Effects of Cold Rolling Deformation and Solution Treatment on Microstructural, Mechanical, and Corrosion Properties of a Biocompatible Ti-Nb-Ta-Zr Alloy
Abstract
1. Introduction
2. Materials and Methods
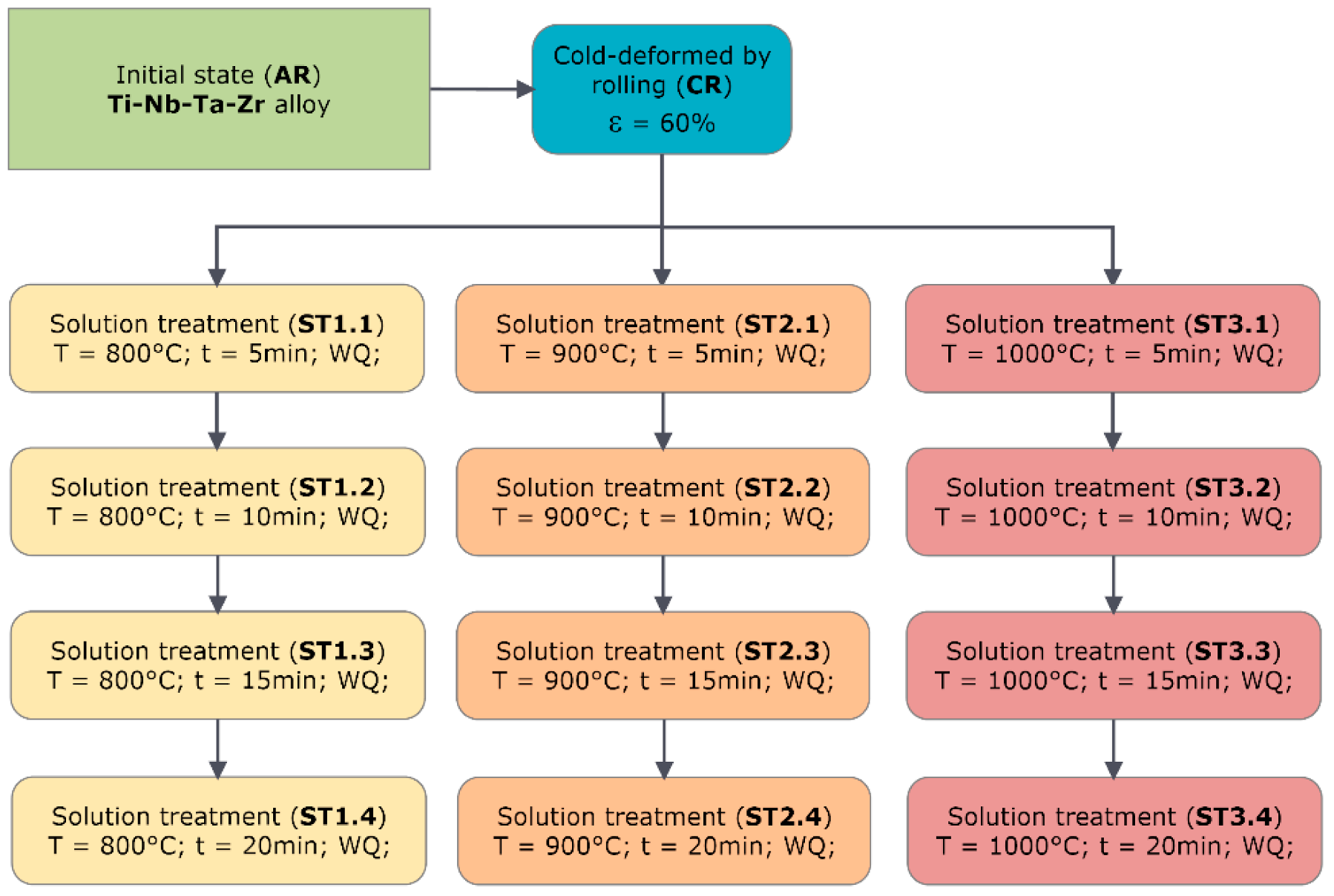
2.1. Alloy Synthesis and Thermo-Mechanical Processing Route
