Effect of Cooling Rate and Sulfur Content on Sulfide Inclusions in Invar Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Procedures
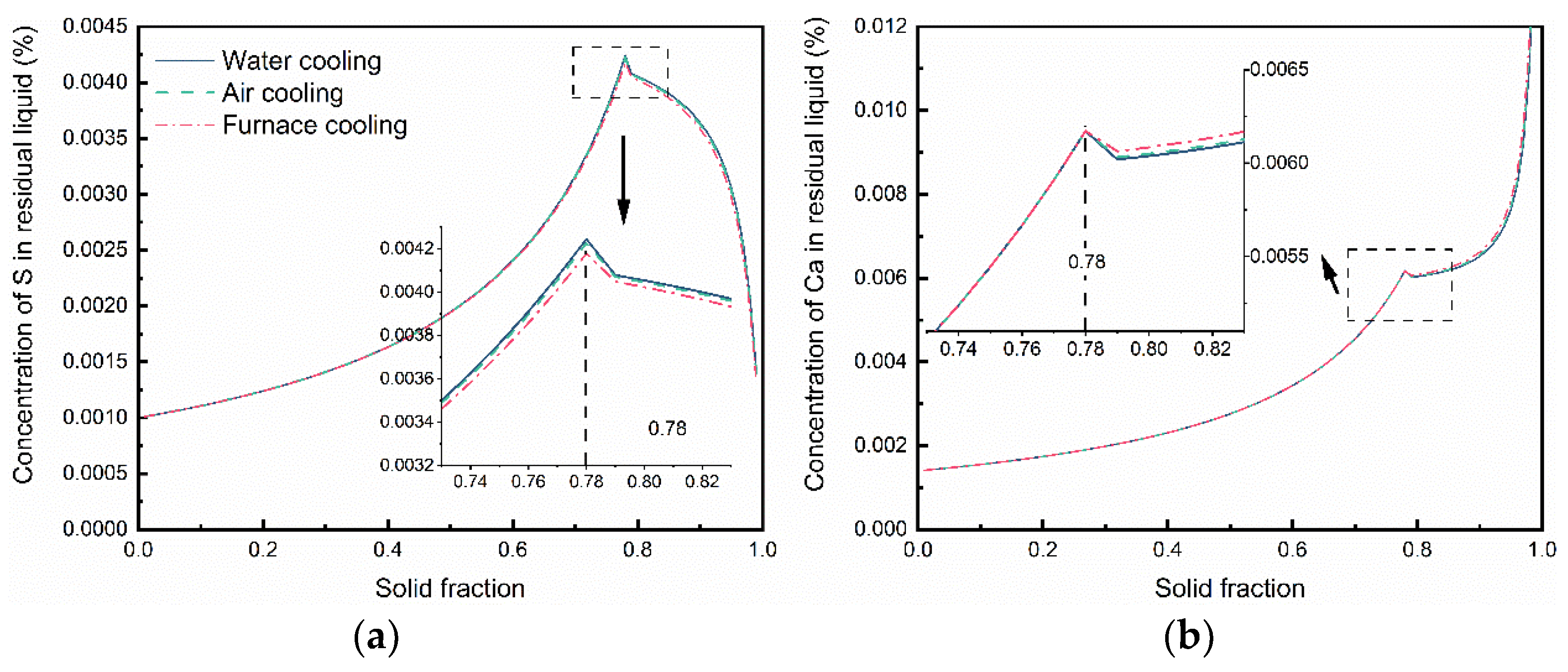
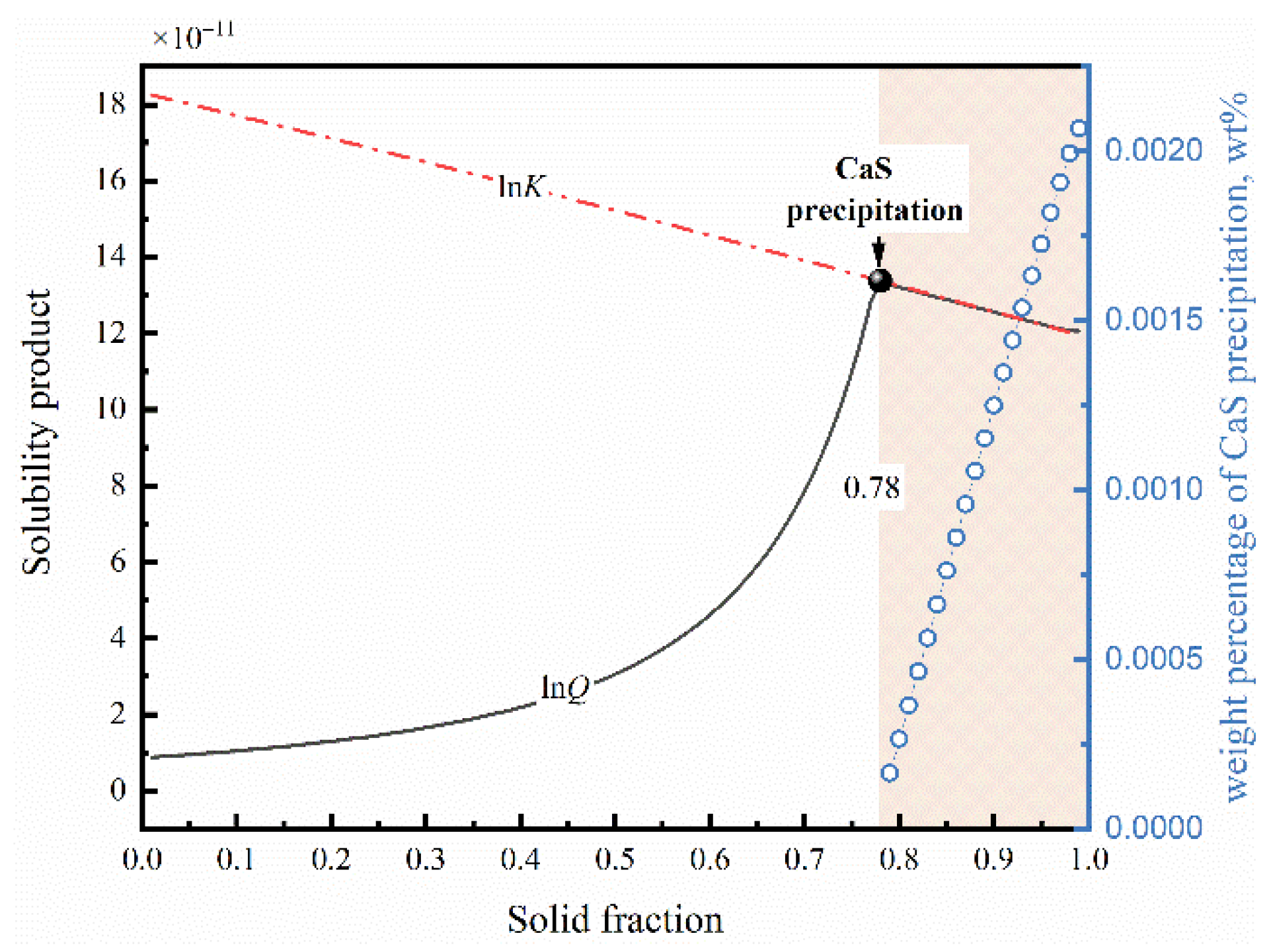
2.2. Thermodynamics of Sulfide Precipitation Process
3. Results and Discussion
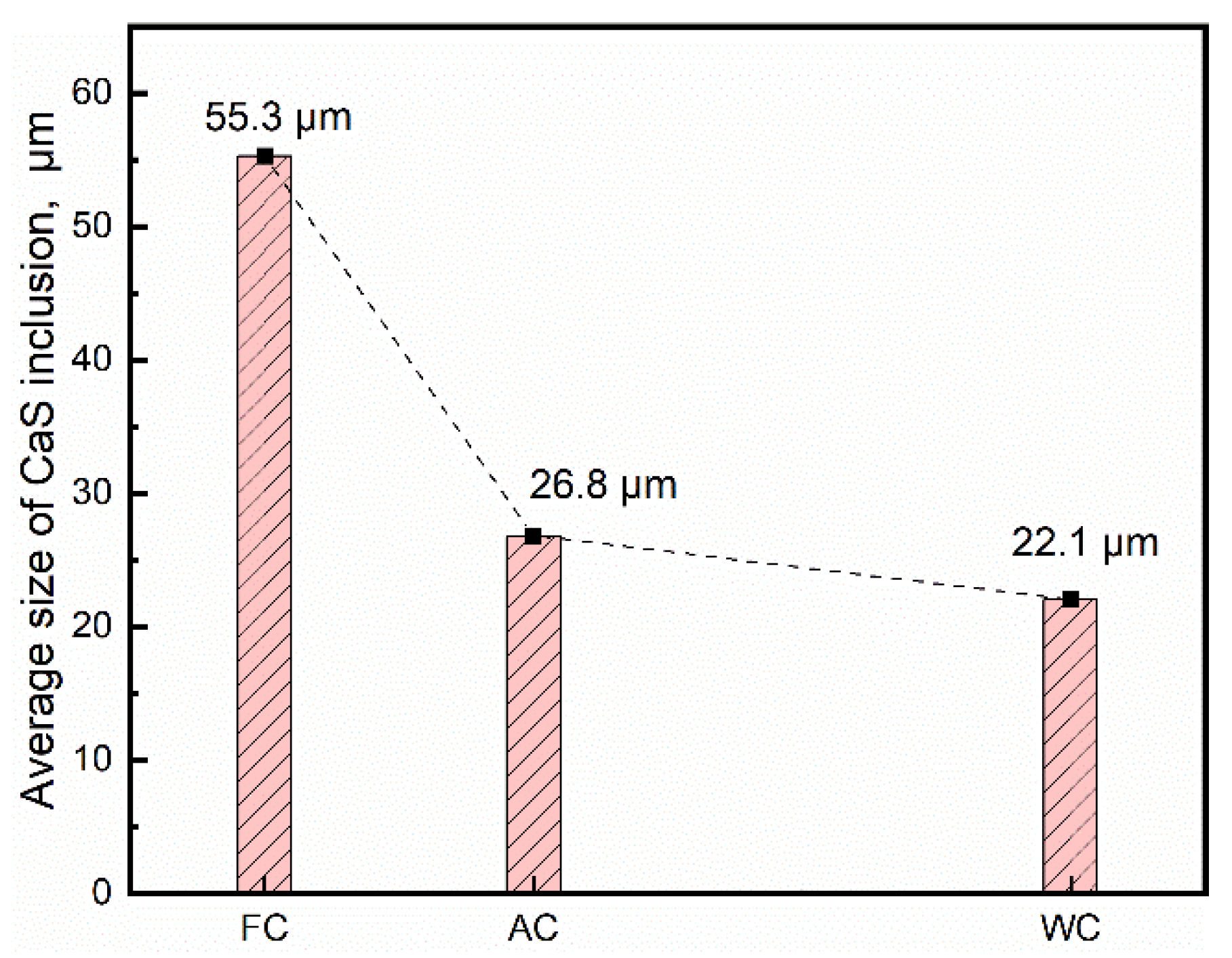
3.1. Effect of Cooling Rate on the Size of CaS Inclusion
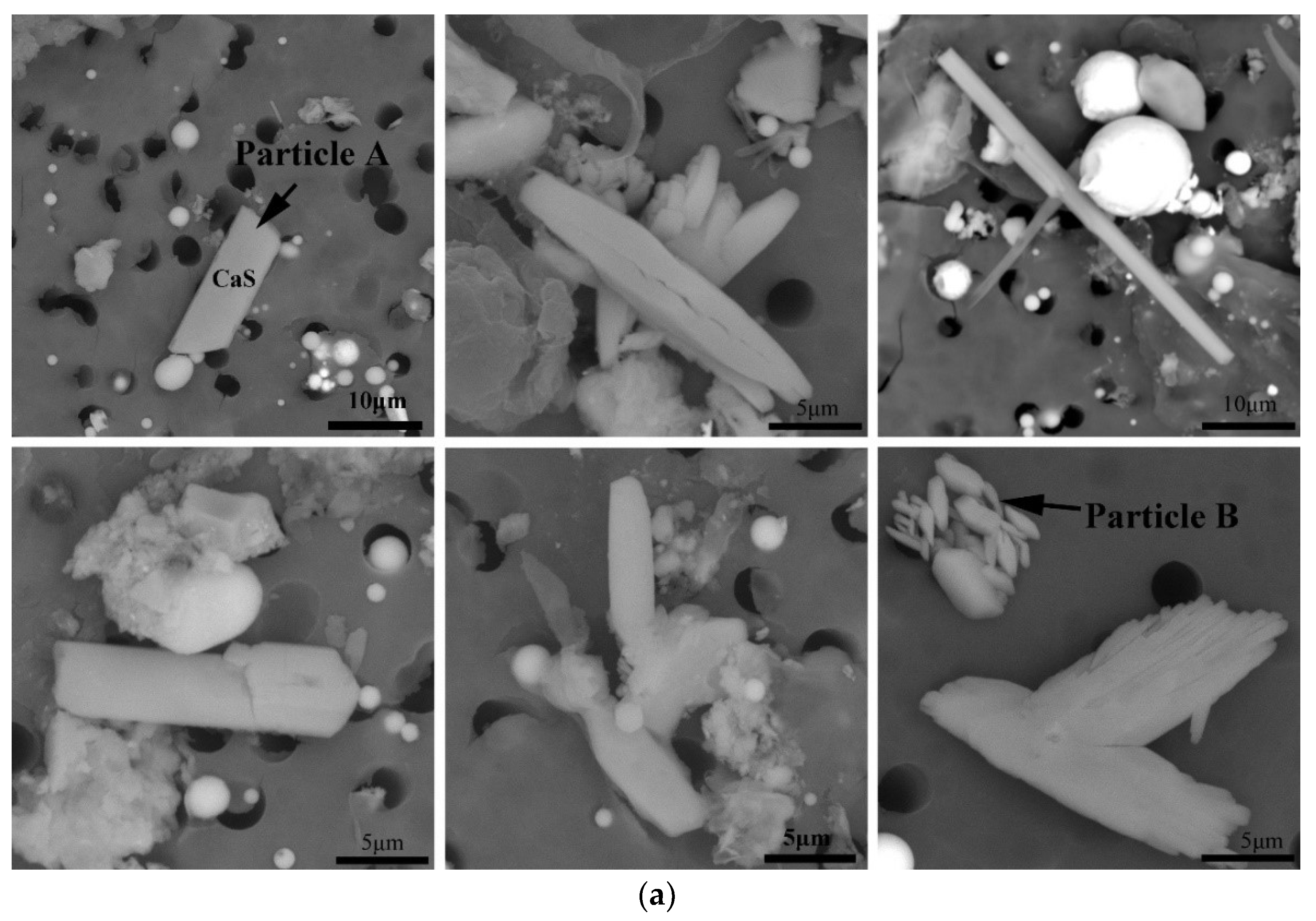
