Potentiostatic Dealloying Fabrication and Electrochemical Actuation Performance of Bulk Nanoporous Palladium
Abstract
1. Introduction
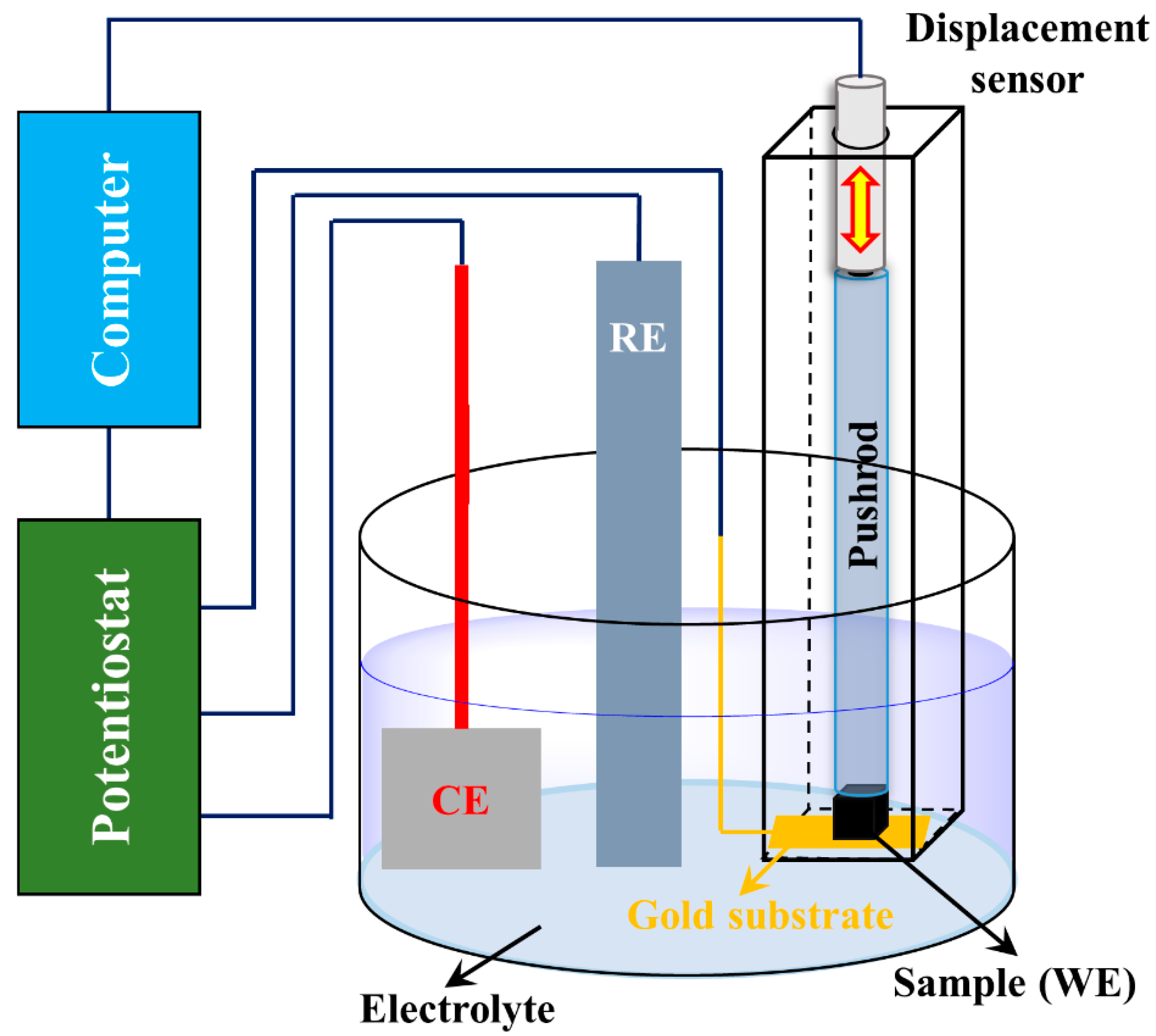
2. Experimental Mehods
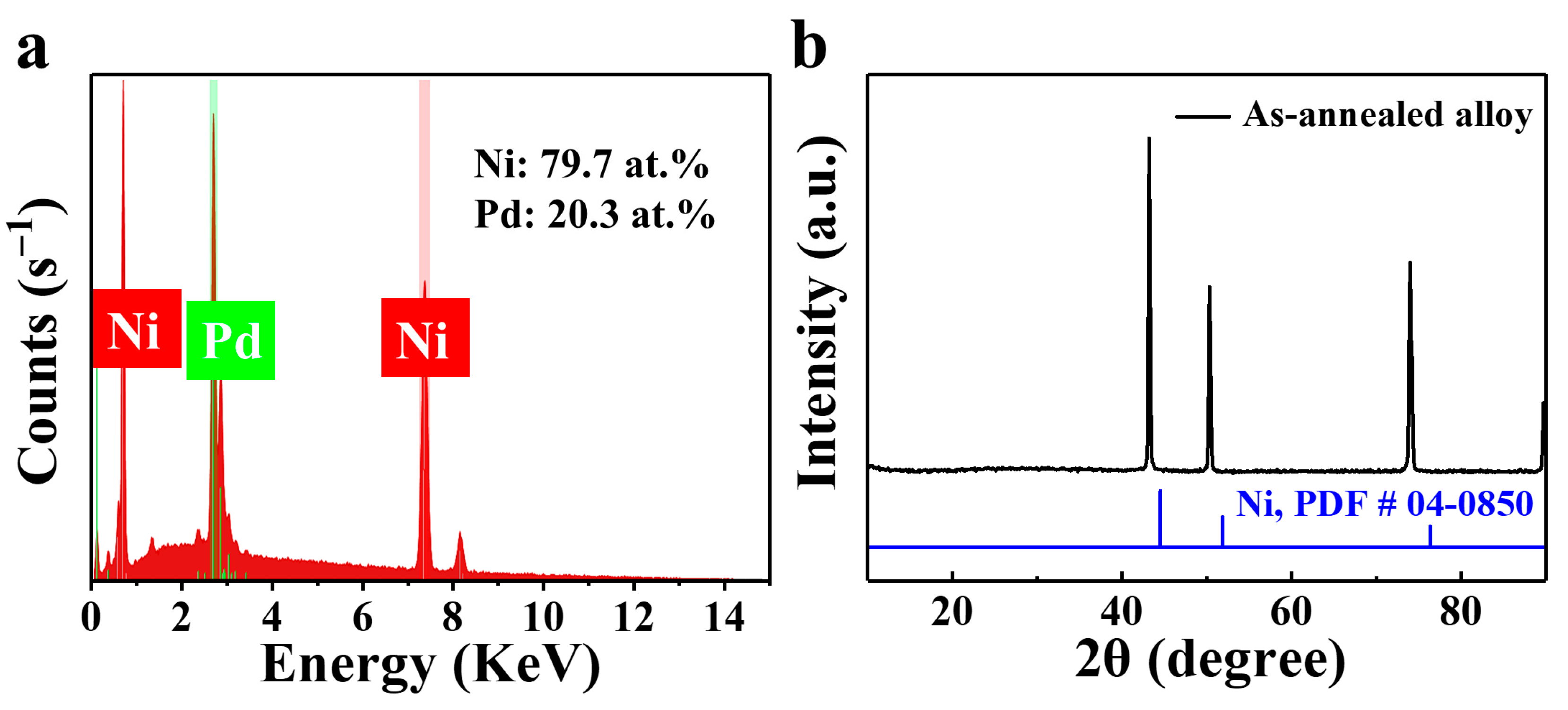
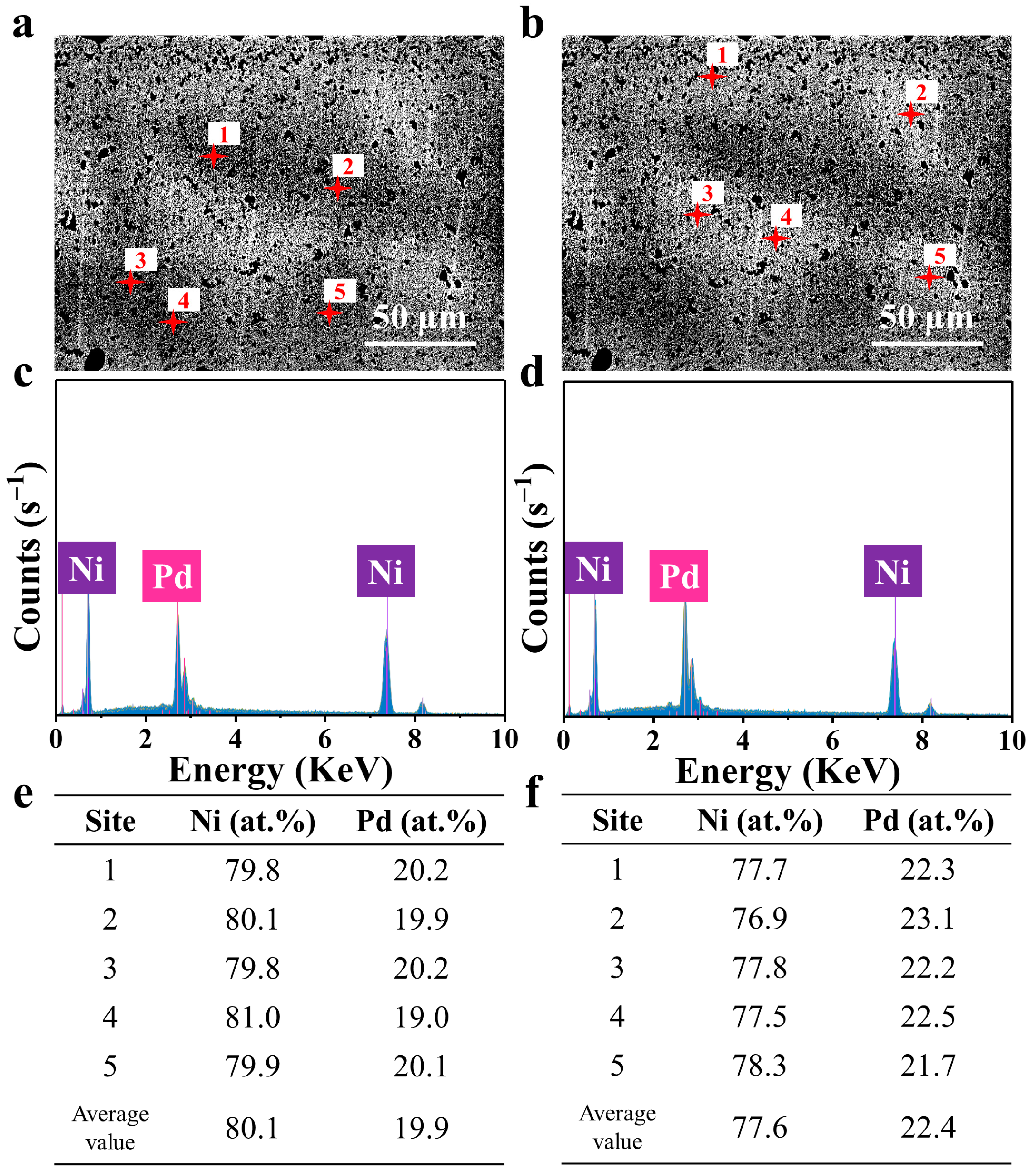
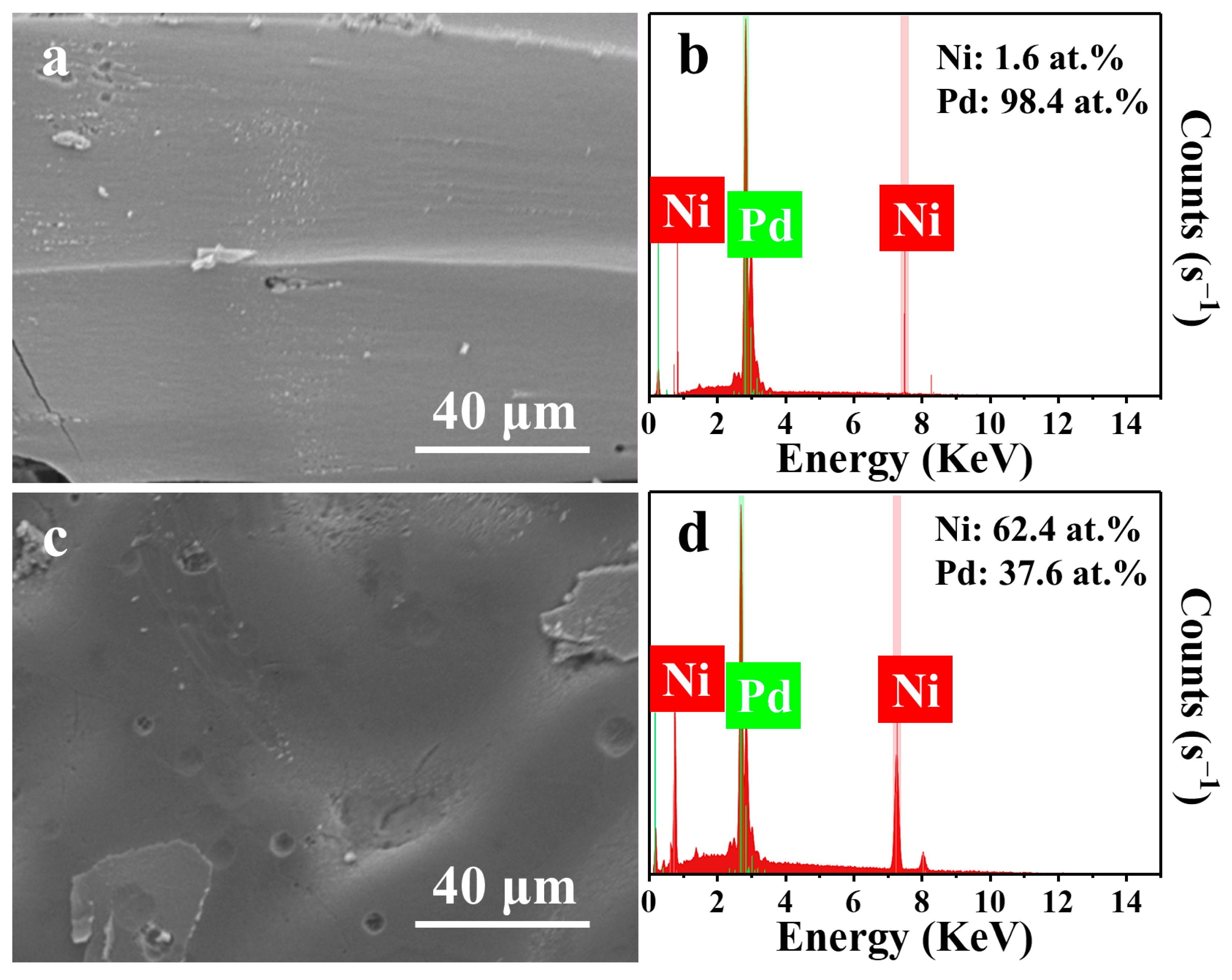
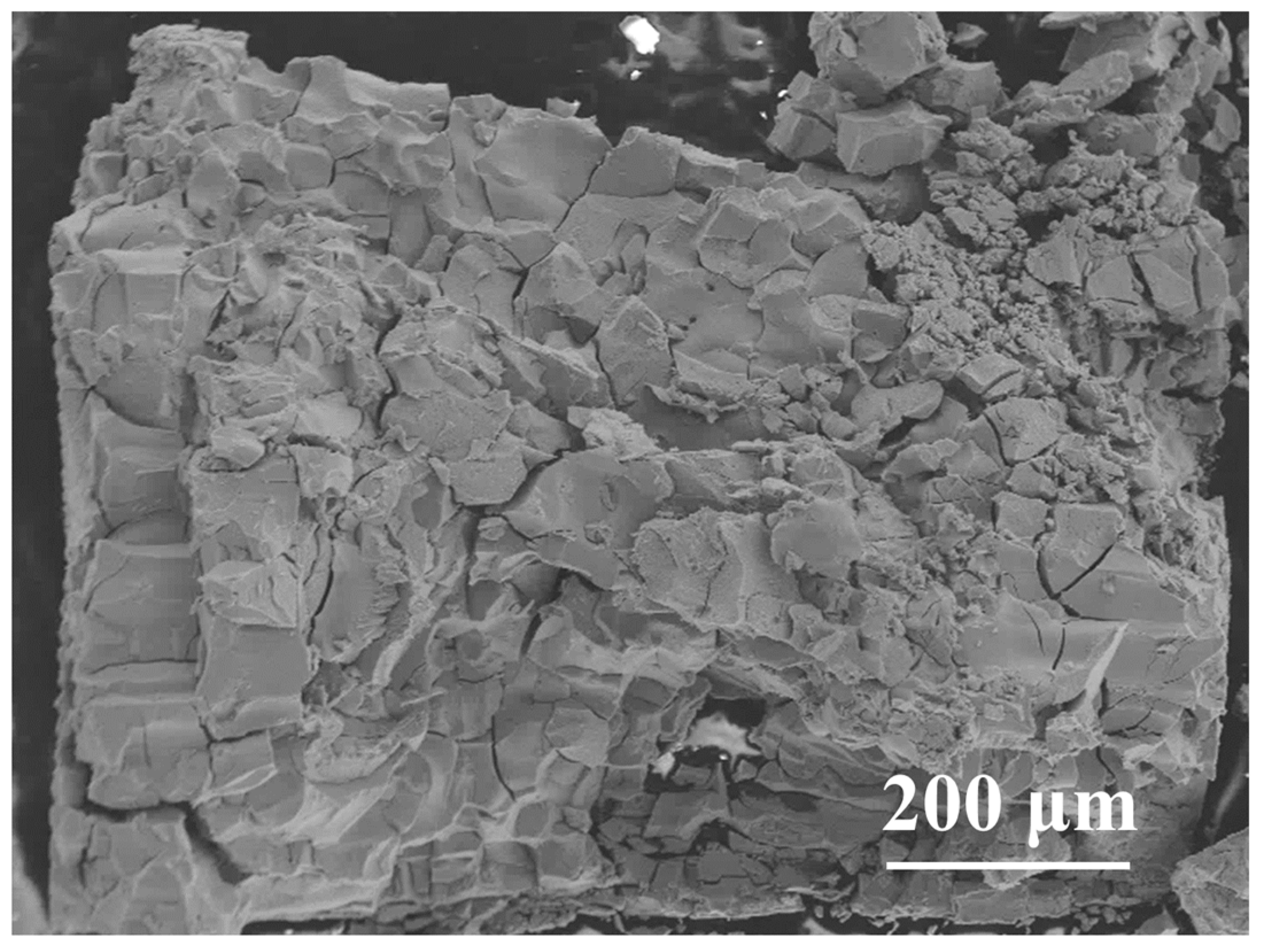
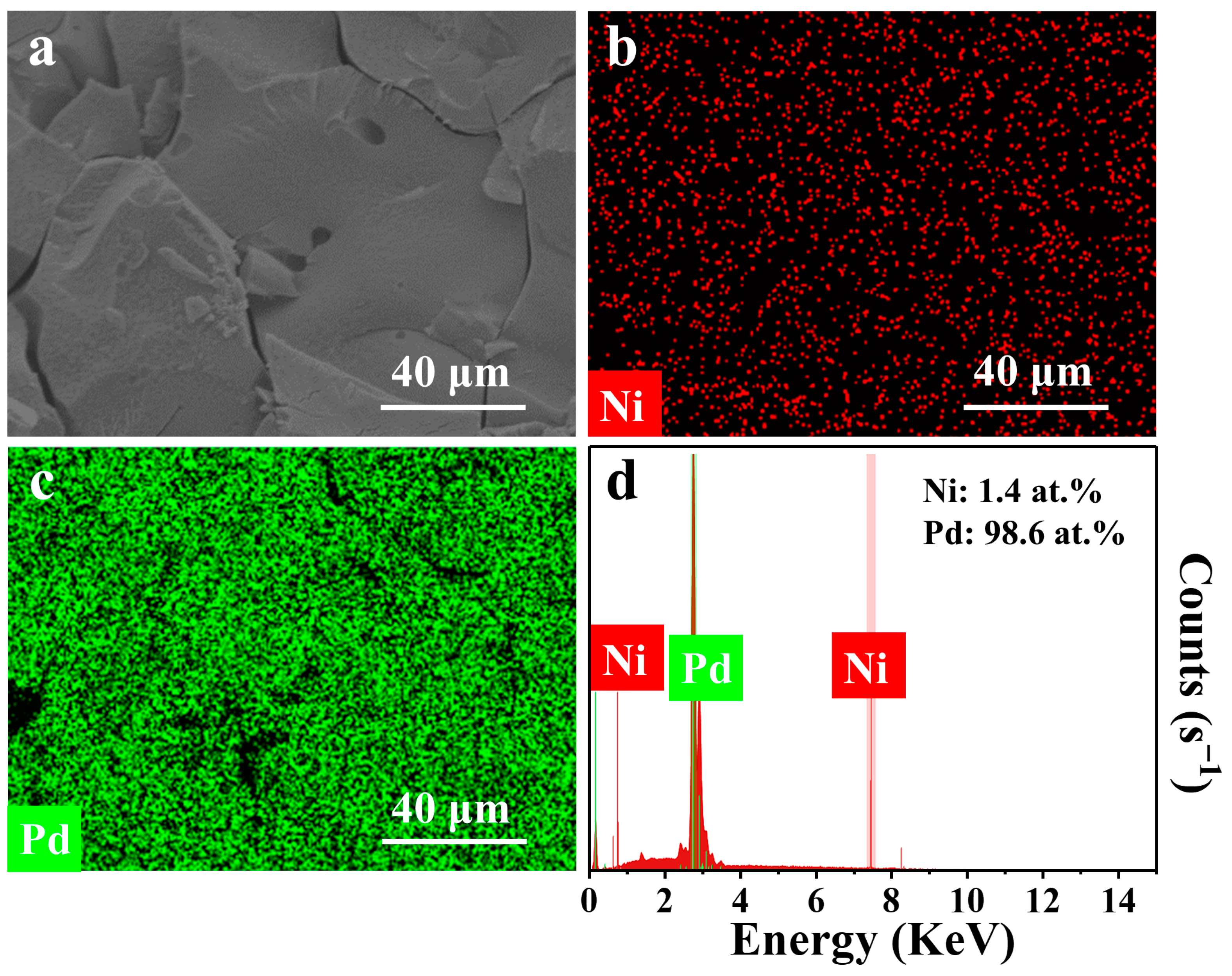
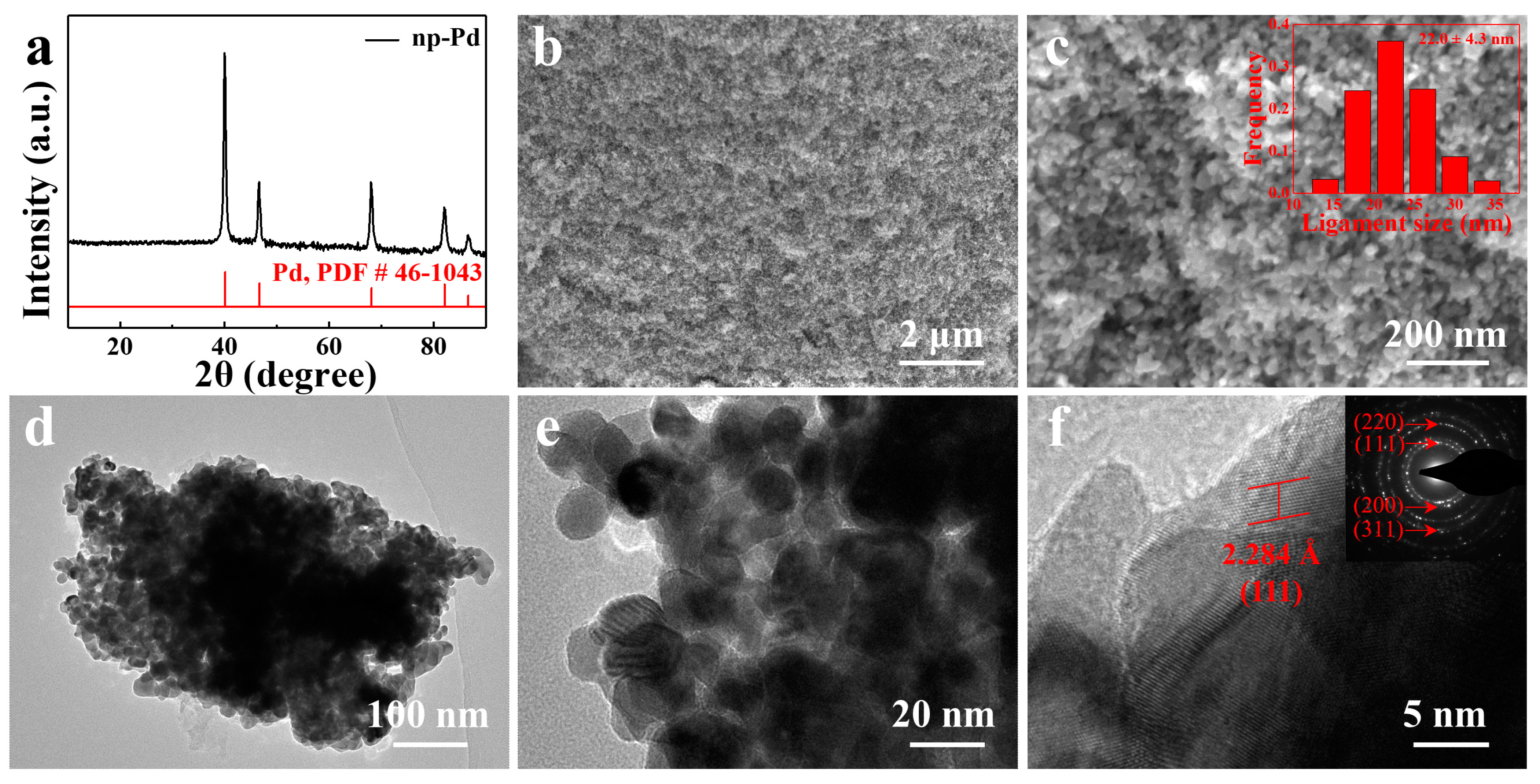
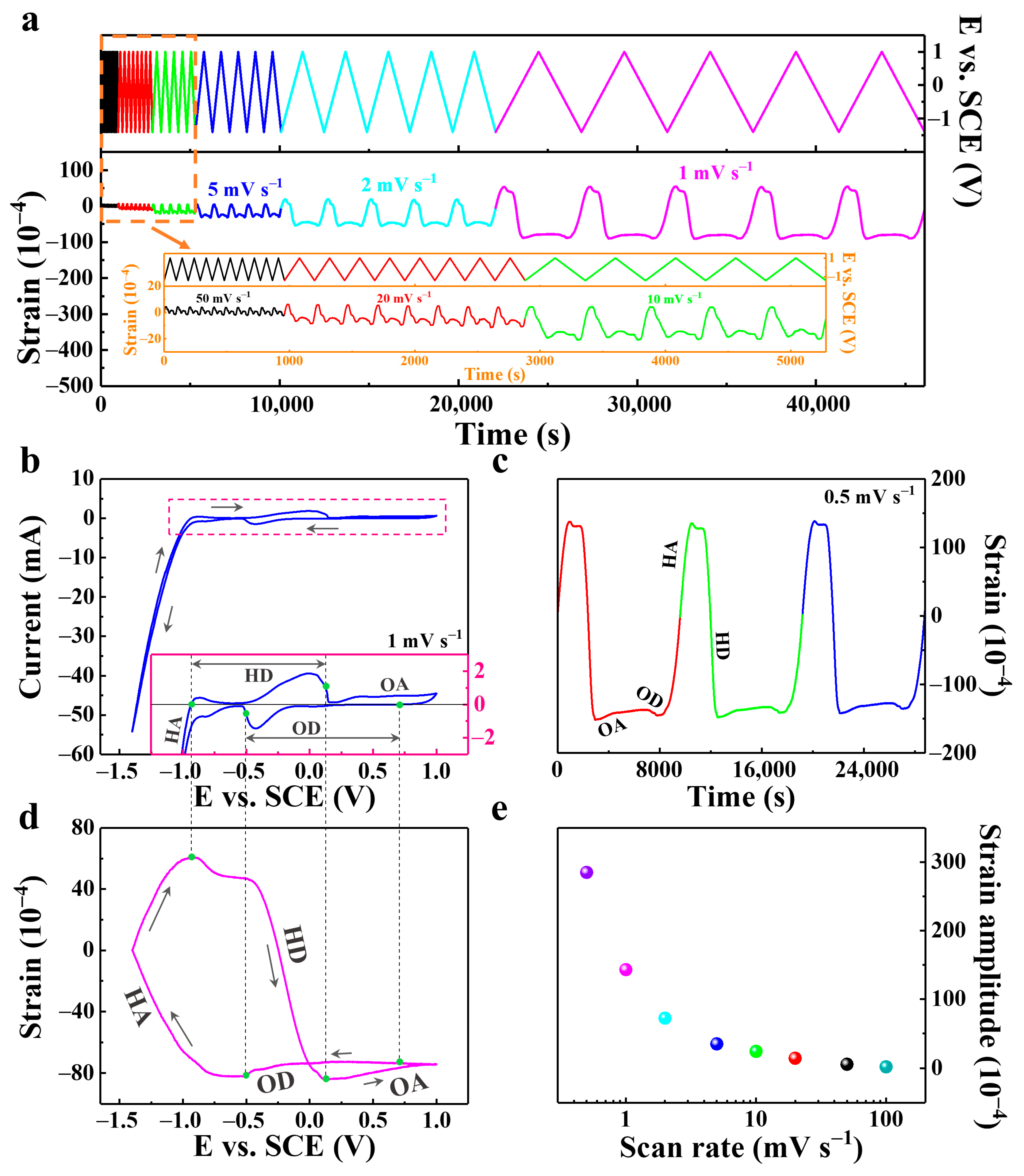
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sang, Q.; Hao, S.; Han, J.; Ding, Y. Dealloyed nanoporous materials for electrochemical energy conversion and storage. EnergyChem 2022, 4, 100069. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, S.; Liang, Y.; Jiang, H.; Li, Z.; Wu, S.; Cui, Z. Self-standing nanoporous NiPd bimetallic electrocatalysts with ultra-low Pd loading for efficient hydrogen evolution reaction. Electrochim. Acta 2022, 411, 140077. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Han, J.; Cheng, C.; Ning, S.; Hirata, A.; Fujita, T.; Chen, M. Engineering the internal surfaces of three-dimensional nanoporous catalysts by surfactant-modified dealloying. Nat. Commun. 2017, 8, 1066. [Google Scholar] [CrossRef]
- Jayathilaka, W.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-Garcia, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of nanomaterials in wearables: A review on wearable actuators and sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Wei, C.; Tao, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Dealloying: An effective method for scalable fabrication of 0D, 1D, 2D, 3D materials and its application in energy storage. Nano Today 2021, 37, 101094. [Google Scholar] [CrossRef]
