Effect of Iron Ore Pellet Size on Metallurgical Properties
Abstract
:1. Introduction
- chemical composition;
- size distribution (which is important for the permeability of the ore burden layers in the furnace);
- cold crushing strength (which is used to characterise the degradation of ore burden materials during transport and handling);
- low-temperature reduction–disintegration (which characterises the effect of the reduction step of hematite to magnetite in the BF shaft);
- reducibility (which characterises the ease of removing oxygen from iron ores);
- swelling (which characterises the vulnerability to change in volume during reduction and is tested to ensure that the volume increase during reduction does not exceed a set maximum);
- softening and melting properties (which are important for the formation of the cohesive and melting zone in the blast furnace).
2. Materials and Methods
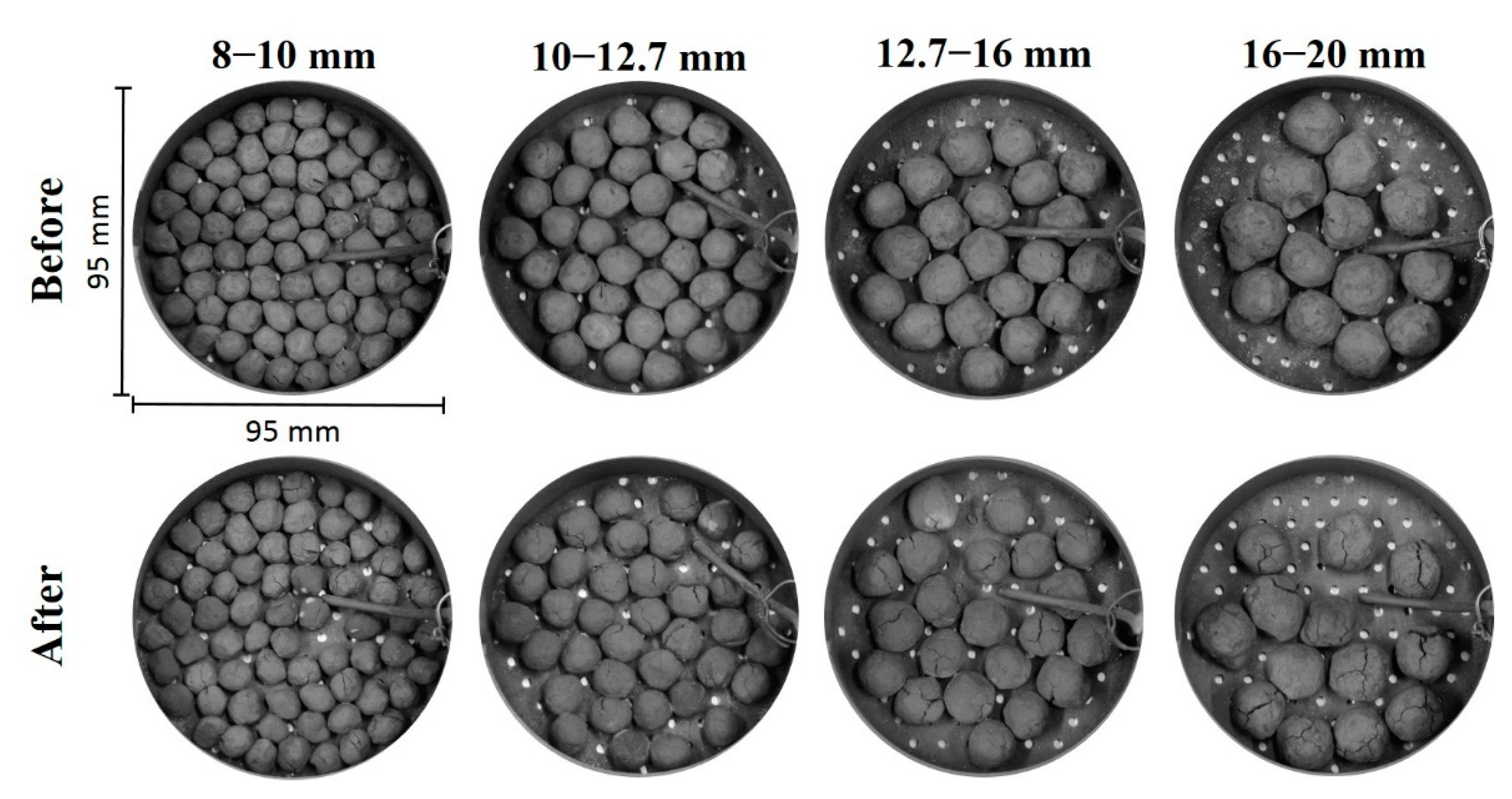
2.1. Iron Ore Pellets
2.2. Reducibility Test
2.3. Reduction-Softening Test
2.4. Cold Crushing Strength Test
2.5. Low-Temperature Reduction-Disintegration Test
3. Results and Discussion
3.1. Chemical Composition
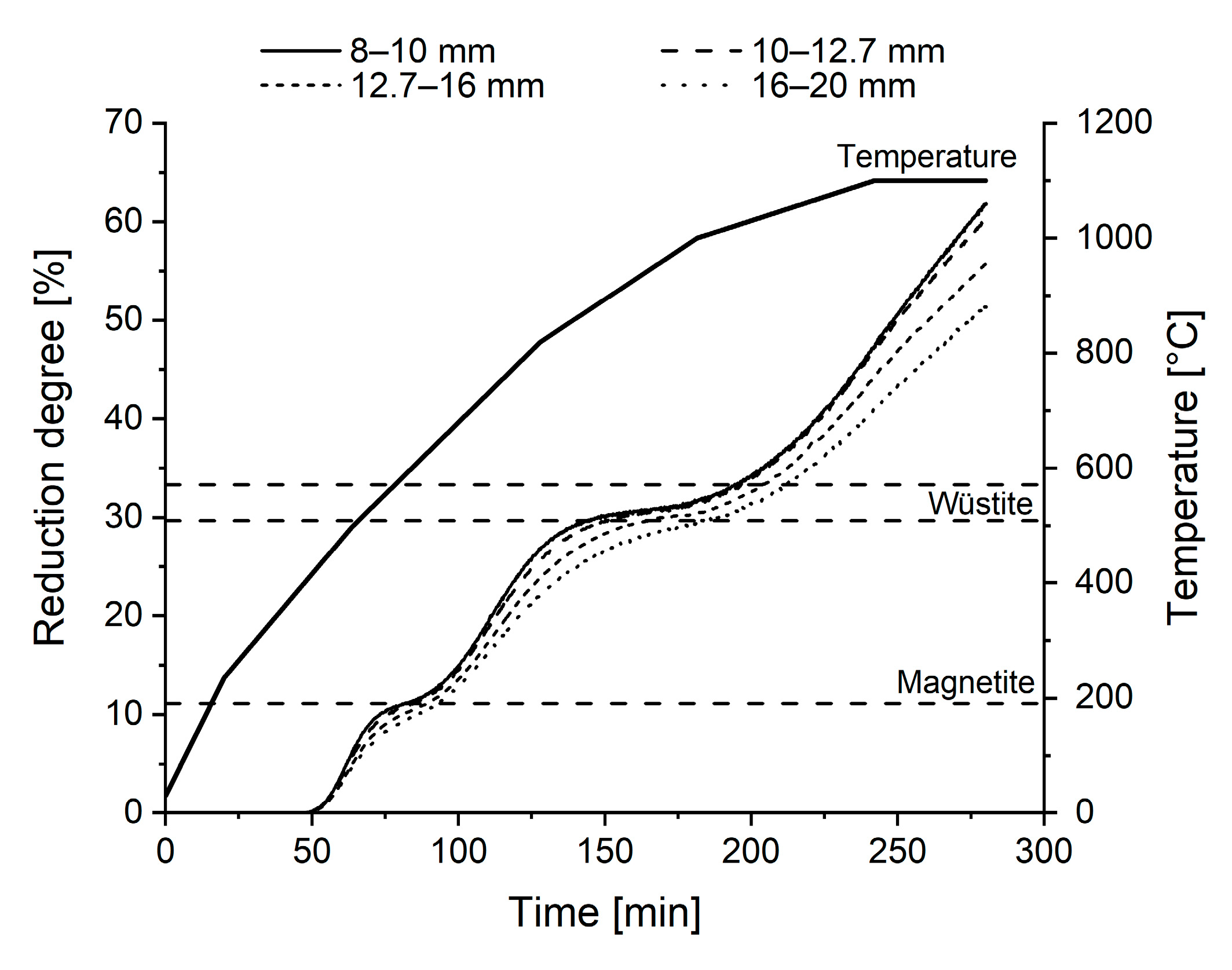
3.2. Reduction Behaviour
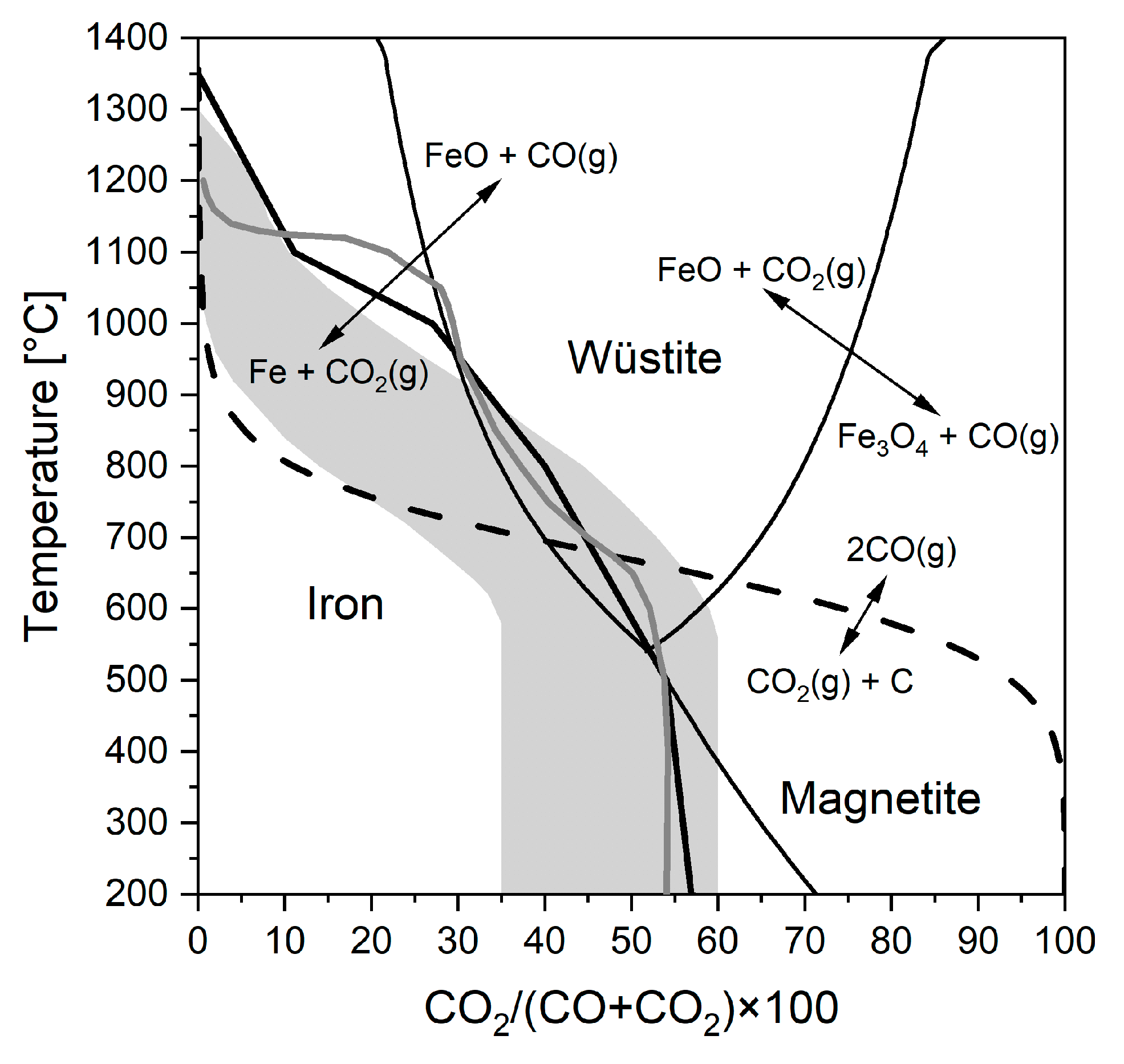
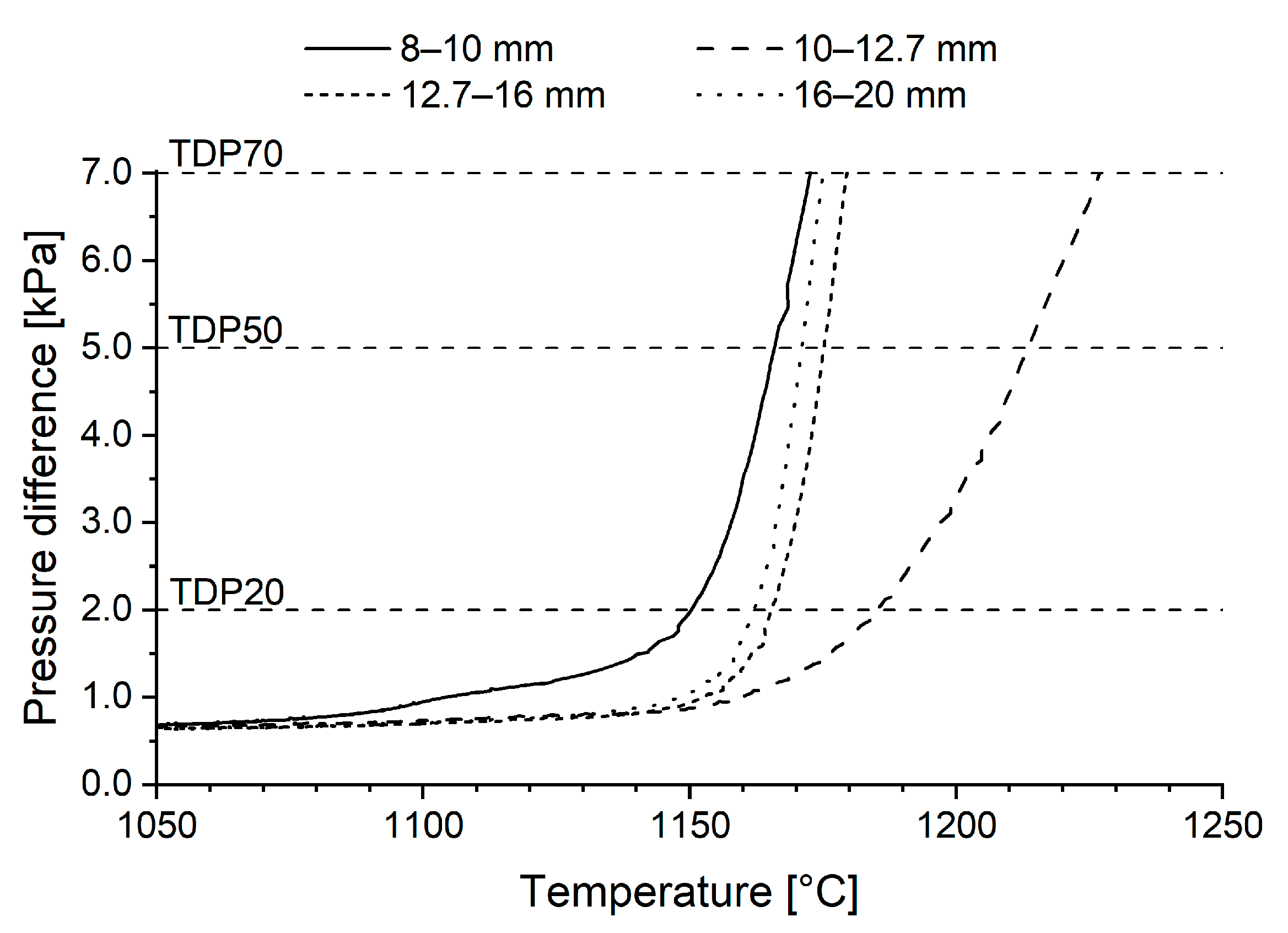
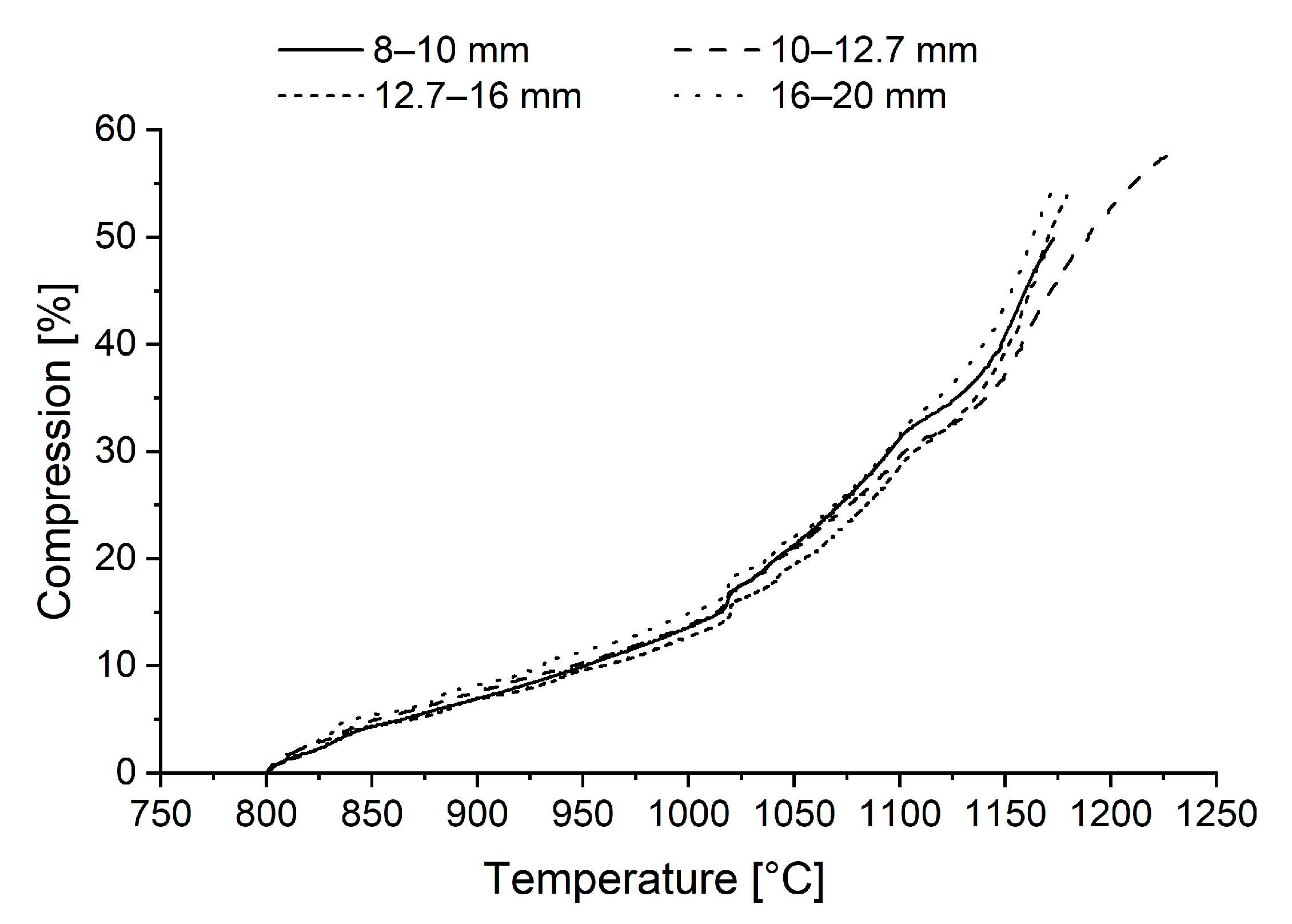
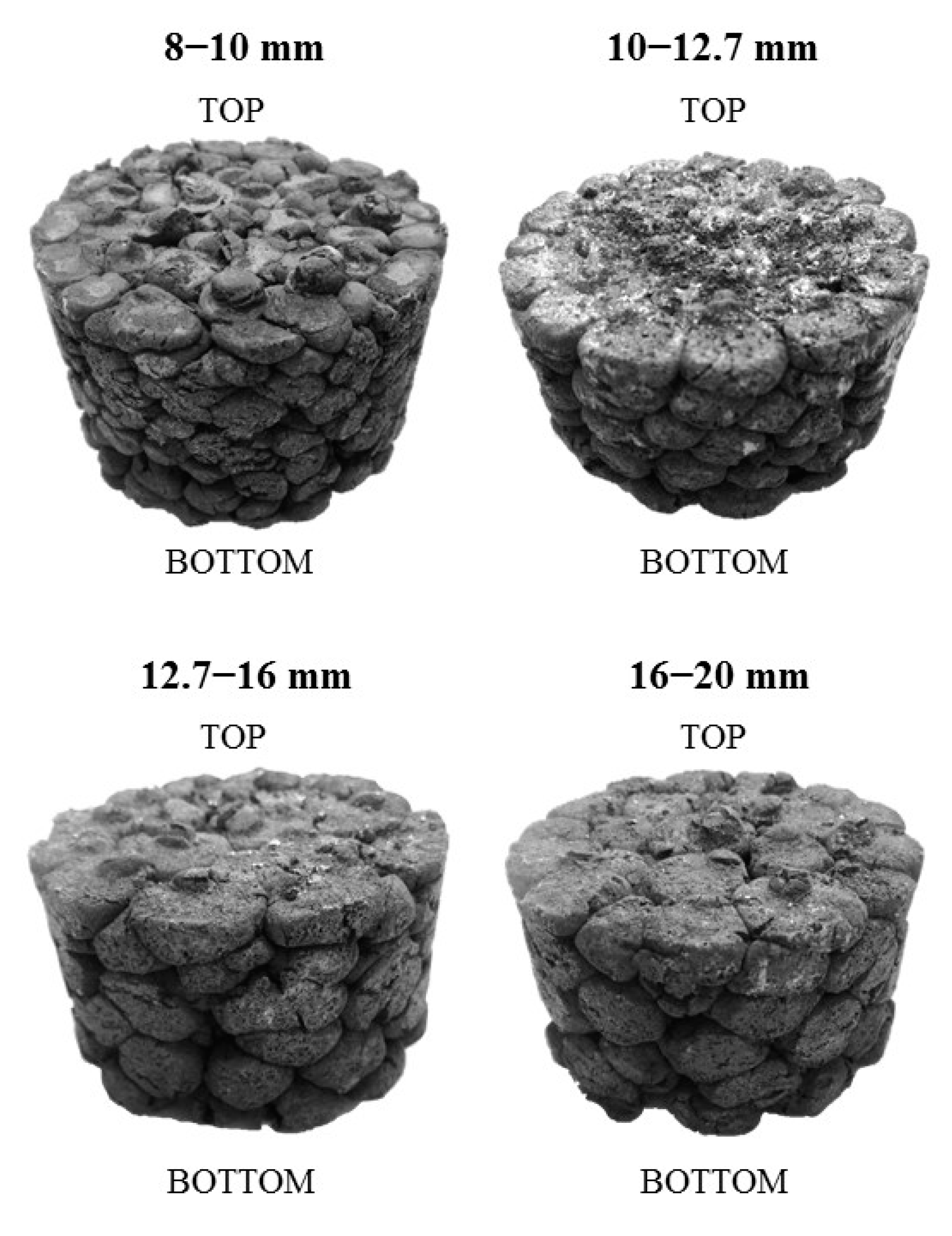
3.3. Reduction-Softening Behaviour
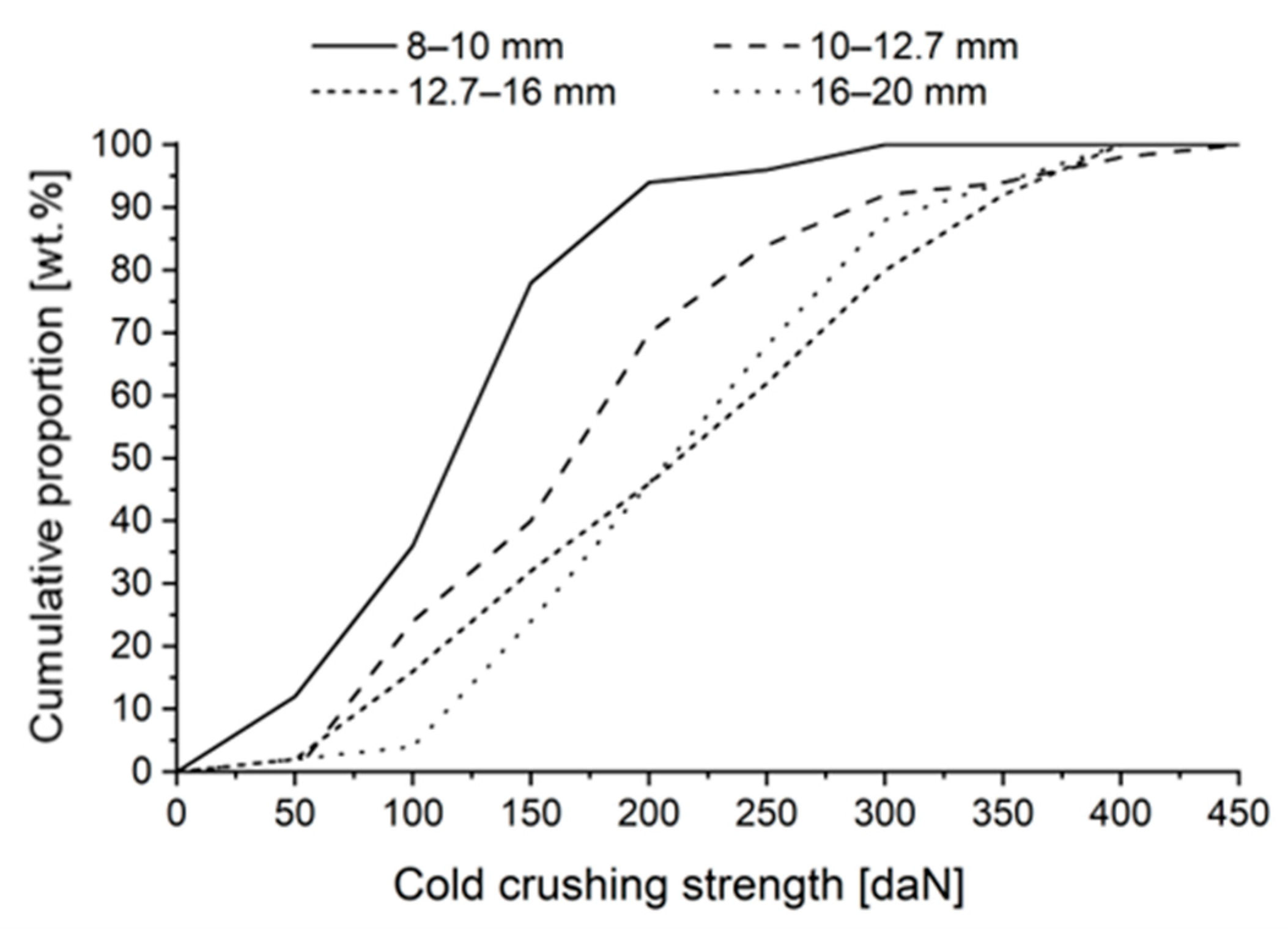
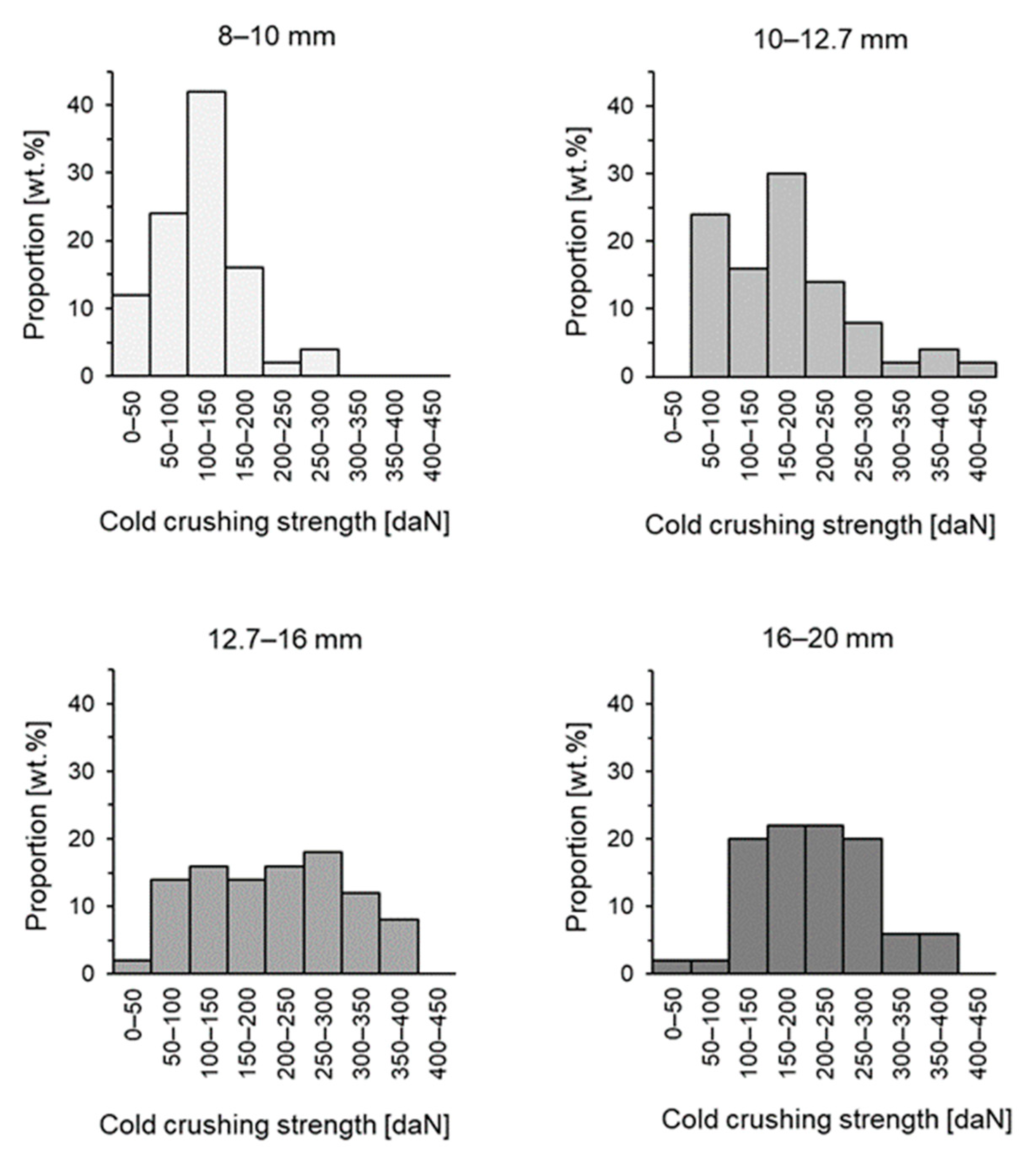
3.4. Cold Crushing Strength
3.5. Low-Temperature Reduction-Disintegration
3.6. Implications for Material Testing and Industrial Relevance
4. Conclusions
- Reducibility under unconstrained conditions decreased as pellet size increased. This is due to the topochemical reduction from the pellet periphery towards the core. However, the difference between 8–10 mm and 10–12.7 mm pellets was not significant.
