Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing
Abstract
1. Introduction
2. Literature Review
2.1. Binary Systems
2.2. Ternary Systems
3. Thermodynamic Modeling
3.1. Pure Elements
3.2. Solution Phases
3.3. Intermetallic Compounds
4. Thermodynamic Optimization
4.1. Ti-Fe-V System
4.2. Ti-Al-Fe-V System
5. Alloy Design
- (1)
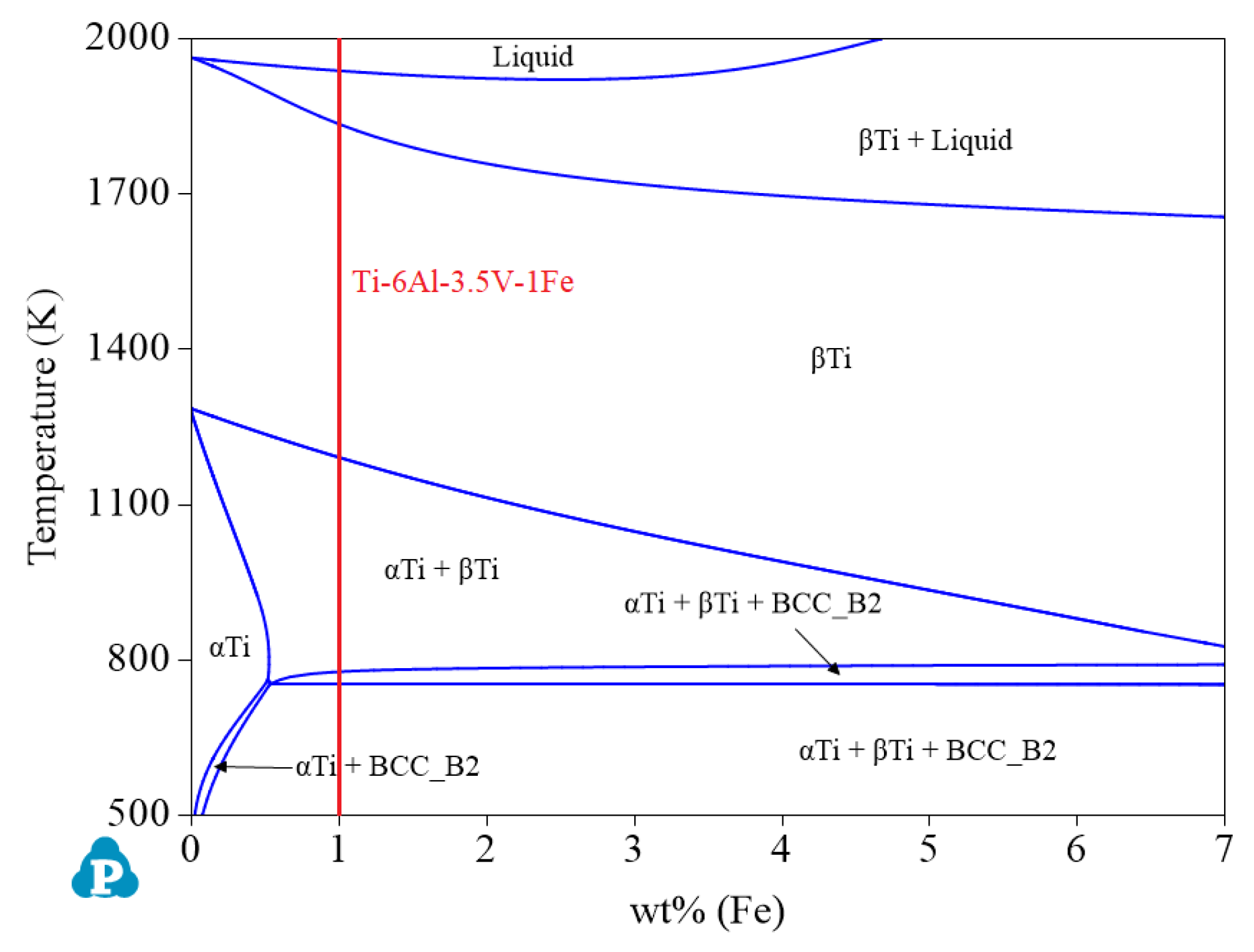
- As a strengthening element of the α phase, the α single-phase region of a keeps expanding with increasing Al content. Figure 12 shows that when the Al content is 6.0 wt%, the largest α single-phase region is observed. Therefore, if you want to design α titanium alloys of the Ti-Al-Fe-V system, 6.0 wt% Al can be preferred. At this time, the ranges of Fe and V are 0–3.05 wt% and 0–10.68 wt%, respectively. The recommended alloy composition is Ti-6Al-3.5V-1Fe, and its actual Mo equivalent is −0.755, which is considered to be 0.
- (2)
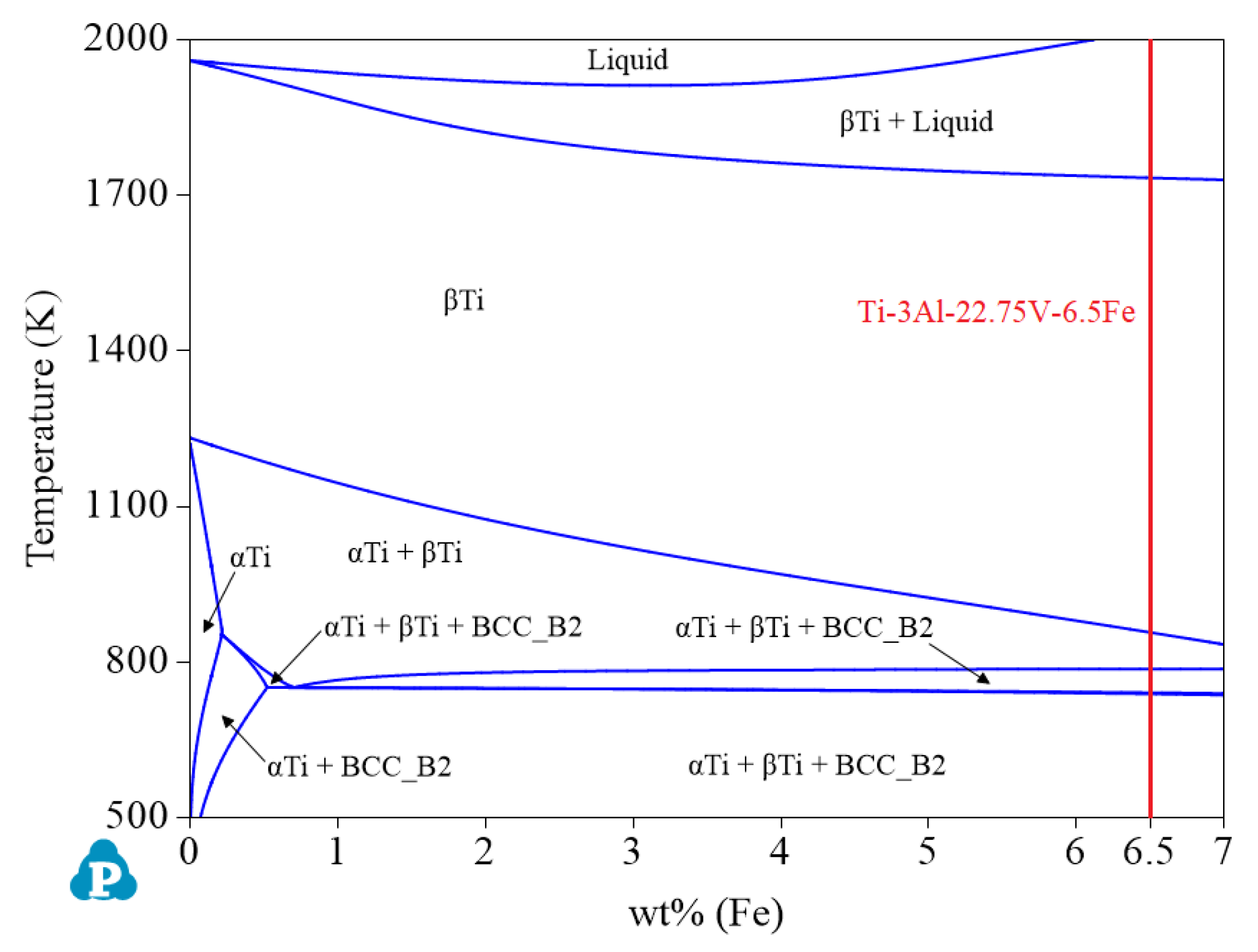
- V and Fe, as β phase reinforcing elements, can enlarge the β phase region. To obtain β titanium alloy, the content of elements V and Fe should be within the appropriate range as much as possible. Figure 10 shows that when the Al content is 3.0 wt%, the largest β single-phase region is observed. Therefore, if you want to design β titanium alloys of the Ti-Al-Fe-V system, 3.0 wt% Al can be preferred. At this time, the ranges of Fe and V are greater than 6.29 wt% and 22.02 wt%, respectively. The recommended alloy composition is Ti-3Al-22.75V-6.5Fe, and its Mo equivalent is 31.