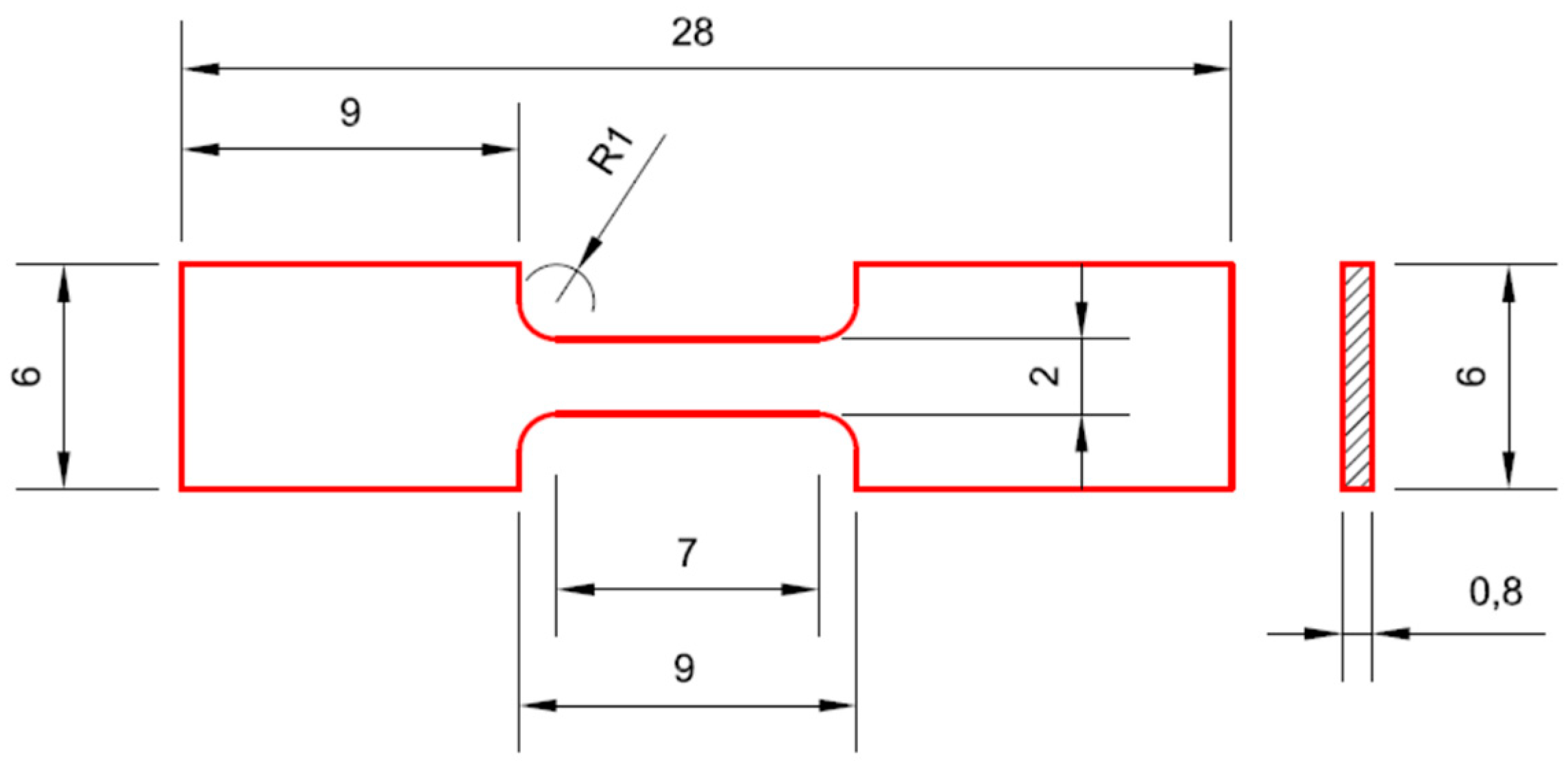
2.2. Microstructural and Mechanical Characterization
2.3. Corrosion Testing in Simulated Body Fluids (SBF)
3. Results and Discussion
3.1. Chemical, Microstructural, and Mechanical Properties of Initial/As-Received (AR) TNTZ Alloy
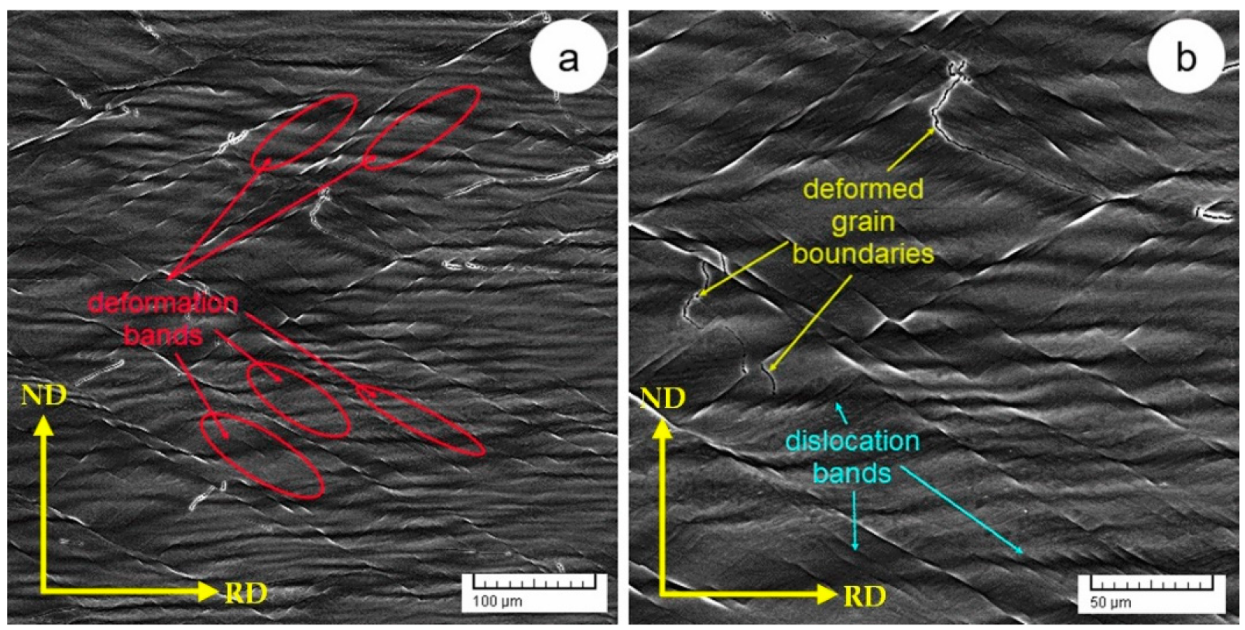
