Effect of Zr on Microstructure and Mechanical Properties of the Al–Cu–Yb and Al–Cu–Gd Alloys
Abstract
:1. Introduction
2. Materials and Methods
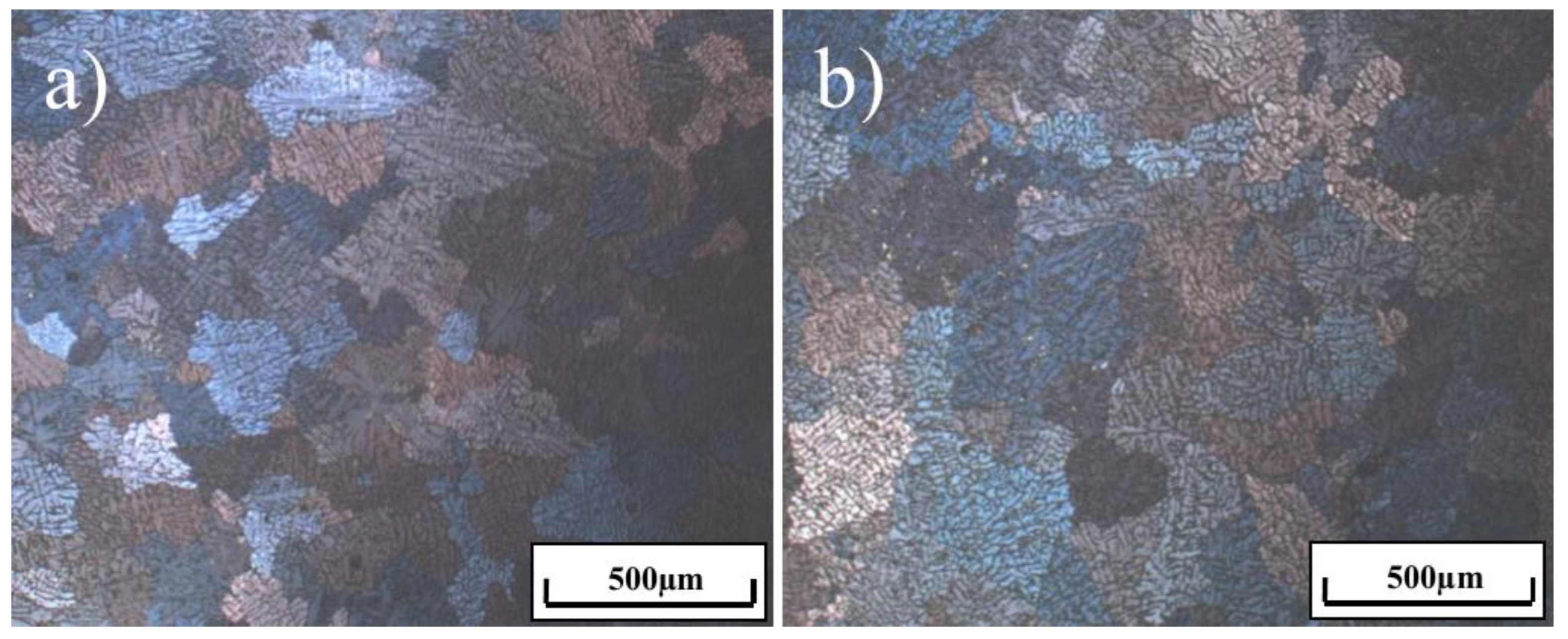
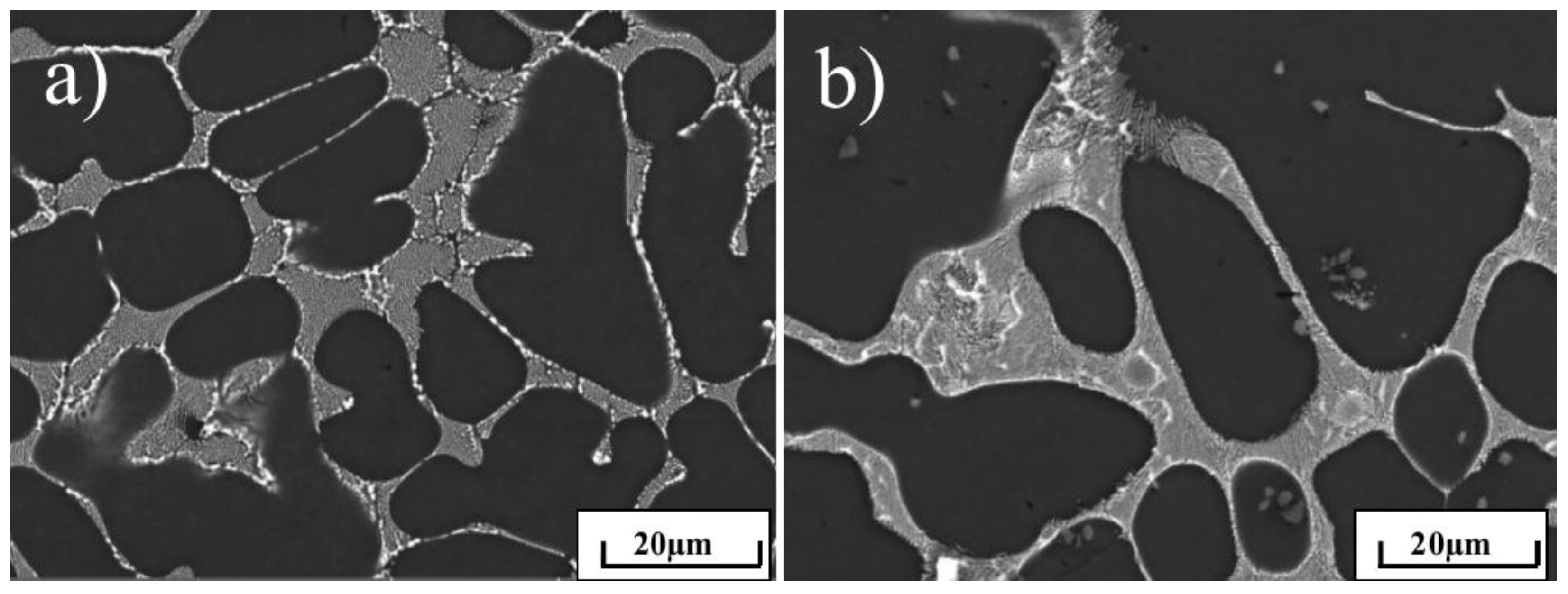
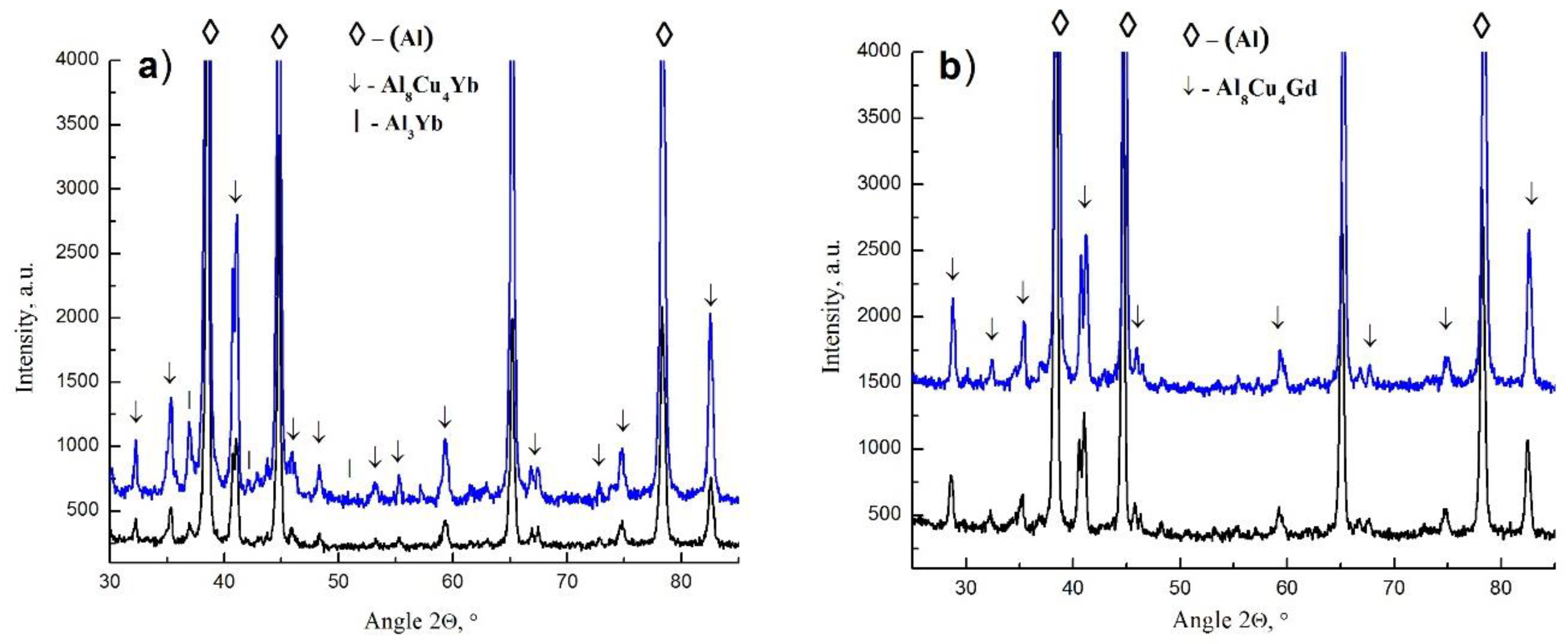
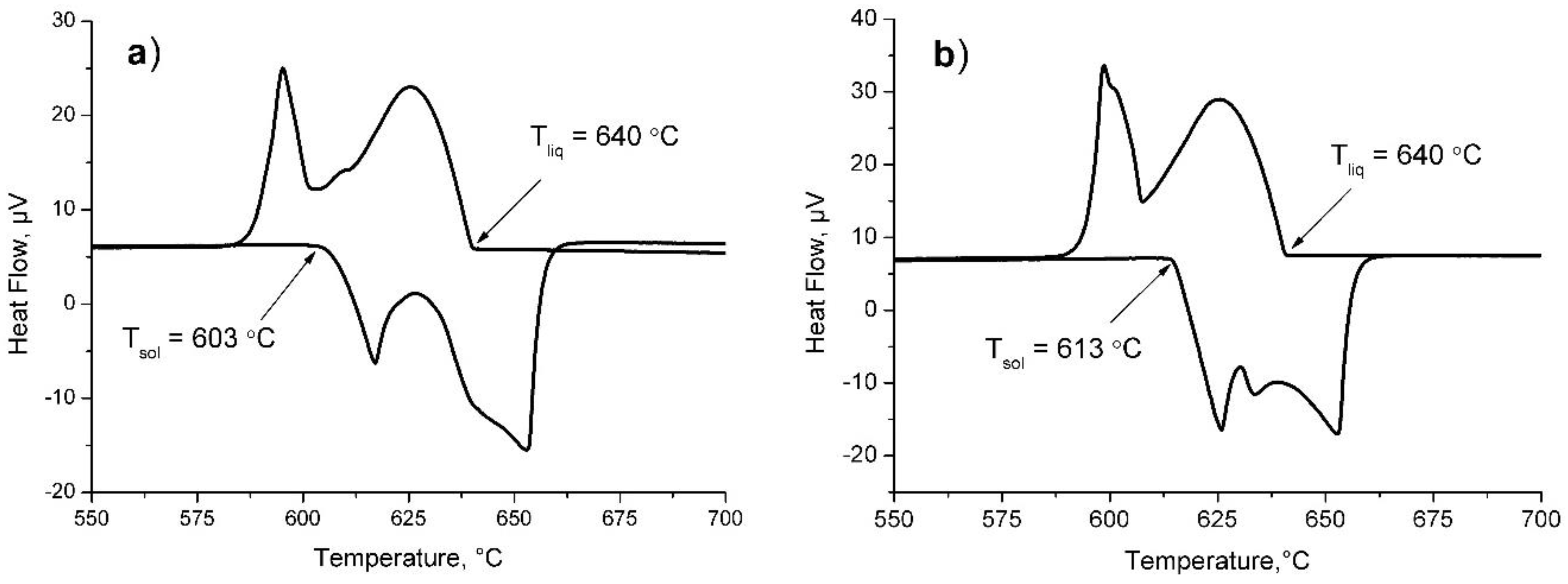
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ASM International Committee Heat Treating of Aluminum; ASM International: Materials Park, OH, USA, 1990; Volume 4, pp. 841–879. [CrossRef]
- Gao, L.; Ou, X.; Ni, S.; Li, K.; Du, Y.; Song, M. Effects of θ′ precipitates on the mechanical performance and fracture behavior of an Al–Cu alloy subjected to overaged condition. Mater. Sci. Eng. A 2019, 762, 138091. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Liu, K.; Pan, L.; Breton, F.; Chen, X.G. Enhanced mechanical properties of high-temperature-resistant Al–Cu cast alloy by microalloying with Mg. J. Alloys Compd. 2020, 827, 154305. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Lan, Y.; Pei, R.; Feng, X.; Xia, T.; Liu, D. Effect of scandium addition on microstructure and mechanical properties of as-cast Al-5%Cu alloys. Vacuum 2020, 177, 109385. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, F.; Ai, F.; Yan, H. Effects of rare earth Er additions on microstructure development and mechanical properties of die-cast ADC12 aluminum alloy. J. Alloys Compd. 2012, 538, 21–27. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, P.; Ma, C. Microstructures and mechanical properties of Al-Cu-Mn alloy with La and Sm addition. Rare Met. 2012, 31, 332–335. [Google Scholar] [CrossRef]