- (3)
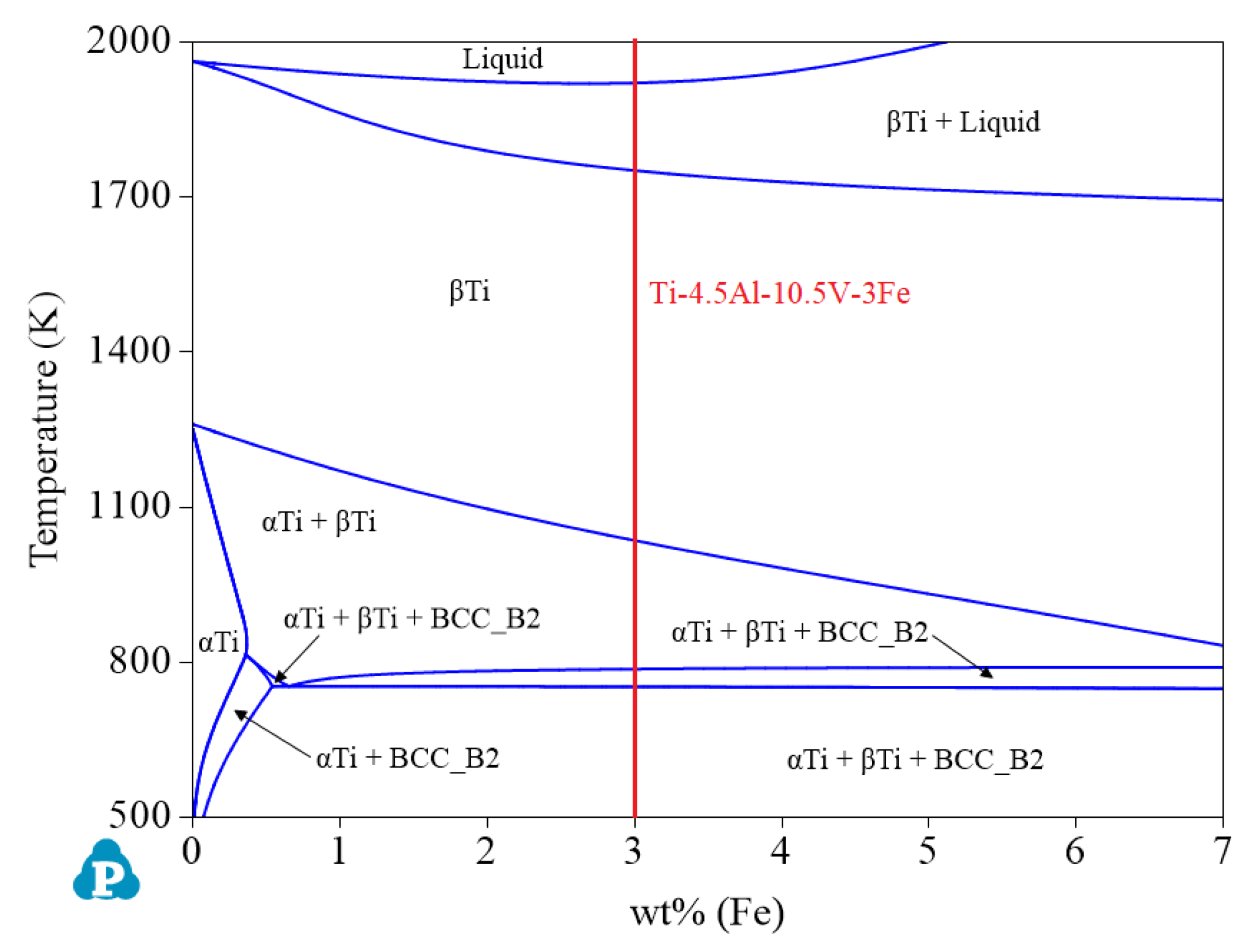
- Although α titanium alloys and β titanium alloys can also be designed when the Al content is 4.5 wt%, the range of options did not reach the optimal when compared with the other two concentrations. Therefore, when the Al content is 4.5 wt%, as shown in Figure 11, priority should be given to design α + β titanium alloys. At this time, the ranges of Fe and V are 2.76–6.58 wt% and 9.66–23.03 wt%, respectively. The recommended alloy composition is Ti-4.5Al-10.5V-3Fe, and its Mo equivalent is 11.2.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, K. The future of metals. Science 2010, 328, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Boyer, R.R. Attributes, characteristics, and applications of titanium and its alloys. JOM 2017, 3, 15–18. [Google Scholar] [CrossRef]
- Qiu, C.; Fones, A.; Hamilton, H.G.C.; Adkins, N.J.E.; Attallah, M.M. A new approach to develop palladium-modified Ti-based alloys for biomedical applications. Mater. Des. 2016, 109, 98–111. [Google Scholar] [CrossRef]
- He, P.Z.; Huang, S.M.; Guo, W.; Wang, Y.F.; He, L. The present situation of the word sponge titanium industry and the reflection on the future development of China. Prog. Titan. Ind. 2017, 34, 1–4. [Google Scholar]
- Gazder, A.A.; Vu, V.Q.; Saleh, A.A.; Markovsky, P.E.; Ivasishin, O.M.; Davies, C.H.J.; Pereloma, E.V. Recrystallisation in a cold drawn low cost beta titanium alloy during rapid resistance heating. J. Alloys Compd. 2014, 585, 245–259. [Google Scholar] [CrossRef]
- Zadra, M.; Girardini, L. High-performance, low-cost titanium metal matrix composites. Mater. Sci. Eng. A 2014, 608, 155–163. [Google Scholar] [CrossRef]
- Santos, P.F.; Niinomi, M.; Cho, K.; Nakai, M.; Liu, H.; Ohtsu, N.; Hirano, M.; Ikeda, M.; Narushima, T. Microstructures, mechanical properties and cytotoxicity of low cost beta Ti-Mn alloys for biomedical applications. Acta Biomater. 2015, 26, 366–376. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.N.; Omotoyinbo, J.A. Development of low-cost titanium alloys: A chronicle of challenges and opportunities. Mater. Today Proc. 2021, 38, 5–7. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Review of densification of titanium based powder systems in press and sinter processing. Powder Metall. 2010, 53, 146–162. [Google Scholar] [CrossRef]
- Bryan, D. ATI 425® Alloy Formability: Theory and Application. Mater. Sci. Forum 2014, 783–786, 543–548. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, H.; Zhang, R.; Jiang, X.; Liu, G.; Du, Y. Experimental and thermodynamic investigation of gradient zone formation for Ti(C,N)-based cermets sintered in nitrogen atmosphere. Ceram. Int. 2017, 43, 12089–12094. [Google Scholar] [CrossRef]
- Kattner, U.R.; Lin, J.C.; Chang, Y.A. Thermodynamic assessment and calculation of the Ti-Al system. Metall. Mater. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ti (aluminum-titanium). J. Phase Equilib. 1993, 14, 764. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.L.; Chang, Y.A.; Kattner, U.R. A thermodynamic description of the Ti-Al system. Intermetallics 1997, 5, 471–482. [Google Scholar] [CrossRef]
- Braun, J.; Ellner, M. Phase equilibria investigations on the aluminum-rich part of the binary system Ti-Al. Metall. Mater. Trans. A 2001, 32, 1037–1047. [Google Scholar] [CrossRef]
- Ohnuma, I.; Fujita, Y.; Mitsui, H.; Ishikawa, K.; Kainuma, R.; Ishida, K. Phase equilibria in the Ti-Al binary system. Acta Mater. 2000, 48, 3113–3123. [Google Scholar] [CrossRef]
- Kostov, A.; Friedrich, B.; Zivkovic, D. Thermodynamic calculations in alloys Ti-Al, Ti-Fe, Al-Fe and Ti-Al-Fe. J. Min. Metall. Sect. B 2008, 44, 49–61. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Rex, S.; Velikanova, T.Y. The Al-B-Nb-Ti system III: Thermodynamic re-evaluation of the constituent binary system Al-Ti. J. Alloys Compd. 2008, 465, 64–77. [Google Scholar] [CrossRef]
- Ohtani, H.; Hillert, M. A thermodynamic assessment of the Fe-N-Ti system. Calphad 1991, 15, 41–52. [Google Scholar] [CrossRef]
- Kumar, K.C.H.; Wollaiits, P.; Delaey, L. Thermodynamic reassessment and calculation of Fe-Ti phase diagram. Calphad 1994, 18, 223–234. [Google Scholar] [CrossRef]
- Dumitrescu, L.F.S.; Hillert, M.; Saunders, N. Comparison of Fe-Ti assessments. J. Phase Equilib. 1998, 19, 441–448. [Google Scholar] [CrossRef]
- Jonsson, S. Assessment of the Fe-Ti system. Metall. Mater. Trans. B 1998, 29, 361–370. [Google Scholar] [CrossRef]
- Keyzer, J.D.; Cacciamani, G.; Dupin, N.; Wollants, P. Thermodynamic modeling and optimization of the Fe-Ni-Ti system. Calphad 2009, 33, 109–123. [Google Scholar] [CrossRef]
- Bo, H.; Wang, J.; Duarte, L.; Leinenbach, C.; Liu, L.B.; Liu, H.S.; Jin, Z.P. Thermodynamic re-assessment of Fe-Ti binary system. Trans. Nonferr. Met. Soc. China 2012, 22, 2204–2211. [Google Scholar] [CrossRef]
- Zeng, K.; Schmid-Fetzer, R. Thermodynamic assessment and applications of Ti-V-N system. Mater. Sci. Technol. 1998, 14, 1083–1091. [Google Scholar] [CrossRef]
- Lindwall, G.; Wang, P.; Kattner, U.R.; Campbell, C.E. The effect of oxygen on phase equilibria in the Ti-V system: Impacts on the AM processing of Ti alloys. JOM 2018, 70, 1692–1705. [Google Scholar] [CrossRef]
- Ghosh, G. Thermodynamic and kinetic modeling of the Cr-Ti-V system. J. Phase Equilib. 2002, 23, 310–328. [Google Scholar] [CrossRef]
- Jacobs, M.H.G.; Schmid-Fetzer, R. Phase behavior and thermodynamic properties in the system Fe-Al. Calphad 2009, 33, 170–178. [Google Scholar] [CrossRef]
- Phan, A.T.; Paek, M.K.; Kang, Y.B. Phase equilibria and thermodynamics of the Fe-Al-C system: Critical evaluation, experiment and thermodynamic optimization. Acta Mater. 2014, 79, 1–15. [Google Scholar] [CrossRef]
- Sundman, B.; Ohnuma, I.; Dupin, N.; Kattner, U.R.; Fries, S.G. An assessment of the entire Al-Fe system including D03 ordering. Acta Mater. 2009, 57, 2896–2908. [Google Scholar] [CrossRef]
- Kroupa, A.; Mazalova, M.; Richter, K.W. The reassessment of the Al-V system and new assessment of the Al-Si-V system. Calphad 2017, 59, 47–60. [Google Scholar] [CrossRef]
- Gong, W.P.; Du, Y.; Huang, B.Y.; Schmid-Fetzer, R.; Zhang, C.F.; Xu, H.H. Thermodynamic reassessment of the Al-V system. Z. Met. 2004, 95, 978–986. [Google Scholar] [CrossRef]