3.2. Microstructural and Mechanical Properties of Cold-Rolled (CR) TNTZ Alloy
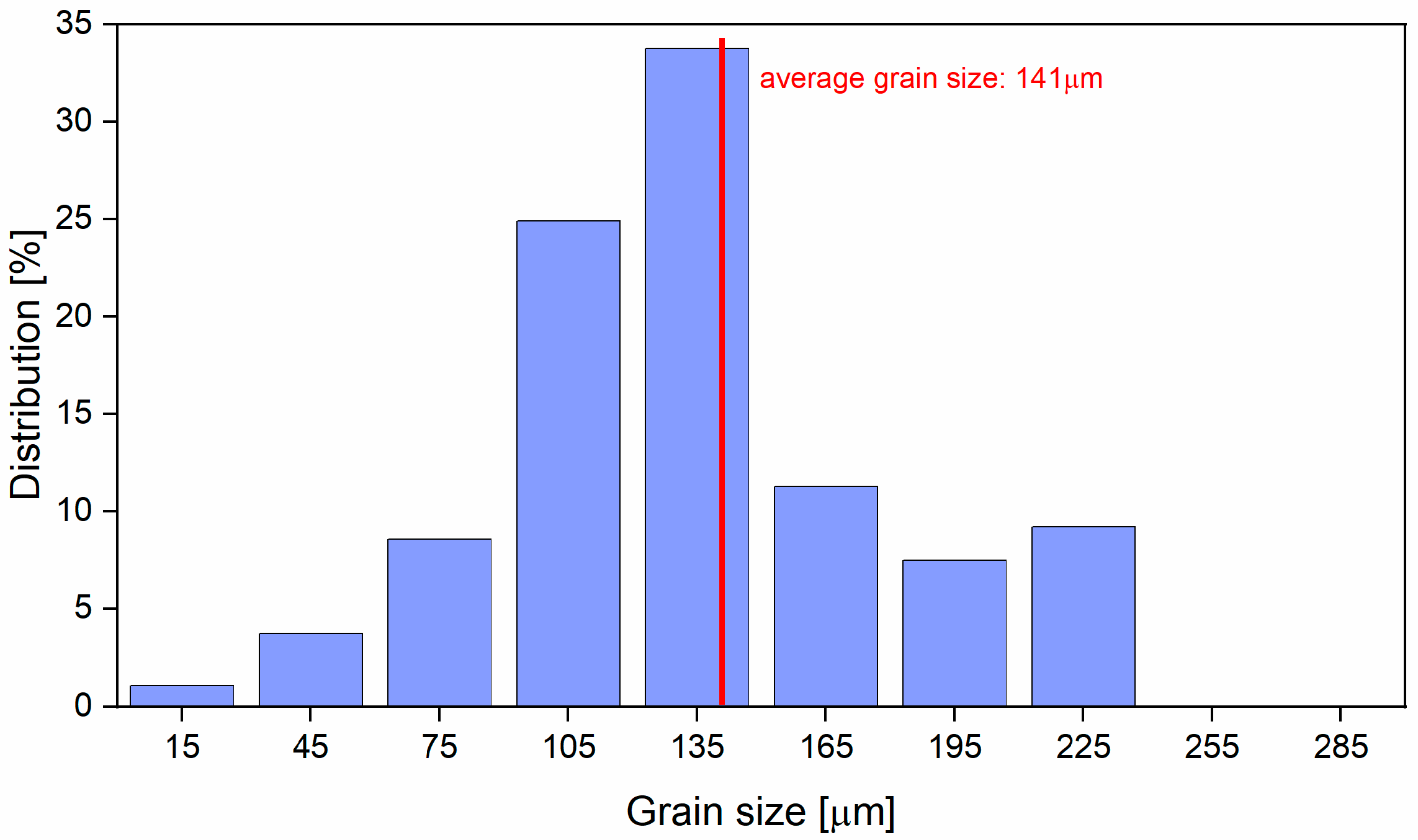
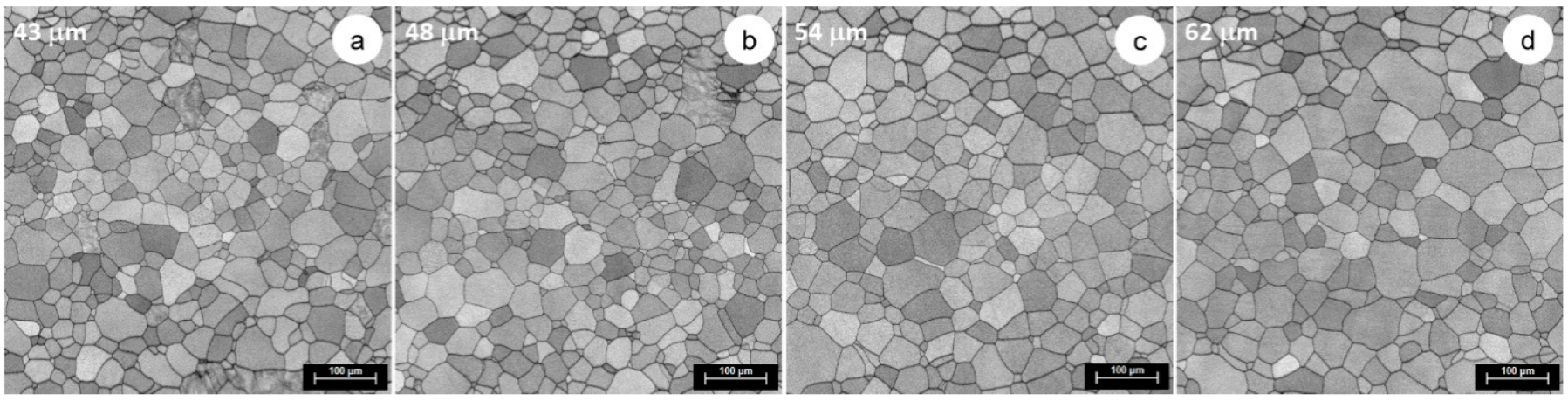
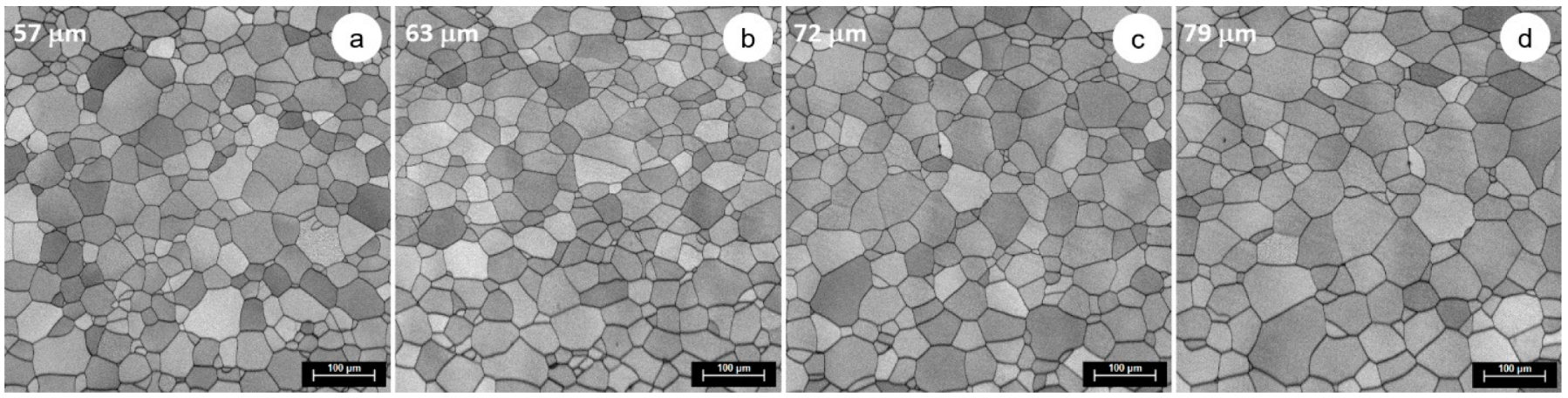
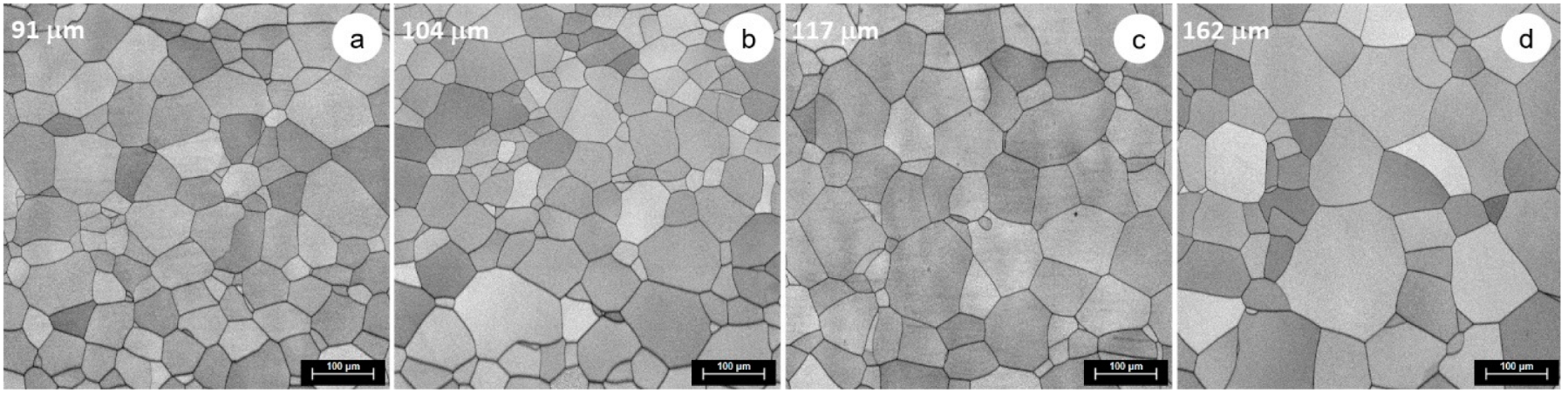
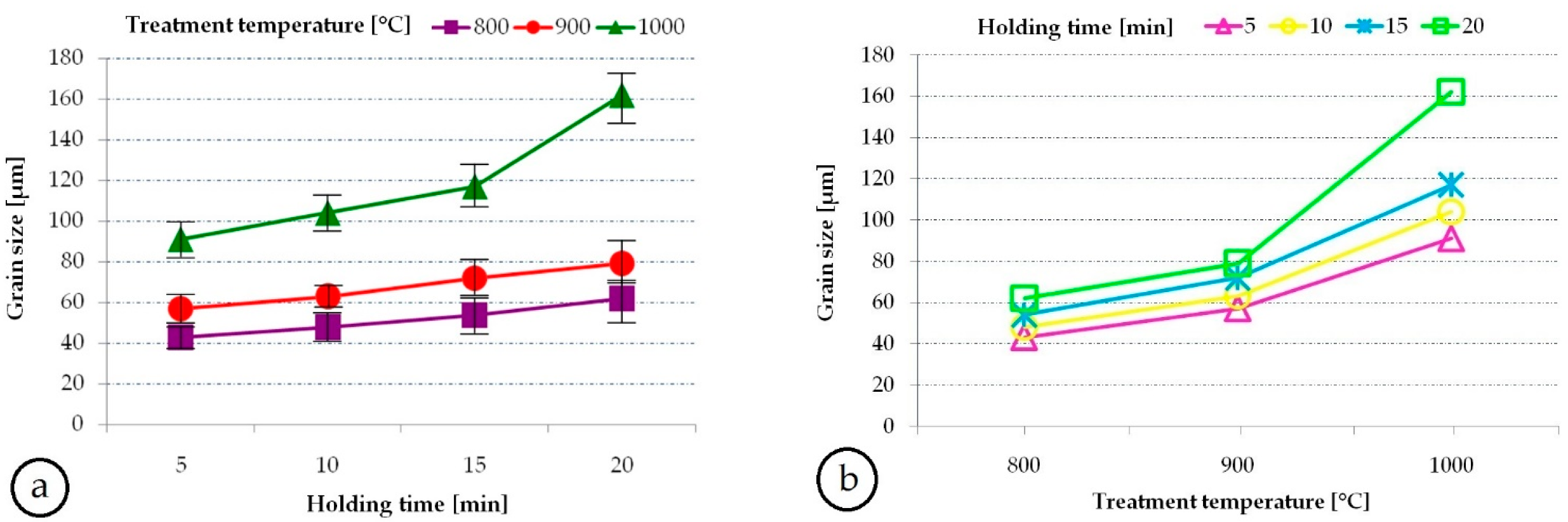