- Belov, N.A.; Khvan, A.V. The ternary Al-Ce-Cu phase diagram in the aluminum-rich corner. Acta Mater. 2007, 55, 5473–5482. [Google Scholar] [CrossRef]
- Belov, N.A.; Khvan, A.V.; Alabin, A.N. Microstructure and phase composition of Al–Ce–Cu alloys in the Al-rich corner. In Materials Science Forum; Trans Tech Publications: Bäch, Switzerland, 2006; Volume 519–521, pp. 395–400. [Google Scholar]
- Amer, S.M.; Barkov, R.Y.; Yakovtseva, O.A.; Pozdniakov, A.V. Comparative Analysis of Structure and Properties of Quasibinary Al–6.5Cu–2.3Y and Al–6Cu–4.05Er Alloys. Phys. Met. Metallogr. 2020, 121, 476–482. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y. Microstructure and materials characterisation of the novel Al–Cu–Y alloy. Mater. Sci. Technol. 2018, 34, 1489–1496. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Iron and Silicon Impurities on Phase Composition and Mechanical Properties of Al–6.3Cu–3.2Y Alloy. Phys. Met. Metallogr. 2020, 121, 1002–1007. [Google Scholar] [CrossRef]
- Pozdnyakov, A.V.; Barkov, R.Y.; Sarsenbaev, Z.; Amer, S.M.; Prosviryakov, A.S. Evolution of Microstructure and Mechanical Properties of a New Al–Cu–Er Wrought Alloy. Phys. Met. Metallogr. 2019, 120, 614–619. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Impurities on the Phase Composition and Properties of a Wrought Al–6% Cu–4.05% Er Alloy. Phys. Met. Metallogr. 2020, 121, 495–499. [Google Scholar] [CrossRef]
- Amer, S.; Barkov, R.; Pozdniakov, A. Microstructure and mechanical properties of novel quasibinary al-cu-yb and al-cu-gd alloys. Metals 2021, 11, 476. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Yu.; Yakovtseva, O.A.; Loginova, I.S.; Pozdniakov, A.V. Effect of Zr on microstructure and mechanical properties of the Al–Cu–Er alloy. Mater. Sci. Technol. 2020, 36, 453–459. [Google Scholar] [CrossRef]
