A Study of the Mechanisms and Kinetics of the Localized Corrosion Aggravation of Ductile Iron in a Harsh Water Quality Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Environmentally Simulated Solution
2.3. Autoclave Immersion Experiment
2.4. Electrochemical Test
2.5. Simulation Model Calculations
3. Results and Discussion
3.1. Exploration of the Corrosion Mechanisms of Ductile Iron Shrinkage Holes
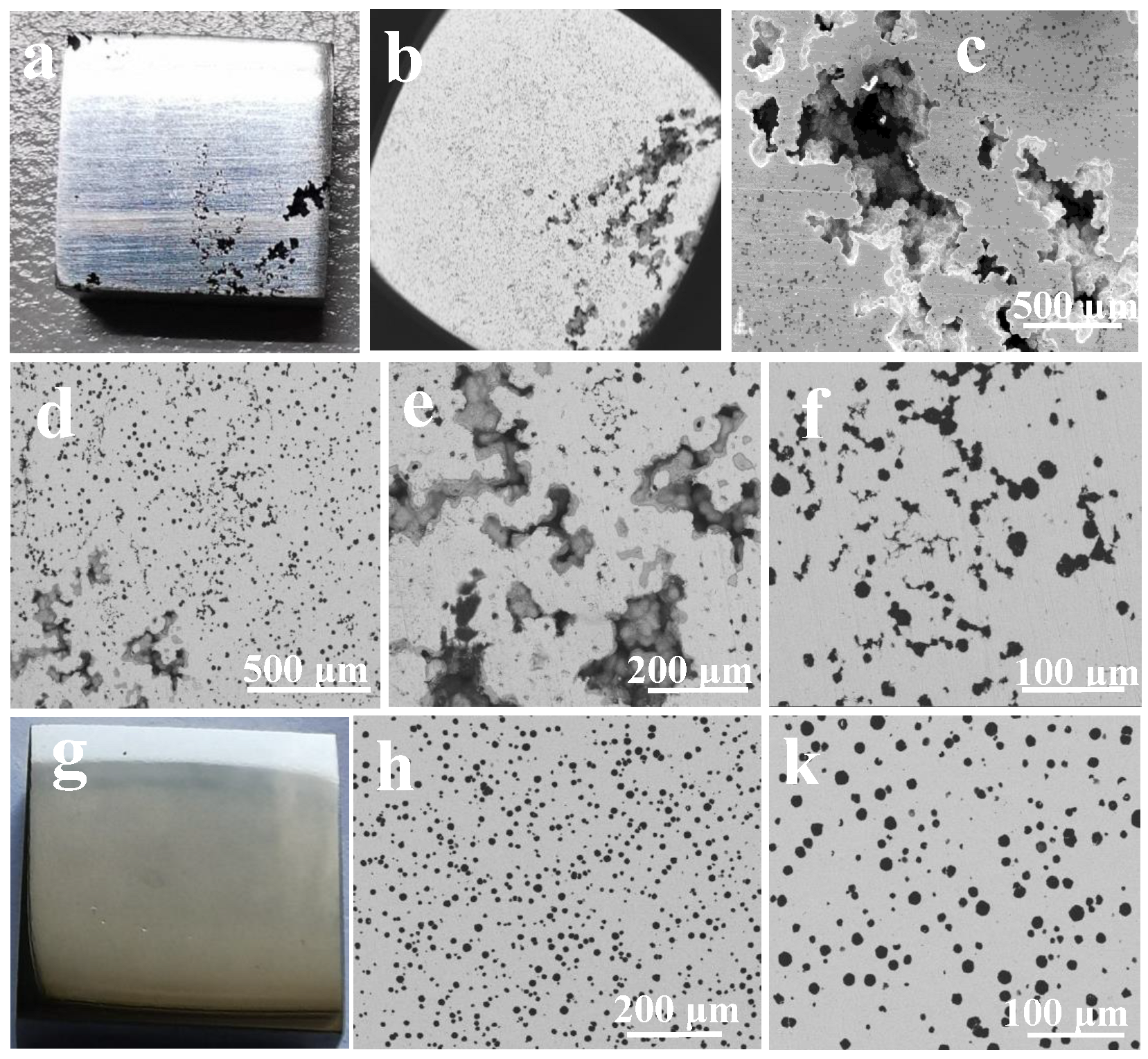
3.1.1. Microstructure Characteristics
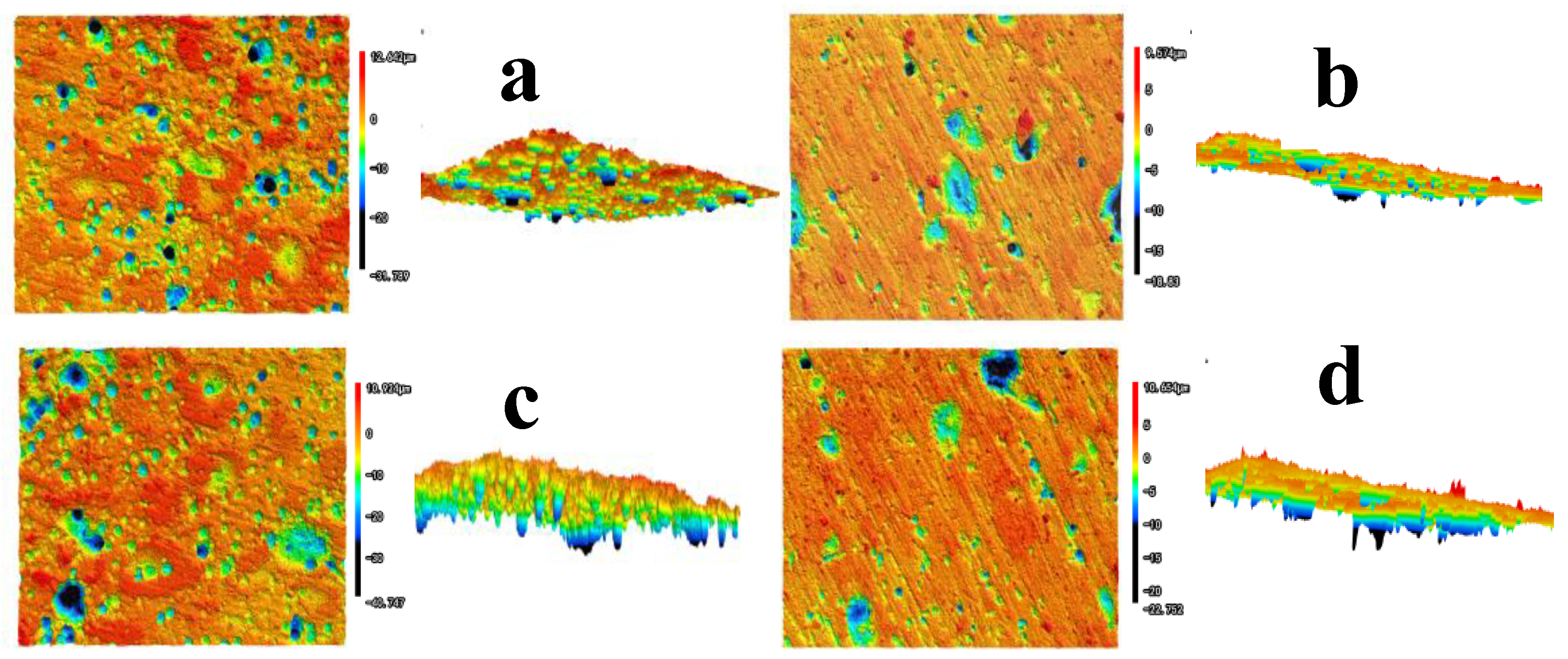
3.1.2. Corrosion Morphology
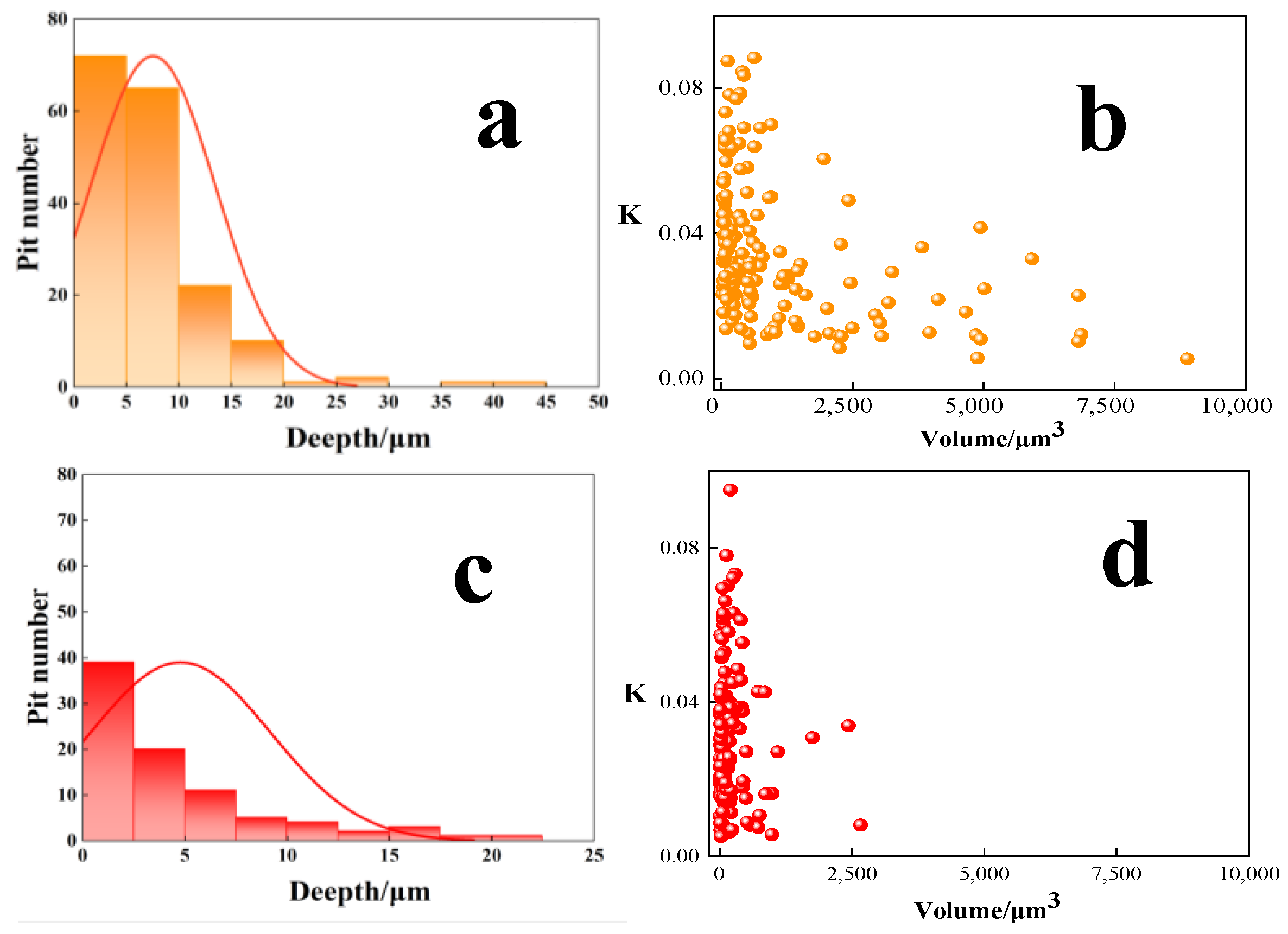
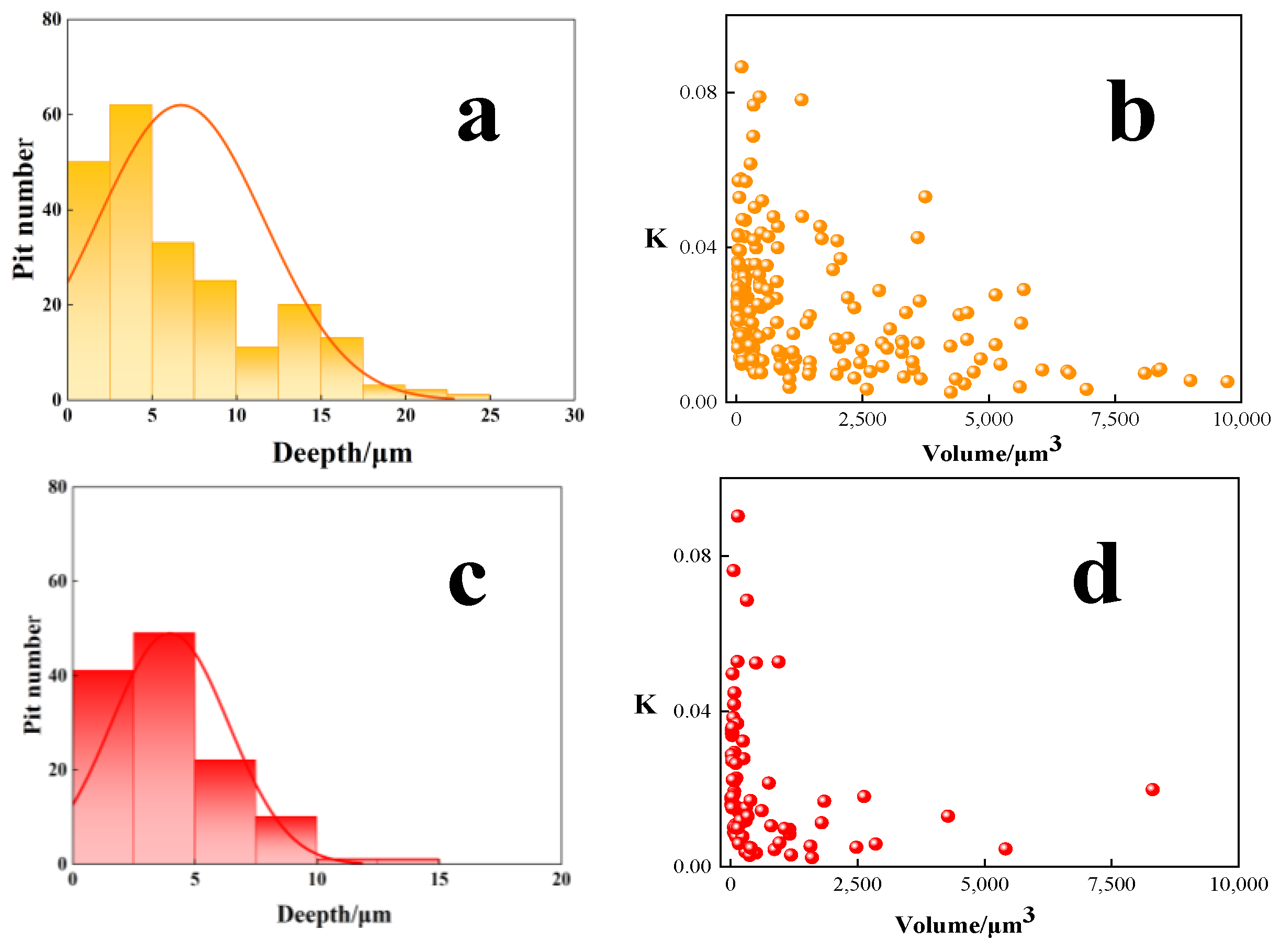
3.1.3. Mechanisms of the Localized Corrosion Initiation
3.1.4. Simulation of the Corrosion Process
3.2. The Effect of the Water Environment on the Corrosion Kinetics of Ductile Iron
3.2.1. Corrosion Rate Analysis
3.2.2. Electrochemical Test
3.2.3. The Influence of Environmental Factors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abubakar, S.A.; Mori, S.; Sumner, J. A Review of Factors Affecting SCC Initiation and Propagation in Pipeline Carbon Steels. Metals 2022, 12, 1397. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 2010, 52, 2716–2724. [Google Scholar] [CrossRef]
- Cassineri, S.; Duff, J.; Cioncolini, A.; Curioni, M.; Banks, A.; Scenini, F. Deposition of corrosion products under pressurised water nuclear reactor conditions: The effect of flow velocity and dissolved hydrogen. Corros. Sci. 2019, 159, 108113. [Google Scholar] [CrossRef]
- Koike, M.H. Erosion corrosion in stainless steel pipe under water vapour two-phase flow conditions. Corros. Sci. 2006, 48, 617–624. [Google Scholar] [CrossRef]