- Jin, H.J.; Weissmüller, J. Bulk nanoporous metal for actuation. Adv. Eng. Mater. 2010, 12, 714–723. [Google Scholar] [CrossRef]
- Jin, H.J.; Wang, X.L.; Parida, S.; Wang, K.; Seo, M.; Weissmüller, J. Nanoporous Au-Pt alloys as large strain electrochemical actuators. Nano Lett. 2010, 10, 187–194. [Google Scholar] [CrossRef]
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer artificial muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Weissmüller, J.; Viswanath, R.N.; Kramer, D.; Zimmer, P.; Würschum, R.; Gleiter, H. Charge-induced reversible strain in a metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef]
- Viswanath, R.N.; Kramer, D.; Weissmüller, J. Adsorbate effects on the surface stress-charge response of platinum electrodes. Electrochim. Acta 2008, 53, 2757–2767. [Google Scholar] [CrossRef]
- Jin, H.J.; Parida, S.; Kramer, D.; Weissmüller, J. Sign-inverted surface stress-charge response in nanoporous gold. Surf. Sci. 2008, 602, 3588–3594. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L. Robust metallic actuators based on nanoporous gold rapidly dealloyed from gold-nickel precursors. Adv. Funct. Mater. 2021, 31, 2107241. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, Y.; Zhang, J.; Ding, Y.; Peng, Z.; Zhang, Z. Hierarchically nanoporous nickel-based actuators with giant reversible strain and ultrahigh work density. J. Mater. Chem. C 2016, 4, 45–52. