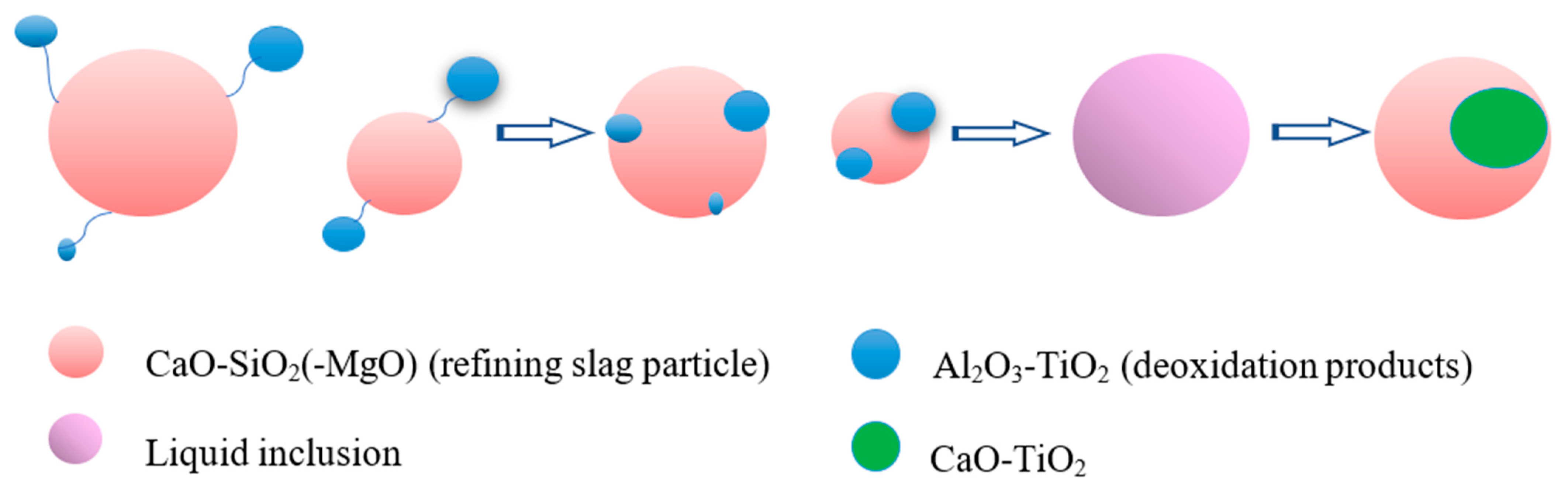
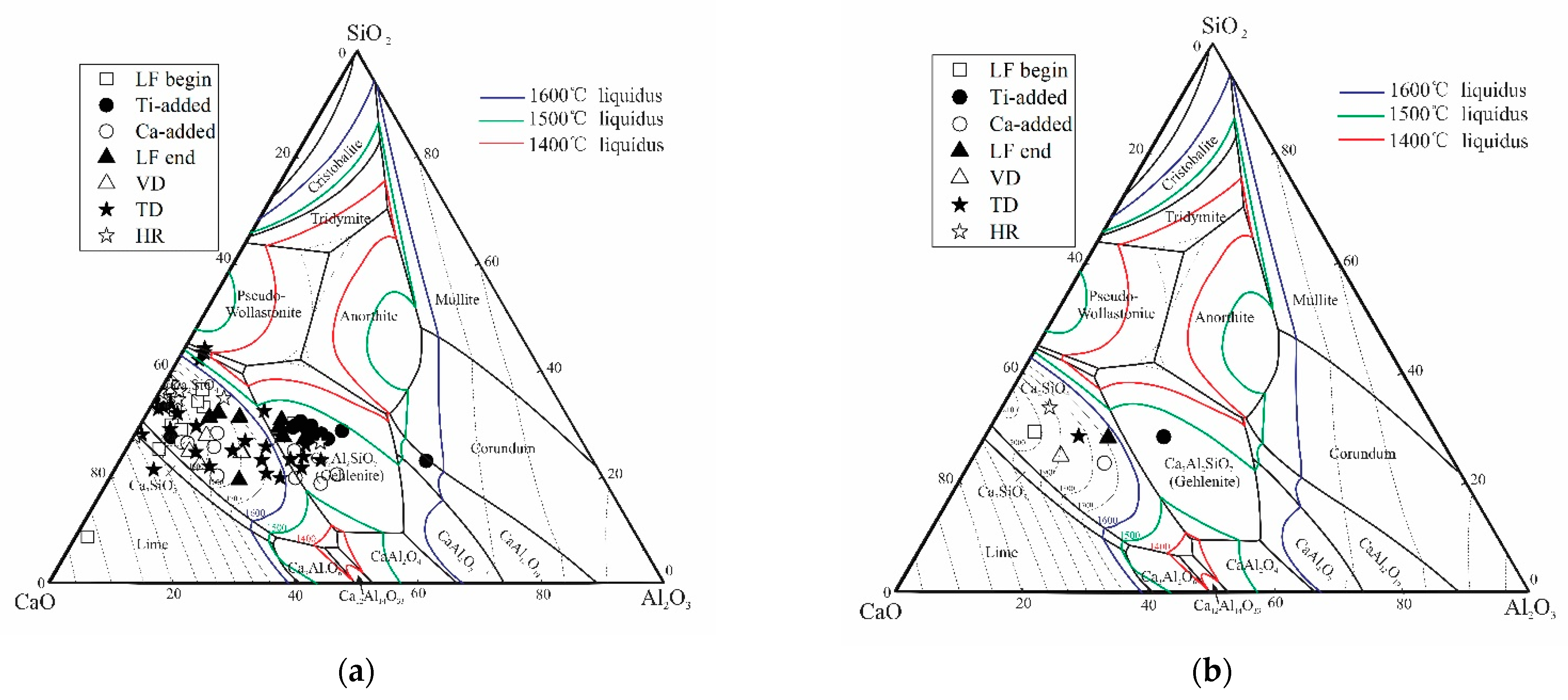
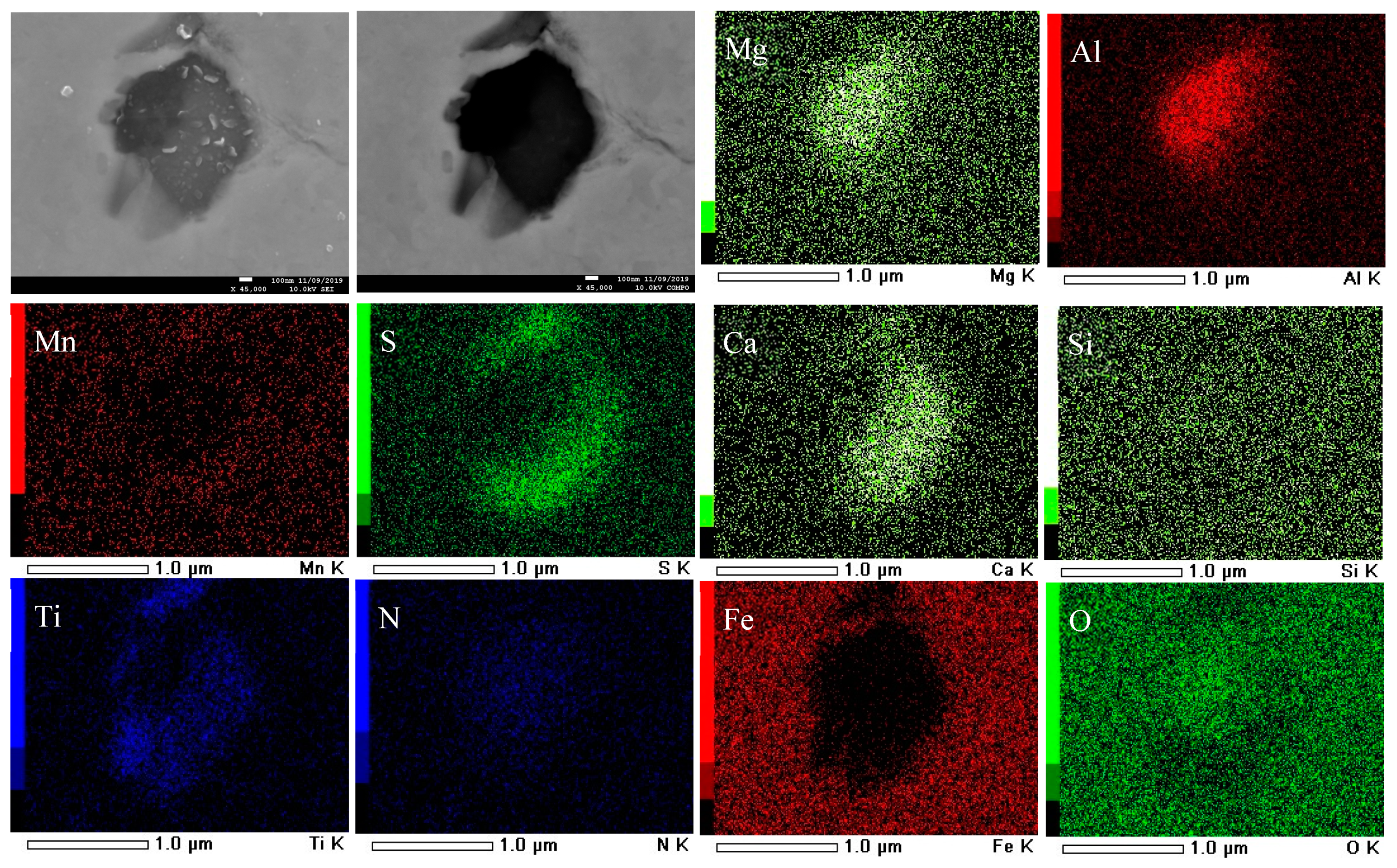
3.3. Formation Mechanism and Evolution of CaO-Al2O3-SiO2-(MgO)-TiOx Inclusion
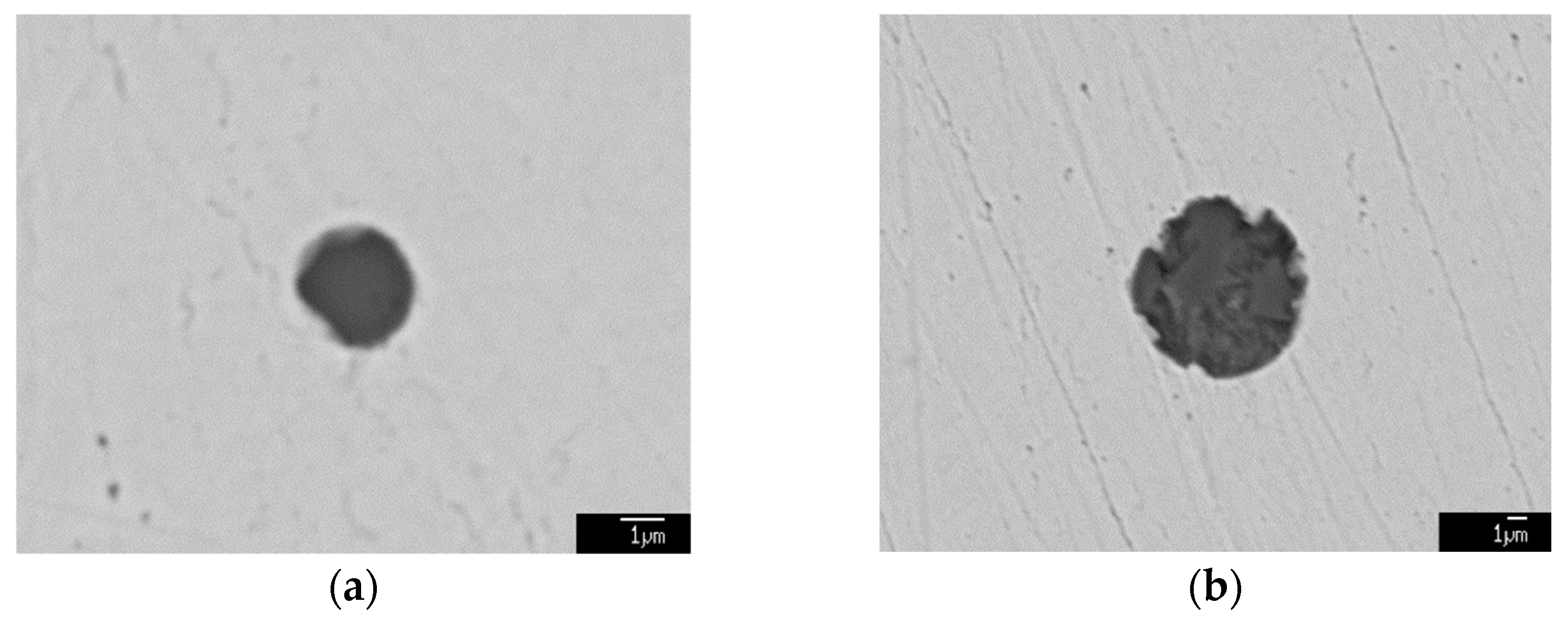
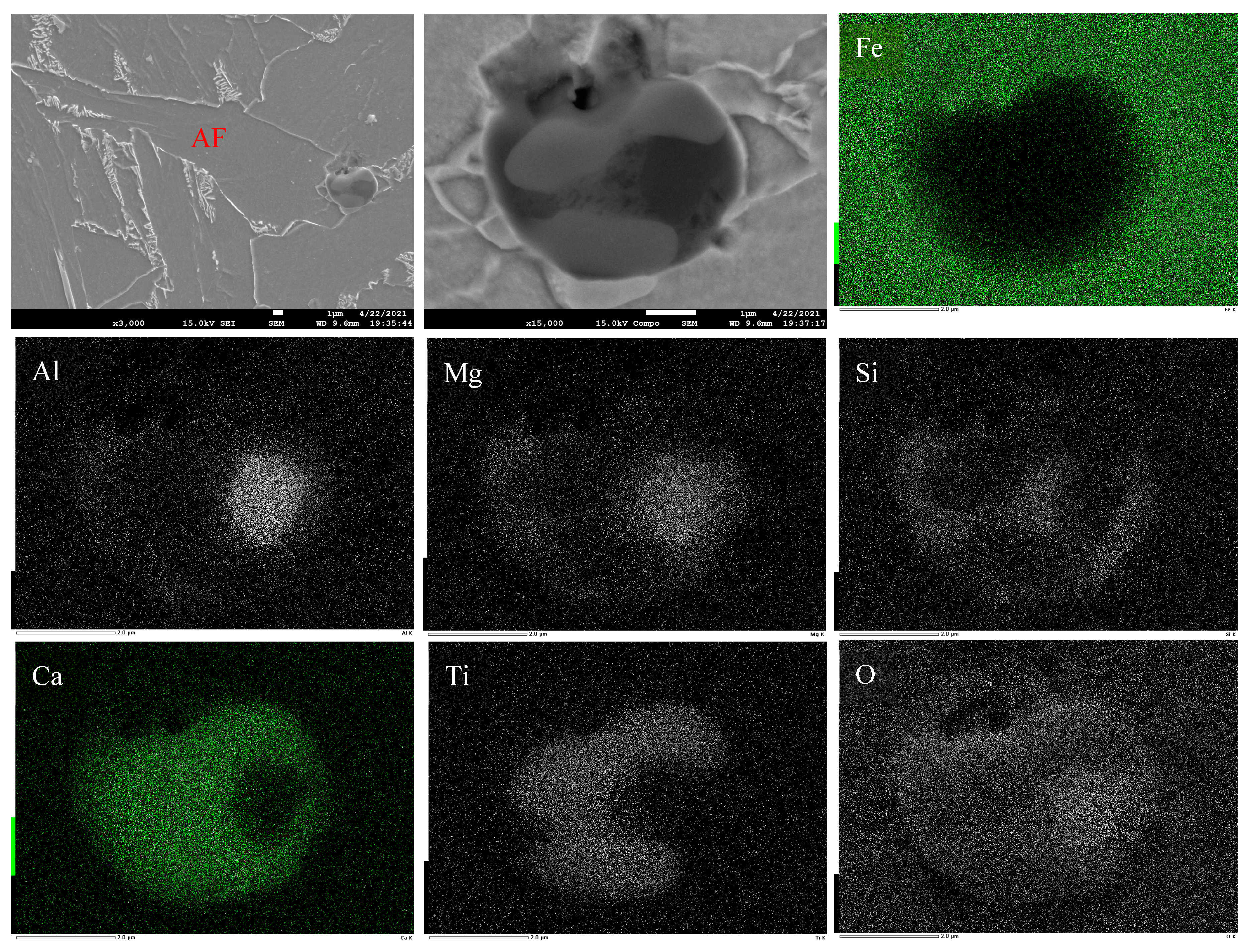
3.4. Formation Mechanism and Evolution of CaO-Al2O3-SiO2 Inclusion
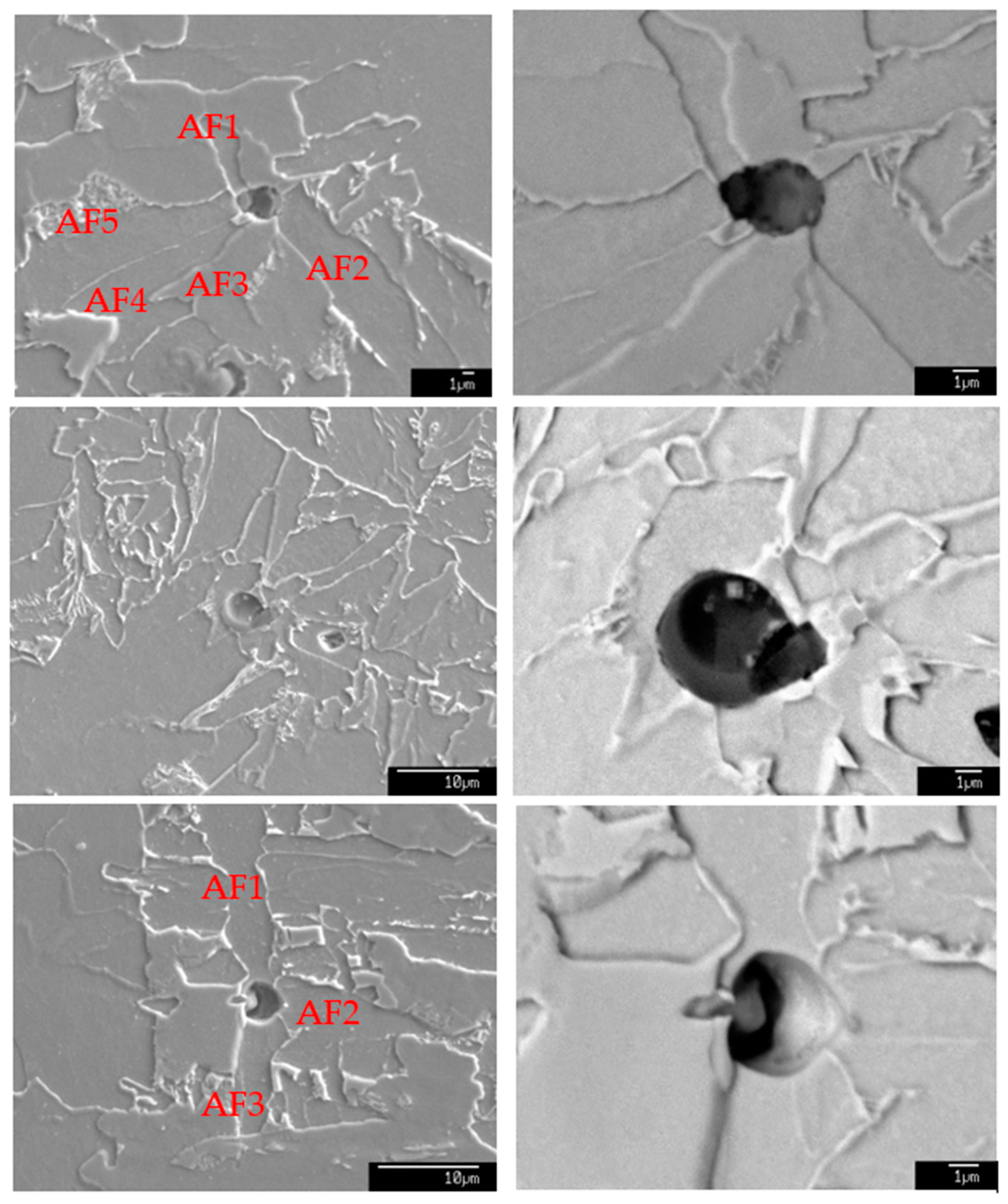
3.5. Effect of Inclusions on IAF Formation
4. Conclusions
- The evolution of inclusions during different stages in Ti-Ca-treated offshore structural steel is from primary deoxidization products (Al2O3-MnO, Al2O3-SiO2-MnO) and their combination with lime (CaO-Al2O3-SiO2-MnO and CaO-SiO2)→CaO-Al2O3-SiO2-(MgO)-TiOx and CaO-Al2O3-SiO2→CaO-Al2O3-SiO2-(MgO)-TiOx, CaO-Al2O3-SiO2, and secondary deoxidization products (Al2O3-MnO-TiOx and SiO2-MnO)→CaO-Al2O3-SiO2-(MgO)-TiOx and CaO-Al2O3-SiO2. The number density of the inclusions in Ti-Ca-treated industrial steel generally dropped from LF, VD, and Tundish to the final product, except for the Ti-Fe and Si-Ca addition in the LF, the number density slightly increased. The total decrease in the inclusion number density was mainly due to the significantly decreasing number density of small inclusions (<1 µm) during the refining process.
