A Review on Phase Field Modeling for Formation of η-Cu6Sn5 Intermetallic
Abstract
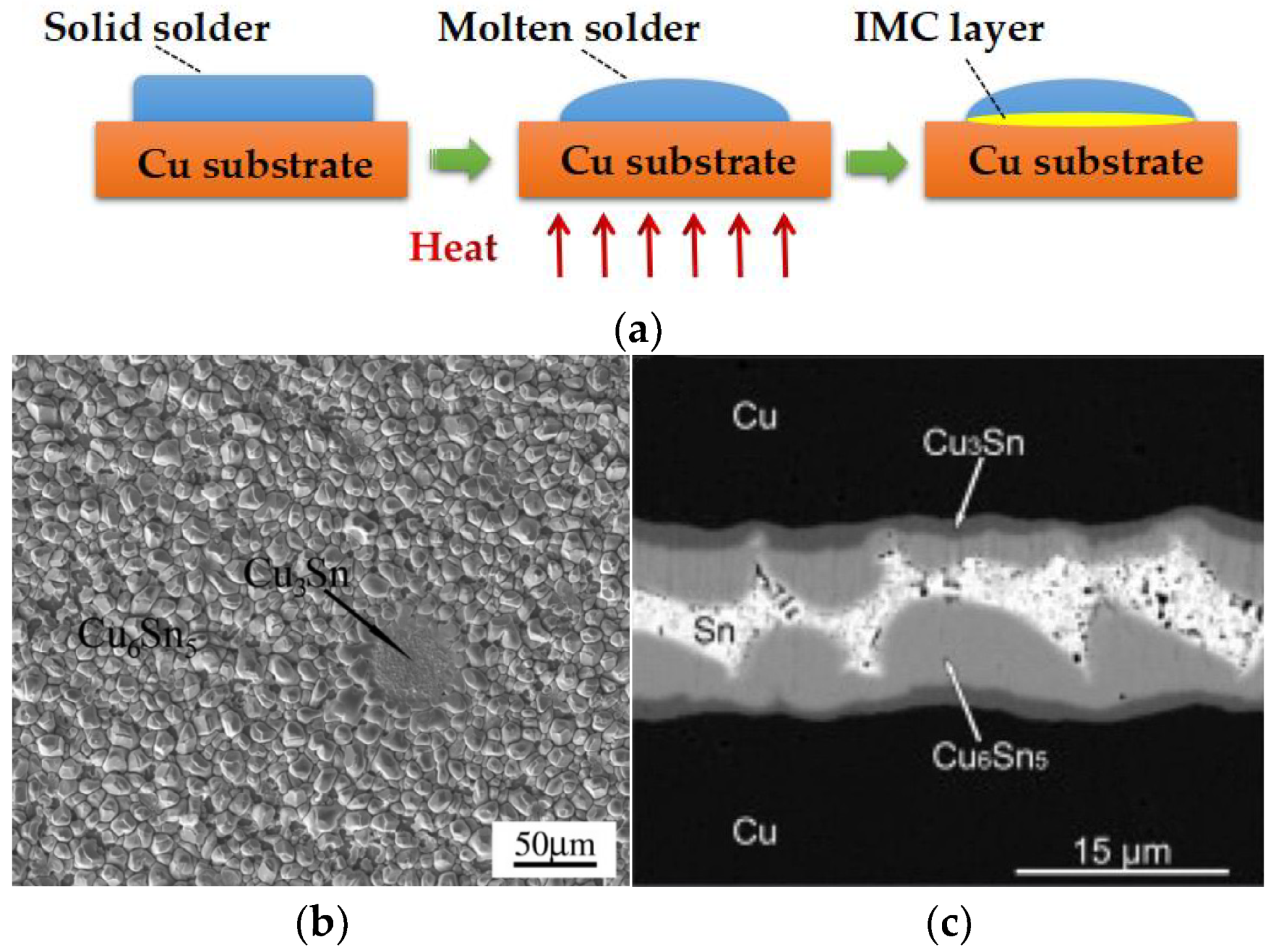
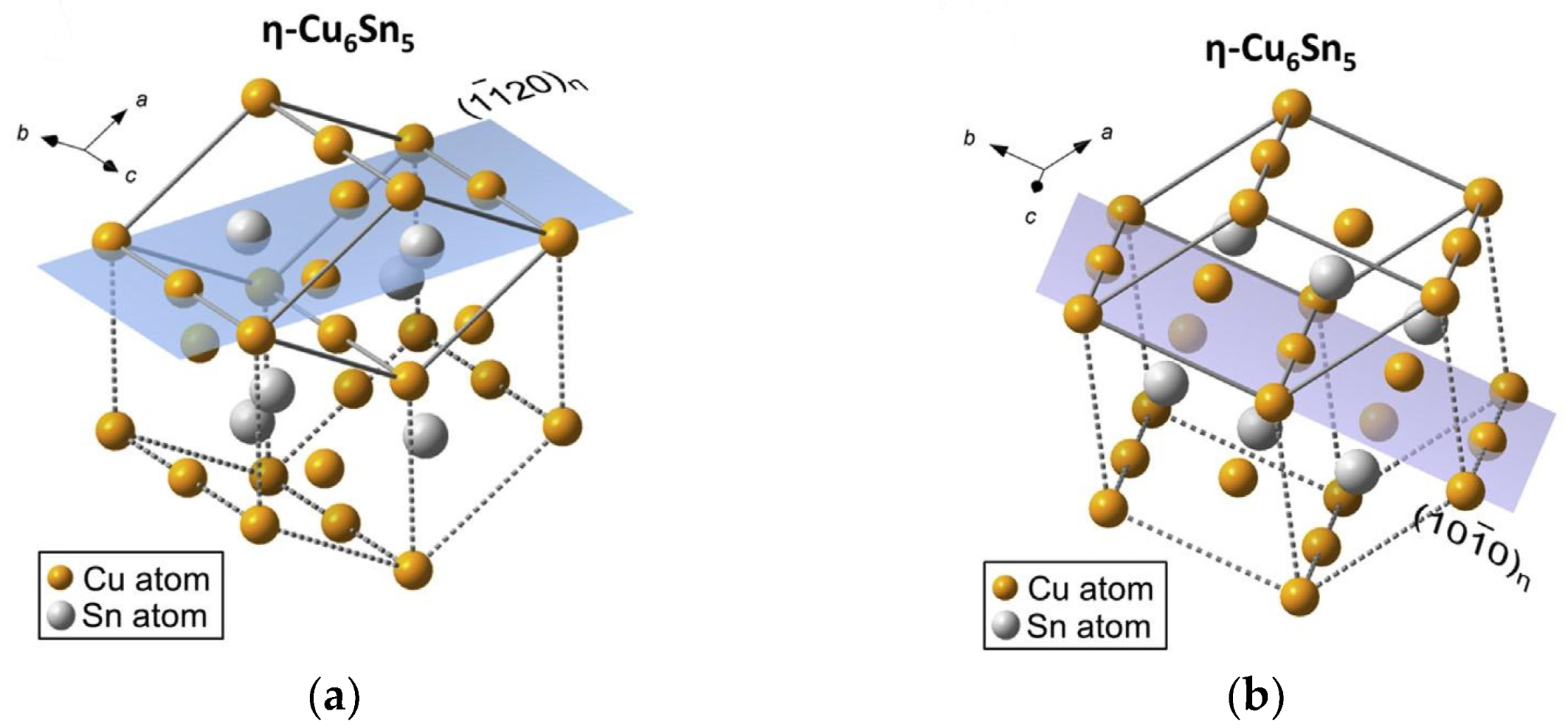
:1. Introduction
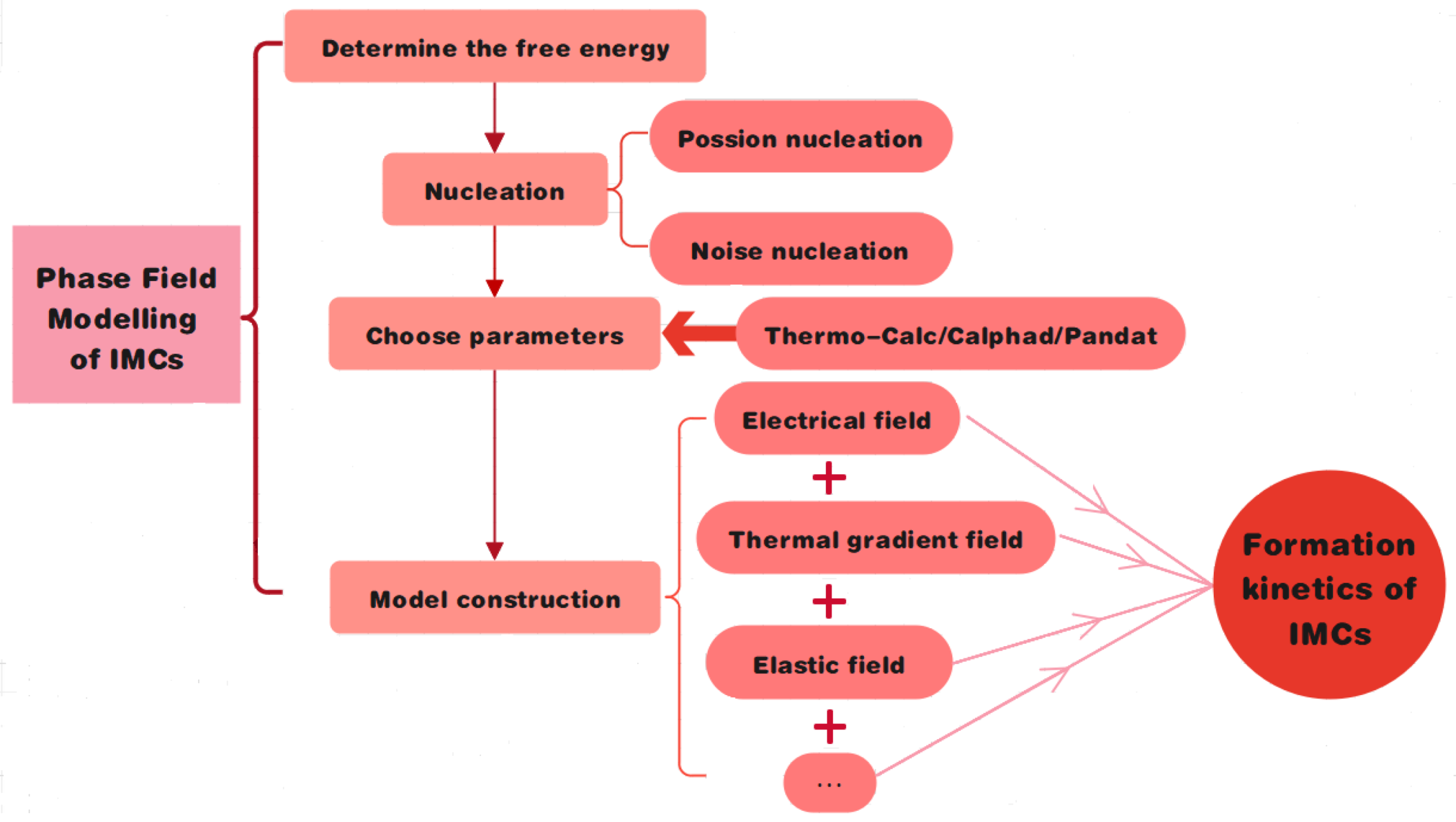
2. Phase Field Method for Formation and Growth of IMCs
2.1. Free Energy Curves of Cu(S), Sn(L) and IMCs
2.2. Thermodynamic Parameters
2.3. Phase Field Modeling
2.3.1. Nucleation of IMCs
2.3.2. Evolution Equations
3. Future Research Aspects
3.1. Interactions between IMCs and Micro-Voids
3.2. Couple Multiple Simulation Methods
3.3. Quantitative Prediction of Growth of IMCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dušek, K.; Bušek, D.; Veselý, P.; Pražanová, A.; Plaček, M.; Re, J.D. Understanding the effect of reflow profile on the metallurgical properties of tin–bismuth solders. Metals 2022, 12, 121. [Google Scholar] [CrossRef]
- Rahim, K.; Mian, A. A review on laser processing in electronic and MEMS packaging. J. Electron. Packag. 2017, 139, 030801. [Google Scholar] [CrossRef]
- Attari, V.; Ghosh, S.; Duong, T.; Arroyave, R. On the interfacial phase growth and vacancy evolution during accelerated electromigration in Cu/Sn/Cu microjoints. Acta. Mater. 2018, 160, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Ahn, B. Emerging interconnection technology and Pb-Free solder materials for advanced microelectronic packaging. Metals 2021, 11, 1941. [Google Scholar] [CrossRef]
- Wieser, C.; Walnsch, A.; Hügel, W.; Leineweber, A. The monoclinic lattice distortion of η′-Cu6Sn5. J. Alloy. Compd. 2019, 794, 491–500. [Google Scholar] [CrossRef]
- Roy, A.; Luktuke, A.; Chawla, N.; Ankit, K. Predicting the Cu6Sn5 growth kinetics during thermal aging of Cu-Sn solder joints using simplistic kinetic modeling. J. Electron. Mater. 2022, 51, 4063–4072. [Google Scholar] [CrossRef]
- Kunwar, A.; Hektor, J.; Nomoto, S.; Coutinho, Y.A.; Moelans, N. Combining multi-phase field simulation with neural network analysis to unravel thermomigration accelerated growth behavior of Cu6Sn5 IMC at cold side Cu–Sn interface. Int. J. Mech. Sci. 2020, 184, 105843. [Google Scholar] [CrossRef]