- Pellets sized 10–12.7 mm reached the highest final temperature and the highest reduction degree in the ARUL reduction-softening test. The highest proportions of MgO in the silicate slag and in the wüstite were verified with FESEM-EDS in the 10–12.7 mm size, increasing the softening temperature of the pellets. A higher reduction degree in the cohesive zone indicates less direct reduction occurring in the blast furnace hearth and a lower need for reducing agents in the process.
- Cold crushing strength increased, on average, as pellet size increased. Simultaneously, the proportion of weak pellets (<150 daN) and very weak pellets (<60 daN) decreased, which is favourable in a blast furnace operation. Almost all pellets sized −10 mm were classified as weak.
- Small pellets showed better resistance against reduction-disintegration compared to large pellets in the LTD test. LTD values were very low for +12.7 mm pellets. High resistance against reduction-disintegration in the upper BF shaft is favourable in a blast furnace operation.
- No abnormal swelling was detected in any of the size fractions of pellets during non-isothermal reduction up to 1100 °C.
- Among the samples studied, pellets sized 10–12.7 mm had the highest metallurgical properties, on average. Therefore, a pellet batch should consist of pellets in that size range as much as possible for a smooth and efficient blast furnace operation.
- To conclude, pellet size influences metallurgical properties of iron ore pellets, and is therefore of the utmost importance to standardise particle size when comparing metallurgical properties among different pellet qualities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousa, E.A. Modern Blast Furnace Ironmaking Technology: Potentials to Meet the Demand of High Hot Metal Production and Lower Energy Consumption. Metall. Mater. Eng. 2019, 25, 69–104. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Pan, J.; Lu, L.; Holmes, R.J. Iron Ore Pelletization. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 435–473. [Google Scholar] [CrossRef]
