Study on the Behavior Mechanism of K and Na during the Synthesis of VN Alloy
Abstract
:1. Introduction
2. Experimental Method
2.1. Experimental Materials and Instruments
2.2. Vanadium-Nitrogen Alloy Production Process
3. Results and Discussion
3.1. Analyze the Source of Impurities K and Na
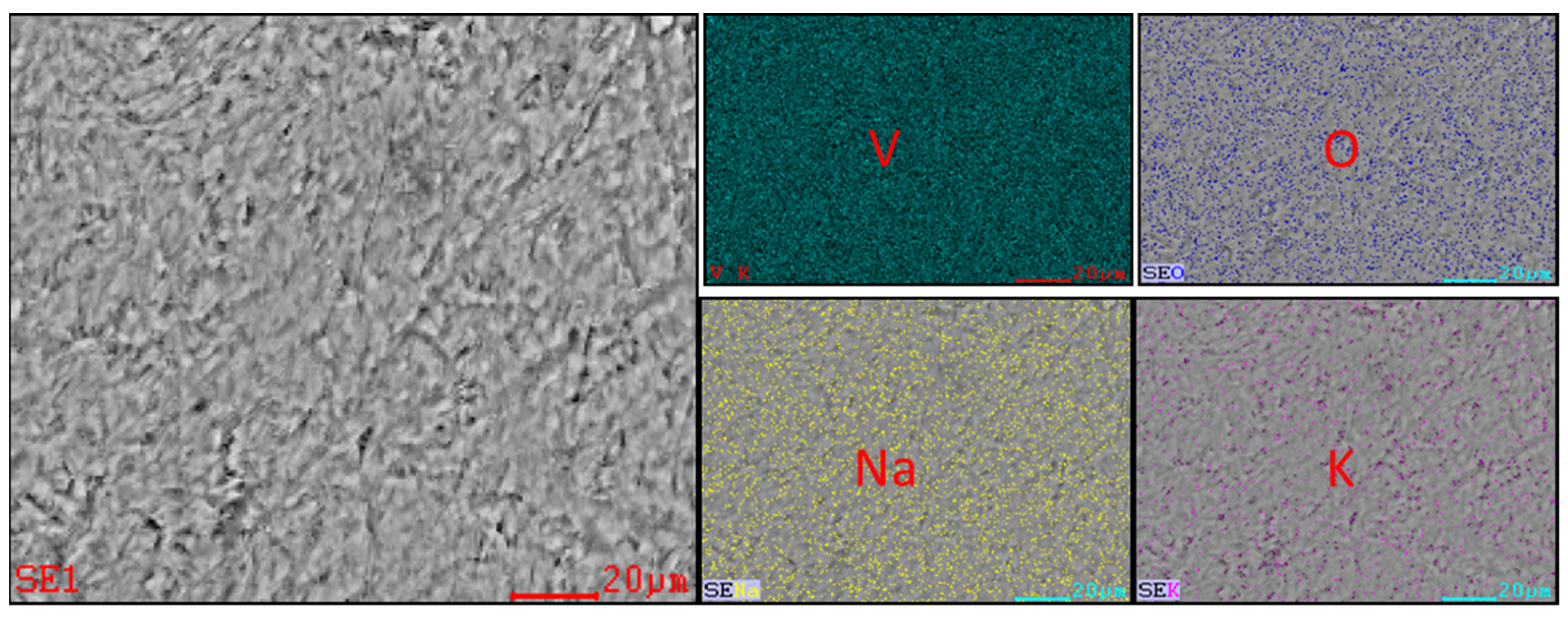
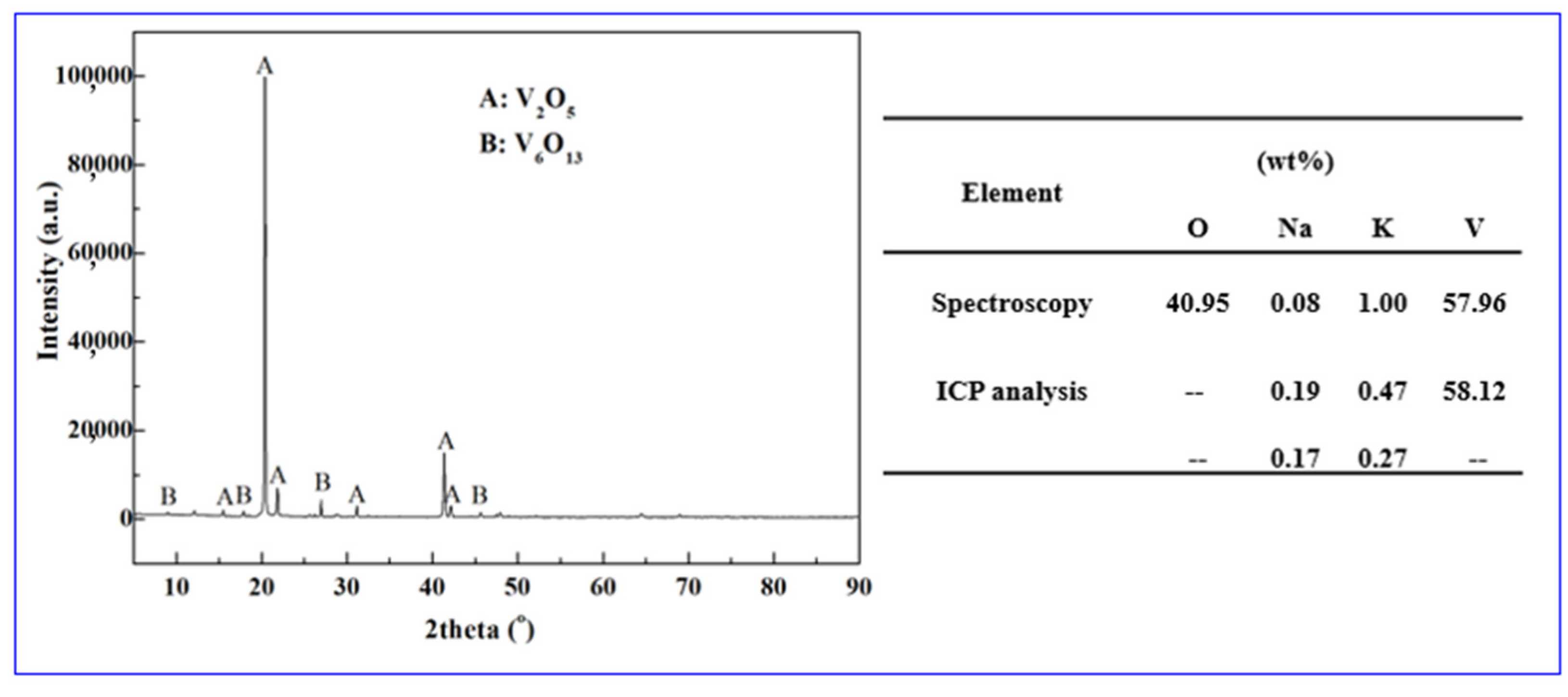
3.2. Occurrence Rule of K and Na in Tablet Vanadium Pentoxide
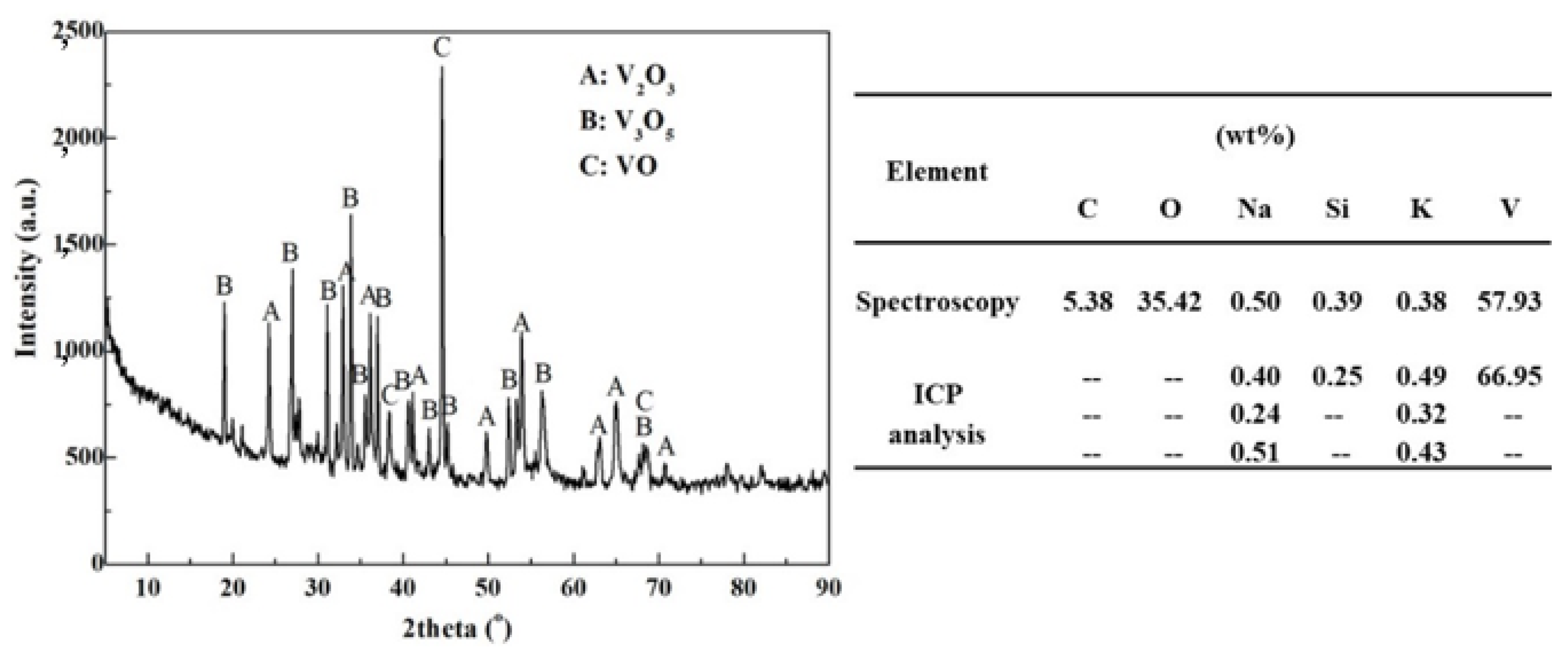
3.3. Sources and Occurrence Rules of K and Na in Vanadium Trioxide
