Microstructural and Interfacial Characterization of Ti–V Diffusion Bonding Zones
Abstract
:1. Introduction
2. Materials and Methods
+ a3/(1 + exp (−(X − b3)/c3)),
3. Results
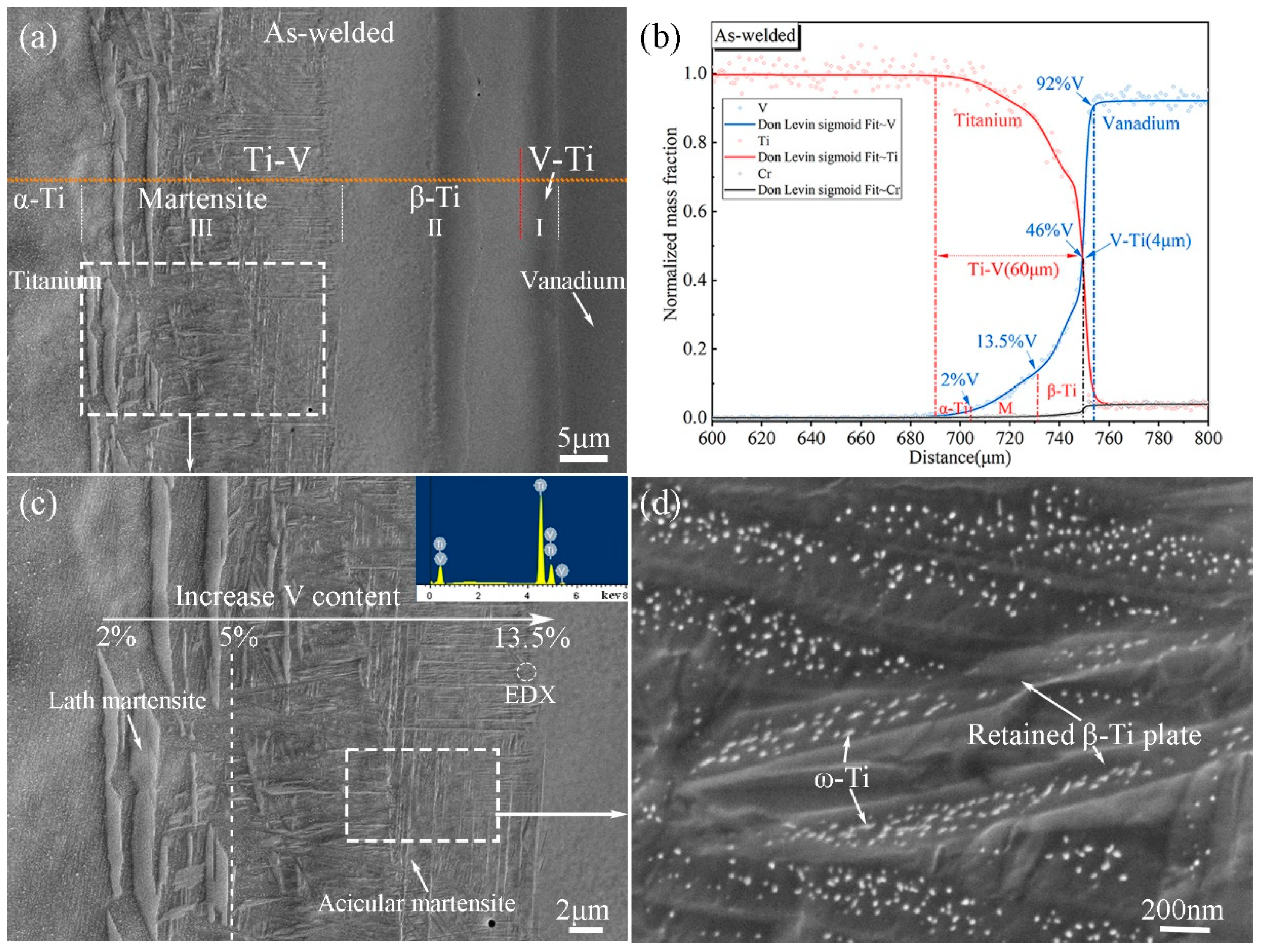
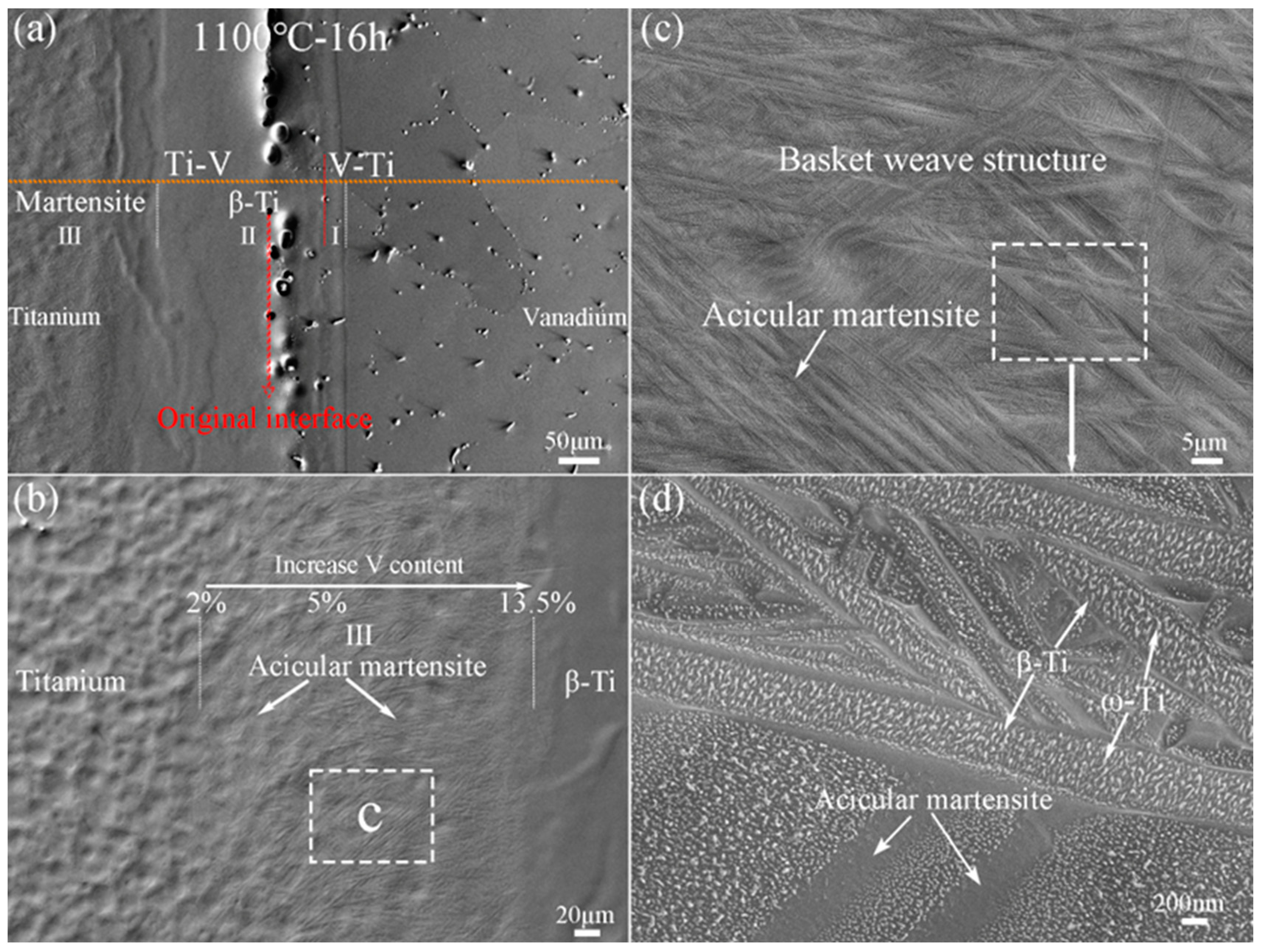