3.2. Effect of Cooling Rate on Morphology of CaS Inclusion
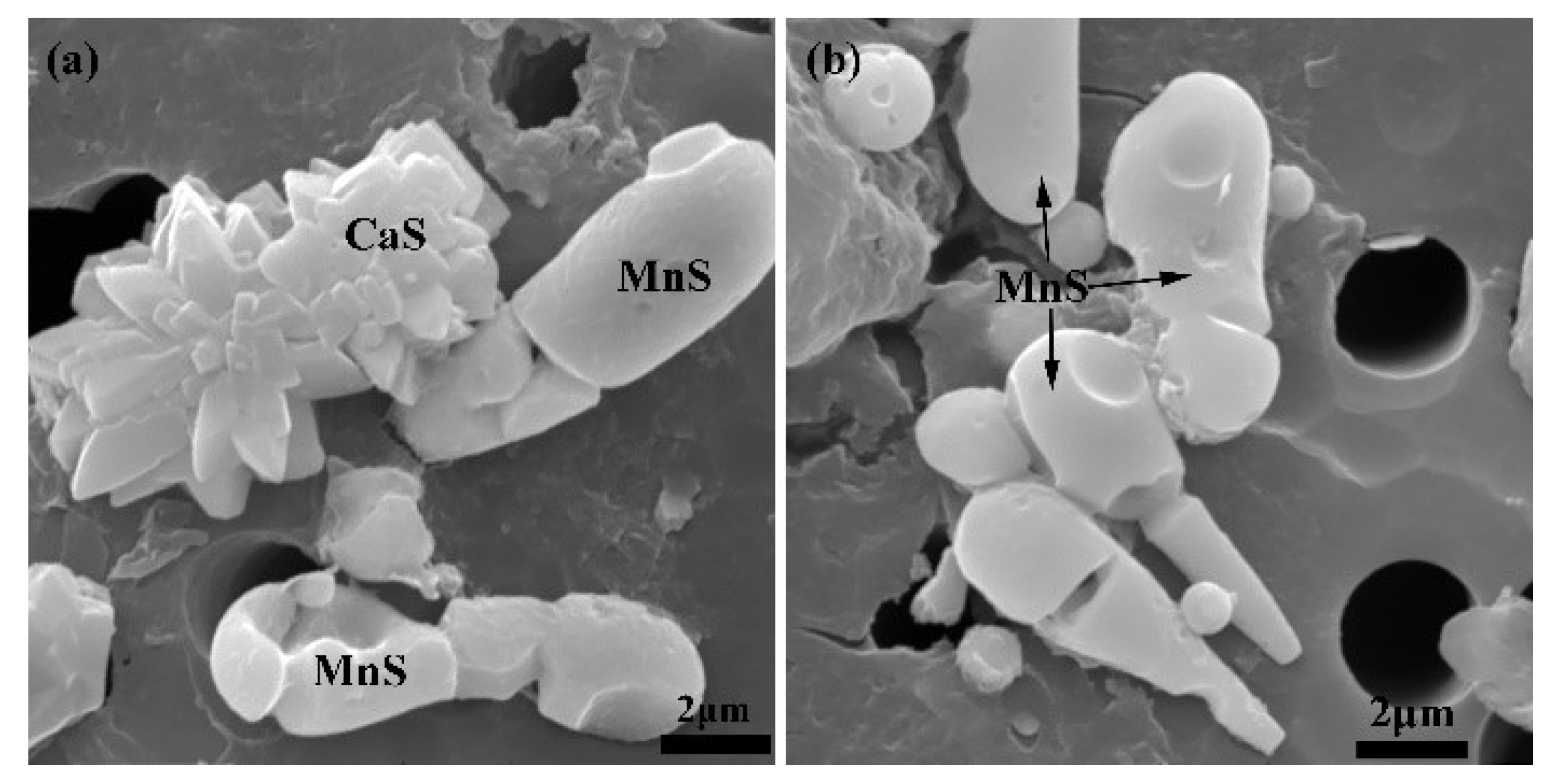
3.3. Effect of S Content on the Type of Sulfide Inclusion
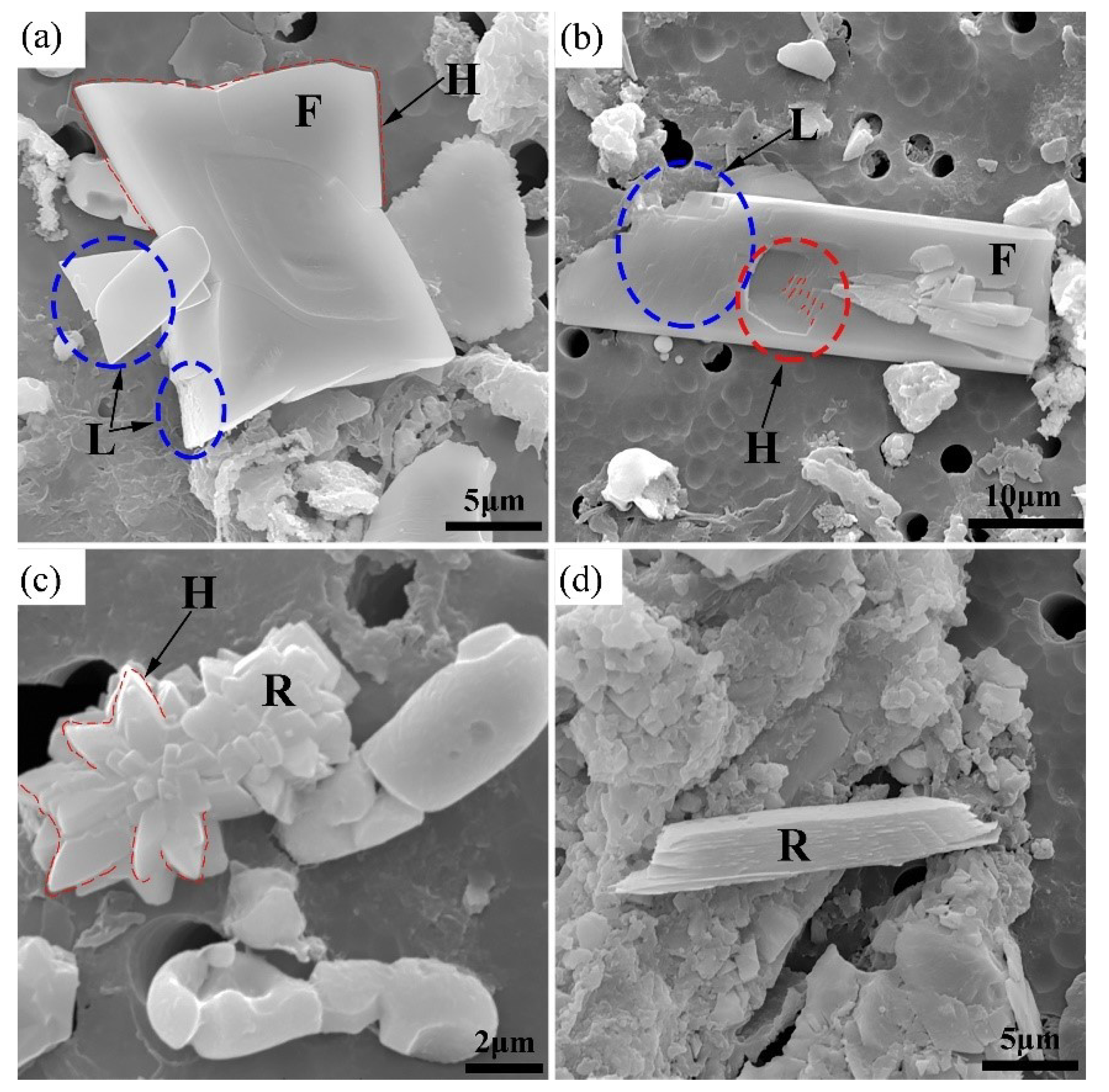
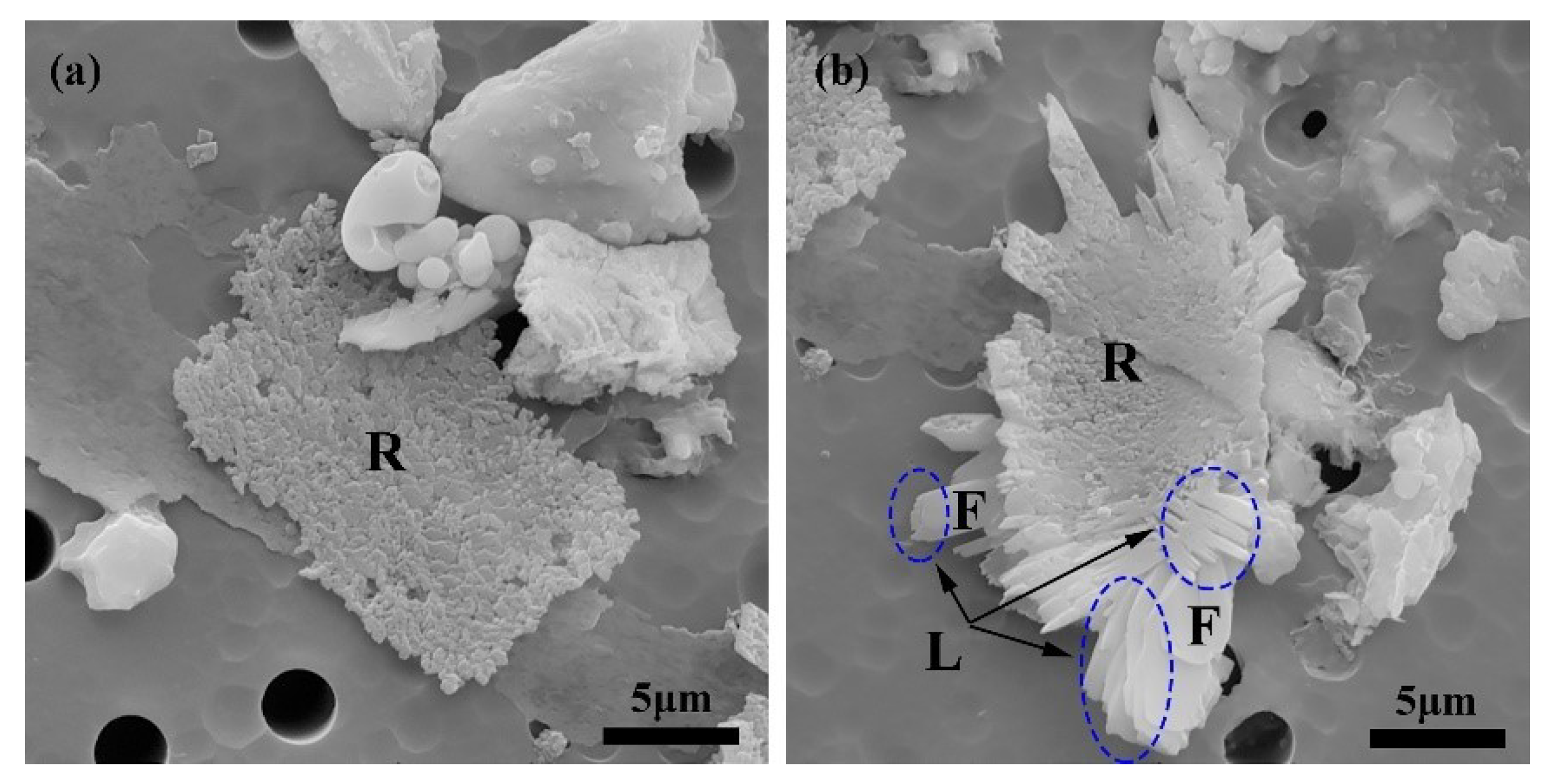
3.4. Effect of S Content on the Morphology of CaS Inclusion
4. Conclusions
- (1)
- In the case of low S content, the cooling rate has no distinct effect on the microsegregation of solutes in the front of the solid–liquid interface, whereas the size and morphology of sulfide inclusions were susceptible to the cooling rate. The smaller the cooling rate, the longer the local solidification time, which allows the precipitated CaS inclusions to grow up, leading to a larger size of CaS inclusions: 22.1 μm with 43.1 K/s and 55.3 μm with 3.1 K/s. The thermodynamic calculations confirmed that CaS is formed during solidification. The later precipitated CaS grows cooperatively with the earlier precipitated Fe matrix, and the morphology of the CaS inclusions is affected by the shape of the matrix. The morphology of CaS inclusion is transformed as well: from round to flat gradually, with the decreasing trends of the cross-section aspect ratio.
- (2)
- In this study, CaS is the preferred precipitated sulfide over MnS. When [S] < [Ca], only CaS precipitates in steel. When [S] > [Ca], where the Ca in the steel cannot completely react off of S, MnS begins to precipitate.