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L.; Krekeler, T.; Ritter, M.; Weissmüller, J. Semiordered hierarchical metallic network for fast and large charge-induced strain. Nano Lett. 2017, 17, 4774–4780. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L.; Krekeler, T. Simultaneous enhancement of actuation strain and mechanical strength of nanoporous Ni-Mn actuators. Adv. Electron. Mater. 2021, 7, 2100381. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Q.; Zhang, Z. Dealloying-driven nanoporous palladium with superior electrochemical actuation performance. Nanoscale 2016, 8, 7287–7295. [Google Scholar] [CrossRef]
- Shi, S.; Markmann, J.; Weissmüller, J. Actuation by hydrogen electrosorption in hierarchical nanoporous palladium. Philos. Mag. 2017, 97, 1571–1587. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, C.; Tan, F.; Wu, F.; Zhang, Z. Nanoporous copper as an inexpensive electrochemical actuator responsive to sub-volt voltages. Electrochem. Commun. 2021, 124, 106940. [Google Scholar] [CrossRef]
- Tan, F.; Yu, B.; Yan, X.; Zhang, Y.; Bai, Q.; Zhang, J.; Zhang, Z. Electrochemical actuation behaviors of bulk nanoporous copper with a hierarchical structure. J. Alloys Compd. 2022, 923, 166469. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, C.; Tan, F.; Zhang, Z. High-performance, low-cost nanoporous alloy actuators by one-step dealloying of Al-Ni-Cu precursors. Intermetallics 2022, 145, 107537. [Google Scholar] [CrossRef]
- Li, J.; Markmann, J.; Weissmüller, J.; Mameka, N. Nanoporous gold-polypyrrole hybrid electrochemical actuators with tunable elasticity. Acta Mater. 2021, 212, 116852. [Google Scholar] [CrossRef]
