Abstract
Bauxite residue, the high alkaline and fine-grained byproduct of Bayer process for alumina’s production, is a material rich in numerous minerals and elements of high value and techno-economical interest in high tech applications such as rare earths, including scandium. Lately, the European Committee has characterized scandium as a critical element because of its risk supply chain. Scandium's high concentration in Greek bauxite residue classifies the waste as a candidate for low cost and high availability of the element, additionally improving its environmental fingerprint. For scandium recovery, hydrometallurgical treatment with inorganic acids is the most common, effective and simple method. In this study, the efficiency of phosphoric acid is investigated for scandium recovery by a direct leaching of bauxite residue without pretreatment. Multi leaching variables, such as acid molarity, solid/liquid ratio, process temperature and leaching time, were examined and optimized individually as well as in a comparative way aiming to scandium selective extraction (mainly with regard to iron) and process viability. A Sc selective recovery of 40% was obtained for phosphoric acid molarity 5 M, solid/liquid ratio 10%, leaching time, 60 min under ambient conditions with low iron leachability, no gel formation and no energy consumption.
1. Introduction
A raw material of great importance is metallic aluminum since its characteristics (lightweight, electrical and thermal conductivity, corrosion resistance) can be used in numerous applications including composite materials, medicine, and food packaging. Aluminum’s global production is the largest one compared to other nonferrous metals, and it consists of two industrial processes: Bayer process followed by Hall–Heroult [1,2].
The main by-product of the Bayer process after bauxite digestion is a fine-grained and highly alkaline sludge waste product, the bauxite residue (BR), also called red mud (RM). Bauxite residue is a complex material, rich in a variety of minerals and metals. It is composed of oxides and salts of main elements (Fe, Si, Al, Ca, Ti, Na), and numerous trace elements (V, Cr, Zn, Ga, Nb, Zr and Ta), while is enriched in techno-economically critical elements such as rare earth elements-REEs (Sc, Y and lanthanides). It also has high specific area, good particle dispersion, characteristics similar to porous materials and good stability in solutions. However, due to its high alkalinity and fine grain size, as well as its poorly designed storage on landfills, many environmental risks arise from the process of its deposition such as water, soil and/or air pollution. Many attempts have been made for the development of environmentally friendly and cost-effective new technologies and processes to dispose of or utilize BR [3,4,5]. The comprehensive utilization of BR is an issue of great economic and environmental importance and can be combined with BR composition and properties. Several reviews refer to bauxite residue valorization as building material [6,7], agent for advanced ceramics [8], coagulant [9,10], adsorbent [9,11,12], catalyst [13], agent for neutralizing acidic wastes or soils remediation [14,15], raw material for metals recovery [16,17,18,19], aiming to a zero waste.
Besides other REEs, scandium (Sc) is the most valuable element with important applications in modern high-tech and green technologies such as Sc-Al alloys used, i.e., in aerospace and defense industry, as well as in fuel cells as an electrolyte improving oxygen ion conductivity [20,21,22]. In recent years, the European Commission has classified Sc, as a critical element, due to its unstable supply chain as a result of its low availability due to its limited exploitable deposits and its low concentrations in easily handled ores [23]. The utilization of BR for the recovery of valuable elements as a secondary, low cost and highly available raw material could be an alternative promising way resulting in circular economy and residue consumption with environmental benefits.
In Greece, the bauxite residue is generated in “Mytilineos S.A.” facilities in Aspra SpitiaViotia, with an annual production of 750,000 tones, and it is considered as chemically stable [24]. A significant fact about Greek BR is the concentration of REEs, which is estimated at ~1 kg REEs/t of dry BR, almost constant over the years with a Sc high content of ~112.9 ± 13 mg/kg of dry BR (~0.02% Sc2O3) close to Sc main resources [25,26]. The residue is enriched by a factor of 2 compared with the original bauxite [25], and it is estimated that a total exploitation of the Greek BR could produce ~1 kg/yr REEs oxides. Thus, this Greek by-product can be considered as a candidate Sc resource.
There are several processes of recovering Sc from bauxite residue, such as pyrometallurgical and hydrometallurgical processes, ionic liquids and bioleaching [18,27,28,29,30,31,32,33]. Among them, hydrometallurgical treatments by direct leaching process with acidic and alkaline solutions, are the most common techniques applied for Sc recovery since they are technologically simple, easy to handle, low energy cost and efficient [18]. There is a great variety of leaching agents which have been studied, including organic or mineral acids [26,29,34,35,36,37,38] as well as alkaline solvents [39]. Mineral acids (HNO3, HCl, H2SO4, aqua regia) have been proven to be most sufficient compared with organic acids, achieving the higher recovery yields for Sc [26,36]. In most cases, Sc is co-extracted with other elements at the expense of selectivity, especially regarding iron.
In the present study, selective extraction of Sc was investigated by a direct acidic leaching of BR using H3PO4 as a leaching agent. Phosphoric acid was used, in this study, as an alternative leaching agent due to its milder environmental impact compared with other mineral acids such as HNO3, HCl or aqua regia. The choice of H3PO4 was based on several criteria such as: its efficiency on Sc/REEs extraction from raw materials like phosphorites and/or by-products; the lack of enough references for a direct BR leaching and most importantly the high selectivity of phosphorus species in Sc extraction [40,41,42,43,44,45,46,47,48,49,50,51,52,53]. A variety of acidic and neutral organophosphorus extractants, such as DEHPA, TBP, TOPO, Cyanex, have been reported to be suitable for Sc isolation from aqueous solutions [40,41,42,43,44,45,46]. The selectivity depends on the type of the acid in aqueous phase, and it is due to the interaction occurring between the donated pair of metal electrons and P-O or P=O bonds [41,42]. The use of phosphorus containing ion exchange resins for selective separation and purification of Sc has also been investigated [47,48,49]. As is concluded, purity of Sc obtained is higher compared with liquid extraction [47]. According to Korovin V. et al. 2011, the functional groups of the resins lead to Sc sorption at the expense of cation exchange, in addition to the formation of coordinate bonds with P=O groups, resulting to chelate complex formation [50].