- Amer, S.M.; Mikhaylovskaya, A.V.; Barkov, R.Y.; Kotov, A.D.; Mochugovskiy, A.G.; Yakovtseva, O.A.; Glavatskikh, M.V.; Loginova, I.S.; Medvedeva, S.V.; Pozdniakov, A.V. Effect of Homogenization Treatment Regime on Microstructure, Recrystallization Behavior, Mechanical Properties, and Superplasticity of Al–Cu–Er–Zr Alloy. JOM 2021, 73, 3092–3101. [Google Scholar] [CrossRef]
- Amer, S.; Yakovtseva, O.; Loginova, I.; Medvedeva, S.; Prosviryakov, A.; Bazlov, A.; Barkov, R.; Pozdniakov, A. The Phase Composition and Mechanical Properties of the Novel Precipitation-Strengthening Al-Cu-Er-Mn-Zr Alloy. Appl. Sci. 2020, 10, 5345. [Google Scholar] [CrossRef]
- Wen, S.P.; Gao, K.Y.; Huang, H.; Wang, W.; Nie, Z.R. Precipitation evolution in Al-Er-Zr alloys during aging at elevated temperature. J. Alloys Compd. 2013, 574, 92–97. [Google Scholar] [CrossRef]
- Wen, S.P.; Gao, K.Y.; Li, Y.; Huang, H.; Nie, Z.R. Synergetic effect of Er and Zr on the precipitation hardening of Al-Er-Zr alloy. Scr. Mater. 2011, 65, 592–595. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y.; Prosviryakov, A.S.; Churyumov, A.Y.; Golovin, I.S.; Zolotorevskiy, V.S. Effect of Zr on the microstructure, recrystallization behavior, mechanical properties and electrical conductivity of the novel Al-Er-Y alloy. J. Alloys Compd. 2018, 765, 1–6. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, J.T.; Cui, X.Y.; Zhang, B.; Zhen, L.; Ringer, S.P. Correlation between precipitates evolution and mechanical properties of Al-Sc-Zr alloy with Er additions. J. Mater. Sci. Technol. 2022, 99, 61–72. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y.; Amer, S.M.; Levchenko, V.S.; Kotov, A.D.; Mikhaylovskaya, A.V. Microstructure, mechanical properties and superplasticity of the Al–Cu–Y–Zr alloy. Mater. Sci. Eng. A 2019, 758, 28–35. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Mn on the Phase Composition and Properties of Al–Cu–Y–Zr Alloy. Phys. Met. Metallogr. 2020, 121, 1227–1232. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Prosviryakov, A.S.; Pozdniakov, A.V. Structure and Properties of New Heat-Resistant Cast Alloys Based on the Al–Cu–Y and Al–Cu–Er Systems. Phys. Met. Metallogr. 2021, 122, 908–914. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Prosviryakov, A.S.; Pozdniakov, A.V. Structure and Properties of New Wrought Al–Cu–Y- and Al–Cu–Er-Based Alloys. Phys. Met. Metallogr. 2021, 122, 915–922. [Google Scholar] [CrossRef]
- Karnesky, R.A.; van Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Effects of substituting rare-earth elements for scandium in a precipitation-strengthened Al-0.08 at. %Sc alloy. Scr. Mater. 2006, 55, 437–440. [Google Scholar] [CrossRef]
- van Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Nanoscale precipitation and mechanical properties of Al-0.06 at.% Sc alloys microalloyed with Yb or Gd. J. Mater. Sci. 2006, 41, 7814–7823. [Google Scholar] [CrossRef]
