Multiple Glass Transitions in Bismuth and Tin beyond Melting Temperatures
Abstract
1. Introduction
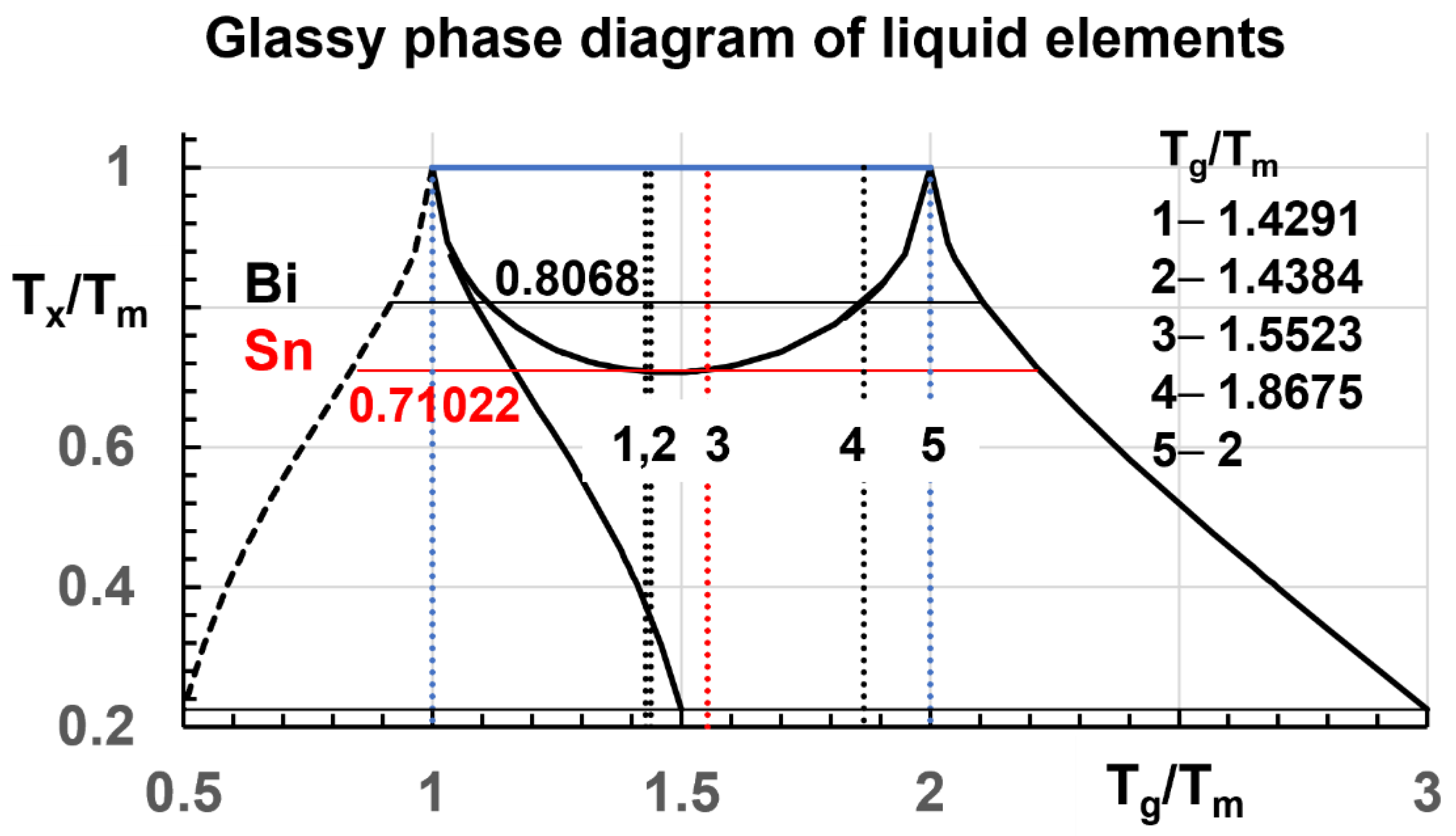
2. Diagram of Glassy Phases
3. Diagram Applications
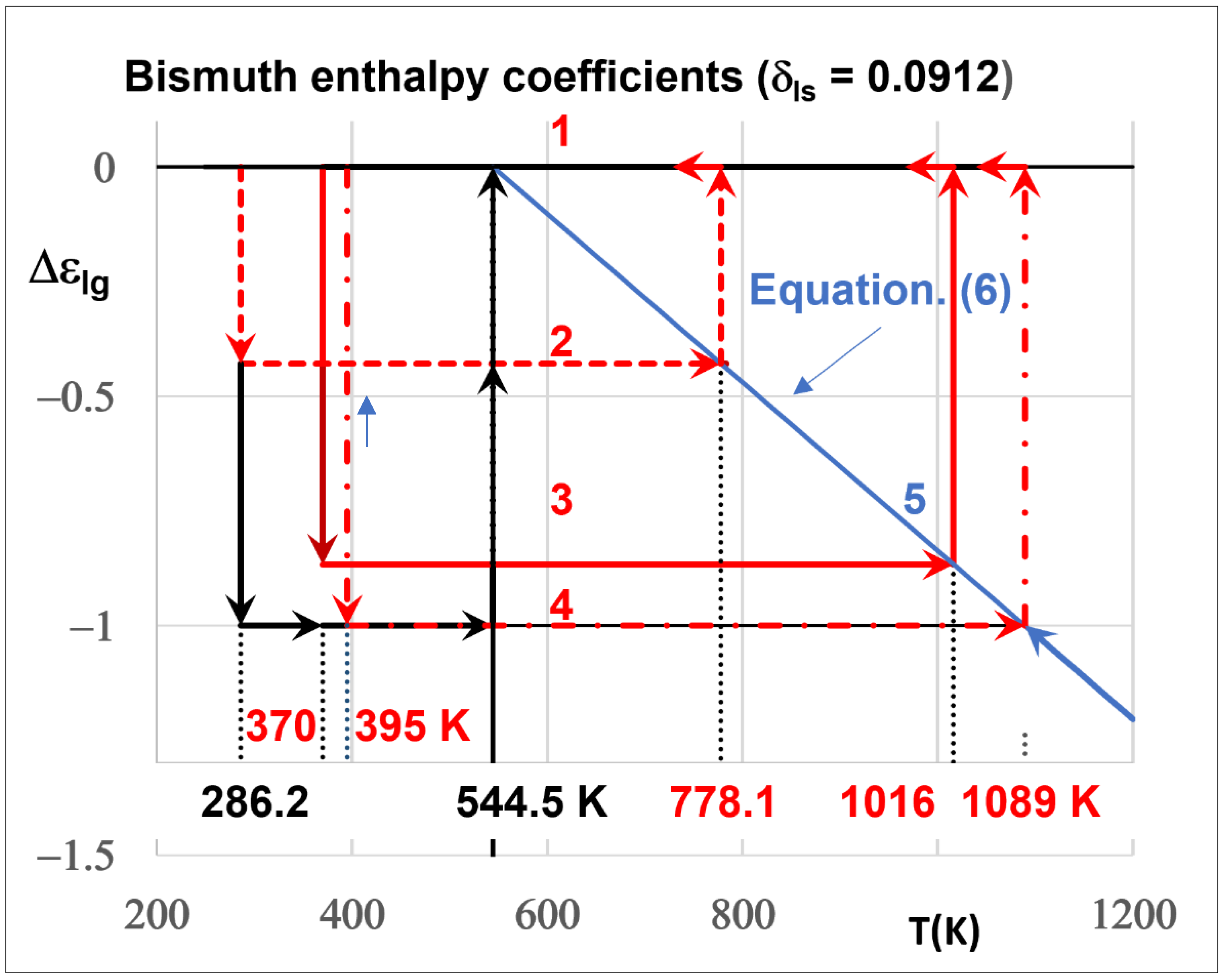
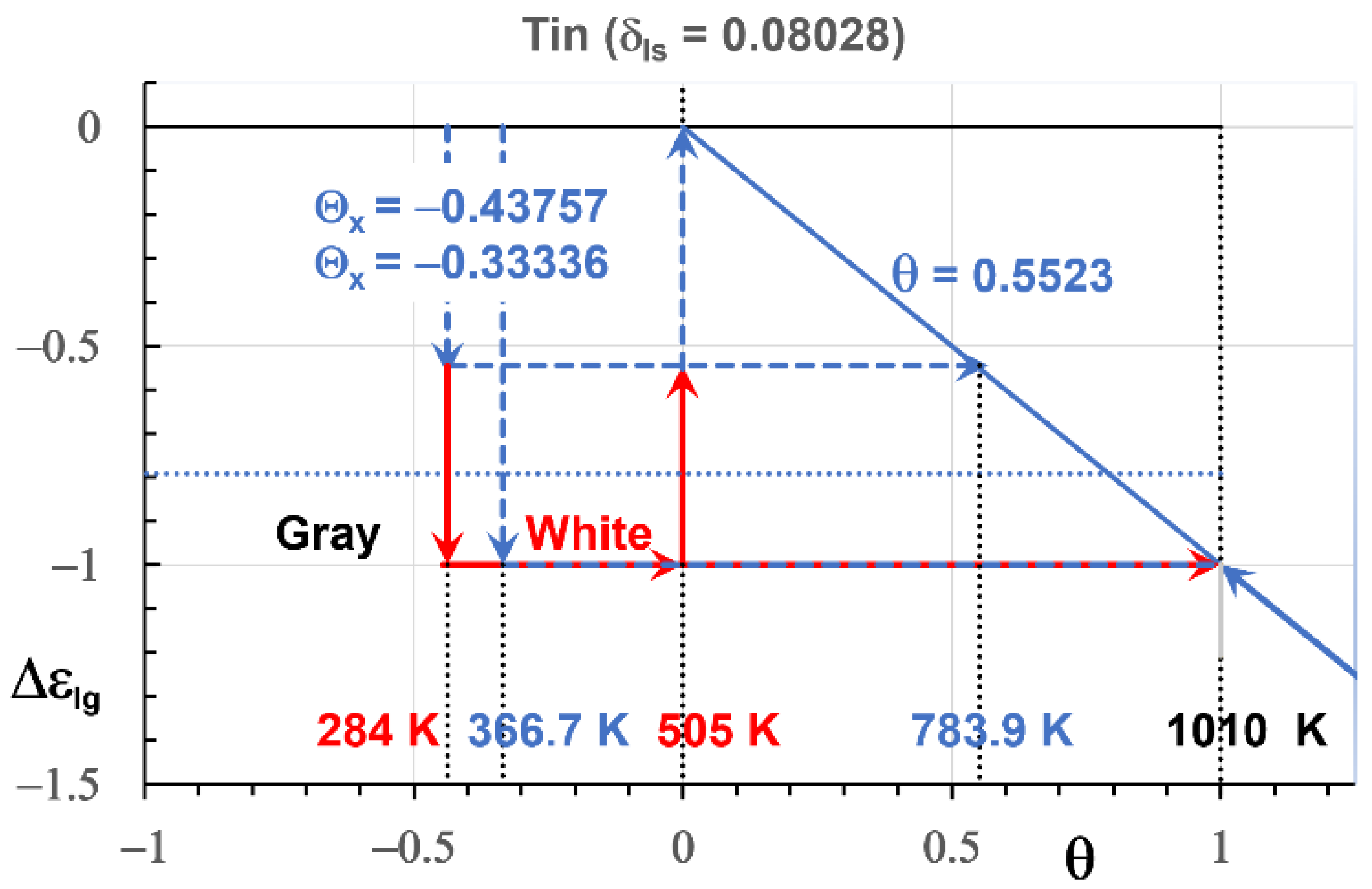
4. Singular Enthalpy Coefficients
4.1. Bismuth
4.2. Tin
5. Experimental Densities of Bi and Sn during Heating
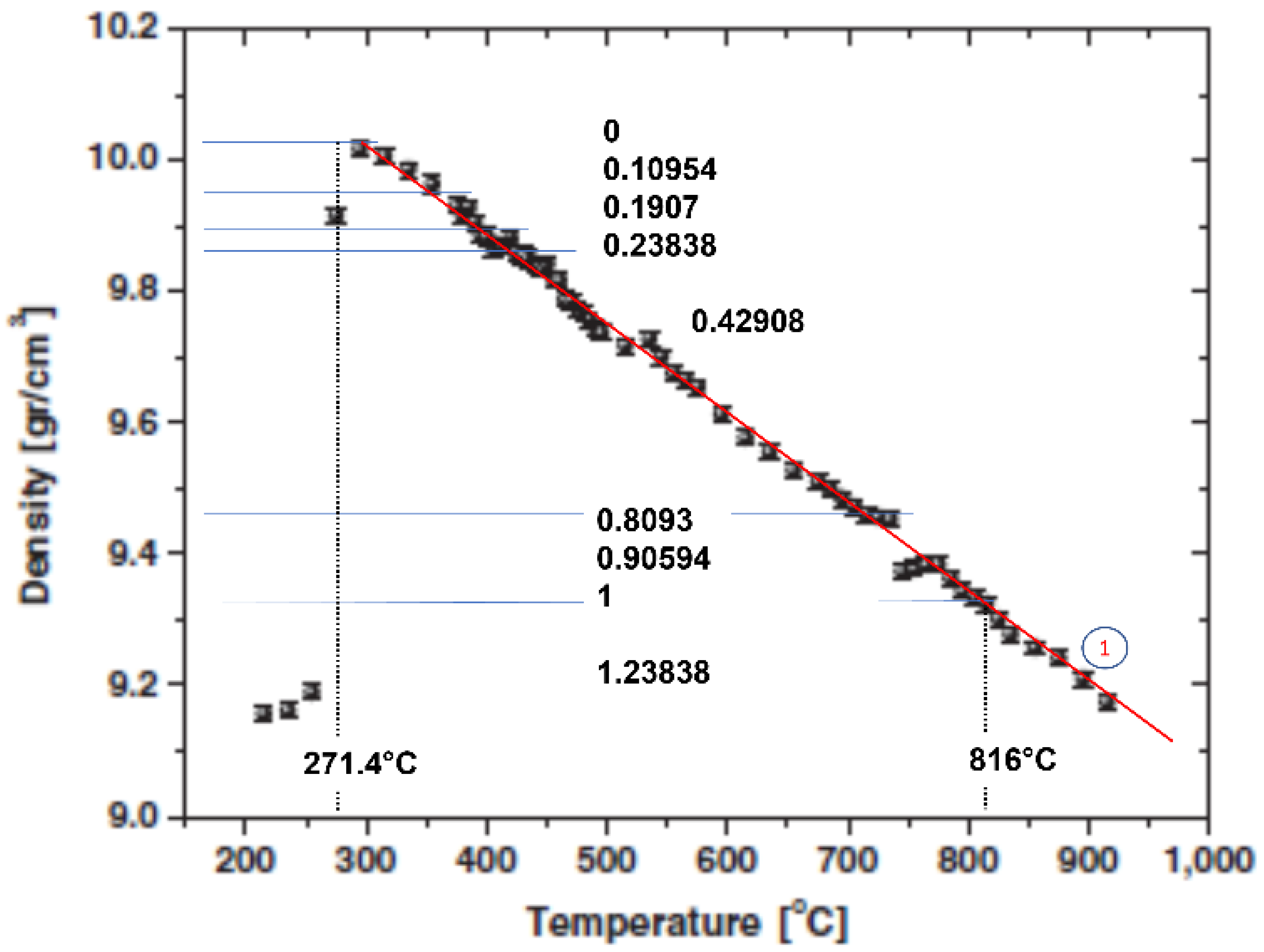
5.1. Bismuth Density
5.2. Tin Density
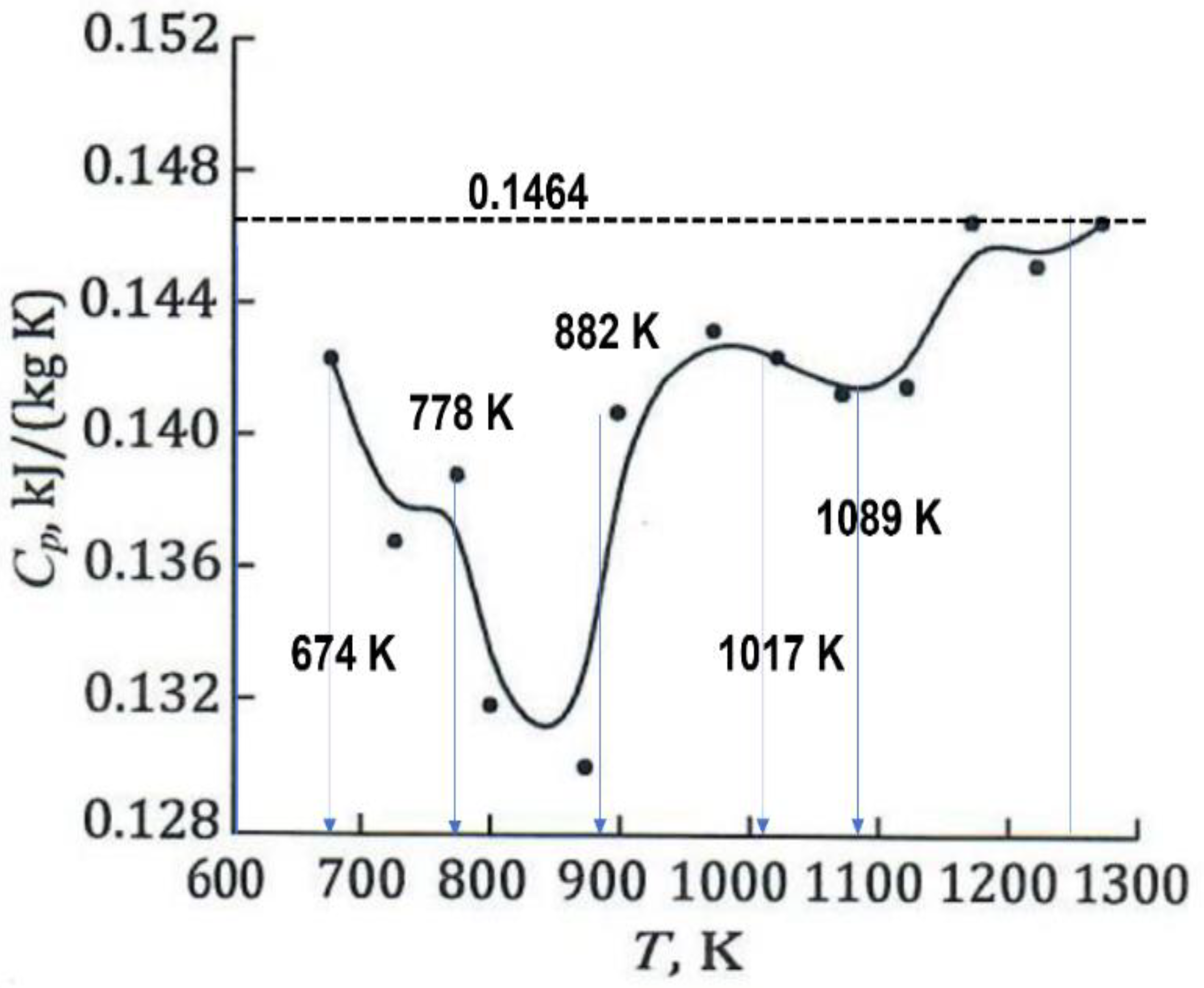
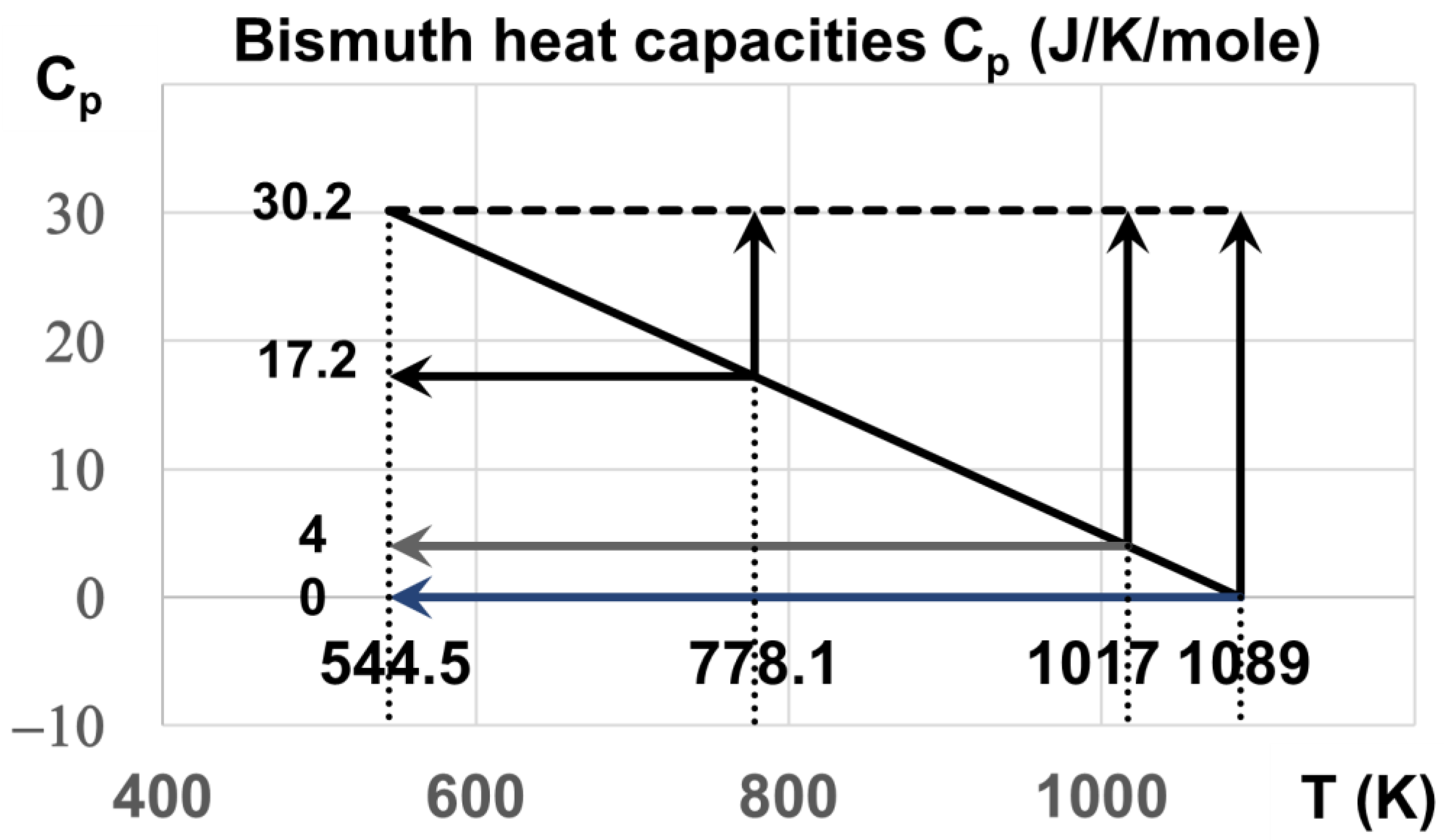
6. The Heat Capacities during Heating
7. Other Experimental Observations of Glassy States above Tm
7.1. In Tin
7.2. In Bismuth
7.3. BiSb20 wt%
7.4. InSn80 wt%
7.5. PbSn61.9 wt%
8. Another Method to Stabilize Glassy States above (2 Tm)