The use of phosphoric acid is reported by Li et al. 2018 [51], on a Sc-enriched material produced after multiple pretreatments of BR for iron and silica removal. In addition to Sc, the final material is enriched in Ca and Ti. Sc, enclosed into perovskite phase, remained in the precipitate due to the acid-resistance of mineral at ambient conditions. Sc leaching is finally achieved under autoclave conditions at 120–140 °C at high acid molarity (6–8 M) and long process duration, resulting to 90% Sc recovery due to dissolution of perovskite by phosphoric acid under the extreme conditions. Further Sc purification with solvent extraction using DE2HPA is performed with the highest separation coefficients achieved for phosphoric acid over other mineral acids. The selective dissolution and removal of Si by phosphoric acid from a low iron BR is described by Deng et al. 2019 [52]. An 80% Si removal is achieved at low acid molarities (1 M) under ambient conditions where Sc and REEs are not affected, resulting to a Sc, Y-enriched precipitate mainly consisted of perovskite. Finally, rare earth recovery from phosphate ores is performed by a wet phosphoric process achieving high extraction yields. Process performance reaches the 61.4% under low temperatures, large L/S ratio: 4/1 and high concentration of phosphoric acid while the presence of surfactant additives enhances further REEs extraction up to 75% [53].
The aim of this work is the study of the direct phosphoric acid leaching of BR, poorly investigated so far [54]. Several parameters were tested and evaluated, such as acid molarity (1–7 M), solid/liquid ratio (S/L) (2–20%), temperature (25–85 °C), final pH, considering high Sc concentration and selective recovery, especially with respect to iron. Taguchi experimental design was performed, followed by statistical evaluation, aiming to optimum leaching conditions for Sc maximization and Fe minimization for a selective and viable process. The results revealed that although there are no common conditions to fully satisfy both limitations, the use of phosphoric acid at ambient conditions results to a viable process.
2. Materials and Methods
2.1. BR Preparation and Characterization
The bauxite residue sample was provided by the Hellenic Aluminium Industry “Mytilineos S.A.” in Aspra Spitia of Viotia in Greece in the form of “ferroalumina”, a compact material after filterpress treatment with a low moisture of ~26%. The pH value of BR was found to be about 11.3. The characterization of the original BR material, leachate solutions and the precipitates after leaching process were conducted by different analytical techniques such as Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES), Atomic Absorption Spectrometry (AAS), X-Ray Fluorescence spectroscopy (XRF), X-Ray Diffraction (XRD), Raman spectroscopy. Bauxite residue (BR) complete characterization has been performed by our team in previous works demonstrating a constant composition of the residue [55,56]. In the present study, only Fe, Al, Si, Ti and Sc were determined in order to confirm their concentration because of their high content and the significant effect on the subsequent processes of ion exchange and solvent extraction for further Sc purification.
The BR’s total dissolution was performed by fusion with lithium tetraborate—Li2B4O7 Spectromelt A10 1.10783 (Merck, Darmstadt, Germany) as flux at a ratio of 3:1 in a platinum crucible at 1100 °C for 45 min. The melt allowed it to cool and then was dissolved under stirring and mild heating on a hotplate, with concentrated nitric acid (p.a., 65%) and deionized water. The solution was placed in a volumetric flask which was filled to the mark with water and was stored for analysis.
2.2. Leaching Experiments
Leaching experiments were performed in conical and sealed spherical flasks under mechanical or magnetic stirring, adding an appropriate amount of dry BR to 200–500 mL of H3PO4 in order to result in different S/L ratios. For the molarity effect study, 1–7 M phosphoric acid solutions were used, while for the S/L effect study, the selected densities were in the range of 2–20%. The evaluation of leaching time effect was carried out via a periodic sample collection. Regarding temperature experiments, H3PO4 was preheated at various temperatures in a sealed spherical flask connected to a findenser air condenser RR31100 Findenser B24 Cone supplied by Randleys (Saffron Walden, UK), before the addition of dry BR. In all cases, the leachates were filtered under vacuum with 0.45 µm cellulose nitrate membranes by Whatman (part of GE Healthcare Life Sciences) (Little Chalfont, Buckinghamshire, UK) and the final pH of the leachates was measured. The leachates were stored for further analysis.
Phosphoric acid (H3PO4) used was of p.a grade, 85% w/w by Riedel-de Haen supplied by Honeywell (Charlotte, NC, USA). For the preparation of the working standards for the elemental analysis, a 1000 mg/L single standard Sc solution TraceCERT 92279 supplied by Fluka Chemie GmbH (Buchs, Switzerland), and a multi element standard solution IV for ICP CertiPUR 1.11355 of 1000 mg/L for each element, supplied by Merck KGaA (Darmstadt, Germany) were used.
2.3. Instrumentation
Mineralogical analysis of the BR batch has been used in the present study and the precipitates after the leaching experiments have been performed by XRD D8 Advanced Twin—Twin with the LYNEXEYE (1D Mode) detector that was supplied by Bruker (Billerica, MA, USA) with CuKα radiation of wavelength 1.54060 Ǻ, the voltage of 40 kV, the current of 40 mA and lower discriminator of 0.18 V. The twin primary was of 1 mm and twin secondary of 5 mm. The scanning range was 2θ = 0–75° with a scanning speed of 0.035 s−1 for about 35 min. The elemental composition of BR and the precipitates was characterized using the energy dispersive XRF Epsilon 1 apparatus by Malvern Panalytical (Malvern, UK) equipped with a silver anode X-ray tube at the power of 10 W (voltage 50 kV and current 1 mA) and time of analysis of 30 min. Furthermore, the BR’s main mineralogical composition was confirmed by Raman analysis using the Renishaw Ramascope RM1000 with a LEICA DMLM microscope, supplied by Renishaw (Gloucestershire, UK) with a HeNe source radiation at 632.8 nm and a grating of 1800 lines/mm. An Edge filter and an inlet slit of 50 μm were used. A CCD camera was used as a radiation collector while for the analysis objective lenses of ×100 and ×50 were applied on an area of less than 2.5 μm and 5 μm of diameter, respectively. The energy of the radiation at the focus point does not exceed 5 mJ.
For the chemical analysis of Sc and main elements (Fe, Al, Si, Ti) in fusion and leachates solutions, the ICP-OES Optima 7000 DV supplied by Perkin Elmer (Waltham, MA, USA) and an AAS 240 FS by Varian (Parent company Agilent Technologies, Palo Alto, Santa Clara, CA, USA) were used. For ICP-OES a power of 1450 W was applied with an argon gas flow rate of 15 L/min, auxiliary gas flow rate of 0.2 L/min and cooling gas flow rate of 18–25 L/min. A nebulizer of Meinhard mode was used. The sample uptake was adjusted at 1.5 ml/min with a nebulizer gas flow rate of 0.7 L/min. The analysis was performed thrice with an integration time 1–5 s. The wavelengths used were: Sc 361.383 nm, Fe 259.939 nm, Al 396.153, Ti 334.940 nm, Si 251.611 nm. Regarding AAS analysis, the operating conditions were chosen as follows: Fe 248.3 nm (flame mode Air/C2H2 and slit width 0.2 nm), Al 309.3 nm (flame mode N2O/C2H2 and slit width 0.5 nm), Ti 364.3 nm (flame mode N2O/C2H2 and slit width 0.5 nm), Si 251.6 nm (flame mode N2O/C2H2 and slit width 0.2 nm) for AAS. For the determination of all the elements, the calibration curve method with external standards was applied in both cases.