- Ma, W.L.; Wang, H.X.; Barker, R.; Kapur, N.; Hua, Y.; Neville, A. Corrosion behaviour of X65 carbon steel under the intermittent oil/water wetting: A synergic effect of flow velocity and alternate immersion period. Corros. Sci. 2021, 187, 109507. [Google Scholar] [CrossRef]
- Vasyliev, G.S. The influence of flow rate on corrosion of mild steel in hot tap water. Corros. Sci. 2015, 98, 33–39. [Google Scholar] [CrossRef]
- Wu, K.-h.; Zhu, L.-q.; Li, W.-p.; Liu, H.-c. Effect of Ca2+ and Mg2+ on corrosion and scaling of galvanized steel pipe in simulated geothermal water. Corros. Sci. 2010, 52, 2244–2249. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Cho, J.-H. Integrated evaluation of mixed surfactant distribution in water-oil-steel pipe environments and associated corrosion inhibition efficiency. Corros. Sci. 2016, 110, 213–227. [Google Scholar] [CrossRef]
- Choo, H.; Choi, Y.; Lee, W.; Lee, C. Effect of pH Variations on the Yield Stress of Calcium Bentonite Slurry Treated with pH-Responsive Polymer. Materials 2020, 13, 2525. [Google Scholar] [CrossRef]
- Ceschini, L.; Campana, G.; Pagano, N.; Angelini, V. Effect of laser surface treatment on the dry sliding behaviour of the EN-GJS400–12 ductile cast iron. Tribol. Int. 2016, 104, 342–351. [Google Scholar] [CrossRef]
- Mojisola, T.; Seidu, S.O.; Olubambi, P.A.; Adediran, A.A. Effect of preconditioning on the microstructure and mechanical properties of ductile cast iron. Mater. Today Proc. 2022, 62, S23–S29. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chen, M.-L. Corrosion behavior of nickel alloyed and austempered ductile irons in 3.5% sodium chloride. Corros. Sci. 2010, 52, 2945–2949. [Google Scholar] [CrossRef]