- Umadevi, T.; Kumar, P.; Lobo, N.F.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Effect of Iron Ore Pellet Size on Its Properties and Microstructure. Steel Res. Int. 2009, 80, 709–716. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ricketts, J. Modern Blast Furnace Ironmaking, 3rd ed.; IOS Press BV: Amsterdam, The Netherlands, 2015; ISBN 978-1-61499-498-5. [Google Scholar]
- Dwarapudi, S.; Ghosh, T.K.; Tathavadkar, V.; Denys, M.B.; Bhattacharjee, D.; Venugopal, R. Effect of MgO in the Form of Magnesite on the Quality and Microstructure of Hematite Pellets. Int. J. Miner. Process. 2012, 112–113, 55–62. [Google Scholar] [CrossRef]
- Niiniskorpi, V. Development of Phases and Structures during Pelletizing of Kiruna Magnetite Ore. Ph.D. Thesis, Åbo Akademi University, Åbo Akademi, Finland, 2004. [Google Scholar]
- Dwarapudi, S.; Umadevi, T.; Rao, S.M.; Ranjan, M. Influence of Pellet Size on Quality and Microstructure of Iron Ore Pellets. ISIJ Int. 2008, 48, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Iljana, M.; Mattila, O.; Alatarvas, T.; Visuri, V.-V.; Kurikkala, J.; Paananen, T.; Fabritius, T. Dynamic and Isothermal Reduction Swelling Behaviour of Olivine and Acid Iron Ore Pellets under Simulated Blast Furnace Shaft Conditions. ISIJ Int. 2012, 52, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Iljana, M.; Mattila, O.; Alatarvas, T.; Kurikkala, J.; Paananen, T.; Fabritius, T. Effect of Circulating Elements on the Dynamic Reduction Swelling Behaviour of Olivine and Acid Iron Ore Pellets under Simulated Blast Furnace Shaft Conditions. ISIJ Int. 2013, 53, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Pisilä, E.; Kondrakov, M.; Fabritius, T. Effect of Adding Limestone on the Metallurgical Properties of Iron Ore Pellets. Int. J. Miner. Process. 2015, 141, 34–43. [Google Scholar] [CrossRef]