9. Conclusions
- (1)
- After melting Bi and Sn at T = Tm, weak fractions (f) were built by slow heating (0.1 °C/min for Bi and one hour between each measurement for Sn) and melted at θg = θn+ = (Tn+ − Tm)/Tm = Δε. Liquid-liquid transitions were observed at 1.8675 Tm for bismuth and 1.5523 Tm for Sn. The glassy character of these transitions is confirmed by structural transitions that we attribute to the melting of configurons. An observed glass transition in Sn was also characterized by a weak specific heat jump predicted by the NCHN model and a peak at 1.5523 Tm due to the thermodynamic character of a transition obeying critical exponents associated with configuron percolation. There is no endothermic latent heat (Δε Hm) during heating.
- (2)
- After melting Bi and Sn at T = Tm, transitions were also observed at T = 2 Tm by DTA and (or) resistivity that we consider as new glass transitions. The transition was reversible only for Sn. A weak endothermic heat instead of Hm was observed for Bi at 2 Tm. The glassy states of Bi and Sn could have a density equal to that of the liquid at Tm only after reversing heating to cooling and a long incubation time close to Tm.
- (3)
- After melting Bi at T = Tm, high-resolution measurements of the density showed the existence of singular values corresponding to those of the enthalpy of Phase 3. The melting heat at Tm corresponds to 1.23838 Hm instead of Hm including, in addition, the latent heat of glassy phase as predicted by the NCHN model.
- (4)