- Roschning, B.; Weissmüller, J. Nanoporous-gold-polypyrrole hybrid materials for millimeter-sized free standing actuators. Adv. Mater. Interfaces 2020, 7, 2001415. [Google Scholar] [CrossRef]
- Wang, K.; Stenner, C.; Weissmüller, J. A nanoporous gold-polypyrrole hybrid nanomaterial for actuation. Sens. Actuators B Chem. 2017, 248, 622–629. [Google Scholar] [CrossRef]
- Detsi, E.; Onck, P.; De Hosson, J.T.M. Metallic muscles at work: High rate actuation in nanoporous gold/polyaniline composites. ACS Nano 2013, 7, 4299–4306. [Google Scholar] [CrossRef]
- Liu, Y.; Bliznakov, S.; Dimitrov, N. Factors controlling the less noble metal retention in nanoporous structures processed by electrochemical dealloying. J. Electrochem. Soc. 2010, 157, K168–K176. [Google Scholar] [CrossRef]
- Li, Y.; Ngo-Dinh, B.-N.; Markmann, J.; Weissmüller, J. Evolution of length scales and of chemical heterogeneity during primary and secondary dealloying. Acta Mater. 2022, 222, 117424. [Google Scholar] [CrossRef]
- Rurainsky, C.; Manjón, A.G.; Hiege, F.; Chen, Y.-T.; Scheu, C.; Tschulik, K. Electrochemical dealloying as a tool to tune the porosity, composition and catalytic activity of nanomaterials. J. Mater. Chem. A 2020, 8, 19405–19413. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z. On the electrochemical dealloying of Al-based alloys in a NaCl aqueous solution. Phys. Chem. Chem. Phys. 2010, 12, 1453–1472. [Google Scholar] [CrossRef]