- (3)
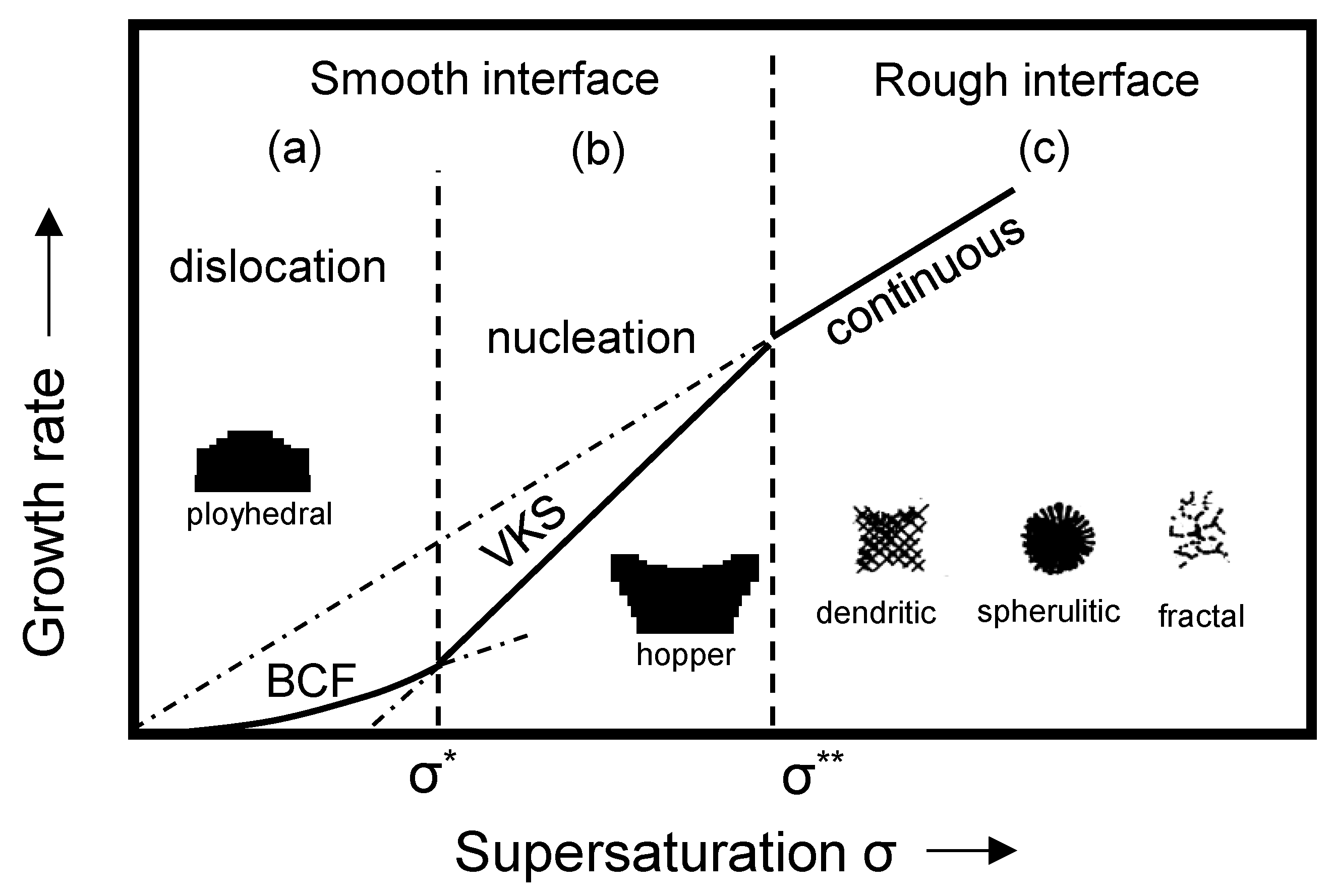
- The various initial content of S diversifies the supersaturation during the formation of CaS inclusions. These differences causes a variety of sulfide precipitations, but also affect the morphological characteristics of precipitated CaS. The increase in supersaturation shapes the morphology from a polyhedron and funnel to a dendritic shape, and roughens the CaS inclusion surfaces.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| T | Temperature(K) |
| TL | Liquidus temperature |
| TS | Solidus temperature |
| T0 | Melting point of pure iron(1809 K) |
| KCaS | The equilibrium constant of Equation (1) |
| a[CaS] | The activity of pure CaS and considered as 1 |
| [%i] | The concentration of solute element i (wt.%) |
| fiT | The activity coefficient of a given solute element at temperature T |
| fi1873K | Activity coefficient at temperature 1873 K |
| TS-L | The temperature at the solid–liquid interface where the solid and liquid coexist in a dendrite |
| fs | Solid fraction |
| Ci:0 | Initial solute concentration |
| Ci,L | Solute concentration at the solid–liquid interface |
| Ci,S-L | Solute concentration at solid–liquid interface |
| ki | Equilibrium partition coefficient |
| α | Back-diffusion parameter |
| DS,i | Diffusion coefficient of solute in the solid phase |
| tf | Local solidification time in seconds |
| λ | Secondary dendrite arm spacing(cm) |
| △CL,i | Consumption of a given solute element caused by the formation of CaS |
| mCaS | The weight percentage of precipitated CaS inclusions. |
| MCa | The atomic weight of Ca |
| MS | The atomic weight of Ca |
References
- Yokoyama, T.; Eguchi, K. Anharmonicity and quantum effects in thermal expansion of an Invar alloy. Phys. Rev. Lett. 2011, 107, 065901. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, T.; Yamamoto, T.; Nakamura, T. Thermal expansions and mechanical properties of electrodeposited Fe–Ni alloys in the Invar composition range. Electrochim. Acta 2016, 205, 178–187. [Google Scholar] [CrossRef]
- Van Schilfgaarde, M.; Abrikosov, I.; Johansson, B. Origin of the Invar effect in iron-nickel alloys. Nature 1999, 400, 46–49. [Google Scholar] [CrossRef]
- Matsushita, M.; Endo, S.; Miura, K.; Ono, F. Pressure induced magnetic phase transition in Fe-Ni Invar alloy. J. Magn. Magn. Mater. 2003, 265, 352–356. [Google Scholar] [CrossRef]
- Vinogradov, A.; Hashimoto, S.; Kopylov, V. Enhanced strength and fatigue life of ultra-fine grain Fe-36Ni Invar alloy. Mater. Sci. Eng. A 2003, 355, 277–285. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.F.; Zhang, L.; Meng, L. Textures of high-strength and low-expansion Fe-Ni alloy wires during cold-drawing processes. Int. J. Miner. Metall. Mater. 2009, 16, 667–671. [Google Scholar]