- Liang, S.B.; Ke, C.B.; Huang, J.Q.; Zhou, M.B.; Zhang, X.P. Phase field simulation of microstructural evolution and thermomigration-induced phase segregation in Cu/Sn58Bi/Cu interconnects under isothermal aging and temperature gradient. Microelectron. Reliab. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Kunwar, A.; Coutinho, Y.A.; Hektor, J.; Ma, H.; Moelans, N. Integration of machine learning with phase field method to model the electromigration induced Cu6Sn5 IMC growth at anode side Cu/Sn interface. J. Mater. Sci. Technol. 2020, 59, 203–219. [Google Scholar] [CrossRef]
- Li, Y.; Fu, G.; Wan, B.; Yan, X.; Zhang, W.; Li, W. Phase-field modelling of lead-free solder joint void growth under thermal-electrical coupled stress. J. Electron. Mater. 2022, 51, 259–272. [Google Scholar] [CrossRef]
- Lee, L.M.; Mohamad, A.A. Interfacial reaction of Sn-Ag-Cu lead-free solder alloy on Cu: A review. Adv. Mater. Sci. Eng. 2013, 2013, 123697. [Google Scholar] [CrossRef] [Green Version]
- Zu, Z.; Chen, D.; Zhang, X.; Bai, H.L.; Leng, C.Y.; Gan, G.Y.; Yan, J.K. On the initial stages and growth process of intermetallic compounds at Cu/Sn interface: A MD simulation and experimental study. Comp. Mater. Sci. 2022, 208, 111349. [Google Scholar] [CrossRef]
- Zou, H.F.; Yang, H.J.; Zhang, Z.F. Morphologies, orientation relationships and evolution of Cu6Sn5 grains formed between molten Sn and Cu single crystals. Acta. Mater. 2008, 56, 2649–2662. [Google Scholar] [CrossRef]
- Yang, A.; Duan, Y.; Li, C.; Yi, J.; Peng, M. Theoretical explorations of structure, mechanical properties, fracture toughness, electronic properties, and thermal conductivity of Ag-doped η′-Cu6Sn5. Intermetallics 2022, 141, 107437. [Google Scholar] [CrossRef]
- Bernal, J.D. The Complex Structure of the Copper–Tin Intermetallic Compounds. Nature 1928, 122, 54. [Google Scholar] [CrossRef]