- Iljana, M. Iron Ore Pellet Properties under Simulated Blast Furnace Conditions. Investigation on Reducibility, Swelling and Softening. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2017. [Google Scholar]
- Kemppainen, A.; Iljana, M.; Heikkinen, E.-P.; Paananen, T.; Mattila, O.; Fabritius, T. Reduction Behavior of Cold-Bonded Briquettes under Simulated Blast Furnace Conditions. ISIJ Int. 2014, 54, 1539–1545. [Google Scholar] [CrossRef] [Green Version]
- Kemppainen, A.; Ohno, K.-I.; Iljana, M.; Mattila, O.; Paananen, T.; Heikkinen, E.-P.; Maeda, T.; Kunitomo, K.; Fabritius, T. Softening Behaviors of Acid and Olivine Fluxed Iron Ore Pellets in the Cohesive Zone of a Blast Furnace. ISIJ Int. 2015, 55, 2039–2046. [Google Scholar] [CrossRef] [Green Version]
- Kemppainen, A. Limiting Phenomena Related to the Use of Iron Ore Pellets in a Blast Furnace. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2015. [Google Scholar]
- Kemppainen, A.; Iljana, M.; Heikkinen, E.-P.; Paananen, T.; Mattila, O.; Fabritius, T. Recent Development in the Evaluation of the High-Temperature Properties of Iron Ore Pellets. In Proceedings of the SCANMET V, 5th International Conference on Process Development in Iron and Steelmaking, Luleå, Sweden, 12–15 June 2016; Swerea MEFOS: Luleå, Sweden , 2016. ISBN 978-91-639-1233-7. [Google Scholar]
- Kemppainen, A.; Mousa, E.; Wang, C.; Suopajärvi, H.; Iljana, M.; Heikkinen, E.-P.; Fabritius, T. Introduction of Biomass Lignin to Blast Furnace Process as Cement Substitute in Cold-Bonded Briquettes. In Proceedings of the 1st International Conference on Energy and Material Efficiency and CO2 Reduction in the Steel Industry (EMECR), Kobe, Japan, 11–13 October 2017; The Iron and Steel Institute of Japan: Tokyo, Japan, 2017; pp. 386–389. [Google Scholar]