- Zhao, Y.; Sato, Y.S.; Kokawa, H.; Wu, A. Microstructure and properties of friction stir welded high strength Fe-36 wt% Ni alloy. Mater. Sci. Eng. A 2011, 528, 7768–7773. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Z.; Wang, G.; Sun, X.; Li, J.; Xue, F.; Peng, H.; Zhang, Y. Effect of aging on microstructures and properties of Mo-alloyed Fe-36Ni Invar alloy. Mater. Sci. Eng. A 2016, 654, 107–112. [Google Scholar] [CrossRef]
- Maciejewski, J. The effects of sulfide inclusions on mechanical properties and failures of steel components. J. Fail. Anal. Prev. 2015, 15, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Raghupathy, V.; Srinivasan, V.; Krishnan, H.; Chandrasekharaiah, M.N. The effect of sulphide inclusions on fracture toughness and fatigue crack growth in 12 wt% Cr steels. J. Mater. Sci. 1982, 17, 2112–2126. [Google Scholar] [CrossRef]
- Ray, G.; Jarman, R.; Thomas, J. Some aspects of crack initiation in mild steel under corrosion fatigue condition. J. Mater. Sci. 1994, 29, 47–53. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The effect of different non-metallic inclusions on the machinability of steels. Materials 2015, 8, 751–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.J.; Wang, X.H.; Ji, C.X.; Li, H.B.; Cui, Y.; Zhu, G.S. Morphology, size and distribution of MnS inclusions in non-quenched and tempered steel during heat treatment. Int. J. Miner. Metall. Mater. 2015, 22, 483–491. [Google Scholar] [CrossRef]
- Kuniya, J.; Anzai, H.; Masaoka, I. Effect of MnS inclusions on stress corrosion cracking in low-alloy steels. Corrosion 1992, 48, 419–425. [Google Scholar] [CrossRef]
- Guo, Y.T.; He, S.P.; Chen, G.J.; Wang, Q. Thermodynamics of complex sulfide inclusion formation in Ca-treated Al-killed structural steel. Metall. Mater. Trans. B 2016, 47, 2549–2557. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Formation mechanism of oxide-sulfide complex inclusions in high-sulfur-containing steel melts. Metall. Mater. Trans. B 2018, 49, 311–324. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K.; Bernhard, C. Acicular ferrite formation and its influencing factors—A review. J. Mater. Sci. Res. 2017, 6, 24–43. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A. Mathematical model for prediction of composition of inclusions formed during solidification of liquid steel. ISIJ Int. 2009, 49, 1819–1827. [Google Scholar]
- Watanabe, K.; Narita, K. Report of the Activity of Joint Research Committee on Theory of Models and Scale-up Method/Isolation and Determination of Sulphides in Steel. Tetsu Hagane 1987, 73, 64–83. [Google Scholar] [CrossRef] [Green Version]
- Karasev, A.; Suito, H. Quantitative evaluation of inclusion in deoxidation of Fe-10 mass pct Ni alloy with Si, Ti, Al, Zr, and Ce. Metall. Mater. Trans. B 1999, 30, 249–257. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Characteristics of particle size distribution of deoxidation products with Mg, Zr, Al, Ca, Si/Mn and Mg/Al in Fe–10mass% Ni alloy. ISIJ Int. 2006, 46, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Hinotani, S.; Endo, J.; Takayama, T.; Mizui, N.; Inokuma, Y. Isolation and determination of sulfides in Ti-bearing ultra low carbon steels. ISIJ Int. 1994, 34, 17–23. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Duan, H.; Zhang, Y.; Luo, Y.; Conejo, A.N. Extraction, Thermodynamic Analysis, and Precipitation Mechanism of MnS-TiN Complex Inclusions in Low-Sulfur Steels. Met. Mater. Trans. A 2016, 47, 3015–3025. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Yang, W.; Wang, Y.; Liu, Y.; Dong, Y. Characterization of the Three-Dimensional Morphology and Formation Mechanism of Inclusions in Linepipe Steels. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2017, 48, 701–712. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Jönsson, P.G. An Investigation of Non-Metallic Inclusions in Different Ferroalloys using Electrolytic Extraction. Metals 2019, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, L.; Yang, W.; Ren, Y.; Conejo, A.N. Precipitation of nitrides in non-oriented silicon steel. Ironmak. Steelmak. Process. Prod. Appl. 2019, 46, 359–367. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, W.; Ren, Q.; Hu, Z.; Li, M.; Zhang, L. Evolution of Non-metallic Inclusions and Precipitates in Oriented Silicon Steel. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2018, 49, 926–932. [Google Scholar] [CrossRef]
- Sims, C.; Dahle, F. Effect of aluminum on the properties of medium carbon cast steel. Trans. Am. Foundrym. Soc. 1938, 46, 65–132. [Google Scholar]
- Sims, C.; Sailer, H.; Boulger, F. Effects of various deoxidizers on the structures of sulphide inclusions. Trans 1949, 1949, 233–248. [Google Scholar]
- Ji, S.; Zhang, L.; Wang, X. Effect of Magnesium on Inclusions in a High Sulfur Steel. Metall. Mater. Trans. B 2022, 53, 848–863. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, Z.; Tian, Q.; Cao, C.W.; Shen, P.; Fu, J.X. Application of fractal theory to study morphology of manganese sulfide inclusion in resulfurized free-cutting steels. J. Iron Steel Res. Int. 2022. [Google Scholar] [CrossRef]
- Valdez, M.E.; Wang, Y.; Sridhar, S. In-Situ Observation of the Formation of MnS during Solidification of High Sulphur Steels. Steel Res. Int. 2004, 75, 247–256. [Google Scholar] [CrossRef]
- Oikawa, K.; Sumi, S.I.; Ishida, K. Morphology control of MnS inclusions in steel during solidification by the addition of Ti and Al. Z. Met. Mater. Res. Adv. Tech. 1999, 90, 13–17. [Google Scholar]
- Ito, Y.; Masumitsu, N.; Matsubara, K. Formation of manganese sulfide in steel. Trans. Iron Steel Inst. Jpn. 1981, 21, 477–484. [Google Scholar] [CrossRef]
- Mohla, P.P.; Beech, J. Effect of cooling rate on morphology of sulfide inclusions. J. Iron Steel Inst. 1969, 207, 177. [Google Scholar]