- Palcut, M.; Ďuriška, L.; Špoták, M.; Vrbovský, M.; Gerhátová, Ž.; Černičková, I.; Janovec, J. Electrochemical corrosion of Al-Pd alloys in HCl and NaOH solutions. J. Min. Metall. Sect. B-Metall. 2017, 53, 333–340. [Google Scholar] [CrossRef]
- Shi, S.; Markmann, J.; Weissmüller, J. Synthesis of uniform bulk nanoporous palladium with tunable structure. Electrochim. Acta 2018, 285, 60–69. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Xu, R.; He, S.; Hua, Z.; Liu, H. One-step rapid synthesis of a 3D porous surface on Zr-based bulk metallic glass. Surf. Coat. Technol. 2021, 418, 127230. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Wang, X.; Ji, H.; Zhao, C.; Wang, Y.; Zhang, Z. Fabrication of bi-modal nanoporous bimetallic Pt-Au alloy with excellent electrocatalytic performance towards formic acid oxidation. Green Chem. 2011, 13, 1914–1922. [Google Scholar] [CrossRef]
- Snyder, J.; Livi, K.; Erlebacher, J. Dealloying silver/gold alloys in neutral silver nitrate solution: Porosity evolution, surface composition, and surface oxides. J. Electrochem. Soc. 2008, 155, C464–C473. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Wang, C.; Liu, Y.; Sun, Y.; Qin, C.; Wang, Z.; Li, Y.; Liu, L.; Liu, S. Controllable nanoporous copper synthesized by dealloying metallic glasses: New insights into the tuning pore structure and applications. Chem. Eng. J. 2022, 427, 130861. [Google Scholar] [CrossRef]
- Erlebacher, J. An atomistic description of dealloying porosity evolution, the critical potential, and rate-limiting behavior. J. Electrochem. Soc. 2004, 151, C614–C626. [Google Scholar] [CrossRef]