3.3. Microstructural and Mechanical Characteristics Evolution during Solution Treatment of TNTZ Alloy
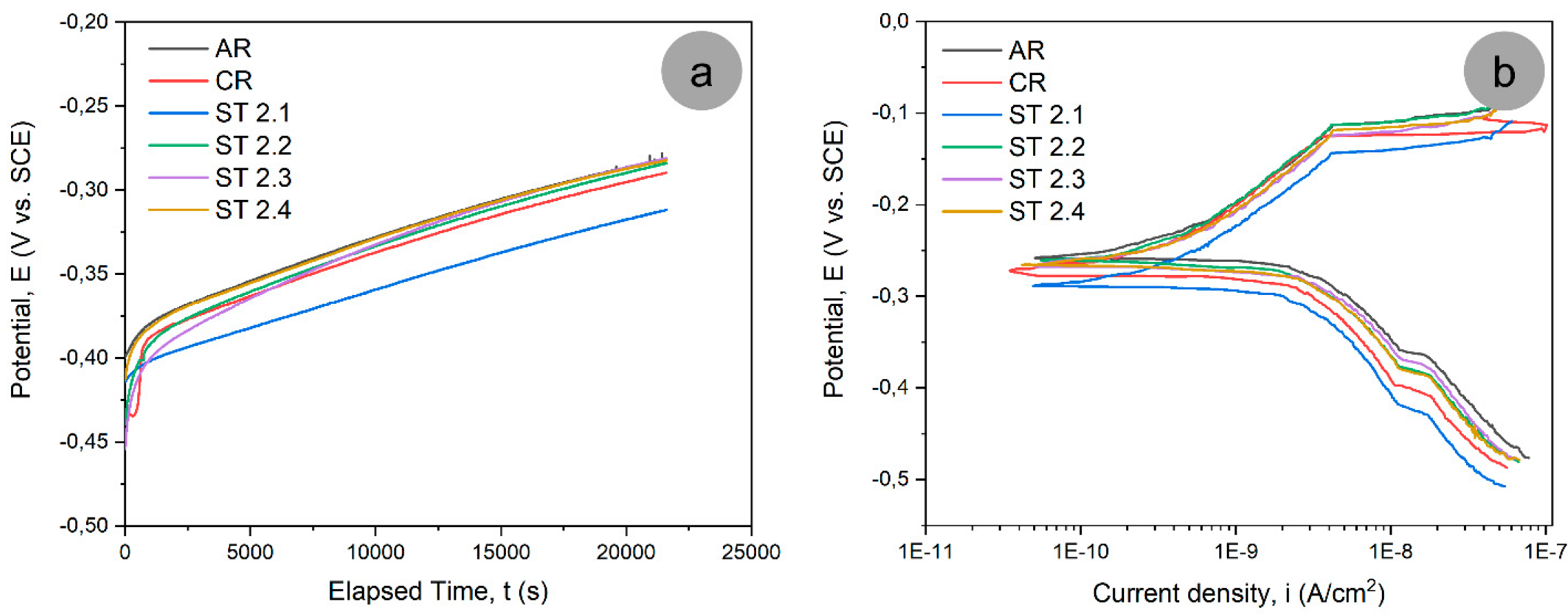
3.4. Corrosion Testing of TNTZ Alloy in SBF
- βa—slope of the anodic curve;
- βC—the slope of the cathode curve;
- icorr—the corrosion current density (µA/cm2).