The mechanical stirring was carried out by the Hei-Torque 100 stirrer supplied by Heidolph (Schwabach, Germany). For the temperature effect experiments, a stirring hotplate RR91226 with Pt1000 S/S Sensor-Tech for temperature monitoring and a 1 L heat on block supplied by Randleys (Saffron Walden, UK) were used. pH measurements were conducted by the multi 350 i pH-meter, with a SenTix® 41 precision electrode supplied by WTW (Weilheim, Germany).
3. Results and Discussion
3.1. BR Batch Characterization
The chemical composition of the bauxite residue (BR) batch for Sc and its main elements is presented in Table 1. A long-term investigation of Greek BR batches has proven that the residue contains a high amount of Sc of 113 mg/kg, which remains constant during the years with a variation of 13 mg/kg [26]. Table 1 presents the chemical composition in Sc and main elements of the BR batch used in this study. The results of XRF semi-quantitative analysis of the same sample for major and minor elements are also presented in Table S1 in Supplementary Materials confirming that Fe, Al, Si, Ti, Ca oxides are the most dominant constituents in Greek BR.

Table 1.
Chemical composition of bauxite residue (BR) batch used in this study.
Mineralogical analysis by XRD confirmed previous studies [26], as is shown in Section 3.3.2. Raman analysis verified XRD results identifying mainly hematite and diaspore (as shown in Figure S1 in Supplementary materials).
3.2. Effect of Time
The leaching process with phosphoric acid was performed at different time intervals up to 4 h. In all cases, the process was integrated rapidly by 90%, within 10–15 min, which is in agreement with other mineral acids such as sulfuric acid, used previously [26]. Leaching time of 60 min was estimated as a safe duration for the process.
3.3. Effect of Acid Molarity and S/L Ratio (Pulp Density)
3.3.1. Sc Extraction
Acid molarity up to 5 M, affects positively both % Sc recovery (Figure 1a) and the concentration (Figure 1b) as shown in Figure 1. However, the effect is more significant for Sc recovery while Sc concentration is increased at high pulp densities (S/L ratios). Sc leachability ranged from 6% with CSc = 1 mg/L (1 M H3PO4 and S/L 20%) to 48% with CSc < 0.5 mg/L (5 M H3PO4 and S/L 2%). Acid molarities higher than 5 M do not enhance Sc extraction further, probably due to the high viscosity of the phosphoric acid solution, resulting in Sc recoveries and concentration decrement which depends on S/L ratio due to the pH value increment (see Section 3.4).
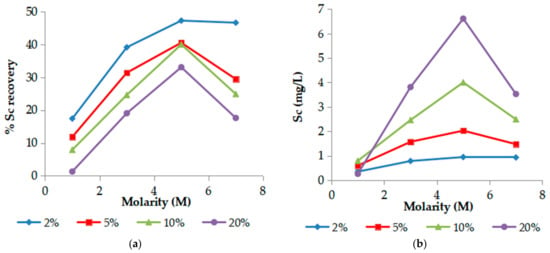
Figure 1.
Effect of acid molarity on (a) % Sc recovery and (b) Sc concentration. (T = 25 °C, t = 60 min).
Regarding the solid to liquid (S/L) ratio effect, the results are demonstrated on graphs in Figure 2. S/L ratio is the critical factor for Sc concentration (Figure 2a) despite the reduction in % Sc recovery (Figure 2b) due to the inefficiency of H3PO4 to neutralize BR alkalinity by S/L increment resulting to high pH values (see Section 3.4). As shown in Figure 2a, higher pulp densities lead to further enrichment of the leachate in Sc, and the positive effect is further favored by higher acid molarities, reaching a Sc concentration close to 7 mg/L (recovery 35%) for 5 M phosphoric acid and pulp density, S/L 20%. The only exception was observed at 1 M phosphoric acid and S/L 20% with pH value of 2.8, which negatively affects Sc extraction. Therefore, the combination of 5 M H3PO4 and S/L ratio 10% seems satisfactory, achieving an acceptable efficiency for both Sc recovery and concentration (40%, CSc = 4.0 mg/L).
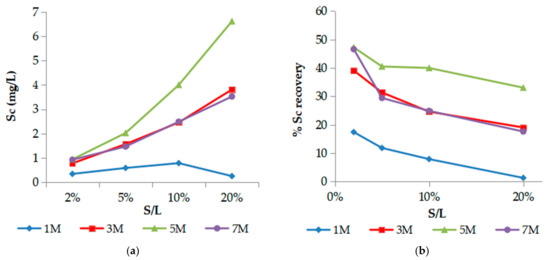
Figure 2.
Effect of S/L ratio (pulp density) on Sc concentration (a) and % Sc recovery (b). (T = 25 °C, t = 60 min).
3.3.2. Main Elements Co-Extraction
Concerning the co-extraction of the main elements, Ti, Al and Fe present a similar behavior with Sc, as is demonstrated in Figure 3a–c, respectively. Acid molarity increment up to 5 M results in higher recoveries and concentrations of the elements at the expense of selectivity, while increased S/L ratios negatively affect main elements’ recovery. Molarities higher than 5 M do not further improve the extraction.