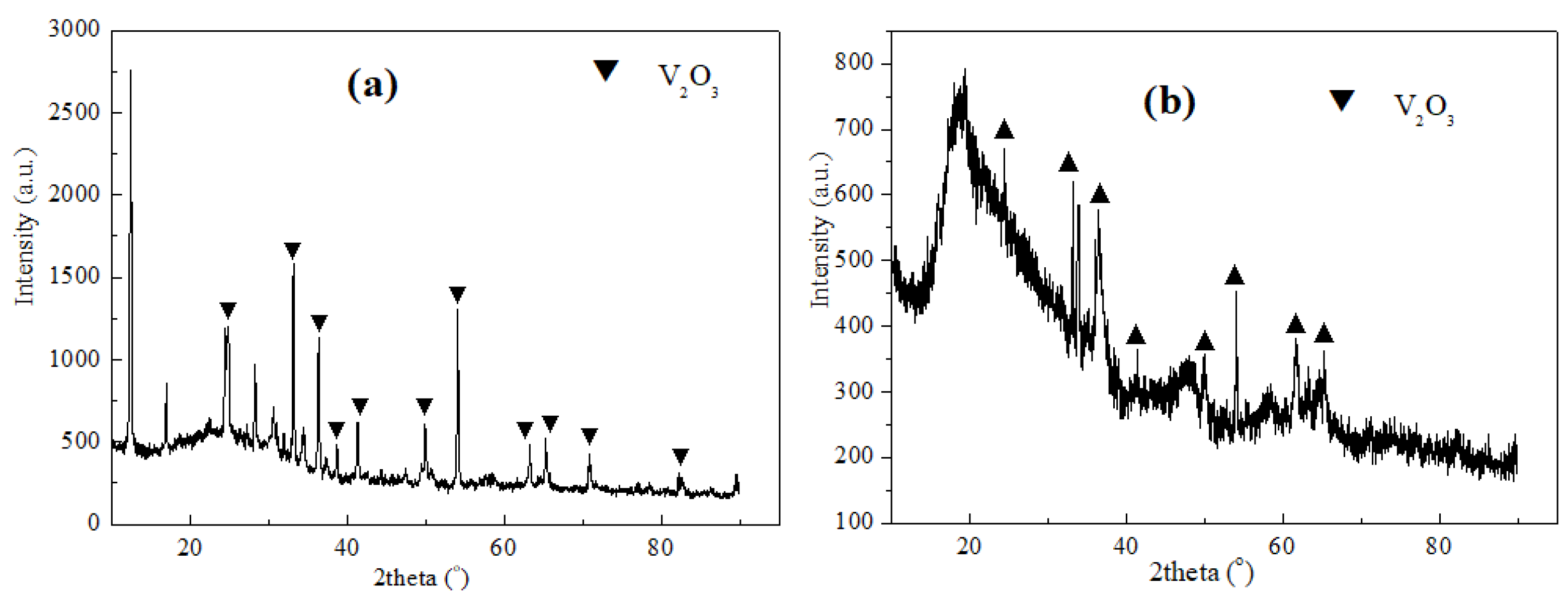
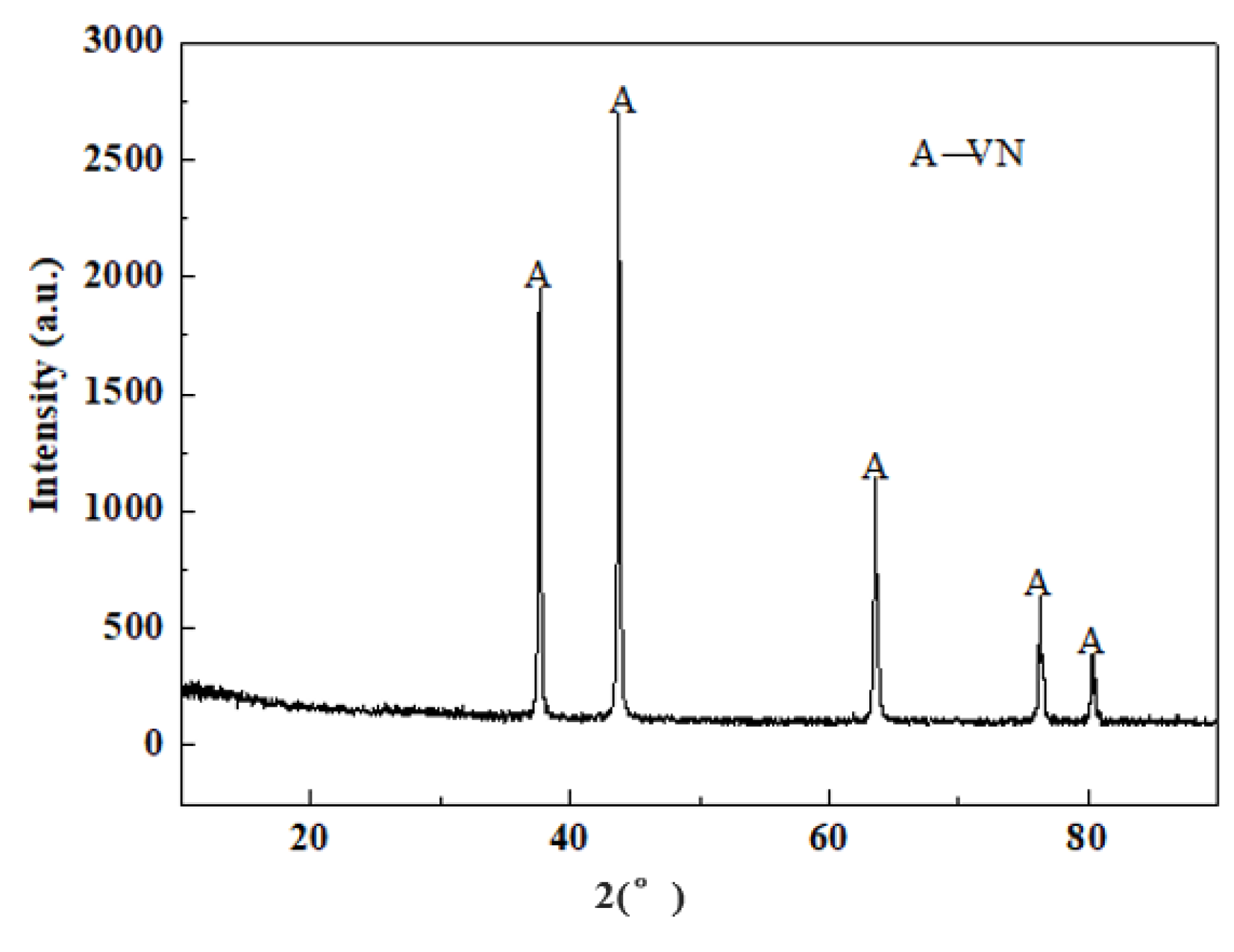
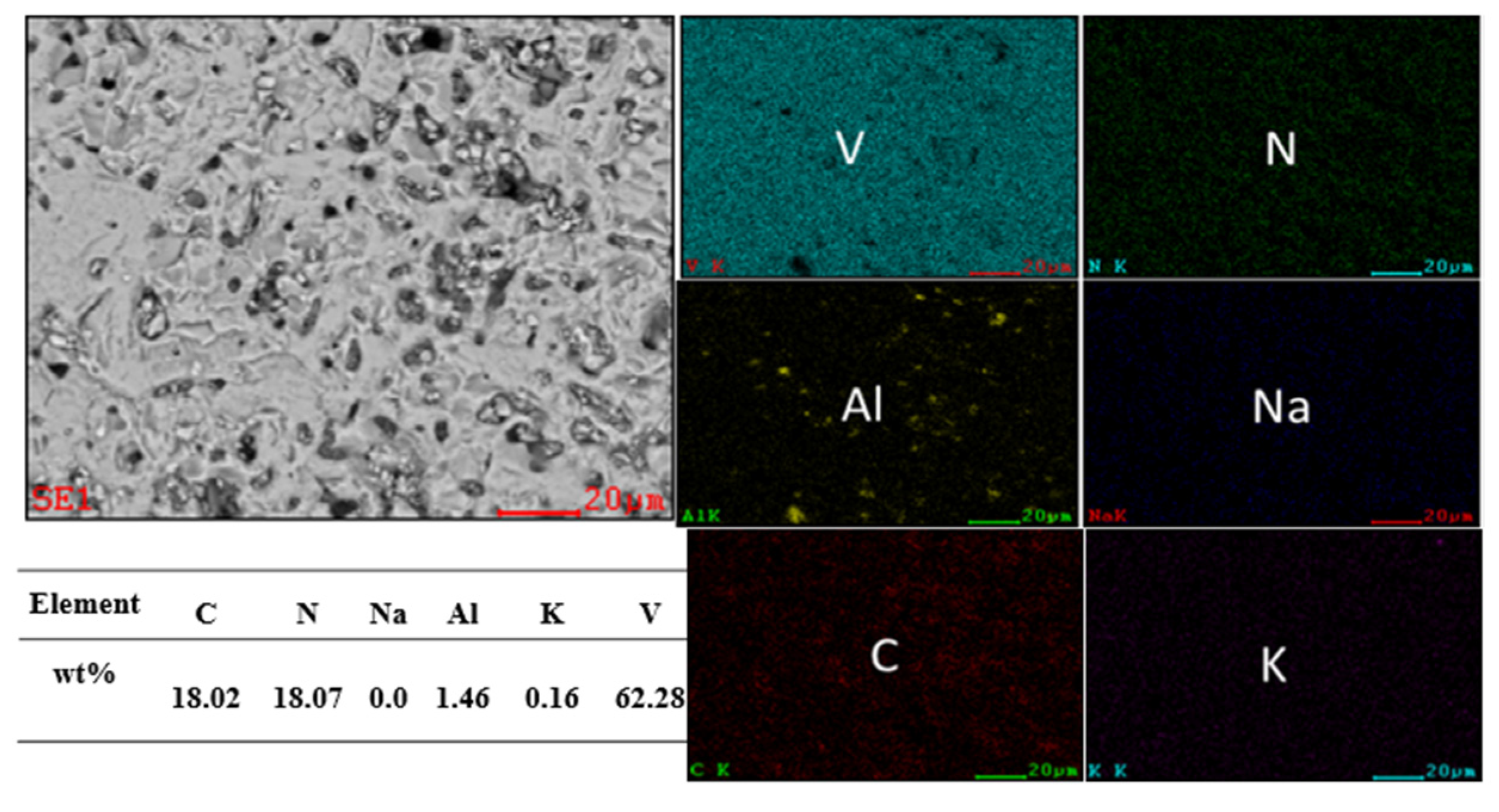
3.4. Characterization of Vanadium Nitride Samples and Occurrence Rules of Internal K and Na
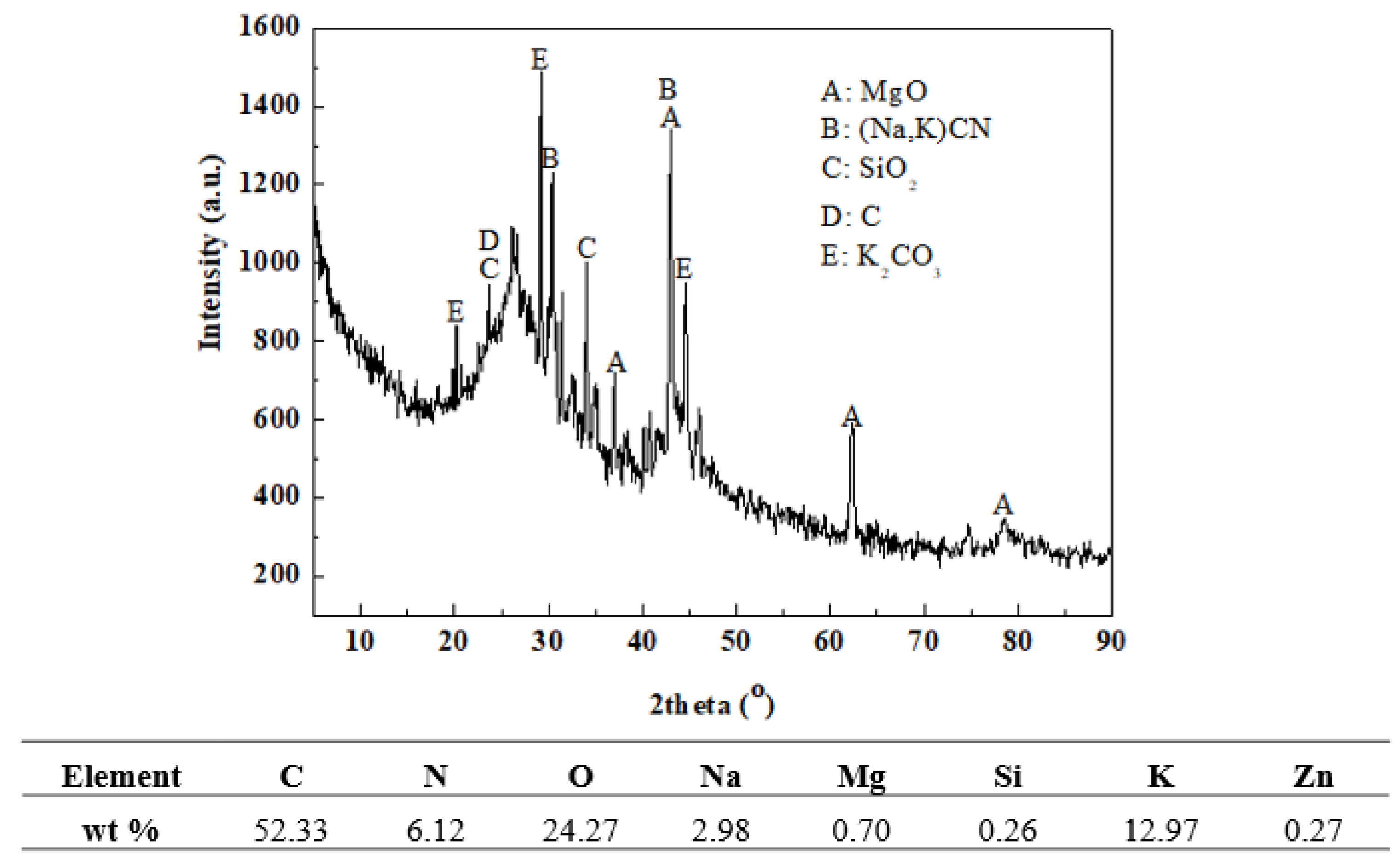
3.5. Analysis of Volatile Compounds in the Preparation of VN
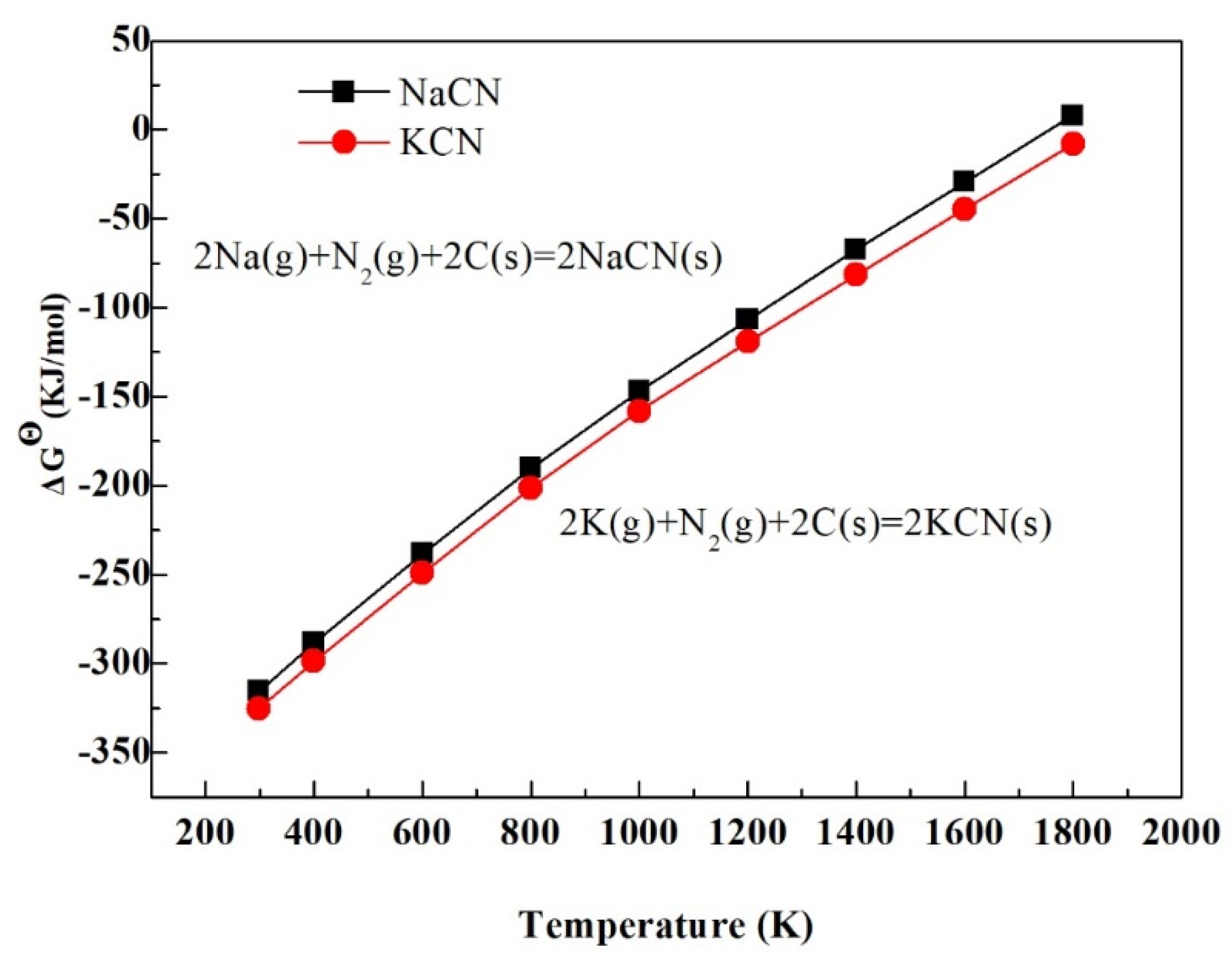
3.6. Study on the Behavior Mechanism of K and Na in the Synthesis of VN
4. Conclusions
- (1)
- The elements K and Na in APV exist in the form of decavanadate and dodecanadate, and due to the adsorption