- Van Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Microstructural evolution and creep properties of precipitation-strengthened Al-0.06Sc-0.02Gd and Al-0.06Sc-0.02Yb (at.%) alloys. Acta Mater. 2011, 59, 5224–5237. [Google Scholar] [CrossRef]
- Barkov, R.Y.; Yakovtseva, O.A.; Mamzurina, O.I.; Loginova, I.S.; Medvedeva, S.V.; Proviryakov, A.S.; Mikhaylovskaya, A.V.; Pozdniakov, A.V. Effect of Yb on the Structure and Properties of an Electroconductive Al–Y–Sc Alloy. Phys. Met. Metallogr. 2020, 121, 604–609. [Google Scholar] [CrossRef]
- Barkov, R.Y.; Mikhaylovskaya, A.V.; Yakovtseva, O.A.; Loginova, I.S.; Prosviryakov, A.S.; Pozdniakov, A.V. Effects of thermomechanical treatment on the microstructure, precipitation strengthening, internal friction, and thermal stability of Al–Er-Yb-Sc alloys with good electrical conductivity. J. Alloys Compd. 2021, 855. [Google Scholar] [CrossRef]
- Pozdnyakov, A.V.; Barkov, R.Y.; Levchenko, V.S. Influence of Yb on the Phase Composition and Mechanical Properties of Low-Scandium Al–Mg–Mn–Zr–Sc and Al–Mg–Cr–Zr–Sc Alloys. Phys. Met. Metallogr. 2020, 121, 84–88. [Google Scholar] [CrossRef]
- Song, M.; Wu, Z.; He, Y. Effects of Yb on the mechanical properties and microstructures of an Al-Mg alloy. Mater. Sci. Eng. A 2008, 497, 519–523. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Fang, H.; Chen, S. A study of nanoscale Al 3(Zr,Yb) dispersoids structure and thermal stability in Al-Zr-Yb alloy. Mater. Sci. Eng. A 2012, 535, 311–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.; Gao, H.; Han, Y.; Wang, K.; Wang, J.; Sun, B.; Gu, S.; You, W. Precipitation evolution of Al-Zr-Yb alloys during isochronal aging. Scr. Mater. 2013, 69, 477–480. [Google Scholar] [CrossRef]