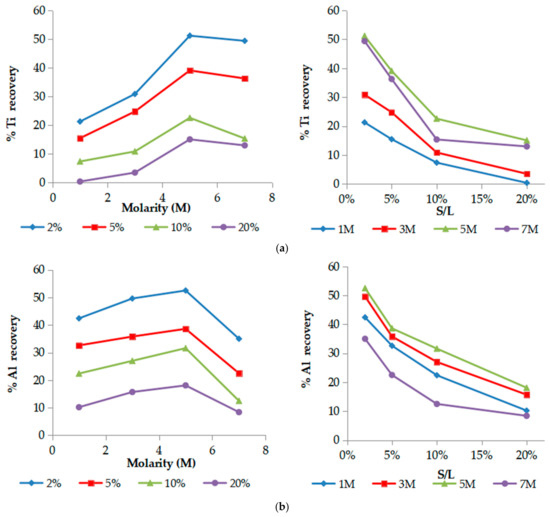
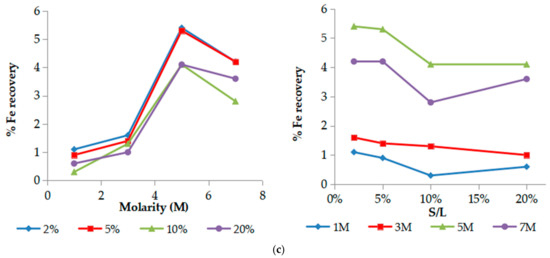
Figure 3.
Effect of acid molarity (left) and S/L ratio (pulp density) (right) on % Ti recovery (a), % Al recovery (b), % Fe recovery (c). (T = 25 °C, t = 60 min).
Among all main elements, iron presence in the leachate is of great importance. Its high co-extraction is undesirable because of its similar chemical properties with Sc, causing problems with the efficiency of the subsequent processes (ion exchange and solvent extraction) and hindering further purification and isolation of Sc in high purity. As iron is the major constituent in BR (Fe2O3 ~ 40–45%), the process selectivity is focused mainly on the leachability of this element.
An opposite trend was observed for Si (Figure 4). As is demonstrated below in Figure 4, Si recovery is favored by the lower molarities of phosphoric acid. 1 M H3PO4 and S/L 2% results to a high yield of Si extraction ~80% with low Sc co-extraction lower than 10% while it is reduced for 5 M H3PO4 where Sc is positively affected. The effect is more significant (Si rec < 20%) for higher pulp densities ≥ 10% where Sc concentration is also significantly increased. This is advantageous for the developed process since no gel formation occurs in all leachate solutions for an over 30-day period, in contrast to other mineral acids, such as sulfuric [26]. However, a white precipitate was observed after long time period mainly consisting of titanium with calcium impurities as resulted with ICP-OES analysis after appropriate precipitate dissolution. This agrees with the literature [51], since phosphoric acid promotes the dissolution of Ca and Ti minerals leading to CaHPO4 and (TiO)3 (PO4)2 formation and then TiO2+ hydrolysis precipitation as TiO2. The presence of precipitate depended on acid molarity and pulp density.
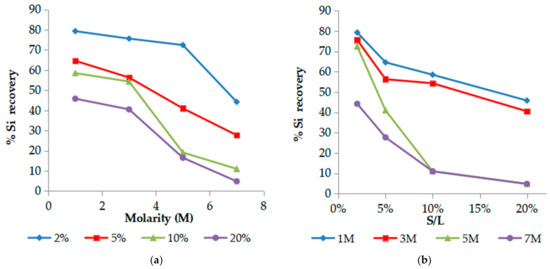
Figure 4.
Effect of (a) acid molarity and (b) S/L ratio (pulp density) on % Si recovery. (T = 25 °C, t = 60 min).
The XRF analysis of the precipitates after leaching with different acid molarities confirmed the trend observed in the leachate solutions for all the elements. As illustrated on the graph (Figure 5), Fe remains the major element in the precipitates confirming its low extraction in all leachates.
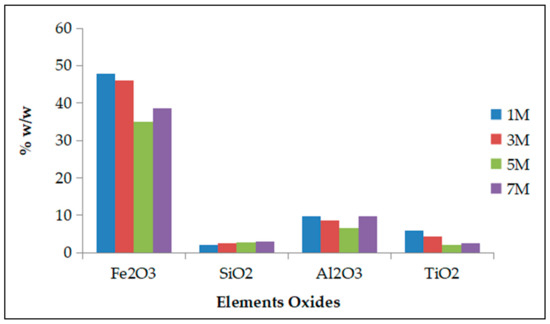
Figure 5.
X-ray fluorescence spectroscopy (XRF) analysis of main elements oxides in the precipitates after leaching process with different acid molarities (effect of molarity).
Mineralogical analysis of the initial raw material by XRD showed that the dominant mineralogical phase in Greek BR is hematite, accompanied by smaller amounts of gibbsite, diaspore, quartz, tohdite, calcite, cancrinite, montmorilonite, rutile and kaolinite (Figure 6a). After the leaching process (Figure 6b), the dominate phase is hematite (2θ = 33° and 2θ = 35.5°) followed by diaspore (2θ = 22°) while calcium silicate oxide was also detected in smaller amounts.
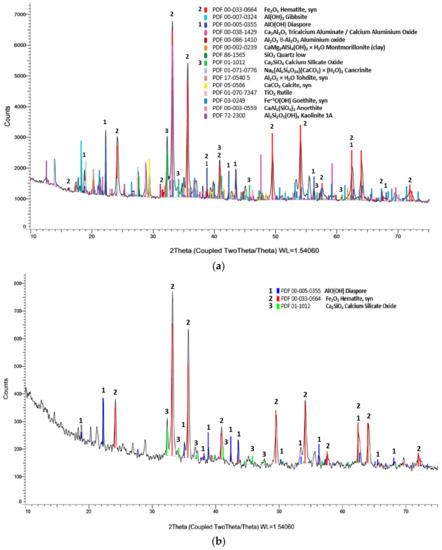
Figure 6.
BR mineralogical analysis (a) before and (b) after leaching process, 1: Diaspore, 2: Hematite, 3: Ca2SiO4 (Leaching conditions: 1 M H3PO4, S/L 10%, 25 °C, 1 h).
As is presented in Figure 6b, several minerals are dissolved even with mild phosphate solutions (1 M H3PO4). Acid molarity increment favors further dissolution of hematite (He) and calcium silicate oxide (Ca2SiO4) while diaspore (D) is not affected even by more concentrated phosphoric acid solutions (Figure 7).
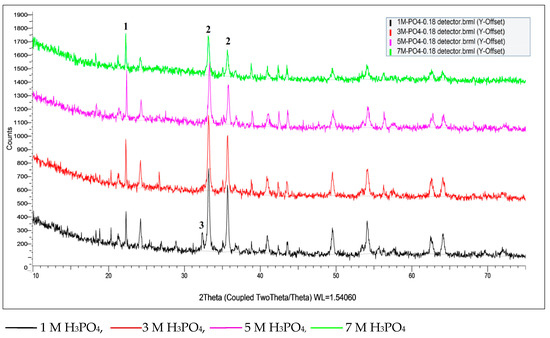
Figure 7.