- The formation mechanism of CaO-Al2O3-SiO2-(MgO)-TiOx inclusion was due to CaO-SiO2-(MgO) from refining slag and refractory combining with the deoxidization product Al2O3 and TiOx. With the refining process proceeding, Ti-oxide continuously increased and gradually “entered” the inclusion and formed the core of the multiphase inclusions while the Al2O3 component generally decreased due to the reduction of Ca, so the average content of CaO showed an adverse trend when compared with that of Al2O3.
- The formation mechanism of CaO-Al2O3-SiO2 inclusions is the initial 2CaO∙Al2O3∙SiO2 inclusion came from the combination of CaO-SiO2 particles in refining slag and the deoxidization product Al2O3, and its liquidus was lower than that of molten steel, so it presented a liquid state in steel and had a smooth surface. After Ca addition, the initial 2CaO∙Al2O3∙SiO2 was gradually transferred to 2CaO∙ SiO2 with Al2O3 continuously reduced by Ca. 2CaO∙ SiO2 had a higher liquidus than that of molten steel, so it presented as a solid state in steel and had a rough surface.
- In Ti-Ca-treated offshore structural steel, after welding simulation, CaO-Al2O3-SiO2 inclusions were not effective at inducing IAFs while CaO-Al2O3-SiO2-(MgO)-TiOx inclusions were proven to be effective nucleation sites for promoting IAFs. The Al2O3-MgO spinel component in welding samples may have different formation mechanisms: one is that it formed directly in molten steel as a solid state, and other phases and inclusions, such as CaO-TiOx and MnS, precipitated on Al2O3-MgO spinel, so the interface between each phase was clear. Another is that CaO-Al2O3-SiO2-(MgO)-TiOx as a whole formed in molten steel as a liquid state, and Al2O3-MgO spinel firstly precipitated due to its highest melting point and was followed by other phases, so the interface between each phase was not clear.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, X.L.; Wu, K.M.; Wang, H.H.; Li, G.Q. Applications of Oxide Metallurgy on High Heat Input Welding Steel. China Metall. 2015, 25, 6–12. [Google Scholar]