- Ke, J.H.; Gao, Y.; Kao, C.R.; Wang, Y. Pattern formation during interfacial reaction in-between liquid Sn and Cu substrates—A simulation study. Acta. Mater. 2016, 113, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; An, R.; Ma, H.; Wang, C.Q. Low-temperature-solderable intermetallic nanoparticles for 3D printable flexible electronics. Acta. Mater. 2019, 162, 163–175. [Google Scholar] [CrossRef]
- Shang, P.J.; Liu, Z.Q.; Pang, X.Y.; Li, D.X.; Shang, J.K. Growth mechanisms of Cu3Sn on polycrystalline and single crystalline Cu substrates. Acta. Mater. 2009, 57, 4697–4706. [Google Scholar] [CrossRef]
- Liu, B.; Tian, Y.; Wang, C.; An, R.; Wang, C.Q. Ultrafast formation of unidirectional and reliable Cu3Sn-based intermetallic joints assisted by electric current. Intermetallics 2017, 80, 26–32. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, S.; Li, Z.; Hou, C.; Li, G. Morphological Evolution and Growth Kinetics of Interfacial Cu6Sn5 and Cu3Sn Layers in Low-Ag Sn-0.3Ag-0.7Cu-xMn/Cu Solder Joints During Isothermal Ageing. J. Electron. Mater. 2018, 47, 5913–5929. [Google Scholar] [CrossRef]
- Hektor, J.; Ristinmaa, M.; Hallberg, H.; Hall, S.A.; Iyengar, S. Coupled diffusion-deformation multiphase field model for elastoplastic materials applied to the growth of Cu6Sn5. Acta. Mater. 2016, 108, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Durga, A.; Wollants, P.; Moelans, N. Phase-field study of IMC growth in Sn-Cu/Cu solder joints including elastoplastic effects. Acta. Mater. 2020, 188, 241–258. [Google Scholar] [CrossRef]
- Park, M.S.; Arróyave, R. Concurrent nucleation, formation and growth of two intermetallic compounds (Cu6Sn5 and Cu3Sn) during the early stages of lead-free soldering. Acta. Mater. 2012, 60, 923–934. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.X.; Zhang, J.H.; Wang, G.; Yang, M.; Wang, H.; Xu, D.S. Phase field modeling of formation mechanism of grain boundary allotriomorph in β→α phase transformation in Ti-6Al-4V alloy. Acta. Metall. Sin. 2020, 56, 1113–1122. [Google Scholar]