- Lee, B.J.; Lee, D.N. A thermodynamic study on the V-C and Fe-V systems. Calphad 1991, 15, 283–291. [Google Scholar]
- Andersson, J.O. A thermodynamic evaluation of the iron-vanadium system. Calphad 1983, 7, 305–315. [Google Scholar] [CrossRef]
- Kumar, K.C.H.; Raghavan, V. A thermodynamic reassessment of the Fe-V system. Calphad 1991, 15, 307–314. [Google Scholar] [CrossRef]
- Zeng, L.J.; Xu, G.L.; Liu, L.B.; Bai, W.M.; Zhang, L.G. Experimental investigation of phase equilibria in the Ti-Fe-Zr system. Calphad 2018, 61, 20–32. [Google Scholar] [CrossRef]
- Hu, B.; Yao, B.; Wang, J.; Zhao, J.R.; Min, F.F.; Du, Y. Thermodynamic assessment of the Al-Mo-V ternary system. J. Min. Metall. Sect. B 2017, 53, 95–106. [Google Scholar] [CrossRef]
- Zhao, C.C.; Yang, S.Y.; Lu, Y.; Guo, Y.H.; Wang, C.P.; Liu, X.J. Experimental investigation and thermodynamic calculation of the phase equilibria in the Fe-Ni-V system. Calphad 2014, 46, 80–86. [Google Scholar] [CrossRef]
- Palm, M.; Inden, G.; Thomas, N. The Fe-Al-Ti system. J. Phase Equilib. 1995, 16, 209–222. [Google Scholar] [CrossRef]
- Mabuchi, H.; Nagayama, H.; Tsuda, H.; Matsui, T.; Morii, K. Formation of ternary L1_2 intermetallic compound and phase relation in the Al-Ti-Fe system. Mater. Trans. JIM 2000, 41, 733–738. [Google Scholar] [CrossRef]
- Kainuma, R.; Ohnuma, I.; Ishikawa, K.; Ishida, K. Stability of B2 ordered phase in the Ti-rich portion of Ti-Al-Cr and Ti-Al-Fe ternary systems. Intermetallics 2000, 8, 869–875. [Google Scholar] [CrossRef]
- Ducher, R.; Stein, F.; Viguier, B.; Palm, M.; Lacaze, J. A re-examination of the liquidus surface of the Al-Fe-Ti system. Z. Met. 2003, 94, 396–410. [Google Scholar] [CrossRef]
- Palm, M.; Lacaze, J. Assessment of the Al-Fe-Ti system. Intermetallics 2006, 14, 1291–1303. [Google Scholar] [CrossRef]
- Takahashi, T.; Minamino, Y. Ternary diffusion and thermodynamic interaction in the beta solid solutions of Ti-Al-Fe alloys at 1423 K. J. Alloys Compd. 2012, 545, 168–175. [Google Scholar] [CrossRef]
- Rafiei, M.; Enayati, M.H.; Karimzadeh, F. Thermodynamic analysis of solid solution formation in the nanocrystalline Fe-Ti-Al ternary system during mechanical alloying. J. Chem. Thermodyn. 2013, 59, 243–249. [Google Scholar] [CrossRef]
- Hu, R.M. Thermodynamic Evaluation of Ti-Al-Fe-Mn Quaternary System and Its Application in the Design of Novel Titanium Alloys. Master’s Thesis, Shanghai University, Shanghai, China, 2015. [Google Scholar]
- Hayes, F.H. The Al-Ti-V (aluminum-titanium-vanadium) system. J. Phase Equilib. 1995, 16, 163–176. [Google Scholar] [CrossRef]
- Farrar, P.A.; Margolin, H. The Titanium-rich region of the Titanium-Aluminum-Vanadium system. Trans. Am. Inst. Min. Metall. Pet. Eng. 1961, 221, 1214–1221. [Google Scholar]
- Ahmed, T.; Flower, H. Partial isothermal sections of Ti-Al-V ternary diagram. Mater. Sci. Technol. 1994, 10, 272–288. [Google Scholar] [CrossRef]
- Shao, G.; Miodownik, A.P.; Tsakiropoulos, P. ω-Phase formation in V-Al and Ti-Al-V alloys. Philos. Mag. A 1995, 71, 1389–1408. [Google Scholar] [CrossRef]
- Shao, G.; Tsakiropoulos, P.; Miodownik, A.P. Ordering and decomposition of the β phase in melt-spun TiAl1−xVx alloys. Mater. Sci. Eng. A 1996, 216, 1–10. [Google Scholar] [CrossRef]
- Chang, W.S.; Muddle, B.C. Structure and stability of a new ternary hexagonal phase in Al3Ti-based Al-Ti-V alloys. Metall. Mater. Trans. A 2003, 34, 491–501. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Du, Y. 1100 ℃ isothermal section of Ti-Al-V ternary system. Mater. Sci. Eng. Powder Metall. 2006, 11, 146–148. [Google Scholar] [CrossRef]
- Kostov, A.; Zivkovic, D. Thermodynamic analysis of alloys Ti-Al, Ti-V., Al-V and Ti-Al-V. J. Alloys Compd. 2008, 460, 164–171. [Google Scholar] [CrossRef]
- Wang, H.; Warnken, N.; Reed, R.C. Thermodynamic and kinetic modeling of bcc phase in the Ti-Al-V ternary system. Mater. Sci. Eng. A 2010, 528, 622–630. [Google Scholar] [CrossRef]
- Lu, X.G.; Gui, N.; Qiu, A.T.; Wu, G.X.; Li, C.H. Thermodynamic Modeling of the Al-Ti-V Ternary System. Metall. Mater. Trans. A 2014, 45, 4155–4164. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kozakai, T. Experimental and theoretical investigations on phase diagram of Fe-base ternary ordering alloys. J. Chim. Phys. Chim. Biol. 1997, 94, 844–848. [Google Scholar] [CrossRef]
- Schoo, K.L.; Sivaramakrishnan, C.S.; Chakrabarti, A.K. Solidification characteristics of the Al-8.3Fe-0.8V-0.9Si alloy. Metall. Mater. Trans. A 2000, 31, 1599–1610. [Google Scholar] [CrossRef]
- Zhao, P.Z.; Kozakai, T.; Miyazaki, T. Phase separations into A2 + DO3 two phases in Fe-Al-V ternary ordering alloys. J. Jpn. Inst. Met. Mater. 1989, 53, 266–272. [Google Scholar] [CrossRef][Green Version]
- Maebashi, T.; Kozakai, T.; Doi, M. Phase equilibria in iron-rich Fe-Al-V ternary alloy system. Z. Met. 2004, 95, 1005–1010. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Q.S.; Wang, S.H.; Lu, X.G.; Li, C.H. Thermodynamic modeling of Al-Fe-V ternary system. Mater. Res. Express 2019, 6, 126539. [Google Scholar] [CrossRef]
- Tsin-Khua, B.; Kornilov, I.I. Fe-V-Ti (iron-vanadium-Titanium). J. Inorg. Chem. 1960, 5, 434. (In Russian) [Google Scholar]
- Ghosh, G.; Raghavan, V. Progress in metallurgical research: Fundamental and applied aspects. In Proceedings of the International Conference, Indian Institute of Technology, Kampur, India, 11 February 1986; pp. 403–408. [Google Scholar]
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagrams; ASM International: Cleveland, OH, USA, 1995; Volume 7, pp. 10927–10934. [Google Scholar]
- Prima, S.B.; Tretyachenko, L.A. Area of homogeneity of Laves phase in the Ti-V-Fe ternary system. Sov. Powder Metall. Met. Ceram. 1987, 26, 414–415. [Google Scholar] [CrossRef]
- Massicot, B.; Joubert, J.M.; Latroche, M. Phase equilibria in the Fe-Ti-V system. Int. J. Mater. Res. 2010, 101, 1414–1423. [Google Scholar] [CrossRef]
- Guo, G.P.; Li, C.R.; Zheng, X.; Du, Z.M. Thermodynamic modeling of the Fe-Ti-V system. Calphad 2012, 38, 155–160. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Available online: https://computherm.com/ (accessed on 16 November 2021).