- Villegas, R.; Redjaimia, A.; Confente, M.; Perrot-Simonetta, M.T. Fractal Nature of Acicular Ferrite, and Fine Precipitation in Medium Carbon Micro-Alloyed Forging Steels. Proc. New Developments on Metallurgy and Applications of High Strength Steels. Ed. Asoc. Argentina de Materiális. Buenos Aires. 2008. Available online: https://www.researchgate.net/publication/301840861_Proc_of_New_Developments_on_Metallurgy_and_Applications_of_High_Strength_Steels_Conference_Minerals_Wiley_John_Sons_Incorporated (accessed on 15 February 2022).
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the Role of Non-metallic Inclusions in the Nucleation of Acicular Ferrite in Steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef] [Green Version]
- wan Kim, J.; koo Kim, S.; sik Kim, D.; deuk Lee, Y.; keun Yang, P. Formation Mechanism of Ca-Si-Al-Mg-Ti-O Inclusions in Type 304 Stainless Steel. ISIJ Int. 1996, 36, 140–143. [Google Scholar] [CrossRef]
- Kanazawa, S.; Nakashima, A.; Okamoto, K.; Kanaya, K. Improved Toughness of Weld Fussion Zone by Fine TiN Particles and Development of a Steel for Large Heat Input Welding. Tetsu Hagané 1975, 61, 2589–2603. [Google Scholar] [CrossRef] [Green Version]
- Takamura, J.; Mizoguchi, S. Roles of Oxides in Steels Performance. In Proceedings of the 6th International Iron and Steel Congress, ISIJ, Nagoya, Japan, 21–26 October 1990; Volume I, pp. 591–597. [Google Scholar]
- Mizoguchi, S.; Takamura, J. Control of Oxides as Inoculants-Metallurgy of Oxides in Steels. In Proceedings of the 6th International Iron and Steel Congress, ISIJ, Nagoya, Japan, 21–26 October 1990; Volume I, pp. 598–604. [Google Scholar]
- Sawai, T.; Wakoh, M.; Ueshima, Y.; Mizoguchi, S. Effects of Zirconium on the Precipitation of MnS in Low Carbon Steels. In Proceedings of the 6th International Iron and Steel Congress, ISIJ, Nagoya, Japan, 21–26 October 1990; Volume I, pp. 605–611. [Google Scholar]
- Ogibayashi, S.; Yamaguchi, K.; Hirai, M.; Goto, H. The Features of Oxides in Ti-deoxidized Steel. In Proceedings of the 6th International Iron and Steel Congress, ISIJ, Nagoya, Japan, 21–26 October 1990; Volume I, pp. 612–617. [Google Scholar]
- Kojima, A.; Yoshii, K.I.; Hada, T.; Saeki, O.; Ichikawa, K.; Yoshida, Y.; Shimura, Y.; Azuma, K. Development of High Toughness Steel Plates for Box Columns with High Heat Input Welding. Shinnittetsu Giho. 2004, 90, 39–44. [Google Scholar]
- Kojima, A.; Kiyose, A.; Uemori, R.; Minagawa, M.; Hoshino, M.; Nakashima, T.; Ishida, K.; Yasui, H. Super High HAZ Toughness Technology with Fine Microstructure Imparted by Fine Particles. Shinnittetsu Giho. 2004, 380, 2–6. [Google Scholar]
