Thermal Stability of Microstructure of High-Entropy Alloys Based on Refractory Metals Hf, Nb, Ta, Ti, V, and Zr
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Details
4. Results and Discussion
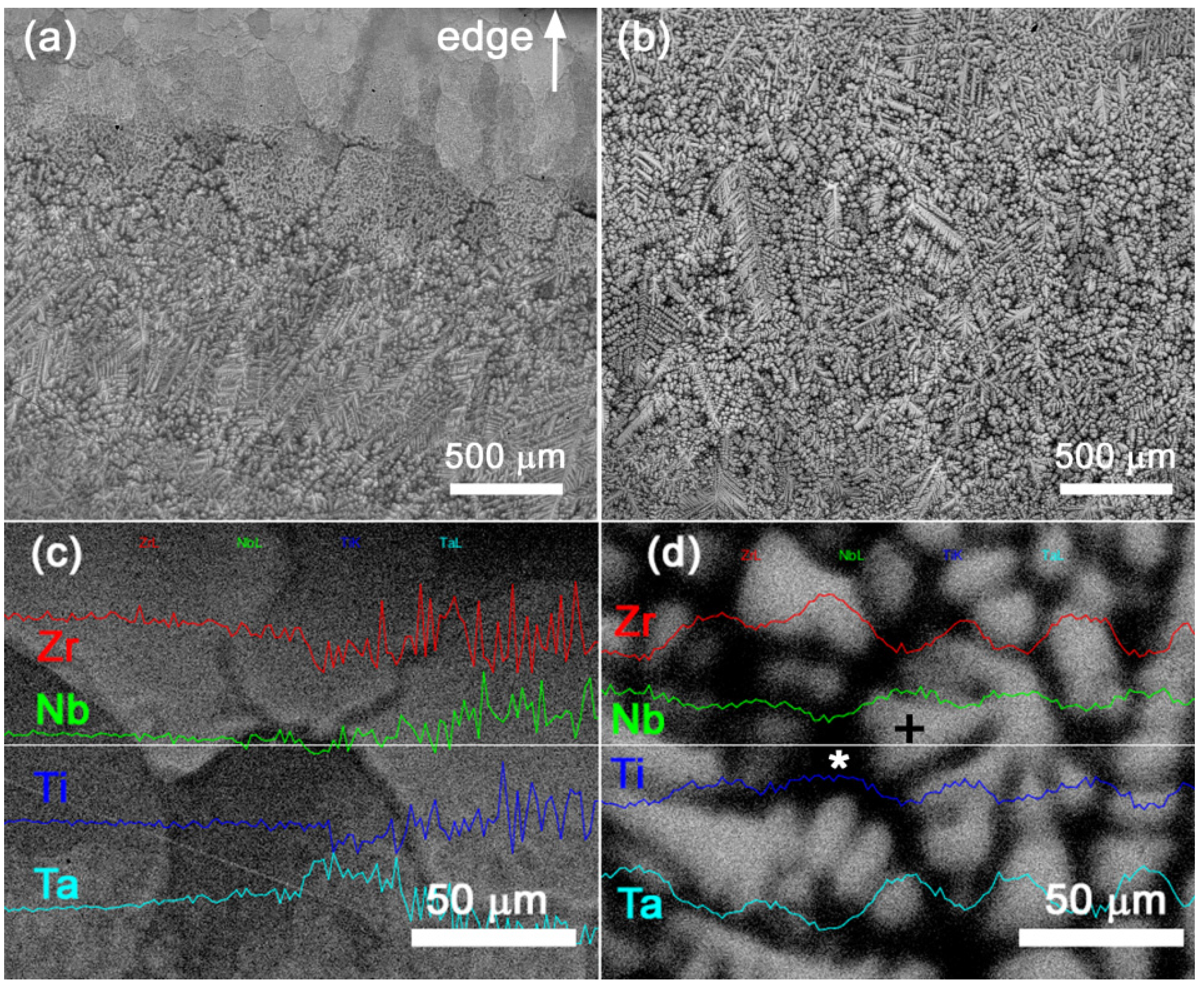
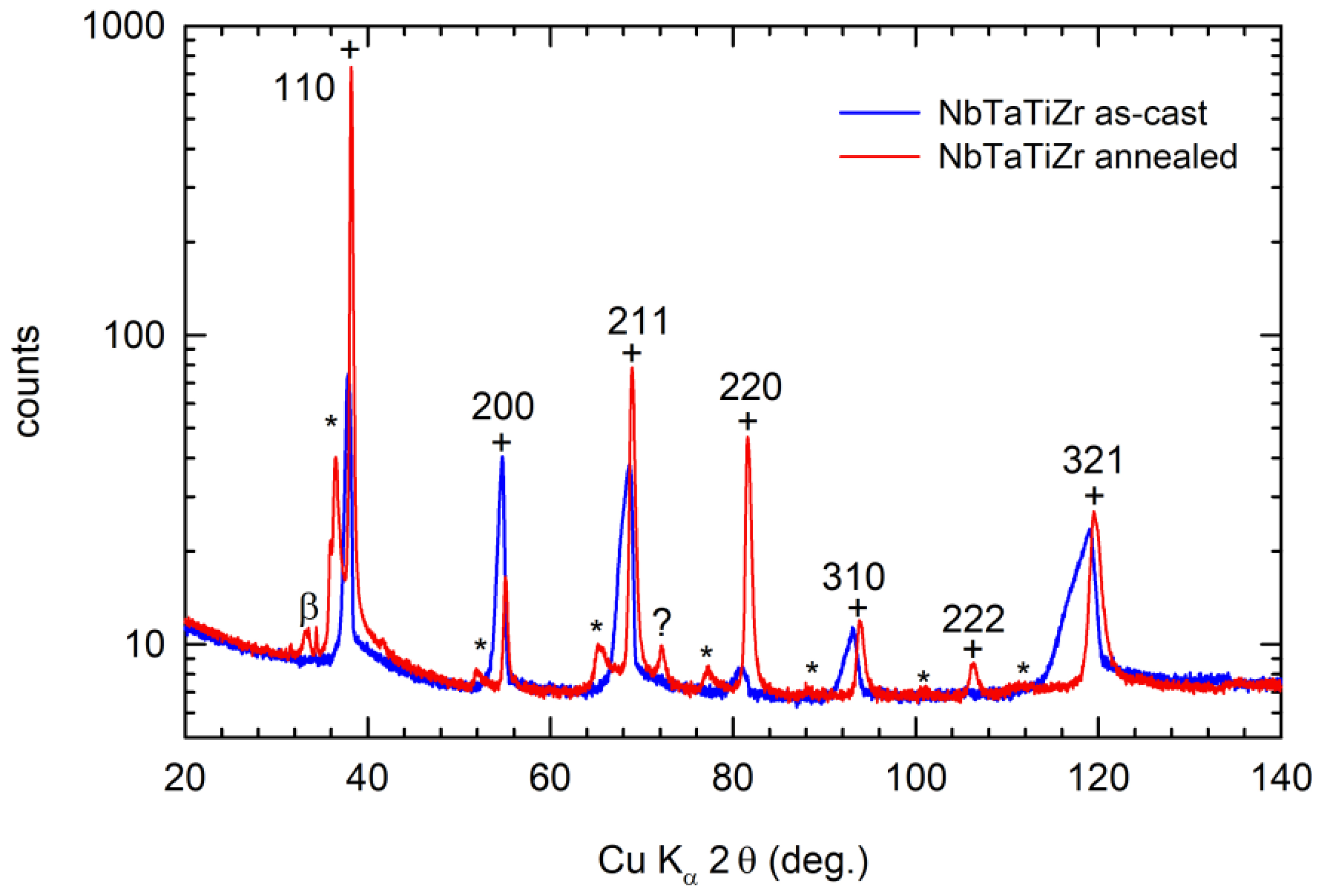
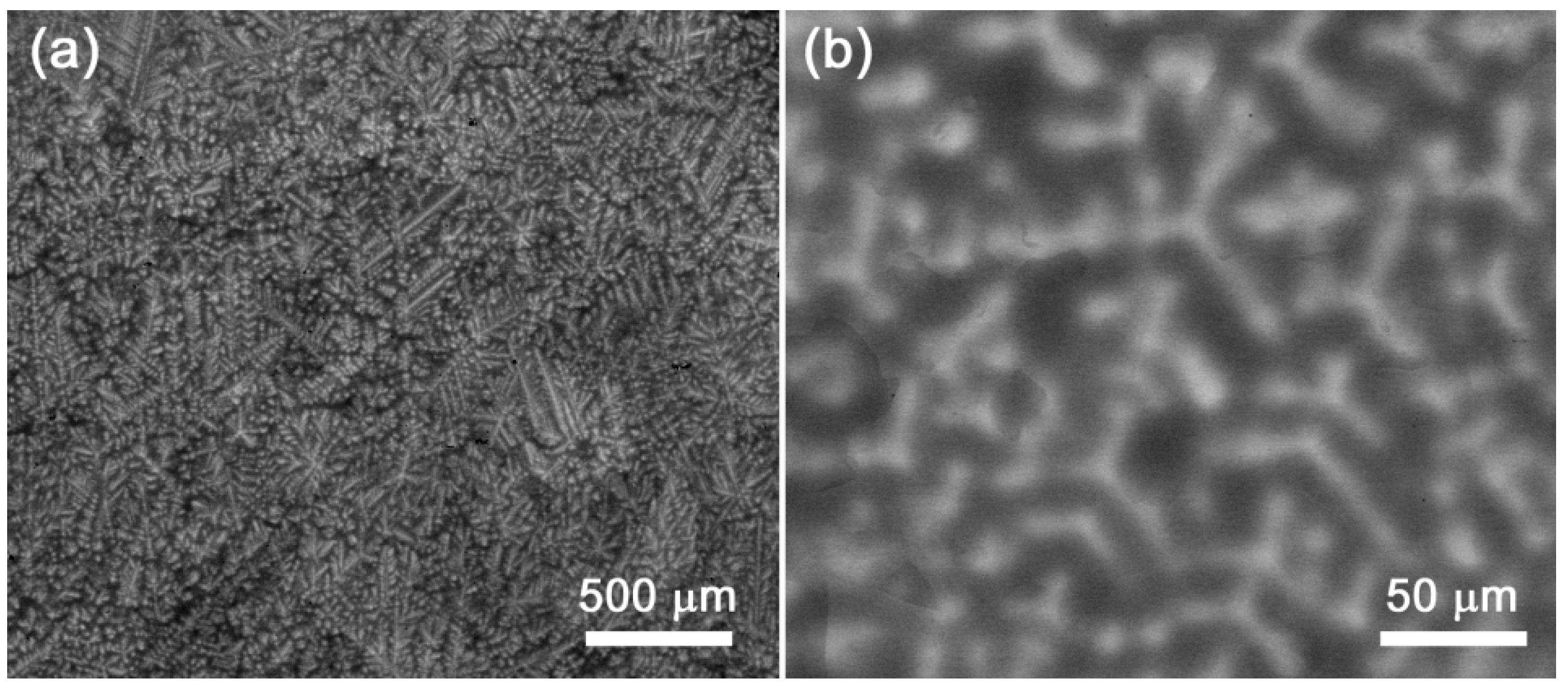
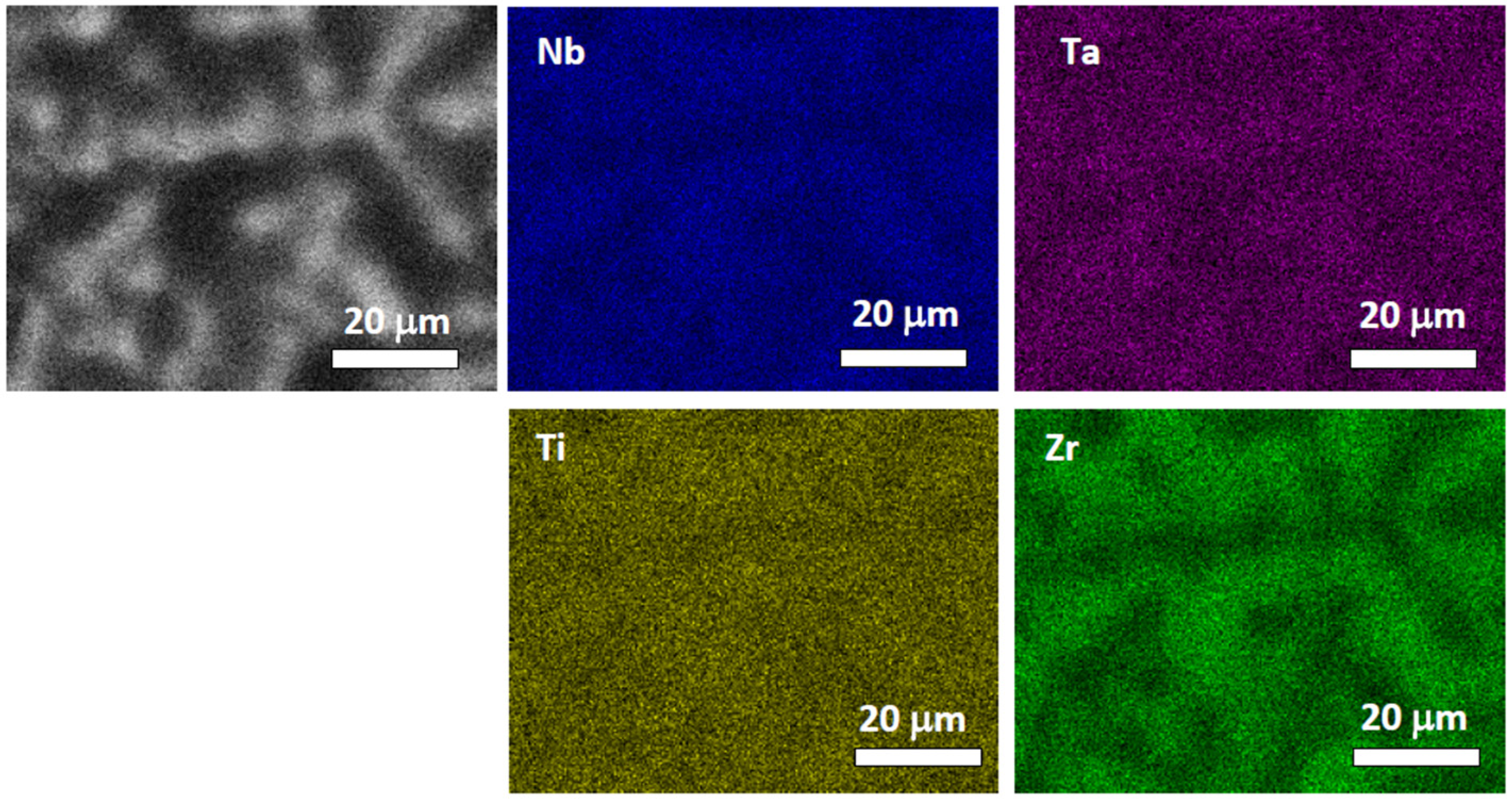
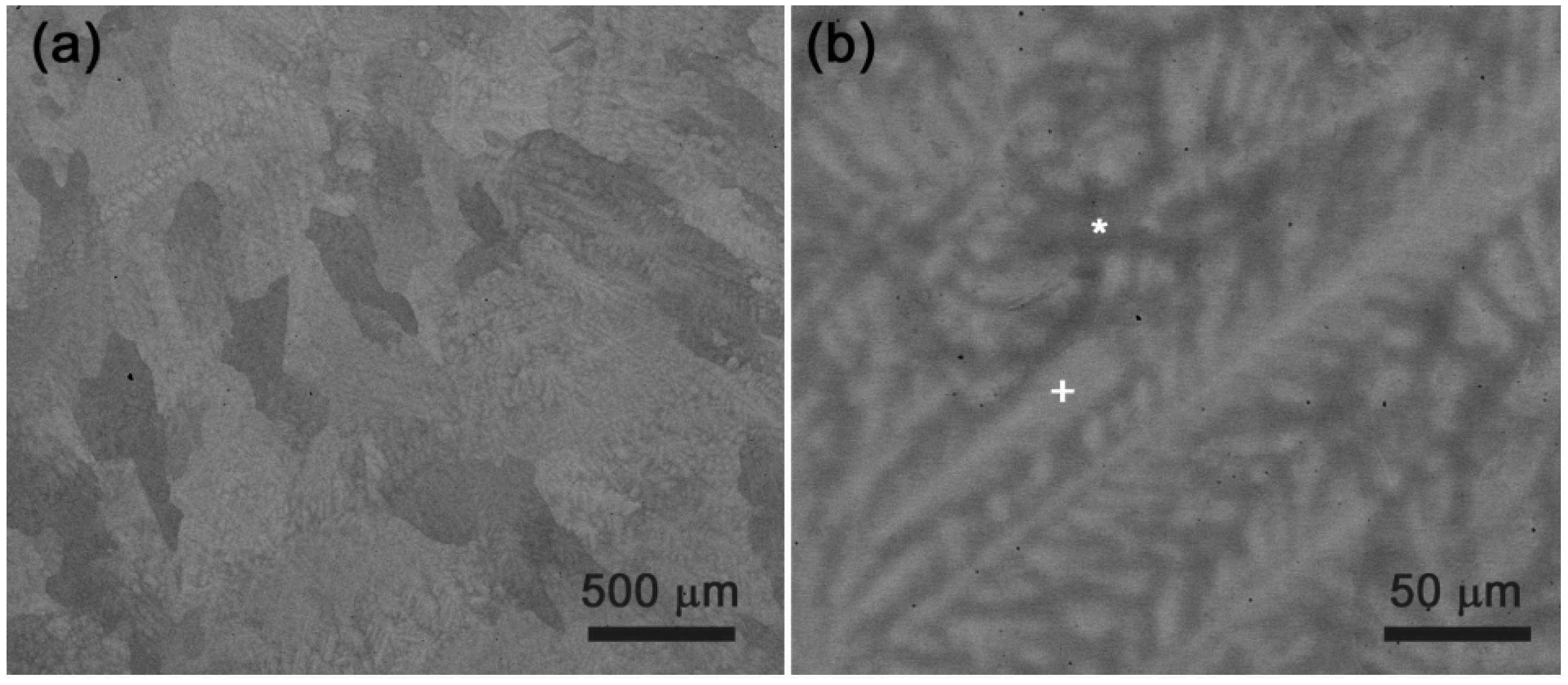
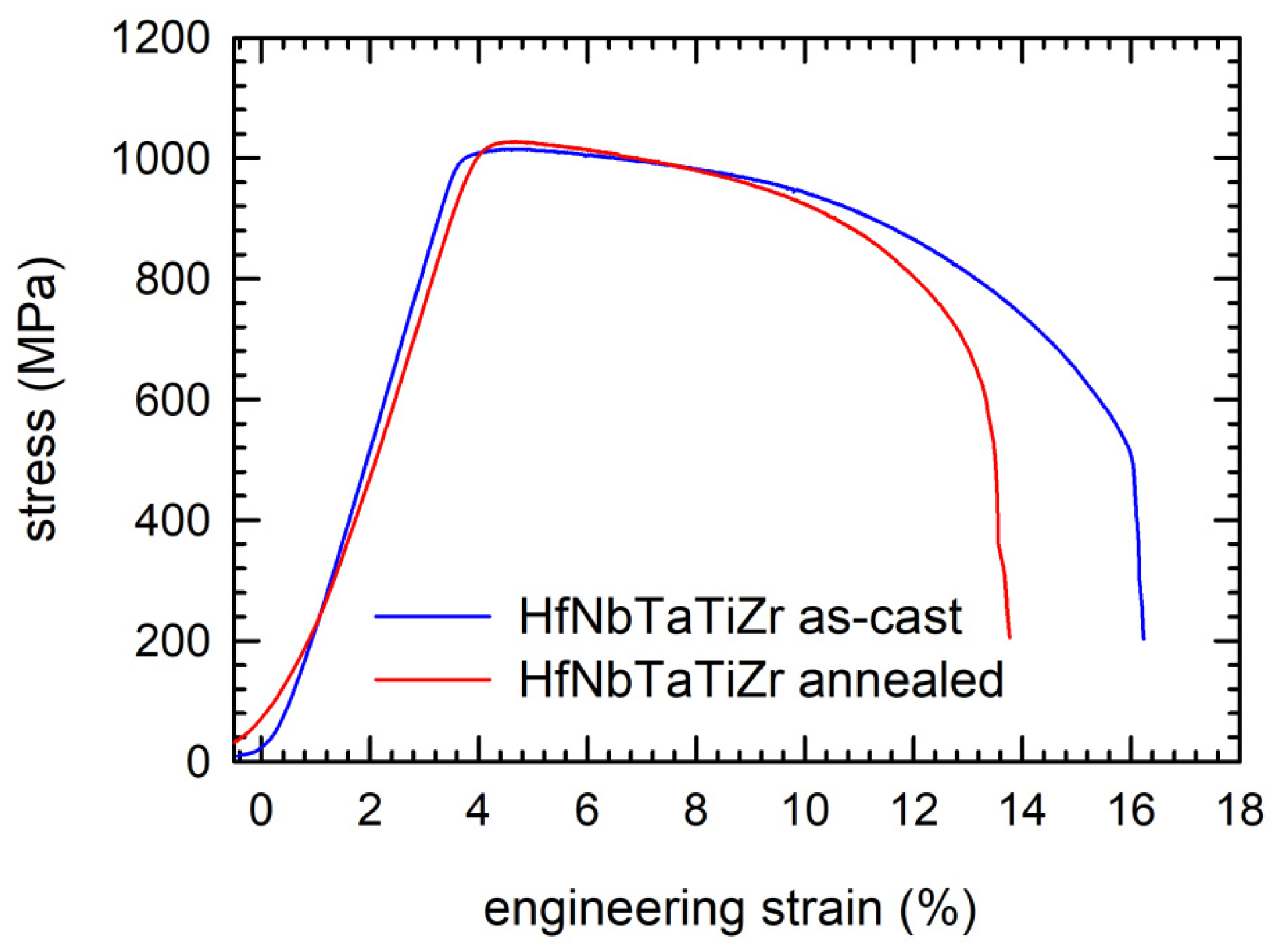
4.1. NbTaTiZr Alloy
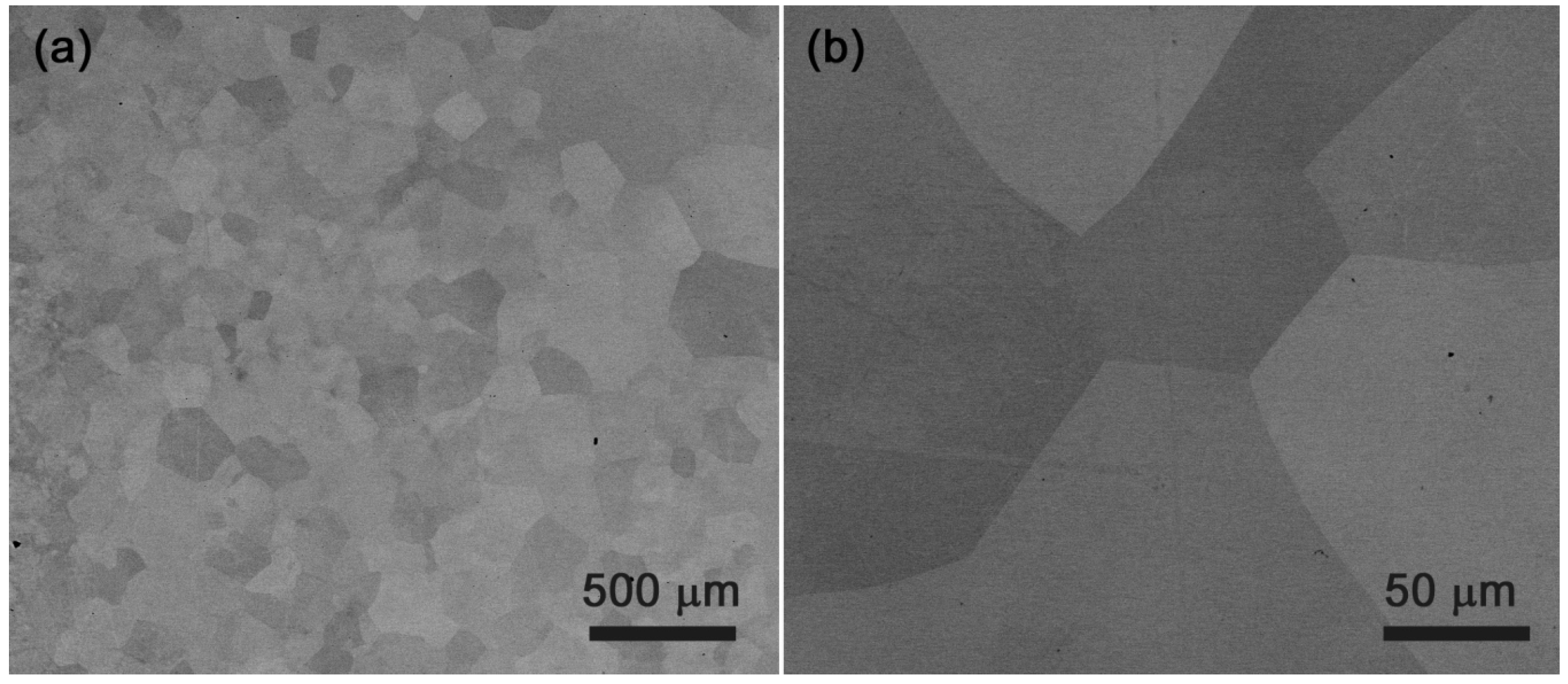
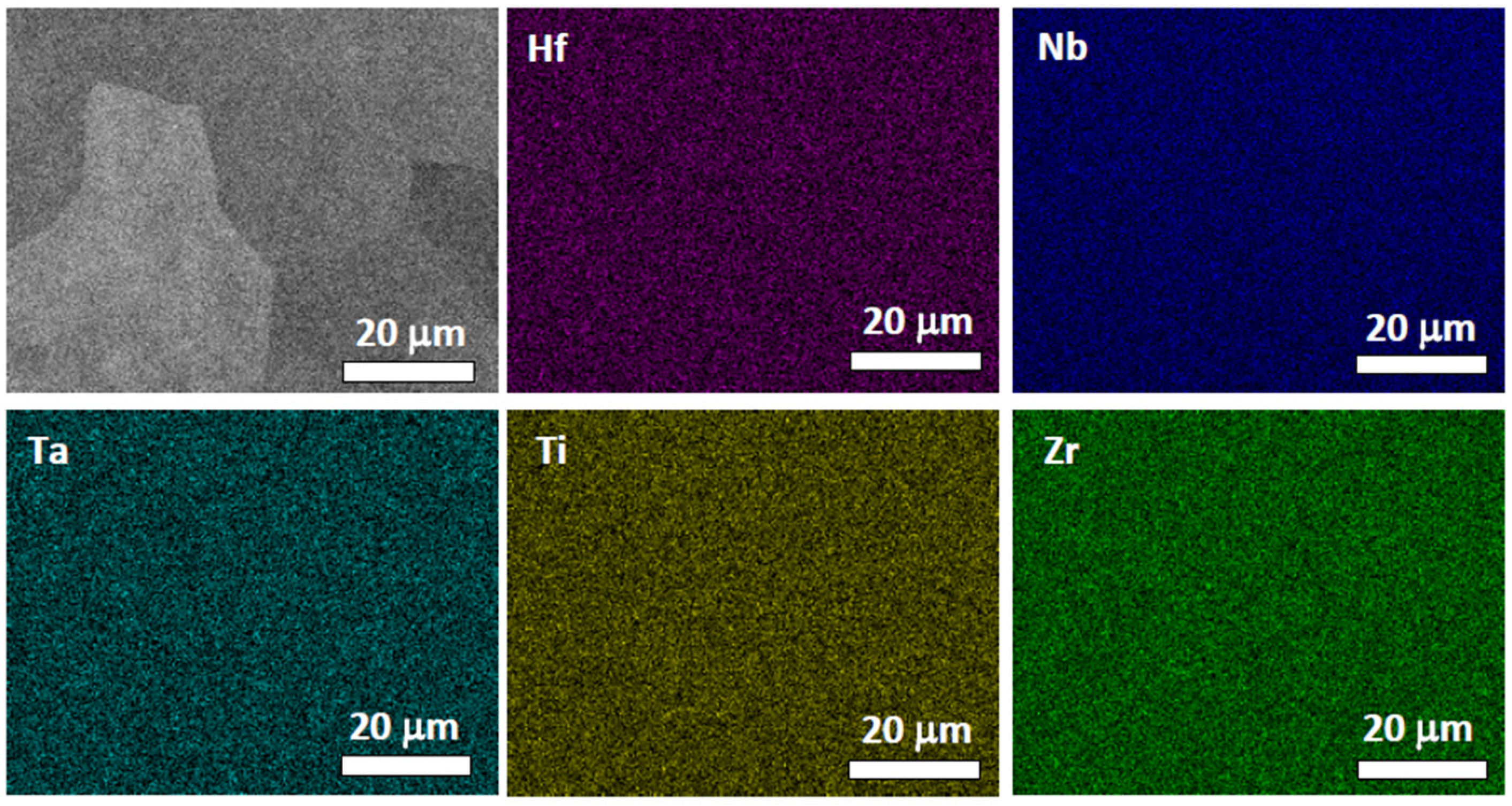
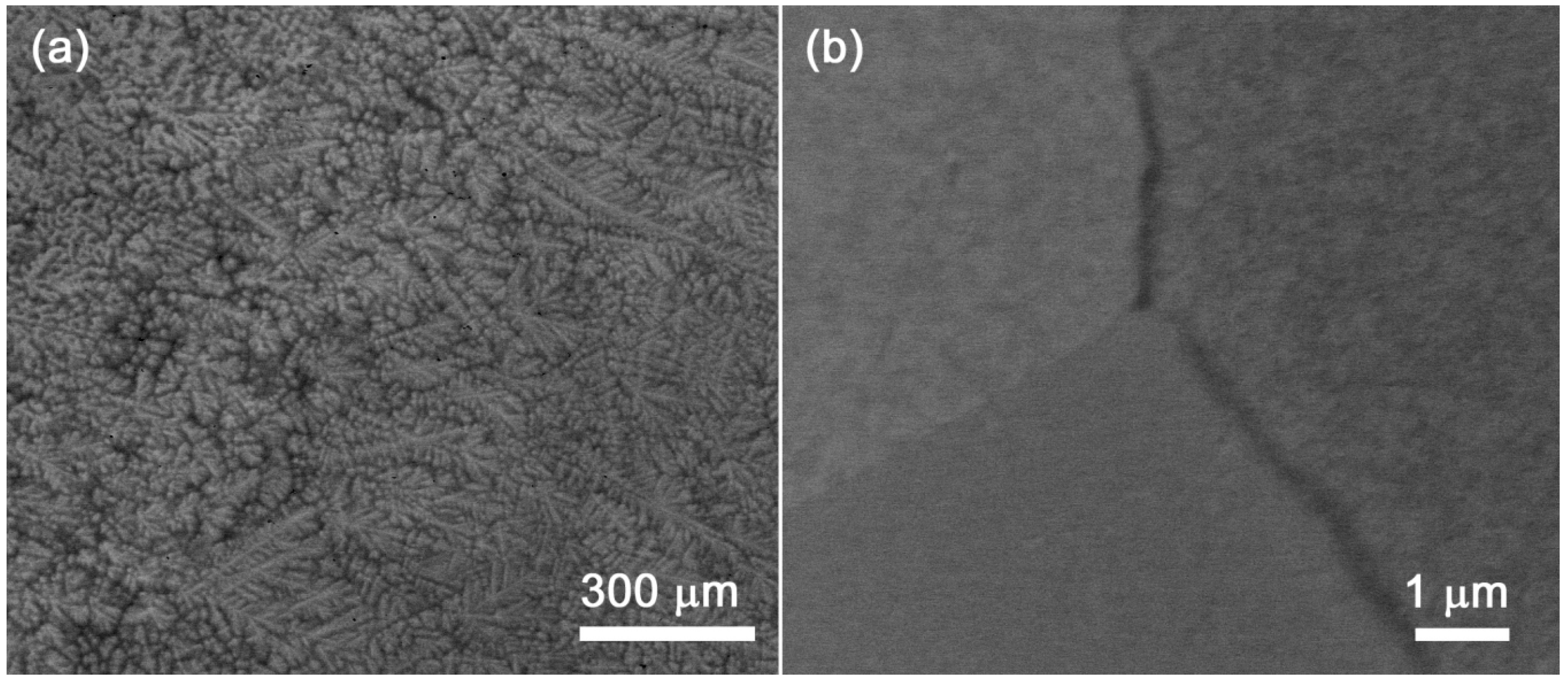
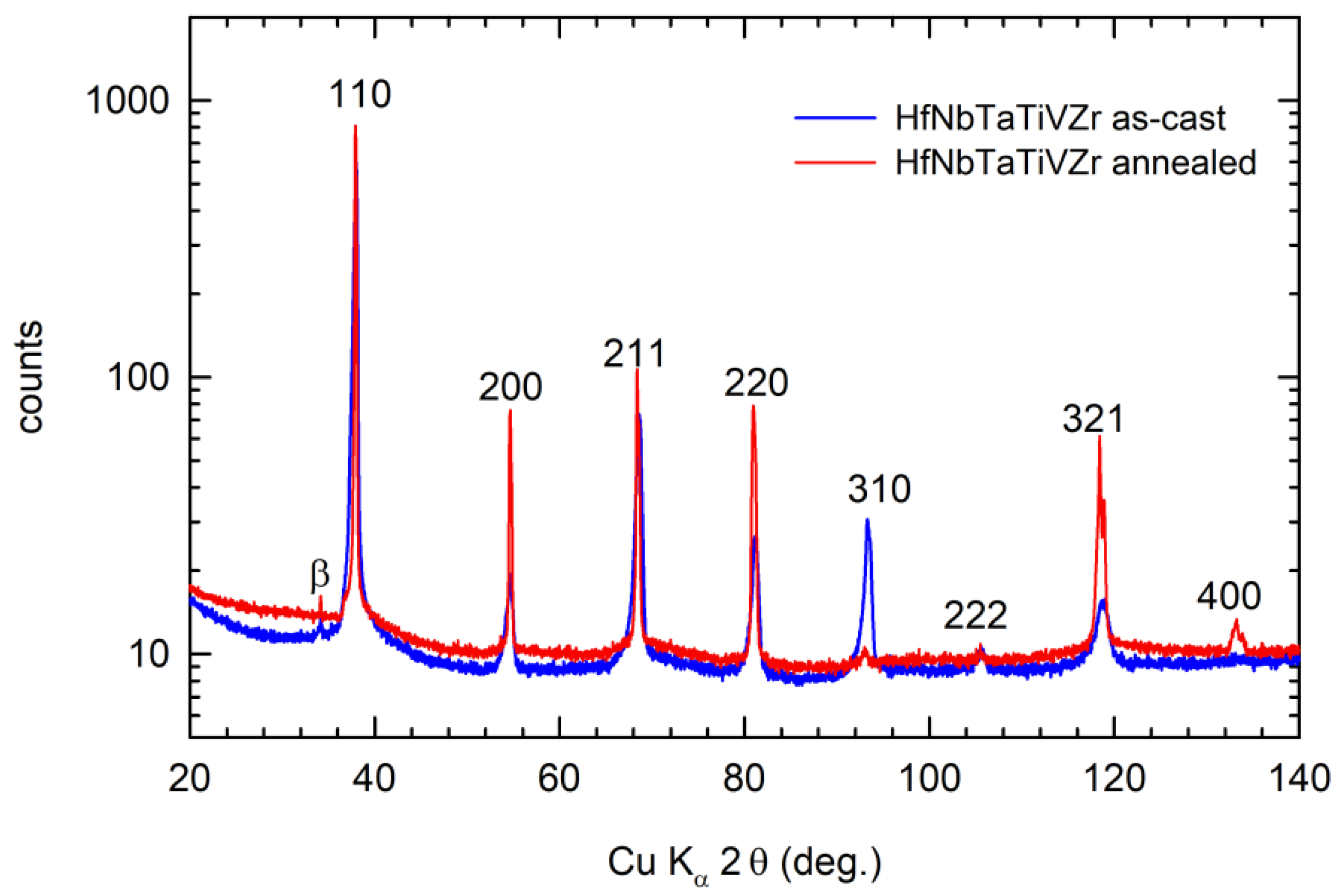
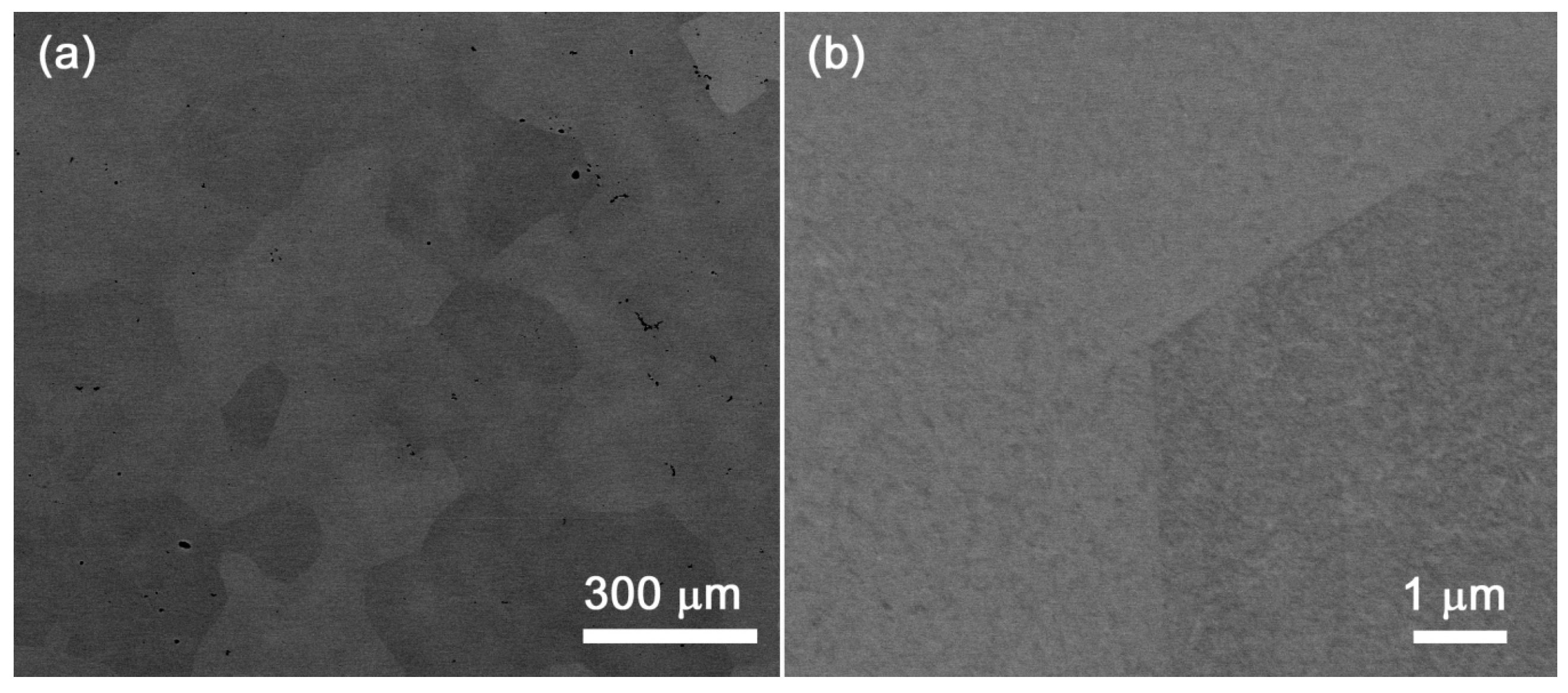
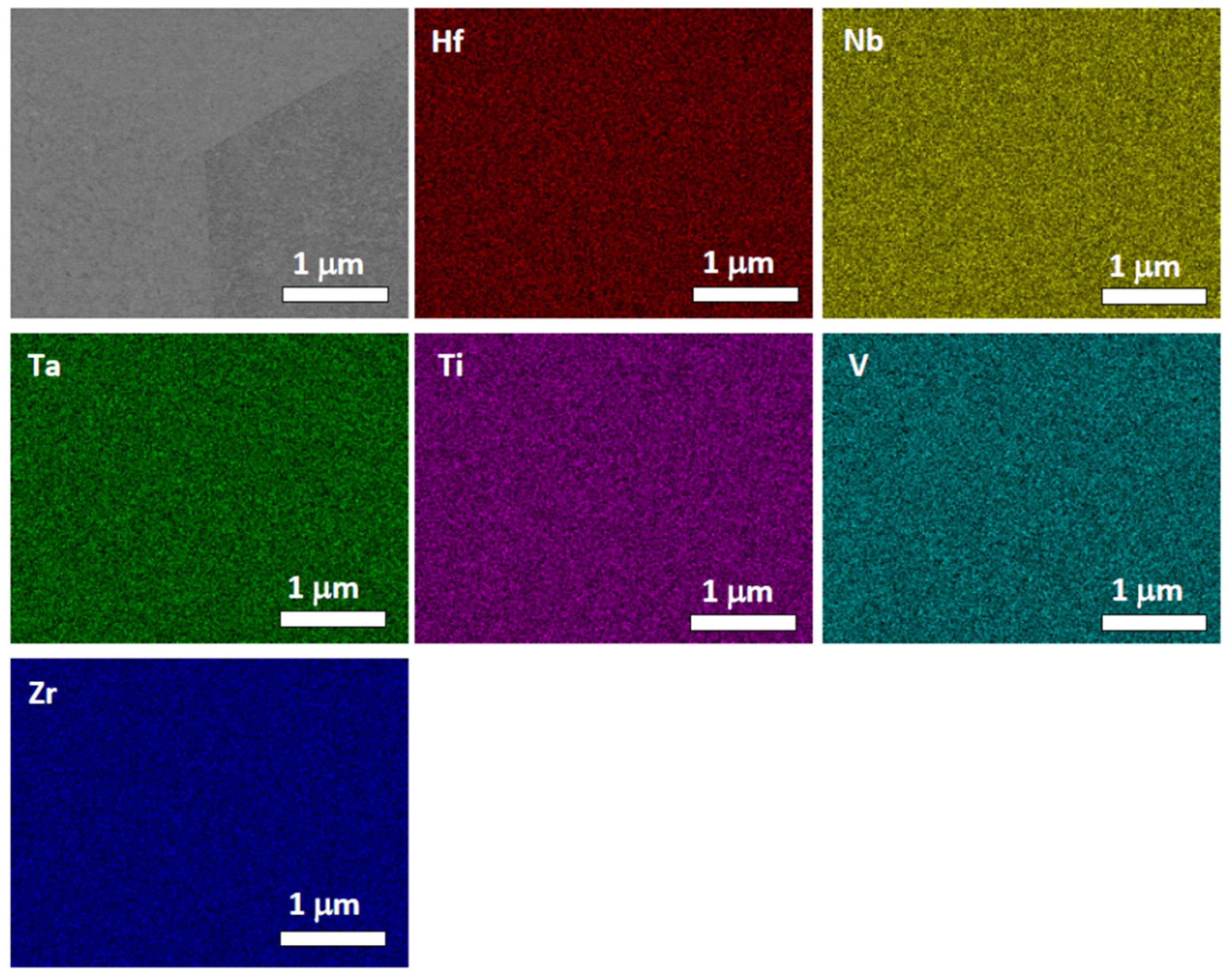
4.2. HfNbTaTiZr Alloy
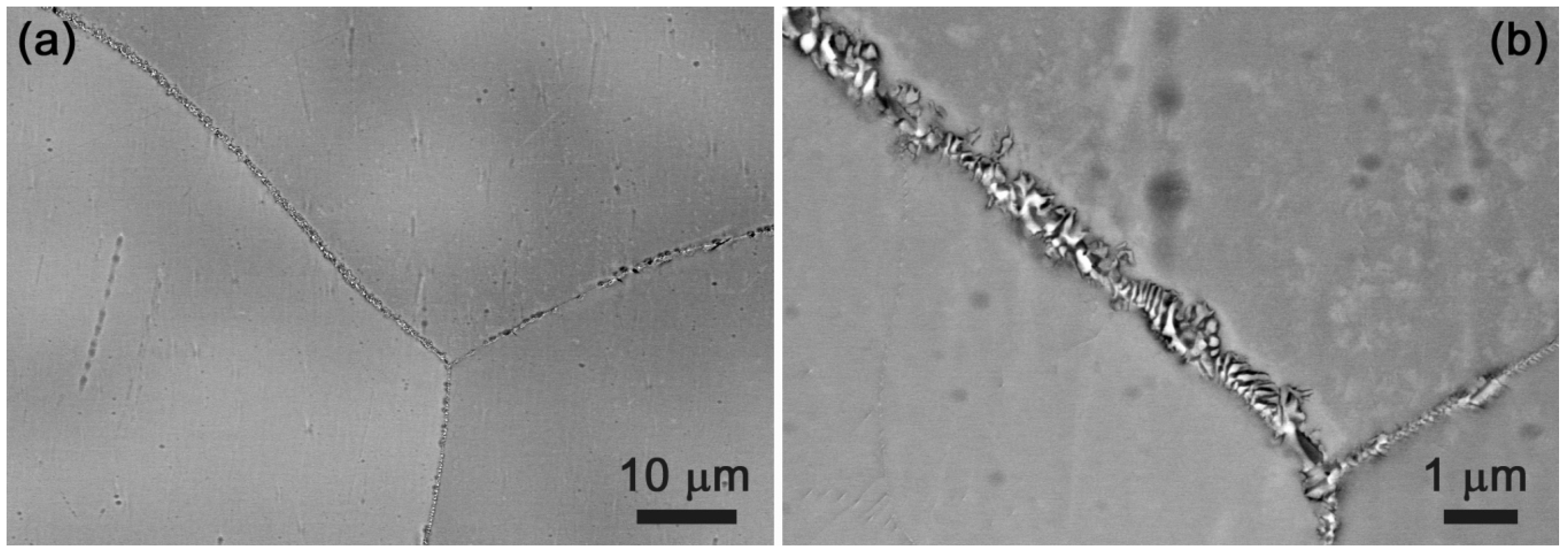
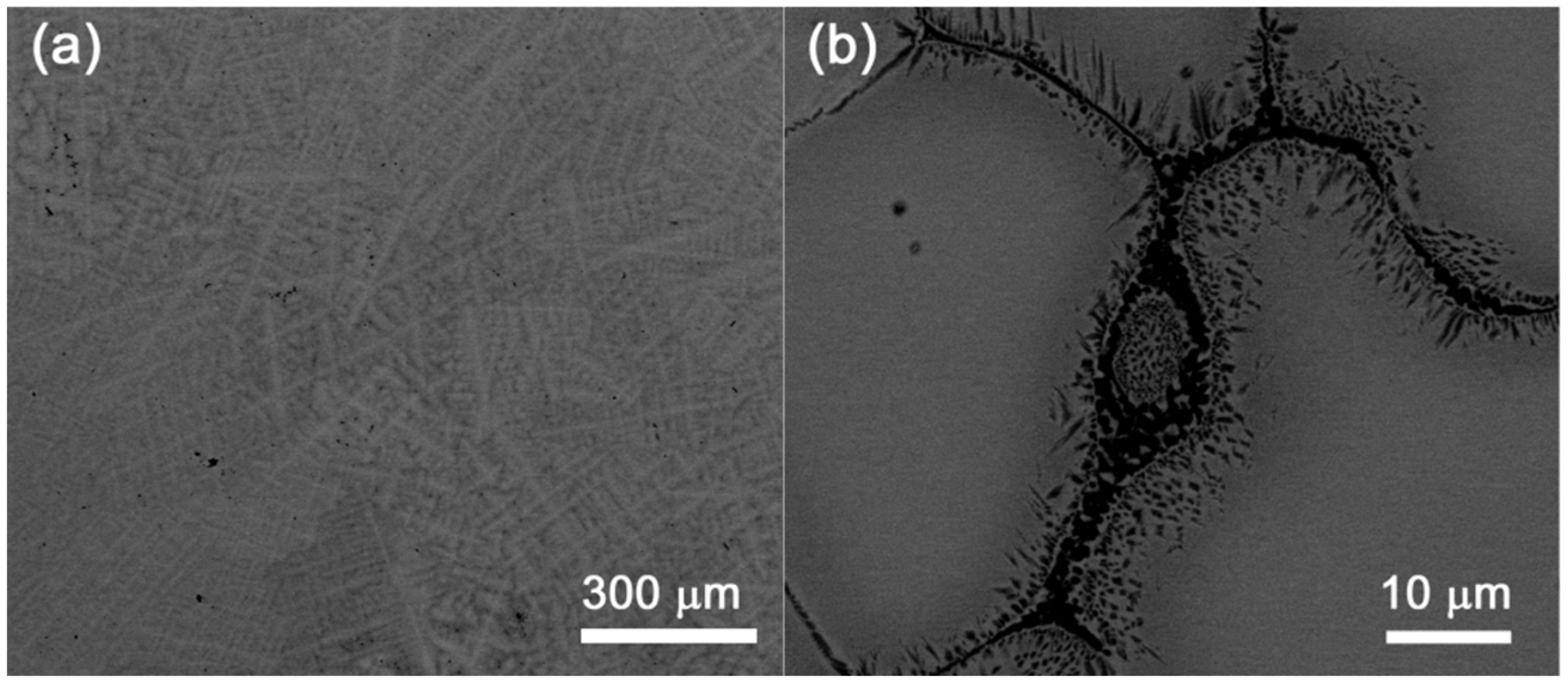
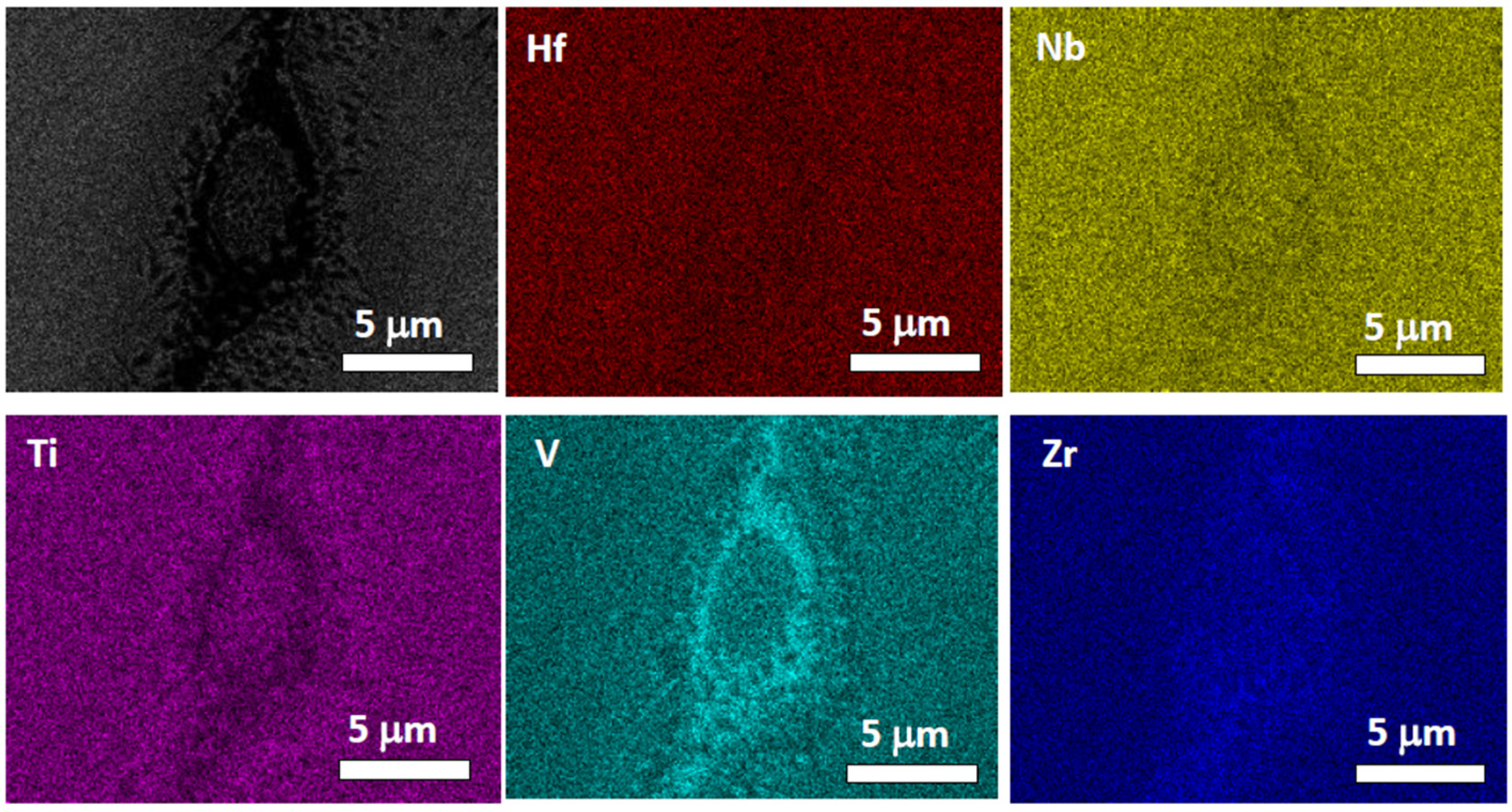
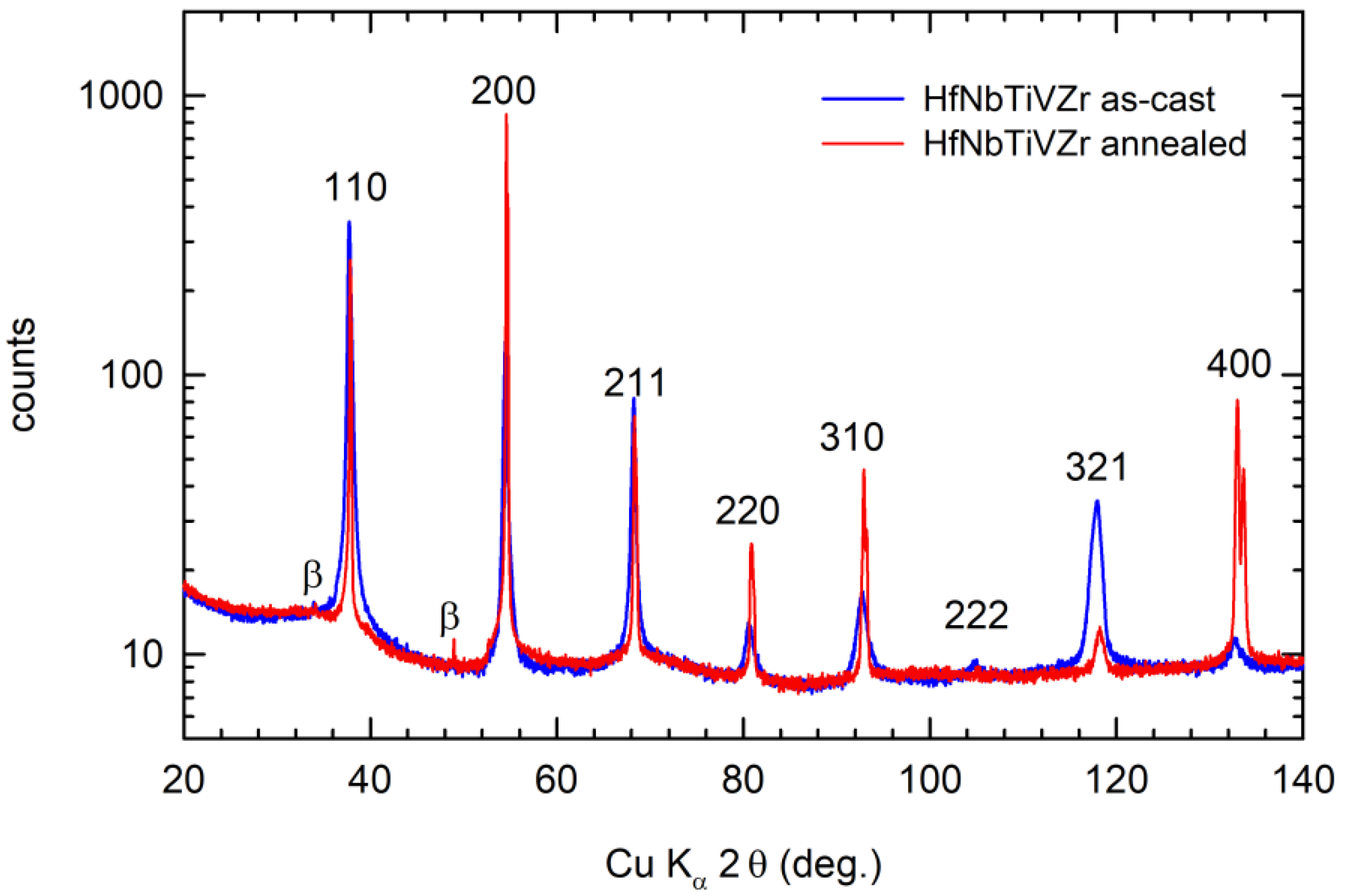
4.3. HfNbTiVZr Alloy
4.4. HfNbTaTiVZr Alloy
4.5. Mechanical Properties of Alloys
