Si-Al-N-O Multi-Layer Coatings with Increased Corrosion Resistance Deposited on Stainless Steel by Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
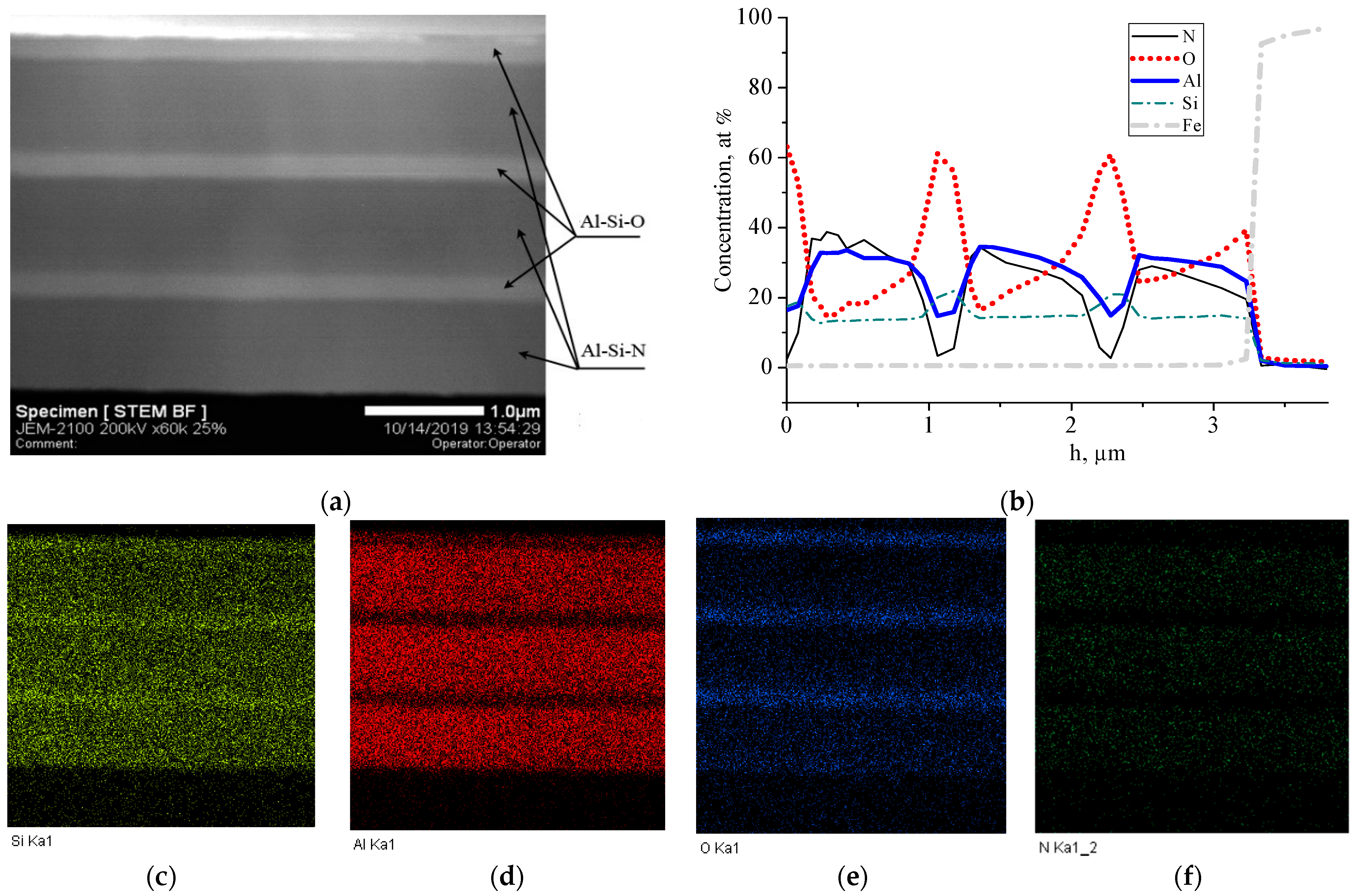
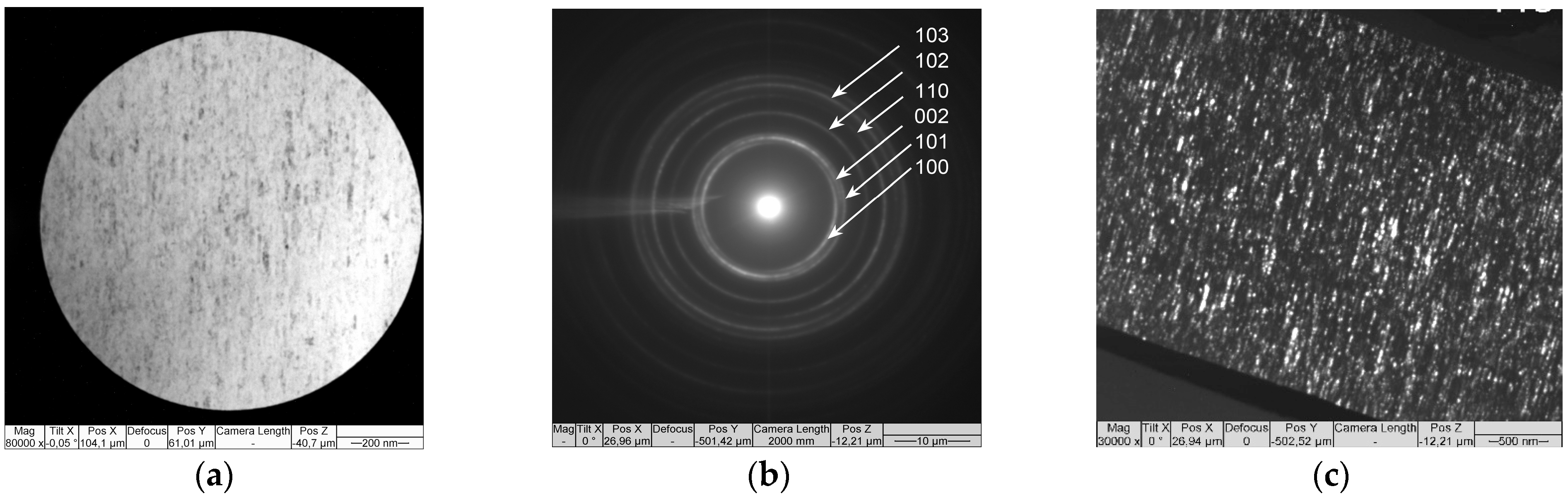
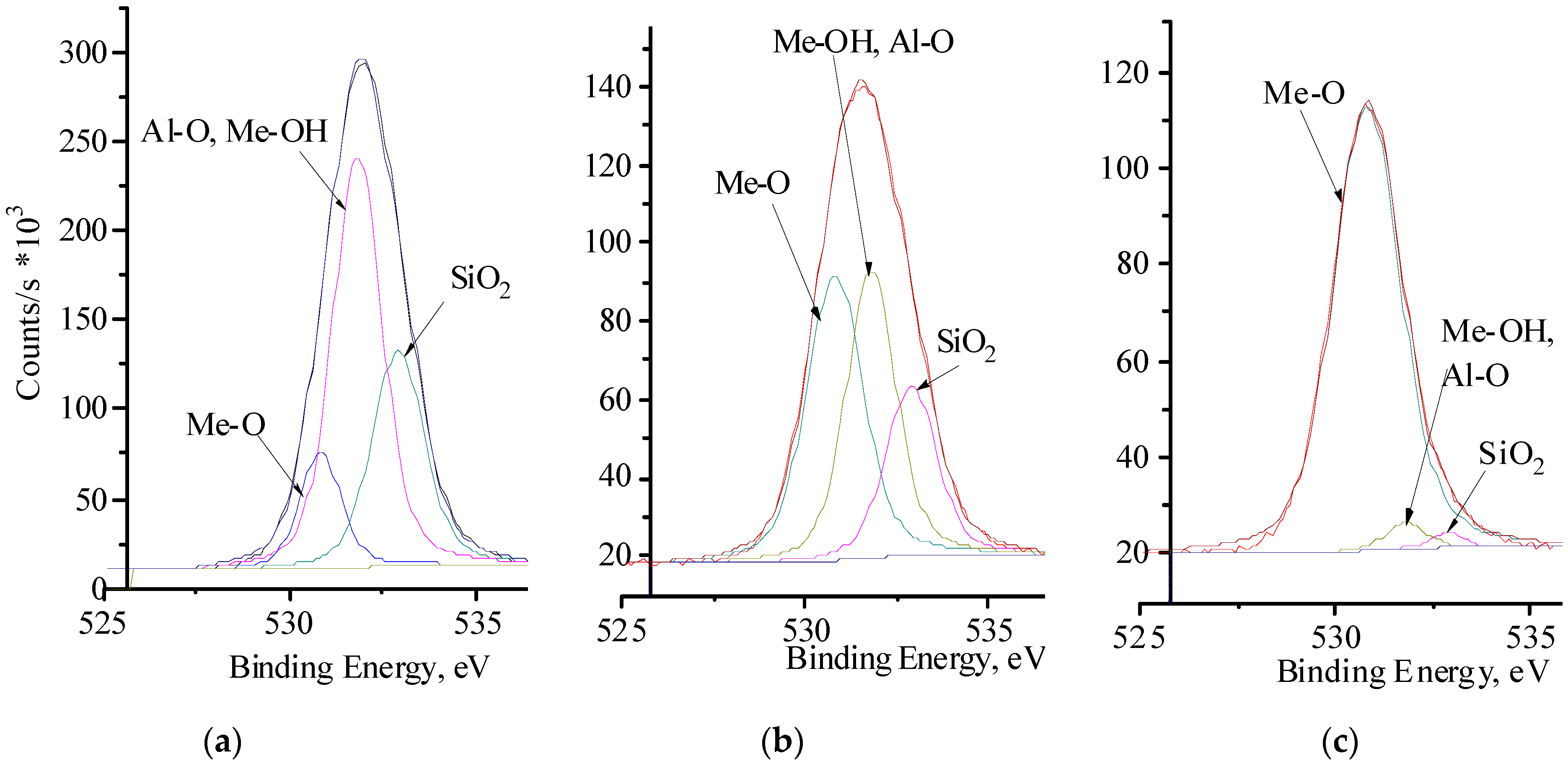
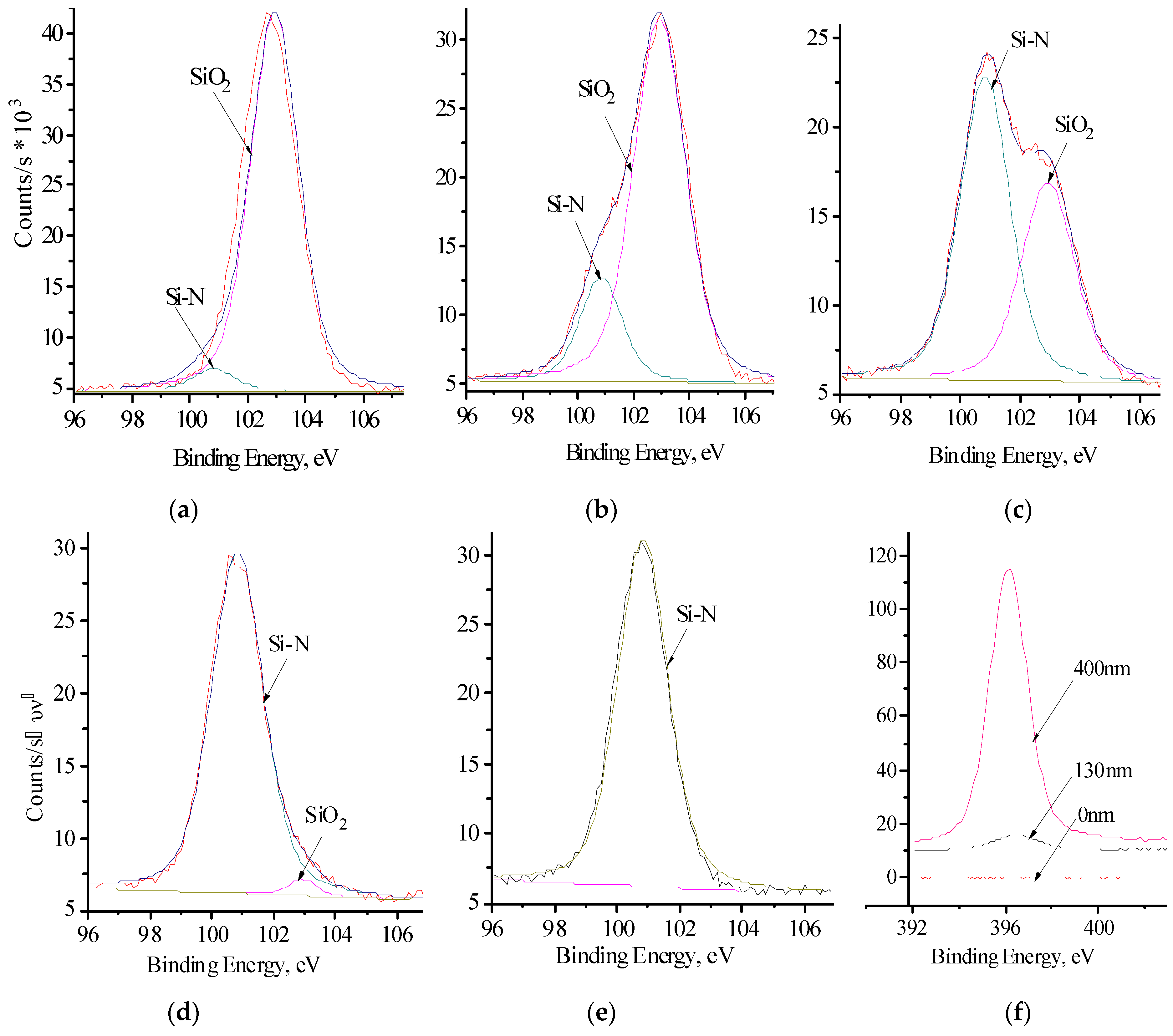
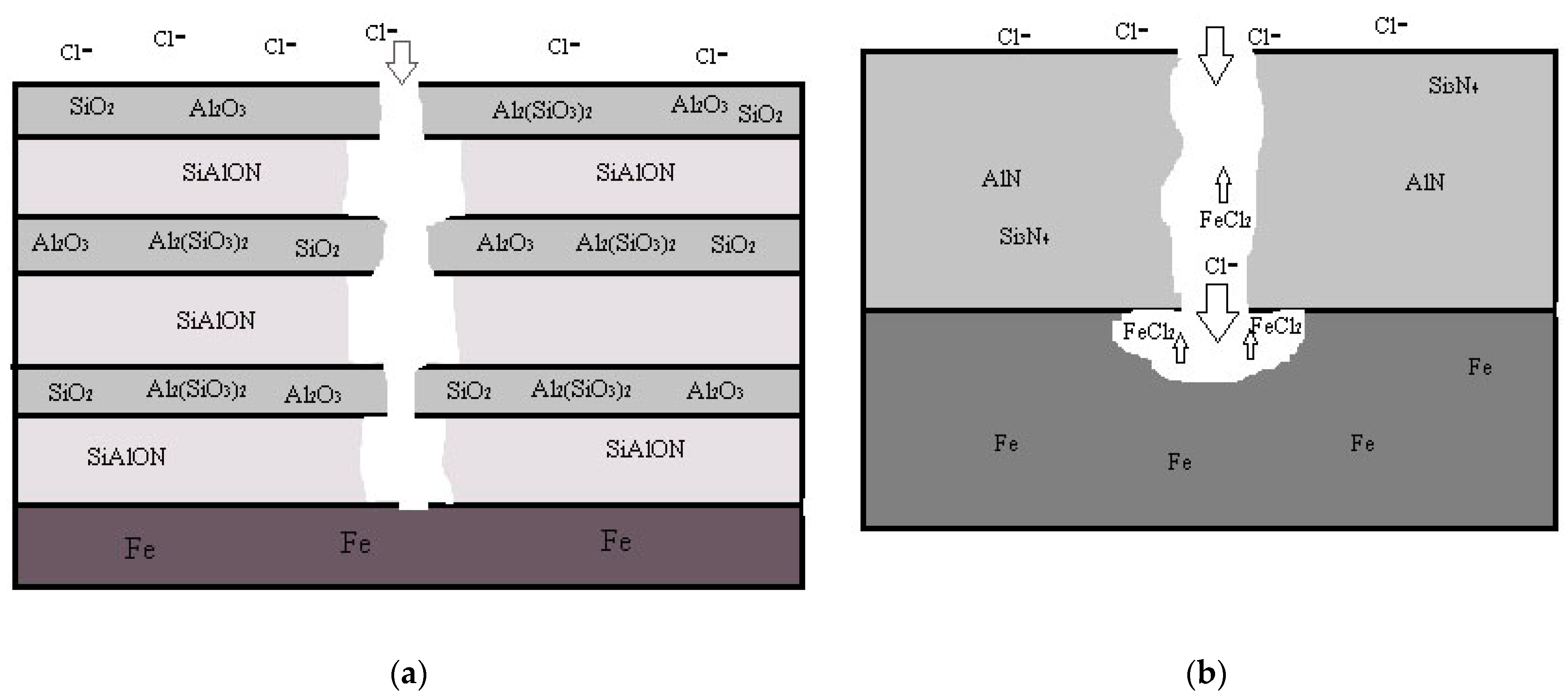
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Xu, Y.; Qu, W. Evaluation of atmospheric corrosion damage to steel space structures in coastal areas. Int. J. Solids Struct. 2005, 42, 4673–4694. [Google Scholar] [CrossRef]
- Allam, I.M.; Maslehuddin, M.; Saricimen, H.; Al-Mana, A.I. Influence of atmospheric corrosion on the mechanical properties of reinforcing steel. Constr. Build. Mater. 1994, 8, 35–41. [Google Scholar] [CrossRef]
- Hou, W.; Liang, C. Atmospheric Corrosion Prediction of Steels. Corrosion 2004, 60, 313–322. [Google Scholar] [CrossRef]
- Cowell, D.; Apsimon, H. Estimating the cost of damage to buildings by acidifying atmospheric pollution in Europe. Atmosp. Environ. 1996, 30, 2959–2968. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Li, Y.; Nie, J.; Liang, Z.; Bai, P.; Yang, Y.; Chen, B.; Liu, S.; Guan, Q.; Cai, J. Microstructure evolution and high-temperature oxidation behavior of FeCrAlNbNi alloyed zone prepared by laser surface alloying on 304 stainless steel. J. Alloy. Compd. 2021, 888, 161468. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Antunes, R.A. Electronic properties of the passive films formed on CoCrFeNi and CoCrFeNiAl high entropy alloys in sodium chloride solution. J. Mater. Res. Technol. 2020, 9, 13879–13892. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of Al CoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Bender, R.; Schütze, M. The role of alloying elements in commercial alloys for corrosion resistance in oxidizing-chloridizing atmospheres. Part I: Literature evaluation and thermodynamic calculations on phase stabilities. Mater. Corros. 2003, 54, 567–586. [Google Scholar] [CrossRef]