- Uemori, R. Analysis of the Crystallographic Direction of the Intra-granular Ferrite in Low Alloy Steel. CAMP-ISIJ 2001, 14, 1174. [Google Scholar]
- Kojima, A.; Tomoya, K.; Noboru, H. Effect of Microstructure and Chemical Composition on Laser Weld Metal Toughness. Welded Structure Symposium 2002. Collect. Lect. Pap. 2002, 11, 327. [Google Scholar]
- Kojima, A. High HAZ Toughness Steels with Fine Microstructure Imparted by Fine Particles. CAMP-ISIJ 2003, 16, 360. [Google Scholar]
- Shigesato, G. Progress of High-Performance Steel Plates with Excellent HAZ Toughness. Nippon. Steel Sumitomo Met. Tech. Rep. 2018, 119, 22–25. [Google Scholar]
- Ye, G.; Jönsson, P.; Lund, T. Thermodynamics and Kinetics of the Modification of Al2O3 Inclusions. ISIJ Int. 1996, 36, 105–108. [Google Scholar] [CrossRef]
- Ito, Y.I.; Suda, M.; Kato, Y.; Nakato, H.; Sorimachi, K.I. Kinetics of Shape Control of Alumina Inclusions with Calcium Treatment in Line Pipe Steel for Sour Service. ISIJ Int. 1996, 36, S148–S150. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, Y.; Numata, M.; Fukagawa, S.; Shinme, K. Inclusion Modification by Calcium Treatment. ISIJ Int. 1996, 36, S151–S154. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Sato, S.; Ohta, H.; Shiwaku, T. Effects of Ca Addition on Formation Behavior of TiN Particles and HAZ Toughness in Large-Heat-Input-Welding. Kobelco Technol. Rev. 2011, 30, 76–79. [Google Scholar]
- Wang, K.; Jiang, M.; Wang, X.; Wang, Y.; Zhao, H.; Cao, Z. Formation Mechanism of CaO-SiO2-Al2O3-MgO Inclusions in Si-Mn-Killed Steel with Limited Aluminum Content during Low Basicity Slag Refining. Metall. Mater. Trans. B 2016, 47, 282–290. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Kang, J.; Yuan, G.; Wang, G. Effect of Thermomechanical Treatment on Acicular Ferrite Formation in Ti-Ca Deoxidized Low Carbon Steel. Metals 2019, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, C.; Kang, J.; Yuan, G.; Misra, R.D.K.; Wang, G. Improved Toughness of Double-pass Welding Heat Affected Zone by Fine Ti-Ca Oxide Inclusions for High-strength Low-alloy Steel. Mater Sci. Eng. A 2020, 780, 139198. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hasegawa, T.; Takamura, J.I. Effect of Boron on Intra-granular Ferrite Formation in Ti-Oxide Bearing Steels. ISIJ Int. 1996, 36, 80–86. [Google Scholar] [CrossRef]