- Abushosha, R.; Ayyad, S.; Mintz, B. Influence of cooling rate and MnS inclusions on hot ductility of steels. Mater. Sci. Technol. 1998, 14, 227–235. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, F.M.; Li, C.R.; Yang, Z.B.; Meng, Q.Y.; Tao, S.F. Effects of cooling rate and Al on MnS formation in medium-carbon non-quenched and tempered steels. Int. J. Miner. Metall. Mater. 2015, 22, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, C.; Zhang, L. Effects of cooling rate and isothermal holding on the characteristics of MnS particles in high-carbon heavy rail steels. Metall. Res. Technol. 2020, 117, 110. [Google Scholar] [CrossRef]
- Sigworth, G.; Elliott, J.F. The thermodynamics of liquid dilute iron alloys. Met. Sci. 1974, 8, 298–310. [Google Scholar] [CrossRef]
- Fahlman, B.D. What is “materials chemistry”. In Materials Chemistry; Springer: New York, NY, USA, 2018; pp. 1–21. [Google Scholar]
- Clyne, T.; Kurz, W. Solute redistribution during solidification with rapid solid state diffusion. Metall. Trans. A 1981, 12, 965–971. [Google Scholar] [CrossRef]
- Dekkers, R.; Blanpain, B.; Wollants, P. Crystal growth in liquid steel during secondary metallurgy. Metall. Mater. Trans. B 2003, 34, 161–171. [Google Scholar] [CrossRef]
- Sunagawa, I. Morphology of minerals, in Morphology of Crystals, part, B. Terra. Sci. Pub. 1988, 1988, 511–587. [Google Scholar]
- Sunagawa, I. Growth and morphology of crystals. Forma 1999, 14, 147–166. [Google Scholar]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1951, 243, 299–358. [Google Scholar]
- Ghez, R. Coverage effects on the BCF surface diffusion model. J. Cryst. Growth 1974, 22, 333–334. [Google Scholar] [CrossRef]
- Faure, F.; Schiano, P.; Trolliard, G.; Nicollet, C.; Soulestin, B. Textural evolution of polyhedral olivine experiencing rapid cooling rates. Contrib. Mineral. Petrol. 2007, 153, 405–416. [Google Scholar] [CrossRef]


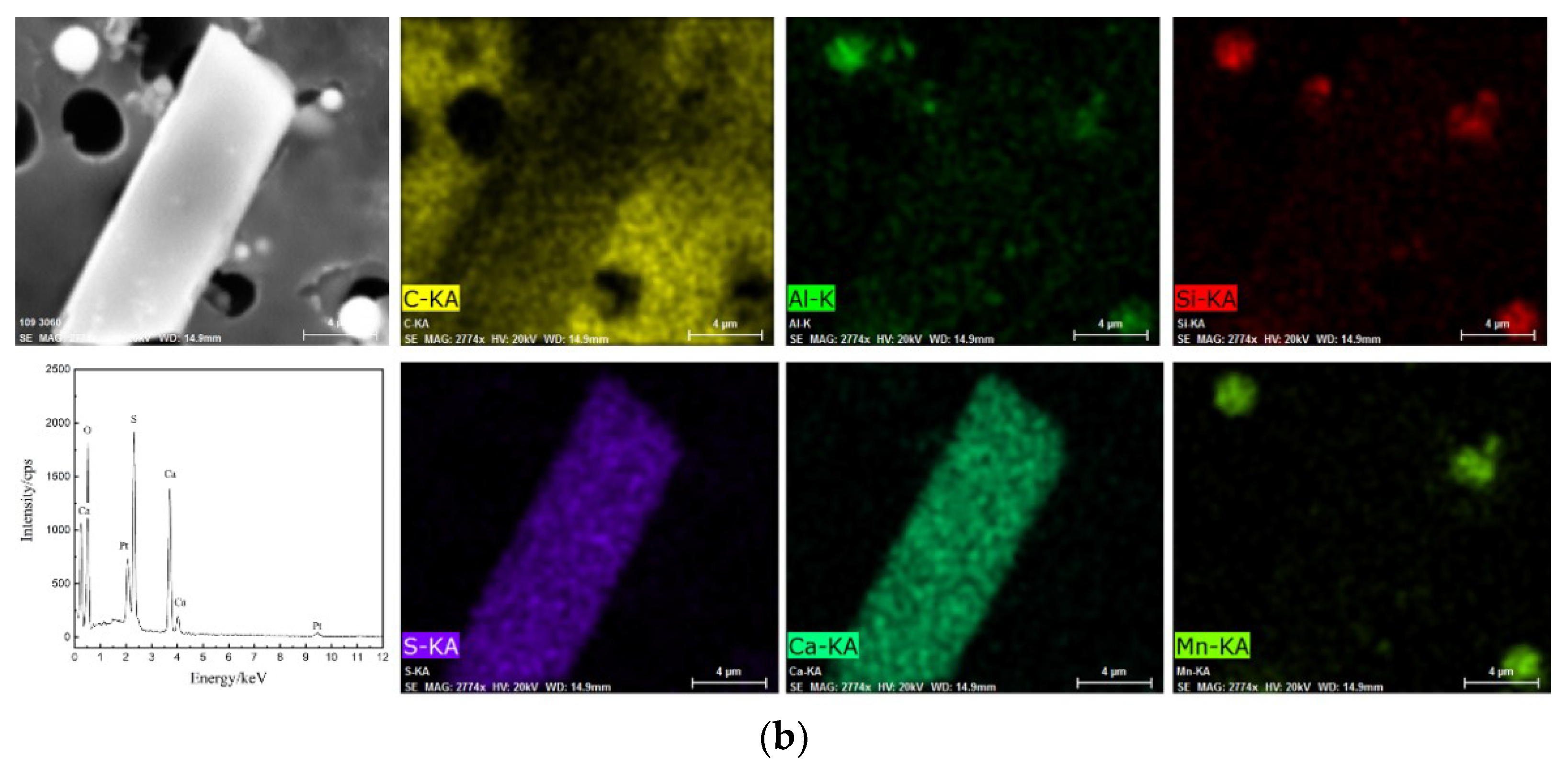
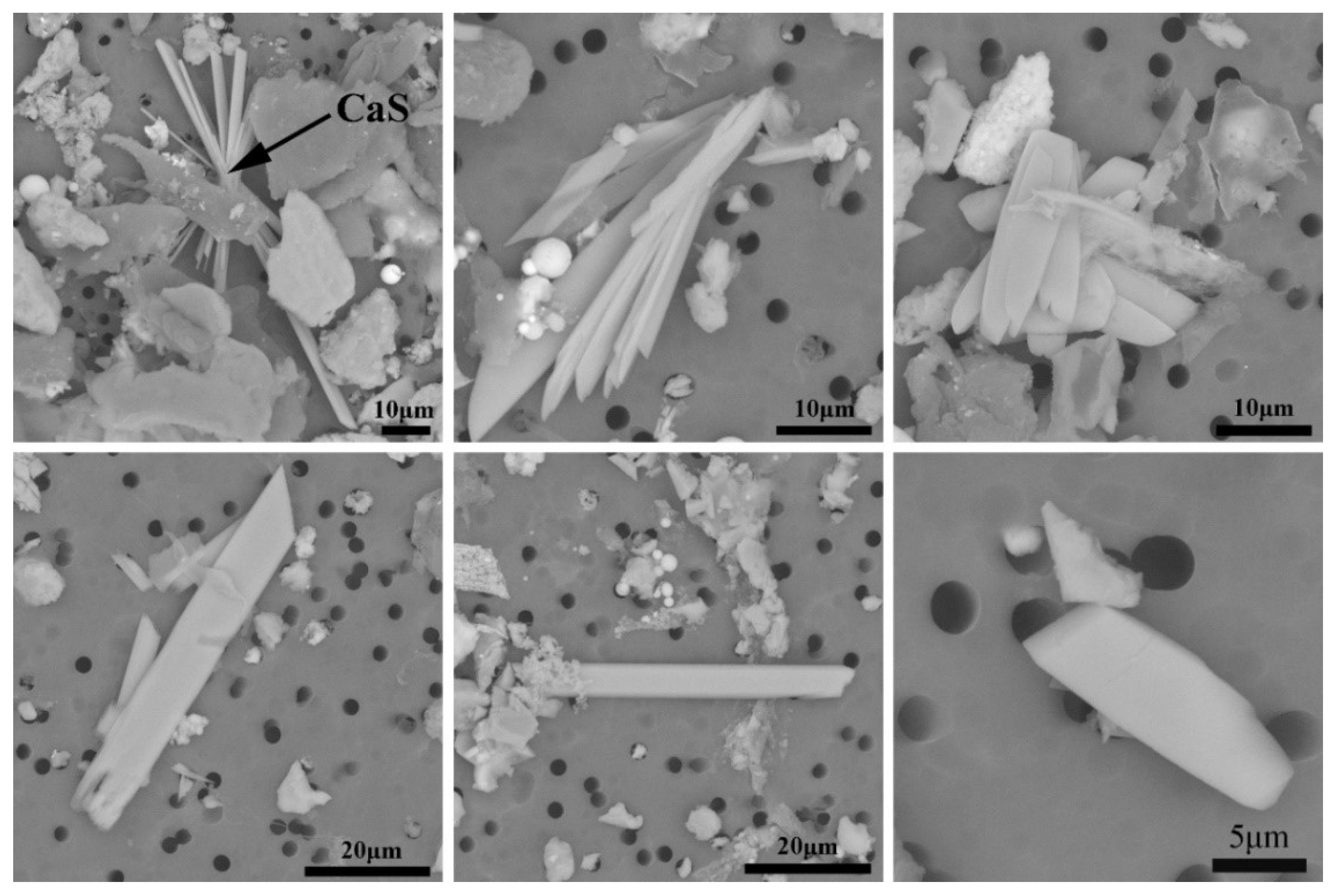
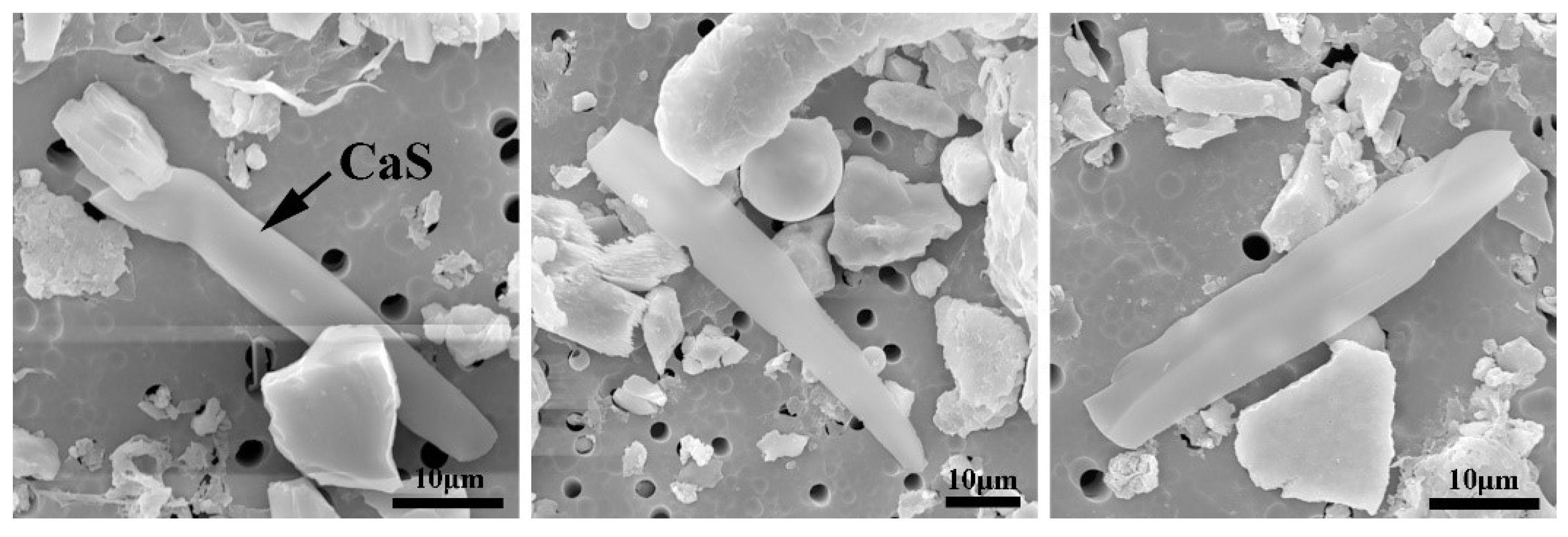
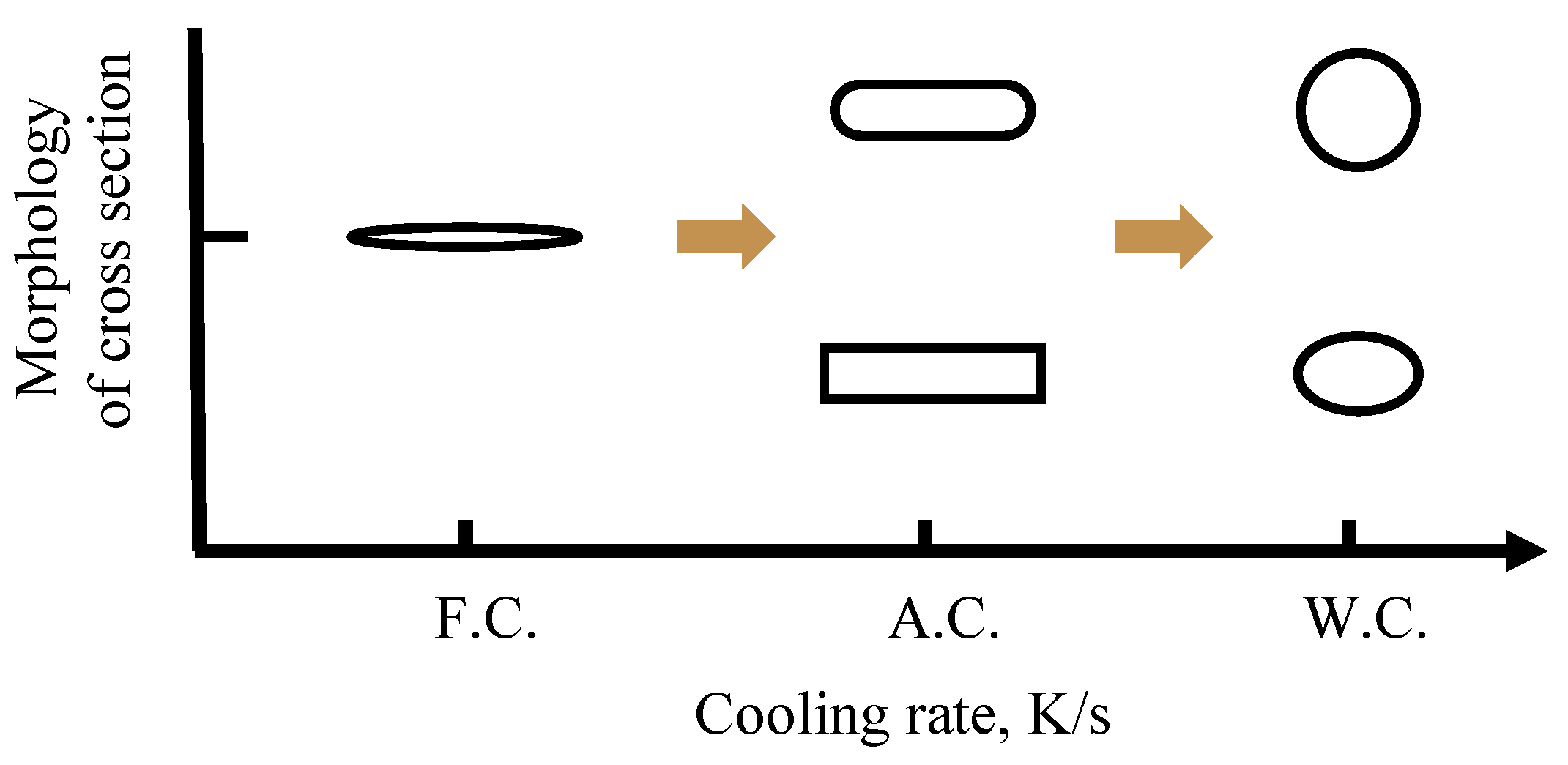

| C | Mg | Al | Si | Ca | Cr | Mn | Ni | T.O | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.019 | 0.0023 | 0.0053 | 0.0939 | 0.0014 | 0.12 | 0.29 | 35.98 | 0.0019 | 0.001 | Bal. |
| Sample Number | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|
| Cooling condition | Water cooling | Air cooling | Furnace cooling | Air cooling | Air cooling |
| S/wt% | 0.001 | 0.001 | 0.001 | 0.0070 | 0.0170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Feng, Y.; Zheng, S. Effect of Cooling Rate and Sulfur Content on Sulfide Inclusions in Invar Alloy. Metals 2022, 12, 2191. https://doi.org/10.3390/met12122191
Chen J, Feng Y, Zheng S. Effect of Cooling Rate and Sulfur Content on Sulfide Inclusions in Invar Alloy. Metals. 2022; 12(12):2191. https://doi.org/10.3390/met12122191
Chicago/Turabian StyleChen, Jing, Yanbiao Feng, and Shaobo Zheng. 2022. "Effect of Cooling Rate and Sulfur Content on Sulfide Inclusions in Invar Alloy" Metals 12, no. 12: 2191. https://doi.org/10.3390/met12122191
APA StyleChen, J., Feng, Y., & Zheng, S. (2022). Effect of Cooling Rate and Sulfur Content on Sulfide Inclusions in Invar Alloy. Metals, 12(12), 2191. https://doi.org/10.3390/met12122191