- Gawel, R.; Kyzioł, K.; Jurasz, Z.; Grzesik, Z. Oxidation resistance of valve steels covered with thin SiC coatings, obtained by RF CVD. Corros. Sci. 2018, 145, 16–25. [Google Scholar] [CrossRef]
- Szymański, K.; Hernas, A.; Moskal, G.; Myalska, H. Thermally sprayed coatings resistant to erosion and corrosion for power plant boilers—A review. Surf. Coat. Technol. 2015, 268, 153–164. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. High temperature erosion of Cr3C2-NiCr thermal spray coatings—The role of phase microstructure. Surf. Coatings Technol. 2009, 203, 1144–1153. [Google Scholar] [CrossRef]
- Formanek, B.; Szymański, K.; Szczucka-Lasota, B.; Włodarczyk, A. New generation of protective coatings intended for the power industry. J. Mater. Process. Technol. 2005, 164–165, 850–855. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Liu, Y.; Cai, Y.; Li, J.; Chen, H.; Wan, Q.; Yang, D.; Tan, J.; Liu, H.; et al. Lead-bismuth eutectic (LBE) corrosion behavior of AlTiN coatings at 550 and 600 °C. J. Nucl. Mater. 2020, 539, 152280. [Google Scholar] [CrossRef]
- Sadeghi, E.; Markocsan, N.; Joshi, S. Advances in corrosion-resistant thermal spray coatings for renewable energy power plants. Part I: Effect of composition and microstructure. J. Therm. Spray Technol. 2019, 28, 1749–1788. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Tang, J.; Wang, Y.; Wang, H.; Normand, B.; Zuo, Y. Electrodeposition of a Pd-Ni/TiO2 composite coating on 316L SS and its corrosion behavior in hot sulfuric acid solution. Coatings 2018, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Syed, J.A.; Gao, Y.; Lu, H.; Meng, X. Electrodeposition of Ni(OH)2 reinforced polyaniline coating for corrosion protection of 304 stainless steel. Appl. Surf. Sci. 2018, 440, 1011–1021. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Han, H.; Zhou, Y.; Hu, S.; He, C.; Yan, Q. Studies on the formation process and anti-corrosion performance of polypyrrole film deposited on the surface of Q235 steel by an electrochemical method. Surf. Coatings Technol. 2018, 341, 95–102. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Zand, R.Z.; Verbeken, K.; Adriaens, A. The corrosion resistance of 316L stainless steel coated with a silane hybrid nanocomposite coating. Prog. Org. Coat. 2011, 72, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.; Matin, A.; Kumar, A.M.; Ibrahim, A.; Laoui, T. Enhancement of anticorrosion property of 304 stainless steel using silane coatings. Appl. Surf. Sci. 2018, 440, 1286–1297. [Google Scholar] [CrossRef]
- Dorofeeva, T.I.; Gubaidullina, T.A.; Gritsenko, B.P.; Sergeev, V.P. Structural phase state and thermal cyclic stability of the thermal barrier Zr-Si-O coatings deposited on a copper substrate by the microplasma method. Prot. Met. Phys. Chem. Surf. 2019, 55, 695–699. [Google Scholar] [CrossRef]
- Salem, A.A.; Grgur, B.N. The influence of the polyaniline initial oxidation states on the corrosion of steel with composite coatings. Prog. Org. Coat. 2018, 119, 138–144. [Google Scholar] [CrossRef]
- Sambyal, P.; Ruhi, G.; Dhawan, S.; Bisht, B.; Gairola, S. Enhanced anticorrosive properties of tailored poly(aniline-anisidine)/chitosan/SiO2 composite for protection of mild steel in aggressive marine conditions. Prog. Org. Coat. 2018, 119, 203–213. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, Z.; Wei, B.; Wu, Z.; Wang, Z. Anticorrosive yet conductive Hf/Si3N4 multilayer coatings on AZ91D magnesium alloy by magnetron sputtering. Surf. Coa Technol. 2017, 309, 12–20. [Google Scholar] [CrossRef]