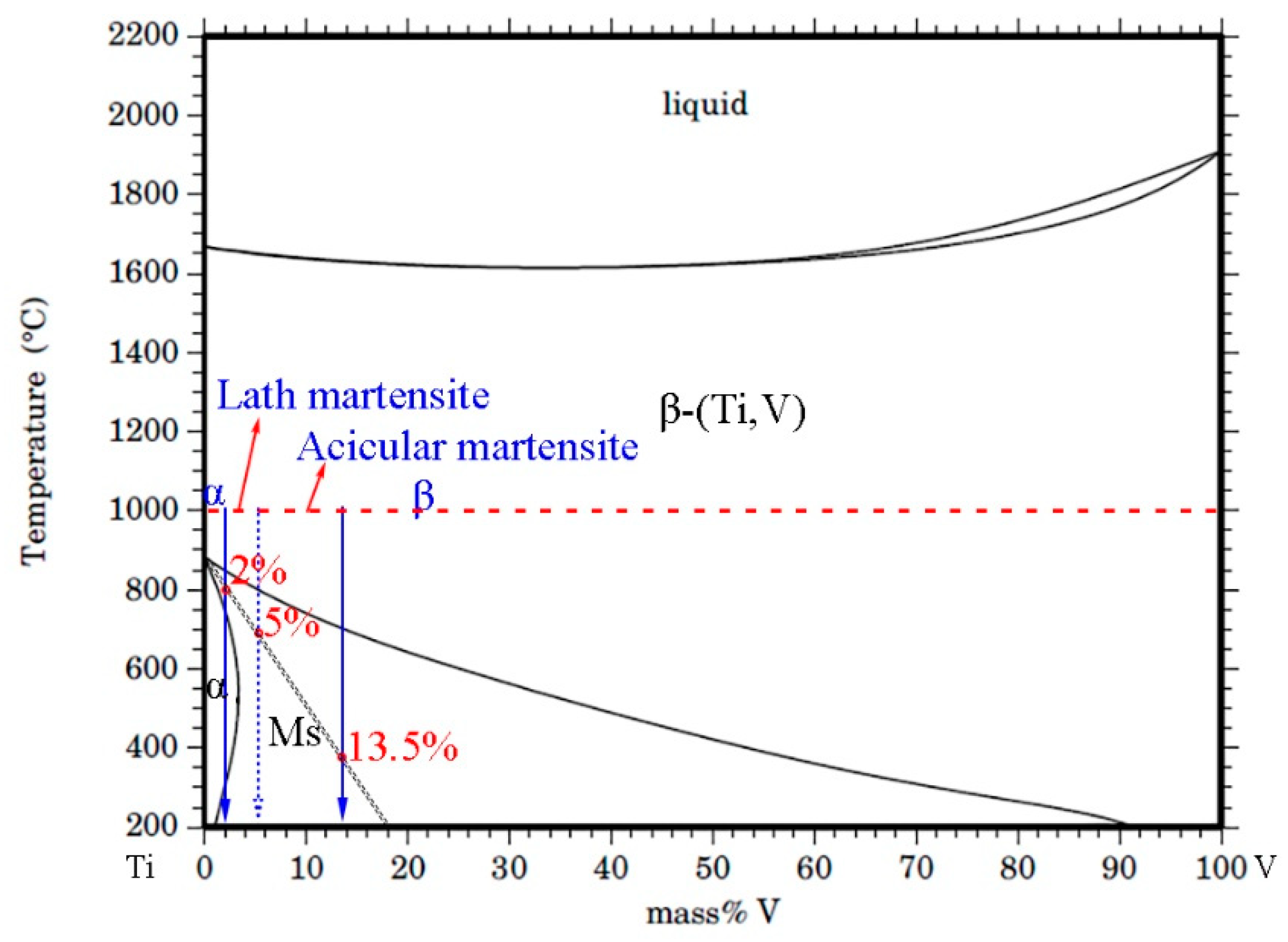
3.1. Microstructural Evolution of Ti–V Diffusion Interface Zones Subjected to High-Temperature Heat Treatment
3.2. Interface Characteristics and Recrystallization of Ti–V Diffusion Interface Zones
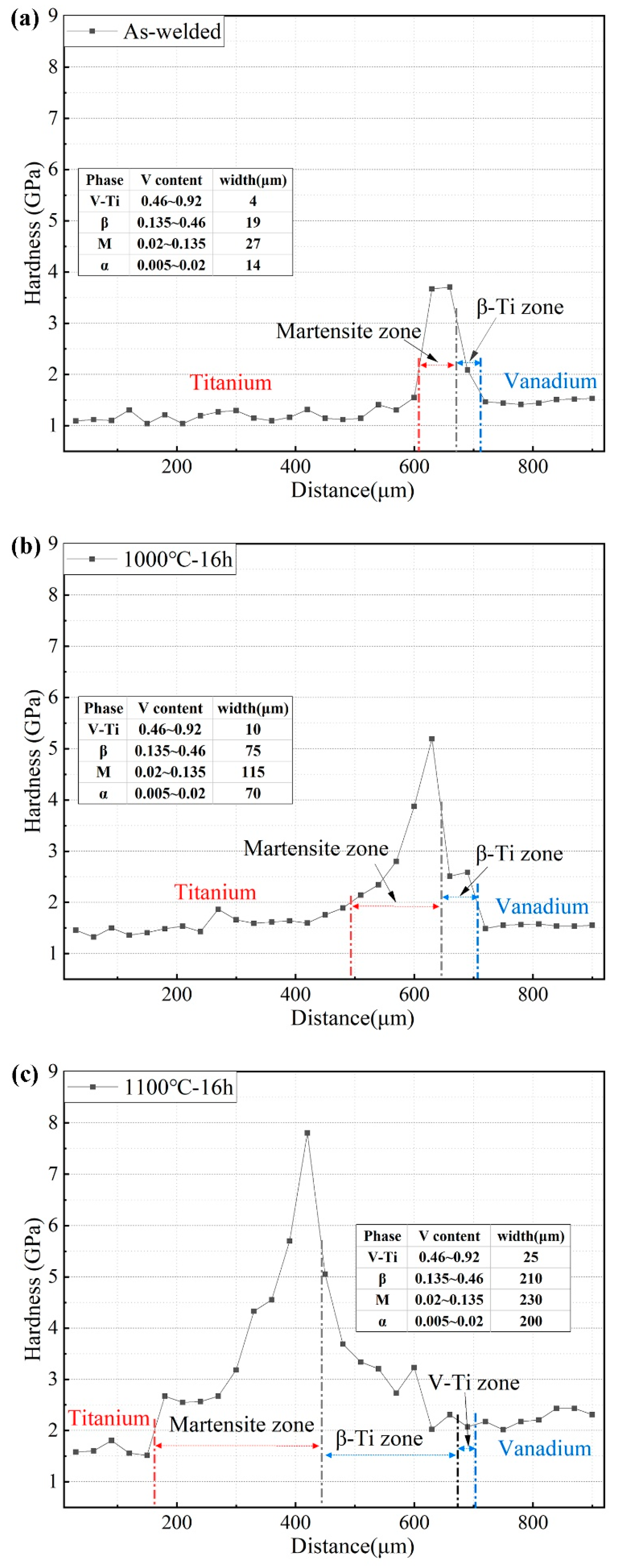
3.3. Mechanical Response to the Microstructural Evolution of Ti–V Diffusion Interface Zones
4. Discussion
5. Conclusions
- (1)
- Ti–V interface bonding zones consist of a martensite Ti (α′-Ti) zone, a BCC structure Ti (β-Ti) zone, and a relatively small V–Ti alloy zone. They are divided by two composition interfaces (V ~13.5% and ~46%) and distinguished by contrasting EBSD data. The original interface falls within the β-Ti zone, as revealed by inert Y2O3 markers in the original V alloy substrate.