Effect of acid molarity on mineralogical composition of the precipitates (1: Diaspore, 2: Hematite, 3: Ca2SiO4).
3.4. Effect of Leachate pH
Final pH value depends on acid molarity and S/L ratio selected. It was found that the pH value of the leachate is critical for Sc leachability. As is observed in Figure 8, pH values close to zero (0–0.4) affect positively Sc recovery which is increased to almost 50%. Low acid molarities and high S/L ratios show pH values of about 2.5 due to the incomplete neutralization of BR, resulting to reduced Sc recoveries (<5%).
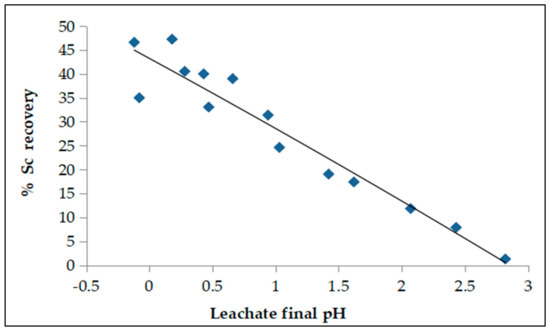
Figure 8.
Effect of pH value (T = 25 °C, t = 60 min).
3.5. Effect of Temperature
Elevated temperatures favor Sc recovery but also strongly affect Fe recovery from 5% (25 °C) to 50% (65 °C) at the expense of process selectivity (Figure 9). Autoclave aggressive conditions, suggested by other researchers because of the almost total Sc recovery [51], is not applicable in the present study due to Fe presence and its total dissolution. The temperature effect on the Fe recovery is more significant in the case of the phosphoric acid than of other mineral acids such as sulfuric acid, where a milder increment occurs [26]. However, an opposite effect was observed for the other main elements since higher temperatures slightly suppress Al recovery and to a higher degree Si and Ti recoveries, probably due to their thermal hydrolysis leading to lower recoveries, compared also to other mineral acids. Since the selectivity criteria focus on the Fe content, ambient conditions have been selected, considering both Sc selectivity against iron and energy consumption cost.
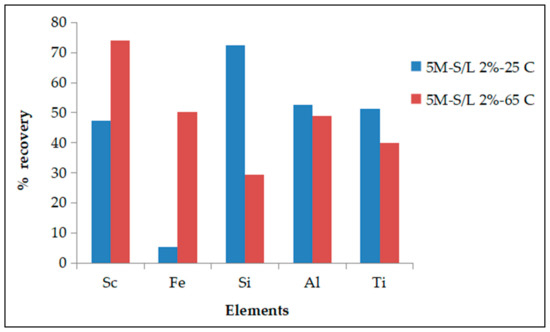
Figure 9.
Effect of process temperature.
3.6. Statistical Evaluation—Experimental Design
In order to assess the synergy effect of the major parameters of the leaching process, the Taguchi experimental design combined with statistical evaluation of the results was applied using Minitab 16 Statistical Software with 95% confidence level [38]. The Taguchi methodology uses orthogonal arrays to conduct a limited amount of experiments LA (BC). In this study, four different factors (acid molarity, solid/liquid ratio, temperature and time) with values of four different levels were investigated, in which the proper orthogonal array is LA (BC), divides the parameters in an equitable way L16 (44). The final amount of the conducted experiments is 16 instead of 256 that would be needed if the traditional design had been employed. The combinations among factors and levels are represented in the Table 2 below.

Table 2.
The 16 leaching experiments resulted from Taguchi experimental design.
The results of the experiments in Table 2 were evaluated statistically with the ANOVA method and the relative importance of each factor was determined. The response factors were Sc and Fe recovery and concentration. Fe was evaluated, since its high presence in the leachate impedes any following ion exchange and extraction processes for further purification of Sc. Thus, as a goal, the requirements set were Sc maximization and Fe minimization, aiming for a selective leaching process.
The extraction of metals was expressed as % metal recovery from the initial raw material, as well as the concentration of the metal in the leachate. The significance of each factor in Sc extraction (and Fe co-extraction) was determined for both cases, in which several differences can be mentioned (Table 3 and Table 4). As is shown in Table 3, for % Sc recovery, the most significant parameter is acid molarity (65.30%), followed by temperature (17.26%), solid to liquid ratio (16.56%) and time (0.88%). For Sc concentration the most important factor is solid to liquid ratio (56.10%), while the contribution of acid molarity is significantly lower (23.00%) followed by temperature (15.45%) and time (5.45%).

Table 3.
% factors contribution for Sc recovery and concentration.

Table 4.
% factors contribution for Fe recovery and concentration.
Regarding Sc recovery, the most crucial factor is by far the acid molarity, in contrast with Sc concentration, in which solid to liquid ratio has large contribution. In both cases, temperature has an important effect, while leaching time has the lowest contribution.
Furthermore, the significance of each parameter for % Fe recovery and concentration is presented in Table 4. Similar to Sc, molarity (41.31%) and solid to liquid ratio (45.41%) are the most significant factors for Fe recovery and concentration, respectively. However, in the case of Fe recovery acid molarity is followed by temperature (37.95%) and solid to liquid ratio (20.00%) while for Fe concentration molarity is the second important factor (30.77%) followed by the temperature (21.08%). Finally, time is the least important parameter in each case (0.74–2.74%).
The effects of each parameter associated with their levels on Sc and Fe recovery are presented in the plots shown in Figure 10 and Figure 11. Since higher S/N ratio corresponds to higher Sc recovery, the optimal conditions can be found either from the maximum values in each graph or from the optimized values calculated from the experimental design analysis. Concerning the data in Figure 10, the maximum point for acid molarity is reached at 7 M (36.79), while for S/L ratio, maximum S/N ratio is found at 2% (34.60). As for temperature, the maximum value of S/N appears at 85 °C (34.63), while time presents no effect, in agreement with the results of individual experiments (see Section 3.2). It was estimated that the optimum leaching parameters for Sc recovery are M = 7 M, S/L = 2%, Temp = 85 °C, time = 1 h, achieving a maximum Sc yield of 95%. For Fe, the identification of the optimum values of the factors was based on the model “smaller is better”, in which the values of the maximum point of each parameter corresponds to the level of minimum Fe recovery. Similarly, for the Sc evaluation mentioned before, and regarding the data in Figure 11, the optimum leaching parameters for Fe recovery minimization correspond to: M = 1 M, S/L = 20%, Temp = 25 °C and time = 1 h, resulting to a minimum Fe recovery of 1.2%.