- Escobar, C.; Villarreal, M.; Caicedo, J.; Aperador, W.; Prieto, P. Novel performance in physical and corrosion resistance HfN/VN coating system. Surf. Coat. Technol. 2013, 221, 182–190. [Google Scholar] [CrossRef]
- Musil, J.; Remnev, G.; Legostaev, V.; Uglov, V.; Lebedynskiy, A.; Lauk, A.; Procházka, J.; Haviar, S.; Smolyanskiy, E. Flexible hard Al-Si-N films for high temperature operation. Surf. Coat. Technol. 2016, 307, 1112–1118. [Google Scholar] [CrossRef]
- Liu, H.; Tang, W.; Hui, D.; Hei, L.; Lu, F. Characterization of (Al, Si)N films deposited by balanced magnetron sputtering. Thin Solid Films 2009, 517, 5988–5993. [Google Scholar] [CrossRef]
- Chang, C.-L.; Huang, C.-S. Effect of bias voltage on microstructure, mechanical and wear properties of Al-Si-N coatings deposited by cathodic arc evaporation. Thin Solid Films 2011, 519, 4923–4927. [Google Scholar] [CrossRef]
- Sergeev, V.P.; Panin, V.E.; Rizakhanov, R.N.; Koroteev, A.S.; Fedorischeva, M.V.; Neufeld, V.V.; Kalashnikov, M.P. Thermal cycle durability of heat-shielding coatings on the basis of Zr-Y-O/Si-Al-N under ion treatment of copper substrates. Adv. Mater. Res. 2014, 880, 146–150. [Google Scholar] [CrossRef]
- Ho, W.Y.; Tsai, C.H.; Hsu, C.H. Corrosion behavior of CrN/AlSiN multilayer coatings on AISI 304 stainless steel in aluminum alloy melt. Adv. Mater. Res. 2011, 415–417, 1938–1941. [Google Scholar] [CrossRef]
- Voevodin, A.; Yerokhin, A.; Lyubimov, V.; Donley, M.; Zabinski, J. Characterization of wear protective Al-Si-O coatings formed on Al-based alloys by micro-arc discharge treatment. Surf. Coat. Technol. 1996, 86–87, 516–521. [Google Scholar] [CrossRef]