- Sun, J.; Zhang, Y.T.; Wang, P.; Ye, Z.F.; Li, D.Z. Effect of N on the microstructure and mechanical properties of high Si martensitic heat-resistant steels. Acta. Metall. Sin. (Engl. Lett.) 2014, 27, 573–584. [Google Scholar] [CrossRef]
- Dinsdale, A.T. Sgte data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Moon, K.-W.; Boettinger, W.J.; Kattner, U.R.; Biancaniello, F.S.; Handwerker, C.A. Experimental and thermodynamic assessment of Sn-Ag-Cu solder alloys. J. Electron. Mater. 2000, 29, 1122–1136. [Google Scholar] [CrossRef]
- Simmons, J.P.; Shen, C.; Wang, Y. Phase field modeling of simultaneous nucleation and growth by explicitly incorprating nucleation events. Scripta. Mater. 2000, 43, 935–942. [Google Scholar] [CrossRef]
- Qi-Sen, H.; Li, L.; Xiu-Xun, W.; Jin-Fu, L. Solidification behaviors of undercooled Ni-P alloys. Acta. Phys. Sin. 2012, 61, 166401–166407. [Google Scholar] [CrossRef]
- Park, M.S.; Arroyave, R. Formation and growth of intermetallic compound Cu6Sn5 at early stages in lead-free soldering. J. Electron. Mater. 2010, 39, 2574–2582. [Google Scholar] [CrossRef] [Green Version]
- Najafi, A. The fluctuation-dissipation theorem of colloidal particle’s energy on 2D periodic substrates: A monte carlo study of thermal noise-like fluctuation and diffusion like Brownian motion. J. Phys. Conf. Ser. 2014, 510, 012025. [Google Scholar] [CrossRef]
- Steinbach, I.; Pezzolla, F.; Nestler, B.; Sedklberg, M.; Ptieler, R.; Schmitz, G.J.; Rezende, J.L.L. A phase field concept for multiphase systems. Phys. D 1996, 94, 135–147. [Google Scholar] [CrossRef]
- Steinbach, I.; Pezzolla, F. A generalized field method for multiphase transformations using interface fields. Phys. D 1999, 134, 385–393. [Google Scholar] [CrossRef]
- Xiong, H.; Huang, Z.; Conway, P. Effects of Stress and Electromigration on Microstructural Evolution in Microbumps of Three-Dimensional Integrated Circuits. IEEE Trans. Device Mater. Reliab. 2014, 14, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Z.; Li, J. Phase field modeling of defects and deformation. Acta. Mater. 2010, 58, 1212–1235. [Google Scholar] [CrossRef]