- (2)
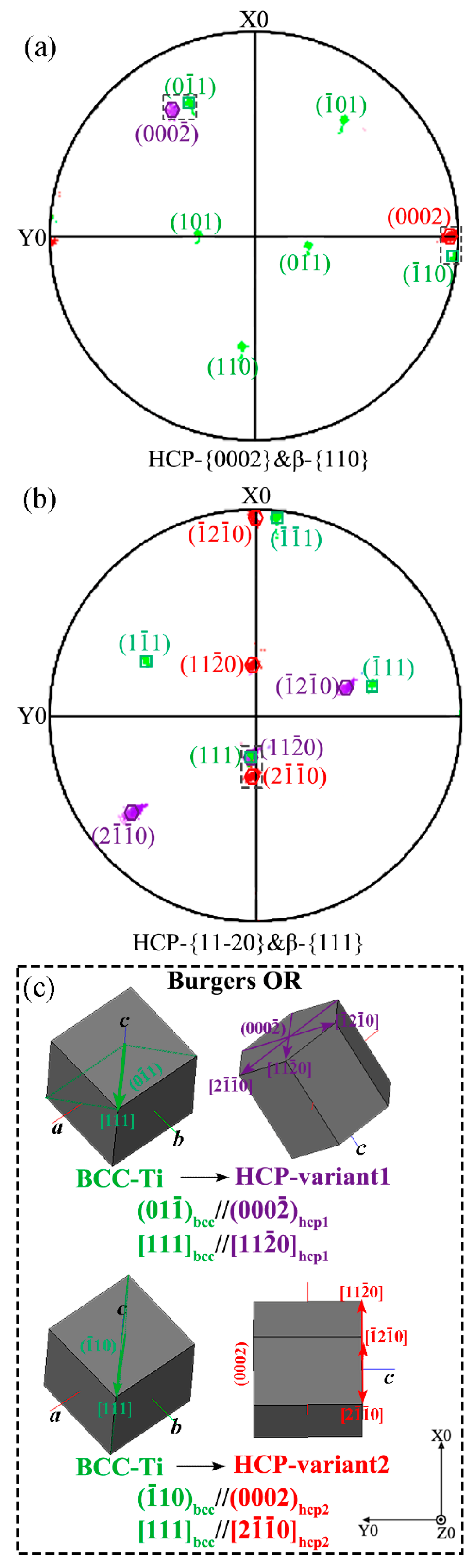
- The Ti–V interface bonding zones exhibit two key microstructural evolution processes during rapid cooling. The first is the β→α′ martensite transformation, which is affected by accumulated strain energy generated by β-to-α phase transformation in the adjacent pure Ti. The ORs between martensite variants and β-Ti conform to Burger’s orientations relationship. The second is the recrystallization of β-Ti, which is affected by these two successive phase transformations. This is apparent from the appearance of deformed and recrystallized grains along the boundaries of the β-Ti grains.
- (3)
- The hardness varies across the Ti–V interface bonding zones from pure Ti to V alloy. With increasing V content, the hardness of the martensite zone increases due to the increasing volume fraction of the hard ω-Ti phase. Upon further increasing the V content, the mechanical stability of the β-Ti increases and depresses stress-induced martensite transformation, which leads to decreased hardness in the β-Ti zone.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Knowles, J.; Choi, S.; Kang, H.; Park, S.; Park, S.M.; Kim, H.; Park, J.T.; Lee, J.; et al. Mechanophysical and biological properties of a3D-printed Titanium alloy for dental applications. Dental Mater. 2020, 36, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2003; pp. 333–350. [Google Scholar]
- Ding, J.; Yang, S.; Zhu, B.; Liu, H.; Liu, G.; Zhou, L.; Zhan, Q.; Wan, F. Twins induced by high-temperature ion irradiation in body-centered cubic V-4Cr-4Ti alloy. Scr. Mater. 2019, 162, 377–381. [Google Scholar] [CrossRef]
- Ding, J.; Yang, S.; Liu, G.; Li, Q.; Zhu, B.; Zhang, M.; Zhou, L.; Shang, C.; Zhan, Q.; Wan, F. Recrystallization nucleation in V-4Cr-4Ti alloy. J. Alloys Compd. 2019, 777, 663–672. [Google Scholar] [CrossRef]
- Jiang, N.; Zhou, F.; Zhang, G.; Yi, X.; Yu, C.; Wang, X.; Rao, W. Recent progress of vanadium-based alloys for fusion application. Tungsten 2021, 3, 382–392. [Google Scholar] [CrossRef]
- Zhang, G.; Han, W.; Yi, X.; Wan, F. Interface Characteristics of Ti-Clad V–4Cr–4Ti Alloy Diffusion-Bonded Joint Produced by Hot Forging. Appl. Sci. 2018, 8, 577. [Google Scholar] [CrossRef] [Green Version]
- Tomashchuk, I.; Grevey, D.; Sallamand, P. Dissimilar laser welding of AISI 316L stainless steel to Ti6–Al4–6V alloy via pure vanadium interlayer. Mater. Sci. Eng. A 2015, 622, 37–45. [Google Scholar] [CrossRef]
- Bobbio, L.; Bocklund, B.; Otis, R.; Borgonia, J.; Dillon, R.; Shapiro, A.; McEnerney, B.; Liu, Z.; Beese, A. Characterization of a functionally graded material of Ti-6Al-4V to 304L stainless steel with an intermediate V section. J. Alloys Compd. 2018, 742, 1031–1036. [Google Scholar] [CrossRef]
- Chen, J.; Muroga, T.; Qiu, S.; Nagasaka, T.; Huang, W.; Tu, M.; Chen, Y.; Xu, Y.; Xu, Z. The development of advanced vanadium alloys for fusion applications. J. Nucl. Mater. 2004, 329–333, 401–405. [Google Scholar] [CrossRef]