- Krawiec, H.; Stypuła, B.; Stoch, J.; Mikołajczyk, M. Corrosion behaviour and structure of the surface layer formed on austempered ductile iron in concentrated sulphuric acid. Corros. Sci. 2006, 48, 595–607. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Lelito, J.; Krystianiak, A.; Ozga, P. In-situ monitoring of the corrosion behaviour of austempered ductile iron (ADI) under cyclic salt spray exposure. Corros. Sci. 2021, 185, 109437. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, G.; Chen, Y.; Zhao, P.; Tian, Y. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, Z.; Zhan, M. An investigation of the erosion–corrosion characteristics of ductile cast iron. Mater. Des. 2007, 28, 260–265. [Google Scholar] [CrossRef]
- Wang, P.J.; Ma, L.W.; Cheng, X.Q.; Li, X.G. Influence of grain refinement on the corrosion behavior of metallic materials: A review. Int. J. Miner. Metall. 2021, 28, 1112–1126. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Zhang, Y.; Wang, C.; Fan, W. A method of atmospheric corrosion prediction for aircraft structure. Mater. Corros. 2018, 70, 79–90. [Google Scholar] [CrossRef]
- Salleh, S. Modelling Pitting Corrosion in Carbon Steel Materials. Ph.D. Thesis, University of Manchester, Manchester, UK, 2013. [Google Scholar]
- Zhao, J.; Liu, Y.; Yang, X.; Xin, H.E.; Wang, L.; Xiong, D.; Yanhong, G.U. Corrosion Behavior of Pipeline Steel in Oilfield Produced Water under Dynamic Corrosion System. J. Wuhan Univ. Technol. 2022, 37, 15. [Google Scholar] [CrossRef]
- Valor, A.; Caleyo, F.; Alfonso, L.; Rivas, D.; Hallen, J.M. Stochastic modeling of pitting corrosion: A new model for initiation and growth of multiple corrosion pits. Corros. Sci. 2007, 49, 559–579. [Google Scholar] [CrossRef]