- Ki—constant that defines the units for the corrosion rate (3.27 × 10−3);
- ρ—density (g/cm3); icorr—the corrosion current density (µA/cm2);
- EW—equivalent weight (grams/equivalent).
4. Conclusions
- A β-type Ti-25.5Nb-4.5Ta-8.0Zr wt%—TNTZ alloy was fabricated by melting in a cold crucible furnace (in levitation) and then subjected to different thermo-mechanical processing schemes (cold rolling and solution treatments), in order to improve biomechanical properties;
- SEM-EDS analysis showed a fine uniform distribution for all alloying elements, indicating a good chemical homogeneity;
- SEM analysis revealed that the microstructure of TNTZ alloy after solution treatment consists of a homogeneous β-Ti single-phase with equiaxed polyhedral grains and tight grain-size distribution;
- 60% cold rolling deformation decreased the elastic modulus from 59 to 50 GPa, which can be attributed to deformation texture formation;
- During solution treatment the most influential factor on grain-size was the heating temperature;
- TMP schemes consisting of cold deformation, followed by solution treatment at 900 °C (variants ST 2.1–ST 2.4), with water quenching (WQ), were the most appropriate TMP routes, as the alloy presented a fully recrystallized equiaxed microstructure, with an average grain size between 57–79 µm, and the best combination of mechanical properties: high strength (σUTS = 767–1004 MPa), good ductility (εf = 5–13%), and low elastic modulus (E = 54–55 GPa);
- The corrosion tests show that the cold-rolled sample had higher electrochemical values (the lowest corrosion current density, the highest polarization resistance, and the smallest corrosion rate) and thus a better corrosion behavior in SBF than all the other investigated samples, being closely followed by the sample CR and ST at 900 °C with a treatment duration of 5 min (ST 2.1) and water quenched (WQ);
- Based on registered biomechanical properties (microstructural, mechanical, and corrosion resistance), the best candidate as implant material for medical applications is the one resulted after a TMP route consisting of a cold deformation (CR) followed by solution treatment (ST) at 900 °C, with a treatment duration of 5 min (ST 2.1) and water quenching (σUTS = 1004 MPa, εf = 10%, E = 56 GPa).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bălţatu, M.S.; Ţugui, C.A.; Perju, M.C.; Benchea, M.; Spataru, M.C.; Sandu, A.V.; Vizureanu, P. Biocompatible Titanium Alloys used in Medical Applications. Rev. Chim.-Buchar.-Orig. Ed. 2019, 70, 1302–1306. [Google Scholar] [CrossRef]
- Piotrowski, B.; Baptista, A.A.; Patoor, E.; Bravetti, P.; Eberhardt, A.; Laheurte, P. Interaction of bone–dental implant with new ultra low modulus alloy using a numerical approach. Mater. Sci. Eng. C 2014, 38, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Goshulak, P.; Samiezadeh, S.; Aziz, M.S.R.; Bougherara, H.; Zdero, R.; Schemitsch, E.H. The biomechanical effect of anteversion and modular neck offset on stress shielding for short-stem versus conventional long-stem hip implants. Med. Eng. Phys. 2016, 38, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Yamako, G.; Chosa, E.; Totoribe, K.; Watanabe, S.; Sakamoto, T. Trade-off between stress shielding and initial stability on an anatomical cementless stem shortening: In-vitro biomechanical study. Med. Eng. Phys. 2015, 37, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Haase, K.; Rouhi, G. Prediction of stress shielding around an orthopedic screw: Using stress and strain energy density as mechanical stimuli. Comput. Biol. Med. 2013, 43, 1748–1757. [Google Scholar] [CrossRef]
- Sumner, D.R. Long-term implant fixation and stress-shielding total hip replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.D.A.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Khanna, R.; Kokubo, T.; Matsushita, T.; Nomura, Y.; Nose, N.; Oomori, Y.; Yoshida, T.; Wakita, K.; Takadama, H. Novel artificial hip joint: A layer of alumina on Ti-6Al-4V alloy formed by micro-arc oxidation. Mater. Sci. Eng. C 2015, 55, 393–400. [Google Scholar] [CrossRef]
- Choudhury, D.; Lackner, J.M.; Major, L.; Morita, T.; Sawae, Y.; Bin Mamat, A.; Stavness, I.; Roy, C.K.; Krupka, I. Improved wear resistance of functional diamond like carbon coated Ti-6Al-4V alloys in an edge loading conditions. J. Mech. Behav. Biomed. Mater. 2016, 59, 586–595. [Google Scholar] [CrossRef]