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W. Recent progress in high entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2014, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W. High-entropy alloys with a hexagonal close-packed structure designed by equi-atomic alloy strategy and binary phase diagrams. JOM 2014, 66, 1984–1992. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Srikanth, M.; Raja Annamalai, A.; Muthuchamy, A.; Jen, C.-P. A Review of the Latest Developments in the Field of Refractory High-Entropy Alloys. Crystals 2021, 11, 612. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Lee, C.; Maresca, F.; Feng, R.; Chou, Y.; Ungar, T.; Widom, M.; An, K.; Poplawsky, J.D.; Chou, Y.-C.; Liaw, P.K.; et al. Strength can be controlled by edge dislocations in refractory high-entropy alloys. Nat. Comm. 2021, 12, 5474. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Phase stability of low-density, multiprincipal component alloys containing aluminum, magnesium, and lithium. JOM 2014, 66, 2009–2020. [Google Scholar] [CrossRef]
- Nutor, R.K.; Cao, Q.; Wang, X.; Zhang, D.; Fang, Y.; Zhang, Y.; Jiang, J.-Z. Phase Selection, Lattice Distortions, and Mechanical Properties in High-Entropy Alloys. Adv. Eng. Mater. 2020, 22, 2000466. [Google Scholar] [CrossRef]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-high strength WNbMoTaV high-entropy alloys with fine grain structure fabricated by powder metallurgical process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructureon the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Meisenkothen, F.; Miracle, D.B.; Woodward, C.F. Microstructure and elevated temperature properties of a refractory TaNbHfZrTi alloy. J. Mater. Sci. 2012, 47, 4062–4074. [Google Scholar] [CrossRef]
- Chen, S.Y.; Tong, Y.; Tseng, K.-K.; Yeh, J.-W.; Poplawsky, J.D.; Wen, J.G.; Gao, M.C.; Kim, G.; Chen, W.; Ren, Y.; et al. Phase transformations of HfNbTaTiZr high-entropy alloy at intermediate temperatures. Scr. Mater. 2019, 158, 50–56. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Vrtnik, S.; Koželj, P.; Meden, A.; Maiti, S.; Steurer, W.; Feuerbacher, M.; Dolinšek, J. Superconductivity in thermally annealed Ta-Nb-Hf-Zr-Ti high-entropy alloys. J. Alloy. Compd. 