- Wen, S.P.; Gao, K.Y.; Huang, H.; Wang, W.; Nie, Z.R. Role of Yb and Si on the precipitation hardening and recrystallization of dilute Al-Zr alloys. J. Alloys Compd. 2014, 599, 65–70. [Google Scholar] [CrossRef]
- Ilyasov, R.R.; Avtokratova, E.V.; Krymskiy, S.V.; Sitdikov, O.S.; Markushev, M.V. Effect of preliminary heterogenization on the structure and hardness of cryorolled aluminum alloy D16. IOP Conf. Ser. Mater. Sci. Eng. 2018, 447, 012047. [Google Scholar] [CrossRef]
- Lefebvre, W.; Masquelier, N.; Houard, J.; Patte, R.; Zapolsky, H. Tracking the path of dislocations across ordered Al3Zr nano-precipitates in three dimensions. Scr. Mater. 2014, 70, 43. [Google Scholar] [CrossRef]
- Souza, P.H.L.; de Oliveira, C.A.S.; Quaresma, J.M.V. Precipitation hardening in dilute Al–Zr alloys. J. Mater. Res. Technol. 2018, 7, 66. [Google Scholar] [CrossRef]
- Zhu, A.W.; Starke, E.A. Strengthening effect of unshearable particles of finite size: A computer experimental study. Acta Mater. 1999, 47, 3263. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. A model for the yield strength of overaged Al–Zn–Mg–Cu alloys. Acta Mater. 2003, 51, 5131. [Google Scholar] [CrossRef] [Green Version]
- Mochugovskiy, A.G.; Mikhaylovskaya, A.V.; Zadorognyy, M.Yu.; Golovin, I.S. Effect of heat treatment on the grain size control, superplasticity, internal friction, and mechanical properties of zirconium-bearing aluminum-based alloy. J. Alloys Compd. 2021, 856, 157455. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Z. Für Met. 2006, 97, 246–265. [Google Scholar] [CrossRef] [Green Version]