- Arevalo, J.L.M.; Perez-Trujillo, F.J.; Castañeda, S.I. Aluminum-silicon coatings on austenitic stainless steel (AISI 304 and 317) deposited by chemical vapor deposition in a fluidized bed. Ingeniería e Investigación 2014, 34, 5–10. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, J.; Huang, J.; Wang, Q.; Zhang, X.; Wang, J. A comprehensive investigation of phase evolution of Al-Si coating during the prolonged aging at 650 °C. Corros. Sci. 2021, 189, 109605. [Google Scholar] [CrossRef]
- Gao, M.; Lu, W.; Yang, B.; Zhang, S.; Wang, J. High corrosion and wear resistance of Al-based amorphous metallic coating synthesized by HVAF spraying. J. Alloys Compd. 2018, 735, 1363–1373. [Google Scholar] [CrossRef]
- Castañeda, S.; Perez-Trujillo, F.J. Al-Mn CVD-FBR coating on P92 steel as protection against steam oxidation at 650 °C: TGA-MS study. J. Nucl. Mater. 2018, 499, 419–430. [Google Scholar] [CrossRef]
- Fu, G.; Wu, Y.; Liu, Q.; Li, R.; Su, Y. Hot corrosion behavior of stainless steel with Al-Si/Al-Si-Cr coating. High Temp. Mater. Process. 2016, 36, 243–248. [Google Scholar] [CrossRef]
- Musil, J.; Šašek, M.; Zeman, P.; Čerstvý, R.; Heřman, D.; Han, J.; Šatava, V. Properties of magnetron sputtered Al–Si–N thin films with a low and high Si content. Surf. Coat. Technol. 2008, 202, 3485–3493. [Google Scholar] [CrossRef]
- Musil, J.; Jílek, R.; Meissner, M.; Tölg, T.; Čerstvý, R. Two-phase single layer Al-O-N nanocomposite films with enhanced resistance to cracking. Surf. Coat. Technol. 2012, 206, 4230–4234. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, L.; Duan, L.; Tuan, W.-H. Preparation of Cr-based multilayer coating on stainless steel as bipolar plate for PEMFCs by magnetron sputtering. Int. J. Hydrogen Energy 2011, 36, 2184–2189. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Z.; Liu, W.; Zhang, D.; Wang, Y.; Zhao, H.; Wang, L.; Li, X. Bias design of amorphous/nanocrystalline Cr Al Si N films for remarkable anti-corrosion and anti-wear performances in seawater. Tribol. Int. 2018, 121, 410–419. [Google Scholar] [CrossRef]