- Höhn, S.; Virtanen, S. Effect of inflammatory conditions and H2O2 on bare and coated Ti-6Al-4V surfaces: Corrosion behaviour, metal ion release and Ca-P formation under long-term immersion in DMEM. Appl. Surf. Sci. 2015, 357, 101–111. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- You, L.; Song, X. A study of low Young’s modulus Ti–Nb–Zr alloys using electrons alloy theory. Scr. Mater. 2012, 67, 57–60. [Google Scholar] [CrossRef]
- Yang, R.; Hao, Y.; Liet, S. Development and application of low-modulus biomedical titanium alloy Ti2448. In Biomedical Engineering, Trends in Materials Science; InTechOpen: London, UK, 2011; pp. 225–248. [Google Scholar] [CrossRef]
- You, L.; Song, X. First principles study of low Young’s modulus Ti–Nb–Zr alloy system. Mater. Lett. 2012, 80, 165–167. [Google Scholar] [CrossRef]
- Karre, R.; Niranjan, M.K.; Dey, S.R. First principles theoretical investigations of low Young’s modulus beta Ti–Nb and Ti–Nb–Zr alloys compositions for biomedical applications. Mater. Sci. Eng. C 2015, 50, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.X.; Feng, X.J.; Yin, L.X.; Liu, X.; Ma, M.; Liu, R. Development of a new beta Ti alloy with low modulus and favorable plasticity for material implant. Mater. Sci. Eng. C 2016, 61, 338–343. [Google Scholar] [CrossRef]
- Zhao, X.; Niinomi, M.; Nakai, M.; Ishimoto, T.; Nakano, T. Development of high Zr-containing Ti-based alloys with low Young’s modulus for use in removable implants. Mater. Sci. Eng. C 2011, 31, 1436–1444. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.; Li, Y.; Ipek, R.; Wen, C. Development of Ti-Nb-Zr alloys with high elastic admissible strain for temporary orthopedic devices. Acta Biomater. 2015, 20, 176–187. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Zhao, X.; Zhao, X. Self-adjustment of Young’s modulus in biomedical titanium alloys during orthopedic operation. Mater. Lett. 2011, 65, 688–690. [Google Scholar] [CrossRef]
- Tan, M.H.C. New Generation Titanium Alloys with Low Elastic Modulus for Orthopaedic Implants. Ph.D. Thesis, Flinders University College of Science and Engineering, Bedford Park, Australia, 2018. Available online: https://flex.flinders.edu.au/file/f544a81b-728f-443d-9263-c29b0944f215/1/ThesisTan2018.pdf (accessed on 3 December 2021).
- Xie, K.Y.; Wang, Y.; Zhao, Y.; Chang, L.; Wang, G.; Chen, Z.; Cao, Y.; Liao, X.; Lavernia, E.J.; Valiev, R.Z.; et al. Nanocrystalline β-Ti alloy with high hardness, low Young’s modulus and excellent in vitro biocompatibility for biomedical applications. Mater. Sci. Eng. C 2013, 33, 3530–3536. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Hu, J.; Li, S.; Zou, Q.; Li, Y.; Jiang, N.; Li, H.; Li, J. Modified surface morphology of a novel Ti-24Nb-4Zr-7.9Sn titanium alloy via anodic oxidation for enhanced interfacial biocompatibility and osseointegration. Colloids Surf. B Biointerfaces 2016, 144, 265–275. [Google Scholar] [CrossRef]
- Xue, P.; Li, Y.; Li, K.; Zhang, D.; Zhou, C. Superelasticity, corrosion resistance and biocompatibility of the Ti-19Zr-10Nb-1Fe alloy. Mater. Sci. Eng. C 2015, 50, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, B.; Johansson, A.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Bone response to a novel Ti-Ta-Nb-Zr alloy. Acta Biomater. 2015, 20, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M.; Ito, H.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Nagai, A.; Hanawa, T. The Effects of Various Metallic Surfaces on Cellular and Bacterial Adhesion. Metals 2019, 9, 1145. [Google Scholar] [CrossRef]
- Surmeneva, M.; Grubova, I.; Glukhova, N.; Khrapov, D.; Koptyug, A.; Volkova, A.; Ivanov, Y.; Cotrut, C.M.; Vladescu, A.; Teresov, A.; et al. New Ti–35Nb–7Zr–5Ta Alloy Manufacturing by Electron Beam Melting for Medical Application Followed by High Current Pulsed Electron Beam Treatment. Metals 2021, 11, 1066. [Google Scholar] [CrossRef]