2017, 695, 3530–3540. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Tong, X.; Zhang, H.; Wang, H.; Liu, X.J.; Ma, L.; Suo, H.L.; Lu, Z.P. Rare-earth high-entropy alloys with giant magnetocaloric effect. Acta Mater. 2007, 125, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef] [Green Version]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sørby, M.H.; Hauback, B.C. Hydrogen storage in high-entropy alloys with varying degree of local lattice strain. Int. J. Hydrog. Energy 2019, 44, 29140–29149. [Google Scholar] [CrossRef] [Green Version]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.-P.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; Sahlberg, M. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloy. Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Čížek, J.; Kalivodová, J.; Janeček, M.; Stráský, J.; Srba, O.; Macková, A. Advanced Structural Materials for Gas-Cooled Fast Reactors—A Review. Metals 2020, 11, 76. [Google Scholar] [CrossRef]
- Nagase, T.; Rack, P.D.; Noh, J.H.; Egami, T. In-situ TEM observation of structural changes in nano-crystalline CoCrCuFeNi multicomponent high-entropy alloy (HEA) under fast electron irradiation by high voltage electron microscopy (HVEM). Intermetallics 2015, 59, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.Q.; Yang, X.; Yang, T.F.; Liu, S.; Zhang, Y. Irradiation resistance in AlxCoCrFeNi high entropy alloy. JOM 2015, 67, 2340–2344. [Google Scholar] [CrossRef]
- He, M.; Wang, S.; Jin, K.; Bei, H.; Yasuda, K.; Matsumura, S. Enhanced damage resistance and novel defect structure of CrFeCoNi under in situ electron irradiation. Scr. Mater. 2016, 125, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Kenik, E.A.; Busby, J.T. Radiation-induced degradation of stainless steel light water reactor internals. Mater. Sci. Eng. R Rep. 2012, 73, 67–83. [Google Scholar] [CrossRef]
- Bruemmer, S.M.; Simonen, E.P.; Scott, P.M.; Andresen, P.L.; Was, G.S.; Nelson, J.L. Radiation-induced material changes and susceptibility to intergranular failure of light-water-reactor core internals. J. Nucl. Mater. 