- Nagasaka, T.; Muroga, T.; Fukumoto, K.; Watanabe, H.; Grossbeck, M.; Chen, J. Development of fabrication technology for low activation vanadium alloys as fusion blanket structural materials. Nucl. Fusion 2006, 46, 618. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, M.; Li, J.; Yan, C.; Qin, Z. Influence of vanadium filler on the properties of Titanium and steel TIG welded joints. J. Mater. Process. Technol. 2017, 240, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhang, Y.; Geng, D.; Zhang, G.; Hideo, W.; Han, W.; Wan, F. Characterization of interface irradiation damage in Ti-clad V-4Cr-4Ti composite material. Acta Phys. Sin. 2019, 68, 216101. [Google Scholar] [CrossRef]
- Banerjee, R.; Collins, P.; Bhattacharyya, D.; Banerjee, S.; Fraser, H. Microstructural evolution in laser deposited compositionally graded α/β titanium-vanadium alloys. Acta Mater. 2003, 51, 3277–3292. [Google Scholar] [CrossRef]
- Murray, J.L. The Ti–V (titanium-vanadium) system. Bull. Alloy. Phase Diagr. 1981, 2, 48–55. [Google Scholar] [CrossRef]
- Aurelio, G.; Guillermet, A.; Cuello, G.; Campo, J. Metastable Phases in the Ti-V System: Part I. Neutron Diffraction Study and Assessment of Structural Properties. Metall. Mater. Trans. A 2002, 33, 1307–1317. [Google Scholar] [CrossRef]
- Sluiter, M.; Turchi, P. Phase stability in Ti-V and Ti-Cr alloys: A theoretical investigation. Phys. Rev. B 1991, 43, 12251–12266. [Google Scholar] [CrossRef]
- Al Hazaa, A.; Haneklaus, N. Diffusion Bonding and Transient Liquid Phase (TLP) Bonding of Type 304 and 316 Austenitic Stainless Steel—A Review of Similar and Dissimilar Material Joints. Metals 2020, 10, 613. [Google Scholar] [CrossRef]
- Zoukel, A.; Khouchaf, L. The Secondary X-Ray Fluorescence and Absorption near the Interface of Multi-Material: Case of EDS Microanalysis. Micron 2014, 67, 81–89. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukhopadhyay, P. Phase Transformations, Examples from Titanium and Zirconium Alloys; Elsevier: Oxford, UK; Britain, UK, 2007; p. 7. [Google Scholar]
- Oka, M.; Lee, C.; Shimizu, K. Transmission electron microscopy study of face-centered orthorhombic Martensite in Ti-12.6 Pct V alloy. Metall. Mater. Trans. B 1972, 3, 37–45. [Google Scholar] [CrossRef]
- Pelleg, J. Diffusion of 51Cr in V. Philos. Mag. A 1995, 71, 431–439. [Google Scholar] [CrossRef]
- Lutjering, G.; Williams, J.C. Titanium, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 29. [Google Scholar]
- Ghosh, C.; Basu, J.; Ramachandran, D.; Mohandas, E. Phase separation and ω transformation in binary V-Ti and ternary V-Ti-Cr alloys. Acta Mater. 2016, 121, 310–324. [Google Scholar] [CrossRef]
- Humphreys, F.J. Characterisation of fine-scale microstructures by electron backscatter diffraction (EBSD). Scr. Mater. 2004, 51, 771–776. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, S.; Chen, Y.; Xu, L.; Wang, X.; Zhang, D.; Li, M. Effects of nano-Y2O3 addition on the microstructure evolution and tensile properties of a near-α titanium alloy. Mater. Sci. Eng. A 2019, 761, 137977. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Viswanathan, G.B.; Fraser, H. Crystallographic and morphological relationships between β phase and the Widmanstatten and allotriomorphic α phase at special β grain boundaries in an α/β titanium alloy. Acta Mater. 2007, 55, 6765–6778. [Google Scholar] [CrossRef]
- Motyka, M. Martensite formation and decomposition during traditional and AM processing of two-phase titanium alloys—An overview. Metals 2021, 11, 481. [Google Scholar] [CrossRef]