capacity of polyamide K+ > Na+, the content of the K element in APV is higher than that of Na. At the same time, K and Na exist in the same form and regularity in the vanadium sheet obtained by APV dehydration-deamination-melting.
- (2)
- The concentration of K in the alloy product is 25 times higher than that of the vanadium raw material, the concentration of Na is 15 times higher than that of the raw material, and the degree of enrichment of K is stronger than that of Na.
- (3)
- During the preparation of the VN alloy, some K and Na vapors promote the reduction in low-valent vanadium oxides to metal V, thereby promoting the formation process of vanadium nitride and improving the formation efficiency of vanadium nitride.
- (4)
- Impurities K and Na are converted into corrosive substances such as KCN, NaCN, K2CO3, Na2CO3, etc. These substances cause the equipment to be corroded; and these impurities are easily deposited on the surface of the product, which affects the equipment life cycle and product quality.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, X.Y.; Zang, M.; Hu, J.V.-N. microalloying Q550D high-strength mid-thick steel plate. J. Northeast. Univ. (Nat. Sci.) 2017, 38, 502–506. [Google Scholar]
- Skobir, D.A.; Godec, M.; Balcar, M. The influence of the microalloying elements of HSLA steel on the microstructure and mechanical properties. Mater. Technol. 2010, 44, 343–347. [Google Scholar]
- Yu, S.S.; Fu, N.X.; Gao, F.; Sui, Z.T. Study on One-step Synthesis of Vanadium Carbonitride. Iron Steel Vanadium Titan. 2007, 28, 1–5. [Google Scholar]