- Van den Steen, N.; Gonzalez-Garcia, Y.; Mol, J.M.C.; Terryn, H.; Van Ingelgem, Y. Predicting the effect of droplet geometry and size distribution on atmospheric corrosion. Corros. Sci. 2022, 202, 11308. [Google Scholar] [CrossRef]
- Messinese, E.; Casanova, L.; Paterlini, L.; Capelli, F.; Bolzoni, F.; Ormellese, M.; Brenna, A. A Comprehensive Investigation on the Effects of Surface Finishing on the Resistance of Stainless Steel to Localized Corrosion. Metals 2022, 12, 1751. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting Corrosion of Metals. Corros. Sci. 1986, 41, 9. [Google Scholar]
- Dastgerdi, A.A.; Brenna, A.; Ormellese, M.; Pedeferri, M.P.; Bolzoni, F. Experimental design to study the influence of temperature, pH, and chloride concentration on the pitting and crevice corrosion of UNS S30403 stainless steel. Corros. Sci. 2019, 159, 108160.1–108160.9. [Google Scholar] [CrossRef]
- Dawson, J.L.; Ferreira, M. Crevice corrosion on 316 stainless steel in 3% sodium chloride solution. Corros. Sci. 1987, 26, 1027–1040. [Google Scholar] [CrossRef]
- Kuhn, R.J. Galvanic Corrosion on Cast Iron Pipes. Ind. Eng. Chem 2002, 22, 335–341. [Google Scholar] [CrossRef]
- Tavakkolizadeh, M. Galvanic Corrosion of Carbon and Steel in Aggressive Environments. J. Compos. Constr. 2001, 5, 200–210. [Google Scholar] [CrossRef]
- Varela, F.E.; Kurata, Y.; Sanada, N. The influence of temperature on the galvanic corrosion of a cast iron-stainless steel couple (prediction by boundary element method). Corros. Sci. 1997, 39, 775–788. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, L.; Wang, C.; Wang, Z. Finite Element Analysis of Multipoint Counter Electrode Sensor in Steel Corrosion Rate Measurement. IEEE Sens. J. 2014, 14, 790–792. [Google Scholar] [CrossRef]