- Desch, P.B.; Schwarz, R.B.; Nash, P. Formation of metastable L12 phases in Al3Zr and Al-12.5%X-25%Zr (X ≡ Li, Cr, Fe, Ni, Cu). J. Less Common Metals 1991, 168, 69–80. [Google Scholar] [CrossRef]
- Buschow, K.H.J. Phase relations and intermetallic compounds in the systems neodymium-aluminium and gadolinium-aluminium. J. Less Common Metals 1965, 9, 452–456. [Google Scholar] [CrossRef]
- Zolotorevsky, N.Y.; Solonin, A.N.; Churyumov, A.Y.; Zolotorevsky, V.S. Study of work hardening of quenched and naturally aged Al–Mg and Al–Cu alloys. Mater. Sci. Eng. A 2009, 502, 111. [Google Scholar] [CrossRef]
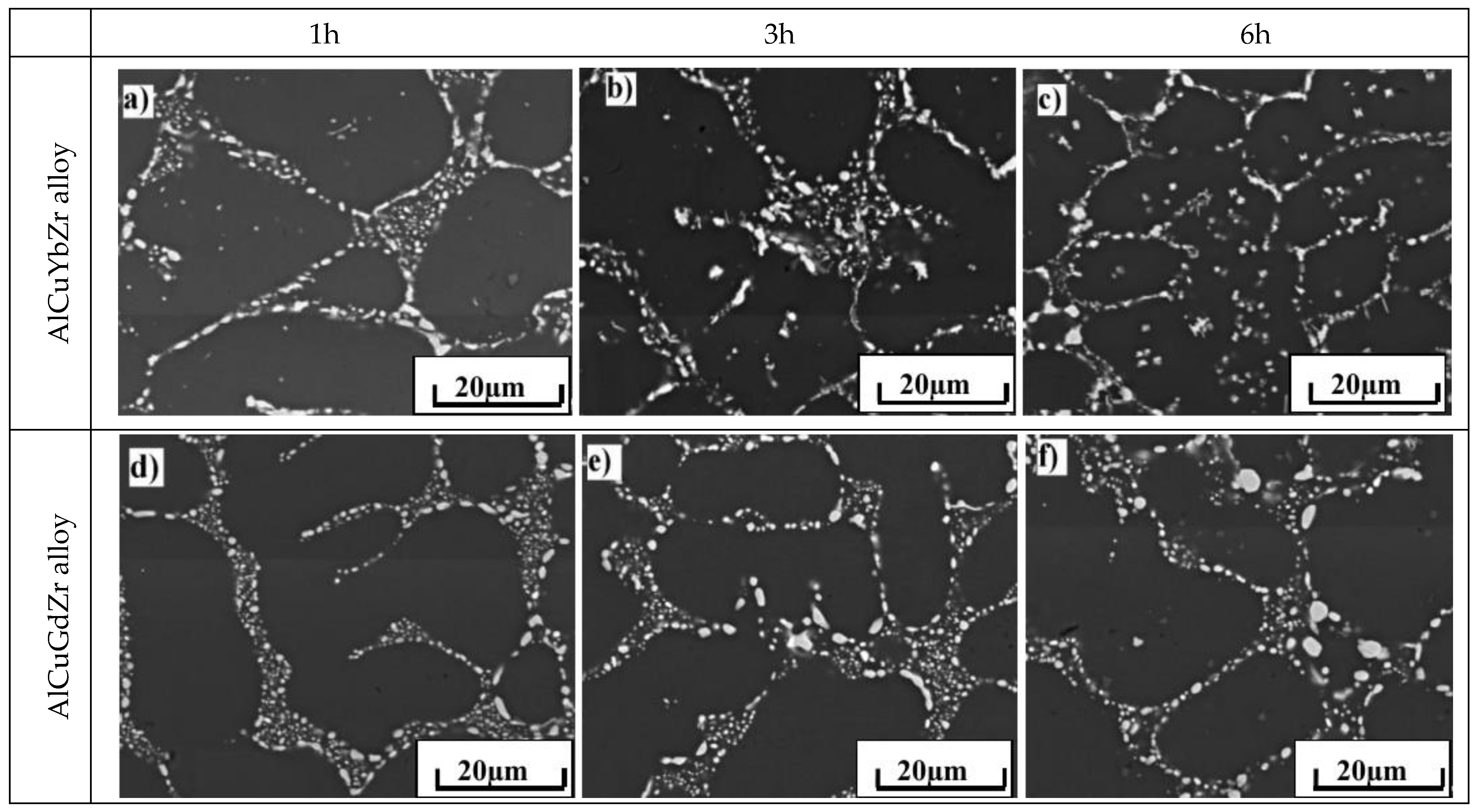
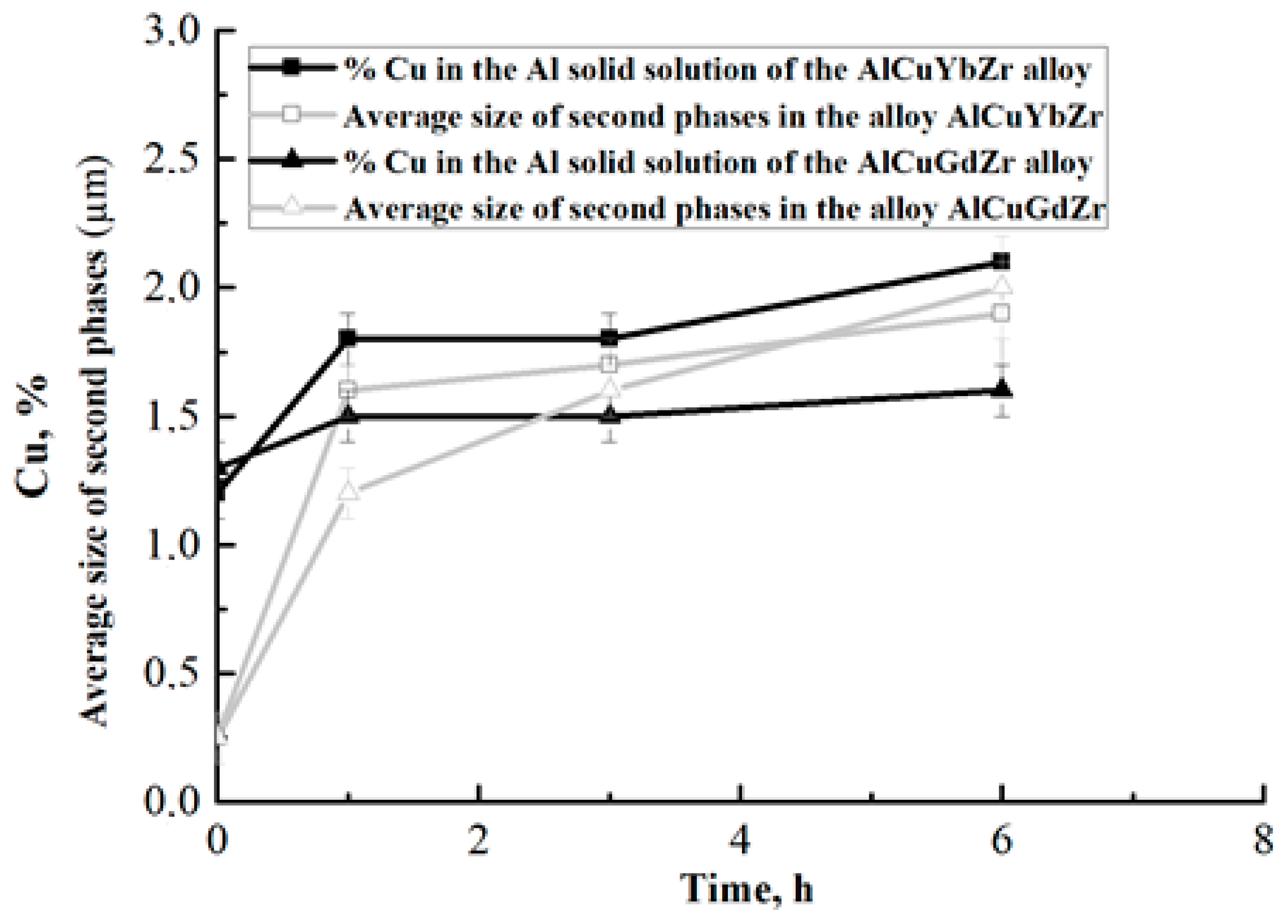
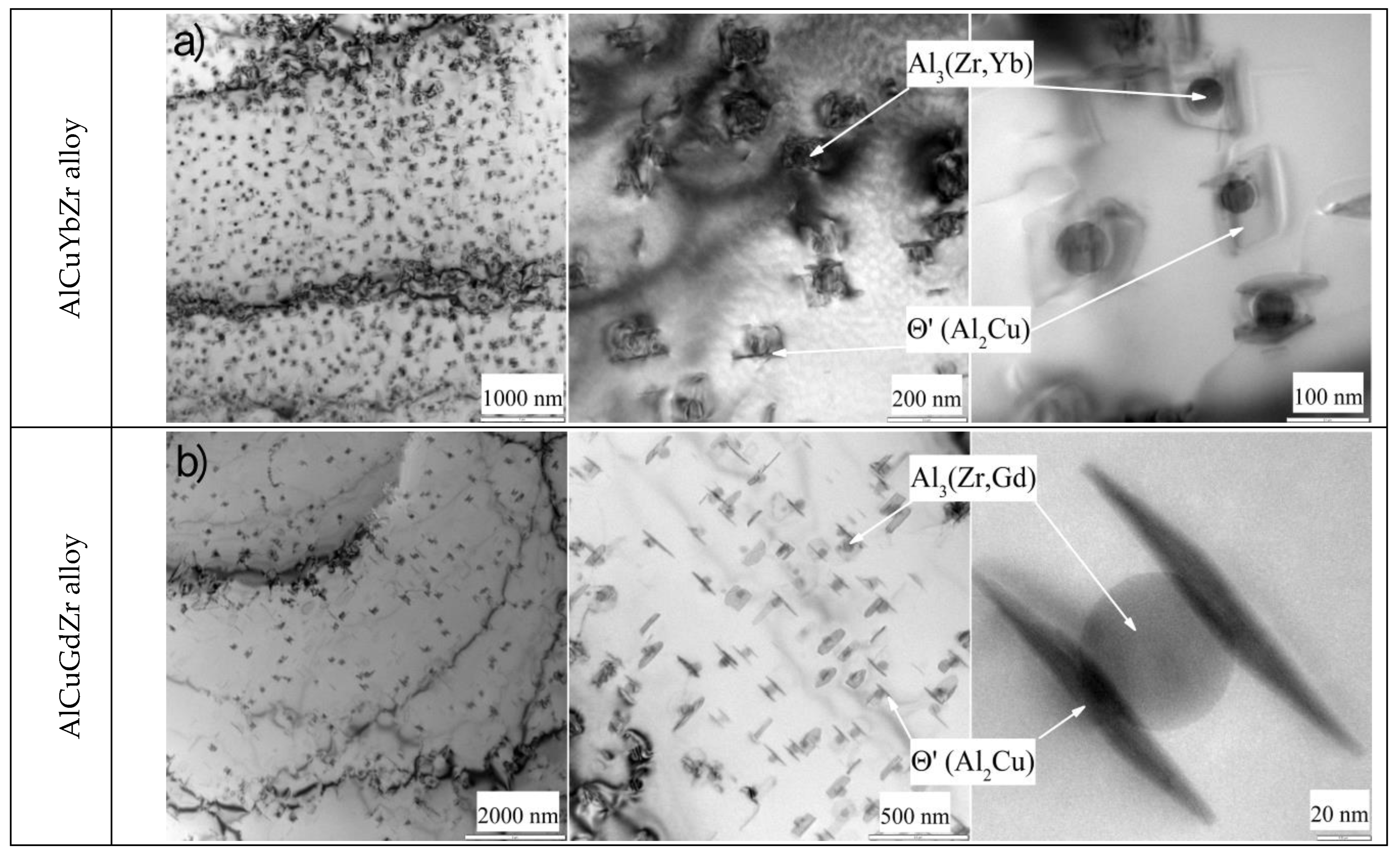
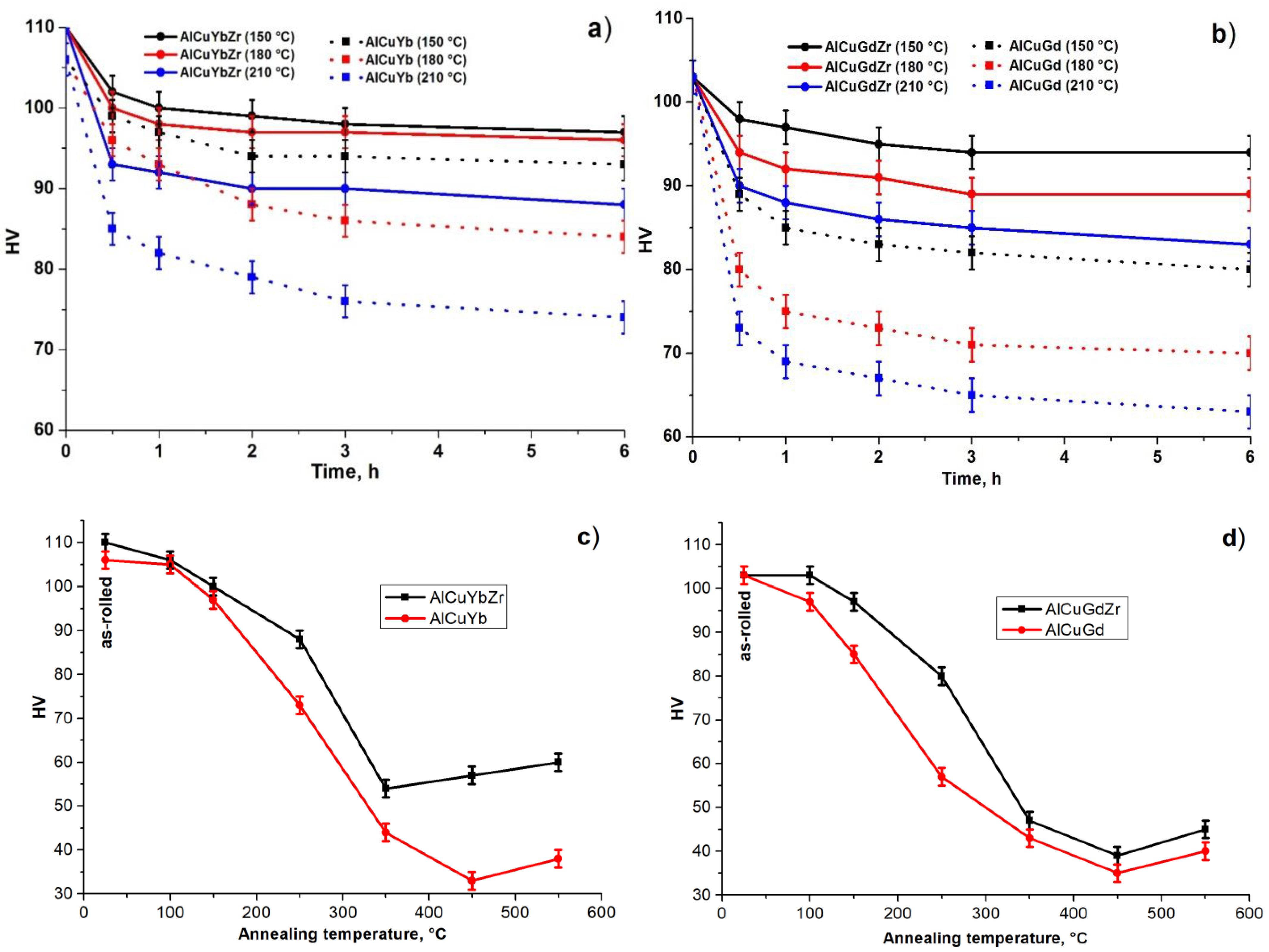

| Alloy | Cu | Yb | Gd | Zr | Al |
|---|---|---|---|---|---|
| AlCuYbZr | 4.1 | 2.2 | - | 0.4 | bal. |
| AlCuGdZr | 4.2 | - | 2.2 | 0.4 | bal. |
| State | YS, MPa | UTS, MPa | El., % | YS, MPa | UTS, MPa | El., % |
|---|---|---|---|---|---|---|
| AlCuYbZr | AlCuGdZr | |||||
| As-rolled * | 288 ± 2 | 325 ± 2 | 2.5 ± 0.5 | 279 ± 1 | 309 ± 3 | 4.8 ± 0.4 |
| 100 °C for 1 h | 276 ± 3 | 312 ± 3 | 3.1 ± 0.4 | 268 ± 2 | 302 ± 2 | 4.1 ± 1.0 |
| 100 °C for 6 h | 272 ± 4 | 307 ± 4 | 2.7 ± 0.3 | 258 ± 3 | 286 ± 4 | 4.6 ± 0.6 |
| 150 °C for 1 h | 265 ± 1 | 295 ± 1 | 4.1 ± 1.3 | 255 ± 2 | 284 ± 3 | 3.8 ± 0.5 |
| 150 °C for 6 h | 262 ± 2 | 287 ± 3 | 2.6 ± 1.0 | 251 ± 2 | 277 ± 2 | 1.8 ± 1.0 |
| 180 °C for 1 h | 254 ± 4 | 281 ± 2 | 5.2 ± 0.3 | 248 ± 2 | 272 ± 3 | 2.5 ± 0.8 |
| 180 °C for 6 h | 252 ± 1 | 274 ± 2 | 3.5 ± 1.0 | 237 ± 2 | 261 ± 2 | 2.5 ± 1.1 |