- Wang, S.S.; Wang, K.; Chen, G.Y.; Li, Z.; Qin, Z.W.; Lu, X.G.; Li, C.H. Thermodynamic modeling of Ti-Fe-Cr ternary system. Calphad 2017, 56, 160–168. [Google Scholar] [CrossRef]
- Muggianu, Y.M.; Bambino, M.; Bros, J.P. Enthalpy of formation of liquid Bi-Sn-Ga alloys at 723 K, choice of an analytical expression of integral and partial excess quantities of mixing. J. Chim. Phys. 1975, 72, 83–88. [Google Scholar] [CrossRef]
- Kolachev, B.; Il’in, A.A.; Ryndenkov, D. Structural diagrams of titanium alloys in the “molybdenum equivalent-aluminum equivalent” coordinates. Russ. Metall. 1997, 1, 118–128. [Google Scholar]
- Wang, H.B.; Wang, S.S.; Gao, P.Y.; Jiang, T.; Lu, X.G.; Li, C.H. Microstructure and mechanical properties of a novel near-α titanium alloy Ti6.0Al4.5Cr1.5Mn. Mater. Sci. Eng. A 2016, 672, 170–174. [Google Scholar] [CrossRef]
- Li, C.L.; Lee, D.; Mi, X.J.; Ye, W.J.; Hui, S.X.; Lee, Y.T. Effect of Al addition on ω precipitation and age hardening of Ti-Al-Mo-Fe alloys. Metall. Mater. Trans. A 2016, 47, 2454–2461. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Tong, X.W.; Wang, J.; Wang, D.C.; Gao, Q. Status quo and development tendency on the research of low-cost titanium alloy. Mater. Rev. 2017, 31, 128–134. [Google Scholar]
- Zhao, Y.Q.; Liu, J.L.; Zhou, L. Segregation law of Cu, Fe and Cr in typical titanium alloy. Rare Met. Mater. Eng. 2005, 34, 531–538. [Google Scholar]
- Shang, G.Q.; Wang, X.N.; Fei, Y.; Li, J.; Zhu, Z.S.; Zhu, L.W. Experimental study on heat yreatment processing of a new low cost titanium alloy used in aviation field. Mater. Sci. Forum 2013, 747–748, 919–925. [Google Scholar] [CrossRef]