- Heikkilä, A.; Iljana, M.; Bartusch, H.; Fabritius, T. Reduction of Iron Ore Pellets, Sinter and Lump Ore under Simulated Blast Furnace Conditions. In Proceedings of the METEC & 4th European Steel Technology and Application Days (ESTAD), Düsseldorf, Germany, 24–28 June 2019; Steel Institute VDEh: Düsseldorf, Germany, 2019. [Google Scholar]
- Heikkilä, A.; Iljana, M.; Bartusch, H.; Fabritius, T. Reduction of Iron Ore Pellets, Sinter, and Lump Ore under Simulated Blast Furnace Conditions. Steel Res. Int. 2020, 91, 2000047. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahim, A.; Iljana, M.; Omran, M.; Vuolio, T.; Bartusch, H.; Fabritius, T. Influence of H2–H2O Content on the Reduction of Acid Iron Ore Pellets in a CO–CO2–N2 Reducing Atmosphere. ISIJ Int. 2020, 60, 2206–2217. [Google Scholar] [CrossRef]
- ISO 7215:2015; Iron Ores for Blast Furnace Feedstocks—Determination of the Reducibility by the Final Degree of Reduction Index. ISO: Geneva, Switzerland, 2015; pp. 1–11.
- Hooey, P.L. Reduction and High Temperature Behaviour of Iron Ore Sinter Made from Magnetite Fines. Ph.D. Thesis, University of Oulu, Oulu, Finland, 1999. [Google Scholar]
- ISO 7992:2015; Iron Ores for Blast Furnace Feedstocks—Determination of Reduction under Load. ISO: Geneva, Switzerland, 2015; pp. 1–12.
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Heikkinen, E.-P.; Fabritius, T. Evaluating the Reduction-Softening Behaviour of Blast Furnace Burden with an Advanced Test. ISIJ Int. 2016, 56, 1705–1714. [Google Scholar] [CrossRef] [Green Version]
- Iljana, M.; Kemppainen, A.; Heikkinen, E.-P.; Fabritius, T.; Paananen, T.; Mattila, O. A New Sophisticated Method for Evaluating the Reduction-Softening Properties of Iron Burden Materials. In Proceedings of the METEC & 2nd European Steel Technology and Application Days (ESTAD), Düsseldorf, Germany, 16–20 June 2015; Steel Institute VDEh: Düsseldorf, Germany, 2015. [Google Scholar]