- Pélisson, A.; Parlinska-Wojtan, M.; Hug, H.; Patscheider, J. Microstructure and mechanical properties of Al-Si-N transparent hard coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2007, 202, 884–889. [Google Scholar] [CrossRef]
- Pélisson-Schecker, A.; Hug, H.J.; Patscheider, J. Morphology, microstructure evolution and optical properties of Al-Si-N nanocomposite coatings. Surf. Coat. Technol. 2014, 257, 114–120. [Google Scholar] [CrossRef]
- Rajendran, S.; Nguyen, T.A.; Kakooei, S.; Yeganeh, M.; Li, Y. Corrosion Protection at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2020; p. 526. [Google Scholar] [CrossRef]
- Gritsenko, B.P.; Rechenko, D.S.; Rogachev, E.A.; Smyrnova, K.V.; Bagdasaryan, A.A.; Sergeev, V.P.; Popov, A.Y.; Nogaibekova, G.Z.; Fedorischeva, M.V.; Pogrebnjak, A.D. Enhancement of the wear resistance of tungsten cobalt carbide plates using ion implantation and Al-Si-N coatings. In Springer Proceedings in Physics; Springer: Singapore, 2020; Volume 240, pp. 279–286. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. Electrochemical behavior and compressive strength of Al-Cu/xCu composites in NaCl solution. J. Solid State Electrochem. 2021, 25, 1303–1317. [Google Scholar] [CrossRef]
- Osório, W.R.; Freitas, E.S.; Garcia, A. EIS and potentiodynamic polarization studies on immiscible monotectic Al-In alloys. Electrochim. Acta 2013, 102, 436–445. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gupta, R.; Gengenbach, T.; Jones, R.; Birbilis, N. A surface study of the native oxide upon a compositionally complex alloy. Corrosion 2018, 74, 1312–1317. [Google Scholar] [CrossRef]
- Dorofeeva, T.I.; Gubaidulina, T.A.; Sergeev, V.P.; Kalashnikov, M.P.; Voronov, A.V. The structural-phase composition of the magnetron-sputtered Al-Si-N-O-based coatings. AIP Conf. Proc. 2020, 2310, 020076. [Google Scholar] [CrossRef]