- Reynolds, A.P.; Hood, E.; Tang, W. Texture in friction stir welds of Timetal 21S. Scr. Mater. 2005, 52, 491–494. [Google Scholar] [CrossRef]
- Srinivasu, G.; Natraj, Y.; Bhattacharjee, A.; Nandy, T.K.; Nageswara Rao, G.V.S. Tensile and fracture toughness of high strength β Titanium alloy, Ti-10V-2Fe-3Al, as a function of rolling and solution treatment temperatures. Mater. Des. 2013, 47, 323–330. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Nakai, M.; Tsutsumi, H.; Aki, S.; Itsumi, Y.; Murakami, S.; Oyama, H. Relationship between microstructures and mechanical properties in Ti-4.5Al-2Mo-1.6V-0.5Fe-0.3Si-0.03C for next-generation aircraft applications. Mater. Trans. 2013, 54, 783–790. [Google Scholar] [CrossRef]
- Wang, Z.G.; Cai, H.J.; Hui, S.X. Microstructure and mechanical properties of a novel Ti-Al-Cr-Fe titanium alloy after solution treatment. J. Alloys Compd. 2015, 640, 253–259. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2003. [Google Scholar]
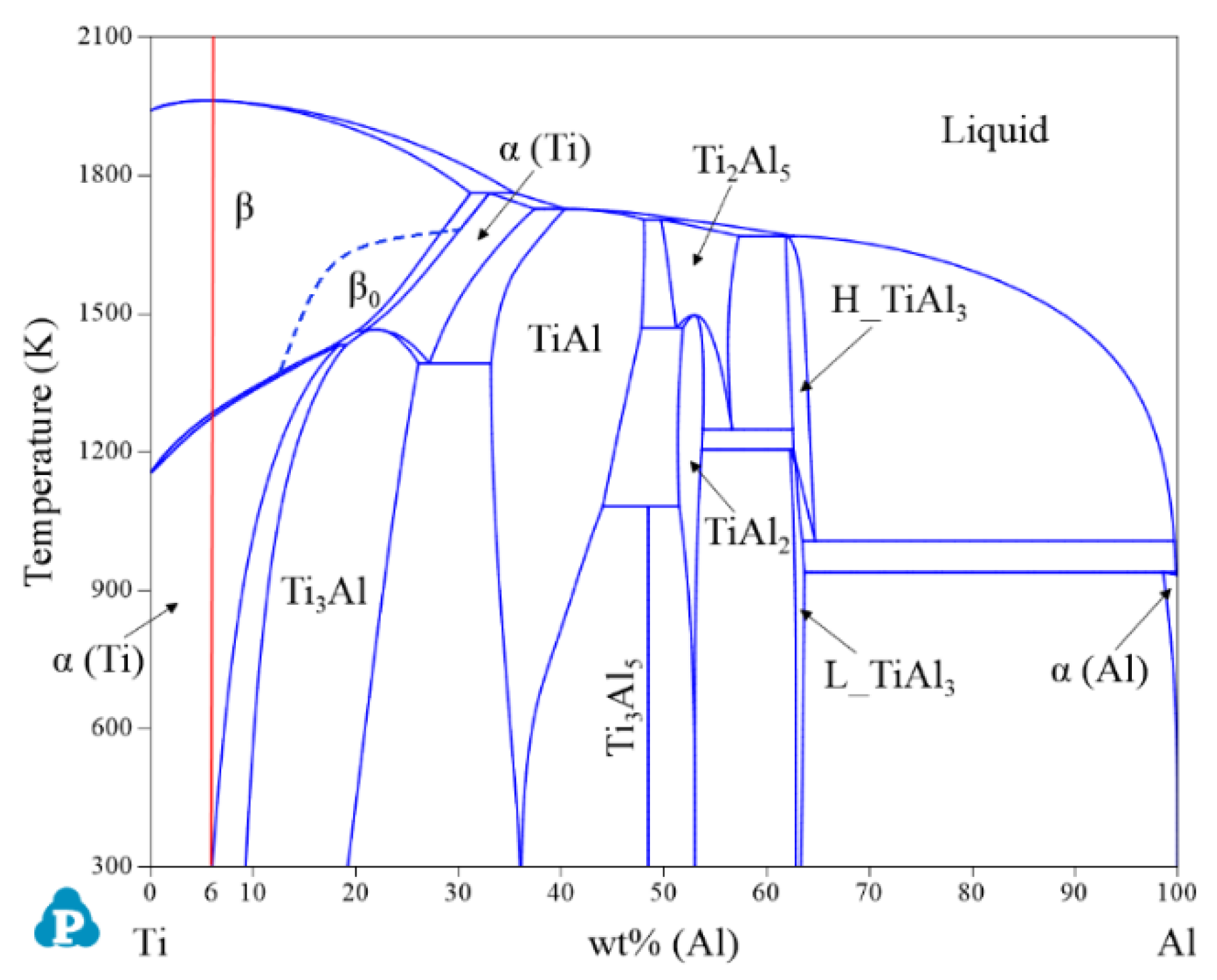
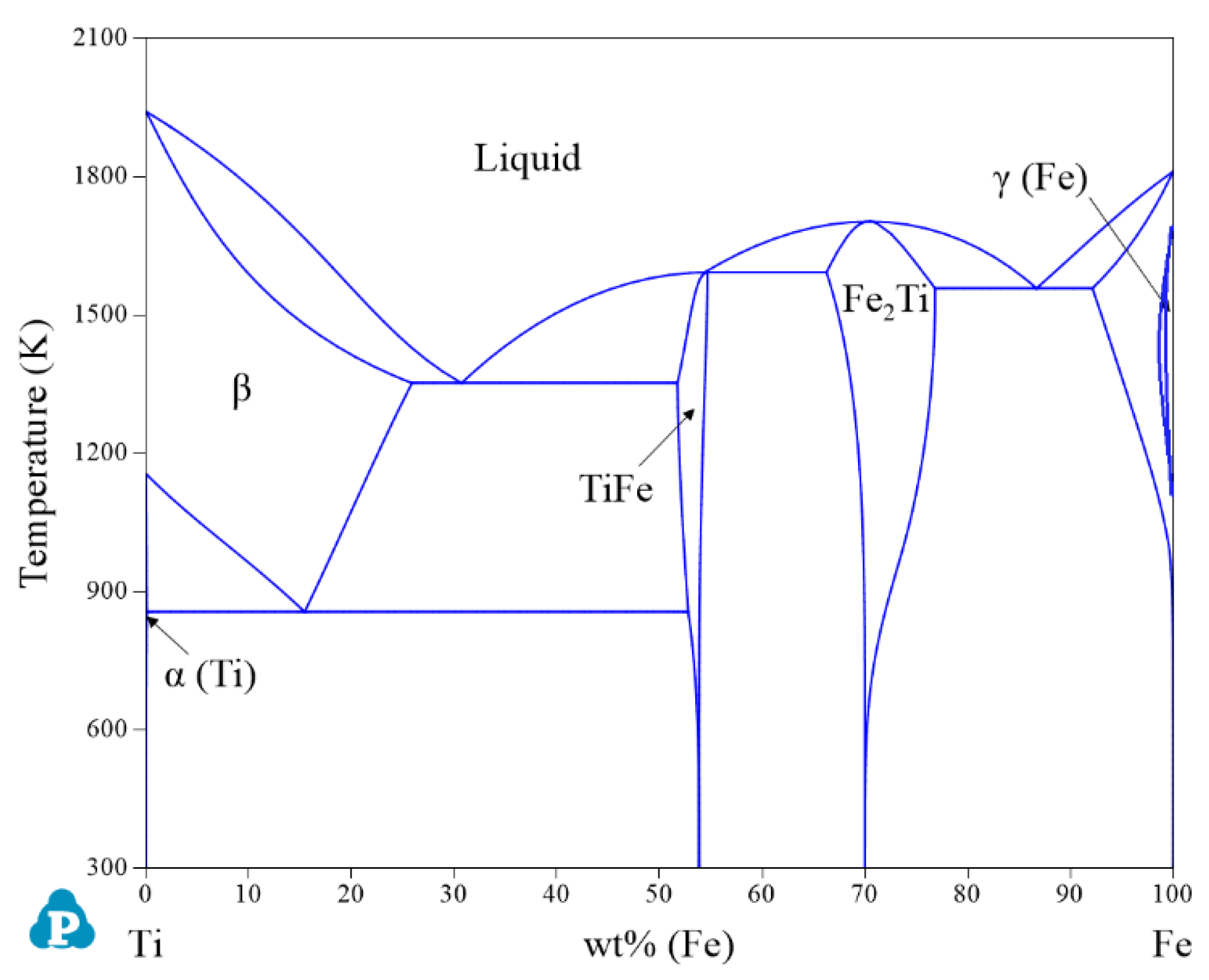
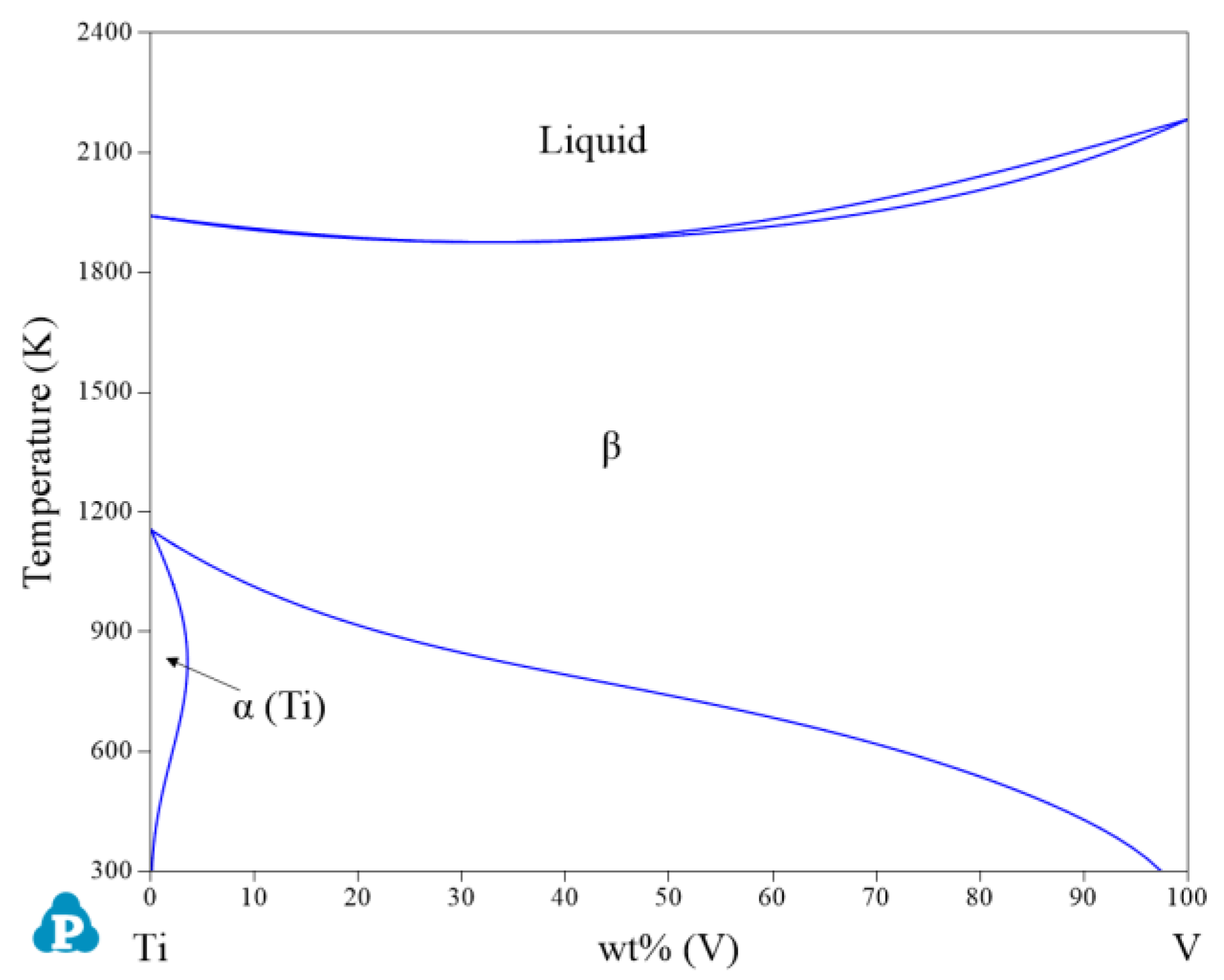
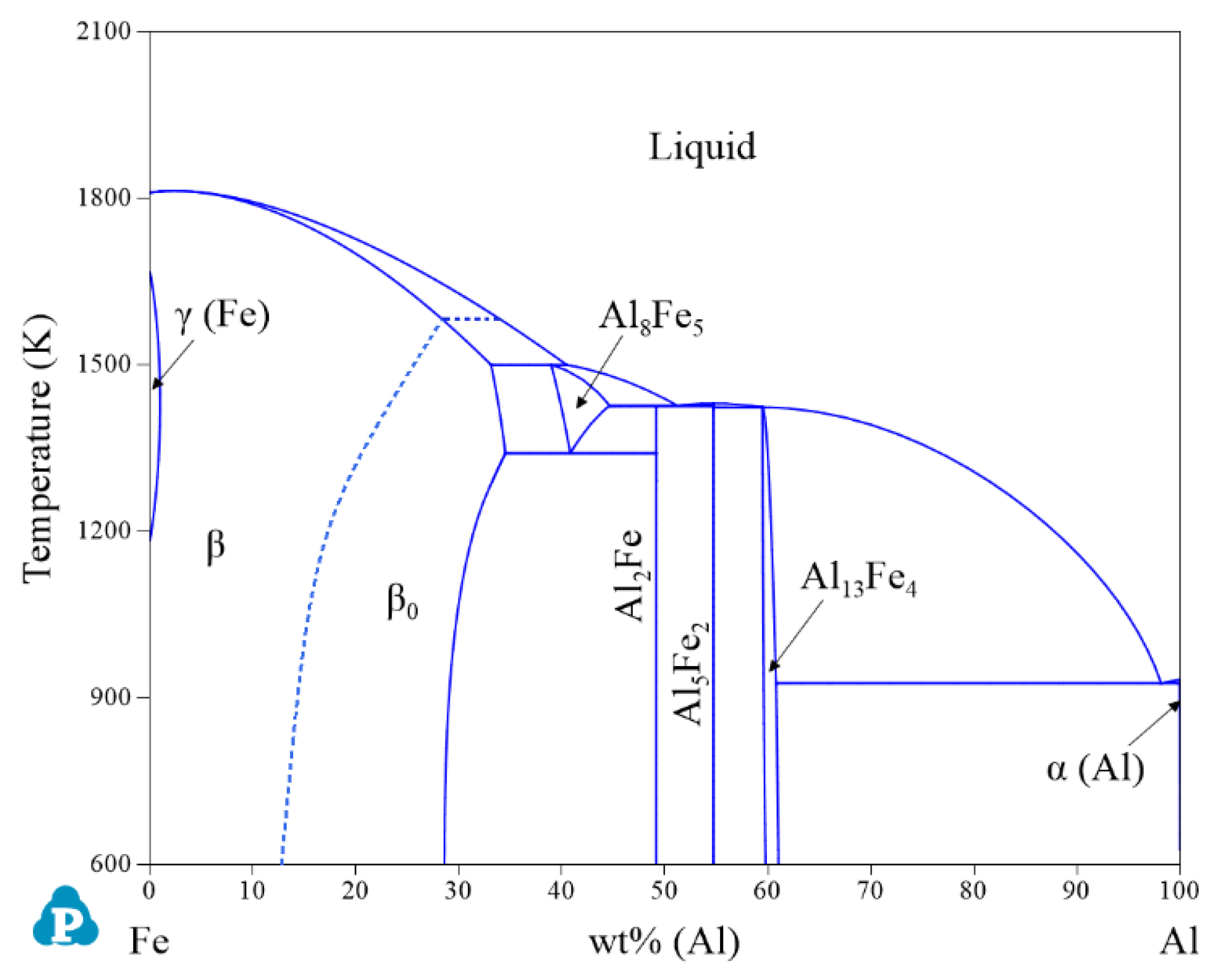
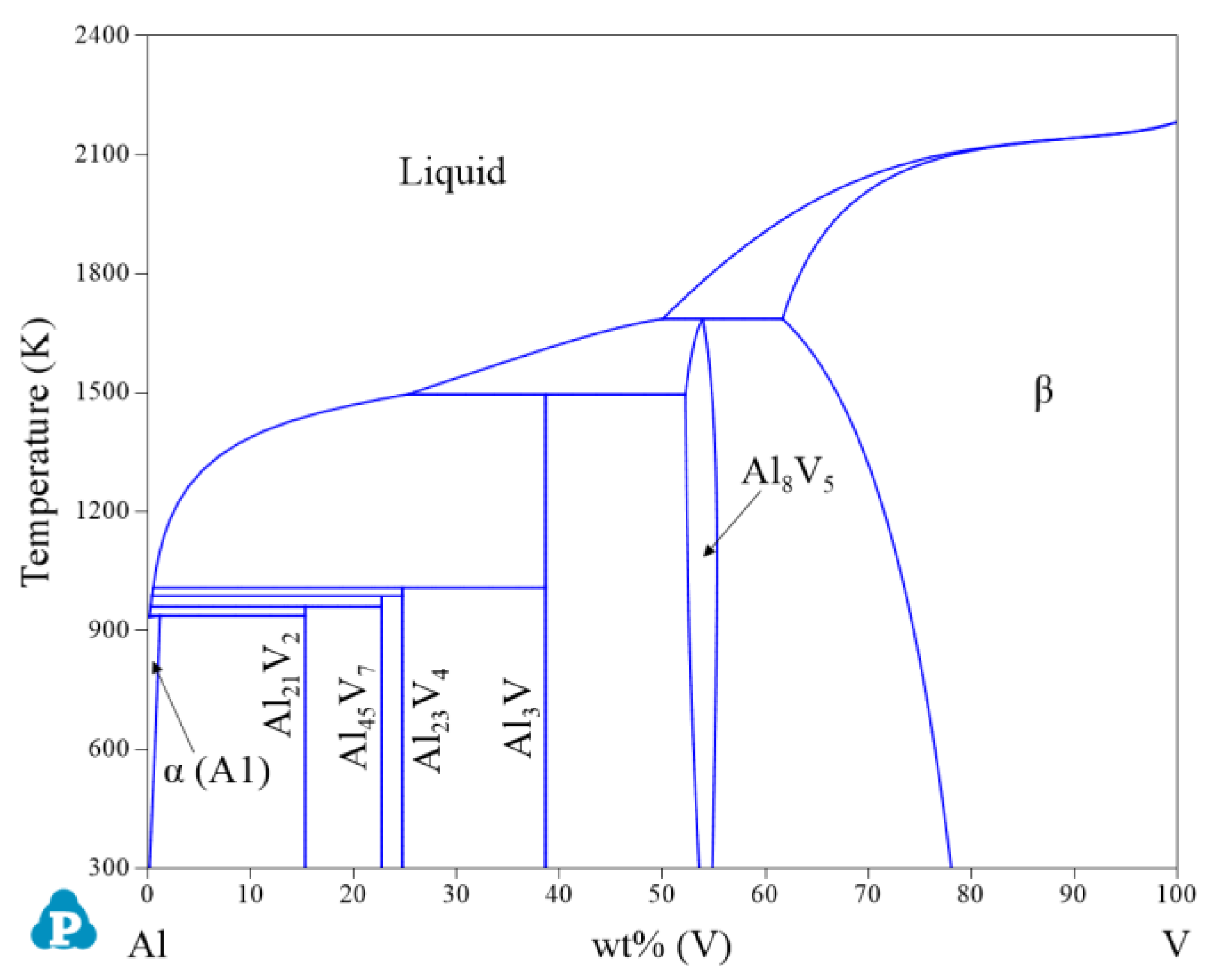
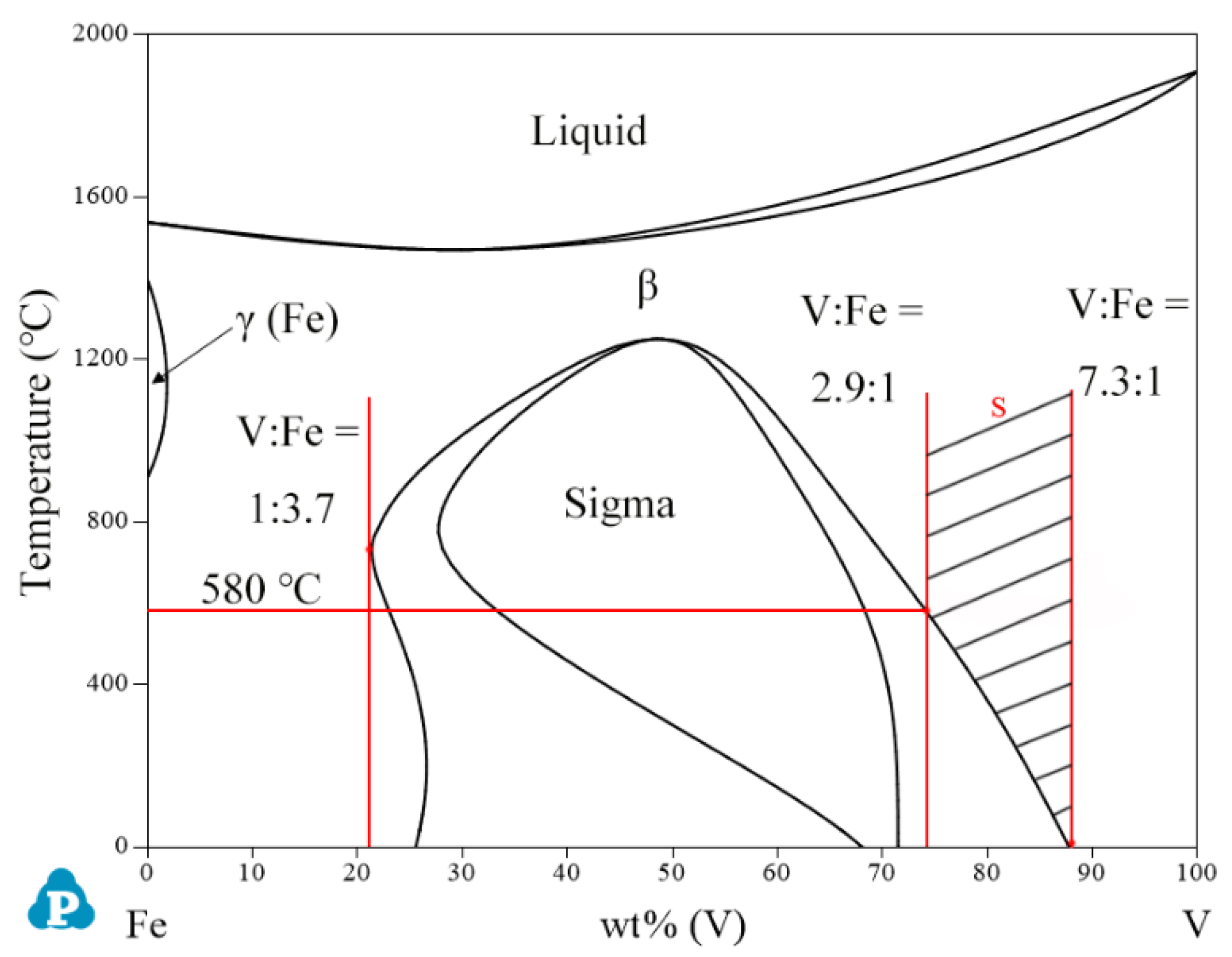
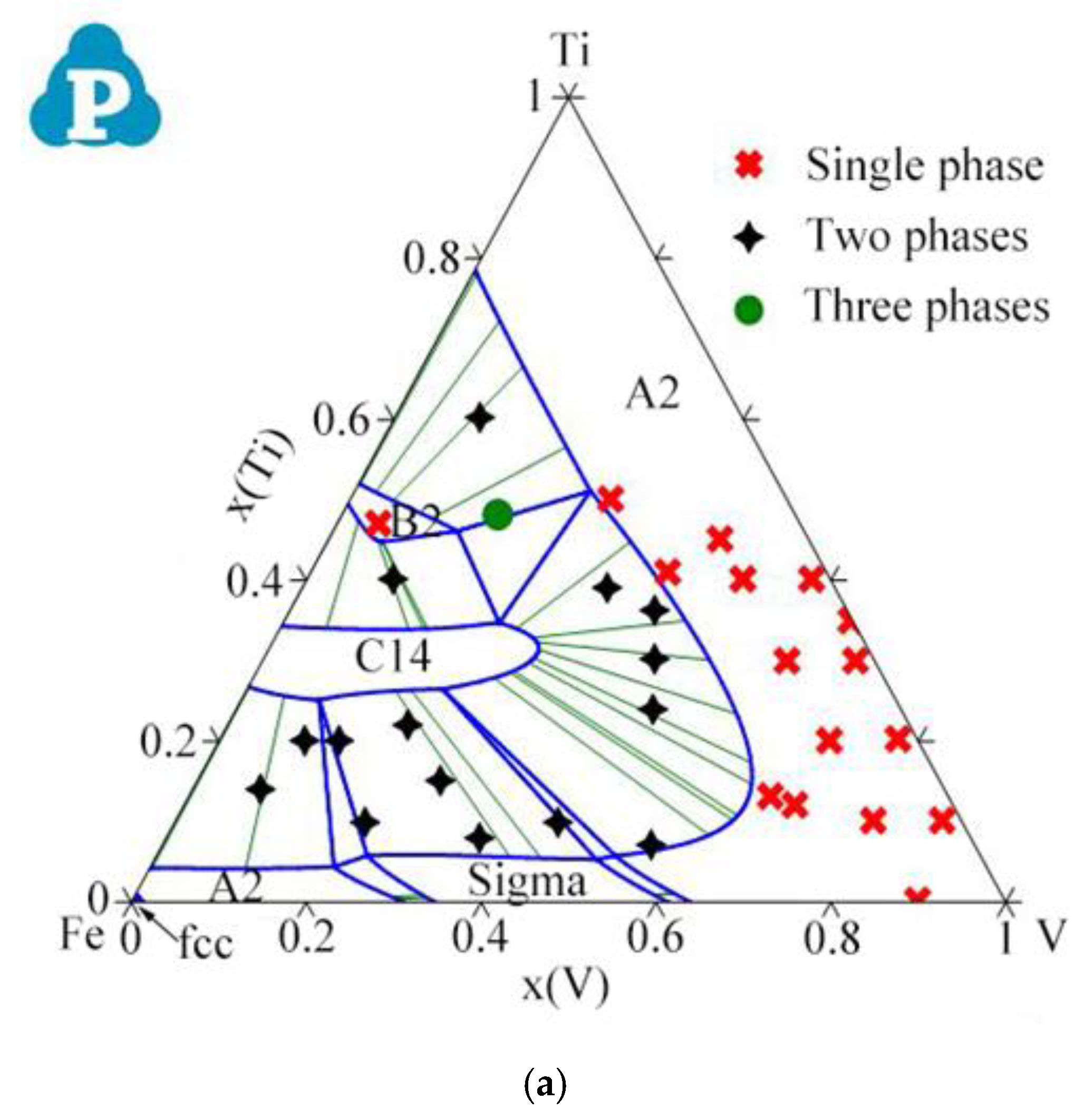
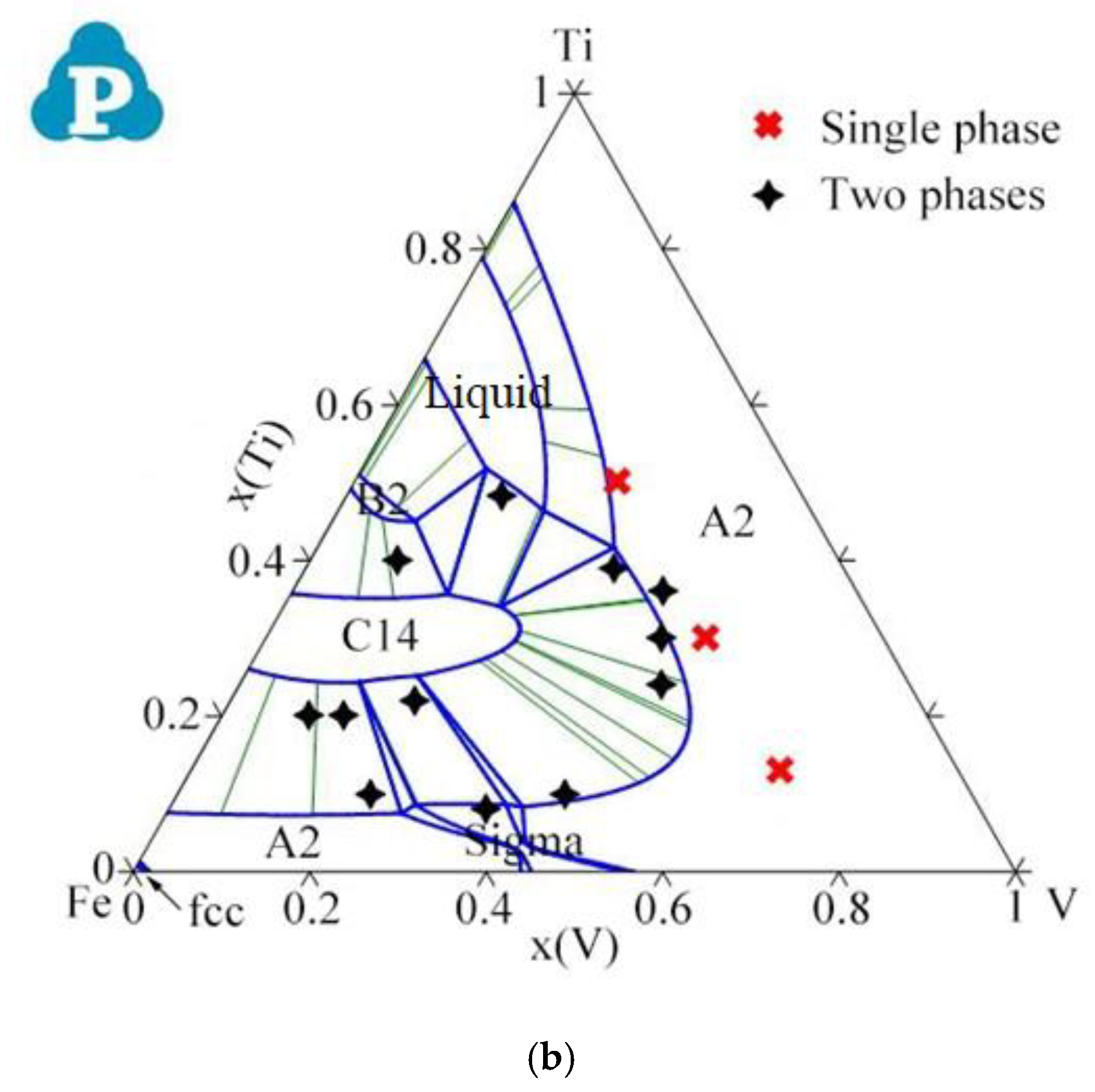
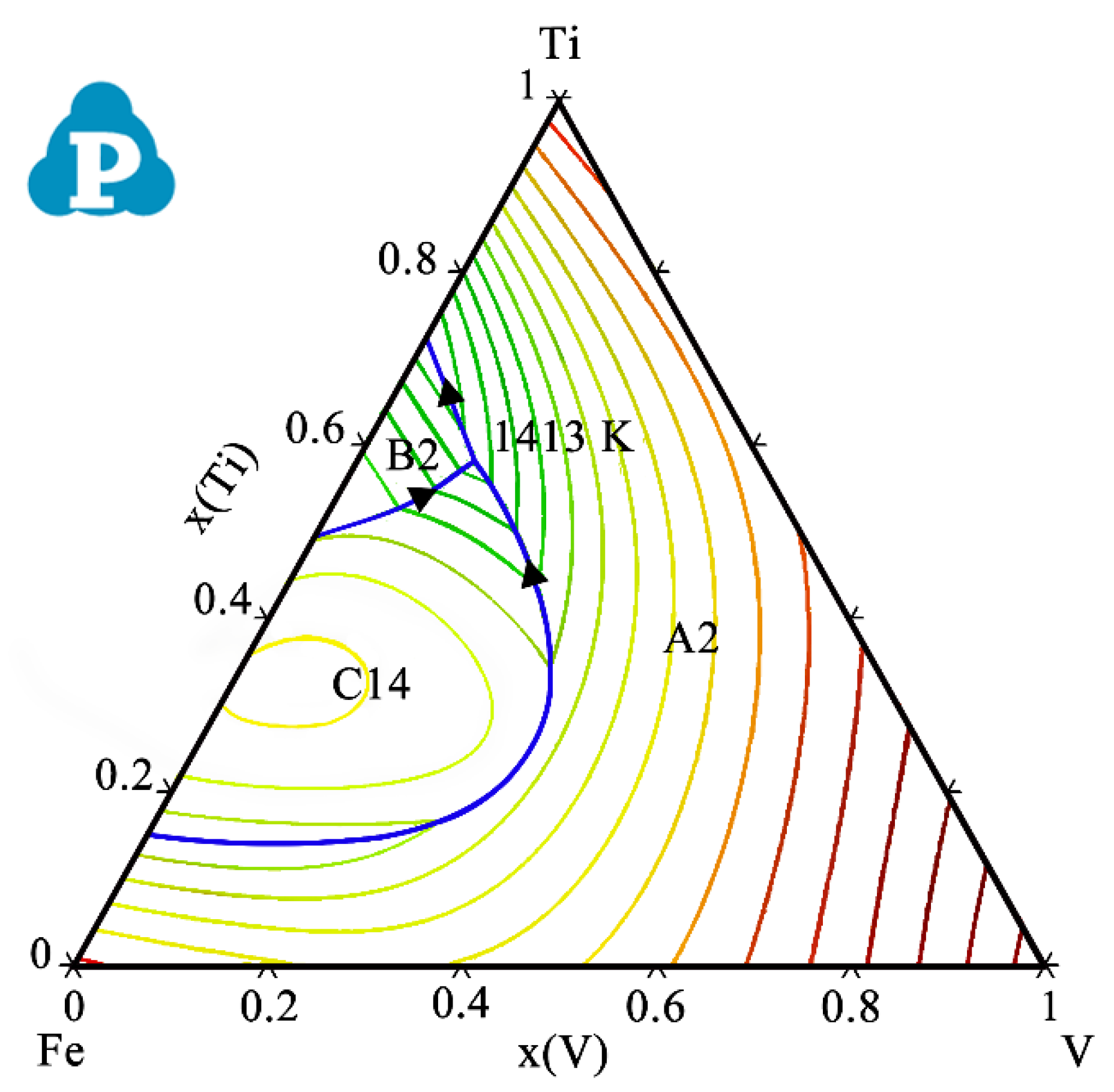
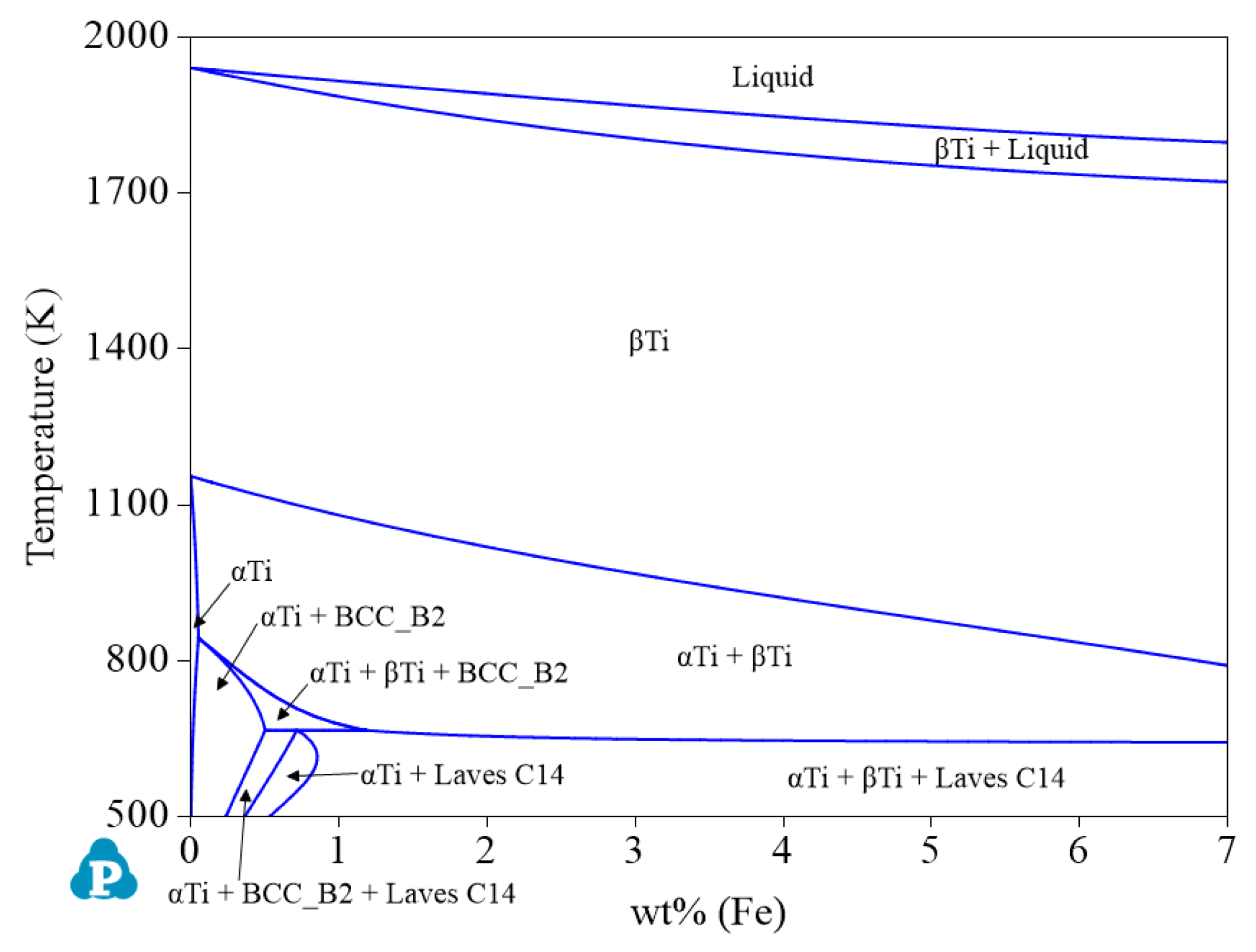
| System | Phase | Struktur-Bericht | Prototype | Person Symbol | Space Group | References |
|---|---|---|---|---|---|---|
| Ti-Al | α(Al) | FCC_A1 | Cu | cF4 | m | [18] |
| β | BCC_A2 | W | cI2 | m | [18] | |