- Mabuchi, H.; Uemori, R.; Fujioka, M. The Role of Mn Depletion in Intra-Granular Ferrite Transformation in the Heat Affected Zone of Welded Joints with Large Heat Input in Strucutral Steels. ISIJ Int. 1996, 36, 1406–1412. [Google Scholar] [CrossRef]
- Fukunaga, K.; Chijiiwa, R.; Watanabe, Y.; Kojima, A.; Nagai, Y.; Mamada, N.; Adachi, T.; Date, A.; Taniguchi, S.; Uemori, R.; et al. Advanced Titanium Oxide Steel with Excellent HAZ Toughness for Offshore Structures. In Proceedings of the ASME 2010 29th International Conference on Ocean, Offshore and Arctic Engineering OMAE2010, Shanghai, China, 6–11 June 2010; pp. 1–9. [Google Scholar]
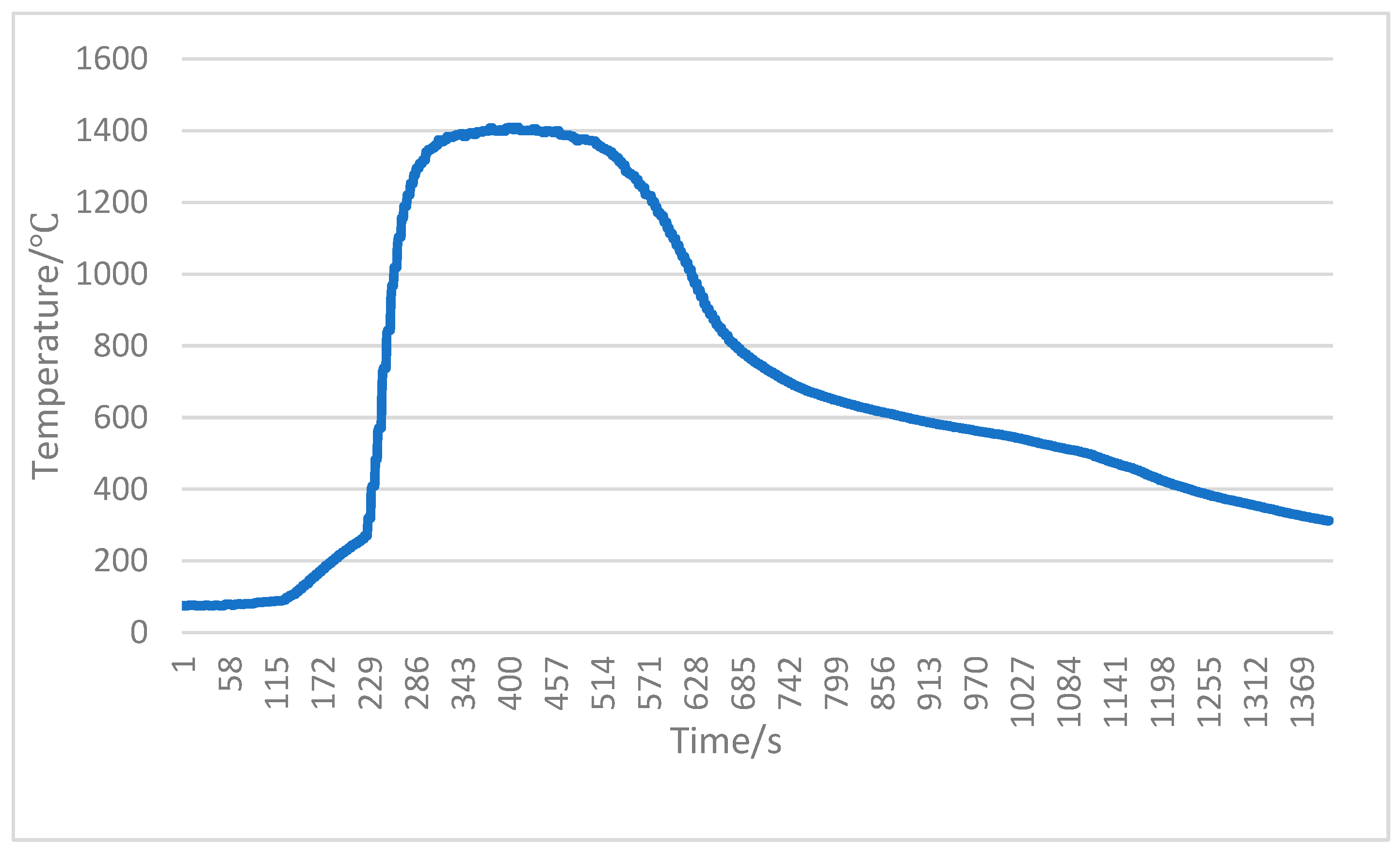


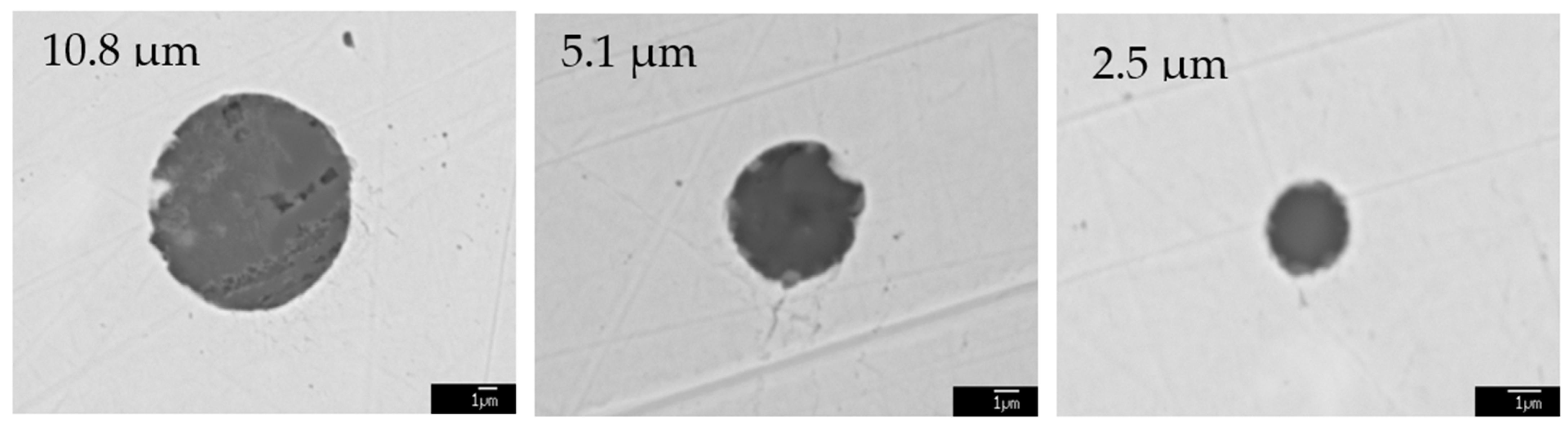
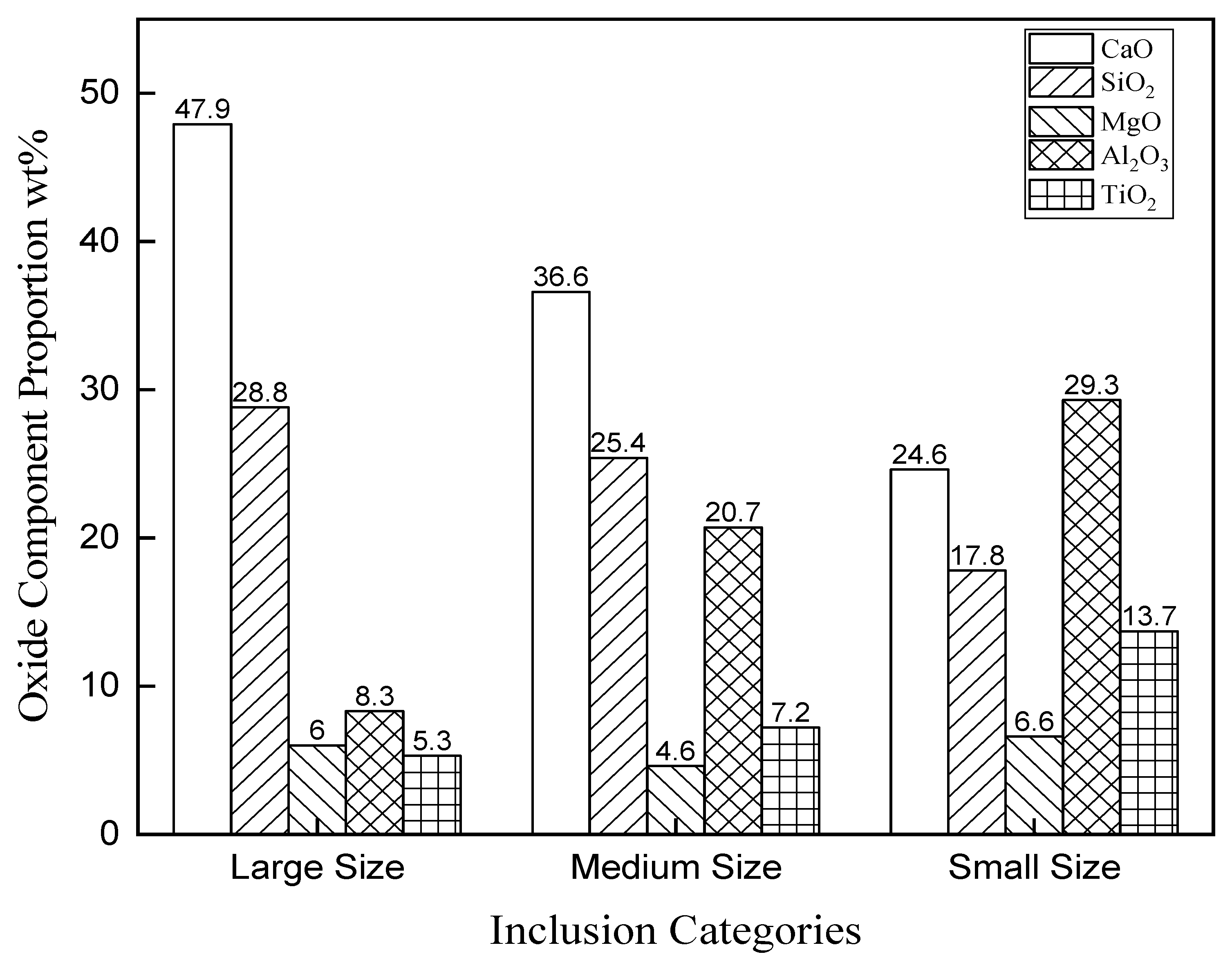
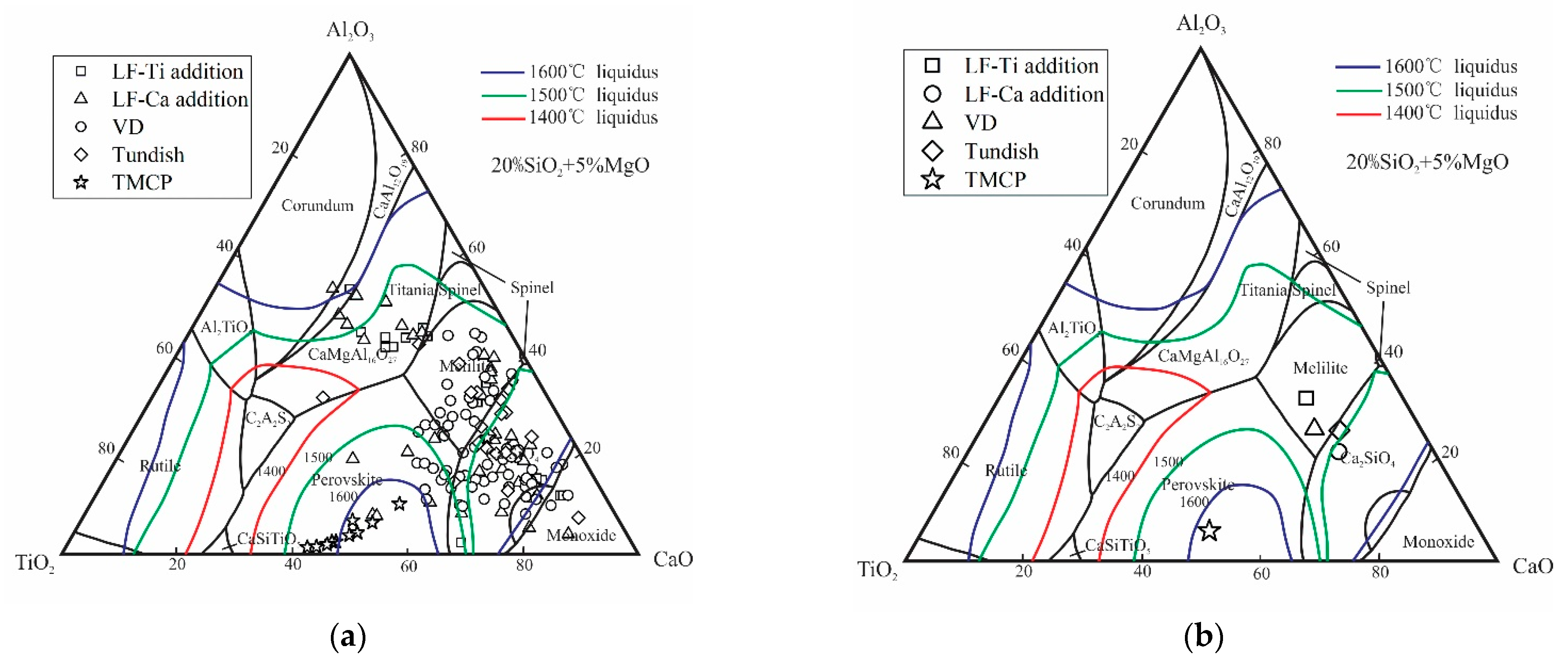



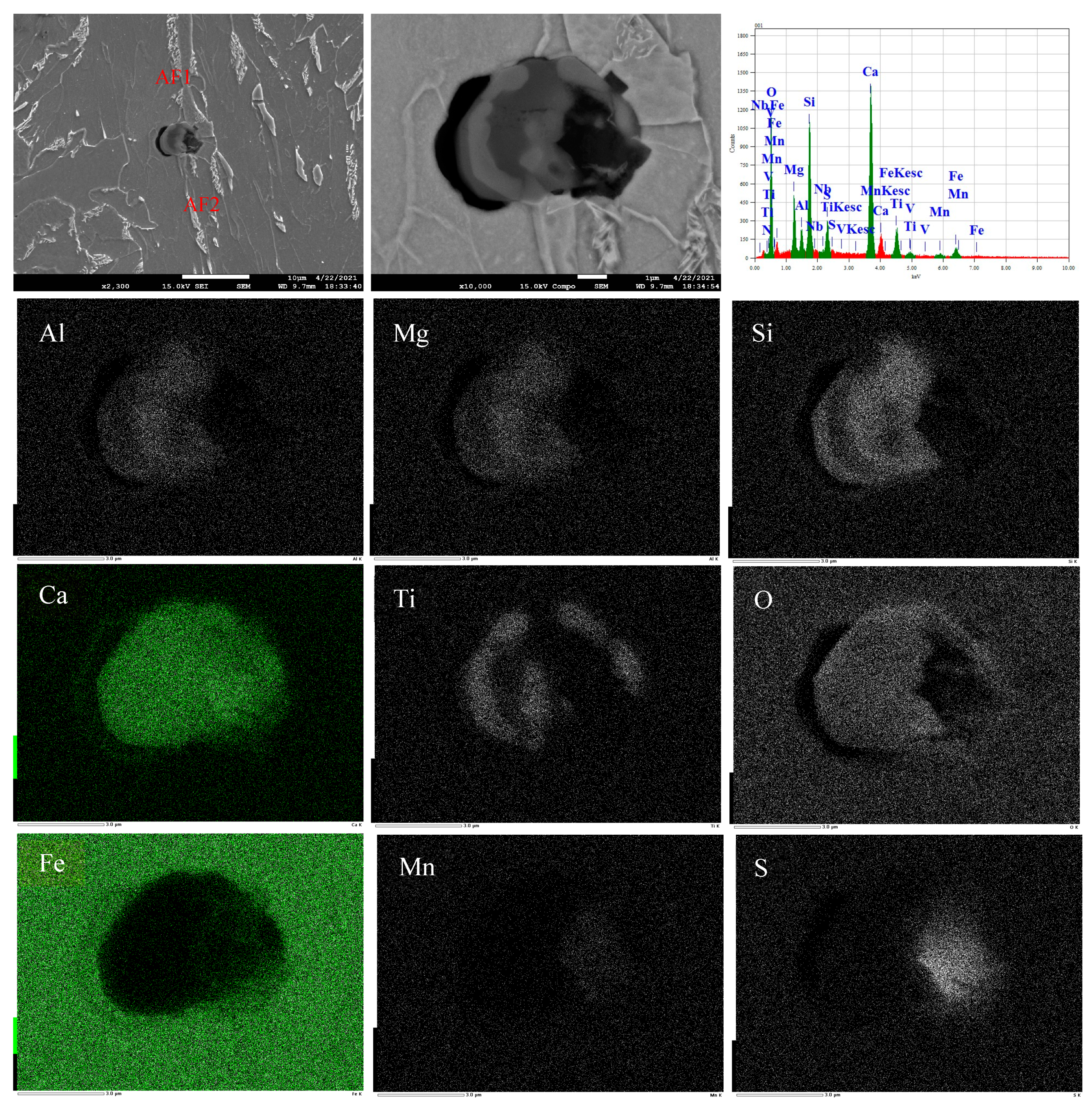
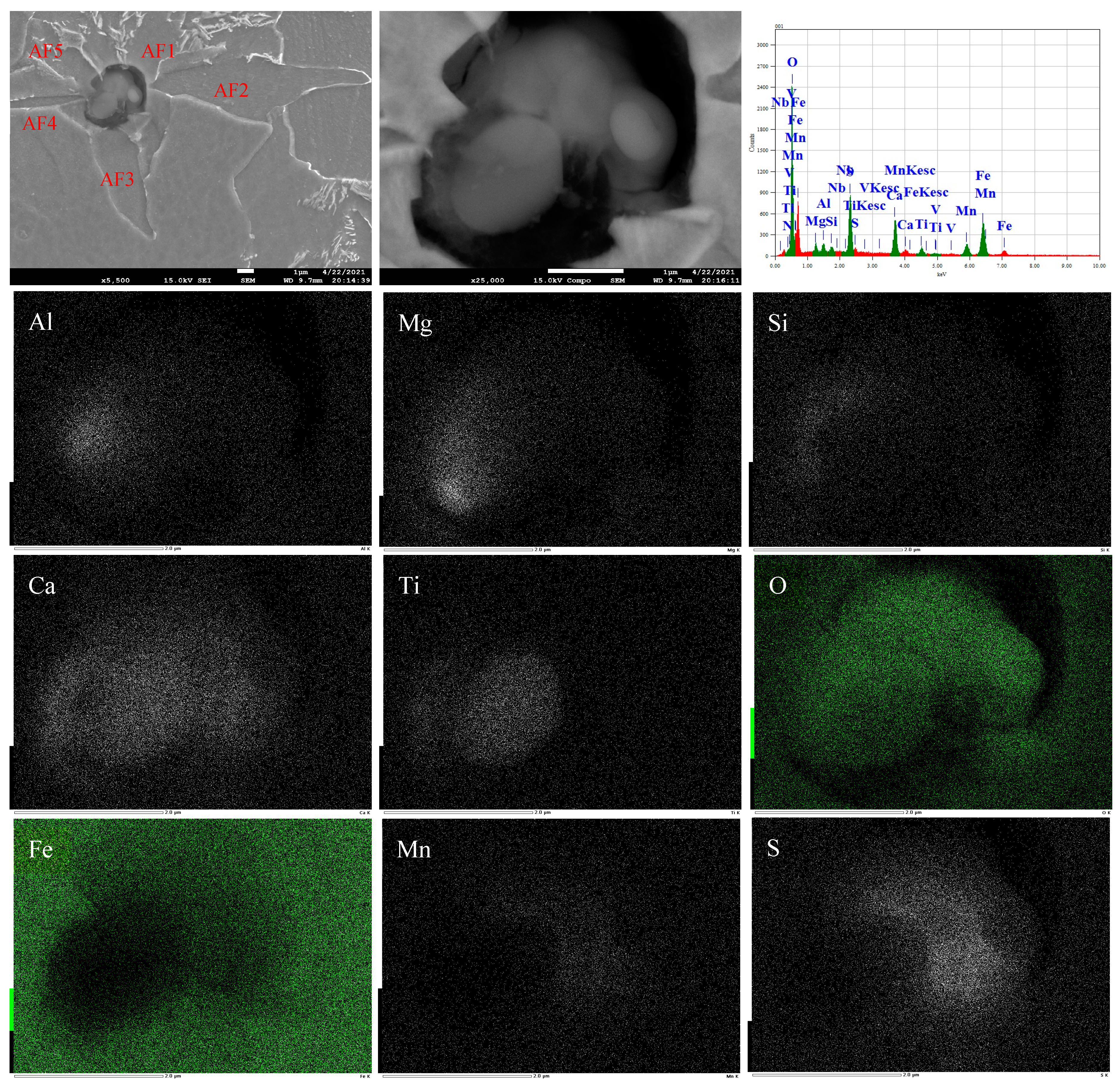
| No. | C | Si | Mn | P | S | Nb | V | Al | O | Ti | Ca |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LF begin | 0.025 | 0.089 | 1.29 | 0.013 | 0.025 | - | - | 0.0062 | 0.0110 | - | - |
| Ti added | 0.032 | 0.201 | 1.31 | 0.013 | 0.0070 | 0.02 | 0.039 | 0.0065 | 0.0068 | 0.0042 | - |
| Ca added | 0.048 | 0.200 | 1.43 | 0.013 | 0.0034 | 0.02 | 0.039 | 0.0058 | 0.0031 | 0.015 | 0.0015 |
| VD | 0.066 | 0.214 | 1.47 | 0.013 | 0.0022 | 0.022 | 0.043 | 0.0052 | 0.0035 | 0.010 | 0.0026 |
| Tundish | 0.065 | 0.216 | 1.47 | 0.013 | 0.0021 | 0.023 | 0.043 | 0.0047 | 0.0032 | 0.010 | 0.0016 |
| TMCP | 0.069 | 0.222 | 1.52 | 0.013 | 0.0021 | 0.023 | 0.044 | 0.0040 | 0.0031 | 0.014 | 0.0014 |
| Inclusions | Chemical Composition | |||||
|---|---|---|---|---|---|---|
| CaO | Al2O3 | SiO2 | MgO | TiO2 | MnO | |
| Al2O3-MnO | <10% | >60% | <10% | <10% | <10% | >10% |
| Al2O3-SiO2-MnO | <10% | >10% | >20% | <10% | <10% | >10% |
| CaO-Al2O3-SiO2-MnO | >10% | >10% | >10% | <10% | <10% | >10% |
| Al2O3-MnO-TiOx | <10% | >20% | <10% | <10% | >30% | >10% |
| SiO2-MnO | <10% | >30% | <10% | <10% | <10% | >30% |
| CaO-SiO2 | >40% | <10% | >10% | <10% | <10% | <10% |
| CaO-Al2O3-SiO2 | >20% | >20% | >20% | <10% | <10% | <10% |
| CaO-Al2O3-SiO2-TiOx | >20% | >10% | >10% | <10% | >10% | <10% |