- Fischer, M.; Trant, M.; Thorwarth, K.; Crockett, R.; Patscheider, J.; Hug, H.J. Understanding the microstructural evolution and mechanical properties of transparent Al-O-N and Al-Si-O-N films. Sci. Technol. Adv. Mater. 2019, 20, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Knotek, O.; Loffler, F.; Beele, W. PVD coating in the system SI-AL-O-N. Key Eng. Mater. 1994, 89, 275–280. [Google Scholar] [CrossRef]
- Luo, J.; Xi, C.; Gu, Y.; Zhang, L.; Zhang, C.; Xue, Y.; Liu, R. Superplastic forging for sialon-based nanocomposite at ultralow temperature in the electric field. Sci. Rep. 2019, 9, 2452. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.Z.; Metselaar, R. α’-Sialon ceramics: A review. Chem. Mater. 1991, 3, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Bobzin, K.; Brögelmann, T.; Kruppe, N.; Carlet, M. Wear behavior and thermal stability of HPPMS (Al,Ti,Cr,Si)ON, (Al,Ti,Cr,Si)N and (Ti,Al,Cr,Si)N coatings for cutting tools. Surf. Coat. Technol. 2020, 385, 125370. [Google Scholar] [CrossRef]
- Whitney, D.L. Coexisting andalusite, kyanite, and sillimanite: Sequential formation of three Al2SiO5 polymorphs during progressive metamorphism near the triple point. Am. Miner. 2002, 87, 405–416. [Google Scholar] [CrossRef]
- Guzmán, P.; Aperador, W.; Yate, L. Enhancement of the pitting corrosion resistance of AISI 316LVM Steel with Ta-Hf-C/Au bilayers for biomedical applications. J. Nanomater. 2017, 2017, 6825250. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Qi, W.; Wang, J. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Dorofeeva, T.I.; Gubaidulina, T.A.; Sergeev, V.P.; Kalashnikov, M.P.; Voronov, A.V. Change in the structure and corrosion resistance of a nickel-chrome coating on stainless steel during implantation of high energy Al+ and B+ Ions. Sov. Phys. J. 2020, 63, 1186–1194. [Google Scholar] [CrossRef]