- Lu, Z.Y.; Luo, D.M.; Dou, X.H.; Chen, H.S.; Sui, Z.T. Study on the Preparation of Vanadium Carbonitride by Vanadium Trioxide. Chin. J. Rare Met. 2003, 27, 193–195. [Google Scholar]
- Hu, J.; Du, L.X.; Wang, W.H. Microstructural control and mechanical properties of 590 MPa grade hot-rolled V-N high strength steel. J. Northeast. Univ. (Nat. Sci.) 2013, 34, 820–823. [Google Scholar]
- Wang, W.Y.; Liu, T.Z.; Liu, J.P. Experimental study on post-fire mechanical properties of high strength Q460 steel. J. Constr. Steel Res. 2015, 114, 100–109. [Google Scholar] [CrossRef]
- Goddard, J.B.; Merket, R.F. Preparation of Low-Carbon Vanadium Nitride. U.S. Patent 4,562,507, 31 December 1985. [Google Scholar]
- Middelhoek, S. Process for the Preparation of Vanadium (Carbon) Nitride. U.S. Patent 3,745,209, 10 July 1973. [Google Scholar]
- Sui, Z.T.; Chen, H.S.; Lu, Z.Y.; Luo, D.M. A Kind of Vanadium Nitrogen Microalloy Additive and Preparation Method Thereof. China Patent 1,480,548, 27 July 2005. [Google Scholar]
- YB/T5304-2011; National Standard of the People’s Republic of China “Vanadium Pentoxide”. Ministry of Industry and Information Technology: Beijing, China, 2012.