- Liu, X.; Honeyands, T.; Evans, G.; Zulli, P.; O’Dea, D. A Review of High-Temperature Experimental Techniques Used to Investigate the Cohesive Zone of the Ironmaking Blast Furnace. Ironmak. Steelmak. 2019, 46, 953–967. [Google Scholar] [CrossRef]
- Peters, K.-H.; Beppler, E.; Gerstenberg, B.; Janhsen, U. Burden Properties and Their Influence on Process in the Blast Furnace. In Proceedings of the 53rd Ironmaking Conference, Chicago, IL, USA, 20–23 March 1994; Iron & Steel Society: Warrendale, PA, USA, 1994; pp. 257–267. [Google Scholar]
- Chaigneau, R.; Bakker, T.; Steeghs, A.; Bergstrand, R. Quality Assessment of Ferrous Burdens: Utopian Dream? In Proceedings of the 60th Ironmaking Conference, Baltimore, MD, USA, 25–28 March 2001; Iron & Steel Society: Warrendale, PA, USA, 2001; pp. 689–703. [Google Scholar]
- Bailly, J.L.; Picard, M.; Sert, D.; Succurro, A.; Rougé, M.; Reboul, J.L. A New Measuring Device for the Simultaneous Evaluation of Heat Pattern and Gas Utilisation Pattern in the Shaft of a Blast Furnace. In Measuring Techniques in Blast Furnace Ironmaking and their Benefits for Industrial Practice; ECSC Workshop: Düsseldorf, Germany, 1998; pp. 139–152. ISBN 92-828-4281-9. [Google Scholar]
- Iljana, M.; Heikkinen, E.-P.; Fabritius, T. Estimation of Iron Ore Pellet Softening in a Blast Furnace with Computational Thermodynamics. Metals 2021, 11, 1515. [Google Scholar] [CrossRef]
- ISO 4700:2015; Iron Ore Pellets for Blast Furnace and Direct Reduction Feedstocks—Determination of the Crushing Strength. ISO: Geneva, Switzerland, 2015; pp. 1–5.
- ISO 13930:2015; Iron Ores for Blast Furnace Feedstocks—Determination of Low-Temperature Reduction-Disintegration Indices by Dynamic Method. ISO: Geneva, Switzerland, 2015; pp. 1–9.