1999, 274, 299–314. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, Q.; Ye, W.; Ren, Y.; Greiner, C.; He, Y.; Wang, H. Design and Characterization of Self-Lubricating Refractory High Entropy Alloy-Based Multilayered Films. ACS Appl. Mater. Interfaces 2021, 13, 55712–55725. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritze, S.; Malinovskis, P.; Riekehr, L.; von Fieandt, L.; Lewin, E.; Jansson, U. Hard and crack resistant carbon supersaturated refractory nanostructured multicomponent coatings. Sci. Rep. 2018, 8, 14508. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Miracle, D.B.; Woodward, C.F. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloy. Compd. 2011, 509, 6043. [Google Scholar] [CrossRef]
- Heidelmann, M.; Feuerbacher, M.; Ma, D.; Grabowski, B. Structural anomaly in the high-entropy alloy ZrNbTiTaHf. Intermetallics 2016, 68, 11–15. [Google Scholar] [CrossRef]
- Zýka, J.; Málek, J.; Veselý, J.; Lukáč, F.; Čížek, J.; Kuriplach, J.; Melikhova, O. Microstructure and Room Temperature Mechanical Properties of Different 3 and 4 Element Medium Entropy Alloys from HfNbTaTiZr System. Entropy 2019, 27, 114. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Semiatin, S.L. Microstructure and properties of a refractory high-entropy alloy after cold working. J. Alloy. Compd. 2015, 649, 1110–1123. [Google Scholar] [CrossRef]
- Couzinié, J.P.; Dirras, G.; Perrière, L.; Chauveau, T.; Leroy, E.; Champion, Y.; Guillot, I. Microstructure of a near-equimolar refractory high-entropy alloy. Mater. Lett. 2014, 126, 285–287. [Google Scholar] [CrossRef]
- Schuh, B.; Völker, B.; Todt, J.; Schell, N.; Perrière, L.; Li, J.; Couzinié, J.P.; Hohenwarter, A. Thermodynamic instability of a nanocrystalline, single-phase TiZrNbHfTa alloy and its impact on the mechanical properties. ActaMater. 2018, 142, 201–212. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS Version 5 (Computer Software), Coelho Software; Brisbane, Australia. 2016. Available online: http://www.topas-academic.net/ (accessed on 1 January 2022).