- Shafei, B.; Alipour, A.; Shinozuka, M. Prediction of corrosion initiation in reinforced concrete members subjected to environmental stressors: A finite-element framework. Cem. Concr. Res. 2012, 42, 365–376. [Google Scholar] [CrossRef]
- Sharland, S.M.; Jackson, C.P.; Diver, A.J. A finite-element model of the propagation of corrosion crevices and pits. Corros. Sci. 1989, 29, 1149–1166. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Sun, M.; Jia, J.; Cheng, X.; Pei, Z.; Li, Q.; Xu, D.; Xiao, K.; Li, X. A new understanding of the effect of Cr on the corrosion resistance evolution of weathering steel based on big data technology. J. Mater. Sci. Technol. 2022, 104, 67–80. [Google Scholar] [CrossRef]
- Sung-Ho, T.; Takafumin, N.; Takumi, U. Corrosion Resistance of Cr-bearing Rebar in Macrocell Corrosion Environments Due to Different Concentrations of Chloride Ions. ISIJ Int. 2006, 46, 1467–1472. [Google Scholar]
- Jeong, J.-Y.; Jeong, C.; Kim, Y.-J.; Jang, C. Effect of Stress Magnitude on Pit Growth Rate of 304 Austenitic Stainless Steel in Chloride Environments. Metals 2021, 11, 1415. [Google Scholar] [CrossRef]
- Chao, L.; Rir, C.; Xuan, L.A.; Zj, A.; Sy, D.; Zc, E.; Dza, B.; Hta, C.; Xla, B. Technology: New insights into the mechanism of localised corrosion induced by TiN-containing inclusions in high strength low alloy steel. J. Mater. Sci. 2022, 124, 141–149. [Google Scholar]
- Liu, C.; Li, X.; Revilla, R.I.; Sun, T.; Li, X. Towards a better understanding of localised corrosion induced by typical non-metallic inclusions in low-alloy steels. Corros. Sci. 2021, 179, 109150. [Google Scholar] [CrossRef]
- Qiao, L.J.; Hsiao, C.M.; Chu, W.Y.; Chen, L.; Zou, J.J. The concentration of hydrogen at crack tip of austenitic stainless steel after stress corrosion and polarization. Scr. Metall. 1988, 22, 627–630. [Google Scholar] [CrossRef]