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.; Sercombe, T. Manufacture by Selective Laser Melting and Mechanical Behavior of a Biomedical Ti-24Nb-4Zr-8Sn Alloy. Scr. Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Bertrand, E.; Gloriant, T.; Gordin, D.M.; Vasilescu, C.; Drob, P.; Drob, S. Synthesis and Characterisation of a New Superelastic Ti-25Ta-25Nb Biomedical Alloy. J. Mech. Behav. Biomed. Mater. 2010, 3, 559–564. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Luo, K.; Xu, H.B. Microstructure and Shape Memory Effect of Ti-20Zr-10Nb Alloy. Mater. Sci. Eng. A 2010, 527, 652–656. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Prima, F.; Yang, R. Controlling reversible martensitic transformation in titanium alloys with high strength and low elastic modulus. Scr. Mater. 2012, 67, 487–490. [Google Scholar] [CrossRef]
- Hanada, S.; Masahashi, N.; Jung, T.K. Effect of stress-induced α″ martensiteon Young’s modulus of β Ti-33.6Nb-4Sn alloy. Mater. Sci. Eng. A 2013, 588, 403–410. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Abbasi, S.M.; Morakabati, M. Deformation-induced martensitic transformation in a new metastable beta titanium alloy. J. Alloys Compd. 2015, 650, 22–29. [Google Scholar] [CrossRef]
- Elmay, W.; Prima, F.; Gloriant, T.; Bolle, B.; Zhong, Y.; Patoor, E.; Laheurte, P. Effects of thermomechanical process on the microstructure and mechanical properties of a fully martensitic titanium-based biomedical alloy. J. Mech. Behav. Biomed. Mater. 2013, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, F.; Liu, P.; Wang, C.; Liu, X.; Li, W.; Han, Q. Effects of Nb on Superelasticity and Low Modulus Properties of Metastable β-Type Ti-Nb-Ta-Zr Biomedical Alloys. J. Mater. Eng. Perform. 2019, 28, 1410–1418. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Raducanu, D.; Gloriant, T.; Gordin, D.M.; Cinca, I. Effects of cold-rolling deformation on texture evolution and mechanical properties of Ti–29Nb–9Ta–10Zr alloy. Mater. Sci. Eng. A 2013, 586, 1–10. [Google Scholar] [CrossRef]
- Cinca, I.; Nocivin, A.; Raducanu, D.; Gloriant, T.; Gordin, D.M.; Dan, I.; Caprarescu, A.; Cojocaru, V.D. XRD and nano indentation testing of thermo-mechanical processed Ti-29Nb-9Ta-10Zr alloy. Met. Mater. 2015, 53, 17–26. [Google Scholar] [CrossRef]
- Tabirca, C.M.; Gloriant, T.; Gordin, D.M.; Thibon, I.; Raducanu, D.; Cinca, I.; Nae, C.I.; Caprarescu, A.; Cojocaru, V.D. Recrystallization temperature influence upon structural changes of 90% Cold rolled Ti-29Nb-9Ta-10Zr biocompatible alloy. Key Eng. Mater. 2014, 592–593, 370–373. [Google Scholar] [CrossRef]
- Nocivin, A.; Raducanu, D.; Vasile, B.; Irimescu, R.; Cojocaru, V.D. Tailoring a low young modulus for a beta titanium alloy by combining severe plastic deformation with solution treatment. Materials 2021, 14, 3467. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.; Zhang, Y.; Li, Y.; Wen, C. Cold rolling deformation and annealing behavior of a β-type Ti–34Nb–25Zr titanium alloy for biomedical applications. J. Mater. Res. Technol. 2020, 9, 2308–2318. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.; Weng, W.; Zhang, Y.; Li, Y.; Wen, C. Effect of thermomechanical treatment on the mechanical and microstructural evolution of a β-type Ti-40.7Zr–24.8Nb alloy. Bioact. Mater. 2019, 4, 303–311. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Serban, N. Effects of Solution Treating on Microstructural and Mechanical Properties of a Heavily Deformed New Biocompatible Ti–Nb–Zr–Fe Alloy. Metals 2018, 8, 297. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Nocivin, A.; Trisca-Rusu, C.; Dan, A.; Irimescu, R.; Raducanu, D.; Galbinasu, B.M. Improving the Mechanical Properties of a β-type Ti-Nb-Zr-Fe-O Alloy. Metals 2020, 10, 1491. [Google Scholar] [CrossRef]
- Peter, I. Investigations into Ti-Based Metallic Alloys for Biomedical Purposes. Metals 2021, 11, 1626. [Google Scholar] [CrossRef]
- Gurau, C.; Gurau, G.; Mitran, V.; Dan, A.; Cimpean, A. The Influence of Severe Plastic Deformation on Microstructure and In Vitro Biocompatibility of the New Ti-Nb-Zr-Ta-Fe-O Alloy Composition. Materials 2020, 13, 4853. [Google Scholar] [CrossRef] [PubMed]
- ASTM G59-97; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM: West Conshohocken, PA, USA, 2014; p. 4. [CrossRef]
- ASTM G102-89 e1; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM: West Conshohocken, PA, USA, 2004; p. 7. [CrossRef]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Corrosion in titanium dental implants/prostheses—A review. Trends Biomater. Artif. Organs 2011, 25, 34–46. [Google Scholar]