| β0 | BCC_B2 | CsCl | cI2 | m | [18] | |
| α(Ti) | HCP_A3 | Mg | hP2 | P63/mmc | [18] | |
| Ti3Al | D019 | Ni3Sn | hP8 | P63/mmc | [18] | |
| TiAl | L10 | AuCu | tP4 | P4/mmm | [18] | |
| Ti3Al5 | - | Ti3Al5 | tP32 | P4/mbm | [18] | |
| Ti2Al5 | - | Ti2Al5 | tP28 | P4/mmm | [18] | |
| TiAl2 | - | HfGa2 | tI24 | I41/amd | [18] | |
| H_TiAl3 | D022 | TiAl3(h) | tI8 | I4/mmm | [18] | |
| L_TiAl3 | - | TiAl3(l) | tI32 | I4/mmm | [18] | |
| Ti-Fe | γ(Fe) | FCC_A1 | Cu | cF4 | m | [36] |
| β | BCC_A2 | W | cI2 | m | [36] | |
| α(Ti) | HCP_A3 | Mg | hP2 | P63/mmc | [36] | |
| TiFe | BCC_B2 | CsCl | cP2 | m | [36] | |
| Fe2Ti | C14 | MgZn2 | hP12 | P63/mmc | [36] | |
| Ti-V | β | BCC_A2 | W | cI2 | m | [27] |
| α(Ti) | HCP_A3 | Mg | hP2 | P63/mmc | [27] | |
| Fe-Al | γ(Fe) and α(Al) | FCC_A1 | Cu | cF4 | m | [30] |
| β | BCC_A2 | W | cI2 | m | [30] | |
| β0 | BCC_B2 | CsCl | cP8 | m | [30] | |
| Al8Fe5 | D82 | Cu5Zn8 | cI52 | m | [30] | |
| Al2Fe | - | Al2Fe | aP18 | P1 | [30] | |
| Al5Fe2 | - | - | oC? | Cmcm | [30] | |
| Al13Fe4 | - | - | mC102 | C2/m | [30] | |
| Al-V | α(Al) | FCC_A1 | Cu | cF4 | m | [37] |
| β | BCC_A2 | W | cI2 | m | [37] | |
| Al21V2 | - | Al21V2 | cF176 | m | [37] | |
| Al45V7 | - | Al45V7 | mC104 | C2/m | [37] | |
| Al23V4 | - | Al23V4 | hP54 | P63/mmc | [37] | |
| Al3V | - | Al3Ti | tI8 | I4/mmm | [37] | |
| Al8V5 | - | Cu5Zn8 | cI52 | 3m | [37] | |
| Fe-V | γ(Fe) | FCC_A1 | Cu | cF4 | m | [38] |
| β | BCC_A2 | W | cI2 | m | [38] | |
| sigma | D8b | σCrFe | tP30 | - | [38] |
| Phase/Model | Thermodynamic Parameters | References |
|---|---|---|
| Liquid (Fe, Ti, V)1 | [24] | |