- Majumdar, S.; Paul, B.; Chakraborty, P.; Kishor, J.; Kain, V.; Dey, G.K. Formation of Al2O3 /FeAl coatings on a 9Cr-1Mo steel, and corrosion evaluation in flowing Pb-17Li loop. J. Nucl. Mater. 2017, 486, 60–65. [Google Scholar] [CrossRef]


| Conditions | Al-Si-N-O Layer | Al-Si-O Layer |
|---|---|---|
| Reactive gas | N2 | O2 |
| Target | AlSix (x = 0.20 ÷ 0.22) | |
| Reactive gas pressure, Pa | 0.08 | 0.075 |
| General gas pressure, Pa | 0.25 | 0.25 |
| Magnetron discharge power, kW | 1.0 | 1.0 |
| Frequency, kHz | 100 | 100 |
| Pulse duration, μs | 5 | 5 |
| Procedure time, min | 18 | 6.5 |
| Layer thickness, μm | 0.9 | 0.2 |
| Temperature substrate, K | 573 | 573 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorofeeva, T.; Gubaidulina, T.; Sergeev, V.; Fedorischeva, M. Si-Al-N-O Multi-Layer Coatings with Increased Corrosion Resistance Deposited on Stainless Steel by Magnetron Sputtering. Metals 2022, 12, 254. https://doi.org/10.3390/met12020254
Dorofeeva T, Gubaidulina T, Sergeev V, Fedorischeva M. Si-Al-N-O Multi-Layer Coatings with Increased Corrosion Resistance Deposited on Stainless Steel by Magnetron Sputtering. Metals. 2022; 12(2):254. https://doi.org/10.3390/met12020254
Chicago/Turabian StyleDorofeeva, Tamara, Tatiana Gubaidulina, Victor Sergeev, and Marina Fedorischeva. 2022. "Si-Al-N-O Multi-Layer Coatings with Increased Corrosion Resistance Deposited on Stainless Steel by Magnetron Sputtering" Metals 12, no. 2: 254. https://doi.org/10.3390/met12020254
APA StyleDorofeeva, T., Gubaidulina, T., Sergeev, V., & Fedorischeva, M. (2022). Si-Al-N-O Multi-Layer Coatings with Increased Corrosion Resistance Deposited on Stainless Steel by Magnetron Sputtering. Metals, 12(2), 254. https://doi.org/10.3390/met12020254