- A glassy phase diagram is proposed for systems having their lowest transition determined by their Lindemann coefficients. Each first order transition at Tx < Tm leads to multiple glass transitions. The possible existence of weak glassy fractions (f) for Tx/Tm < 0.7069, with glass transition temperatures much higher than (2 Tm) is envisaged (beyond (3 Tm) for f < 22.45%). Resistivity measurements showed decreases in Bi and Sn from Tm to 2 Tm and beyond, after thermal cycling between undercooled liquid and solid states.
- (5)
- The glassy phase formations at Tx are accompanied by latent heats, without being recovered at Tn+ with (Tm < Tn+ < 2 Tm) during heating. Predictions of their contribution equal to (θn+ Hm/Tm) are proposed for Tn+ = Tg ≤ 2 Tm. The heat capacity linearly decreases down to zero when Δε increases up to 1 (Tn+ = 2 Tm). An enthalpy equal to zero, down to Tm, would be induced by reversing heating to cooling from a temperature slightly weaker than Tg = Tn+. We show, for the first time, that the liquid-specific heat is constant between Tm and 2 Tm in the absence of glassy phase.
- (6)
- The stability of glassy fractions for Tx/Tm < 0.7069 can be very high because their (Tg) could be much higher than (2 Tm) as proved by resistivity decreases observed up to 2 Tm and beyond in Bi and Sn. The glassy fraction (f) is enhanced by successive thermal cycles between solid and liquid states. Each new glassy fraction could be added and could reinforce the total glassy fraction at very high temperatures up to f ≤ 100%.