- Sieradzki, K.; Dimitrov, N.; Movrin, D.; McCall, C.; Vasiljevic, N.; Erlebacher, J. The dealloying critical potential. J. Electrochem. Soc. 2002, 149, B370–B377. [Google Scholar] [CrossRef]
- Damian, A.; Maroun, F.; Allongue, P. Electrochemical de-alloying in two dimensions: Role of the local atomic environment. Nanoscale 2016, 8, 13985–13996. [Google Scholar] [CrossRef]
- Graf, M.; Roschning, B.; Weissmüller, J. Nanoporous gold by alloy corrosion: Method-structure-property relationships. J. Electrochem. Soc. 2017, 164, C194–C200. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Baumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef]
- dos Santos, C.G.P.; Machado, E.G.; Kiss, I.Z.; Nagao, R. Investigation of the oscillatory electrodissolution of the Nickel-Iron alloy. J. Phys. Chem. C 2019, 123, 24087–24094. [Google Scholar] [CrossRef]
- Hudson, J.L.; Tsotsis, T.T. Electrochemical reaction dynamics: A review. Chem. Eng. Sci. 1994, 49, 1493–1572. [Google Scholar] [CrossRef]
- Schmuki, P. From Bacon to barriers: A review on the passivity of metals and alloys. J. Solid State Electrochem. 2002, 6, 145–164. [Google Scholar] [CrossRef]
- Czerwiński, A.; Kiersztyn, I.; Grdeń, M. The study of hydrogen sorption in palladium limited volume electrodes (Pd-LVE): Part II. Basic solutions. J. Electroanal. Chem. 2000, 492, 128–136. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, X.; Ma, H.; Zhang, J. Electrochemical synthesis, voltammetric behavior, and electrocatalytic activity of Pd nanoparticles. J. Phys. Chem. C 2008, 112, 2456–2461. [Google Scholar] [CrossRef]