| [24] | ||
| [35] | ||
| [35] | ||
| [27] | ||
| [27] | ||
| This work | ||
| FCC_A1 (Fe, Ti, V)1 | [24] | |
| [24] | ||
| [24] | ||
| [24] | ||
| [24] | ||
| [35] | ||
| BCC_A2 (Fe, Ti, V)1 | [24] | |
| [24] | ||
| [24] | ||
| [24] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [27] | ||
| [27] | ||
| This work | ||
| HCP_A3 (Fe, Ti, V)1 | [24] | |
| [27] | ||
| BCC_B2 (Fe, Ti, V)0.5(Fe, Ti, V)0.5 | [24] | |
| [24] | ||
| [24] | ||
| [24] | ||
| [24] | ||
| [24] | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| Sigma (Fe)8(V)4(Fe, Ti, V)18 | [35] | |
| [35] | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| C14 (Fe, Ti, V)2(Fe, Ti, V)1 | [24] | |
| [24] | ||
| This work | ||
| [24] | ||
| [24] | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work |
| System | Source of the Ternary Description | Constituent Binary System | Source of the Constituent Binary Description | Postscript |
|---|---|---|---|---|
| Ti-Al-Fe | Hu et al. [46] | Ti-Al | Witusiewicz et al. [18] | Adopted |
| Ti-Fe | Bo et al. [24] | |||
| Al-Fe | Sundman et al. [30] | |||
| Ti-Al-V | Lu et al. [56] | Ti-Al | Witusiewicz et al. [18] | Adopted |
| Ti-V | Ghosh et al. [27] | |||
| Al-V | Gong et al. [32] | |||
| Al-Fe-V | Wang et al. [61] | Al-Fe | Sundman et al. [30] | Adopted |
| Al-V | Gong et al. [32] | |||
| Fe-V | Kumar et al. [35] | |||
| Ti-Fe-V | Present work | Ti-Fe | Bo et al. [24] | Re-assessed in the present work |
| Ti-V | Ghosh et al. [27] | |||
| Fe-V | Kumar et al. [35] |
| Country | Trade Mark | Nominal Composition (wt%) | References |
|---|---|---|---|
| USA | Timetal 62S | Ti-6Al-1.7Fe-0.1Si | [78] |