- Liang, S.B.; Ke, C.B.; Zhou, M.B.; Zhang, X.P. Coupled Phase Field and Finite Element Modeling of Void Evolution and Physical Property Change of Micro Flip-Chip Solder Joints under Electromigration and Elastic Stress Field. In Proceedings of the 18th International Conference on Electronic Packaging Technology, Harbin, China, 16–19 August 2017; p. 1631. [Google Scholar]
- Liang, S.B.; Ke, C.B.; Ma, W.J.; Zhou, M.B.; Zhang, X.P. Numerical simulations of migration and coalescence behavior of microvoids driven by diffusion and electric field in solder interconnects. Microelectron. Reliab. 2017, 71, 71–82. [Google Scholar] [CrossRef]
- Sun, J.; Qi, M.; Zhang, J.H.; Li, X.X.; Wang, H.; Ma, Y.J.; Xu, D.S.; Lei, J.F.; Yang, R. Formation mechanism of α lamellae during β→α transformation in polycrystalline dual-phase Ti alloys. J. Mater. Sci. Technol. 2021, 71, 98–108. [Google Scholar] [CrossRef]
- Moelans, N. A quantitative and thermodynamically consistent phase-field interpolation function for multi-phase systems. Acta. Mater. 2011, 59, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Moelans, N.; Blanpain, B.; Wollants, P. An introduction to phase-field modeling of microstructure evolution. Calphad 2008, 32, 268–294. [Google Scholar] [CrossRef]
- Moelans, N.; Godfrey, A.; Zhang, Y.; Juul, J.D. Phase-field simulation study of the migration of recrystallization boundaries. Phys. Rev. B 2013, 88, 054103. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Gibbons, S.L.; Arróyave, R. Phase-field simulations of intermetallic compound evolution in Cu/Sn solder joints under electromigration. Acta. Mater. 2013, 61, 7142–7154. [Google Scholar] [CrossRef]