- Vijayaraghavan, V.; Sabane, A.V.; Tejas, K. Hypersensitivity to titanium: A less explored area of research. J. Indian Prosthodont. Soc. 2012, 12, 201–207. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
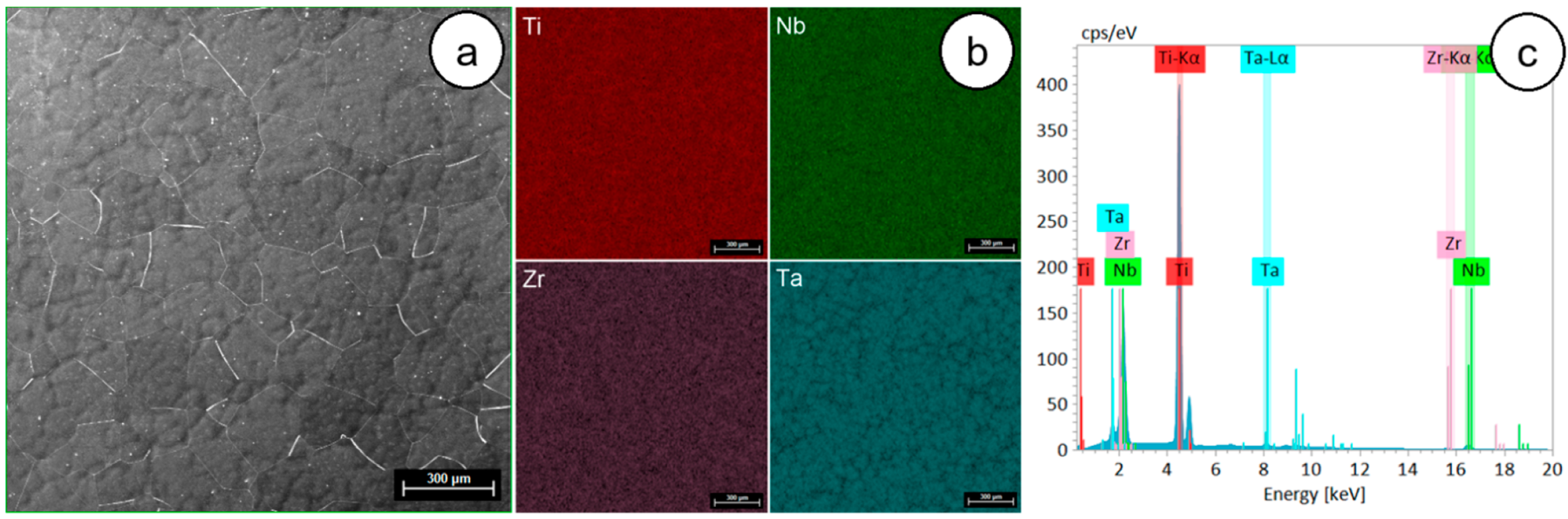

| Element | At. No. | Mass, [% wt.] | Abs. Error, [%] | Rel. Error, [%] |
|---|---|---|---|---|
| Titanium | 22 | 62.29 | 1.61 | 2.77 |
| Niobium | 41 | 25.26 | 0.47 | 2.76 |
| Zirconium | 40 | 7.94 | 0.10 | 3.33 |
| Tantalum | 73 | 4.51 | 0.11 | 3.10 |
| Sum | 100.00 | - | - | |
| Structural State | Ultimate Strength, σUTS [MPa] | Yield Strength, σ0.2 [MPa] | Fracture Strain, εf [%] | Elastic Modulus, E [GPa] |
|---|---|---|---|---|
| AR TNTZ alloy | 708 ± 10 | 512 ± 13 | 10 ± 2 | 59 ± 2 |
| Structural State | Ultimate Strength, σUTS [MPa] | Yield Strength, σ0.2 [MPa] | Fracture Strain, εf [%] | Elastic Modulus, E [GPa] |
|---|---|---|---|---|
| CR TNTZ alloy | 1261 ± 12 | 1166 ± 10 | 4 ± 1 | 50 ± 3 |
| Grain-Size [µm] | ||||
|---|---|---|---|---|
| Treatment Temperature [°C] | Holding Time [min] | |||
| 5 | 10 | 15 | 20 | |
| 800 | 43 | 48 | 54 | 62 |
| 900 | 57 | 63 | 72 | 79 |
| 1000 | 91 | 104 | 117 | 162 |
| Structural State | Ultimate Strength, σUTS [MPa] | Yield Strength, σ0.2 [MPa] | Fracture Strain, εf [%] | Elastic Modulus, E [GPa] |
|---|---|---|---|---|
| Solution treated (ST 1.1) | 1166 ± 10 | 1052 ± 12 | 6 ± 1 | 54 ± 4 |
| Solution treated (ST 1.2) | 1083 ± 12 | 978 ± 12 | 7 ± 1 | 55 ± 3 |
| Solution treated (ST 1.3) | 1034 ± 13 | 916 ± 10 | 7 ± 1 | 55 ± 4 |
| Solution treated (ST 1.4) | 997 ± 11 | 814 ± 12 | 8 ± 2 | 54 ± 4 |
| Structural State | Ultimate Strength, σUTS [MPa] | Yield Strength, σ0.2 [MPa] | Fracture Strain, εf [%] | Elastic Modulus, E [GPa] |
|---|---|---|---|---|
| Solution treated (ST 2.1) | 1004 ± 11 | 720 ± 12 | 10 ± 2 | 56 ± 4 |
| Solution treated (ST 2.2) | 944 ± 10 | 615 ± 14 | 12 ± 2 | 55 ± 2 |
| Solution treated (ST 2.3) | 863 ± 12 | 573 ± 12 | 16 ± 1 | 55 ± 4 |
| Solution treated (ST 2.4) | 767 ± 11 | 531 ± 12 | 21 ± 2 | 56 ± 3 |
| Structural State | Ultimate Strength, σUTS [MPa] | Yield Strength, σ0.2 [MPa] | Fracture Strain, εf [%] | Elastic Modulus, E [GPa] |
|---|---|---|---|---|
| Solution treated (ST 3.1) | 847 ± 14 | 546 ± 10 | 13 ± 2 | 57 ± 4 |
| Solution treated (ST 3.2) | 844 ± 13 | 597 ± 12 | 9 ± 2 | 55 ± 4 |
| Solution treated (ST 3.3) | 859 ± 14 | 613 ± 11 | 5 ± 2 | 54 ± 4 |
| Solution treated (ST 3.4) | 856 ± 12 | 723 ± 13 | 5 ± 2 | 56 ± 3 |
| No. | Sample | EOC (mV) | Ecorr (mV) | icorr (nA/cm2) | βc (mV) | βa (mV) | Rp (MΩ·cm2) | CR (µm/year) |
|---|---|---|---|---|---|---|---|---|
| 1 | AR | −281 | −258 | 3.141 | 176.66 | 787.94 | 19.975 | 0.0257 |
| 2 | CR | −289 | −274 | 1.368 | 155.81 | 298.67 | 32.544 | 0.0112 |
| 3 | ST 2.1 | −311 | −288 | 1.445 | 165.62 | 244.69 | 29.718 | 0.0118 |
| 4 | ST 2.2 | −284 | −260 | 2.227 | 170.04 | 518.33 | 24.997 | 0.0182 |
| 5 | ST 2.3 | −280 | −267 | 2.252 | 172.20 | 245.81 | 19.550 | 0.0184 |
| 6 | ST 2.4 | −282 | −265 | 2.201 | 170.27 | 428.88 | 24.076 | 0.0180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelescu, M.L.; Dan, A.; Ungureanu, E.; Zarnescu-Ivan, N.; Galbinasu, B.M. Effects of Cold Rolling Deformation and Solution Treatment on Microstructural, Mechanical, and Corrosion Properties of a Biocompatible Ti-Nb-Ta-Zr Alloy. Metals 2022, 12, 248. https://doi.org/10.3390/met12020248
Angelescu ML, Dan A, Ungureanu E, Zarnescu-Ivan N, Galbinasu BM. Effects of Cold Rolling Deformation and Solution Treatment on Microstructural, Mechanical, and Corrosion Properties of a Biocompatible Ti-Nb-Ta-Zr Alloy. Metals. 2022; 12(2):248. https://doi.org/10.3390/met12020248
Chicago/Turabian StyleAngelescu, Mariana Lucia, Alexandru Dan, Elena Ungureanu, Nicoleta Zarnescu-Ivan, and Bogdan Mihai Galbinasu. 2022. "Effects of Cold Rolling Deformation and Solution Treatment on Microstructural, Mechanical, and Corrosion Properties of a Biocompatible Ti-Nb-Ta-Zr Alloy" Metals 12, no. 2: 248. https://doi.org/10.3390/met12020248
APA StyleAngelescu, M. L., Dan, A., Ungureanu, E., Zarnescu-Ivan, N., & Galbinasu, B. M. (2022). Effects of Cold Rolling Deformation and Solution Treatment on Microstructural, Mechanical, and Corrosion Properties of a Biocompatible Ti-Nb-Ta-Zr Alloy. Metals, 12(2), 248. https://doi.org/10.3390/met12020248