- Verein Deutscher Eisenhüttenleute (VDEh) (Ed.) Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995; ISBN 3-514-00457-9. [Google Scholar]
- Suzuki, M.; Shinmura, T.; Iimura, K.; Hirota, M. Study of the Wall Effect on Particle Packing Structure Using X-Ray Micro Computed Tomography. Adv. Powder Technol. 2008, 19, 183–195. [Google Scholar] [CrossRef]
- Borinder, T.; Torssell, K. High Temperature Behaviour of Blast Furnace Pellets. In Proceedings of the 45th Ironmaking Conference, Washington, DC, USA, 6–9 April 1986; Iron & Steel Society: Warrendale, PA, USA, 1986; pp. 13–18. [Google Scholar]
- Dwarapudi, S.; Ghosh, T.K.; Shankar, A.; Tathavadkar, V.; Bhattacharjee, D.; Venugopal, R. Effect of Pyroxenite Flux on the Quality and Microstructure of Hematite Pellets. Int. J. Miner. Process. 2010, 96, 45–53. [Google Scholar] [CrossRef]
- Dwarapudi, S.; Ghosh, T.K.; Shankar, A.; Tathavadkar, V.; Bhattacharjee, D.; Venugopal, R. Effect of Pellet Basicity and MgO Content on the Quality and Microstructure of Hematite Pellets. Int. J. Miner. Process. 2011, 99, 43–53. [Google Scholar] [CrossRef]
- ISO 4695:2015; Iron Ores for Blast Furnace Feedstocks—Determination of the Reducibility by the Rate of Reduction Index. ISO: Geneva, Switzerland, 2015; pp. 1–12.
- ISO 4696-1:2015; Iron Ores for Blast Furnace Feedstocks—Determination of Low-Temperature Reduction-Disintegration Indices by Static Method—Part 1: Reduction with CO, CO2, H2 and N2. ISO: Geneva, Switzerland, 2015; pp. 1–11.
- ISO 4696-2:2015; Iron Ores for Blast Furnace Feedstocks—Determination of Low-Temperature Reduction-Disintegration Indices by Static Method—Part 2: Reduction with CO and N2. ISO: Geneva, Switzerland, 2015; pp. 1–10.
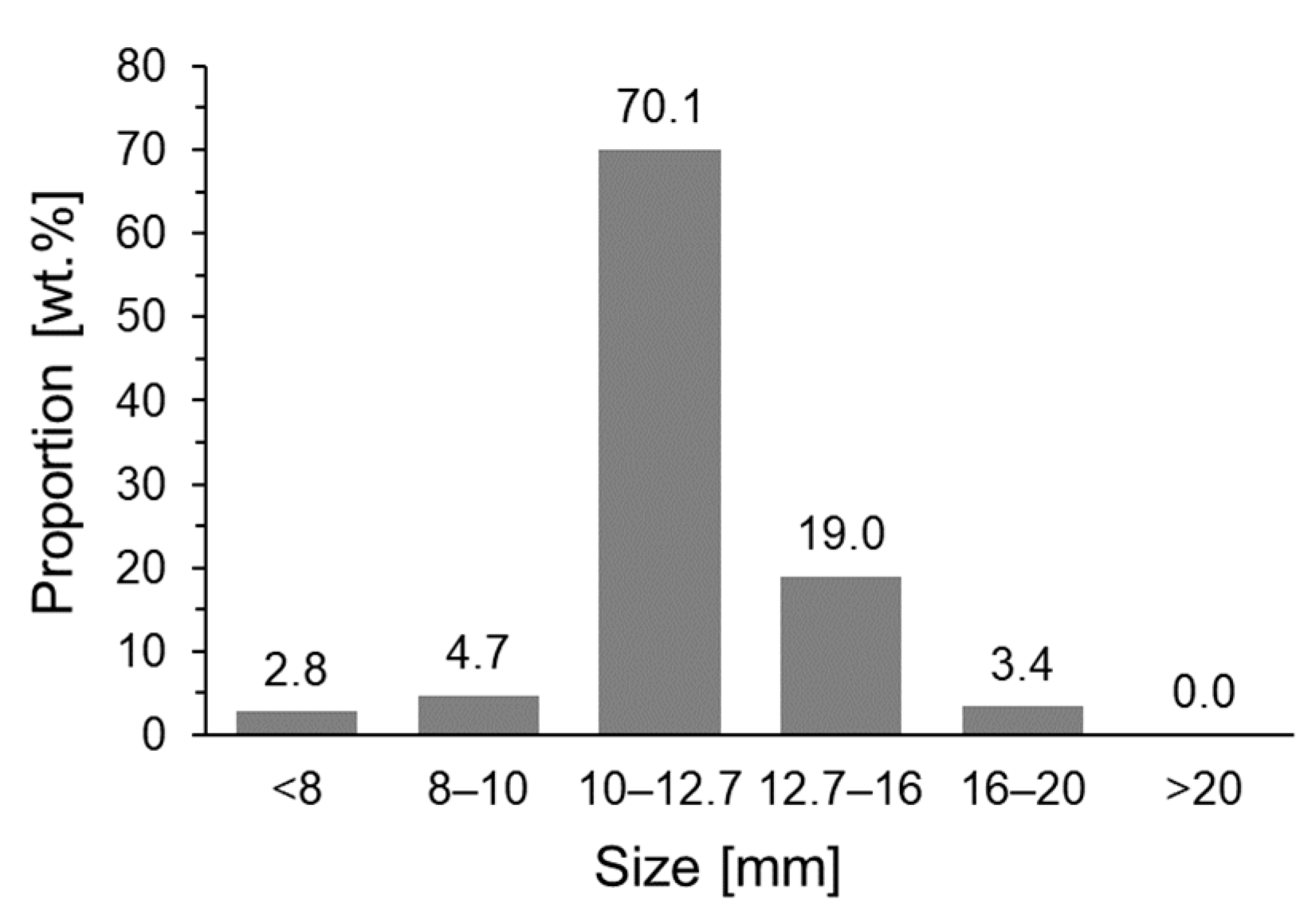
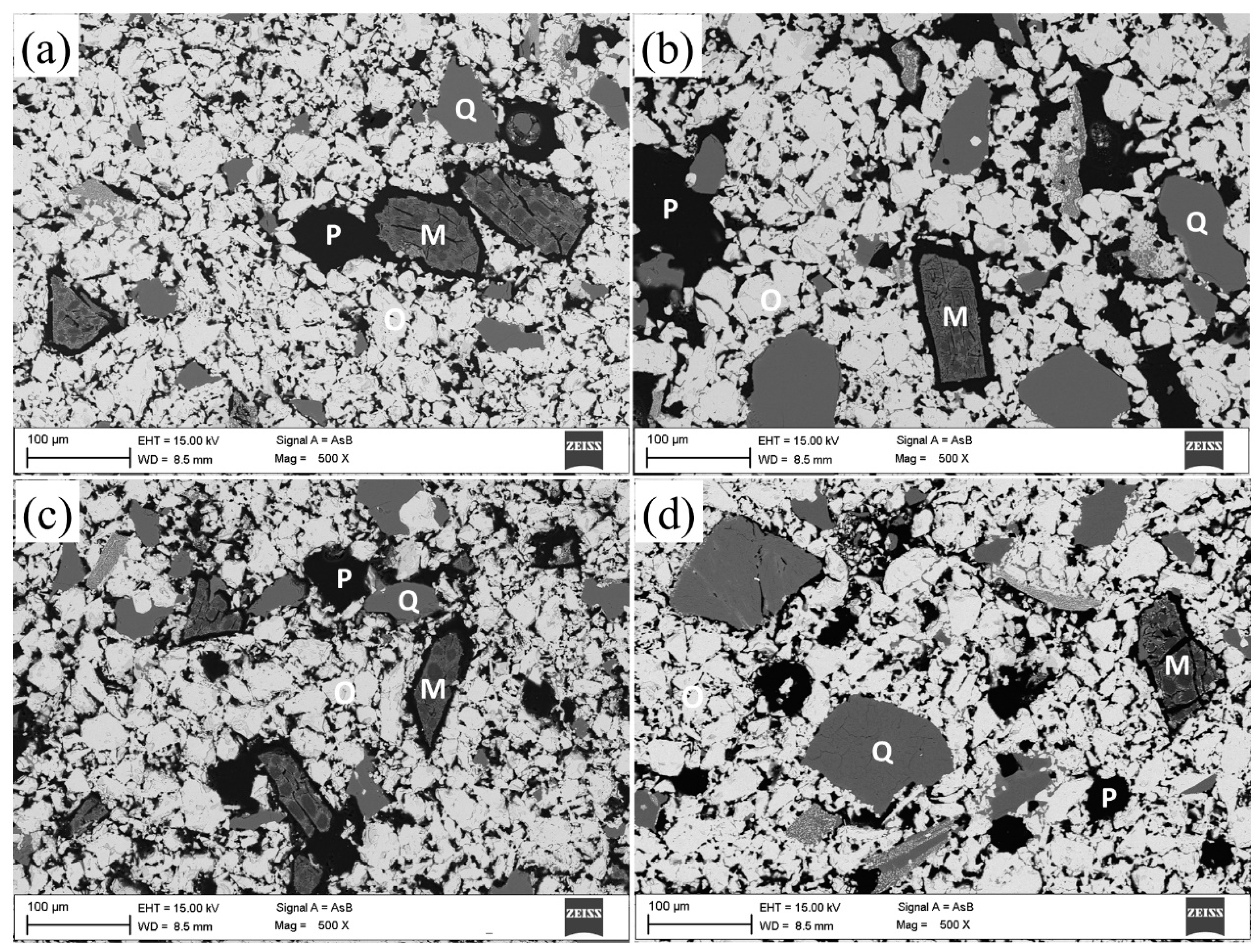



| Component/Basicity | 8–10 mm | 10–12.7 mm | 12.7–16 mm | 16–20 mm |
|---|---|---|---|---|
| Fetot | 64.25 | 64.05 | 64.18 | 64.20 |
| FeO | <0.1 | 0.2 | 0.6 | 0.6 |
| SiO2 | 5.31 | 5.48 | 5.64 | 5.33 |
| MgO | 1.40 | 1.47 | 1.40 | 1.53 |
| CaO | 0.24 | 0.20 | 0.15 | 0.18 |
| Al2O3 | 0.32 | 0.32 | 0.31 | 0.29 |
| S | 0.004 | 0.002 | 0.001 | 0.001 |
| B2 | 0.045 | 0.036 | 0.027 | 0.034 |
| B4 | 0.29 | 0.29 | 0.26 | 0.30 |