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Čížek, J.; Haušild, P.; Cieslar, M.; Melikhova, O.; Vlasák, T.; Janeček, M.; Král, R.; Harcuba, P.; Lukáč, F.; Zýka, J.; et al. Strength enhancement of high entropy alloy HfNbTaTiZr by severe plastic deformation. J. Alloy. Compd. 2018, 768, 924–937. [Google Scholar] [CrossRef]
- Varvenne, C.; Luque, A.; Curtin, W.A. Theory of strengthening in fcc high entropy alloys. Acta Mater. 2016, 118, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Hua, D.; Xia, Q.; Wang, W.; Zhou, Q.; Li, S.; Qian, D.; Shi, J.; Wan, H. Atomistic insights into the deformation mechanism of a CoCrNi medium entropy alloy under nanoindentation. Int. J. Plast. 2021, 142, 102997. [Google Scholar] [CrossRef]
- Zýka, J.; Málek, J.; Pala, Z.; Andršová, I.; Veselý, J. Structure and Mechanical Properties of TaNbHfZrTi High Entropy Alloy. In Proceedings of the Metal 2015—24th International Conference on Metallurgy and Materials, Brno, Czech Republic, 3–5 June 2015. [Google Scholar]
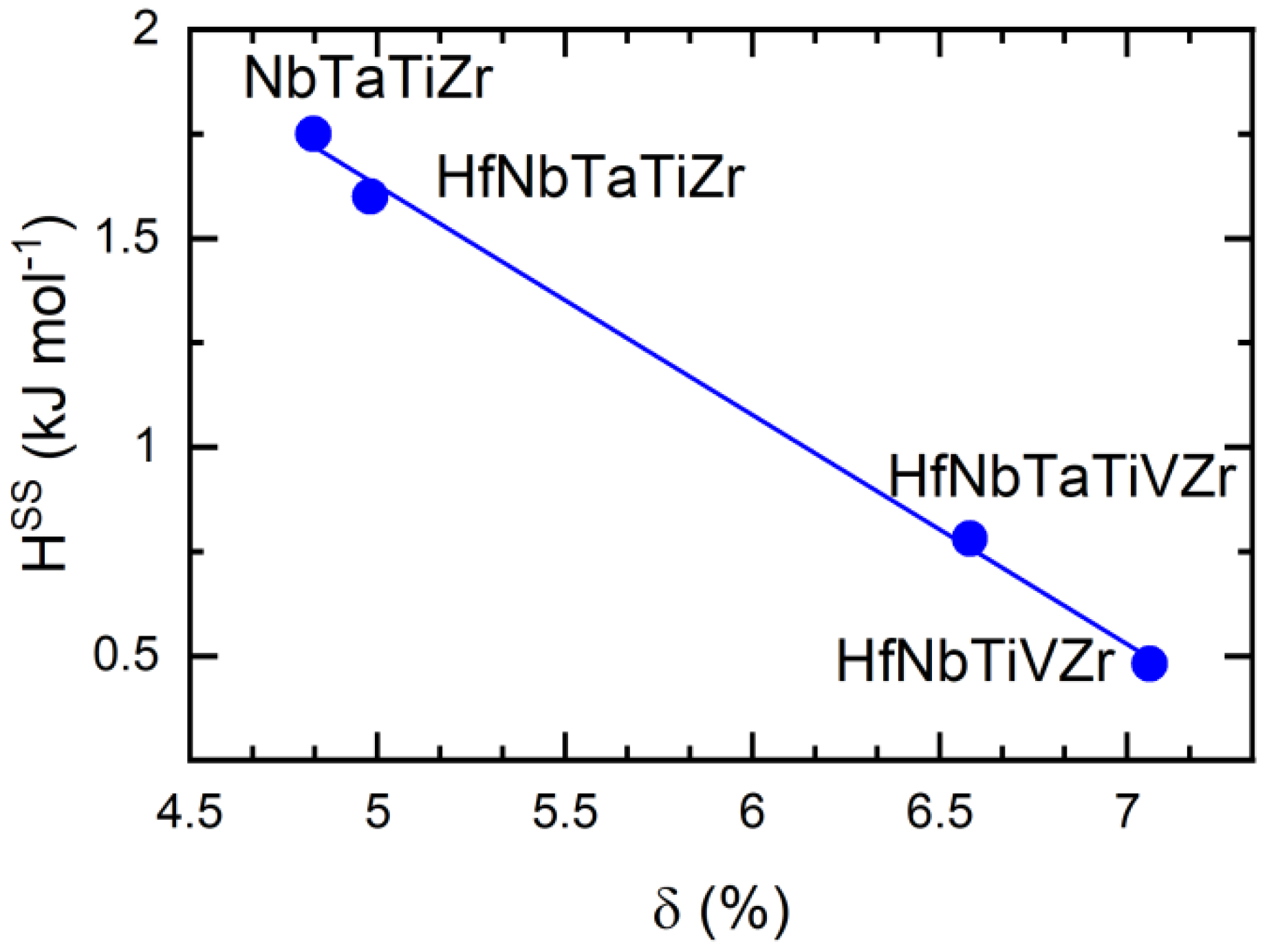




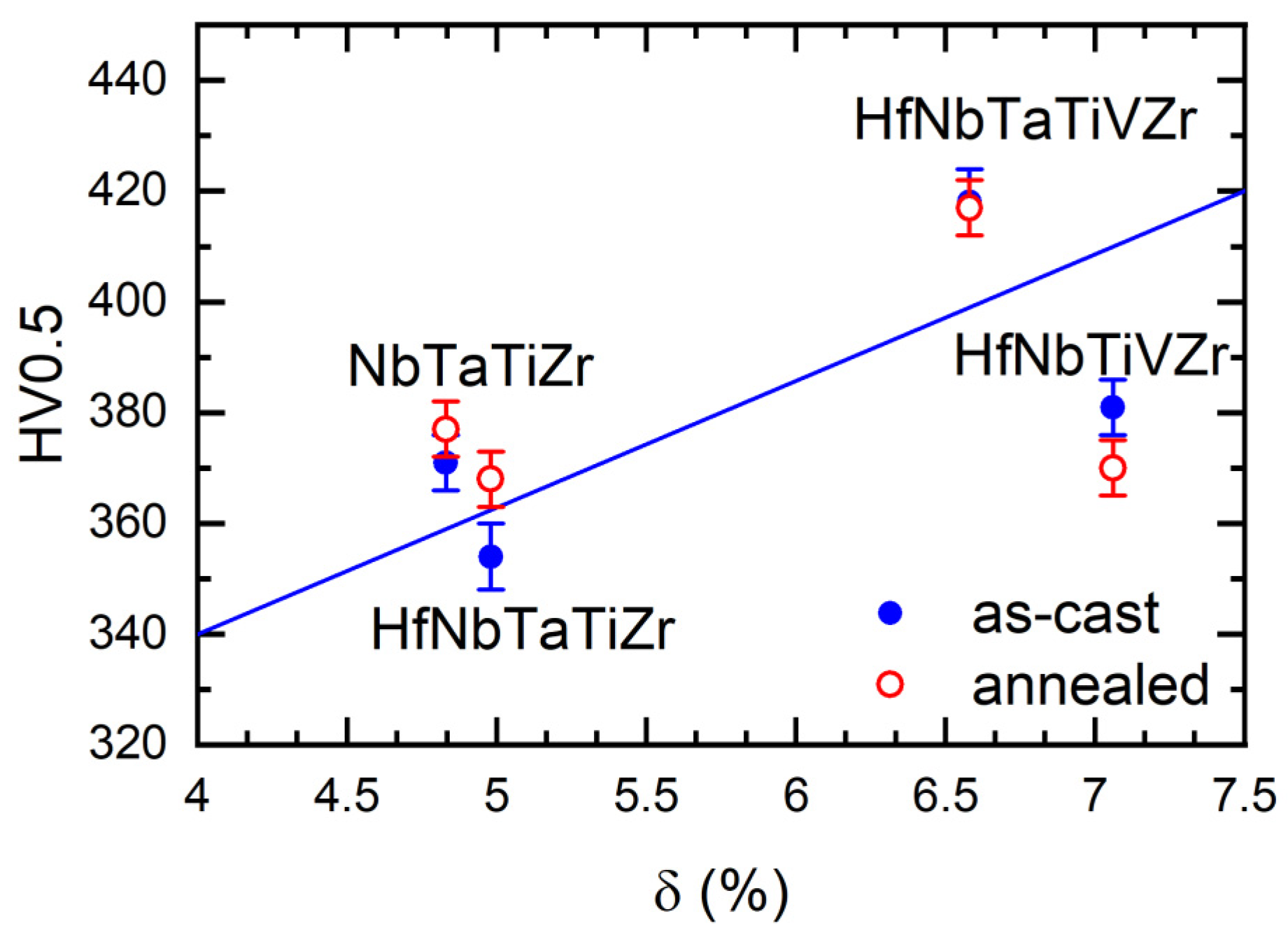
| Composition | δ (%) | VEC | SSS ® | HSS (kJ/mol) | HV0.5 as-Cast | HV0.5 Annealed |
|---|---|---|---|---|---|---|
| NbTaTiZr | 4.83 | 4.5 | 1.39 | 1.75 | 371 ± 5 | 377 ± 5 |
| HfNbTaTiZr | 4.98 | 4.4 | 1.61 | 1.60 | 354 ± 6 | 368 ± 5 |
| HfNbTiVZr | 7.06 | 4.4 | 1.61 | 0.48 | 381 ± 5 | 370 ± 5 |
| HfNbTaTiVZr | 6.58 | 4.5 | 1.79 | 0.78 | 418 ± 6 | 417 ± 5 |
| Element | ri (Å) | Structure | Lattice Parameters (Å) | Temperature Range (°C) |
|---|---|---|---|---|
| Hf (α) | 1.56 | hcp | a = 3.196, c = 5.051 | <1995 °C |
| Hf (β) | 1.56 | bcc | a = 3.610 | >1995 °C |
| Nb | 1.43 | bcc | a = 3.300 | - |
| Ta | 1.43 | bcc | a = 3.301 | - |
| Ti (α) | 1.45 | hcp | a = 2.951, c = 4.686 | <882 °C |
| Ti (β) | 1.43 | bcc | a = 3.306 | >882 °C |
| V | 1.31 | bcc | a = 3.03 | - |
| Zr (α) | 1.59 | hcp | a = 3.232, c = 5.147 | <865 °C |
| Zr (β) | 1.56 | bcc | a = 3.609 | >865 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasák, T.; Čížek, J.; Melikhova, O.; Lukáč, F.; Preisler, D.; Janeček, M.; Harcuba, P.; Zimina, M.; Srba, O. Thermal Stability of Microstructure of High-Entropy Alloys Based on Refractory Metals Hf, Nb, Ta, Ti, V, and Zr. Metals 2022, 12, 394. https://doi.org/10.3390/met12030394
Vlasák T, Čížek J, Melikhova O, Lukáč F, Preisler D, Janeček M, Harcuba P, Zimina M, Srba O. Thermal Stability of Microstructure of High-Entropy Alloys Based on Refractory Metals Hf, Nb, Ta, Ti, V, and Zr. Metals. 2022; 12(3):394. https://doi.org/10.3390/met12030394
Chicago/Turabian StyleVlasák, Tomáš, Jakub Čížek, Oksana Melikhova, František Lukáč, Dalibor Preisler, Miloš Janeček, Petr Harcuba, Mariia Zimina, and Ondřej Srba. 2022. "Thermal Stability of Microstructure of High-Entropy Alloys Based on Refractory Metals Hf, Nb, Ta, Ti, V, and Zr" Metals 12, no. 3: 394. https://doi.org/10.3390/met12030394
APA StyleVlasák, T., Čížek, J., Melikhova, O., Lukáč, F., Preisler, D., Janeček, M., Harcuba, P., Zimina, M., & Srba, O. (2022). Thermal Stability of Microstructure of High-Entropy Alloys Based on Refractory Metals Hf, Nb, Ta, Ti, V, and Zr. Metals, 12(3), 394. https://doi.org/10.3390/met12030394