- Cai, M.; Lee, C.; Lee, Y. Effect of grain size on tensile properties of fine-grained metastable β titanium alloys fabricated by stress-induced martensite and its reverse transformations. Scr. Mater. 2012, 66, 606–609. [Google Scholar] [CrossRef]
- Murdock, J.; Lundy, T.; Stansbury, E. Diffusion of Ti44 and V48 in titanium. Acta Metall. 1964, 12, 1033–1039. [Google Scholar] [CrossRef]
- Murdock, J.; McHargue, C.J. Self-diffusion in body-centered cubic titanium-vanadium alloys. Acta Metall. 1968, 16, 493–500. [Google Scholar] [CrossRef]
- Franke, P.; Seifert, H.J. Ternary Steel Systems: Phase Diagrams and Phase Transition Data, Binary System Ti–V. In Landolt-Börnstein—Group IV Physical Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 19C1. [Google Scholar]
- Neelakantan, S.; Rivera-Díaz-del-Castillo, P.; Zwaag, V. Prediction of the martensite start temperature for β titanium alloys as a function of composition. Scr. Mater. 2009, 60, 611–614. [Google Scholar] [CrossRef]
- Collins, P.; Banerjee, R.; Banerjee, S. Laser deposition of compositionally graded titanium–vanadium and titanium–molybdenum alloys. Mat. Sci. Eng. A 2003, 352, 118–128. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Kim, J.; Chan, H.; Hong, J.; Yeom, J. Effect of prior β grain size on the martensitic transformation of titanium alloys. Mater. Charact. 2021, 182, 11525. [Google Scholar] [CrossRef]
- Liu, G.; Yang, S.; Han, W.; Yan, P.; Wang, M.; Hu, Q.; Misra, R.D.K.; Shang, C.; Wan, F. The simultaneous occurrence of Kurdjumov-Sachs and irrational orientation relationships between delta-ferrite and austenite phase in a 17Cr–5Ni stainless steel. Mater. Sci. Eng. A 2020, 798, 114112. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C.; Morsdorf, L. Predicting the Ms temperature of steels with a thermodynamic based model including the effect of the prior austenite grain size. Acta Mater. 2017, 125, 401–415. [Google Scholar] [CrossRef]
- Massalski, T.B. Massive transformations revisited. Metall. Trans. A 1984, 15, 421–425. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Yu, L.; Liu, C.; Sui, G.; Yang, J. Uniaxial diffusion bonding of CLAM/CLAM steels: Microstructure and mechanical performance. J. Nucl. Mater. 2015, 461, 301–307. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.-L.; Ding, M.-L.; Zhang, K.; Qu, D.-D.; Meng, Y.; Luo, G.-X.; Yang, S.-W. Microstructural and Interfacial Characterization of Ti–V Diffusion Bonding Zones. Metals 2022, 12, 2032. https://doi.org/10.3390/met12122032
Liu G-L, Ding M-L, Zhang K, Qu D-D, Meng Y, Luo G-X, Yang S-W. Microstructural and Interfacial Characterization of Ti–V Diffusion Bonding Zones. Metals. 2022; 12(12):2032. https://doi.org/10.3390/met12122032
Chicago/Turabian StyleLiu, Guo-Liang, Mei-Li Ding, Kun Zhang, Dan-Dan Qu, Yang Meng, Geng-Xing Luo, and Shan-Wu Yang. 2022. "Microstructural and Interfacial Characterization of Ti–V Diffusion Bonding Zones" Metals 12, no. 12: 2032. https://doi.org/10.3390/met12122032
APA StyleLiu, G.-L., Ding, M.-L., Zhang, K., Qu, D.-D., Meng, Y., Luo, G.-X., & Yang, S.-W. (2022). Microstructural and Interfacial Characterization of Ti–V Diffusion Bonding Zones. Metals, 12(12), 2032. https://doi.org/10.3390/met12122032