- Grdeń, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwiński, A. Electrochemical behaviour of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Madden, J.D.W.; Vandesteeg, N.A.; Anquetil, P.A.; Madden, P.G.A.; Takshi, A.; Pytel, R.Z.; Lafontaine, S.R.; Wieringa, P.A.; Hunter, I.W. Artificial muscle technology: Physical principles and naval prospects. IEEE J. Ocean. Eng. 2004, 29, 706–728. [Google Scholar] [CrossRef]
- Juarez, T.; Biener, J.; Weissmüller, J.; Hodge, A.M. Nanoporous metals with structural hierarchy: A review. Adv. Eng. Mater. 2017, 19, 1700389. [Google Scholar] [CrossRef]
- Biener, J.; Hodge, A.M.; Hamza, A.V.; Hsiung, L.M.; Satcher, J.H., Jr. Nanoporous Au: A high yield strength material. J. Appl. Phys. 2005, 97, 024301. [Google Scholar] [CrossRef]
- Viswanath, R.N.; Weissmüller, J. Electrocapillary coupling coefficients for hydrogen electrosorption on palladium. Acta Mater. 2013, 61, 6301–6309. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Bharti, V.; Zhao, X. Giant electrostriction and relaxor ferroelectric behavior in electron-irradiated poly(vinylidene fluoride-trifluoroethylene) copolymer. Science 1998, 280, 2101–2104. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, L.; Gao, H.; Bai, Q.; Zhang, C.; Zhang, Z. Electrochemical actuation behaviors and mechanisms of bulk nanoporous Ni-Pd alloy. Scr. Mater. 2017, 137, 73–77. [Google Scholar] [CrossRef]

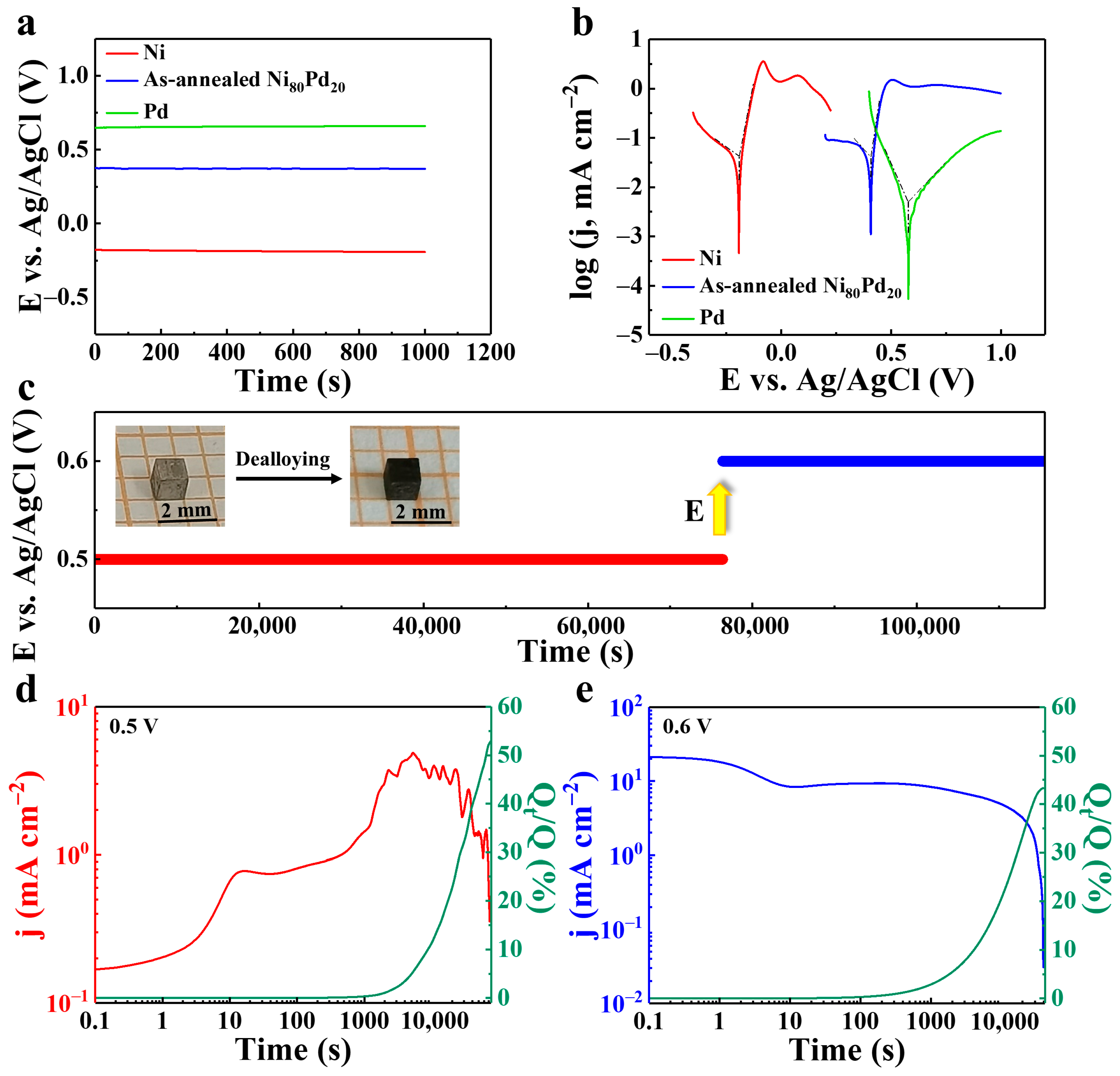

| Metal | Open-Circuit Potential (Eocp vs. Ag/AgCl) | Corrosion Potential (Ecorr vs. Ag/AgCl) | Corrosion Current Density (jcorr, mA cm−2) |
|---|---|---|---|
| Ni | −0.18 | −0.19 | 0.043 |
| As-annealed Ni80Pd20 | 0.37 | 0.41 | 0.042 |