| CaO-TiOx | >30% | <10% | <10% | <10% | >30% | <10% |
| No. | CaO | Al2O3 | SiO2 | MgO | TiO2 | MnO | FeO | S |
|---|---|---|---|---|---|---|---|---|
| a | 40.6 | 25.5 | 20.5 | 2.3 | 2.9 | 0.2 | 7.0 | 0.8 |
| b | 59.2 | 2.8 | 31.3 | 0.3 | 0.9 | 0.2 | 5.1 | 0.1 |
| No. | CaO | Al2O3 | SiO2 | MgO | TiO2 | MnO | FeO | S |
|---|---|---|---|---|---|---|---|---|
| 1 | 22.7 | 15.7 | 0.7 | 6.6 | 15.3 | 16.1 | 12.1 | 11.0 |
| 2 | 27.7 | 19.2 | 6.9 | 9.2 | 22.8 | 2.8 | 6.6 | 4.7 |
| No. | CaO | Al2O3 | SiO2 | MgO | TiO2 | MnO | FeO | S | Composition |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40.5 | 29.2 | 24.9 | 3.0 | 0.3 | 0.1 | 2.4 | 0.0 | CaO-Al2O3-SiO2 |
| 2 | 40.3 | 1.4 | 0.5 | 0.3 | 54.7 | 0.0 | 2.9 | 0.0 | CaO-TiOx |
| 3 | 53.4 | 0.1 | 0.0 | 0.3 | 0.0 | 6.7 | 9.1 | 30.3 | (Ca, Mn) S |
| 4 | 15.1 | 46.2 | 3.2 | 19.6 | 0.9 | 2.8 | 8.1 | 3.6 | CaO-Al2O3-MgO |
| 5 | 53.4 | 0.2 | 27.6 | 1.9 | 0.1 | 0.5 | 16.4 | 0.1 | CaO-SiO2 |
| No. | CaO | Al2O3 | SiO2 | MgO | TiO2 | MnO | FeO | S |
|---|---|---|---|---|---|---|---|---|
| 1 | 13.4 | 29.3 | 8.0 | 24.8 | 19.1 | 1.6 | 10.6 | 0.1 |
| 2 | 11.7 | 16.5 | 16.3 | 20.8 | 30.1 | 1.7 | 3.3 | 0.2 |
| 3 | 3.7 | 18.2 | 0.3 | 3.8 | 36.1 | 3.2 | 37.3 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, Z.; Liu, H.; Zhang, P.; Wang, F.; Wang, G.; Zhao, B.; Tang, F.; Ma, X. The Formation Mechanisms and Evolution of Multi-Phase Inclusions in Ti-Ca Deoxidized Offshore Structural Steel. Metals 2022, 12, 511. https://doi.org/10.3390/met12030511
Rong Z, Liu H, Zhang P, Wang F, Wang G, Zhao B, Tang F, Ma X. The Formation Mechanisms and Evolution of Multi-Phase Inclusions in Ti-Ca Deoxidized Offshore Structural Steel. Metals. 2022; 12(3):511. https://doi.org/10.3390/met12030511
Chicago/Turabian StyleRong, Zhe, Hongbo Liu, Peng Zhang, Feng Wang, Geoff Wang, Baojun Zhao, Fengqiu Tang, and Xiaodong Ma. 2022. "The Formation Mechanisms and Evolution of Multi-Phase Inclusions in Ti-Ca Deoxidized Offshore Structural Steel" Metals 12, no. 3: 511. https://doi.org/10.3390/met12030511
APA StyleRong, Z., Liu, H., Zhang, P., Wang, F., Wang, G., Zhao, B., Tang, F., & Ma, X. (2022). The Formation Mechanisms and Evolution of Multi-Phase Inclusions in Ti-Ca Deoxidized Offshore Structural Steel. Metals, 12(3), 511. https://doi.org/10.3390/met12030511