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Bian, X.; Liu, J. Discontinuous structural phase transition of liquid metal and alloys. Phys. Lett. A 2004, 326, 429–435. [Google Scholar] [CrossRef]
- Yue, Y. Experimental evidence for the existence of an ordered structure in a silicate liquid above its liquidus temperature. J. Non-Cryst. Sol. 2004, 345, 523–527. [Google Scholar] [CrossRef]
- Greenberg, Y.; Yahel, E.; Caspi, E.M.; Benmore, C.; Beuneu, B.; Dariel, M.P.; Makov, G. Evidence for a temperature-driven structural transition in liquid bismuth. EPL 2009, 86, 36004. [Google Scholar] [CrossRef]
- Zu, F.-Q. Temperature-Induced Liquid-Liquid Transition in Metallic Melts: A Brief Review on the New Physical Phenomenon. Metals 2015, 5, 395–417. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Chen, J.; Chen, S.; Yang, W.; Ren, Y.; Zuo, X.; Zeng, J.; Wu, Q.; Sheng, H. Folded network and structural transition in molten tin. Nat. Commun. 2022, 13, 126. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Hu, R.; Kou, H.; Beaugnon, E. Evidence for the structure transition in a liquid Co-Sn alloy by in-situ magnetization measurement. Mater. Lett. 2015, 145, 261–263. [Google Scholar] [CrossRef]
- He, Y.-X.; Li, J.; Wang, J.; Kou, H.; Beaugnon, E. Liquid-liquid structure transition and nucleation in undercooled Co-B eutectic alloys. Appl. Phys. A 2017, 123, 391. [Google Scholar] [CrossRef]
- He, Y.-X.; Li, J.-S.; Wang, J.; Beaugnon, E. Liquid-liquid structure transition in metallic melt and its impact on solidification: A review. Trans. Nonferr. Met. Soc. China 2020, 30, 2293–2310. [Google Scholar] [CrossRef]
- Qiu, X.; Li, J.; Wang, J.; Guo, T.; Kou, H.; Beaugnon, E. Effect of liquid-liquid structure transition on the nucleation in undercooled Co-Sn eutectic alloy. Mater. Chem. Phys. 2016, 170, 261–265. [Google Scholar] [CrossRef]
- Bennett, T.D.; Yue, Y.; Li, P.; Qiao, A.; Tao, H.Z.; Greaves, N.G.; Richards, T.; Lanpronti, G.I.; Redfern, S.A.T.; Blanc, F.; et al. Melt-quenched glasses of metal-organic frameworks. J. Am. Chem. Soc. 2016, 138, 3484–3492. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Li, J.; Li, C.; Kou, H.; Zhang, P.; Beaugnon, E. Nucleation of supercooled Co melts under a high magnetic field. Mater. Chem. Phys. 2019, 225, 133–136. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, L.; Sun, Q.; Qin, J.; Brian, X.; Yue, Y. Indication of liquid-liquid phase transition in CuZr-based melts. Appl. Phys. Lett. 2013, 103, 171904. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kiraga, K.; Inoue, A.; Masumoto, T.; Jo, H.H. Crystallization and high mechanical strengthof Al-based amorphous alloys. Mater. Trans. 1994, 35, 293–302. [Google Scholar] [CrossRef]
- Hu, Q.; Sheng, H.C.; Fu, M.W.; Zeng, X.R. Influence of melt temperature on the Invar effect in (Fe71.2B.024Y4.8)96Nb4 bulk metallic glasses. J. Mater. Sci. 2019, 48, 6900–6906. [Google Scholar]
- Popel, P.S.; Chikova, O.A.; Matveev, V.M. Metastable colloidal states of liquid metallic solutions. High Temp. Mater. Proc. 1995, 4, 219–233. [Google Scholar] [CrossRef]
- Jiang, H.-R.; Bochtler, B.; Riegler, X.-S.; Wei, S.S.; Neuber, N.; Frey, M.; Gallino, I.; Busch, R.; Shen, J. Thermodynamic and kinetic studies of the Cu-Zr-Al(-Sn) bulk metallic glasses. J. Alloy. Compd. 2020, 844, 156126. [Google Scholar] [CrossRef]
- Wei, S.; Yang, F.; Bednarcik, J.; Kaban, I.; Shuleshova, O.; Meyer, A.; Busch, R. Liquid-liquid transition in a strong bulk metallic glass-forming liquid. Nat. Commun. 2013, 4, 2083. [Google Scholar] [CrossRef]
- Lan, S.; Ren, Y.; Wei, X.Y.; Wang, B.; Gilbert, E.P.; Shibayama, T.; Watanabe, S.; Ohnuma, M.; Wang, X.-L. Hidden amorphous phase and reentrant supercooled liquid in Pd-Ni-P metallic glass. Nat. Commun. 2017, 8, 14679. [Google Scholar] [CrossRef]
- Yang, B.; Perepezko, J.H.; Schmeltzer, J.W.P.; Gao, Y.; Schick, C. Dependence of crystal nucleation on prior liquid overheating by differential fast scanning calorimeter. J. Chem. Phys. 2014, 140, 104513. [Google Scholar] [CrossRef]
- Xu, W.; Sandor, M.T.; Yu, Y.; Ke, H.-B.; Zhang, H.P.; Li, M.-Z.; Wang, W.-H.; Liu, L.; Wu, Y. Evidence of liquid-liquid transition in glass-forming La50Al35Ni15 melt above liquidus temperature. Nat. Commun. 2015, 6, 7696. [Google Scholar] [CrossRef]
- Chen, E.-Y.; Peng, S.-X.; Michiel, M.D.; Vaughan, G.B.M.; Yu, Y.; Yu, H.-B.; Ruta, B.; Wei, S.; Liu, L. Glass-forming ability correlated with the liquid-liquid transition in Pd42.5Ni42.5P15 alloy. Scr. Mater. 2021, 193, 117–121. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Building and breaking bonds by homogenous nucleation in glass-forming melts leading to three liquid states. Materials 2021, 14, 2287. [Google Scholar] [CrossRef] [PubMed]
- Tournier, R.F.; Ojovan, M.I. Multiple melting temperatures in glass-forming melts. Sustainability 2022, 14, 2351. [Google Scholar] [CrossRef]