- Eškinja, M.; Moshtaghi, M.; Hönig, S.; Zehethofer, G.; Mori, G. Investigation of the effects of temperature and exposure time on the corrosion behavior of a ferritic steel in CO2 environment using the optimized linear polarization resistance method. Results Mater. 2022, 14, 100282. [Google Scholar] [CrossRef]
- Choi, Y.S.; Shim, J.J.; Kim, J.G. Effects of Cr, Cu, Ni and Ca on the corrosion behavior of low carbon steel in synthetic tap water. J. Alloys Compd. 2005, 391, 162–169. [Google Scholar] [CrossRef]
- García, I.; Damborenea, J. Corrosion properties of tin prepared by laser gas alloying of ti and ti6al4v. Corros. Sci. 1998, 40, 01419. [Google Scholar] [CrossRef]
- Parapurath, S.; Jacob, L.; Gunister, E.; Vahdati, N. Effect of Microstructure on Electrochemical Properties of the EN S275 Mild Steel under Chlorine-Rich and Chlorine-Free Media at Different pHs. Metals 2022, 12, 1386. [Google Scholar] [CrossRef]
- Jia, S.; Gao, J.; Guo, H. Influence of Water Quality on Corrosion of Cast Iron Pipe in Reclaimed Water. J. Chin. Soc. Corros. Prot. 2020, 40, 569–576. [Google Scholar]
- Aziz, I.; Qi, Z.; Min, X. Using EIS to evaluate anti-corrosion properties of the SiC_p/5A06 aluminium MMC treated by cerium conversion coatings. J. Rare Earths 2010, 28, 109–116. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, L.; Zhu, Y.; Ai, F.; Li, H.; Li, Y.; Jiang, Z. The Effect of Immersion Corrosion Time on Electrochemical Corrosion Behavior and the Corrosion Mechanism of EH47 Ship Steel in Seawater. Metals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Improvement of Corrosion Properties of Microarc Oxidation Coating on Magnesium Alloy by Optimizing Current Density Parameters. Appl. Surf. Sci. 2007, 253, 6939–6945. [Google Scholar] [CrossRef]