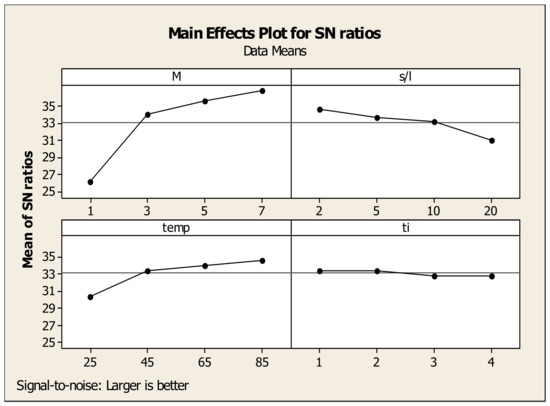
Figure 10.
Effects of each factor associated with their levels on the statistical performance of S/N ratio for Sc recovery.
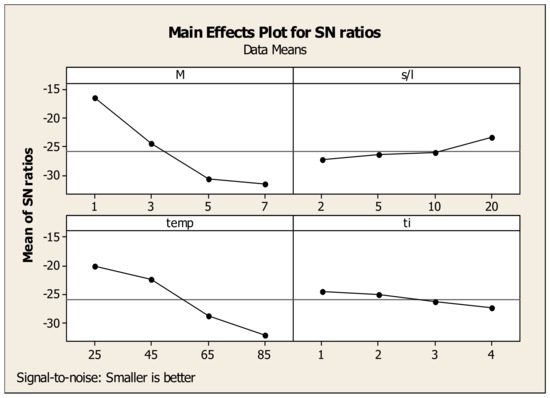
Figure 11.
Effects of each factor associated with their levels on the statistical performance of S/N ratio for Fe recovery.
The same approach was followed for the evaluation of optimum parameters for Sc and Fe concentration (Figure 12 and Figure 13). In the case of Sc (“larger is better” model), the maximum point for acid molarity (M) is 10.30, which corresponds to 7 M. As for the solid to liquid ratio, the highest value of S/N ratio is 11.87 for pulp density 20%. For the temperature, the maximum is observed at 11.14 for 85 °C, while for time the optimum value is found at 9.88 corresponding to 1 h since it does not affect further Sc concentration. According to the results, the optimized leaching parameters for maximum Sc concentration are: M = 7 M, S/L = 20%, Temp = 85 °C and time = 1 h achieving a maximum Sc concentration of 11.3 mg/L but a high Fe dissolution of about 51,500 mg/L resulting to a non-selective leaching. Concerning Fe concentration (“smaller is better” model), the graphs showed that the optimum values for acid molarity, solid to liquid ratio, temperature and time were reached at 1 M (−61.74), 2% (−62.54), 25 °C (−65.85) and 1 h (−70.09), respectively. Thus, the optimized leaching parameters for Fe minimum concentration are: M = 1 M, S/L = 2%, Temp = 25 °C and time = 1 h which lead to a minimum Fe concentration of 150 mg/L.
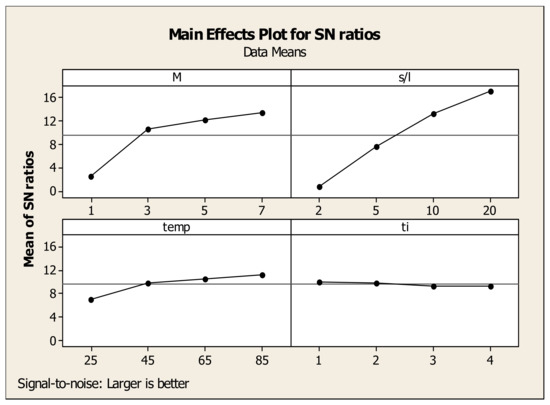
Figure 12.
Effects of each factor associated with their levels on the statistical performance of S/N ratio for Sc concentration.
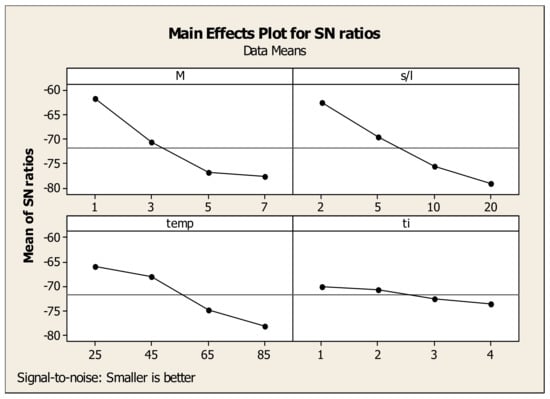
Figure 13.
Effects of each factor associated with their levels on the statistical performance of S/N ratio for Fe concentration.
For the verification of the statistical results from the Taguchi methodology, confirmation experiments under the optimum conditions have been performed. The predicted values as well as the measured values from the confirmation experiments are shown in the Table 5 below. As is shown, a good agreement was achieved with a deviation 1.42–5.51% confirming Taguchi values.

Table 5.
Comparison of Taguchi predictions and confirmation experiment results.
According to the results mentioned above, it is clear that there are no uniform conditions satisfying both requirements, Sc maximization and Fe minimization. Sc maximization is achieved by aggressive conditions where an almost total dissolution of Fe minerals, about 85%, occurs, leading to a non-selective leaching (with regard to iron) and it is in agreement with the case of sulfuric acid [38]. For this reason, taking into account the limitations of the method selectivity (low Fe leachability) and viability (low energy consumption, good Sc extraction, no gel formation), milder conditions were selected as optimum, more specifically: acid molarity = 5 M, solid/liquid ratio = 10%, process temperature = 25 °C, leaching time = 60 min. Under these conditions, a Sc recovery of 40% is obtained with low Fe leachability ~4%, and avoidance of high Si impurities.
Table 6 reports a comparison between the efficiency of different mineral acids under the optimum conditions selected in previous studies for selective Sc recovery with respect also to Sc concentration. Considering data, it is concluded that phosphoric acid leads to almost comparable recoveries for Sc and Fe with sulfuric and nitric acid. Nitric acid is proved as the most effective but its use is subject to restrictions due to nitrate’s serious environmental impact. For sulfuric and phosphoric acid, a similar recovery is observed for 1 M H2SO4 which is increased close to HNO3 performance by acid molarity 2 M with simultaneous increment of main elements content. Especially for Ti and Si this issue is important since high concentrations cause problems with the subsequent processes for further Sc isolation. Concerning HCl and aqua regia although they significantly enhance Sc recovery compared with other mineral acids, they are considered insufficient due to high Fe co-extraction up to 12 and 16%, respectively and their serious environmental impact [26].