| Index | 8–10 mm | 10–12.7 mm | 12.7–16 mm | 16–20 mm |
|---|---|---|---|---|
| Number of pellets | 69 | 36 | 21 | 13 |
| Initial weight [g] | 120.20 | 120.20 | 120.08 | 120.23 |
| RDfinal [%] | 61.9 | 60.2 | 55.7 | 51.4 |
| Index | 8–10 mm | 10–12.7 mm | 12.7–16 mm | 16–20 mm |
|---|---|---|---|---|
| TDP20 [°C] | 1151 | 1186 | 1165 | 1163 |
| TDP50 [°C] | 1166 | 1214 | 1176 | 1172 |
| TDP70 [°C] | 1173 | 1227 | 1179 | 1175 |
| TDP70—TDP20 [°C] | 22 | 41 | 14 | 12 |
| Compressionfinal [%] | 49.9 | 58.0 | 53.8 | 55.2 |
| RDfinal [%] | 51.6 | 61.8 | 52.1 | 52.4 |
| Size Fraction | MgO | FeO | Others |
|---|---|---|---|
| 8–10 mm | 0.0 | 100.0 | 0.0 |
| 10–12.7 mm | 1.0 | 98.6 | 0.4 |
| 12.7–16 mm | 0.2 | 99.7 | 0.1 |
| 16–20 mm | 0.1 | 99.7 | 0.2 |
| Size Fraction | MgO | FeO | SiO2 | Others |
|---|---|---|---|---|
| 8–10 mm | 6.0 | 62.8 | 30.3 | 0.9 |
| 10–12.7 mm | 9.5 | 59.2 | 31.0 | 0.3 |
| 12.7–16 mm | 6.5 | 62.9 | 30.2 | 0.4 |
| 16–20 mm | 6.5 | 63.0 | 30.3 | 0.2 |
| Index | 8–10 mm | 10–12.7 mm | 12.7–16 mm | 16–20 mm |
|---|---|---|---|---|
| Average [daN] | 118 | 171 | 211 | 211 |
| Standard deviation [daN] | 53.7 | 85.8 | 92.1 | 76.4 |
| Proportion of <150 daN [wt.%] | 78 | 40 | 32 | 24 |
| Proportion of <60 daN [wt.%] | 16 | 4 | 4 | 2 |
| Index | 8–10 mm | 10–12.7 mm | 12.7–16 mm | 16–20 mm |
|---|---|---|---|---|
| Proportion of >6.3 mm [wt.%] | 80.7 | 67.9 | 50.6 | 47.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iljana, M.; Paananen, T.; Mattila, O.; Kondrakov, M.; Fabritius, T. Effect of Iron Ore Pellet Size on Metallurgical Properties. Metals 2022, 12, 302. https://doi.org/10.3390/met12020302
Iljana M, Paananen T, Mattila O, Kondrakov M, Fabritius T. Effect of Iron Ore Pellet Size on Metallurgical Properties. Metals. 2022; 12(2):302. https://doi.org/10.3390/met12020302
Chicago/Turabian StyleIljana, Mikko, Timo Paananen, Olli Mattila, Mikhail Kondrakov, and Timo Fabritius. 2022. "Effect of Iron Ore Pellet Size on Metallurgical Properties" Metals 12, no. 2: 302. https://doi.org/10.3390/met12020302
APA StyleIljana, M., Paananen, T., Mattila, O., Kondrakov, M., & Fabritius, T. (2022). Effect of Iron Ore Pellet Size on Metallurgical Properties. Metals, 12(2), 302. https://doi.org/10.3390/met12020302