- Tian, Z.L.; Zhang, T.; Wei, C.J.; Lai, Y.Q.; Li, J. Effect of current density on corrosion of NiFe2O4 based cermet inert anode for aluminum electrolysis. Chin. J. Nonferrous Met. 2014, 24, 2360–2365. [Google Scholar]

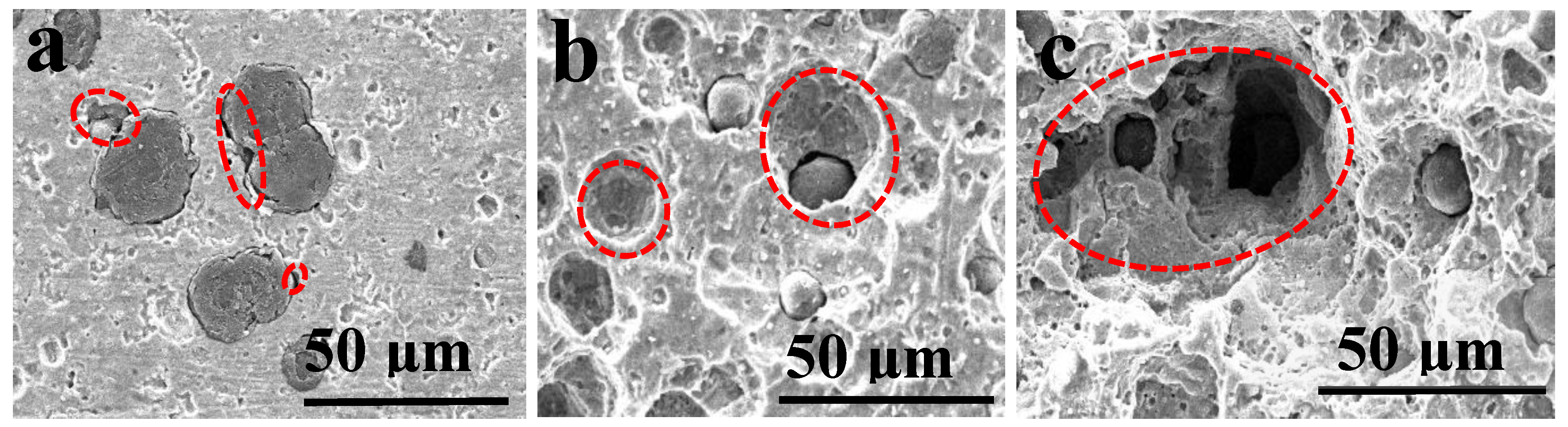
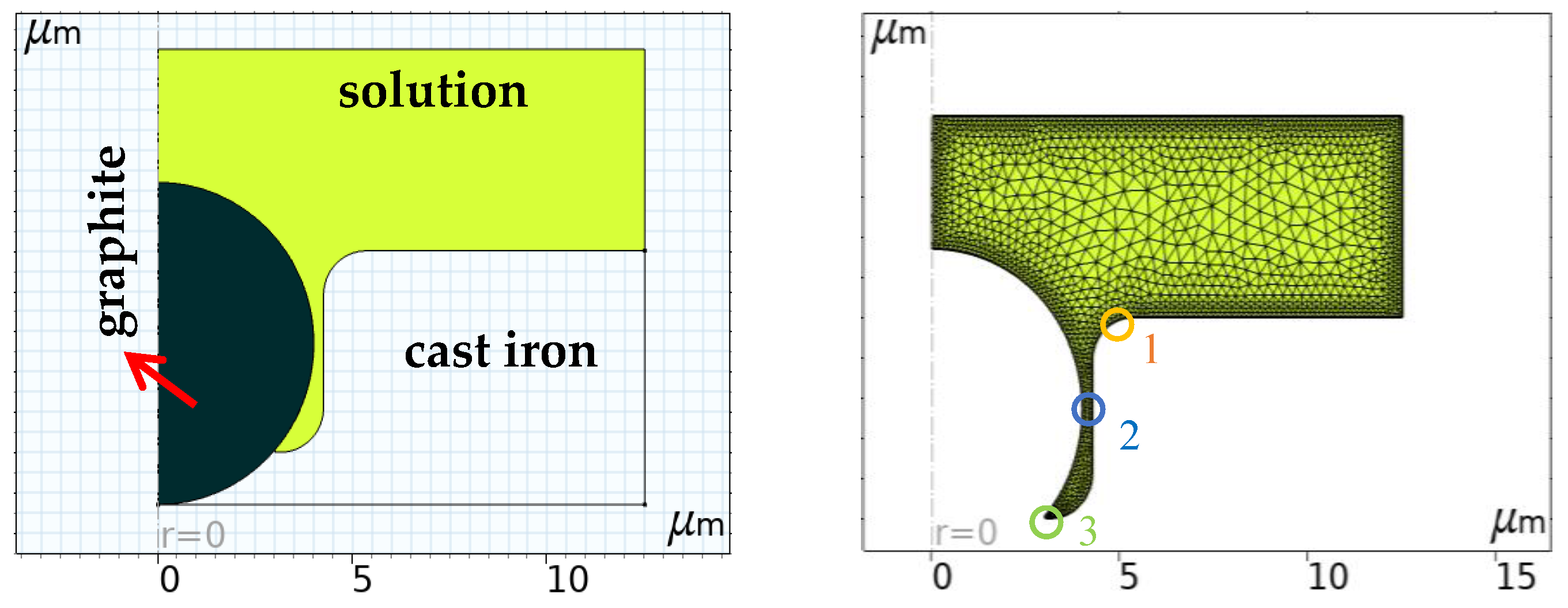
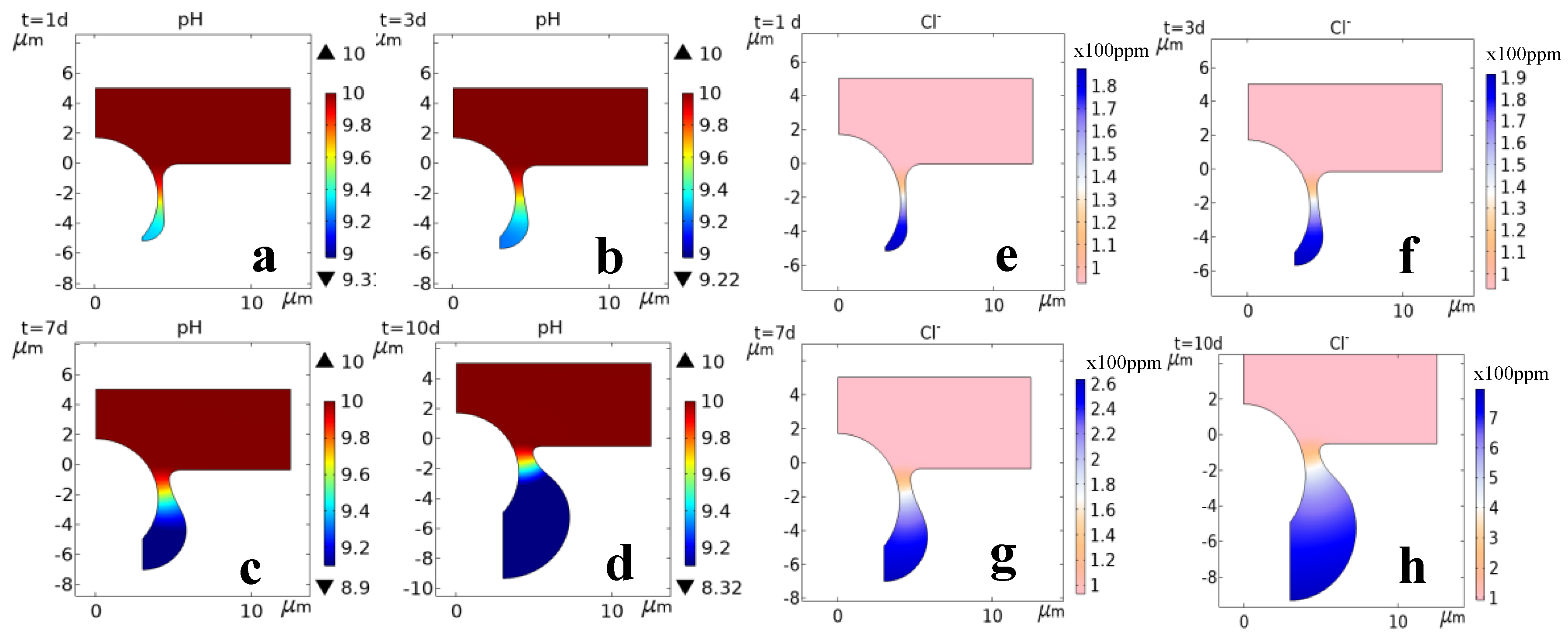
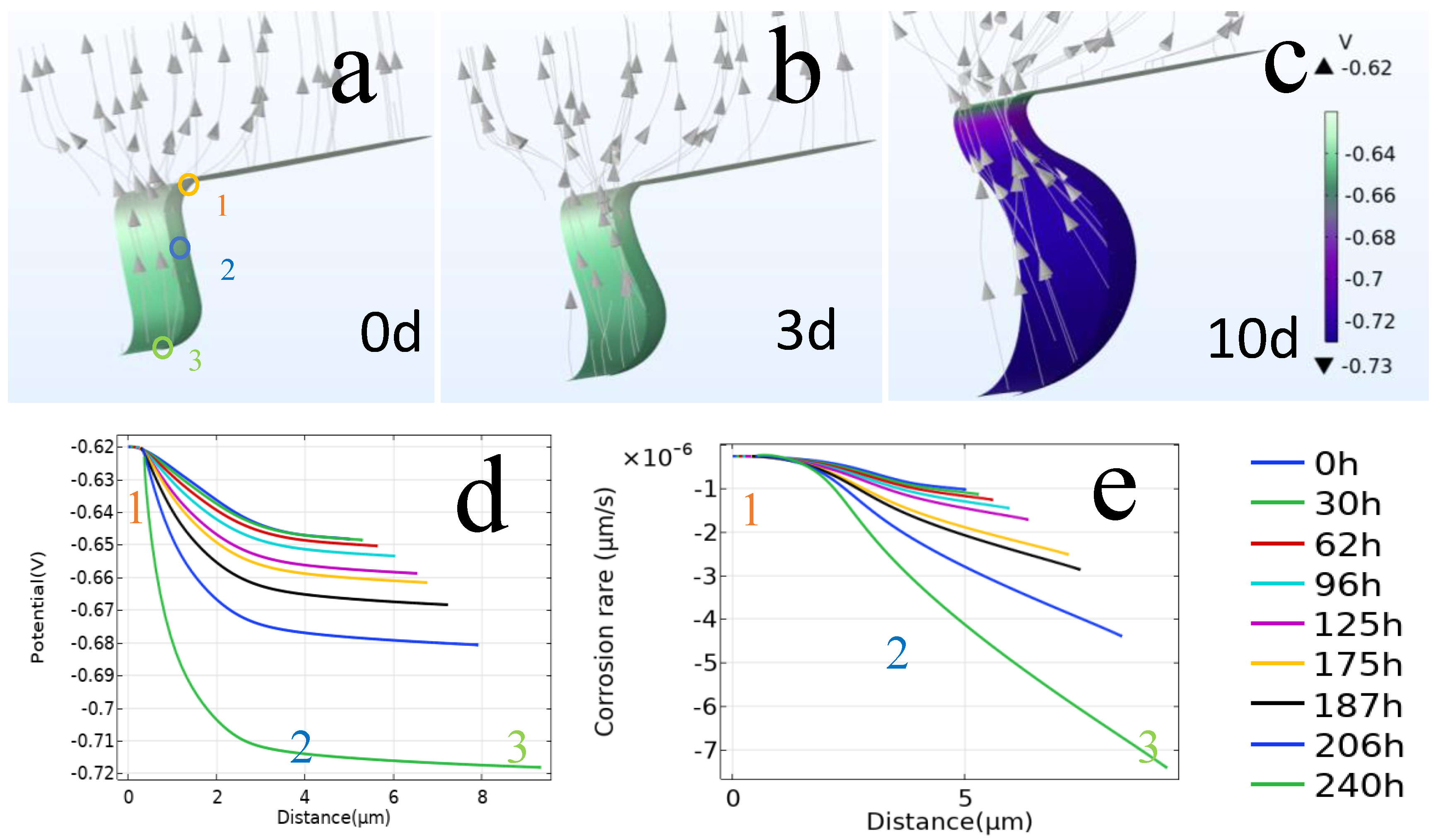

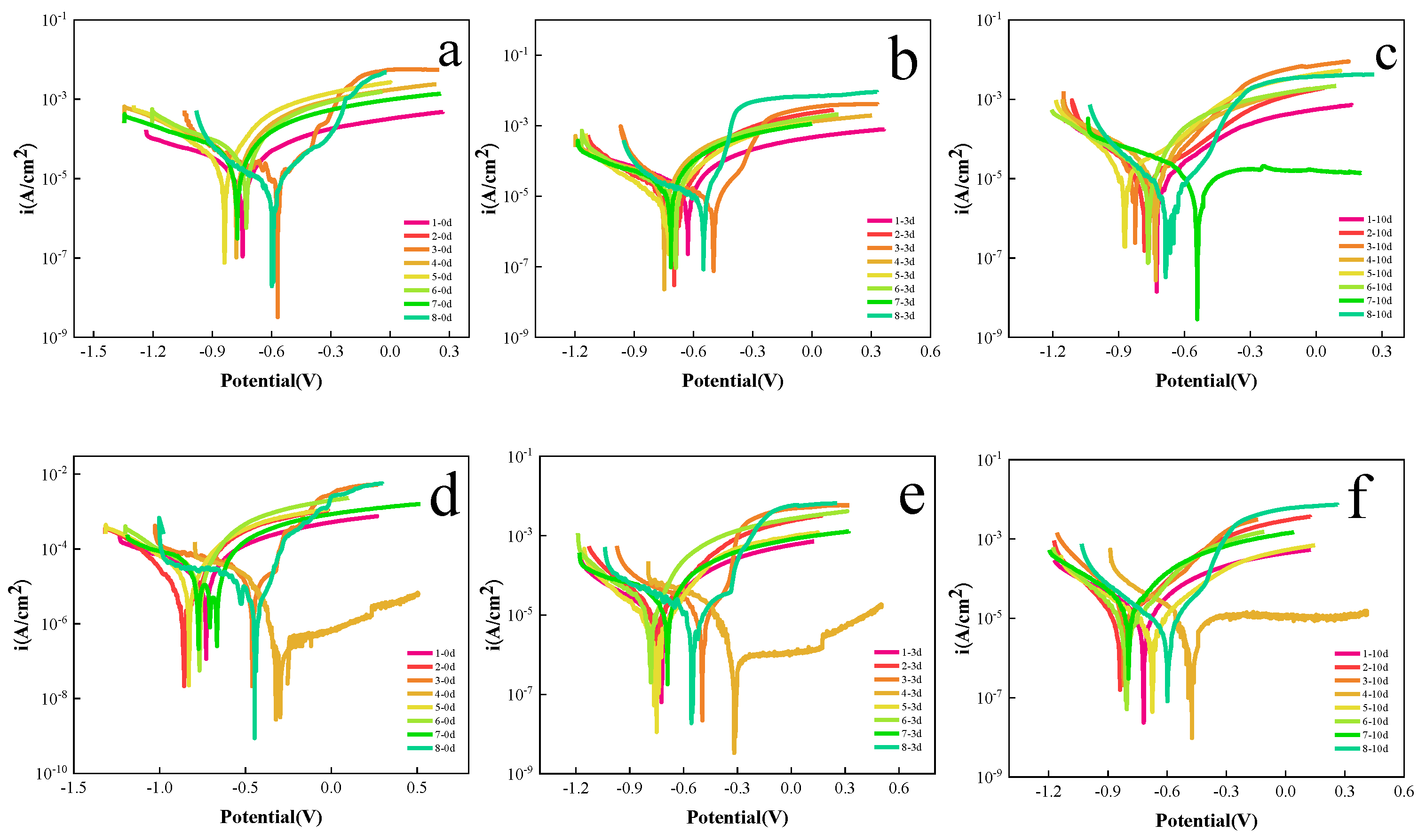
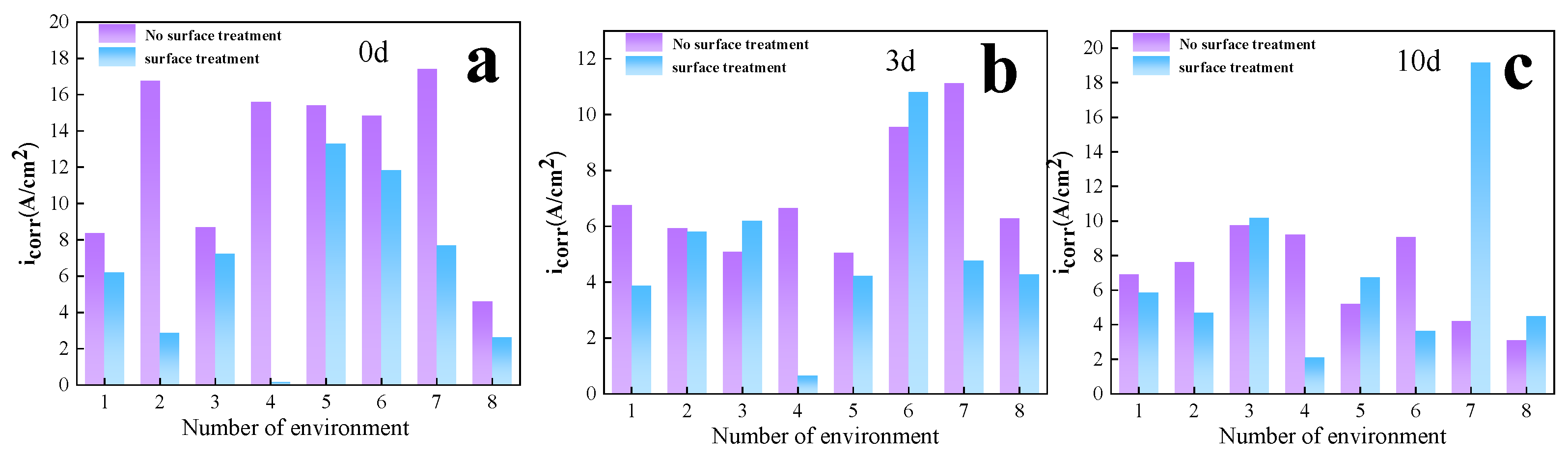
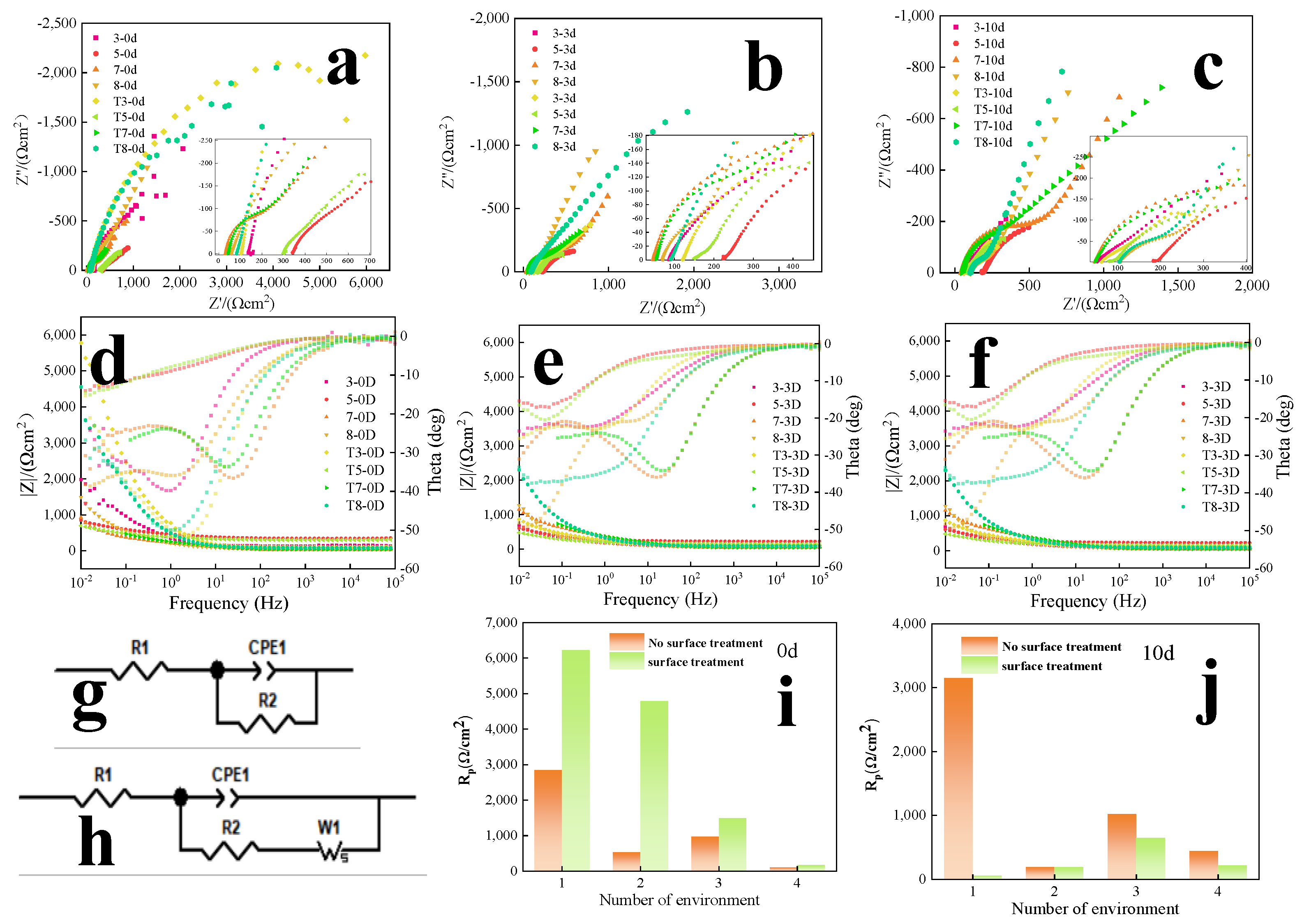
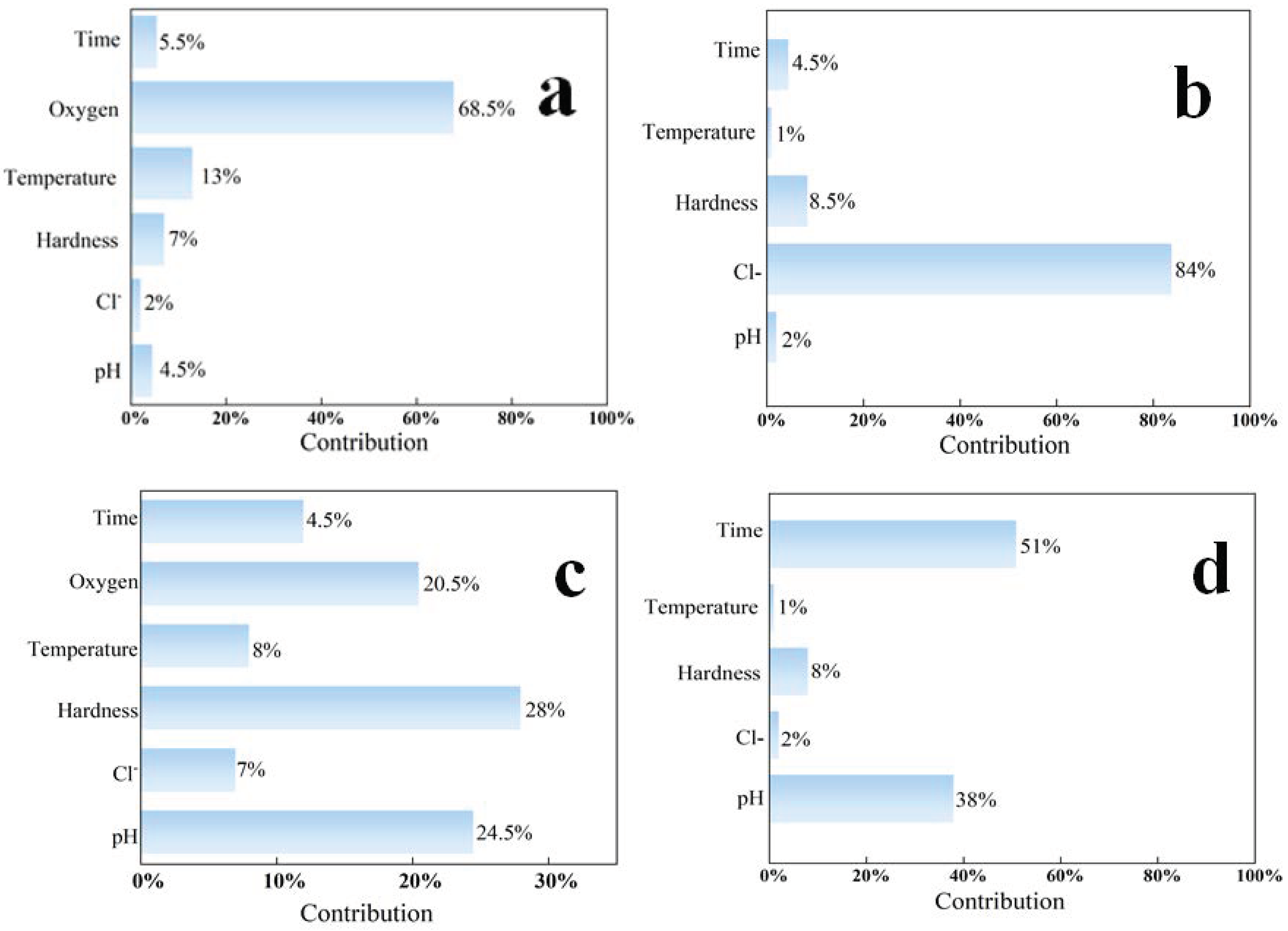
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Hardness/ppm | 20 | 20 | 20 | 20 | 80 | 80 | 80 | 80 |
| pH | 7 | 7 | 10 | 10 | 7 | 7 | 10 | 10 |
| Temperature/°C | 60 | 90 | 90 | 60 | 90 | 60 | 60 | 90 |
| Cl−/ppm | 20 | 120 | 20 | 120 | 20 | 120 | 20 | 120 |
| Oxygen concentration/mg∗L−1 | 0.3 | 0.3 | 6.5 | 6.5 | 6.5 | 0.3 | 6.5 | 0.3 |
| Times(d) | icorr without Mechanical Treatment(A/cm2) | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 0 | 8.3 | 16.8 | 8.7 | 15.6 | 15.4 | 14.8 | 17.4 | 4.6 |
| 3 | 6.8 | 5.9 | 5.1 | 6.7 | 5.0 | 9.6 | 11.1 | 6.3 |
| 10 | 6.9 | 7.6 | 9.7 | 9.2 | 5.2 | 9.1 | 4.2 | 3.1 |
| Average | 7.3 | 10.1 | 7.8 | 10.5 | 8.5 | 11.1 | 10.9 | 4.6 |
| icorr with mechanical treatment(A/cm2) | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 0 | 6.2 | 2.9 | 7.2 | 0.2 | 13.3 | 11.8 | 7.7 | 2.6 |
| 3 | 3.9 | 5.8 | 6.2 | 0.6 | 4.2 | 10.8 | 4.8 | 4.2 |
| 10 | 5.9 | 4.7 | 10.2 | 2.1 | 6.7 | 3.6 | 19.2 | 4.5 |
| Average | 5.3 | 4.5 | 7.9 | 1.0 | 8.1 | 8.7 | 10.6 | 3.8 |