Table 6.
Comparison of H3PO4 efficiency with other mineral acids for selective Sc recovery under optimum condition in each case and one stage leaching.
4. Assessment and Conclusions
Phosphoric acid was tested, as an alternative mineral acid, for Sc leaching from bauxite residue due to Sc selectivity of several phosphorus species. The effect of time, solid to liquid ratio, acid molarity and temperature was evaluated against concentration and recovery of Sc and recovery of iron. The direct leaching process was completed rapidly since, as confirmed, a duration of over 60 min does not favor further Sc leachability while increasing the process cost. Acid molarity and solid/liquid ratio were proven to be critical factors for process efficiency. Increasing acid molarity raises Sc leachability reaching a maximum at 5 M acid concentration. Further increment shows the contrary effect due to acid viscosity increment at higher molarities. Pulp density enhances Sc concentration while Sc recovery is adversely affected due to the incomplete neutralization of BR alkalinity and higher pH values. Final pH values close to zero have a significant positive effect on Sc recovery, similar to other, previously studied acids. Main elements such as Ti, Al and Fe show a trend similar to Sc versus acid molarity and solid/liquid ratio opposed to silicon leachability which is improved at weak acidic solutions. Temperature effect is considerably positive for Sc compared with other acids but spawns stronger Fe dissolution. Si, Al and Ti are adversely affected due to their hydrolysis in high temperatures.
The major influential factors for Sc maximization are molarity and S/L suggesting aggressive conditions while for Fe minimization mild conditions of temperature and molarity are required. Therefore, for a selective and viable Sc recovery by phosphoric acid leaching, the proposed optimum conditions are: 5 M, S/L 10%, 25 °C, 1 h resulting to 40% Sc and ~4% Fe recoveries with low impurities of Si, Al, Ti and prevention of gel formation. The results are comparable with other mineral acids. Future perspective is oriented towards the use of phosphoric acid for the quantitative silicon removal from bauxite residue prior to any further leaching process in order to avoid gel formation.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/met12020228/s1, Figure S1: Raman analysis of BR batch and Table S1: XRF analysis of BR batch used in this study.
Author Contributions
This work has been performed by the members of the Laboratory of Inorganic and Analytical Chemistry of School of Chemical Engineering (L.-A.T., G.P., T.L., K.O. and M.O.-P.) in collaboration with Laboratory of Mineralogy, Petrology and Economic Geology, School of Mining and Metallurgical Engineering (E.C.) of NTUA. G.P., L.-A.T. and T.L. performed the experiments; L.-A.T. and T.L. integrated ICP-OES measurements; L.-A.T. and G.P. evaluated the data and wrote the paper; M.O.-P., T.L., K.O. and E.C. reviewed the paper; M.O.-P. supervised the whole work. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the European Community’s Horizon 2020 Program (H2020/2014-2020), under Grant Agreement number 730105, EC/EASME (SCALE project).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taberaux, A.T.; Peterson, R.D. Chapter 2.5—Aluminium Production. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 839–917. ISBN 9780080969886. [Google Scholar] [CrossRef]
- Ezema, I.C. Chapter 9—Materials. In Sustainable Construction Technologies; Tam, V.W.Y., Le, K.N., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 237–262. ISBN 9780128117491. [Google Scholar]
- Rai, S.; Bahadure, S.; Chaddha, M.J.; Agnihotri, A. Disposal Practices and Utilization of Red Mud (Bauxite Residue): A Review in Indian Context and Abroad. J. Sustain. Metall. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A Review on Comprehensive Utilization of Red Mud and Prospect Analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in red mud utilization—A review. Miner. Process. Extr. Metall. Rev. 2004, 26, 1–29. [Google Scholar] [CrossRef]
- Yadav, H.; Garg, A. Study of Red Mud as an Alternative Building Material for Interlocking Block Manufacturing in Construction Industry. Int. J. Mater. Sci. Eng. 2018, 3, 295–300. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053–1063. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Huang, H.; Meng, K.; Hu, K.; Hu, P.; Wang, X.; Zhang, Z.; Meng, X. Novel glass ceramic foams materials based on red mud. Ceram. Int. 2014, 40, 6677–6683. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef]
- Orescanin, V.; Tibljas, D.; Valkovic, V. A study of coagulant production from red mud and its use for heavy metals removal. J. Trace Microprobe Tech. 2002, 20, 233–245. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, P.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Xu, Y.; Chen, Z.; Yin, F. Green process for supercritical water oxidation of sewage sludge with red mud as CO2 absorbent. J. Environ. Chem. Eng. 2016, 4, 3065–3074. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Catalytic applications of red mud, an aluminium industry waste: A review. Appl. Catal. B 2008, 81, 64–77. [Google Scholar] [CrossRef]
- Keller, V.; Stopić, S.; Xakalashe, B.; Ma, Y.; Ndlovu, S.; Mwewa, B.; Simate, G.S.; Friedrich, B. Effectiveness of Fly Ash and Red Mud as Strategies for Sustainable Acid Mine Drainage Management. Minerals 2020, 10, 707. [Google Scholar] [CrossRef]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E.; Markopoulos, C. Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek “red mud” residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process. J. Hazard. Mater. 2013, 254, 193–205. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Scandium-Scale Project. Available online: http://scale-project.eu/scandium/ (accessed on 15 November 2021).
- Dorin, T.; Ramajayam, M.; Vahid, A.; Langan, T. Chapter 12—Aluminium Scandium Alloys. In Woodhead Publishing Series in Metals and Surface Engineering, Fundamentals of Aluminium Metallurgy; Lumley, R.N., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 439–494. [Google Scholar]
- Scandium Aluminium Alloy Al-Sc Alloy. Available online: htttps://www.nanotrun.com/scandium-aluminum-alloy-al-sc-alloy-p00327p1.html (accessed on 18 November 2021).
- Tagawa, Y.; Ohba, F.; Takei, C.; Ihara, M. Carbon Deposition and Power Density in Rechargeable Direct Carbon Fuel Cells with Gadolinium-doped Ceria and Scandium-stabilized Zirconia. ECS Trans. 2009, 25, 1133. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials. Third List of Critical Raw Materials for the EU of 2017. Available online: https://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 20 November 2021).
- Aluminium of Greece. Available online: https://www.alhellas.com/ (accessed on 18 November 2021).