- Tournier, R.F. Liquid-liquid transitions due to melting temperatures of residual glassy phases expected in Pt57Cu23P20. Asp. Min. Miner. Sc. 2022, 8, 979–989. [Google Scholar]
- An, Q.; Johnson, W.L.; Samwer, K.; Corona, S.L.; Goddard, W.A., III. First-order transition in liquid Ag to the heterogeneous G-Phase. J. Phys. Chem. Lett. 2020, 11, 632–645. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Johnson, W.L.; Samwer, K.; Corona, S.L.; Goddard, W.A., III. Formation of two glass phases in binary Cu-Ag liquid. Acta Mater. 2020, 195, 274–281. [Google Scholar] [CrossRef]
- Tournier, R.F. Lindemann’s rule applied to the melting of crystals and ultra-stable glasses. Chem. Phys. Lett. 2016, 651, 198–202, Erratum in: 2017, 675, 174. [Google Scholar] [CrossRef][Green Version]
- Vopson, M.M.; Rugers, N.; Hepburn, I. The generalized Lindemann Melting coefficient. Sol. State Commun. 2020, 318, 113977. [Google Scholar] [CrossRef]
- Tournier, R.F. Validation of non-classical homogeneous nucleation model for G-glass and L-glass formations in liquid elements with recent molecular dynamics simulations. Scr. Mater. 2021, 199, 113859. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Prediction of second melting temperatures already observed in pure elements by molecular dynamics simulations. Materials 2021, 14, 6509. [Google Scholar] [CrossRef]
- Becker, S.; Devijver, E.; Molinier, R.; Jakse, N. Glass-forming ability of elemental zirconium. Phys. Rev. B 2020, 102, 104205. [Google Scholar] [CrossRef]
- Bazlov, A.I.; Louzguine-Luzguin, D.V. Crystallization of FCC and BCC liquid metals studied by molecular dynamics simulation. Metals 2020, 10, 1532. [Google Scholar]
- Ojovan, M.I. Ordering and structural changes at the glass-liquid transition. J. Non-Cryst. Sol. 2013, 382, 79. [Google Scholar] [CrossRef]
- Hasmy, A.; Ispas, S.; Hehlen, B. Percolation transitions in compressed SiO2 Glasses. Nature 2021, 599, 65. [Google Scholar] [CrossRef] [PubMed]
- Vinet, P.; Magnusson, V.; Frederikssen, H.; Desré, P.J. Correlations between surface and interface energies with respect to crystal nucleation. J. Colloid Interface Sci. 2002, 255, 363–374. [Google Scholar] [CrossRef]
- Tournier, R.F. Fragile-to-fragile liquid transition at Tg and stable-glass phase nucleation rate maximum at the Kauzmann temperature. Physica B 2014, 454, 253–271. [Google Scholar] [CrossRef]
- Tournier, R.F. First-order transitions in glasses and melts induced by solid superclusters nucleated by homogeneous nucleation instead of surface melting. Chem. Phys. 2019, 524, 40–54. [Google Scholar] [CrossRef]
- Jiang, Q.K.; Wang, X.D.; Nie, X.P.; Zhang, G.Q.; Ma, H.; Fecht, H.J.; Bendnarck, J.; Franz, H.; Liu, Y.G.; Cao, Q.P.; et al. Zr-(Cu,Ag)-Al bulk metallic glasses. Acta Mater. 2008, 56, 1785–1796. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, C.; Yue, Y. Thermodynamic anomaly of the sub-Tg relaxation in hyperquenched metallic glasses. J. Chem. Phys. 2013, 138, 174508. [Google Scholar] [CrossRef]
- Wang, L.-M.; Borick, S.; Angell, C.A. An electrospray technique for hyperquenched glass calorimetry studies: Propylene glycol and di-n-butylphthalate. J. Non-Cryst. Sol. 2007, 353, 3829–3837. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. The structural relaxation and glass transition of La-Al-Ni and Zr-Al-Cu amorphous alloys with a significant supercooled liquid region. J. Non-Cryst. Sol. 1992, 150, 396. [Google Scholar] [CrossRef]
- Hornboll, L.; Yue, Y. Enthalpy relaxation in hyperquenched glasses of different fragility. J. Non-Cryst. Sol. 2008, 354, 1832–1870. [Google Scholar] [CrossRef]
- Tournier, R.F. Presence of intrinsic growth nuclei in overheated and undercooled liquid elements. Physica B 2007, 392, 79–91. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Paik, J.S. Rapidly Solidified Amorphous and Crystalline Alloys; Kear, B.H., Gessen, B.G., Coehen, M., Eds.; Elsevier Science: New York, NY, USA, 1982; pp. 42–63. [Google Scholar]
- Tournier, R.F. Predicting glass-to-glass and liquid-to-liquid phase transitions in supercooled water using non-classical nucleation theory. Chem. Phys. 2018, 500, 45–53. [Google Scholar] [CrossRef]
- Tournier, R.F. Amorphous ices. In Encyclopedia of Glass Science, Technology, History, and Culture; Richet, P., Ed.; Wiley & Sons: Hoboken, NJ, USA, 2021; Volume 1, Chapter 3.14. [Google Scholar]
- Tournier, R.F. Homogeneous nucleation of phase transformations in supercooled water. Physica B 2020, 579, 411895. [Google Scholar] [CrossRef]
- Angell, C.A.; Rao, K.J. Configurational excitations in condensed matter and the “bond lattice”. Model for the liquid-glass transition. J. Chem. Phys. 1972, 57, 470–481. [Google Scholar] [CrossRef]