| Times(d) | Rs without Mechanical Treatment (Ω/cm2) | Rp/WR without Mechanical Treatment (Ω/cm2) | ||||||
| 3 | 8 | 5 | 7 | 3 | 8 | 5 | 7 | |
| 0 | 112 | 58 | 327 | 31 | 2848 | 526/ 3 | 967 | 100 /2 |
| 3 | 84 | 69 | 226 | 46. | 989 | 139.4/0.5 | 821 | 163 /4 |
| 10 | 49 | 108 | 187 | 45 | 3149 | 193/ | 1020 | 445 /0.2 |
| Times(d) | Rs with mechanical treatment (Ω/cm2) | Rp/WR with mechanical treatment (Ω/cm2) | ||||||
| 3 | 8 | 5 | 7 | 3 | 8 | 5 | 7 | |
| 0 | 102 | 83 | 297 | 52 | 6222 | 4785 | 1493 | 171/ 36 |
| 3 | 118 | 91 | 154 | 35 | 1409 | 366.1 /62 | 547 | 182 /14 |
| 10 | 58 | 109 | 133 | 47 | 58.26 | 150/1.1 | 650 | 220 /4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Liu, T.; Tao, K.; Zhu, L.; Liu, C.; Yong, X.; Cheng, X. A Study of the Mechanisms and Kinetics of the Localized Corrosion Aggravation of Ductile Iron in a Harsh Water Quality Environment. Metals 2022, 12, 2103. https://doi.org/10.3390/met12122103
Wang B, Liu T, Tao K, Zhu L, Liu C, Yong X, Cheng X. A Study of the Mechanisms and Kinetics of the Localized Corrosion Aggravation of Ductile Iron in a Harsh Water Quality Environment. Metals. 2022; 12(12):2103. https://doi.org/10.3390/met12122103
Chicago/Turabian StyleWang, Bingqin, Tao Liu, Kai Tao, Lingsheng Zhu, Chao Liu, Xingyue Yong, and Xuequn Cheng. 2022. "A Study of the Mechanisms and Kinetics of the Localized Corrosion Aggravation of Ductile Iron in a Harsh Water Quality Environment" Metals 12, no. 12: 2103. https://doi.org/10.3390/met12122103
APA StyleWang, B., Liu, T., Tao, K., Zhu, L., Liu, C., Yong, X., & Cheng, X. (2022). A Study of the Mechanisms and Kinetics of the Localized Corrosion Aggravation of Ductile Iron in a Harsh Water Quality Environment. Metals, 12(12), 2103. https://doi.org/10.3390/met12122103