- Daluz, A.D.J.; da Silva, T.V.S.; Tôrres, B.O.; Costa, D.F.N.; Santos, L.A.M. Long-termair way evolution after orthognathic surgery: Systematic Review. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, 191–198. [Google Scholar] [CrossRef] [PubMed]
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrão, G.; Gonçalves, J.; Sánchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915. [Google Scholar] [CrossRef] [PubMed]
- Buggia-Prévot, V.; Fernandez, C.G.; Udayar, V.; Vetrivel, K.S.; Elie, A.; Roseman, J.; Sasse, V.A.; Lefkow, M.; Meckler, X.; Bhattacharyya, S.; et al. A Function for EHD Family Proteins in Unidirectional Retrograde Dendritic Transport of BACE1 and Alzheimer’s Disease Aβ Production. Cell Rep. 2013, 5, 1552–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.L.; Liu, G.Q.; Chen, H.S. Vanadium Titanium Material; Metallurgical Industry Press: Beijing, China, 2009; pp. 109–110. [Google Scholar]
- Ivakin, A.A.; Yatsenko, A.P.; Pletnev, R.N.; Gorshkov, V.V. Ion exchange of alkali metal and ammonium cations on dodecavanadium acid. Zhurnal Prikladnoj Khimii 1978, 51, 1993–1996. [Google Scholar]
- Evans, H.T.; Hughes, J.M. Crystal chemistry of natural vanadium bronzes. Amer. Miner. 1990, 75, 508–521. [Google Scholar]
- Xu, J.N.; Yang, G.Y.; Sun, H.R.; Wang, T.G.; Xu, J.Q.; Wu, Q.J. Synthesis and Structure of Na6V10O28·12H2O. Struct. Chem. 1996, 253–256. [Google Scholar]
- Xu, J.N.; Yang, G.Y.; Sun, H.R.; Wang, T.G.; Xu, J.Q. Synthesis and Structure of K6V10O28·9H2O. Chem. Res. Appl. 1997, 36–41. [Google Scholar]
- Liang, Y.J.; Che, Y.C. Handbook of Inorganic Thermodynamics; Northeastern University Press: Shenyang, China, 1993; pp. 449–479. [Google Scholar]
- Ihsan, B. Thermochemical Data of Pure Substances; Printed in the Federal Republic of Germany; VCh: Weinheim, Germany, 1995; pp. 285–1815. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, X.; Zhang, C.; Lu, M.; Wang, H.; Wan, H. Study on the Behavior Mechanism of K and Na during the Synthesis of VN Alloy. Metals 2022, 12, 2111. https://doi.org/10.3390/met12122111
Li L, Wang X, Zhang C, Lu M, Wang H, Wan H. Study on the Behavior Mechanism of K and Na during the Synthesis of VN Alloy. Metals. 2022; 12(12):2111. https://doi.org/10.3390/met12122111
Chicago/Turabian StyleLi, Lanjie, Xindong Wang, Caidong Zhang, Mingliang Lu, Haixu Wang, and Heli Wan. 2022. "Study on the Behavior Mechanism of K and Na during the Synthesis of VN Alloy" Metals 12, no. 12: 2111. https://doi.org/10.3390/met12122111
APA StyleLi, L., Wang, X., Zhang, C., Lu, M., Wang, H., & Wan, H. (2022). Study on the Behavior Mechanism of K and Na during the Synthesis of VN Alloy. Metals, 12(12), 2111. https://doi.org/10.3390/met12122111