- Ochsenkühn-Petropoulou, M.; Lyberopulu, T.; Parissakis, G. Direct determination of lanthanides yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- Ochsenkuehn-Petropoulou, M.; Tsakanika, L.-A.; Lymperopoulou, T.; Ochsenkuehn, K.-M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals 2018, 8, 915. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Metall. 2015, 2, 28–37. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Developing New Process for Selective Extraction of Rare Earth Elements from Bauxite Residue Based on Functionalized Ionic Liquids. In Light Metals 2018; TMS 2018; The Minerals, Metals & Materials Series; Martin, O., Ed.; Springer: Cham, Switzerland, 2018; pp. 149–156. [Google Scholar] [CrossRef]
- Bonomi, C.; Alexandri, A.; Vind, J.; Panagiotopoulou, A.; Tsakiridis, P.; Panias, D. Scandium and titanium recovery from bauxite residue by direct leaching with a Brønsted acidic ionic liquid. Metals 2018, 8, 834. [Google Scholar] [CrossRef]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kiskira, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Pavlopoulos, C.; Papadopoulou, K.; Ochsenkühn, K.-M.; Lyberatos, G.; Ochsenkühn-Petropoulou, M. Study of Microbial Cultures for the Bioleaching of Scandium from Alumina Industry By-Products. Metals 2021, 11, 951. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Ochsenkühn, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropoulou, M.; Hatzilyberis, K.; Mendrinos, L.; Salmas, C. Pilot Plant Investigation of the Leaching Process for the Recovery of Scandium from Red Mud. Ind. Eng. Chem. Res. 2002, 41, 5794–5801. Available online: https://pubs.acs.org/doi/abs/10.1021/ie011047b (accessed on 20 November 2021). [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Hatzilyberis, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Ochsenkühn, K.-M.; Georgiou, P.; Defteraios, N.; Tsopelas, F.; Ochsenkühn-Petropoulou, M. Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Mineral Acids. Minerals 2018, 8, 79. [Google Scholar] [CrossRef]
- Lymperopoulou, T.; Georgiou, P.; Tsakanika, L.-A.; Hatzilyberis, K.; Ochsenkuehn-Petropoulou, M. Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology. Minerals 2019, 9, 236. [Google Scholar] [CrossRef]
- Petrakova, O.V.; Panov, A.V.; Gorbachev, S.N.; Klimentenok, G.N.; Perestoronin, A.V.; Vishnyakov, S.E.; Anashkin, V.S. Improved efficiency of red mud processing through scandium oxide recovery. In Light Metals 2015; Hyland, M., Ed.; Wiley: New York, NY, USA, 2015; pp. 91–96. [Google Scholar] [CrossRef]
- Ferizoglu, E.; Kaya, Ş.; Topkaya, Y.A. Solvent extraction behaviour of scandium from lateritic nickel-cobalt ores using different organic reagents. Physicochem. Probl. Miner. Process. 2018, 54, 538–545. [Google Scholar] [CrossRef]
- Ye, Q.; Li, G.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Solvent extraction behavior of metal ions and selective separation Sc3+ in phosphoric acid medium using P204. Sep. Purif. Technol. 2019, 209, 175–181. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, C.Y. Separation and purification of scandium by solvent extraction and related technologies: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1237–1246. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Zlobina, E.; Ismailova, A.; Tassibekov, K. Extractive Separation of Scandium from Rare Earth Elements. MATEC Web Conf. 2017, 96, 00001. [Google Scholar] [CrossRef][Green Version]
- Junior, A.B.B.; Espinosa, D.C.R.; Tenório, J.A.S. Selective separation of Sc(III) and Zr(IV) from the leaching of bauxite residue using trialkylphosphine acids, tertiary amine, tri-butyl phosphate and their mixtures. Sep. Purif. Technol. 2021, 279, 119798. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Parissakis, G. Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal. Chim. Acta 1995, 315, 231–237. [Google Scholar] [CrossRef]
- Korovin, V.; Shestak, Y.; Pogorelov, Y. Scandium extraction by neutral organo-phosphorus compounds supported on a porous carrier. Hydrometallurgy 1999, 52, 1–8. [Google Scholar] [CrossRef]
- Korovin, V.; Shestak, Y. Scandium extraction from hydrochloric acid media by Levextrel-type resins containing di-isooctyl methyl phosphate. Hydrometallurgy 2009, 95, 346–349. [Google Scholar] [CrossRef]
- Wakui, Y.; Matsunaga, H.; Suzuki, T.M. Selective recovery of trace scandium from acid aqueous with (2-ethylhexyl hydrogen 2-ethylhexylphosphonate)-impregnated resin. Anal. Sci. 1989, 5, 189–193. [Google Scholar] [CrossRef][Green Version]
- Korovin, V.; Shestak, Y.; Pogorelov, Y. Comparison of scandium recovery mechanisms by phoshporus-containing sorbents, solvent extractants and extractants supported on porous carrier. In Scandium: Compounds, Productions and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 77–100. [Google Scholar]
- Li, G.; Ye, Q.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Extraction of scandium from scandium-rich material derived from bauxite ore residues. Hydrometallurgy 2018, 176, 62–68. [Google Scholar] [CrossRef]
- Deng, B.; Li, G.; Luo, J.; Ye, Q.; Liu, M.; Rao, M.; Jiang, T.; Bauman, L.; Zhao, B. Selectively leaching the iron-removed bauxite residues with phosphoric acid for enrichment of rare earth elements. Sep. Purif. Technol. 2019, 227, 115714. [Google Scholar] [CrossRef]
- Wang, L.; Long, Z.; Huang, X.; Yu, Y.; Cui, D.; Zhang, G. Recovery of rare earths from wet-process phosphoric acid. Hydrometallurgy 2010, 101, 41–47. [Google Scholar] [CrossRef]
- Tsakanika, L.-A.; Lymperopoulou, T.; Ochsenkuhn Petropoulou, M. Investigation of Sc leaching from bauxite residue using phosphoric acid. In Proceedings of the International Conference Instrumental Methods of Analysis Modern Trends and Applications-IMA 2017, Heraklion, Greece, 17–21 September 2017; p. 270. [Google Scholar]
- Lyberopulu, T. Determination and Recovery of Rare Earths from Bauxites and Red Mud. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 1996. [Google Scholar]
- Tsakanika, L.-A. Separation and Recovery of Lanthanides from Red Mud by Use of Selective Extraction and Chromatographic Techniques. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