| 210 °C for 1 h | 252 ± 2 | 272 ± 2 | 1.9 ± 0.4 | 237 ± 1 | 260 ± 2 | 3.7 ± 1.2 |
| 250 °C for 0.5 h | 236 ± 2 | 252 ± 1 | 4.5 ± 0.5 | 225 ± 5 | 235 ± 4 | 5.2 ± 0.4 |
| 300 °C for 10 min | 207 ± 2 | 226 ± 2 | 10.5 ± 1 | 207 ± 3 | 212 ± 2 | 8 ± 1 |
| AlCuYbZr [14] | AlCuGdZr [14] | |||||
| 210 °C for 1 h | 238 ± 2 | 253 ± 1 | 5.9 ± 0.1 | 202 ± 1 | 210 ± 1 | 6.7 ± 0.7 |
| 250 °C for 0.5 h | 216 ± 2 | 229 ± 1 | 6 ± 2 | 175 ± 4 | 182 ± 3 | 16.0 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamzurina, O.I.; Amer, S.M.; Loginova, I.S.; Glavatskikh, M.V.; Mochugovskiy, A.G.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Zr on Microstructure and Mechanical Properties of the Al–Cu–Yb and Al–Cu–Gd Alloys. Metals 2022, 12, 479. https://doi.org/10.3390/met12030479
Mamzurina OI, Amer SM, Loginova IS, Glavatskikh MV, Mochugovskiy AG, Barkov RY, Pozdniakov AV. Effect of Zr on Microstructure and Mechanical Properties of the Al–Cu–Yb and Al–Cu–Gd Alloys. Metals. 2022; 12(3):479. https://doi.org/10.3390/met12030479
Chicago/Turabian StyleMamzurina, Olga I., Sayed M. Amer, Irina S. Loginova, Maria V. Glavatskikh, Andrey G. Mochugovskiy, Ruslan Yu. Barkov, and Andrey V. Pozdniakov. 2022. "Effect of Zr on Microstructure and Mechanical Properties of the Al–Cu–Yb and Al–Cu–Gd Alloys" Metals 12, no. 3: 479. https://doi.org/10.3390/met12030479
APA StyleMamzurina, O. I., Amer, S. M., Loginova, I. S., Glavatskikh, M. V., Mochugovskiy, A. G., Barkov, R. Y., & Pozdniakov, A. V. (2022). Effect of Zr on Microstructure and Mechanical Properties of the Al–Cu–Yb and Al–Cu–Gd Alloys. Metals, 12(3), 479. https://doi.org/10.3390/met12030479