- Liu, G.P.; Zhang, J.Y.; Lei, M.; Li, Y.L.; Li, X.W. A comparative study of the diffuse-interface model and sharp-interface model in the soldering related wetting spreading systems. Metals 2019, 9, 944. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Gibbons, S.L.; Arróyave, R. Computational investigation of the evolution of intermetallic compounds affected by microvoids during the solid-state aging process in the Cu-Sn system. J. Electron. Mater. 2013, 42, 999–1009. [Google Scholar] [CrossRef]
- Sun, J.; Liang, H.X.; Sun, S.F.; Hu, J.T.; Teng, C.Y.; Zhao, L.Y.; Bai, H.L. Pattern formation by spinodal decomposition in ternary lead-free Sn-Ag-Cu solder alloy. Metals 2022, 12, 1640. [Google Scholar] [CrossRef]
- Koleňák, R.; Augustin, R.; Martinkovič, M.; Chachula, M. Comparison study of SAC405 and SAC405+0.1%Al lead free solders. Solder. Surf. Mt. Technol. 2013, 25, 175–183. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Guo, Q.; Li, G. A diffusion model and growth kinetics of interfacial intermetallic compounds in Sn-0.3Ag-0.7Cu and Sn-0.3Ag-0.7Cu-0.5CeO2 solder joints. J. Alloy. Compd. 2020, 818, 152893. [Google Scholar] [CrossRef]


| Phases | Temperature Range | Free Energy |
|---|---|---|
| Sn (Pure) | ||
| Cu (Pure) | ||
| Sn (Liquid) | ||
| Sn * (F.C.C.) | ||
| Sn (B.C.C.) | ||
| Sn (H.C.P.) | ||
| Cu (Liquid) | ||
| Cu (B.C.C.) | ||
| Cu (B.C.T.) | ||
| Cu6Sn5 | -- | |
| Cu3Sn | -- |
| Phases | Thermodynamic Parameters |
|---|---|
| Liquid | |
| F.C.C | |
| B.C. T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhao, L.; Liang, H.; Zhang, Y.; Li, X.; Teng, C.; Wang, H.; Bai, H. A Review on Phase Field Modeling for Formation of η-Cu6Sn5 Intermetallic. Metals 2022, 12, 2043. https://doi.org/10.3390/met12122043
Sun J, Zhao L, Liang H, Zhang Y, Li X, Teng C, Wang H, Bai H. A Review on Phase Field Modeling for Formation of η-Cu6Sn5 Intermetallic. Metals. 2022; 12(12):2043. https://doi.org/10.3390/met12122043
Chicago/Turabian StyleSun, Jia, Lingyan Zhao, Huaxin Liang, Yao Zhang, Xuexiong Li, Chunyu Teng, Hao Wang, and Hailong Bai. 2022. "A Review on Phase Field Modeling for Formation of η-Cu6Sn5 Intermetallic" Metals 12, no. 12: 2043. https://doi.org/10.3390/met12122043