| Timetal 21S | Ti-15Mo-2.7Nb-3Al-0.2Si | [79] | |
| Ti-1023 | Ti-10V-2Fe-3Al | [80] | |
| Japan | SP-700 | Ti-4.5Al-3V-2Mo-2Fe | [78] |
| KSTi-9 | Ti-4.5Al-2Mo-1.6V-0.5Fe-0.3Si-0.03C | [81] | |
| China | Ti8LC | Ti-6Al-1Fe-1Mo | [78] |
| Ti12LC | Ti-4.5Al-1.5Fe-6.8Mo | [78] | |
| - | Ti-3Al-3.7Cr-2Fe | [82] |
| The Content of Al (wt%) | The Content of Fe and V in α Titanium Alloys | The Content of Fe and V in α + β Titanium Alloys | The Content of Fe and V in β Titanium Alloys | |||
|---|---|---|---|---|---|---|
| Fe (wt%) | V (wt%) | Fe (wt%) | V (wt%) | Fe (wt%) | V (wt%) | |
| 3.0 | <2.48 | <8.68 | 2.48–6.29 | 8.68–22.02 | >6.29 | >22.02 |
| 4.5 | <2.76 | <9.66 | 2.76–6.58 | 9.66–23.03 | >6.58 | >23.03 |
| 6.0 | <3.05 | <10.68 | 3.05–6.86 | 10.68–24.01 | >6.86 | >24.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Duan, B.; Mao, L.; Jiao, L.; Chen, G.; Lu, X.; Li, C. Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing. Metals 2022, 12, 444. https://doi.org/10.3390/met12030444
Feng Q, Duan B, Mao L, Jiao L, Chen G, Lu X, Li C. Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing. Metals. 2022; 12(3):444. https://doi.org/10.3390/met12030444
Chicago/Turabian StyleFeng, Qisheng, Baohua Duan, Lu Mao, Lina Jiao, Guangyao Chen, Xionggang Lu, and Chonghe Li. 2022. "Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing" Metals 12, no. 3: 444. https://doi.org/10.3390/met12030444
APA StyleFeng, Q., Duan, B., Mao, L., Jiao, L., Chen, G., Lu, X., & Li, C. (2022). Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing. Metals, 12(3), 444. https://doi.org/10.3390/met12030444