- Ozhovan, M.I. Topological characteristics of bonds in SiO2 and GeO2 oxide systems at glass-liquid transition. J. Exp. Theor. Phys. 2006, 103, 819–829. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Undercooled phase behind the glass phase with superheated medium-eange order above glass transition temperature. Physica B 2021, 602, 412542. [Google Scholar] [CrossRef]
- Iwashita, T.; Micholson, D.M.; Egami, T. Elementary excitations and crossover phenomenon in liquids. Phys. Rev. Lett. 2013, 110, 205504. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Connectivity and glass transition in disordered oxide systems. J. Non-Cryst. Sol. 2010, 356, 2534–2540. [Google Scholar] [CrossRef]
- Ojovan, M.I. The modified random network (MRN) model within the configuron theory percolation (CPT) of glass transition. Ceramics 2021, 4, 121–134. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Tournier, R.F. On structural rearrangements near the glass transition temperature in amorphous silica. Materials 2021, 14, 5235. [Google Scholar] [CrossRef] [PubMed]
- Ojovan, M.I. Glass formation. In Encyclopedia of Glass Science, Technology, History, and Culture; Conradt, P., Takada, R., Dyon, A., Richet, J., Eds.; Wiley: Hoboken, NJ, USA, 2021; Chapter 3.1; pp. 249–259. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Louzguine Luzgin, D.V. Revealing Structural Changes at Glass Transition via Radial Distribution Functions. J. Phys. Chem. 2020, 124, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Khvan, A.V.; Babkina, T.; Dinsdale, A.T.; Usprenskaya, I.A.; Farthushna, I.V.; Druzhinina, A.T.; Sysdikova, A.B.; Belov, M.P.; Belov, M.P.; Abrikosov, I.A. Thermodynamic properties of Tin: Part I: Experimental investigations, ab-initio modelling of alpha-, beta-phase and a thermodynamic description for pure metal in solid and liquid state from 0 K. Calphad 2019, 65, 50–72. [Google Scholar] [CrossRef]
- Kozyrev, N.V.; Gordeev, V.V. Thermodynamic characterization and equation of state for solid and liquid lead. Metals 2022, 12, 16. [Google Scholar] [CrossRef]
- Alchagirov, B.B.; Chochaeva, A. Temperature dependence of the density of liquid tin. High Temp. 2000, 38, 44–48. [Google Scholar] [CrossRef]
- Kamiya, A.; Terasaki, H.; Kondo, T. Precise determination of the effect of temperature on the density of solid and liquid iron; nickel, and tin. Am. Mineral. 2021, 106, 1077–1082. [Google Scholar] [CrossRef]
- Assael, M.J.; Armyra, I.J. Reference data for the density and viscosity of liquid cadmium, cobalt, gallium, indium, mercury, silicon, thallium, and zinc. J. Phys. Chem. 2012, 42, 033101. [Google Scholar] [CrossRef]
- Stankus, S.V.; Savchenko, I.V.; Yatsuk, O.S. The caloric properties of liquid bismuth. High Temp. 2018, 56, 33–37. [Google Scholar] [CrossRef]
- Froberg, M.C.; Weber, R. Dichtemessugen an eisen-kupfe legierungen. Prch. Eisenhuttenw. 1964, 35, 877–899. [Google Scholar]
- Gronvold, F. Enthalpy of fusion and temperature of fusion of indium, and redetermination of the enthalpy of fusion of tin. J. Chem. Therm. 1993, 25, 1133–1144. [Google Scholar] [CrossRef]
- Chen, H.S.; Turnbull, D. The specific heat of tin and gallium in their stable and undercooled pure liquid states. Acta Metall. 1968, 16, 369–373. [Google Scholar] [CrossRef]
- Bryant, C.A.; Keesom, P.H. Low temperature specific heat of indium and tin. Phys. Rev. 1961, 123, 491. [Google Scholar] [CrossRef]
- Badawi, W.A.; Brown-Acquaye, H.A.; Eid, A.E. Heat capacity and thermodynamic properties of bismuth in the range 333 to 923 K. Bull. Chem. Soc. Jpn. 1987, 60, 3765–3769. [Google Scholar] [CrossRef]
- Miller, R.R. Liquid Metal Handbook; OECD 2015 NEA N°7268; Nuclear Energy Agency: Paris, France, 1954. [Google Scholar]
- Ojovan, M.I. Viscosity and glass transition in amorphous oxides. Adv. Condens. Matter Phys. 2008, 2008, 817829. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Louzguine-Luzgin, D.V. On structural rearrangement during the vitrification of molten copper. Materials 2022, 15, 1313. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Zu, F.Q.; Guo, L.J.; Zhang, B. Internal friction method: Suitable also for structural changes of liquids. Mater. Sci. Eng. A. 2004, 370, 427–430. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tournier, R.F. Multiple Glass Transitions in Bismuth and Tin beyond Melting Temperatures. Metals 2022, 12, 2085. https://doi.org/10.3390/met12122085
Tournier RF. Multiple Glass Transitions in Bismuth and Tin beyond Melting Temperatures. Metals. 2022; 12(12):2085. https://doi.org/10.3390/met12122085
Chicago/Turabian StyleTournier, Robert F. 2022. "Multiple Glass Transitions in Bismuth and Tin beyond Melting Temperatures" Metals 12, no. 12: 2085. https://doi.org/10.3390/met12122085
APA StyleTournier, R. F. (2022). Multiple Glass Transitions in Bismuth and Tin beyond Melting Temperatures. Metals, 12(12), 2085. https://doi.org/10.3390/met12122085