| Pd | 0.65 | 0.58 | 0.005 |
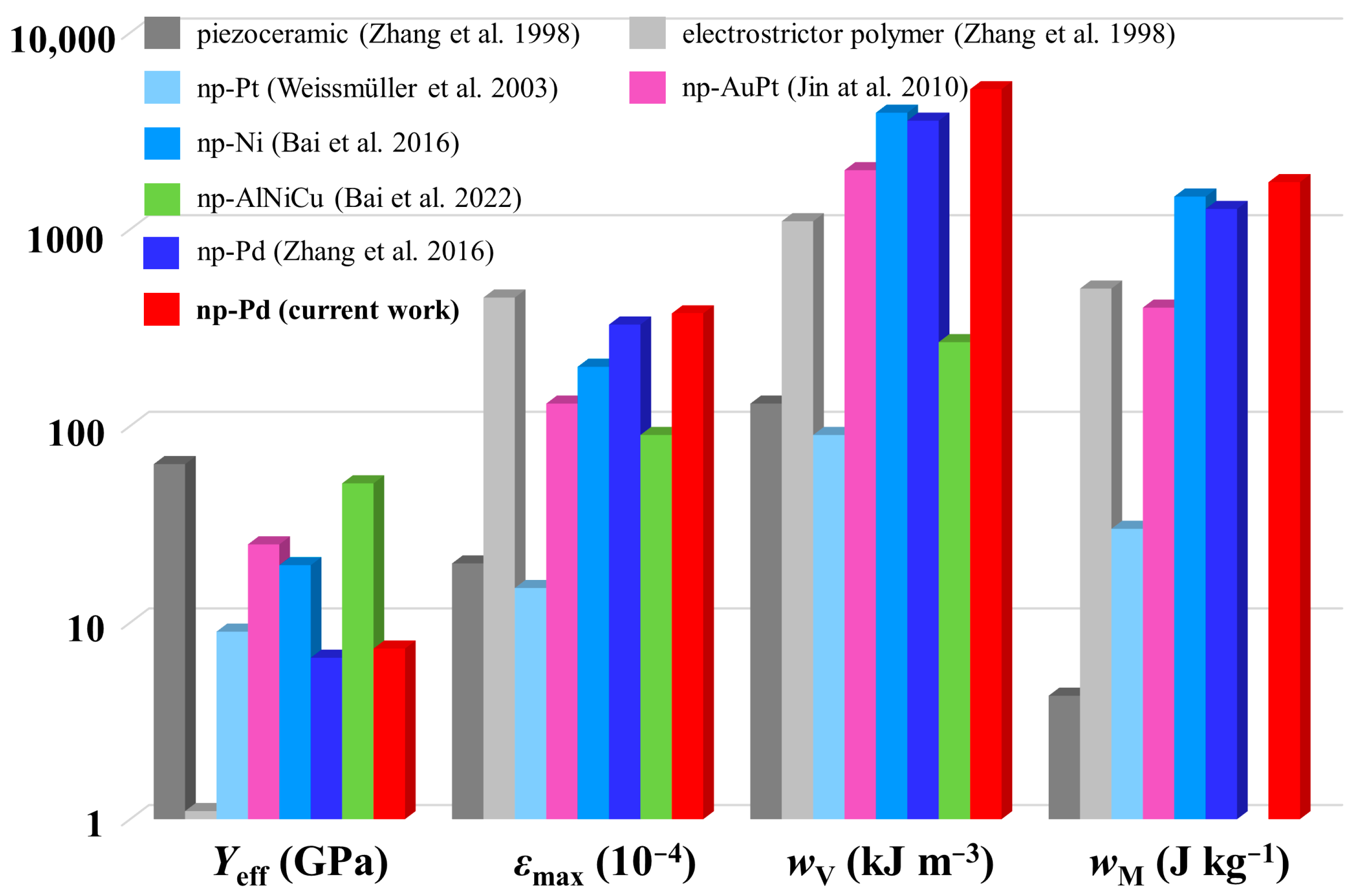
| Materials | Yeff (GPa) | εmax (10−4) | wV (kJ m−3) | wM (J kg−1) |
|---|---|---|---|---|
| piezoceramic [50] | 64 | 20 | 130 | 4.25 |
| electrostrictor polymer [50] | 1.1 | 450 | 1100 | 500 |
| np-Pt [9,10] | 9 | 15 | 90 | 30 |
| np-Au [12] | — | 10 | — | — |
| np-AuPt [7] | 25 | 130 | 2000 | 400 |
| np-Ni [13] | 19.6 | 200 | 3920 | 1468 |
| np-NiMn [15] | — | 194 | — | — |
| np-NiPd [51] | — | 47 | — | — |
| np-Pd [16] | 6.63 | 328 | 3570 | 1270 |
| np-Pd [17] | — | 400 | — | — |
| np-AlNiCu [20] | 51.1 | 90 | 267 | — |
| np-Pd (current work) | 7.38 | 374 | 5161 | 1738 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Yu, B.; Bai, Q.; Zhang, Z. Potentiostatic Dealloying Fabrication and Electrochemical Actuation Performance of Bulk Nanoporous Palladium. Metals 2022, 12, 2153. https://doi.org/10.3390/met12122153
Tan F, Yu B, Bai Q, Zhang Z. Potentiostatic Dealloying Fabrication and Electrochemical Actuation Performance of Bulk Nanoporous Palladium. Metals. 2022; 12(12):2153. https://doi.org/10.3390/met12122153
Chicago/Turabian StyleTan, Fuquan, Bin Yu, Qingguo Bai, and Zhonghua Zhang. 2022. "Potentiostatic Dealloying Fabrication and Electrochemical Actuation Performance of Bulk Nanoporous Palladium" Metals 12, no. 12: 2153. https://doi.org/10.3390/met12122153
APA StyleTan, F., Yu, B., Bai, Q., & Zhang, Z. (2022). Potentiostatic Dealloying Fabrication and Electrochemical Actuation Performance of Bulk Nanoporous Palladium. Metals, 12(12), 2153. https://doi.org/10.3390/met12122153







